Abstract
Background:
The burden of respiratory syncytial virus (RSV)-associated acute respiratory illnesses among healthy infants (<1 year) in the inpatient setting is well established. The focus on RSV-associated illnesses in the outpatient (OP) and emergency department (ED) settings are however understudied. We sought to determine the spectrum of RSV illnesses in infants at three distinct healthcare settings.
Methods:
From 16 December 2019 through 30 April 2020, we performed an active, prospective RSV surveillance study among infants seeking medical attention from an inpatient (IP), ED, or OP clinic. Infants were eligible if they presented with fever and/or respiratory symptoms. Demographics, clinical characteristics, and illness histories were collected during parental/guardian interviews, followed by a medical chart review and illness follow-up surveys. Research nasal swabs were collected and tested for respiratory pathogens for all enrolled infants.
Results:
Of the 627 infants screened, 475 were confirmed eligible; 360 were enrolled and research tested. Within this final cohort, 101 (28%) were RSV-positive (IP = 37, ED = 18, and OP = 46). Of the RSV-positive infants, the median age was 4.5 months and 57% had ⩾2 healthcare encounters. The majority of RSV-positive infants were not born premature (88%) nor had underlying medical conditions (92%). RSV-positive infants, however, were more likely to have a lower respiratory tract infection than RSV-negative infants (76% vs 39%, p < 0.001). Hospitalized infants with RSV were younger, 65% required supplemental oxygen, were more likely to have lower respiratory tract symptoms, and more often had shortness of breath and rales/rhonchi than RSV-positive infants in the ED and OP setting.
Conclusion:
Infants with RSV illnesses seek healthcare for multiple encounters in various settings and have clinical difference across settings. Prevention measures, especially targeted toward healthy, young infants are needed to effectively reduce RSV-associated healthcare visits.
Keywords: RSV across healthcare settings, RSV in infants
Introduction
Globally, respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections (LRTIs) and hospitalizations in infants (<1 year) and young children.1–3 In the United States, more than 57,000 hospitalizations with direct medical costs of $400 million have been attributed to RSV infections each year in children younger than 5 years.1,2,4 More than three-quarters of annual hospitalizations in children younger than 5 years are among infants.5,6 Known risk factors for hospitalization are primarily in preterm infants and those with congenital heart disease or chronic lung disease of prematurity; however, the majority of RSV-associated hospitalizations are among children without underlying medical conditions.3,7,8 RSV burden is not exclusive to the inpatient (IP) setting, yet the relative impact of visits attributed to RSV in the emergency department (ED) and ambulatory care settings are unclear and understudied,7,8 especially among infants.
At present, palivizumab prophylaxis (monthly intramuscular injections during RSV season) is the only product available to prevent LRTI hospitalization caused by RSV and is only indicated in high-risk infants.9,10 Broader prevention, such as vaccines and long-acting monoclonal RSV-neutralizing antibodies targeting infants and children are under development, with multiple phase 1–3 trials being conducted.11–13 Despite the development of pharmaceutical prevention, RSV epidemiology among infants primarily focus on palivizumab-eligible populations and the IP setting and is dependent on RSV detection through clinical testing. This focus has limited the evidence on the burden of RSV in the ED and outpatient (OP) clinic settings; and for full-term, otherwise healthy infants. Therefore, we aimed to (1) assess infant RSV infections across three distinct healthcare settings, (2) evaluate the differences in infant RSV sociodemographic characteristics and clinical presentations across healthcare settings, and 3) determine the number of healthcare encounters attributed to an infant’s RSV illness.
Methods
Study design
We conducted an active, prospective RSV surveillance study among infants in Davidson County, Tennessee, from 16 December 2019 to 30 April 2020. Infants who presented to an IP setting, ED, or one of four OP clinics with fever (⩾100.4°F) and/or at least one respiratory symptom (i.e. wheezing, crackles, rales, diminished breath sounds, shortness of breath, cough, earache, nasal congestion, rhinorrhea, coryza, and/or sore throat) within 14 days of illness onset were eligible for enrollment. 14 Written informed consent was obtained from parents or legal guardians in English, Spanish, or Arabic prior to enrollment.
Infants were excluded if they were hospitalized for more than 48 hours, were newborns never discharged from the hospital, presented with both fever and neutropenia (absolute neutrophil count <500 × 103/µL), had a known non-infectious cause for their symptoms, and/or had been previously enrolled in the past 7 days.
Enrollment locations
IP/ED surveillance
Surveillance was conducted in a 307-bed academic children’s hospital and ED. Enrollment was initiated on 16 December 2019 and occurred seven and three (i.e. Tuesday, Friday, and Saturday) days a week (2:00–10:00 p.m.) in the IP and ED settings, respectively. In the United States, EDs are typically reserved and used for emergencies or after hours when OP clinics are closed.
Pediatric OP clinic surveillance
Surveillance was conducted at four distinct pediatric OP clinics: one academic, one private/academic, and two private. Enrollment commenced on 16 and 19 of December 2019 at the academic clinic and the private/academic pediatric clinic, respectively; and subjects were approached for eligibility Monday through Thursday each week. At the private clinics, one began enrollment on 9 January 2020, and the other on 21 January 2020, with enrollment occurring 6 days per week (i.e. Monday to Saturday). On 16 March 2020, the study ceased enrollment at all OP clinics, except the academic clinic, because of coronavirus disease 2019 (COVID-19) pandemic-related restrictions. OP clinics in the United States generally include family practitioners and private care.
Clinical setting distribution was determined by the highest admission status of the infant (i.e. infants enrolled from the ED or OP clinics and later hospitalized within 7 days of enrollment were considered as IP).
Data and specimen collection
Trained staff interviewed parents or legal guardians of the enrolled infant for demographic, social, illness, and epidemiologic histories using a standardized questionnaire in commonly spoken languages (i.e. English, Spanish, or Arabic). A research nasal swab and a medical chart review after discharge for clinical outcomes, antibiotic use, provider-ordered RSV testing, and clinical microbiological data were collected from all infants. Fourteen days following enrollment, parents or legal guardians were administered a follow-up interview to assess illness duration, and additional healthcare encounters (e.g. OP clinic, urgent care, ED visits, etc.). Parents who reported ongoing symptoms on the follow-up survey were administered a questionnaire every 7 days until the infant’s symptoms resolved. All data and specimen information were maintained in a secure REDCapTM (Research Electronic Data Capture, Vanderbilt University, Nashville, TN, USA) database. 15
Definitions
An infant was defined to have LRTI if they had at least one of the following findings reported during their physical assessment: (1) wheezing; (2) crackles; (3) rales; (4) diminished breath sounds; or (5) shortness of breath/rapid or shallow breathing. Infants were considered to have an underlying medical condition if they had at least one of the following: asthma/reactive airway disease, atopic/allergic conditions, blood disorders, cancer, cerebral palsy, chronic lung conditions, cystic fibrosis, diabetes, Down’s syndrome, eczema, endocrine diseases, food allergies, genetic/metabolic disorders, heart disease, immunocompromised conditions, kidney disease, liver disease, neurological disorders, sickle cell disease/trait, seizure disorder, and/or other (e.g. neonatal abstinence syndrome, congenital defects, etc.). 14
Dates of enrollment were reported using the Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Reporting (MMWR) weeks of the epidemiological year.16,17 Our study enrollment period took place from MMWR weeks 50 through 18 (i.e. 16 December 2019 to 30 April 2020).16,17
Laboratory testing and pathogen detection
Research nasal swabs were placed in viral transport medium and further aliquoted into a tissue lysis buffer (ATL Lysis Buffer, QIAGEN), snap-frozen, and stored at −80°C. All specimens were tested for RSV A and B, adenovirus, four human coronaviruses [(HCoVs): OC43, 229E, HKU1 and NL63)], human metapneumovirus, human rhinovirus/enterovirus, influenza (A and B), bocavirus, and parainfluenza virus (1, 2, 3, and 4), Mycoplasma pneumonia and Chlamydophila pneumoniae using Luminex® NxTAG Respiratory Pathogen Panel. Luminex NxTAG CoV extended panel was used to test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Statistical analysis
Differences in demographic and clinical characteristics between RSV-positive and RSV-negative infants and between RSV-positive infants by settings (IP, ED, and OP clinic) were evaluated using Pearson’s χ2 test for categorical variables and linear regression with robust standard errors for continuous variables. Statistical tests were based on a significance level (α) of 0.05 (two-sided, where applicable). All analyses were conducted using statistical software Stata/IC 16.0 (StataCorp LLC, College Station, TX).
Results
Study population
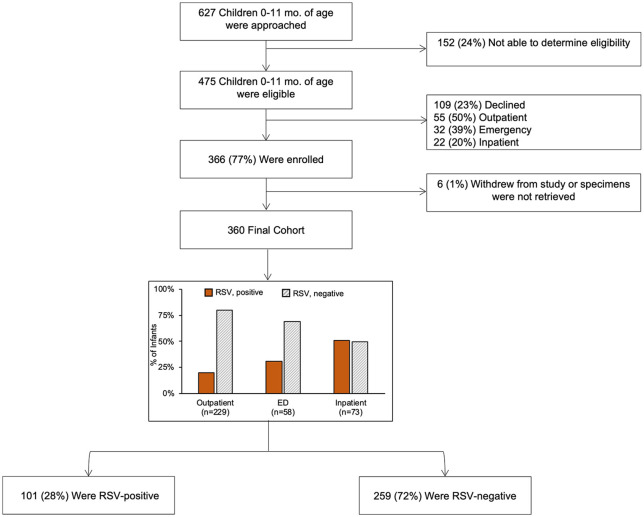
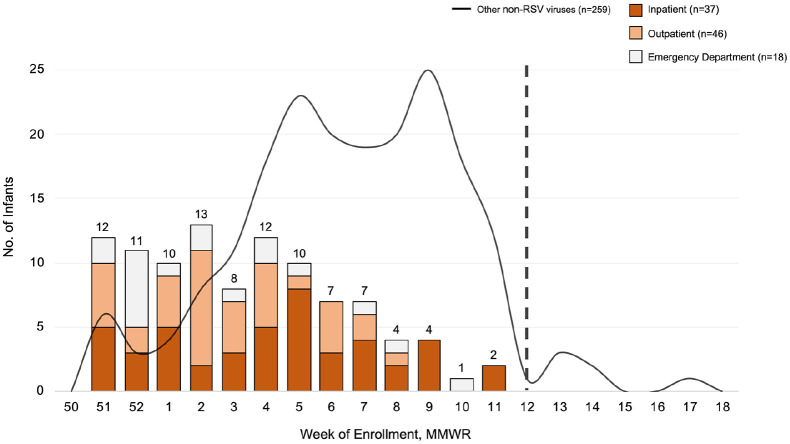
Of the 627 infants screened, 475 (76%) were confirmed eligible and of those 360 (76%) were enrolled and tested (IP = 73, ED = 58, OP clinics = 229; Figure 1). Primary reasons for not being able to determine eligibility were language barriers (47%) and missed patients due to multiple appointments scheduled at the same time (24%). Among the 360 infants in the final cohort, 101 (28%) were RSV-positive (RSV A = 80; RSV B = 22), with distribution of RSV cases by setting over time displayed in Figure 2.
Figure 1.
Consort diagram of RSV surveillance study in Davidson County infants, December 2019 through April 2020.
Figure 2.
Davidson County infants with RSV compared to other respiratory viruses by MMWR week and setting, December 2019 to April 2020. Our study enrollment period took place from Morbidity and Mortality Weekly Report (MMWR) weeks 50–18 (i.e. 9 December 2019 to 30 April 2020). The dashed line denotes that the MMWR week enrollment was halted in the outpatient settings due to COVID-19.
Comparison of RSV-positive vs RSV-negative infants
RSV-positive infants had a lower mean age (RSV+, mean: 4.9 months, SD: 3.2 vs RSV–, mean: 6.6 months, SD: 3.2, p < 0.001) and fewer had an underlying medical conditions (8% vs 21%, p = 0.004) than RSV-negative infants. Compared to RSV-negative infants, RSV-positive infants were more likely to have LRTI (76% vs 39%, p < 0.001), be hospitalized (37% vs 14%, p < 0.001), and require supplemental oxygen (65% vs 36%, p < 0.001). No infants with RSV were born younger than 29 weeks gestational age, and there were no statistically significant differences of prematurity (<37 weeks) between RSV-positive and RSV-negative infants (p = 0.496).
Among RSV-positive infants, those with LRTI were primarily seen in the IP and OP clinics than the ED (IP: 47% and OP: 37% vs ED: 17%, respectively, p < 0.001), and more often presented with irritability (91% vs 71%, p = 0.013) than infants without LRTI. No RSV-positive infants received palivizumab; only one 7-month old, born 26 weeks gestational age, who was RSV-negative received palivizumab. Moreover, 90% of infants in our cohort were born at normal birth weight (2500+ grams [RSV-positive: 95%; RSV-negative: 88%]), with 9% born weighing 1500 to <2500 g (RSV-positive: 4%; RSV-negative: 11%), and 1% weighing <1500 g (RSV-positive: 1%; RSV-negative: 1%).
RSV by healthcare setting
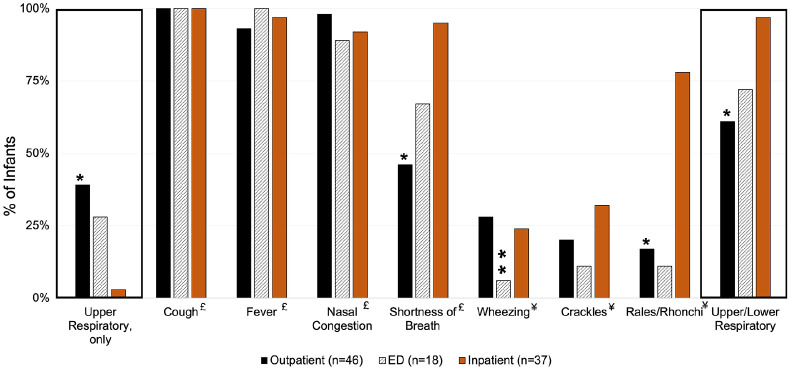
Hospitalized infants with RSV were younger (i.e. 0–3 months) and more likely to have LRTI symptoms compared to RSV-positive infants seen in the ED and OP clinics (Table 1). Of note, provider-ordered RSV testing was primarily conducted in the IP setting, as compared to the ED and OP clinic (IP: 73% vs ED: 39% and OP: 28%, p < 0.001; Table 1). RSV-positive children in the ED had higher proportion of public insurance, secondhand smoke exposure, maternal education with high school, and shorter duration of illness compared to RSV-positive children in the IP and OP settings (Table 1). No differences of antibiotic or bronchodilator prescription/administration practices were observed among RSV-positive infants across healthcare settings. In addition, RSV-positive infants who presented to the OP setting were more likely to present with only upper respiratory symptoms (Figure 3). Infants who presented to the ED or were hospitalized had a higher proportion of shortness of breath than infants in the OP setting.
Table 1.
Sociodemographic and clinical characteristics of infants with respiratory syncytial virus (RSV) by setting, December 2019 through April 2020.
| Characteristic | All RSV + (n = 101) | Inpatient (n = 37) | ED (n = 18) | Outpatient (n = 46) | p value a |
|---|---|---|---|---|---|
| Age, months – mean (SD) | 4.9 (3.2) | 3.8 (2.7) | 4.5 (3.3) | 5.9 (3.2) | <0.001 |
| Age, group – n (%) | |||||
| 0–3 months | 48 (47) | 23 (62) | 10 (56) | 15 (33) | 0.036 |
| 4–6 months | 26 (26) | 9 (24) | 2 (11) | 15 (33) | |
| 7–11 months | 27 (27) | 5 (14) | 6 (33) | 16 (35) | |
| Sex, male – no. (%) | 57 (56) | 22 (59) | 8 (44) | 27 (59) | 0.526 |
| Race – no. (%) | |||||
| White | 45 (45) | 18 (49) | 10 (56) | 17 (37) | 0.620 |
| Black | 29 (29) | 9 (24) | 5 (28) | 15 (33) | |
| Other | 27 (27) | 10 (27) | 3 (17) | 14 (30) | |
| Insurance – no. (%) | |||||
| Private | 28 (28) | 10 (27) | 0 | 18 (39) | 0.025 |
| Public | 67 (66) | 26 (70) | 16 (89) | 25 (54) | |
| Self-pay/none | 6 (6) | 1 (3) | 2 (11) | 3 (7) | |
| Ethnicity, Hispanic – no. (%) | 31 (31) | 15 (41) | 7 (40) | 9 (20) | 0.085 |
| Premature, <37 weeks – no. (%) | 12/100 (12) | 4 (11) | 3 (17) | 5/45 (11) | 0.797 |
| Underlying medical condition – no. (%) | 8 (8) | 2 (5) | 2 (11) | 4 (9) | 0.737 |
| Breastfeeding history – no.(%) | 84 (83) | 32 (86) | 14 (78) | 38 (83) | 0.714 |
| Daycare attendance – no. (%) | 29 (29) | 9 (24) | 3 (17) | 17 (37) | 0.207 |
| Second-hand smoke exposure – no. (%) | 19 (19) | 5 (14) | 9 (50) | 5 (11) | 0.001 |
| Maternal education – no. (%) | |||||
| Less than high school | 20 (20) | 11 (30) | 4 (22) | 5 (11) | 0.010 |
| High school | 38 (37) | 12 (32) | 12 (67) | 14 (30) | |
| 2- to 4-year college degree | 28 (28) | 10 (27) | 2 (11) | 16 (35) | |
| Graduate degree | 15 (15) | 4 (11) | 0 | 11 (24) | |
| LRTI – no. (%) | 77 (76) | 36 (97) | 13 (72) | 28 (61) | <0.001 |
| Supplemental oxygen – no. (%) | 24 (24) | 24 (65) | – | – | – |
| Illness duration, days – mean (SD) | 19 (39) | 14 (10) | 12 (8) | 28 (62) | 0.205 |
| Prescription/administration – no. (%) | |||||
| Antibiotic | 28 (28) | 9 (24) | 3 (17) | 16 (35) | 0.293 |
| Bronchodilator | 9 (9) | 6 (16) | 1 (6) | 2 (4) | 0.145 |
| Co-detection – no. (%) | |||||
| Virus | 12 (12) | 4 (11) | 0 | 8 (17) | 0.149 |
| Bacteria | 3 (3) | 3 (8) | 0 | 0 | 0.069 |
| Provider-ordered RSV testing – no. (%) | 47 (47) | 27 (73) | 7 (39) | 13 (28) | <0.001 |
| Positive | 46 (98) | 26 (96) | 7 (100) | 13 (100) | 0.685 |
ED, emergency department; RSV, respiratory syncytial virus; SD, standard deviation.
Definitions: Underlying medical conditions had at least one of the following conditions: asthma/reactive airway disease, atopic/allergic conditions, blood disorders, cancer, cerebral palsy, chronic lung conditions, cystic fibrosis, diabetes, Down’s syndrome, eczema, endocrine diseases, food allergies, genetic/metabolic disorders, heart disease, immunocompromised conditions, kidney disease, liver disease, neurological disorders, sickle cell disease/trait, seizure disorder, and/or other.
P values were calculated using linear regression with robust standard errors for continuous variables and Pearson χ2 test for categorical variables, alpha set at <0.05.
P values are across healthcare settings (i.e. inpatient, ED, outpatient). P values <0.05 are bolded.
Figure 3.
Proportion of Davidson County infants by respiratory syncytial virus by healthcare setting, December 2019 to April 2020. No infant presented with only lower respiratory symptoms.
Definitions: Lower respiratory symptoms: wheezing, crackles, rales/rhonchi, diminished breath sounds, shortness of breath; upper respiratory symptoms: fever, cough, earache, nasal congestion, rhinorrhea, and sore throat.
ED, emergency department.
*p value < 0.05 for the pairwise comparison between outpatient and inpatient setting.
**p value < 0.05 for the pairwise comparison between outpatient and emergency department.
£Symptoms collected during parent/guardian interview.
¥Symptoms collected during physical exam.
Spectrum of healthcare encounters
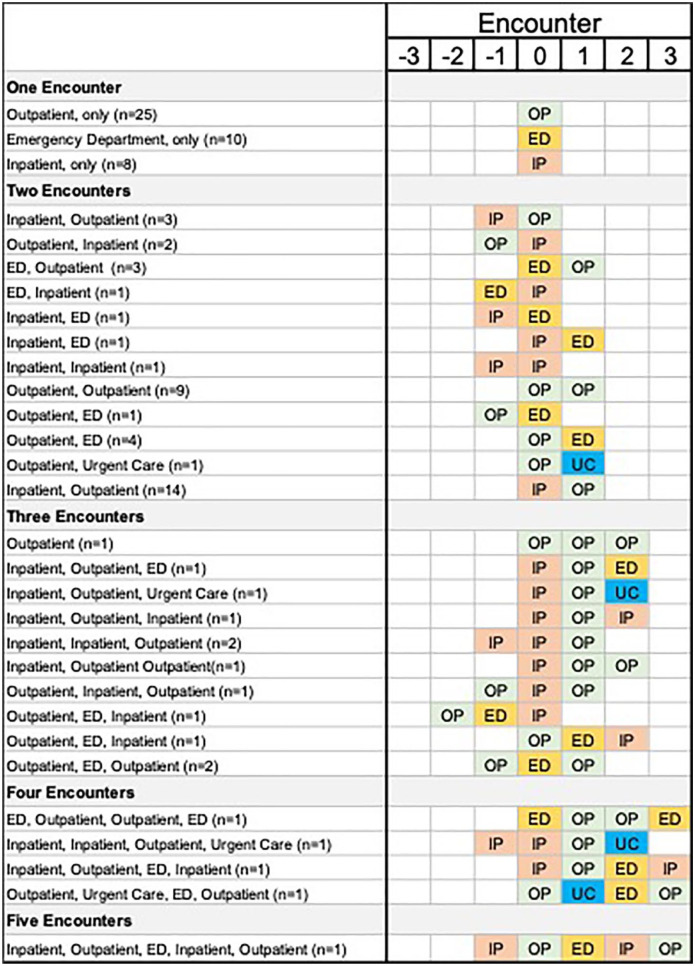
Of the RSV-positive infants, 57% (58/101) had more than one healthcare encounter, and among those, 66% (38/58) were hospitalized at some point during their illness [among those with only one visit: 19% (8/43 were hospitalized), Figure 4)]. In contrast, 13% (41/259) of RSV-negative infants reported more than one healthcare visit for their illness, and among those, 49% (20/41) were hospitalized [among those with only one visit: 11% (25/218) were hospitalized].
Figure 4.
Healthcare encounters associated with the initial respiratory syncytial virus illness. Infants who were enrolled as an inpatient, but first sought medical care through the emergency department on ‘encounter 0’ were classified as an inpatient. 0 = enrollment visit; 1 to 3 = healthcare encounters after enrollment reported in the follow-up survey; −3 to −1 = healthcare encounters before enrollment reported during the initial interview.
ED, emergency department; IP, inpatient; OP, outpatient; UC, urgent care.
Discussion
In our active RSV surveillance study, among 360 infants who sought medical attention at an OP clinic, ED, or IP setting during the 2019–2020 respiratory season with fever and/or respiratory symptoms, nearly one-third were RSV-positive. RSV-positive infants were younger, presented with more severe illness as indicated by higher proportion being hospitalized with LRTI and receiving supplemental oxygen, yet were less likely to have an underlying medical condition compared to RSV-negative infants. In addition, we noted the majority of these children sought more than one healthcare encounter for their illness through our 14-day follow-up survey. Our findings highlight that RSV impacts young healthy children who seek medical attention across all clinical settings, including multiple visits for the same illness. Prevention strategies are essential to reduce illness and the attributed burden in our healthcare systems.
Our study included a follow-up survey to better understand the full spectrum of illness and to document if additional healthcare visits were sought for the same illness. Infants within our study had a median of two healthcare visits for a single RSV infection. Among these healthcare encounters, 45% of infants were hospitalized at least once throughout the course of their illness. Literature evaluating the burden of RSV typically groups healthcare encounters that are within 14 days of each other into the highest level of care.1,18,19 This methodology is widely practiced, and we followed a similar approach in our initial analyses.1,18,19 Given that the vast majority of prior surveillance studies are cross-sectional, they potentially underestimate the full illness spectrum of RSV-associated healthcare utilization across clinical settings in the United States.1,6,18,20–22 For example, in a population-based surveillance study among children under 5 years, 61% of RSV-positive children were hospitalized and 39% were seen in ambulatory care (i.e. EDs, pediatric offices). 1 Owing to the cross-sectional nature of the study design however, the authors were unable to establish whether those hospitalizations required additional OP follow-up visits. Therefore, our approach of following infants until their illness subsided provided an opportunity to define the subsequent visits required. While we show that the majority of RSV visits were in the OP and ED settings, infants hospitalized also required further medical attention in the OP settings, thus highlighting the importance of surveillance studies with extended follow-up across multiple settings to understand the burden of a single RSV infection in infants and the healthcare infrastructure. In addition, each subsequent healthcare visit also contributes to additional family and healthcare burden and costs23,24 and further studies are needed to document the true healthcare resource use and costs attributable to RSV illness.
We also noted RSV patients were younger, more likely to have severe illness, including LRTI symptoms and higher frequency of oxygen use, yet less likely to have underlying medical conditions compared to infants without RSV. These findings are consistent with other studies noting that children with RSV had more severe illness than RSV-negative children.1,5,6,18,20,21 We also found that provider-ordered RSV testing was minimally performed in the ED and OP clinics, which is consistent with previous studies finding only 3% of RSV illnesses in the OP setting being clinically diagnosed. 1 Thus, if the estimated burden of RSV is dependent on provider-ordered viral testing, the prevalence of RSV in the ED and OP settings will be substantially underrecognized.1,21,25 Therefore, our study highlights the importance of active, prospective, surveillance in all settings to accurately document the burden of RSV illness and the need for new prevention interventions.
Currently, the only licensed preventive strategy is palivizumab, which is recommended only for high-risk children under 2 years and premature infants <29 weeks gestational age and is not widely available worldwide due to multiple injections and the associated financial burden.9,10 Prematurity has consistently been identified as a risk factor associated with severe RSV infection;21,22,26–28 however, in our cohort only 12% of the RSV-positive infants were born earlier than 37 weeks’ gestational age. Among the premature RSV-positive infants, we did not identify a difference between healthcare settings but rather found higher proportions of infants younger than 3 months in the IP setting. Notably, none of the RSV-positive infants enrolled in our study were born younger than 29 weeks’ gestation and only one infant in our entire cohort received palivizumab. Our findings do not elucidate the impact palivizumab has on preventing RSV infections in high-risk infants but does highlight that the development of additional prophylactic interventions is essential to prevent RSV infections among otherwise healthy, full-term infants.
The strengths of our study include the active, prospective study design, the assessment of RSV across three distinct healthcare settings, and the inclusion of a follow-up period after enrollment to capture the full spectrum of illness. Although our study had several strengths, we also acknowledge its limitations. First, our findings may not be generalizable to other counties or regions outside of Nashville, Tennessee, as our study population was restricted to infants in Davidson County and only evaluated a single IP and ED setting. Second, the number of healthcare encounters for a single illness was captured by a 2-week follow-up survey. Thus, our healthcare encounters potentially are impacted by differential recall bias. We do not, however, believe this largely impacted our study as we had a 91% (329/360) response rate. Third, due to language barriers, we were unable to approach all infants’ parents for study enrollment, thus potentially introducing selection bias. Finally, we report 45% of infants had at least one hospitalization during our study period. This high proportion may be explained by a higher RSV circulation compared to prior RSV seasons. Additional population-based studies are needed to establish RSV-attributed healthcare visit rates among infants.
In summary, RSV contributes to substantial burden of healthcare visits, including in the ambulatory care settings. Infants with RSV are more likely to be previously healthy, yet are more likely to present with LRTI symptoms and have more severe illnesses compared to RSV-negative children. Robust surveillance and accessibility to point-of-care testing would potentially assist in defining the true prevalence of RSV across all clinical settings during the respiratory seasons further underscoring the importance of preventive measures for young healthy infants.
Acknowledgments
The authors would like to thank Yesenia Romero Herazo, Joan Eason, and Angela Mendoza who enrolled the infants into our study and the families who participated. They would also like to thank Laura Stewart for her advice and suggestions in study operations.
Footnotes
ORCID iD: Danielle A. Rankin  https://orcid.org/0000-0003-3018-3373
https://orcid.org/0000-0003-3018-3373
Contributor Information
Danielle A. Rankin, Vanderbilt Epidemiology PhD Program, Vanderbilt University School of Medicine, 1161 21st Ave South, D7232 MCN, Nashville, TN 37232, USA; Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Zaid Haddadin, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Loren Lipworth, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Anna L. Stahl, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA
Jon Fryzek, EpidStrategies, Rockville, MD, USA.
Mina Suh, EpidStrategies, Rockville, MD, USA.
Donald S. Shepard, Heller School for Social Policy and Management, Brandeis University, Waltham, MA, USA
Rebekkah Varjabedian, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Kailee N. Fernandez, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA
Seifein Salib, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Jessica Villarreal, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Mercedes Bruce, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Rendie McHenry, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Andrew J. Spieker, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA
Christopher B. Nelson, Sanofi Pasteur, Swiftwater, PA, USA
Natasha B. Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA
Declarations
Ethics approval and consent to participate: This study was approved by the Institutional Review Board at Vanderbilt University (Ref no. 151683). Written informed consent was obtained from parents or legal guardians prior to enrollment.
Consent for publication: Not Applicable.
Author contributions: Danielle A. Rankin: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Zaid Haddadin: Conceptualization; Data curation; Methodology; Writing – review & editing.
Loren Lipworth: Conceptualization; Investigation; Project administration; Supervision; Writing – review & editing.
Anna L. Stahl: Data curation; Formal analysis; Validation; Writing – review & editing.
Jon Fryzek: Conceptualization; Methodology; Supervision; Writing – review & editing.
Mina Suh: Conceptualization; Methodology; Supervision; Writing – review & editing.
Donald S. Shepard: Conceptualization; Methodology; Supervision; Writing – review & editing.
Rebekkah Varjabedian: Data curation; Project administration; Writing – review & editing.
Kailee N. Fernandez: Data curation; Writing – review & editing.
Seifein Salib: Data curation; Writing – review & editing.
Jessica Villarreal: Data curation; Writing – review & editing.
Mercedes Bruce: Data curation; Writing – review & editing.
Rendie McHenry: Data curation; Investigation; Validation; Writing – review & editing.
Andrew J. Spieker: Conceptualization; Methodology; Supervision; Writing – review & editing.
Christopher B. Nelson: Conceptualization; Methodology; Resources; Supervision; Writing – review & editing.
Natasha B. Halasa: Conceptualization; Funding acquisition; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This collaborative study with Vanderbilt University Medical Center was funded by Sanofi Pasteur and AstraZeneca. National Center for Advancing Translational Sciences (grant no. UL1TR000445). DAR is supported by the National Institutes of Health (award no. TL1TR002244).
Competing Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NH receives funding from Sanofi Pasteur, Quidel, and speaker compensation from an education grant supported by Genentech. CBN is an employee of Sanofi Pasteur and may hold shares and/or stock options in the company. JF, MS, and DSS received funding from Sanofi Pasteur for this and other contracted work. All other authors report no other conflicts of interest.
Availability of data and materials: The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
References
- 1. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality – United States, 2014-2017. Morb Mortal Wkly Rep 2018; 67: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amand C, Tong S, Kieffer A, et al. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res 2018; 18: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014-15. J Pediatric Infect Dis Soc 2020; 9: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics 2020; 146: e20193611. [DOI] [PubMed] [Google Scholar]
- 7. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383: 415–425. [DOI] [PubMed] [Google Scholar]
- 8. Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets 2012; 12: 92–97. [DOI] [PubMed] [Google Scholar]
- 9. American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee, Brady MT, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134: 415–420. [DOI] [PubMed] [Google Scholar]
- 10. Rainisch G, Adhikari B, Meltzer MI, et al. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine 2020; 38: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18: e295–e311. [DOI] [PubMed] [Google Scholar]
- 12. Neuzil KM. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol 2016; 23: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PATH. RSV vaccine and mAb snapshot, https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (2021, accessed 11 January 2022).
- 14. Haddadin Z, Rankin DA, Lipworth L, et al. Respiratory virus surveillance in infants across different clinical settings. J Pediatr 2021; 234: 164–171. [DOI] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. MMWR weeks, https://ndc.services.cdc.gov/wp-content/uploads/MMWR_Week_overview.pdf (accessed 5 October 2020).
- 17. Centers for Disease Control and Prevention. Weeks ending log 2019-2020, https://stacks.cdc.gov/view/cdc/58590 (2019, accessed 5 October 2020).
- 18. Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019; 8: 284–286. [DOI] [PubMed] [Google Scholar]
- 19. Turi KN, Wu P, Escobar GJ, et al. Prevalence of infant bronchiolitis-coded healthcare encounters attributable to RSV. Health Sci Rep 2018; 1: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haddadin Z, Beveridge S, Fernandez K, et al. Respiratory syncytial virus disease severity in young children. Clin Infect Dis 2021; 73: e4384–e4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132: e341–e348. [DOI] [PubMed] [Google Scholar]
- 22. Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J 2011; 30: 510–517. [DOI] [PubMed] [Google Scholar]
- 23. Chirikov VV, Simões EAF, Kuznik A, et al. Economic-burden trajectories in commercially insured US infants with respiratory syncytial virus infection. J Infect Dis 2020; 221: 1244–1255. [DOI] [PubMed] [Google Scholar]
- 24. Shi N, Palmer L, Chu BC, et al. Association of RSV lower respiratory tract infection and subsequent healthcare use and costs: a Medicaid claims analysis in early-preterm, late-preterm, and full-term infants. J Med Econ 2011; 14: 335–340. [DOI] [PubMed] [Google Scholar]
- 25. McGuiness CB, Boron ML, Saunders B, et al. Respiratory syncytial virus surveillance in the United States, 2007-2012: results from a national surveillance system. Pediatr Infect Dis J 2014; 33: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi GA, Silvestri M, Lanari M. Should the American Academy of Pediatrics respiratory syncytial virus guidelines be modified? Pediatrics 2010; 125: e1021; author reply e1022. [DOI] [PubMed] [Google Scholar]
- 27. Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr 2003; 143(Suppl. 5): S118–S126. [DOI] [PubMed] [Google Scholar]
- 28. Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J 2011; 5: 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]