Abstract
Purpose
In a post-hoc analysis of the CATNON trial (NCT00626990), we explored whether adding temozolomide to radiotherapy improves outcome in patients with IDH1/2wt anaplastic astrocytomas with molecular features of glioblastoma (redesignated as glioblastoma, IDH-wildtype in the 2021 WHO classification of CNS tumors).
Experimental Design
From the randomized phase 3 CATNON study examining the addition of adjuvant and concurrent temozolomide to radiotherapy in anaplastic astrocytomas, we selected a subgroup of IDH1/2wt and H3F3Awt tumors with presence of TERT promoter mutations and/or EGFR amplifications and/or combined gain of chromosome 7 and loss of chromosome 10. Molecular abnormalities including MGMT promoter methylation status were determined by next-generation sequencing, DNA methylation profiling, and SNaPshot analysis.
Results
Of the 751 patients entered in the CATNON study, 670 had fully molecularly characterized tumors. 159 of these tumors met the WHO 2021 molecular criteria for glioblastoma, IDH-wildtype. Of these patients, 47 received radiotherapy only and 112 received a combination of radiotherapy and temozolomide. There was no added effect of temozolomide on either overall survival (HR 1.19, 95% CI 0.82–1.71) or progression-free survival (HR 0.87, 95% CI 0.61–1.24). MGMT promoter methylation was prognostic for overall survival, but was not predictive for outcome to temozolomide treatment either with respect to overall survival or progression-free survival.
Conclusions
In this cohort of patients with glioblastoma, IDH-wildtype temozolomide treatment did not add benefit beyond that observed from radiotherapy, regardless of MGMT promoter status. These findings require a new well-powered prospective clinical study to explore the efficacy of temozolomide treatment in this patient population.
Keywords: IDH-wildtype, anaplastic astrocytoma, glioblastoma, temozolomide, MGMT, radiotherapy, IDH1, IDH2
Introduction
The benefit of the addition of temozolomide to radiotherapy in people with newly diagnosed glioblastoma was first demonstrated in 2005 in the pivotal EORTC 26981/22981-NCIC CE3 randomized clinical trial.1 The efficacy of temozolomide in combination with radiotherapy was confirmed in a study on elderly patients with glioblastoma.2 In both studies, the clinical benefit of temozolomide treatment was largely confined to patients with glioblastomas with a methylated O6-methylguanine DNA methyltransferase (MGMT) promoter.2,3 In other clinical trials, a survival benefit of single agent treatment with temozolomide was demonstrated in patients with MGMT-promoter methylated high-grade gliomas.4–6 In the CATNON trial, the efficacy of the addition of temozolomide during and after radiotherapy was investigated in patients with grade 3 astrocytoma. In the recently published 2nd interim analysis of the study, the benefit of temozolomide was found to be restricted to patients with astrocytoma grade 3 with isocitrate dehydrogenase 1 and 2 (IDH1/2) mutations (mt), and only for adjuvant temozolomide treatment.7 There was no clinical benefit of temozolomide in patients with IDH1/2 wildtype (wt) gliomas regardless of MGMT promoter status.7 However, IDH1/2wt gliomas are not a single entity, and molecular subtyping of grade 2 and 3 IDH1/2wt gliomas has identified prognostically significant patient subgroups.8–10 In particular a major subgroup of grade 2 and 3 IDH1/2wt glioma has emerged with molecular features of glioblastoma. These are characterized by either (i) a mutation of the telomerase reverse transcriptase promoter (pTERT), and/or (ii) paired chromosome 7 trisomy and loss of heterozygosity of chromosome 10 (7+/10− signature), and/or (iii) amplification of epidermal growth factor receptor (EGFRamp).11–13 With outcomes resembling those of glioblastoma, the 2021 world health organization (WHO) classification of central nervous system (CNS) tumors now labels these tumors as glioblastoma, IDH-wildtype and consequently most guidelines recommend to treat them with radiotherapy and both concurrent and adjuvant temozolomide.14 However, the benefit of adding temozolomide to radiation therapy has not been proven in patients with tumors meeting the molecular criteria of glioblastoma, IDH-wildtype but not the histological criteria.
The randomized CATNON trial (NCT00626990) with a control arm of radiotherapy alone allows the retrospective analyses of the effect of adjuvant and concurrent temozolomide in patients with histologically grade 3 astrocytomas with molecular features of glioblastoma (in the WHO 2021 classified as glioblastoma, IDH-wildtype), also in relation to the MGMT promoter methylation status.
Materials and Methods
Patient Population
Patients with tumors meeting the molecular criteria for glioblastoma, IDH-wildtype were identified in the European Organization for Research and Treatment of Cancer (EORTC), non-blinded, multicenter, randomized CATNON trial. This trial examined the effect of concurrent and adjuvant temozolomide given in addition to radiotherapy in adult patients with a diagnosis of primary 1p/19q non-codeleted anaplastic glioma (n=751) according to the 2007 WHO classification of CNS tumors.7 Patient randomization (1:1:1:1) was based on a 2×2 factorial design; after primary surgery patients were treated with radiotherapy (59.4 Gy in 33 fractions of 1.8 Gy) without any temozolomide, or radiotherapy with concurrent temozolomide (75 mg/m2 daily, max 7 weeks), or radiotherapy with adjuvant temozolomide (12 4-week cycles: 150–200 mg/m2 on day 1–5), or radiotherapy with concurrent and adjuvant temozolomide. Patients were stratified based on MGMT promoter status as determined by quantitative methylation-specific PCR. The collection of formalin-fixed paraffin-embedded (FFPE) tumor material was part of the study design. All institutions obtained ethics approval from their institutional review boards or ethics review committees before enrollment started. All patients gave written informed consent according to local, national, and international guidelines.
Procedures
DNA was isolated from FFPE tumor material.15 For samples with ≥60 ng DNA available, the DNA methylation and sequencing data were produced and reported in a previous study.16 In short, IDH1/2, and H3F3A mutation status were determined by a standard glioma-tailored next-generation sequencing (NGS) panel.17 Mutation status of pTERT was determined with a SNaPshot assay of the two hotspot mutations in gliomas (C228T and C250T).18 DNA methylation profiles were acquired with the Infinium MethylationEPIC BeadChip (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions after using the Infinium FFPE DNA Restoration Kit. Copy number data (presence of EGFRamp, and the 7+/10- signature) were derived and interpreted from the DNA methylation data as previously described.16 MGMT promoter status was assessed from the DNA methylation data with the MGMT-STP27 algorithm.19 For samples with <60 ng DNA available, DNA methylation profiling and the standard NGS panel could not both be performed. Instead, IDH1/2, H3F3A, and pTERT mutation status, and copy number data were determined by an in-house developed NGS panel requiring less DNA for successful analysis.20,21 Two dedicated neuropathologists centrally reviewed all tumor samples (JMK: European and Australian samples, KA: North-American samples). Clinical data such as survival data, sex, age at enrollment, use of corticosteroids at enrollment, type of surgery, mini-mental state examination (MMSE) score at enrollment, and treatment regimen were collected from the study entry forms.
Statistical analysis
For the analyses, the temozolomide treatment arms were combined into several larger cohorts. The ‘temozolomide and radiotherapy’ cohort is comprised of the concurrent arm, the adjuvant arm, and the concurrent/adjuvant arm. The ‘adjuvant temozolomide’ cohort consists of the adjuvant arm and the concurrent/adjuvant arm. The ‘no adjuvant temozolomide’ cohort consists of the concurrent arm and the radiotherapy only arm. The ‘concurrent temozolomide’ cohort consists of the concurrent arm and the concurrent/adjuvant arm. The ‘no concurrent temozolomide’ cohort consists of the adjuvant arm and the radiotherapy only arm. The primary endpoint of overall survival and the secondary endpoint of progression-free survival were measured from the date of randomization until the date of event (death or death/progression respectively) or censored at the date of last follow-up. Survival curves were created using the Kaplan-Meier technique and compared with the log-rank test. The Cox regression model was used for univariable and multivariable analysis to determine hazard ratios (HR) with 95% confidence intervals (CI). Significance was set at p-values below 0.05 unless otherwise specified. Statistical analysis was performed using R version 3.6.3 and packages minfi, and survival.
Results
Cohort distribution
We identified 202 patients with IDH1/2wt and H3F3Awt astrocytomas grade 3 from the 751 patients with astrocytomas grade 3 enrolled in the CATNON study (standard NGS panel: n=194, in-house NGS panel: n=8). The tumors of 159 patients fulfilled the molecular characteristics of glioblastoma, IDH-wildtype i.e. presence of EGFRamp, and/or pTERTmt, and/or the 7+/10- signature (EGFRamp: n=83, pTERTmt: n=144, 7+/10- signature: n=105, Appendix Table A1). Of this patient cohort, 47 (29.6%) patients received radiotherapy alone and 112 (70.4%) patients received radiotherapy with adjuvant and/or concurrent temozolomide. These data are summarized in Figure 1.
Figure 1.
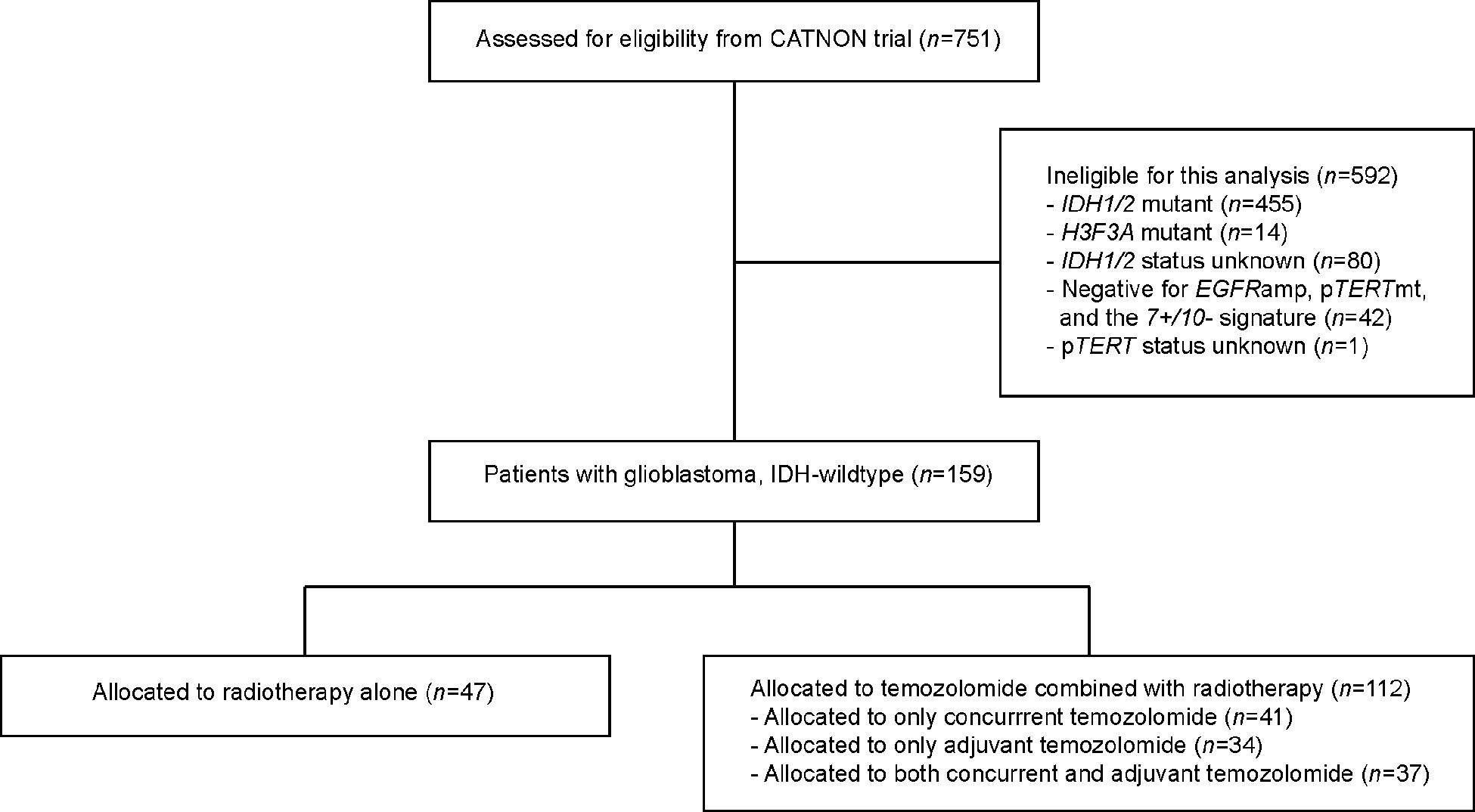
Consort diagram.
Baseline characteristics
Table 1 illustrates the baseline characteristics of the temozolomide and radiotherapy cohort, and the patient cohort treated with radiotherapy only. No significant differences were found between the two cohorts based on age, sex, type surgery, corticosteroid use, WHO performance score, MMSE score, and presence of necrosis and/or microvascular proliferation. There was a trend towards more tumors with unmethylated MGMT promoter in the temozolomide and radiotherapy cohort (p=0.066).
Table 1.
Baseline characteristics of the 159 patients with glioblastoma, IDH-wildtype of the CATNON trial. P values compare patients that received only radiotherapy (n=47) with patients that received radiotherapy with concurrent and/or adjuvant temozolomide (n=112).
| Characteristics | Radiotherapy only (n=47) | Temozolomide and radiotherapy (n=112) | p value |
|---|---|---|---|
| Age | >0.9a | ||
| Median | 55 | 55 | |
| IQR | 46–67 | 48–63 | |
| Sex | 0.4b | ||
| Female | 19 (40%) | 37 (33%) | |
| Male | 28 (60%) | 75 (67%) | |
| WHO performance score | 0.2b | ||
| 0 | 28 (60%) | 53 (47%) | |
| ≥1 | 19 (40%) | 59 (53%) | |
| MMSE score | 0.2b | ||
| 27–30 | 32 (68%) | 90 (80%) | |
| ≤26 | 11 (23%) | 17 (15%) | |
| Unknown | 4 (9%) | 5 (4%) | |
| Type of surgery | 0.6b | ||
| Resection | 35 (74%) | 79 (71%) | |
| Biopsy | 12 (26%) | 33 (29%) | |
| Corticosteroid use | 0.6b | ||
| No use | 33 (70%) | 74 (66%) | |
| Stable/decreasing dose | 14 (30%) | 38 (34%) | |
| MGMT promoter | 0.066b | ||
| Unmethylated | 25 (53%) | 74 (66%) | |
| Methylated | 21 (45%) | 32 (29%) | |
| Unknown | 1 (2%) | 6 (5%) | |
| Necrosis and/or microvascular proliferation | 0.5b | ||
| Absent | 36 (77%) | 90 (80%) | |
| Present | 10 (21%) | 18 (16%) | |
| Unknown | 1 (2%) | 4 (4%) |
Wilcoxon rank sum test.
Pearson’s Chi-squared test. IQR = interquartile range. WHO = World Health Organization. MMSE = mini-mental state examination.
Survival analysis
At the time of database lock (May 7th, 2019), 143 out of 159 patients (89.9%) with tumors meeting the criteria of glioblastoma, IDH-wildtype, were deceased, and in 154 patients (96.9%) progression of disease was reported. The median overall survival of this patient cohort was 1.4 years (95% CI 1.3–1.8 years, Appendix Figure A1A) and the median progression-free survival was 0.5 years (95% CI 0.5–0.7 years, Appendix Figure A1B). In this cohort of patients, there was no added effect of temozolomide in relation to radiotherapy alone on either overall survival (HR 1.19, 95% CI 0.82–1.71, Figure 2A), or progression-free survival (HR 0.87, 95% CI 0.61–1.24, Figure 2B). This lack of effect in overall and progression-free survival was not associated with the timing of the temozolomide treatment i.e. concurrent, adjuvant, or both concurrent and adjuvant temozolomide. Neither the adjuvant temozolomide nor the concurrent temozolomide was associated with clinical benefit in this cohort of patients. Moreover, no significant differences in overall survival or progression-free survival were found between the radiotherapy alone treatment arm and the radiotherapy with both concurrent and adjuvant temozolomide treatment arm (Appendix Figure A2).
Figure 2.
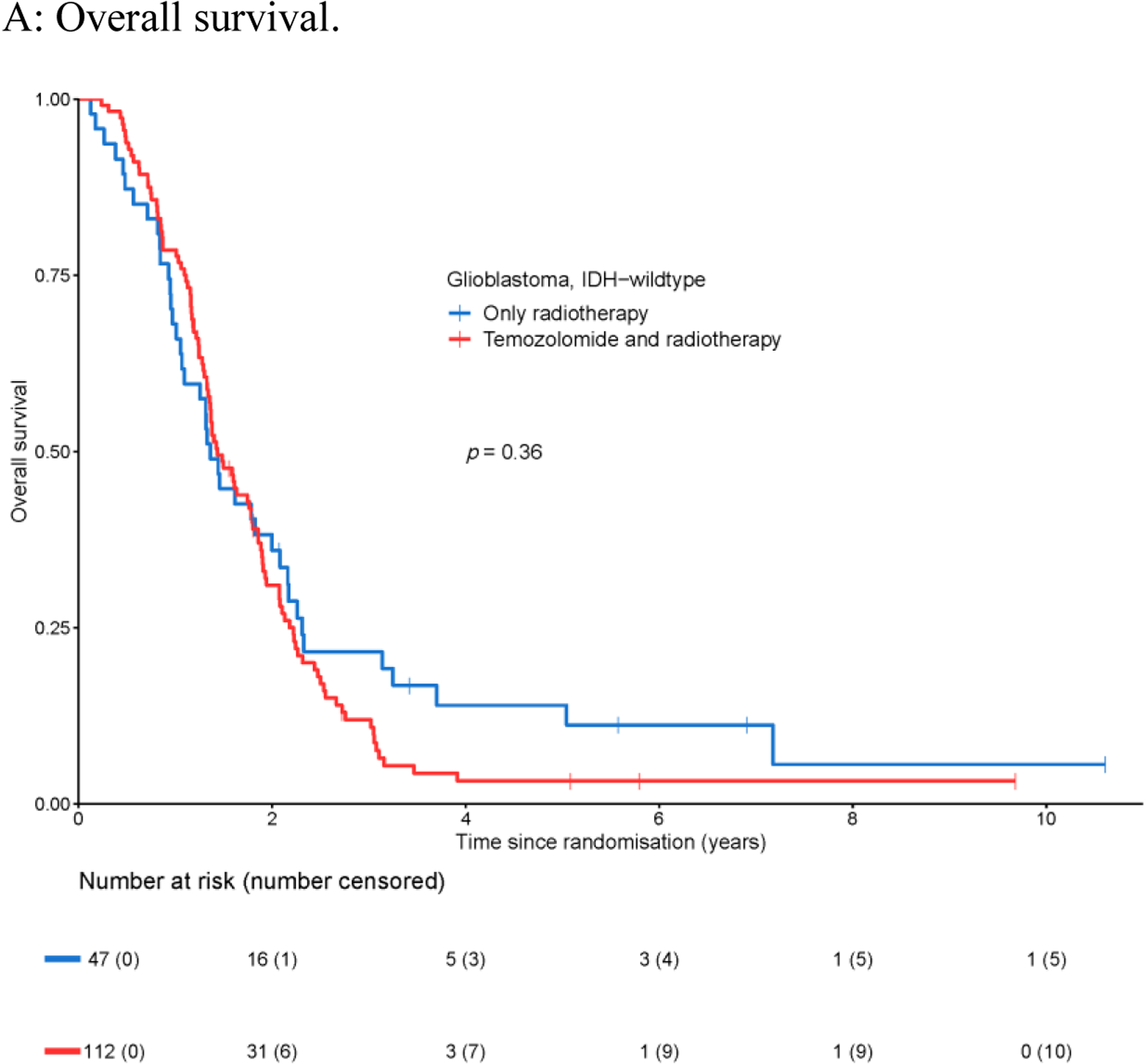
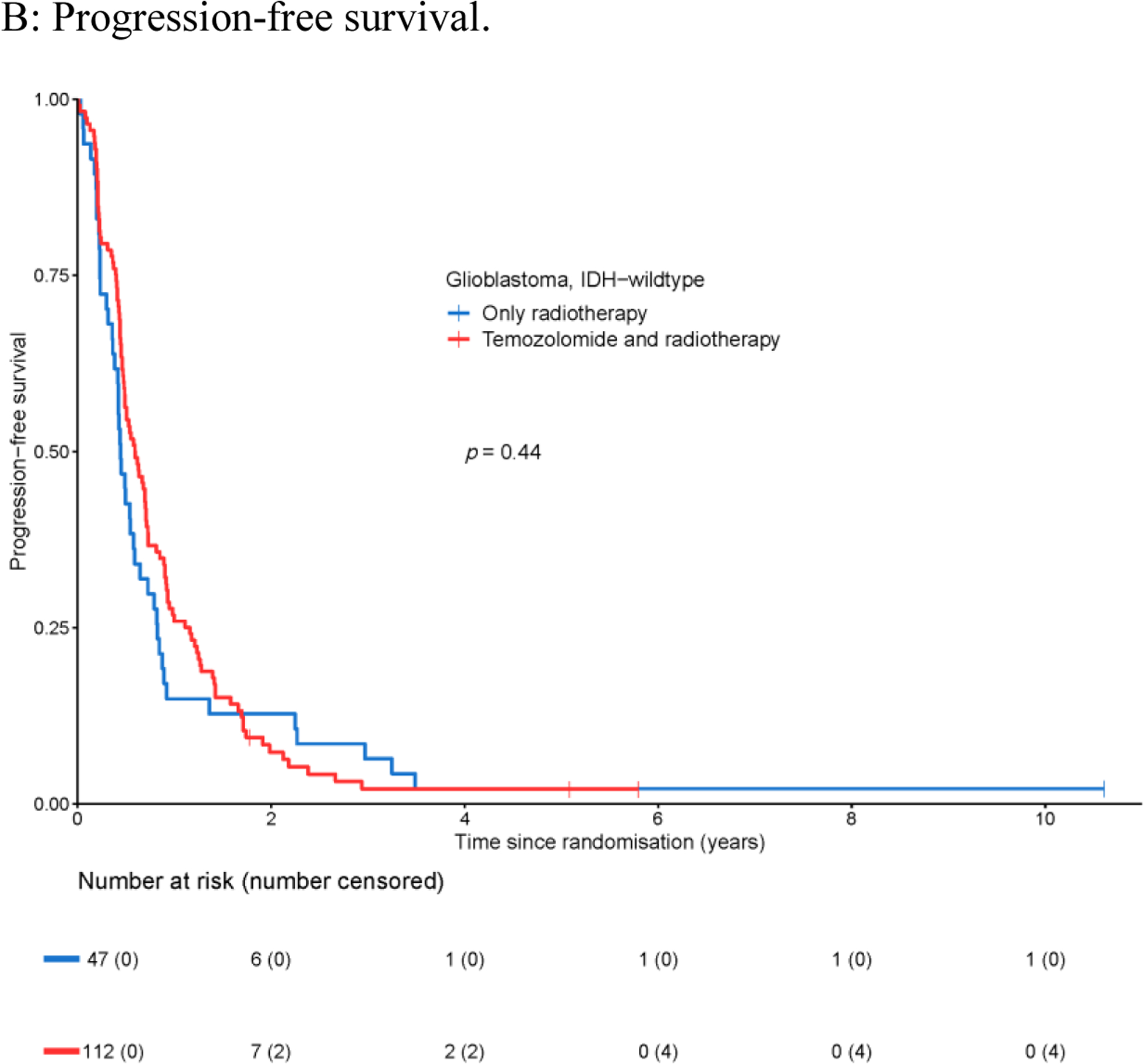
Survival of patients with glioblastoma, IDH-wildtype with respect to treatment regimen: temozolomide and radiotherapy vs. only radiotherapy.
MGMT promoter methylation status and survival
For 152 of the 159 patients in this cohort, tumor DNA methylation data were available allowing the determination of the MGMT promoter methylation status by the MGMT-STP27 algorithm. Of these, 53 (34.9%) of tumors were MGMT-methylated, and the remaining 99 tumors (65.1%) were MGMT-unmethylated. Patients with MGMT-methylated tumors had superior overall survival (HR 0.65, 95% CI 0.45–0.92, Figure 3A); the median overall survival for the cohort with MGMT-methylated tumors was 1.8 years (95% CI 1.4–2.2 years), and for the cohort with MGMT-unmethylated tumors was 1.4 years (95% CI 1.2–1.6 years). MGMT promoter methylation was not prognostic for progression-free survival (HR 0.95, 95% CI 0.68–1.34, Appendix Figure A3A) with a median progression-free survival of 0.5 years for both the cohort with MGMT-methylated tumors (95% CI 0.4–0.8 years), and the cohort with MGMT-unmethylated tumors (95% CI 0.5–0.7 years). No survival benefit was detected for temozolomide in addition to radiotherapy on overall survival in either the cohort with MGMT-methylated tumors (HR 1.36, 95% CI 0.75–2.48, Figure 3B), or the cohort with MGMT-unmethylated tumors (HR 0.88, 95% CI 0.54–1.42, Figure 3C). Similarly, no predictive effect for temozolomide efficacy was identified on progression-free survival in patients with MGMT-methylated and MGMT-unmethylated tumors (Appendix Figure A3B–C). The lack of predictive effect of MGMT promoter methylation extended to overall survival and progression-free survival and to each temozolomide cohort i.e. in none of the three temozolomide arms a clinical benefit was observed (Appendix Figure A4).
Figure 3.
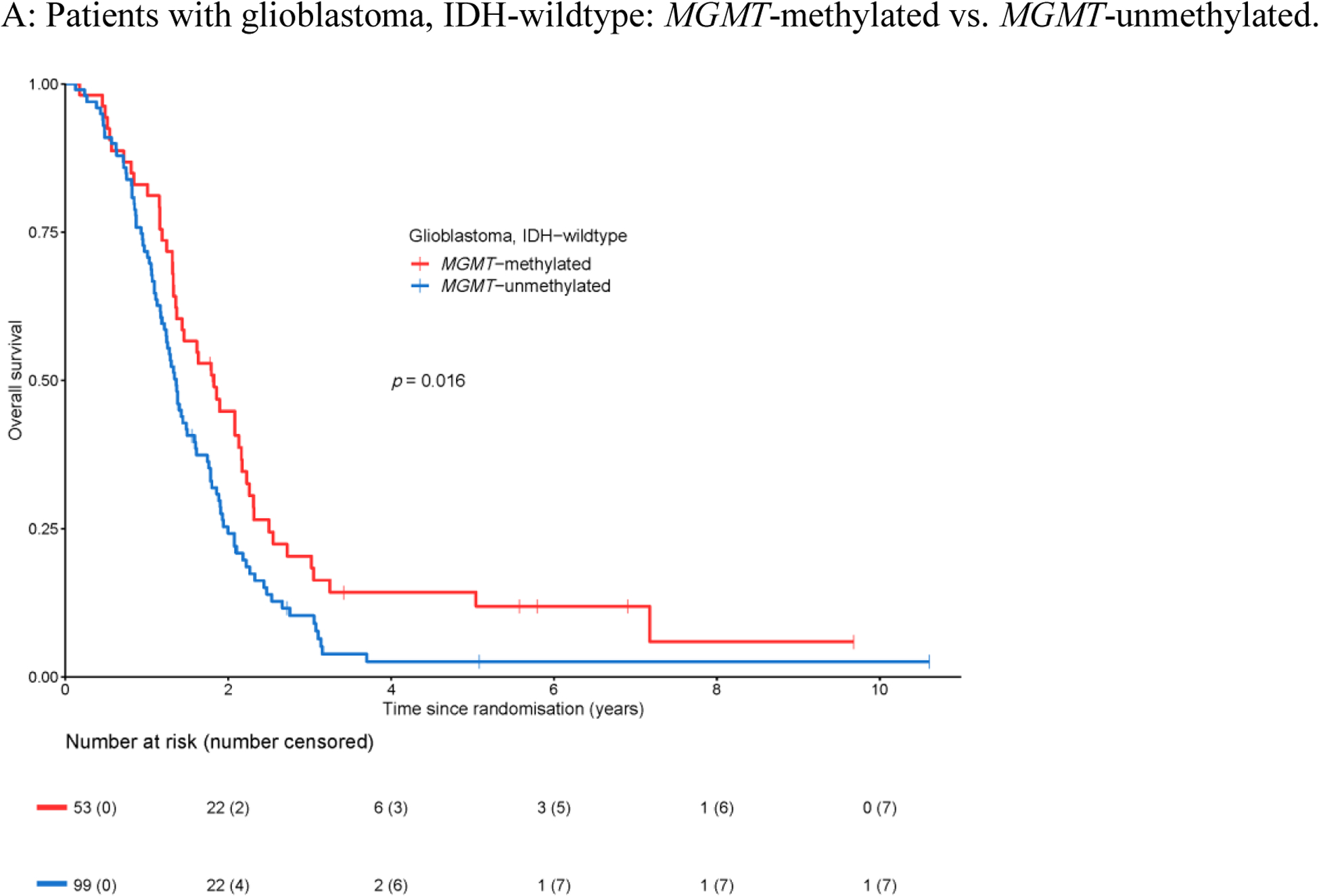
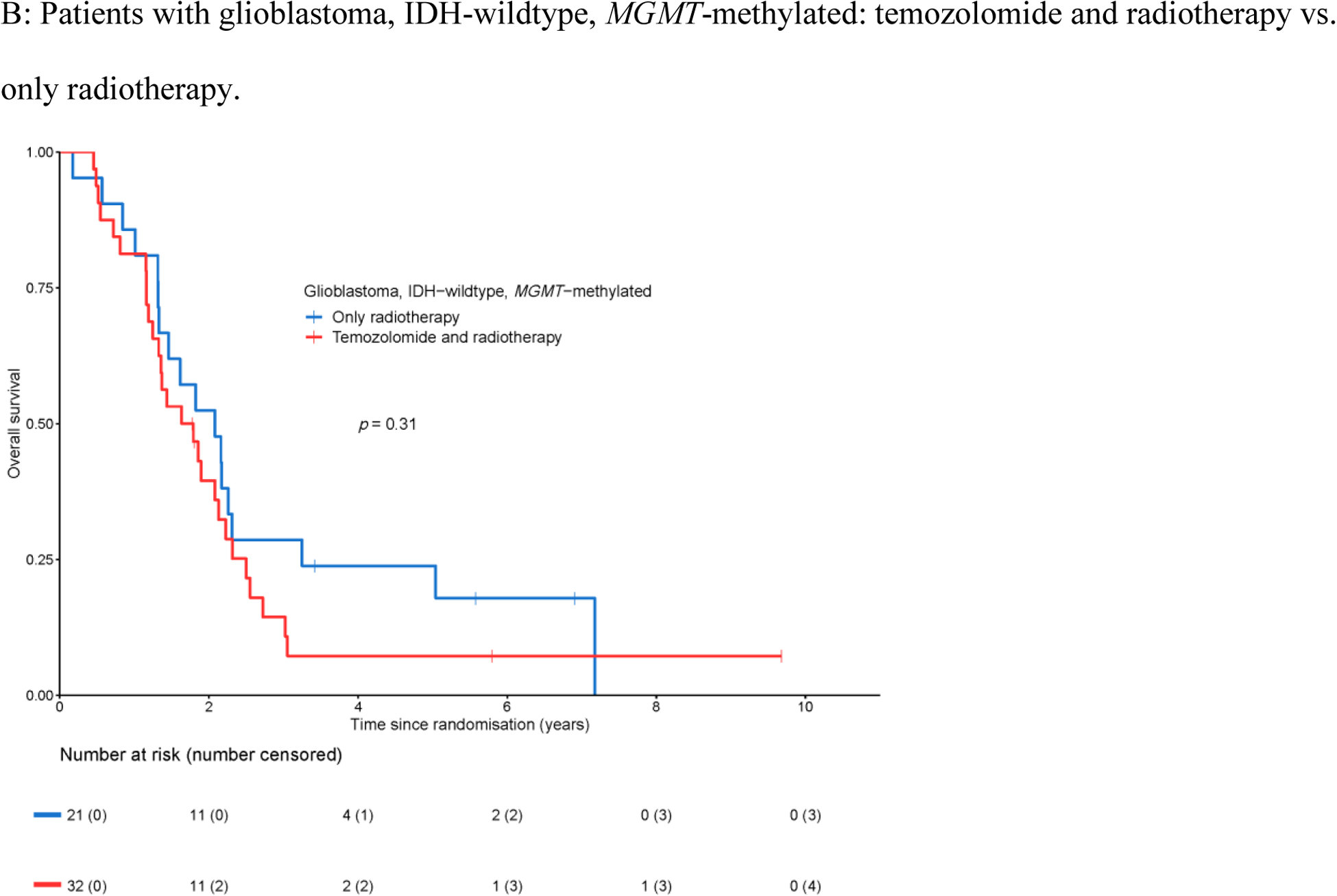
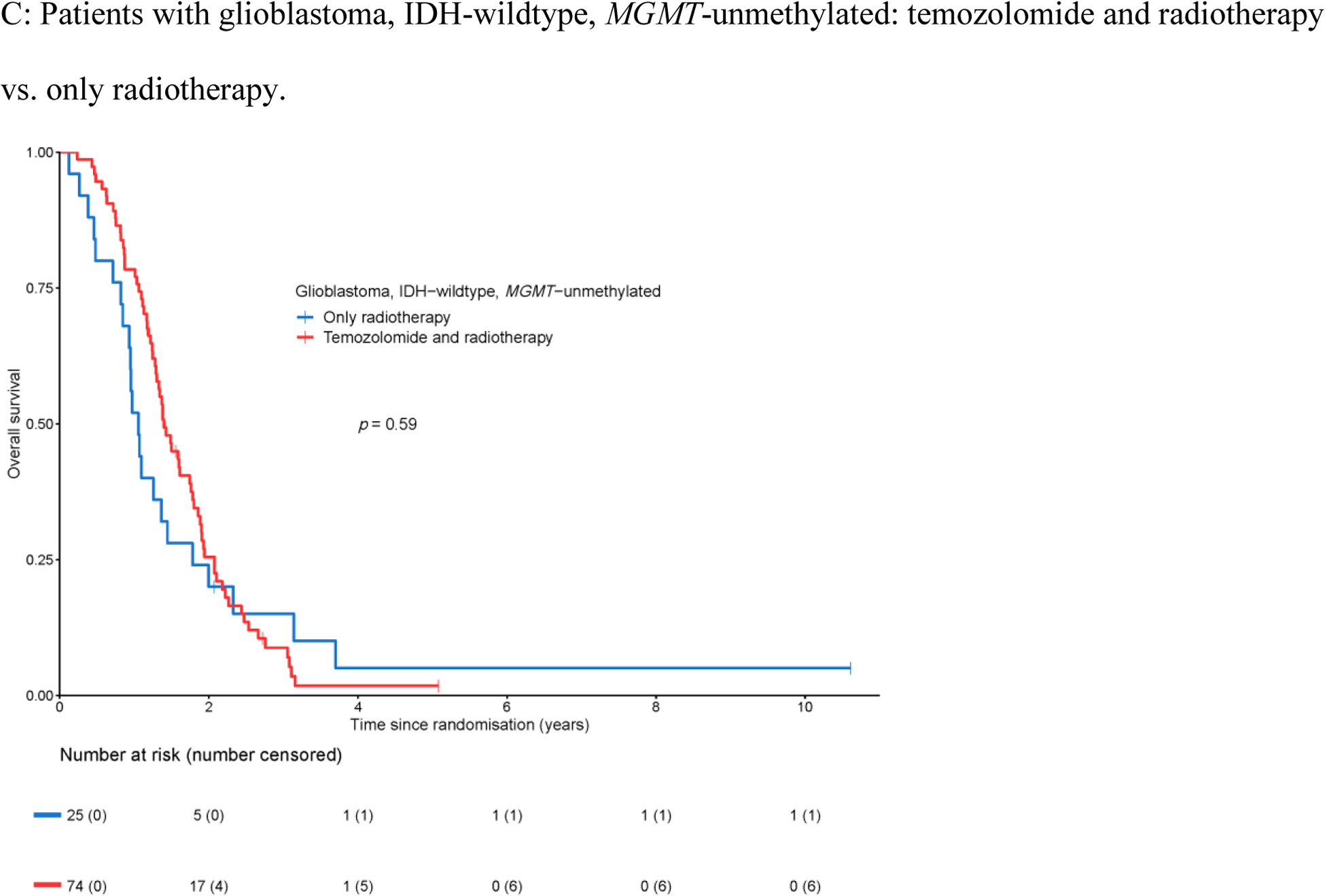
Overall survival with respect to MGMT promoter methylation.
Multivariable analysis
To correct for possible confounding factors, we selected all available factors from univariable analyses with likelihood ratio test p values ≤ 0.10. For overall survival, these factors included age group (<50 years vs. ≥50 years), MMSE score (≤26 vs. 27–30), type of surgery (biopsy vs. resection), use of corticosteroids at randomization (yes vs. no), and MGMT promoter status (methylated vs. unmethylated) (Appendix Table A2). For progression-free survival, the significant factors included age group, type of surgery, and use of corticosteroids at randomization (Appendix Table A3). Lack of clinical benefit of temozolomide was still apparent after correction for these factors in this cohort of patients on overall survival (temozolomide and radiotherapy vs. radiotherapy only: HR 1.03, 95% CI 0.69–1.53, Table 2), and in progression-free survival (HR 0.79, 95% CI 0.55–1.13, Appendix Table A4).
Table 2.
Multivariable analysis for overall survival of 143 patients with glioblastoma, IDH-wildtype (16 patients excluded due to missing data) by Cox proportional hazards model. The effect of the treatment regimen is adjusted for significant covariables (univariable analysis p <0.10) excluding histological factors.
| Variables | n | HR (95%CI) | p valuea |
|---|---|---|---|
| Treatment regimen | 0.57 | ||
| Temozolomide and radiotherapy vs. radiotherapy only | 101 vs. 42 | 0.89 (0.60–1.33) | |
| Age group | 0.009 | ||
| ≥50 years vs. <50 years | 101 vs. 42 | 1.75 (1.15–2.67) | |
| MMSE score | 0.22 | ||
| ≤26 vs. 27–30 | 27 vs. 116 | 1.33 (0.84–2.38) | |
| Type of surgery | 0.095 | ||
| Biopsy vs. resection | 42 vs. 101 | 1.40 (0.94–2.07) | |
| Corticosteroid use | 0.019 | ||
| Stable/decreasing dose vs. no use | 47 vs. 96 | 1.61 (1.08–2.38) | |
| MGMT promoter | 0.14 | ||
| Methylated vs. unmethylated | 50 vs. 93 | 0.75 (0.52–1.10) |
Wald test. HR = hazard ratio. CI = confidence interval. MMSE = mini-mental state examination.
Tumors with TERT promoter mutations only
Twenty-nine of the 159 patients with tumors meeting the criteria of glioblastoma, IDH-wildtype (18.2%) had a mutation of pTERT, and were negative for EGFRamp and the 7+/10- signature (pTERTmt only). Although this cohort is limited in size, we examined this subgroup in more detail. Patients with pTERTmt only tumors did not differ from other patients with tumors meeting the criteria of glioblastoma, IDH-wildtype with respect to overall survival (median overall survival pTERTmt only 1.4 years vs other glioblastoma, IDH-wildtype 1.4 years, HR 0.78, 95% CI 0.50–1.21, Appendix Figure A5A), or in progression-free survival (median progression-free survival pTERTmt only 0.6 years vs other glioblastoma, IDH-wildtype 0.5 years, HR 0.79, 95% CI 0.52–1.19, Appendix Figure A5B). We also failed to identify a beneficial effect of temozolomide in the patient cohort with pTERTmt only tumors on either overall survival or progression-free survival, though this analysis is based on few patients (radiotherapy only: n=9, temozolomide and radiotherapy: n=20, Appendix Figure A5C–D).
Histological features
As per study protocol, all samples included in the CATNON trial were diagnosed as an astrocytoma grade 3 by at least one central dedicated neuropathologist according to the 2007 WHO classification of CNS tumors. Therefore, all glioblastoma, IDH-wildtype analyzed in the current study were also diagnosed as such. Despite the strict histological criteria, subtle signs of necrosis and/or microvascular proliferation were reported at central histology review in 28 tumor samples (necrosis only: n=4, microvascular proliferation only: n=14, both necrosis and microvascular proliferation: n=10) which are considered histological features of glioblastoma but did not lead to exclusion from the study. Presence of these histological features of glioblastoma in the patients with molecularly defined glioblastoma, IDH-wildtype was associated with shorter overall survival (HR 1.62, 95% CI 1.06–2.48, Appendix Figure A6A), but no significant difference was found in progression-free survival (HR 1.46, 95% CI 0.96–2.22, Appendix Figure A6B). After adjustment for significant factors from univariable analysis including histological factors for glioblastoma, futility of temozolomide treatment remained for this cohort of patients with glioblastoma, IDH-wildtype in overall survival (HR 0.90, 95% CI 0.60–1.35, Appendix Table A5), and progression-free survival (HR 0.79, 95% CI 0.55–1.14, Appendix Table A6).
Discussion
This is the first dataset investigating temozolomide treatment efficacy in patients with IDH1/2wt astrocytomas grade 3 with molecular features of glioblastoma treated in a randomized clinical trial with a control arm of radiotherapy only. Of 202 IDH1/2wt and H3F3Awt tumors present in the CATNON trial, 159 tumors fulfilled the WHO 2021 molecular criteria of glioblastoma, IDH-wildtype.14 We did not observe benefit of the addition of temozolomide to radiotherapy in this cohort of patients, neither for overall nor progression-free survival. Similarly, no benefit of temozolomide was observed in the subgroup of patients with tumors harboring a methylated MGMT promoter. Moreover, the timing of the temozolomide treatment (concurrent, adjuvant or both) was not related to survival outcome.
Our data conflict with the results from earlier randomized clinical trials examining the efficacy of temozolomide in combination with radiotherapy in glioblastoma.1,2 The study by Stupp et al. showed efficacy of concurrent temozolomide followed by 6 cycles of adjuvant temozolomide.1 However, during enrollment and primary analysis of this trial the role of IDH1/2 in glioma had not yet been described. The exact percentage of patients with IDH1/2mt tumors in that study is unspecified, although in a later subgroup analysis of 160 tumor samples only 8% had an IDH1 mutation.22 In the study by Perry et al. on elderly patients with glioblastoma, the percentage of patients with IDH1/2mt tumors is described, comprising less than 1% of patients.2 Intriguingly, there is a possibility that age-based selection could have had an effect on outcome in this study; patients younger than 70 years had no survival benefit of additional temozolomide in combination with radiotherapy, whereas patients older than 70 years did show a prolonged survival due to temozolomide treatment.2 Thus, the results we find from the CATNON trial might not be as easily comparable to these previous studies due to a younger patient population in our study and a well-documented molecular subtyping of the tumors of the investigated patients.
With the possible exception of 28 samples with subtle signs of necrosis and/or microvascular proliferation, we prognosticated patients solely on molecular data. For patients with an IDH1/2wt lower-grade glioma, both EGFRamp and the 7+/10- signature are now indicators of a grade 4 diagnosis on their own.9,23,24 Conversely, cohorts of patients with pTERTmt only tumors have been described with variable patient outcome.13,23,25 In our cohort, we did not observe a difference in overall survival or progression-free survival between patients with pTERTmt only tumors and patients with the typical glioblastoma, IDH-wildtype. However, the number of patients with IDH-wildtype, pTERTmt only tumors was limited, and we emphasize the importance of excluding other pTERTmt tumor diagnoses (e.g. 1p/19q codeleted tumors, pleomorphic xanthoastrocytomas) when performing similar analyses, or when setting up prospective trials.12 It is also important to note, that the CATNON trial only included patients with a grade 3 tumor, and it remains to be determined if patients with pTERTmt only tumors, grade 2 have a comparable prognosis.25
The major limitations of this study are the modest sample size, and the post-hoc design. The CATNON trial was not specifically powered to answer the clinical questions of the present study; the lack of predictive effect in this post-hoc study of a subset of tumors now meeting the criteria for glioblastoma, IDH-wildtype and the lack of impact of MGMT promoter methylation on overall survival could be due to the limited small sample size. However, a recent randomized study in 37 patients with grade 2 or 3 IDH-wildtype glioma showing TERT promoter mutations did observe a survival benefit of the addition of temozolomide to radiotherapy.26 Despite the mentioned limitations of our study, we extracted a 159-patient cohort by clearly defined and now official diagnostic criteria from the entire CATNON dataset without an indication of temozolomide efficacy. At the minimum, our study therefore questions whether the addition of temozolomide treatment to radiotherapy is beneficial for patients with molecularly defined glioblastoma, IDH-wildtype.
In short, we found no effect of adjuvant and concurrent temozolomide treatment in patients with anaplastic astrocytomas now meeting the molecular criteria for glioblastoma, IDH-wildtype, regardless of MGMT promoter status. At present, these findings are insufficient to warrant a change in the management of these patients; i.e. given the outcome of other studies we believe these patients should be offered radiotherapy in combination with temozolomide chemotherapy. However, these findings do warrant a well-powered prospective study on the effectiveness of temozolomide when added to radiotherapy in tumors meeting the contemporary WHO 2021 molecular criteria for glioblastoma, IDH-wildtype. The choice for a trial design will depend on whether the trial should demonstrate that adding temozolomide will improve patient outcome as compared to radiotherapy alone, or whether it should demonstrate that temozolomide can safely be left out.
Supplementary Material
Translational relevance.
The practice changing randomized phase III CATNON trial has established the role for adjuvant temozolomide in patients with IDH-mutant astrocytoma, grade 3. In this study on the efficacy of temozolomide in anaplastic glioma without 1p/19q codeletion, patients were also included with tumors that are redesignated as IDH-wildtype glioblastomas by the 2021 WHO classification of CNS tumors. In this manuscript, we describe an absence of clinical benefit of temozolomide treatment in the IDH-wildtype glioblastoma patient population. Moreover, this lack of clinical benefit is not related to MGMT promoter methylation status, or timing of the temozolomide treatment i.e. concurrent, adjuvant, or both concurrent and adjuvant temozolomide. Our data raise important questions about the current treatment for IDH-wildtype glioblastoma patients. A new well-powered prospective clinical study is required to explore the efficacy of temozolomide treatment in patient with histological anaplastic astrocytoma that meet the molecular criteria for IDH-wildtype glioblastoma.
Acknowledgements
This study was funded by Merck, Sharp and Dohme (MSD) formerly Schering-Plough by an educational grant, and by the provision of temozolomide. The clinical study was also supported by the NRG (grants U10CA180868 and U10CA180822), Cancer Research UK grant CRUK/07/028, and Cancer Australia (project grants 1026842 and 1078655). The molecular study was funded by grant GN-000577 from The Brain Tumour Charity, grant 10685 from the Dutch Cancer Society, and financial support from the Vereniging Heino ‘Strijd van Salland’. We thank our patients and their relatives for their willingness to participate to this study. We also thank all sites and their staff for contributing to this study. We further acknowledge the support of this study by the staff at the EORTC Headquarters, Brussels, Belgium, the NRG Oncology (formerly the Radiation Therapy Oncology Group) staff at the American College of Radiology; the staff at the Australian National Health and Medical Research Council (NHMRC) Clinical Trials Centre (COGNO Coordinating Centre); and the staff at MRC Clinical Trials Unit, London UK.
Declarations of interest
MS reports consulting fees from Genenta, consulting fees from Abbvie, consulting fees from Taiho Oncology, consulting fees from Orion Pharma, consulting fees from Mundipharma, research funding from AstraZeneca, outside the submitted work. WW reports grants from Apogenix, grants from Pfizer, grants from Roche, outside the submitted work. PMC reports personal fees from Bristol-Meyers Squibb, personal fees from Abbvie, personal fees from Merck Serono, personal fees from Merck Sharp & Dohme, personal fees from Vifor Pharma, personal fees from Daiichi Sankyo, personal fees from LEO Pharma, personal fees from AstraZeneca, personal fees from Takeda, outside the submitted work. MAV reports royalty rights and indirect equity from Infuseon Therapeutics, personal fees from Cellinta, personal fees from Celgene, outside the submitted work. AKN reports grants and travel funding from Astra Zeneca, grants, consulting fees and payment to institution from Douglas Pharmaceuticals, consulting fees from Bayer Pharmaceuticals, steering committee fees from Roche Pharmaceuticals, steering committee fees and travel funding from Boehringer Ingelheim, consulting fees from Pharmabcine, consulting fees and payment to institution from Atara Biotherapeutics, consulting fees from Trizell Ltd, outside the submitted work. JFB reports consulting fees from Bristol-Meyers Squibb, consulting fees from MSD, consulting fees from Novartis, consulting fees from Sanofi, consulting fees from Regeneron, consulting fees from Merck, consulting fees from Pfizer, consulting fees from Pierre-Fabre, consulting fees from Sun Pharma, consulting fees from AstraZeneca, consulting fees from Immunocore, outside the submitted work. OLC reports personal fees and non-financial support from Abbvie, non-financial support from Immatics, non-financial support from BMS, outside the submitted work. RR reports advisory board fees from UCB and Novocure, outside the submitted work. MW reports personal fees from Adastra, grants from Apogenix, grants and personal fees from Merck, Sharp and Dohme (MSD), grants and personal fees from Merck (EMD), personal fees from Bristol Meyer Squibb (BMS), personal fees from Medac, personal fees from Nerviano Medical Sciences, personal fees from Novartis, personal fees from Orbus, personal fees from Philogen, grants from Quercis, personal fees from yMabs, outside the submitted work. AvD reports patents for “DNA-methylation based method for classifying tumor species” (EP16710700 and EP15158660), and is receiving royalties for diagnostic use of IDH1 R132H mutant specific antibody H09 and BRAF V600E mutant specific antibody VE1 of which all terms are being managed by the German Cancer Research Center in accordance with its conflict of interest policies. HJD reports personal fees from Abbvie, outside the submitted work. BGB reports personal fees and non-financial support from Roche, outside the submitted work. MJvdB reports grants from Dutch Cancer Foundation, grants from Brain Tumor Charity, grants from Strijd van Salland, grants from MSD formerly Schering Plough, during the conduct of the study; personal fees from Carthera, personal fees from Nerviano, personal fees from Bayer, personal fees from Celgene, personal fees from Agios, personal fees from Abbvie, personal fees from Karyopharm, personal fees from Boston Pharmaceuticals, personal fees from Genenta, outside the submitted work. All other authors declare no competing interests.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–96, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Perry JR, Laperriere N, O’Callaghan CJ, et al. : Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med 376:1027–1037, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens AC, Gorlia T, et al. : MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom A, Gronberg BH, Marosi C, et al. : Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–26, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Wick W, Platten M, Meisner C, et al. : Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–15, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Wick W, Roth P, Hartmann C, et al. : Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol 18:1529–1537, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Bent MJ, Tesileanu CMS, Wick W, et al. : Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol 22:813–823, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. : Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med 372:2499–508, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller M, Weber RG, Willscher E, et al. : Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129:679–93, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Aibaidula A, Chan AK, Shi Z, et al. : Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol 19:1327–1337, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Wesseling P, Aldape K, et al. : cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol 30:844–856, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brat DJ, Aldape K, Colman H, et al. : cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol 136:805–810, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. : Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol 22:515–523, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis DN, Perry A, Wesseling P, et al. : The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draaisma K, Chatzipli A, Taphoorn M, et al. : Molecular Evolution of IDH Wild-Type Glioblastomas Treated With Standard of Care Affects Survival and Design of Precision Medicine Trials: A Report From the EORTC 1542 Study. J Clin Oncol 38:81–99, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Tesileanu CMS, van den Bent MJ, Sanson M, et al. : Prognostic significance of genome-wide DNA methylation profiles within the randomised, phase 3, EORTC CATNON trial on non-1p/19q deleted anaplastic glioma. Neuro Oncol, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahm F, Schrimpf D, Jones DT, et al. : Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131:903–10, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Koopmans AE, Ober K, Dubbink HJ, et al. : Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci 55:6024–30, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Bady P, Delorenzi M, Hegi ME: Sensitivity Analysis of the MGMT-STP27 Model and Impact of Genetic and Epigenetic Context to Predict the MGMT Methylation Status in Gliomas and Other Tumors. J Mol Diagn 18:350–361, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Dubbink HJ, Atmodimedjo PN, van Marion R, et al. : Diagnostic Detection of Allelic Losses and Imbalances by Next-Generation Sequencing: 1p/19q Co-Deletion Analysis of Gliomas. J Mol Diagn 18:775–786, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Dubbink HJ, Atmodimedjo PN, Kros JM, et al. : Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol 18:388–400, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegi ME, Janzer RC, Lambiv WL, et al. : Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol 123:841–52, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Stichel D, Ebrahimi A, Reuss D, et al. : Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol 136:793–803, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Wijnenga MMJ, Dubbink HJ, French PJ, et al. : Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol 134:957–959, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Berzero G, Di Stefano AL, Ronchi S, et al. : IDH-wildtype lower-grade diffuse gliomas: the importance of histological grade and molecular assessment for prognostic stratification. Neuro Oncol 23:955–966, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Chen Y, Bao Z, et al. : Chemoradiotherapy with temozolomide vs. radiotherapy alone in patients with IDH wild-type and TERT promoter mutation WHO grade II/III gliomas: A prospective randomized study. Radiother Oncol 167:1–6, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


