Abstract
Because Cryptosporidium parvum oocysts are very resistant to conventional water treatment processes, including chemical disinfection, we determined the kinetics and extent of their inactivation by monochromatic, low-pressure (LP), mercury vapor lamp UV radiation and their subsequent potential for DNA repair of UV damage. A UV collimated-beam apparatus was used to expose suspensions of purified C. parvum oocysts in phosphate-buffered saline, pH 7.3, at 25°C to various doses of monochromatic LP UV. C. parvum infectivity reductions were rapid, approximately first order, and at a dose of 3 mJ/cm2 (=30 J/m2), the reduction reached the cell culture assay detection limit of ∼3 log10. At UV doses of 1.2 and 3 mJ/cm2, the log10 reductions of C. parvum oocyst infectivity were not significantly different for control oocysts and those exposed to dark or light repair conditions for UV-induced DNA damage. These results indicate that C. parvum oocysts are very sensitive to inactivation by low doses of monochromatic LP UV radiation and that there is no phenotypic evidence of either light or dark repair of UV-induced DNA damage.
Cryptosporidium parvum is an important health-related waterborne pathogen that is ubiquitous in surface and source waters (16) and very resistant to conventional water treatment processes (9). Although conventional water filtration systems achieve some removal (9), chemical disinfection by chlorination is incapable of achieving appreciable inactivation of C. parvum oocysts at practical disinfectant doses and contact times (7, 23). Previous studies using the in vitro viability assays of excystation and vital dye staining suggested that C. parvum oocysts are also very resistant to monochromatic low-pressure (LP) UV radiation (5, 21) and much more resistant than the enteric viruses that have been proposed as the basis for determining UV dosimetry in water and wastewater treatment (11, 12, 19). However, recent studies using in vivo animal bioassays indicate that polychromatic medium-pressure mercury lamp UV as well as LP UV extensively reduce C. parvum oocyst infectivity at relatively low doses (4, 6).
Animal bioassays are considered the “gold standard” for assessing Cryptosporidium oocyst infectivity (14). However, they are costly, require tedious and lengthy procedures for handling animals and scoring for infection, give variable and imprecise estimates of infectious dose and infectivity concentrations, and raise ethical concerns about the use of experimental animals when alternative infectivity assays are available (24). In vitro cell culture infectivity assays using fluorescent antibodies against living stages of C. parvum (27, 32) are reliable and convenient alternatives to animal infectivity assays, but comparisons of cell culture and animal infectivity assays for assessing UV inactivation of Cryptosporidium oocysts have not yet been reported in the literature. In this study, we compared mouse and cell culture infectivity assays in LP UV disinfection experiments on C. parvum oocysts. The kinetics and extent of inactivation of C. parvum oocysts also were compared to those for coliphage MS2, a widely used viral indicator of the efficacy of UV radiation disinfection in water.
Nucleic acids are considered the primary targets of UV radiation (13), and some health-related microorganisms in water, including most indicator bacteria (10, 30), and at least some pathogenic bacteria (8, 28) have one or more DNA repair pathways. However, it is not known if a coccidian protozoan such as C. parvum has DNA repair pathways for UV-induced damage. Therefore, in addition to developing the UV dose-response relationship for C. parvum oocysts exposed to LP UV radiation, comparing its inactivation using animal and cell culture infectivity assays, and assessing coliphage MS2 as a surrogate indicator for UV inactivation of C. parvum oocysts, we evaluated the potential for C. parvum oocysts to repair UV-induced DNA damage through light and dark repair pathways.
MATERIALS AND METHODS
C. parvum and coliphage MS2.
C. parvum oocysts (Iowa strain) were purchased from Pat Mason, Pleasant Hills Farm, Troy, Idaho. Shed oocysts collected daily from experimentally infected 3-day-old calves were screened to remove large debris and hair and then purified and dispersed by processing through discontinuous sucrose gradients, followed by cesium chloride (CsCl) gradients (1.15 g/ml; specific gravity, 1.15). Recovered oocysts were washed in phosphate-buffered saline (PBS; pH 7.2), resuspended in PBS containing 1,000 U penicillin/ml and 1,000 mg of streptomycin/ml, and stored at 4°C.
Bacteriophage MS2, a male-specific (F+) RNA coliphage, was grown and assayed in Escherichia coli C3000 by the double-agar-layer plaque technique (11, 12, 20). Virus stock was produced by scraping the top agar layer of plates having confluent lysis of host cells into a small amount of PBS, extracting it with an equal volume of chloroform, centrifuging it at 4,000 × g for 30 min at 4°C, and recovering the supernatant fluid.
LP UV irradiation system and radiometry.
A collimated-beam UV apparatus consisted of two 15-W LP mercury vapor germicidal lamps emitting nearly monochromatic UV radiation at 253.7 nm that was directed through a circular opening to provide incident radiation normal to the surface of the test suspension in a 60- by 15-mm cell culture petri dish. UV irradiance at 253.7 nm was measured with a radiometer and UV 254 detector (IL500; International Light) that had been factory calibrated, traceable to National Institute of Standards and Technology standards, just prior to this study.
Petri factor and dose determinations.
The measured incident irradiance at the surface of the test liquid (approximately 0.06 mW/cm2) was corrected for any nonhomogeneity of irradiation across the surface area of the petri dish to provide a value for the average incident irradiance. The average irradiance in the mixed suspension was determined mathematically from an integration of the Beer Lambert law over the sample depth, accounting for UV absorbance and incident average irradiance (18). Target doses were computed as the product of average irradiance and time (in seconds), and required exposure times were calculated by dividing the desired UV dose by the average UV irradiance.
UV disinfection experiments.
Five-milliliter volumes of PBS containing purified C. parvum oocysts or coliphage MS2 at concentrations of ∼106 organisms/ml in 60- by 15-mm cell culture petri dishes were irradiated with a UV collimated-beam system while the samples were magnetically stirred slowly at room temperature (23 to 25°C). After predetermined exposure times, the samples were removed from the UV irradiation system and serially diluted 10-fold for subsequent infectivity assays.
DNA repair experiments.
Five samples, each with ∼5 × 106 C. parvum oocysts in 5 ml of PBS, in 60- by 15-mm cell culture petri dishes, were exposed to low doses of UV irradiation (1.2 or 3 mJ/cm2) and then wrapped with aluminum foil immediately after UV exposure. One sample was immediately serially diluted 10-fold and inoculated into cell cultures as an experimental, nonrepair control. Duplicate samples were transferred to 25 and 37°C incubators. One dish at each temperature was illuminated by a 15-W fluorescent lamp at a distance of ∼25 to 50 cm with slow stirring (light repair), and the other was just stirred while kept wrapped with aluminum foil (dark repair). Conditions were 25°C for 2 to 4 h and 37°C for 1 to 2 h as commonly used in bacterial and mammalian cell DNA repair of UV damage (8, 10, 25). After incubation, the samples were immediately serially diluted and inoculated into cell cultures for infectivity assay.
C. parvum infectivity assays. (i) Mouse bioassays.
Oocysts in samples were concentrated by centrifugation at 3,500 × g for 20 min. resuspended in small volumes of water, and administered to week-old, neonatal BALB/c mice at doses of 50,000, 5,000, and 500 oocysts in 25-μl volumes. Mice were housed for approximately 1 week postinoculation and then examined for evidence of infection. Mouse infection was determined by flow cytometric enumeration of intestinal oocysts. Each neonatal mouse was killed by CO2 inhalation, the terminal colon (below the cecum) was removed to a siliconized microcentrifuge tube containing 400 μl of 2.5% aqueous potassium dichromate, and the intestinal contents were expressed and homogenized into potassium dichromate using the wooden applicator and extensive vortexing. The intestinal homogenates were subjected to a microscale discontinuous sucrose gradient method for oocyst purification and suspended in PBS with 0.1% bovine serum albumin (1, 3). For immunofluorescence, the partially purified stool concentrate was incubated for 30 min at 37°C with 5 μl of an oocyst-specific monoclonal antibody (OW50) conjugated with fluorescein isothiocyanate (1/50 dilution in PBS). Samples were adjusted to 600 μl with PBS, stored at 4°C, and protected from light until analyzed for the presence of oocysts by flow cytometry using a FACScan (Becton Dickinson, Mountain View, Calif.) (2). The resulting data files collected were stored on floppy disk and subsequently analyzed for the presence of fluorescing oocyst signals using provided software (Lysis II; Becton Dickinson). Mouse infectivity data, i.e., the number of positive mice out of five or six mice per sample dilution, were analyzed, and the titers were calculated as most probable numbers.
(ii) Cell culture infectivity assay.
Cell culture infectivity assays for C. parvum were done in Madin-Darby canine kidney (MDCK) cell cultures (ATCC CCL-34) grown in well slides and used C. parvum living-stage-specific monoclonal antibodies (C3C3) fluorescently labeled with either the red fluorochrome Cy3 or fluorescein isothiocynate to detect the parasite living stages according to methods similar to those previously described (22, 29). Infectivity assay was quantal and based on scoring the presence or absence of living stages (sexual gamonts and asexual meronts) in 50 to 100 sequential, nonoverlapping fields at a magnification of ×250 or ×400 by epifluorescent microscopy. Fields containing one or more fluorescent development stages of Cryptosporidium were scored as positive, and fields containing no fluorescent life stages were scored as negative. From the number of positive and negative test units (fields), the oocyst infectivity titer was calculated as a most probable number with the Thomas equation.
Data presentation and statistical analysis.
Microorganism concentrations in control samples were computed and taken as No, the initial concentrations. For each test sample, the average microorganism concentrations were computed for each dose (d) as Nd. The proportions of initial microorganisms remaining at each dose, Nd/No, were log10 transformed [log10 (Nd/No)], and the values of replicate experiment were averaged. These average values were then paired with the data for dose and plotted. The extent of log10 reductions of C. parvum oocyst infectivity by different treatments, including DNA repair treatments, was statistically compared by paired t tests and either one-way or repeated-measures analysis of variance using a statistics software package (Statistica; StatSoft, Tulsa, Okla.) on a personal computer.
RESULTS
Table 1 shows a comparison of mouse and cell culture infectivity assays of C. parvum to determine inactivation by 2-, 5-, and 10-mJ/cm2 doses of LP UV radiation. Cell culture and animal infectivity assays provided comparable results, because a UV dose of 2 mJ/cm2 gave reductions of C. parvum oocyst infectivity (1.7 and 1.5 log10, respectively) that were not significantly different (by paired t test at the 5% level).
TABLE 1.
Comparison of inactivation of C. parvum oocysts by LP UV based on cell culture and mouse infectivity assays
| UV dose (mJ/cm2) |
C. parvum reduction (log10 value)a
|
|||||
|---|---|---|---|---|---|---|
| Cell culture infectivity assay
|
Mouse infectivity assay
|
|||||
| Expt 1 | Expt 2 | Mean (SD) | Expt 1 | Expt 2 | Mean (SD) | |
| 2 | 1.7 | 1.7 | 1.7 (0.01) | 1.2 | 1.9 | 1.5 (0.45) |
| 5 | >2.7 | >3.2 | 2.7 | >3.9 | ||
| 10 | >2.7 | >3.2 | >2.6 | 4.3 | ||
A “greater than” sign indicates that no living stages of C. parvum were observed in the sample volume analyzed. However, for calculation purposes, it was assumed that one living stage was detected in the total sample volume analyzed, and this was used to calculate an infectivity concentration.
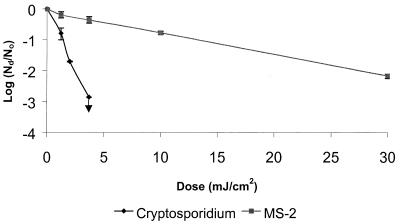
Figure 1 shows the average kinetics of reduction of C. parvum and coliphage MS2 by several different doses of LP UV radiation in PBS at room temperature. The reductions of C. parvum infectivity were very rapid, approximately first order, and reached the detection limits of the infectivity assays (∼3 log10) within a dose of 3 mJ/cm2. The reductions of coliphage MS2 were considerably slower and less extensive than those of C. parvum, with only an ∼2-log10 reduction at a dose of 30 mJ/cm2. These kinetics of MS2 reduction by LP UV radiation are similar to those previously reported in the literature (17, 20, 26, 31).
FIG. 1.
Kinetics of inactivation of C. parvum and coliphage MS2 by monochromatic LP UV radiation. Error bars indicate ranges of data from two to five replicate experiments per dose. ↓, detection limit.
Table 2 summarizes the results of experiments investigating dark and light DNA repair of UV-irradiated C. parvum oocysts dosed with 1.2 and 3 mJ of LP UV/cm2. The reductions of C. parvum oocyst infectivity by both doses were not restored by exposing UV-irradiated oocysts to either light or dark repair conditions. At a UV dose of 1.2 mJ/cm2, the log10 reductions of C. parvum oocyst infectivity for control, dark repair, and light repair samples were not significantly different at the 5% level by one-way or repeated-measures analysis of variance (data not shown). At a UV dose of 3 mJ/cm2, C. parvum inactivation was >2.6 log10 and there was no restoration of this infectivity reduction by exposure of the oocysts to light or dark repair conditions. Therefore, there was no evidence of either light or dark repair of DNA damage caused by LP UV irradiation of C. parvum oocysts at doses causing relatively low (∼1-log10) and high (>2.6-log10) infectivity reductions and associated nucleic acid damage.
TABLE 2.
Effects of dark and light repair conditions on infectivity of C. parvum oocysts dosed with 1.2 and 3 mJ LP UV/cm2a
| Dose and condition | Mean log10C. parvum reduction (1SD)
|
||
|---|---|---|---|
| Control | Dark | Light | |
| 1.2 mJ/cm2 | |||
| 37°C, 1 h | 0.8 (0.21) | 0.8 (0.21) | 0.9 (0.21) |
| 37°C, 2 h | 0.8 (0.08) | 1.7 (0.86) | 1.0 (0.35) |
| 25°C, 2 h | 0.8 (0.21) | 0.9 (0.20) | 1.0 (0.25) |
| 25°C, 4 h | 0.7 (0.04) | 1.4 (0.30) | 0.9 (0.18) |
| 3 mJ/cm2 | |||
| 37°C, 1 h | >2.6 | >2.6 | >2.6 |
| 25°C, 2 h | >2.6 | >2.6 | >2.6 |
Average values of duplicate or triplicate experiments.
DISCUSSION
In vitro cell culture infectivity assays provided a level of sensitivity similar to that of in vivo animal infectivity assays for C. parvum and gave comparable results for oocyst inactivation by LP UV. Cell culture infectivity assays have several advantages over mouse infectivity assays: the former are more precise (because the numbers of test units scored as infected or uninfected per oocyst dose are more than an order of magnitude greater), faster (days instead of weeks), simpler (because mice do not have to be acquired, housed, fed, handled, sacrificed, and necropsied, etc.), less variable (because cell lines are more genetically stable than mice from different litters), and not ethically controversial. Therefore, in vitro cell culture assays are reliable and convenient substitutes for animal assays of C. parvum infectivity.
C. parvum oocysts were very sensitive to UV irradiation from LP UV lamps, a finding which supports a recently published study using a mouse infectivity assay (6). Appreciable inactivation of C. parvum oocysts was achieved at practical doses of LP UV radiation comparable to doses reported for polychromatic medium-pressure UV radiation (4). The enteric bacteriophage MS2 was much more resistant than C. parvum to LP UV radiation. Because C. parvum has a much larger overall size and genome size than MS2 (five chromosomes ranging in size from 1,400 to >3,300 kb [15], compared to about 4 kb of single-stranded RNA), it is a much larger “target” for UV irradiation. Because MS2 is one of the waterborne enteric microorganisms more resistant to UV radiation (17, 31), it and the other male-specific coliphages are useful treatment indicators for UV disinfection processes (11, 12).
There was no detectable phenotypic evidence of either light or dark repair of UV-damaged DNA in C. parvum oocysts under the conditions tested (both bacterial and mammalian DNA repair conditions). The UV doses tested in this study were at least an order of magnitude lower than the UV doses typically used in water and wastewater treatment practice (1 to 3 mJ/cm2, compared to 30 to 40 mJ/cm2). It is unlikely, therefore, that typical UV treatment doses would allow for DNA repair and reactivation, due to the greater extent of UV damage. However, alternative DNA repair mechanisms may exist and be activated under different environmental conditions than those used in this study. Therefore, more thorough biochemical and genetic studies are recommended to further investigate the presence of DNA repair activities in C. parvum under a variety of reactivation conditions. Because C. parvum oocysts are very sensitive to low doses of LP UV radiation, properly designed and operated LP UV disinfection systems should be able to control this pathogen in wastewater effluents and water supplies.
ACKNOWLEDGMENTS
This research was supported by funds from the Water Environment Research Foundation (project no. 98-HHE-2) and from Trojan Technologies Inc., London, Ontario, Canada.
REFERENCES
- 1.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 2.Arrowood M J, Hurd M R, Mead J R. A new method for evaluating experimental cryptosporidial parasite loads using immunofluorescent flow cytometry. J Parasitol. 1995;81:404–409. [PubMed] [Google Scholar]
- 3.Arrowood M J, Donaldson K. Improved purification methods for calf-derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol. 1996;43:S89. doi: 10.1111/j.1550-7408.1996.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 4.Bukhari Z, Hardy T M, Bolton J R, Dussert B, Clancy J L. Medium-pressure UV light for oocyst inactivation. J Am Water Works Assoc. 1999;91(3):86–94. [Google Scholar]
- 5.Campbell A T, Robertson L J, Snowball M R, Smith H V. Inactivation of oocysts of Cryptosporidium parvum by ultraviolet light. Water Res. 1995;29:2583–2586. [Google Scholar]
- 6.Clancy J L, Bukhari Z, Hardy T M, Bolton J, Dussert B, Marshall M M. Using UV to inactivate Cryptosporidium. J Am Water Works Assoc. 2000;92(9):97–104. [Google Scholar]
- 7.Clark R M, Hurst C J, Regli S. Costs and benefits of pathogen control in drinking water. In: Craun G F, editor. Safety of water disinfection: balancing chemical and microbial risks. Washington, D.C.: International Life Sciences Press; 1993. pp. 181–198. [Google Scholar]
- 8.Das G, Kaveri S, Das J. Repair of ultraviolet-light-induced DNA damage in Vibrio cholerae. Biochim Biophys Acta. 1981;655:413–420. doi: 10.1016/0005-2787(81)90053-8. [DOI] [PubMed] [Google Scholar]
- 9.Gibson C J, III, Hass C N, Rose J B. Risk assessment of waterborne protozoa: current status and future trends. Parasitology. 1998;117(Suppl.):S205–S212. doi: 10.1017/s0031182099004746. [DOI] [PubMed] [Google Scholar]
- 10.Harris G D, Adams V D, Sorensen D L, Curtis M S. Ultraviolet inactivation of selected viruses with photoreactivation of the bacteria. Water Res. 1987;21:687–692. [Google Scholar]
- 11.Havelaar A H, Meulemans C C E, Pot Hogeboom W M, Koster J. Inactivation of bacteriophage MS2 in wastewater effluent with monochromatic and polychromatic UV light. Water Res. 1990;24:1387–1394. [Google Scholar]
- 12.Havelaar A H, Nieuwstad T J, Meulemans C C E, Van Olphen M. F-specific RNA bacteriophages as model viruses in UV disinfection of wastewater. Water Sci Technol. 1991;24:347–352. [Google Scholar]
- 13.Jagger J. Introduction to research in ultraviolet photobiology. Englewood Cliffs, N.J: Prentice-Hall; 1967. [Google Scholar]
- 14.Korich D G, Marshall M M, Smith H V, O'Grady J, Bukhari Z, Fricker C R, Rosen J P, Clancy J L. Interlaboratory comparison of the CD-1 neonatal mouse logistic dose-response model for Cryptosporidium parvum oocysts. J Eukaryot Microbiol. 2000;47:294–298. doi: 10.1111/j.1550-7408.2000.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 15.Lally N C, Baird G D, McQuay S J, Wright F, Oliver J J. A 2359-base pair DNA fragment from Cryptosporidium parvum encoding a repetitive oocyst protein. Mol Biochem Parasitol. 1992;56:69–78. doi: 10.1016/0166-6851(92)90155-d. [DOI] [PubMed] [Google Scholar]
- 16.LeChevallier M W, Norton W D. Giardia and Cryptosporidium in raw and finished water. J Am Water Works Assoc. 1995;87(9):54–68. [Google Scholar]
- 17.Meng Q S, Gerba C P. Comparative inactivation of enteric adenovirus, poliovirus and coliphages by ultraviolet radiation. Water Res. 1996;30:2665–2668. [Google Scholar]
- 18.Morowitz H J. Absorption effects in volume irradiation of microorganism. Science. 1950;111:229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwstad T J, Havelaar A H, van Olphen M. Hydraulic and microbiological characterization of reactors for UV disinfection of secondary effluent. Water Res. 1991;25:775–784. [Google Scholar]
- 20.Nieuwstad T J, Havelaar A H. The kinetics of batch ultraviolet inactivation of bacteriophage MS2 and microbiological calibration of an ultraviolet pilot plant. J Environ Sci Health. 1994;29:257–273. [Google Scholar]
- 21.Ransome M E, Whitmore T N, Carrington E G. Effect of disinfectants on the viability of Cryptosporidium parvum. Water Supply. 1993;11:103–117. [Google Scholar]
- 22.Slifko T R, Friedman D, Rose J B, Jakubowski W. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl Environ Microbiol. 1997;63:3669–3675. doi: 10.1128/aem.63.9.3669-3675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobsey M D. Inactivation of health-related microorganisms in water by disinfection processes. Water Sci Technol. 1989;21:179–195. [Google Scholar]
- 24.Sobsey M D, Leland S E., Jr . Methods of testing protozoacides and antihelminthics. In: Block S S, editor. Disinfection, sterilization and preservation. 5th ed. New York, N.Y: Lippincott-Williams and Wilkins; 2001. pp. 1413–1428. [Google Scholar]
- 25.Sutherland B M, Cimino J S, Delihas N, Shih A G, Oliver R P. Ultraviolet light-induced transformation of human cells to anchorage-independent growth. Cancer Res. 1980;40:1934–1939. [PubMed] [Google Scholar]
- 26.Tree J A, Adams M R, Lees D L. Virus inactivation during disinfection of wastewater by chlorination and UV irradiation and the efficacy of F+ bacteriophages as a viral indicator. Water Sci Technol. 1997;35:227–232. [Google Scholar]
- 27.Upton S J, Tilley M, Brillhart D B. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 28.U. S. Environmental Protection Agency. Design manual: municipal wastewater disinfection. EPA/625/1–81/021. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1986. [Google Scholar]
- 29.Venczel L V, Arrowood M J, Hurd M, Sobsey M D. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl Environ Microbiol. 1997;63:1598–1601. doi: 10.1128/aem.63.4.1598-1601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitby G E, Palmateer G, Cook W G, Maarschalkerweerd J, Huber D, Flood K. Ultraviolet disinfection of secondary effluent. J Water Pollut Control Fed. 1984;56:844–850. [Google Scholar]
- 31.Wilson B R, Roessler P F, Van Dellen E, Abbaszadegan M, Gerba C P. Proceedings of Water Quality Technology Conference. Denver, Colo: American Water Works Association; 1992. Coliphage MS-2 as a UV disinfection efficacy test surrogate for bacteria and viral pathogens; pp. 219–235. [Google Scholar]
- 32.You X, Arrowood M J, Lejkowski M, Xie L, Schinazi R F, Mead J R. A chemiluminescence immunoassay for evaluation of Cryptosporidium parvum growth in vitro. FEMS Microbiol Lett. 1996;136:251–256. doi: 10.1111/j.1574-6968.1996.tb08057.x. [DOI] [PubMed] [Google Scholar]