Abstract
Bone tissue engineering (BTE) is a promising method for the repair of difficult-to-heal bone tissue damage by providing three-dimensional structures for cell attachment, proliferation, and differentiation. Traditional Chinese medicine (TCM) has been introduced as an effective global medical program by the World Health Organization, comprising intricate components, and promoting bone regeneration by regulating multiple mechanisms and targets. This study outlines the potential therapeutic capabilities of TCM combined with BTE in bone regeneration. The effective active components promoting bone regeneration can be generally divided into flavonoids, alkaloids, glycosides, terpenoids, and polyphenols, among others. The chemical structures of the monomers, their sources, efficacy, and mechanisms are described. We summarize the use of compounds and medicinal parts of TCM to stimulate bone regeneration. Finally, the limitations and prospects of applying TCM in BTE are introduced, providing a direction for further development of novel and potential TCM.
Graphical Abstract
Keywords: Traditional Chinese medicine, Bone tissue engineering, Bone regeneration, Scaffolds, Osteogenesis
Traditional Chinese medicine (TCM)
Role of TCM
TCM comprises natural products and extracts derived from herbs, animals, and minerals with effective bio-functions for maintaining health and treating disease. During decades, its use has been widespread globally [1–3]. As early as the Eastern Han Dynasty, the classical text on Chinese Medicine, Shen Nong’s Materia Medica (Shen Nong Ben Cao Jing), recorded the use of TCM in the treatment of diseases. Nowadays, TCM has been shown to play a crucial role in the prevention and management of diseases, such as cardiovascular disease, cancer, and diabetes [4–6]. TCM was considered a “miracle” drug for certain major diseases, such as artemisinin in malaria and arsenic trioxide in acute promyelocytic leukemia [7, 8]. Therefore, further understanding and expanding the use of TCM is necessary for continued developments in the field.
Classification of TCM
The classification of TCM is sophisticated and complex. From the ancient books and Pharmacopoeias, such as Shen Nong’s Materia Medica [9], Compendium of Materia Medica (Ben Cao Gang Mu) [10], Yellow Emperor’s Inner Canon and Treatise on Cold Damage, to the latest Chinese Pharmacopoeia [11, 12], the classification standard of TCM are still difference. And some of the scholar different from the traditional classification in China, some scholars divided TCM into Alkaloids, Terpenoids, Flavonoids, Volatile Oils etc. based on the active components (Table 1). The classification of TCM in this review is based on the active components reported for application in bone tissue engineering (BTE) that promote bone formation, including flavonoids, alkaloids, glycosides, terpenoids, and polyphenols, among others. And the drug component has been shown in Table 2. The classification of TCM is significant for guiding clinical application and avoiding instability and unsafety caused by improper combination [13].
Table 1.
The classification standard of TCM
| Classification standard | Category | References |
|---|---|---|
| Shen Nong’s Materia Medica | Top-grade | [9] |
| Medium-grade | ||
| Low-grade | ||
| Compendium of Materia Medica (Ben Cao Gang Mu) | Sources, habitats, colors, parts of plants, and how they were collected, processed, and selected for prescriptions | [10] |
| Four fundamental characters: cold, cool, warm, and hot | [27] | |
| Five fundamental tastes: salty, sour, bitter, sweet, and pungent | ||
| Four toxic states: toxic, nontoxic, very toxic, and slightly toxic | ||
| 12 meridians: bladder, spleen, large intestine, stomach, small intestine, liver, cardiovascular, heart, kidney, gallbladder, pericardium and san jiao | ||
| Yellow Emperor’s Inner Canon | Herb-derived medicine, animal-derived medicine and mineral-derived medicine | [11, 12] |
| Treatise on Cold Damage | ||
| Chinese Pharmacopoeia | ||
| Active components | Alkaloids | [28–30] |
| Terpenoids | ||
| Flavonoids | ||
| Volatile Oils | ||
| Lignanoids | ||
| Coumarins | ||
| Quinones | ||
| Phenols | ||
| Glycosides | ||
| Saponins | ||
| Stilbenes | ||
| Phenols | ||
| Esters |
Table 2.
The basic information of TCM
| Category | Components | Structural forms | Molecular weight | Source | Main function | References |
|---|---|---|---|---|---|---|
| Flavonoids | Icariin |
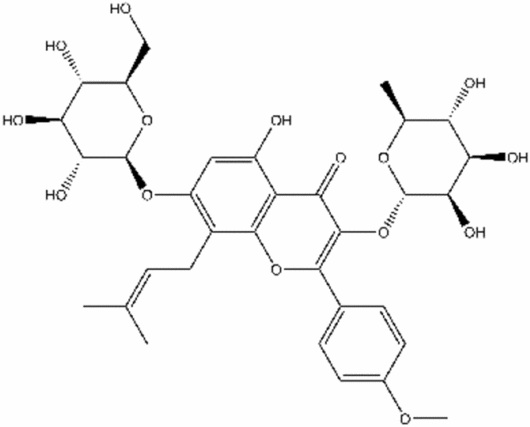
|
676.67 | Epimedium species | Treatment of fractures, joint disease, and gonadal dysfunctions | [31, 32] |
| Icaritin |
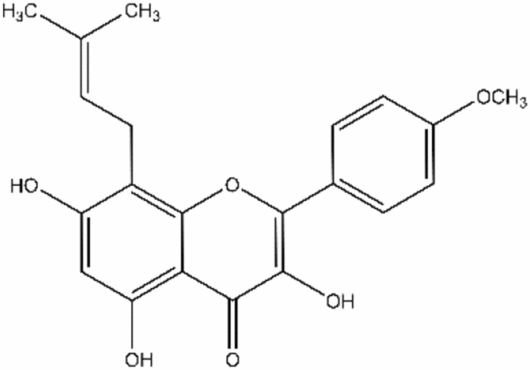
|
386.4 | Epimedium species | Osteoprotective effect, neuroprotective effect, cardiovascular protective effect, anti-cancer effect, anti-inflammation effect, and immune-protective effect | [33] | |
| Hydroxy safflower yellow A |
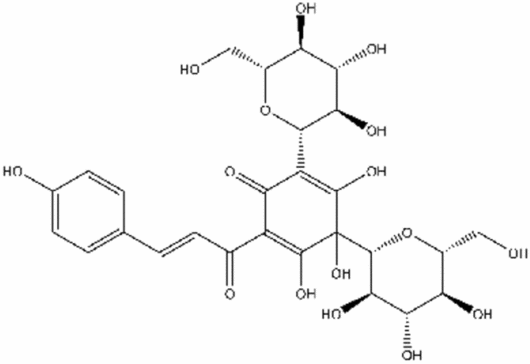
|
612.53 | Safflower | Cardiovascular protection, coronary heart disease treatment and capillary angiogenesis, blood circulation and dispersing blood stasis | [34–38] | |
| Xanthohumol |
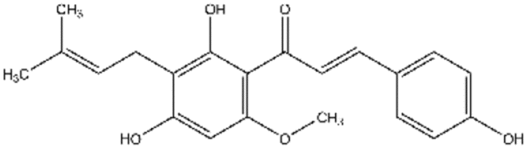
|
354.4 | Humulus lupulus | Stimulate osteogenic differentiation, anti-inflammatory, and inhibits osteoclastogenesis | [39–41] | |
| Kaempferol |
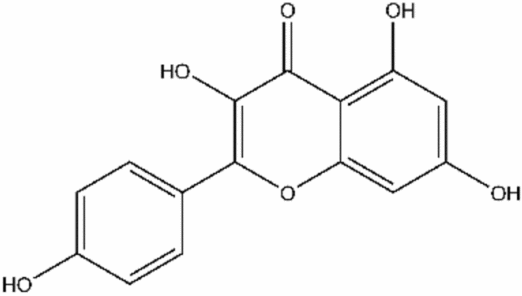
|
286.23 | Kaempferia galangal | Osteoporosis, diabetes, obesity, immune regulation, antiviral, and antidepressant treatments | [42–45] | |
| Cuscuta chinensis Lam. | Not applicable (NA) | N/A | Kaempferia galangal | Osteoporosis treatment | [45] | |
| Baicalin |
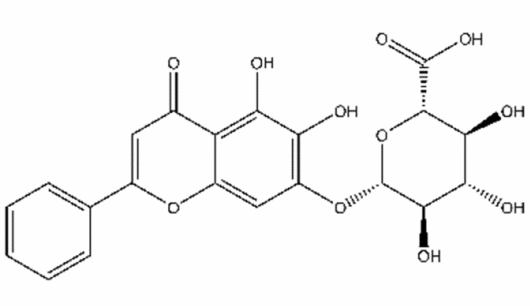
|
446.36 | Radix Scutellariae | Antioxidant, antiapoptotic, and immunoregulatory activities with minimal side-effects | [46, 47] | |
| Baicalein |
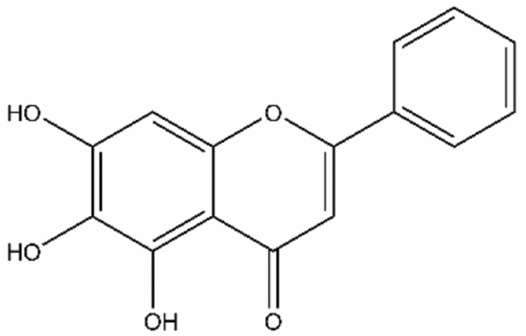
|
270.24 | Radix Scutellariae | Antioxidant, antiapoptotic, and immunoregulatory activities with minimal side-effects | [46, 47] | |
| Naringin |
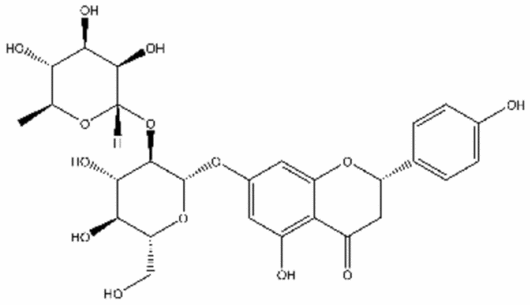
|
580.53 | Tomatoes, grapefruits, and many other citrus fruits | Anti-inflammatory, antiapoptotic activities, and have therapeutic potential cancer, cardiovascular disease, diabetes, and oral disease | [48–50] | |
| Hesperetin |
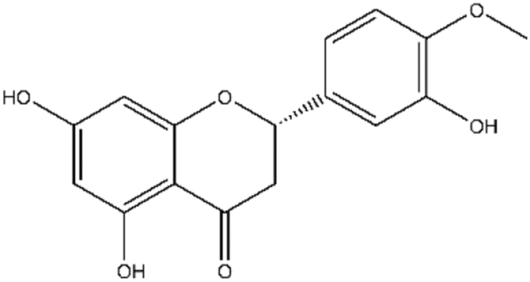
|
302.28 | Chenpi | Antioxidant, anti-inflammatory, and anti‐carcinogenic effects | [51] | |
| Quercetin |
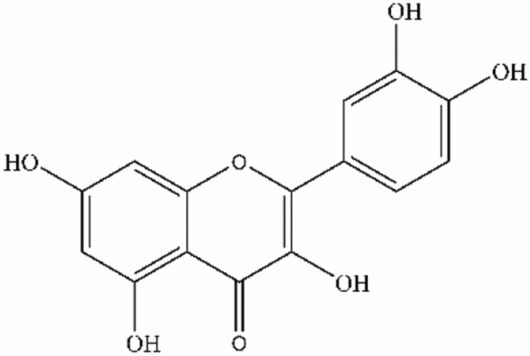
|
302.24 | Quercetum, fruit and vegetables | Anti-inflammatory, anti-viral, anti-oxidant, anti-cancer properties, osteogenesis and angiogenesis | [52, 53] | |
| Silymarin |
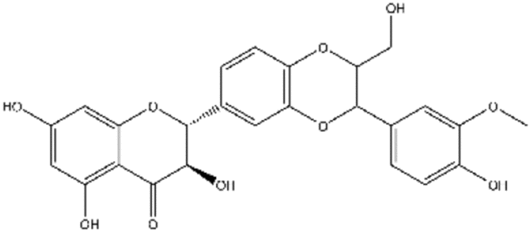
|
482.44 | Silybum marianum | Hepatoprotective effects, anti-viral, anti-Parkinson, anti‐Alzheimer effects, anti-cancer and anti‐inflammatory | [54, 55] | |
| Alkaloids | Tetrandrine |
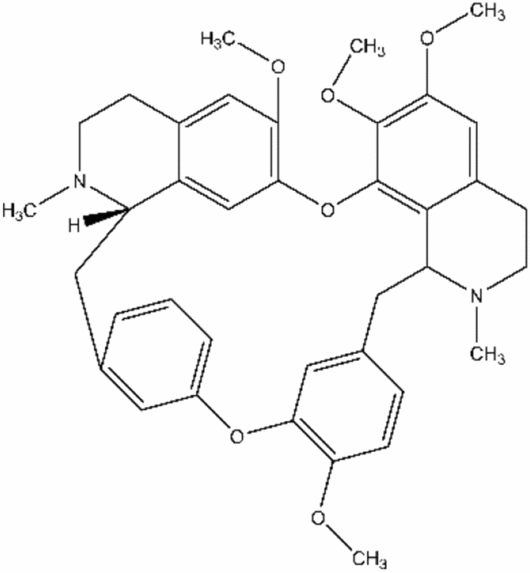
|
622.76 | Stephania tetrandria | Anti-inflammatory, immunosuppressant, anti-allergic effects, anti-oxidant, anti-diabetic and anti-microbial | [56, 57] |
| Berberine |
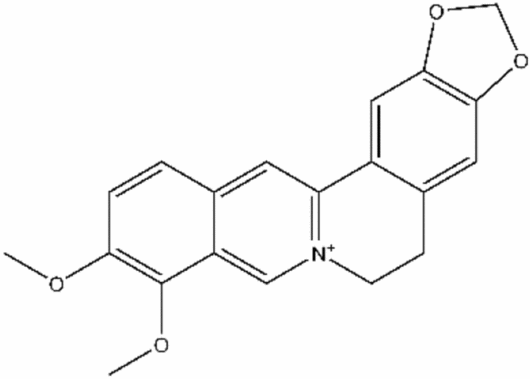
|
336.36 | Rhizoma coptidis | Diabetes, anti-inflammation, anti-cancer therapies, lowing of blood lipids and promote bone formation | [58–60] | |
| Glycosides | Ginsenoside Rg1 |
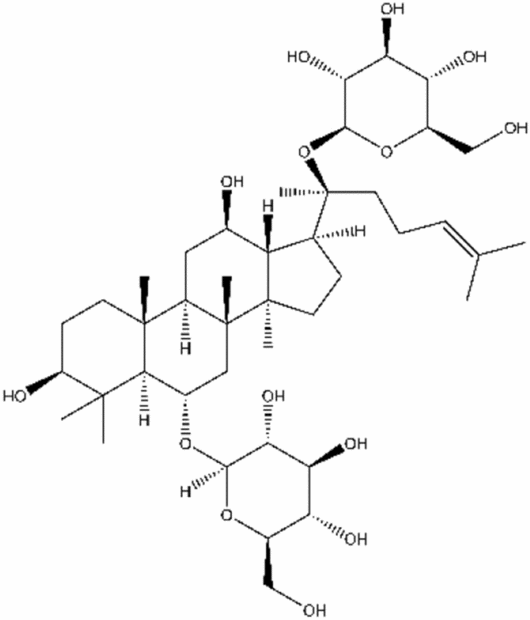
|
801.01 | Ginseng | Cell proliferation and differentiation, anti-apoptosis, and anti-inflammation | [61] |
| Ginsenoside Rb1 |
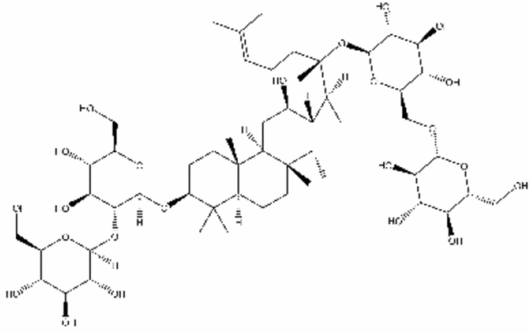
|
1109.31 | Ginseng | Osteogenesis | [62] | |
| Terpenoids | Ursolic acid |
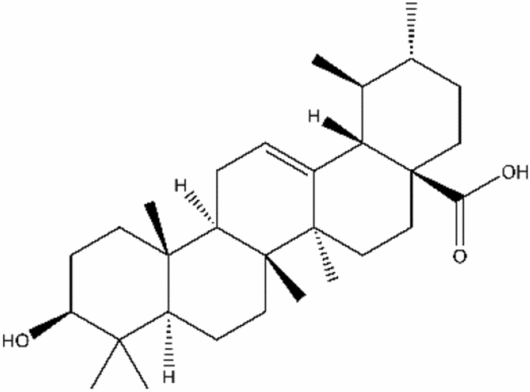
|
456.70 | Fruits and vegetables | Anticancer, antioxidant, and other pharmacological effects | [63] |
| Polyphenols | Resveratrol |
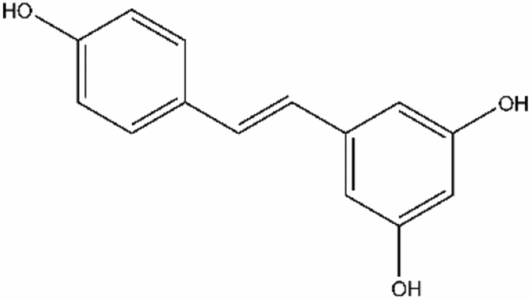
|
228.25 | Veratrum grandiflorum O. Loes | Mediating inflammation, tumerogenesis, and cardioprotective effects | [64–66] |
| Curcumin |
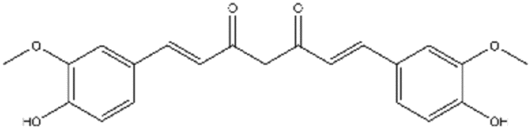
|
368.39 | Curcuma long L. | Anti-oxidant, anti-inflammatory, and anti-cancer effects | [67] | |
| Epigallocatechin gallate |
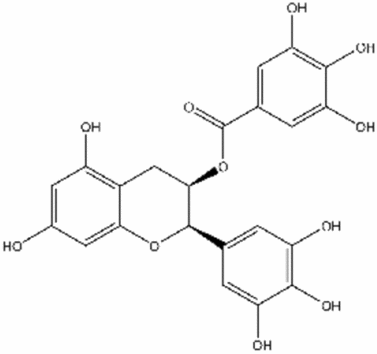
|
458.38 | Camellia sinensis L. | Anti-oxidant and anti-inflammatory effects | [68] |
BTE
Bone remodeling and regeneration are continuous and dynamic physiological processes regulated by two cellular mechanisms, namely bone formation and resorption [14]. The loss of bone tissue can occur following an accident, trauma, cancer, and congenital malformation. Although bone remodeling and regeneration is a lifelong process, a bone defect, especially A large one, severely affects the function of the defunct area and quality of life due to the limited self-repair capacity of bone tissue and some inevitable side effects after surgery [15, 16]. Currently, the clinical “gold standard” for bone transplantation and reconstruction is autogenous bone grafts; however, the risk of donor site morbidity, limited graft supply, and bone formation delay must be seriously considered [17, 18]. To address naturally arising difficulties, BTE has been applied to provide a cell friendly microenvironment for defect repair and tissue regeneration. BTE is an interdisciplinary field that combines the principles of engineering and biology to develop biological substitutes for restoring, maintaining, or improving bone tissue function [19, 20]. BTE has been applied in the treatment of large sections of bone tissue are absent, including traumas, bone cancer tumor resection, congenital malformation, and debridement of infected bone tissue [21]. The ideal characteristics of BTE including non-immunogenic, biocompatibility, controllable, readily available and have the mechanical properties similar to the natural tissue material, and possess suitable structure, architecture, and pore sizes for cells survival and activity [22, 23]. The advent and development of BTE brought about promising approaches for bone regeneration by ways of including the three key factors, namely (1) cells, (2) scaffolds, and (3) growth factors [24–26].
The application of TCM in BTE
The integrate TCM with BTE has a unique advantage in bone regeneration. Oral administration is the typical route for drug delivery, but the drawbacks such as first-pass metabolism would reduce the drug efficacy [69], while the topical delivery would prevention of first pass effect by liver and gut enzymes [70, 71]. To achieve successful and satisfactory therapeutic results, oral delivery requires overcoming the challenges by increasing the permeability of the intestinal epithelial membrane, inhibiting the degrading enzymes or protecting therapeutic by encapsulation [72], while the topical delivery can avoid this problem. Besides, the side effects of large doses of drugs have not been fully elucidated. At present, some drugs, such as BMP-2 and PTH, can be combined with BTE to promote bone defect healing. However, this may lead to extensive spread and subsequent accumulation in different organs, and further induce negative systemic side effects. Meanwhile, the production of these medicines are expensive [73]. Although the systemic administration of TCM shows low toxicity and side effects, it usually takes effect slowly. Therefore, regional TCM may provide a suitable alternative to TCM therapy. By integrating TCM with BTE, the BTE could act as a delivery carriers, and the topically-applied drug cannot only reach the interior target tissue with a greater bioavailability, but also prolong residence time as well as sustain drug release [74]. At the same time, TCM combined with BTE can improve its mechanical properties, such as Young’s modulus, compressive strength, hydrophilicity [75, 76].
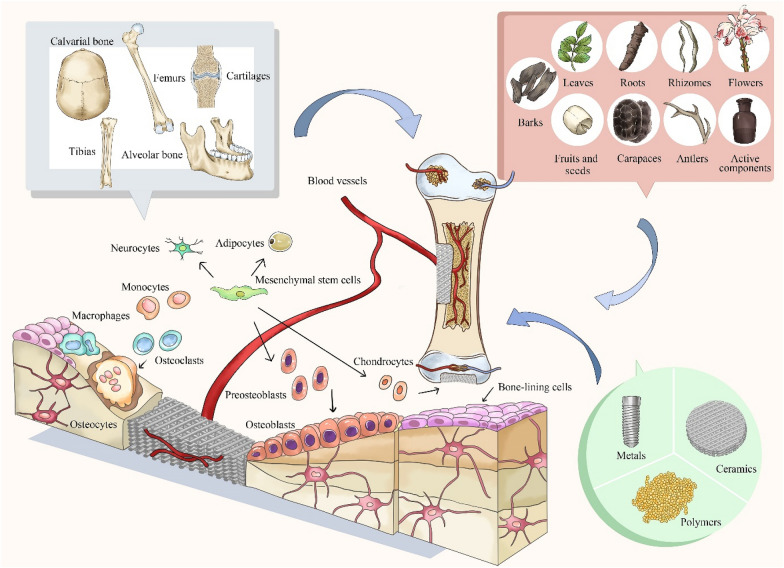
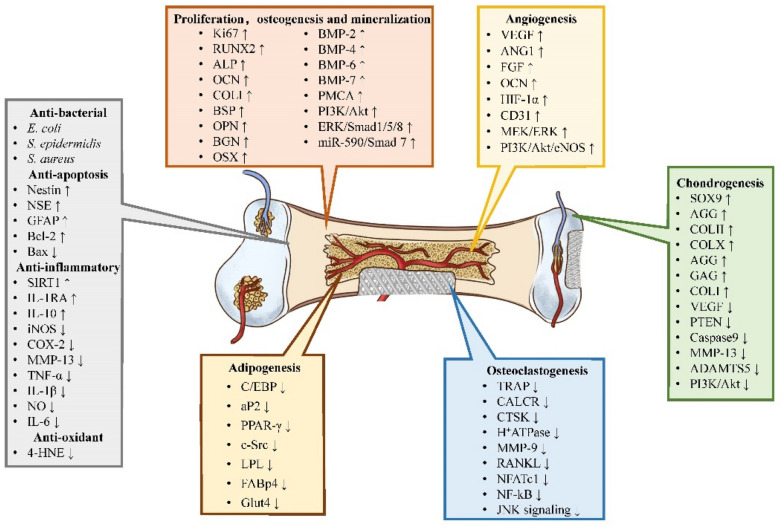
Bone has the capacity of self-renewal; nevertheless, bone tissue regeneration remains a challenge [77]. The different types of TCM summarized here promote bone formation via multiple mechanisms and targets (Fig. 1), for instance, regulating the process of cell proliferation, osteogenesis and mineralization; chondrogenesis; angiogenesis; osteoclastogenesis; adipogenesis; and anti-inflammatory, anti-oxidant, anti-bacterial, and anti-apoptosis (Fig. 2).
Fig. 1.
Schematic diagram representing the application of traditional Chinese medicine in bone tissue engineering to accelerate bone regeneration
Fig. 2.
The osteogenic mechanism of TCM ingredients
Proliferation, osteogenesis and mineralization
Traditional Chinese medicine (TCM) has been praised in the world of medicine due to its effects in promoting cell proliferation, regulating bone metabolism, etc. [78], as shown in Table 3. Based on the variety of biomaterials, the use of TCM exhibits great biocompatibility and low cytotoxicity to the cells seeded on the biomaterial, even at extremely high concentrations, as shown by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromile (MTT) assay and cell counting Kit-8 (CCK-8) assay [76, 79–82]. Icariin loaded PHBV scaffold significantly promoted the proliferation of human osteoblast-like MG-63 cells and the pre-osteoblast MC3T3-E1 cells in a concentration-dependent manner, as shown by Alamar blue assay, and the enhanced cellular proliferation results were due to the upregulating expression of BMP-2, BMP-6, BMP-7 and BGN [83]. Resveratrol and Ang-2 combined with PEGDA/TCS hydrogel showed a good cytocompatibility, and cell density in the resveratrol group was significantly higher than that in the control groups in a hypoxic environment, which was verified by the proliferation marker Ki67 via WB assay [84]. In addition, in the epigallocatechin gallate loaded gelatin scaffold, the chemical modification by epigallocatechin gallate would mitigate MMP-2 and -9 expression, thus slowing down the degradation of gelatin scaffold [85].
Table 3.
Promoting proliferation, osteogenesis and mineralization
| Active components | Biomaterials | Experimental models | Efficacy | References |
|---|---|---|---|---|
| Icariin | SF/SBA15 | Rat BMSC (rBMSC), 38.4 µM | Up-regulating RUNX2, ALP, OCN, and COLI | [94] |
| SF/PLCL nanofibrous membrane | rBMSC, 10−5 mol/L; rat calvarial defects model | Up-regulating ALP activity | [76] | |
| PLGA microspheres | rBMSC, 4 × 10−3 M; rat calvarial defects model | Up-regulating RUNX2, ALP, OCN, COLI, and OPN | [125] | |
| Col/PCL/HAp composite scaffolds | rBMSC; rabbit tibial plateau defects model | Up-regulating ALP, COLI, OCN, and OPN | [86] | |
|
CS-modified halloysite nanotubes |
hASCs, 10−5 M; | Up-regulating ALP | [79] | |
| CS/nHAP | Osteoblast | N/A | [80] | |
| PCL/Gel | MC3T3-E1, 0.05 wt% | Up-regulating ALP, OCN, COLI, and calcium content | [91] | |
| PLGA/TCP | MC3T3-E1, 0.32%; SAON rabbit distal femur defect model; | Up-regulating BSP, OCN, and ALP | [92] | |
| TCP | Ros17/28, 5 × 10−5 M | Up-regulating ALP | [93] | |
| PHBV scaffolds | MC3T3-E1, MG-63, 25 mg/mL | Up-regulating BGN, BMP-2, BMP-6, and BMP-7 | [83] | |
| BioCaP | MC3T3-E1, 5 mg/L; rat calvarial defects model | Up-regulating ALP, OCN, RUNX2, BMP-2, and COLI | [87] | |
| ECM-SIS | MC3T3-E1, 10−5 M; mouse calvarial defect model | Up-regulating ALP, BSP, OCN, and BMP-4 | [117] | |
| Icaritin | PLGA/TCP | Rabbit BMSC, 0.74 g/kg; rabbit bilateral thigh muscles model | Up-regulating ALP and calcium deposition | [88] |
| PLGA/TCP | Rabbit BMSC, 1.4 × 10−3 M; | Up-regulating COLI, ALP, OCN and calcium deposition | [95] | |
| Hydroxy safflower yellow A | BG | rBMSCs, HUVECs; rat calvarial defects model | Up-regulating RUNX2, OPN, OCN, ALP and BMP-2 | [96] |
| Xanthohumol | HA-g-PLLA | MC3T3-E1, 5, 10, 20 wt% | N/A | [81] |
| Kaempferol | TiO2 | rBMSC; rat femur defect | Up-regulating RUNX2, OCN, ALP, OPN and ON | [97] |
| Zn | MG-63, 25 µM; Zebrafish model | Up-regulating RUNX2, COLI, ALP, OCN, and ON | [98] | |
| Baicalin and baicalein | Ca-polyP | Primary human osteoblasts, 3 µg/mL | Up-regulating calcium, calcium efflux channel, PMCA and ALP | [119] |
| Naringin | SF/HAp | hUCMSCs, 0.1 wt%; rabbit femoral distal bone defect | Up-regulating ALP, RUNX2, OSX and COL1A and promoting AKT and PI3K phosphorylation | [49] |
| microsphere/SAIB hybrid depots | Primary osteoblasts, 4% w/w; mouse calvarial defect model | Up-regulating ALP, RUNX2 and OCN | [99] | |
| CS | UMR106, 5 wt%; | Up-regulating ALP | [100] | |
| PLGA/PLLA/PDLLA | MC3T3-E1, 7 wt%; | N/A | [82] | |
| PLGA | Rat calvarial defects model | Up-regulating BMP-2, OSX, OPN, BSP, COLI, OCN and calcium content | [116] | |
| Hesperetin | Gel | hMSC, 1 µM; rat tibial osteotomy model | Up-regulating RUNX2, ALP, OCN and COLI and promoting ERK and Smads-1/5/8 phosphorylation | [89] |
| Quercetin | Ti | hMSCs, 64 ± 10 and 842 ± 361 nmol | Up-regulating ALP activity and calcium content | [126] |
| DC/HAp | Rabbit BMSCs, 25 µM; rat calvarial defect model | Up-regulating RUNX2, OCN, and COLI | [101] | |
| PLLA | MC3T3-E1, 200 µM | Up-regulating RUNX2, ALP, OCN, and COLI | [102] | |
| MSCS/PCL | Wharton’s jelly MSC, 2% | Up-regulating calcium deposit | [127] | |
| Decellularized goat-lung scaffold | BMSC, 100 µM | Up-regulating ALP and calcium deposits | [128] | |
| Ti | hUCMSCs, HGF, 50 nM | Up-regulating RUNX2, COL, OCN, and ALP | [103] | |
| Silymarin | DC/HAp | rBMSCs, 100 µM; rat calvarial bone defect model | Up-regulating RUNX2, COLI, and OCN | [104] |
| PLA/Carbon nanotubes | Wharton’s jelly MSCs; rat calvarial bone defect model | Up-regulating ALP | [105] | |
| Silibinin | Zn | C3H10T1/2, MG-63, 60 µM | Up-regulating RUNX2, COLI, ALP, OCN, and miR-590/Smad-7 pathway | [120] |
| Alginate/Gel | C3H10T1/2, 20, 50, 100 µM | Up-regulating RUNX2, COLI, OCN, ALP, Pre-miR-20b and pre-miR-410 and down-regulating pre-miR-30c-1and pre-miR-221 | [129] | |
| Berberine | PCL/COL | DPSCs, 50 µg/mL; rat calvaria defects model | Up-regulating ALP, BMP-2, COLI and RUNX2 | [58] |
| PCL/PVP-MC/CS bilayer membrane | MC3T3-E1, 10 µM; rat femur defect model | N/A | [106] | |
| Negatively charged O-carboxymethyl chitosan microspheres | MG-63, rBMSCs; rabbit lateral femoral condyle model | Up-regulating ALP and OCN | [90] | |
| Ginsenoside Rg1 | SF/PCL | MC3T3-E1, HUVECs, 5% w/w | Up-regulating ALP, BMP-2, RUNX2 and OCN | [107] |
| Ginsenoside Rb1 | MSCS/PCL | hDPSCs, 5% v/v; rabbit femoral defect model | Up-regulating ALP, OPN and OCN | [62] |
| Ursolic acid | MBG/CS porous scaffolds | hBMSCs, MC3T3-E1, 5 µM; rat calvarial defect model | Up-regulating ALP, COLI, RUNX2 and BMP-2 and promoting Smad1/5 phosphorylation | [118] |
| Resveratrol | PEGDA/TCS hydrogel | rBMSCs, HUVECs, 800 µM; rat tibia defect model | Up-regulating Ki67, RUNX2, OPN, and calcium content | [84] |
| PLGA microsphere | hMSCs, hTHP-1 monocytes, 25 µM | Up-regulating ALP, OCN and calcium content | [108] | |
| SLNs/GelMA | rBMSCs, 0.02% w/v; rat calvarial critical-size defect model | Up-regulating ALP, OCN, RUNX2 and OPN | [109] | |
| PLA/OMMT | hASCs, 0.1 wt% | Up-regulating ALP, OCN and OPN | [110] | |
| Curcumin | PCL | MC3T3-E1, 1 wt% | Up-regulating RUNX2, ALP, BMP-2, OCN, and OPN | [111] |
| Epigallocatechin gallate | Ti-6Al-4 V | hADSCs, Raw264.7, 0.1, 0.5, 1 mg/mL; rabbit tibias defect model | Up-regulating calcium content, RUNX2, OSX, OCN, OPN | [112] |
| PLLA | ADSCs, Raw 264.7, 1 mg/mL; mouse calvarial defect | Up-regulating ALP, RUNX2, and OPN | [113] | |
| Gel sponges | UMR106, 0.07 mg; rat calvarial defects model | Down-regulating MMP-2, and MMP-9 | [85] | |
| POSS | MC3T3-E1, 6 wt% | Up-regulating ALP | [114] | |
| DC/HAp | Rabbit BMSC, 5 µM; nude mouse model | Up-regulating RUNX2, OCN, and COLI | [115] | |
| Gel sponges | Rat calvarial defects model | N/A | [130] | |
| Genipin | CS framework | 5 mg | Up-regulating RUNX2, OCN, OPN, and ALP | [121] |
| CS | 5 mg | Up-regulating RUNX2, OCN, OPN, and ALP | [122] | |
| Silica particles | 20 mM | Up-regulating ALP | [123] | |
| Proanthocyanidins | COLI | 10% | Up-regulating ALP | [124] |
Except the low-cytotoxicity of TCM, which provided the basic condition for the application of TCM in BTE, TCM could also directly stimulate osteogenesis. Most studies adopted the rat calvarial bone defect models, while other studies also used tibial plateau defects model, rabbit bilateral thigh muscles model, rabbit lateral femoral condyle model, rat tibial osteotomy model, etc. [86–90]. The results show that more new bone formation and mineralization was found in the center and periphery of the bone defect area after filling with icariin-loaded bioactive scaffold, which attribute to the upregulating of osteogenic-related factors, such as RUNX2, ALP, OCN, COLI, BSP and OPN [76, 86, 91–94]. Besides, other TCM combined with different scaffold materials have similar effects, for instance, icaritin [88, 95], hydroxy safflower yellow A [96], kaempferol [97, 98], naringin [99, 100], quercetin [101–103], silymarin [104, 105], berberine [58, 90, 106], ginsenoside [62, 107], resveratrol [108–110], curcumin [111], and epigallocatechin gallate [112–115]. The rat calvarial defect almost completely repaired with physiological integrity at 16 weeks in the PLGA scaffold incorporated with gelatin, alendronate, and naringin groups, and this might owing to the inhibitory impact of alendronate on osteoclasts and the positive effect of naringin on osteoblasts, the PLGA + Gelatin/ALD/NG scaffold had a high potential for bone repair, as shown by upregulating BMP-2, OSX, OPN, BSP, COLI, OCN and calcium content, and inhibiting TRAP [116]. BMP family possess diverse biological functions during osteogenic differentiation, and including the maintenance of normal bone and bone regeneration. After the treatment of TCM loaded scaffold, BMP-2 and BMP-4 were significantly increased [58, 87, 96, 107, 111, 116–118]. Some of the research has demonstrated the mechanism under the TCM promoting bone regeneration. Baicalin/baicalein loaded Ca-polyP particles rises the intracellular calcium level through activation of the phospholipase C. Meanwhile, both flavones upregulated the expression of the osteoblast calcium efflux channel, the plasma membrane Ca2+-ATPase (PMCA), and the expression of ALP, which promotes bone mineralization [119]. Naringin-inlaid composite silk fibroin/hydroxyapatite scaffold had no effect on PI3K and Akt expression but strongly promoted PI3K phosphorylation compared to the control groups, indicating that naringin increased PI3K and Akt activity for stimulating osteogenic differentiation [49]. Using a rat osteotomy model, hesperetin/gelatin sponge scaffold combined with mesenchymal stem cells resulted in accelerated fracture healing, which attributed to the activation of ERK and Smad1/5/8 signaling [89]. Similarly, Zn-silibinin complexes showed promising effects on osteoblast differentiation by regulating miR-590/Smad-7 signaling pathway [120], and ursolic acid loaded mesoporous bioglass/chitosan porous scaffolds would promote the bone regeneration in rat calvarial defect model by stimulating Smad1/5 phosphorylation [118]. Both genipin and proanthocyanidins could act as a cross-linker, and promote the process of osteogenesis via upregulating the expression of RUNX2, OCN, OPN, and ALP [121–124].
Chondrogenesis
Chondrocytes and the surrounding dense layers of extracellular matrix (ECM) form the cartilage [131]. Intramembranous ossification and endochondral ossification are two major processes to form new bone during bone repair [132]. It has been reported that endochondral ossification can be supported by biomaterials, and have a great bone regeneration in clinical [133]. Some of the TCM, for instance, icariin, resveratrol and epigallocatechin, can directly upregulate the expression of chondrogenic-related genes (Table 4). Icariin conjugated hyaluronic acid/collagen (HA/Col) hydrogel showed that the expression of SOX9, AGG, COLII, and COLX was remarkably up-regulated, and the matrix synthesis of sGAG and type II collagen was significantly enhanced [134]. In the rabbit osteochondral defect model, the icariin loaded biomaterials promoting the restoration of supercritical-sized osteochondral defects, as shown by the gross morphology examination and histological analysis, such as hematoxylin and eosin (H&E) and toluidine blue (TB) stained [134, 135]. Besides, icariin has the potential to promote stable chondrogenic differentiation of BMSCs without hypertrophy, and this would further accelerate the process of chondrogenesis [136]. Except upregulating the expression of SOX9, AGG, COLII and COLI in rabbit chondrocyte and BMSCs [137], Yu et al. found that in the resveratrol–PLA–gelatin porous nano-scaffold, the expression levels of SIRT1, AKT and type II collagen proteins was increased significantly, while the expression levels of PI3K/AKT signaling pathway-related proteins, including VEGF, PTEN, Caspase9 and MMP13, was decreased significantly compared to the PLA–gelatin nano-scaffold without resveratrol, which was detected by the immunohistochemical staining. According to the H&E, Masson, Gomori, and Picrosirius red staining, the regenerated cartilage was the thickest, and more chondrocytes were observed with distribution and moderate morphology compared to the negative control groups [138]. The expression of cartilage-specific gene expression, such as s COLII, SOX9 and ACAN was detected by Real-time PCR assay, which showed similar trends as biochemical analysis that the epigallocatechin-loaded hyaluronic acid would promote chondrogenesis. COLI and COLX, which are absent in healthy articular cartilage, was also examined and showed lower expression levels of these markers in epigallocatechin-loaded hyaluronic acid group [139].
Table 4.
Chondrogenesis
| Active components | Biomaterials | Experimental model | Efficacy | References |
|---|---|---|---|---|
| Icariin | HA/Col hydrogel | BMSC, 1 µM; rabbit osteochondral defect model | Up-regulating SOX9, AGG, COL II, and COL X | [134] |
| COL | Rabbit chondrocyte, 10−5 M; rabbit distal femora osteochondral defects model | Up-regulating AGG, COLII, SOX9, GAG, and COLI | [135] | |
| Selfassembling peptide nanofiber hydrogel | rBMSC; 1 × 10−6 M | Up-regulating COLII and SOX9 | [136] | |
| Resveratrol | COLI/PAA | Chondrocytes, BMSCs, 0.5%; rabbit osteochondral defects model | Up-regulating SOX9, AGG, COLII and COLI | [137] |
| PLA–Gel porous nano-scaffold | Rat articular cartilage defect model | Up-regulating AKT and COLII and down-regulating PI3K/AKT signaling pathway-related proteins (VEGF, PTEN, Caspase9 and MMP-13) | [138] | |
| Epigallocatechin | Hyaluronic acid | Chondrocytes, 50 µM; mouse osteoarthritis model | Up-regulating GAG, COLII, SOX9 and ACAN and down-regulating ADAMTS5, COLI, and COLX | [139] |
| Genipin | Carbon dot hydrogel | 500 mM | Collagen–genipin–carbon dot nanoparticles improving chondrogenic differentiation and cartilage regeneration | [140] |
Angiogenesis
Bone is a highly vascularized structure. The formation of blood vessel can stimulate and maintain bone cells activity, deliver nutrients and oxygen, and remove metabolites [141]. Notable, a subtype of blood vessel, type H vessels, was provided to be associated with bone formation [142]. TCM would mainly upregulate the expression of angiogenic-related genes, such as VEGF, ANG1, HIF-1α and CD31, to stimulate vascularization Table 5. Liu et al. constructed an osteoporosis model in rats, and the osteogenic and angiogenic differentiation of bone mesenchymal stem cells (BMSCs) treated with icariin was evaluated. Real-time PCR analysis indicated that, similar to the expression of osteogenic genes, the expression of vascular endothelial growth factor (VEGF) and angiopoietin 1 (ANG1) mRNA was promoted by icariin, especially at 20 µM. CPC could act as a suitable icariin delivery system for repairing bone defects, and after implanted into nude mice, the extent of blood vessel growth in the icariin-loaded CPC groups was markedly greater than that in the CPC group. Besides, the systemic administration of icariin has an anti-osteoporotic effect that promotes bone defect repair [143]. Icariin could also induce the osteogenic differentiation in rat ASCs and stimulate the expression of VEGF, which would further promote the process of bone formation, as shown by SEM, micro-CT imaging, H&E and immunohistochemical staining [144]. A phytomolecule icaritin was regarded as a novel osteogenic exogenous growth factor, and a bioactive composite scaffold PLGA/TCP/icaritin was developed. The PLGA/TCP/icariin scaffold was implanted into the bone defect model, and the results of histological staining indicated favorable biocompatibility, rapid bioresorption and more new vessel growth in PLGA/TCP/icaritin scaffolds in contrast to PLGA/TCP scaffolds [145]. Chung BH et al. constructed a similar complex scaffold, and PLGA/TCP/icaritin enhanced new bone formation within the bone defect after core decompression in SAON rabbits and significantly promoted neovascularization in the rabbit muscle pouch experiment, and the mechanism under angiogenesis might due to the upregulating of MEK/ERKand PI3K/Akt/eNOS-dependent signal pathways as previously report [146, 147]. In the rabbit ulnar segmental bone defect model, the blood perfusion within defect sites was detected by dynamic MRI at weeks 2 and 4 post-surgery, which verified the PLGA/TCP/icariin scaffolds induced significant blood vessel ingrowth into the pores of the implanted scaffold in the early stages of bone regeneration compared with that of the control scaffold [148]. Hydroxy-safflower yellow A was loaded into BG scaffolds by coating chitosan/sodium alginate film, and the expression levels of HIF-1α were more pronounced in a dose-dependent manner after 10 days induction in Hydroxy-safflower yellow A loaded groups, as shown by Western blot assay. Hydroxy-safflower yellow A contribute to osteogenesis and angiogenesis definitely, as well as the promotion of repair function and both in vivo and in vitro, the results of high-concentration of Hydroxy-safflower yellow A groups showed the best performance [96]. In the large tibial defect, resveratrol combined with ANG2 could promote new bone formation, and enhance density and size of new blood vessels by increasing autophagy to decrease ANG2 and hypoxia-induced apoptosis, maintaining the growth and proliferation in the endothelial cells, and upregulating the expression of CD31 in the bone defect area [84].
Table 5.
Angiogenesis
| Active components | Biomaterials | Experimental model | Efficacy | References |
|---|---|---|---|---|
| Icariin | CPC | rBMSC, 20 µM; OVX rat calvarial defect model | Up-regulating VEGF and ANG1 | [143] |
| SMC-PHBHHx scaffold | BMSC, 10−6 mol/L; rat calvarial defects model | Up-regulating VEGF, and FGF | [20] | |
| 45S5 Bioglass | rADSC, 10−7 mol/L; rat calvarial defects model | Up-regulating VEGF | [144] | |
| Icaritin | PLGA/TCP | BMSC, BMC 0.052: 100 (powder weight to solution volume); rat calvarial defect model | Up-regulating OCN | [145] |
| PLGA/TCP | Rabbit ulnar segmental bone defect | N/A | [148] | |
| PLGA/TCP | rBMSC, 1 µM; SAON rabbit both distal and proximal femur defect model | MEK/ERK and PI3K/Akt/eNOS-dependent signal pathways | [146, 147] | |
| Hydroxy safflower yellow A | BG | rBMSCs, HUVECs; rat calvarial defects model | Up-regulating HIF-1α | [96] |
| Silibinin | Zn | C3H10T1/2, MG-63; 60 µM; 3, 7 d | Up-regulating VEGF and ANG1 | [120] |
| Resveratrol | PEGDA/TCS Hydrogel | rBMSCs, HUVECs, 800 µM; rat tibia defect model | Up-regulating CD31 | [84] |
| PLGA microsphere | hMSCs, hTHP-1 monocytes, 25 µM | Up-regulating VEGF | [108] |
Osteoclastogenesis
The proper balance of osteoblasts and osteoclasts are essential in the maintenance of bone homeostasis [149]. Of these, osteoclasts, derived from haematopoietic lineage, are multinucleated cells involved in bone resorption. Macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear kappa B ligand (RANKL) is crucial for proliferation and differentiation of osteoclasts, respectively [149]. In the early stage of bone remodeling, osteoclast can remove the dying osteocytes and osteoblasts [150]. This can accelerate the process of bone remodeling. However, the dysregulating between osteoblast and osteoclast would lead to osteoporosis or heterotopic ossification [149]. Icariin and icaritin loaded biomaterials would downregulate the ratio of RANKL/OPG in BMSC and osteoclast, thus promoting osteogenesis by inhibiting osteoclastogenesis [143, 145]. After 7 days of cell culture, osteoclastic markers were evaluated, and quercitrin implant surfaces significantly decreased the expression of osteoclast related genes, including Trap, CalcR, Ctsk, H+ATPase, Mmp9 compared to controls. Besides, the functional osteoclastic markers Ctsk, H+Atpase and Mmp9 was significantly lower for quercitrin implant surfaces as well as the expression of RankL in vivo [151]. Ursolic acid induced dose-dependent attenuation of titanium (Ti) particle-induced mouse calvarial bone loss, and decreased the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts, which attribute to inhibited the expression of NFATc1 in mRNA and protein level, primarily via the suppression of nuclear factor-kB (NF-kB) signaling, and partly through the suppression of c-Jun N-terminal kinase (JNK) signaling [152]. These results have been shown in Table 6.
Table 6.
Osteoclastogenesis
| Active components | Biomaterials | Experimental model | Efficacy | References |
|---|---|---|---|---|
| Icariin | CPC | rBMSC, 20 µM; OVX rat calvarial defect model | Down-regulating RANKL | [143] |
| Icaritin | PLGA/TCP | BMSC, BMC 0.052: 100 (powder weight to solution volume); rat calvarial defect model | Down-regulating RANKL/OPG | [145] |
| Quercetin | Ti | RAW264.7, 1 mM; rabbit tibia model | Down-regulating Trap, CalcR, Ctsk, H+ATPase, MMP-9 and RANKL | [151] |
| Ursolic acid | Ti particle | RAW264.7, BMMs, 5 µM; mouse calvarial bone defect model | Down-regulating NFATc1, NF-kB and JNK signaling | [152] |
| Epigallocatechin | Ti-6Al-4 V | hADSCs, Raw264.7, 0.1, 0.5, 1 mg/mL; rabbit tibias defect model | Down-regulating TRAP, CTSK, and RAW264.7 number | [112] |
| PLLA | ADSCs, Raw 264.7, 1 mg/mL; mouse calvarial defect | Down-regulating RAW264.7 number | [113] |
Adipogenesis
Numerous studies have indicated a reciprocal relationship between osteoblastogenesis and adipogenesis, and adipogenesis-induction factors would inhibit osteoblastogenesis [153]. However, adipose-derived stem cells (ADSCs), similar to BMSCs, is an immune-privileged cell type with low immunogenicity, and can also differentiate into osteogenic and chondrogenic lineages [154, 155]. The using of icaritin loaded PLGA/TCP scaffold would prevent femoral head collapse in a bipedal SAON model, and this might partly attribute to the inhibiting adipogenic effect of icaritin. In SAON, the expression of adipogenic differentiation regulatory genes C/EBP-β, PPAR-γ and aP2group was control group by 15, 10 and 8 times. After treated with icaritin, the C/EBP, PPAR-γ and aP2 expression was reduced by 72%, 67% and 73%. Of these, C/EBP was required for the downstream proteins involved in adipogenesis, PPAR-γ was the key transcription factor in adipocyte differentiation, and aP2 was regarded as a terminal differentiation marker [156]. icaritin loaded PLGA/TCP also demonstrated the downregulating effect of PPAR-γ in rat calvarial defect model [145]. Cell-infiltratable and injectable gelatin hydrogels would be likely capable of mediating sustained delivery of icaritin to maintain a high-concentration in the long term, and the releasing of icaritin would inhibit the expression of adipogenic markers CEBPα and PPARγ after 7 and 14 days of culture, as shown by Real-time PCR and immunohistochemical staining [157]. The curcumin-loaded silk hydrogel exhibits an interconnected porous structure, and the results of adipogenic markers including PPAR-γ, LPL, FABp4, and Glut4, and the oil red O staining shows that film-associated curcumin accelerates hBMSC adipogenesis when the concentration of curcumin was more than 0.25 mg/mL, while adipogenesis of hBMSCs were inhibited when curcumin concentrations exceeded 5 µM [158]. Madhurakkat et al. found that Epigallocatechin gallate coating on nanofibers can serve as an anti-adipogenic platform by preventing differentiation of ADSCs into adipocytes via inhibiting the expression of LPL and PPAR-γ (Table 7) [113].
Table 7.
Adipogenesis
| Active components | Biomaterials | Experimental model | Efficacy | References |
|---|---|---|---|---|
| Icaritin | PLGA/TCP | Rabbit BMSC, 3T3-L1, 10−6 M; SAON emu proximal femur defect model; SAON rabbit distal femur defect model | Down-regulating C/EBP, aP2, PPAR-γ and lipid droplet | [156] |
| PLGA/TCP | BMSC, BMC 0.052: 100 (powder weight to solution volume); rat calvarial defect model | Down-regulating PPAR-γ2 | [145] | |
| Gel hydrogels | hMSC, 100, 200 nM; SAON rat femoral head defect | Down-regulating PPAR-γ and c-Src | [157] | |
| Curcumin | Silk hydrogel | hBMSC; 12.5 µM | Down-regulating PPAR-γ, LPL, FABp4 and Glut4 | [158] |
| Epigallocatechin gallate | PLLA | ADSCs, Raw 264.7, 1 mg/mL; mouse calvarial defect | Down-regulating LPL and PPAR-γ | [113] |
Others
Traditional Chinese medicines exhibit many biological activities, including anti-bacterial, anti-apoptotic, anti-inflammatory and antioxidant effects [159], and these could also contribute to the process of bone formation. Zinc silibinin complex exhibited antibacterial activity in a concentration-dependent manner. There was no significant change in bacterial growth with 1 g/mL of concentration whereas 10 g/mL concentration of Zn-silibinin complexes showed significant against E. coli (Gram-negative) and S. aureus (Gram-positive) strains compared to control, which would minimize the risk of bacterial infection post implantation and accelerate the augment of bone regeneration [120]. The berberine loaded negatively charged O-carboxymethyl chitosan microspheres possessed an ability to reduce the rate of infection caused by S. aureus, which can be ascribed to the burst release and diffuse of the berberine [38]. Except process the capacity of promoting osteogenesis, which was verified by the expression level of ALP and OCN, quercitrin-functionalized porous Ti-6Al-4 V implants also presented a great potential in decreasing bacterial adhesion and viability, which could decrease bacterial adhesion by 75% and produce a bactericidal effect [160]. For anti-apoptotic properties, the hBMSCs were co-cultured with ginsenoside Rg1-loaded alginate-chitosan microspheres groups. Ginsenoside Rg1 promotes hBMSC proliferation, accelerates differentiation into Nestin-, NSE- and GFAP-positive cells, and attenuates apoptosis through upregulating anti-apoptotic protein Bcl-2 and inhibiting pro-apoptotic protein Bax compare to the control groups [161]. Resveratrol–PLA–gelatin porous nano-scaffold has been shown to contribute to protect cartilage tissue. This can be attribute to the upregulating of SIRT1, which would delay the MMP13-induced decomposition of cartilage matrix, such as glycogen and II collagen, thus, the life of chondrocytes was prolonged [138].
In the bone defect model, the using of TCM, such as epigallocatechin gallate, resveratrol, ginsenoside Rb1 and baicalin, mainly attenuated the inflammation level by stimulating the expression of anti-inflammatory cytokine IL-10, and inhibiting the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 [62, 108, 112, 114, 139, 162]. Resveratrol was incorporated into atelocollagen hydrogels to fabricate anti-inflammatory cell-free scaffolds, and then, the scaffolds were transplanted into the rabbit osteochondral defects. After implantation for 2, 4 and 6 weeks, the inflammatory related genes IL-1β, MMP-13, and COX-2 were remarkable decreased compared with the untreated defects, as shown by Real-time PCR. After 12 weeks, the osteochondral defects were completely repaired in scaffold groups, which was detected by immunohistochemical and glycosaminoglycan staining [137]. Tetrandrine loaded PLLA films possess sustained releasing behavior. The degree of inflammatory reaction for the implant with the tetrandrine loaded PLLA films was more moderate than control PLLA films in 4, 12 weeks after operation, due to tetrandrine maintained lower levels of inflammatory factors, such as NO, TNF-a, IL-6, iNOS, COX-2, which suggesting that tetrandrine could regulate the mRNA and protein expression to reduce the inflammatory response in macrophages, and accelerate tissue regeneration [163]. Huang AQ et al. combined the well-known antioxidant epigallocatechin gallate into gelatin sponges, and then, the implanted complex would decrease intracellular ROS levels in macrophage cell lines, as shown by anti-4-hydroxynonenal staining, thereby partially inhibiting the expression of MMPs, and promote bone formation (Table 8) [85].
Table 8.
Others
| Mechanism | Active components | Biomaterials | Experimental models | Efficacy | References |
|---|---|---|---|---|---|
| Anti-bacterial properties | Silibinin | Zn | C3H10T1/2, MG-63, 60 µM | E. coli (Gram-negative) and S. aureus (Gram-positive) strains | [120] |
| Anti-bacterial properties | Berberine | Negatively charged O-carboxymethyl chitosan microspheres | MG-63, rBMSCs; rabbit lateral femoral condyle model | S. aureus | [90] |
| Anti-bacterial properties | Quercetin | Ti-6Al-4 V | MC3T3-E1 | S. epidermidis | [160] |
| Anti-apoptotic properties | Ginsenoside Rg1 | Alginate-CS microspheres | hBMSC, 2 g | Up-regulating Nestin, NSE, GFAP and Bcl-2 and down-regulating Bax | [161] |
| Anti-inflammatory properties | Resveratrol | PLA–Gel porous nano-scaffold | Rat articular cartilage defect model | Up-regulating SIRT1 | [138] |
| Anti-inflammatory and anti-apoptotic properties | Baicalin | TPGS polymeric micelles | Rat gingival fibroblasts, 20 mg/mL; rat periodontal disease model | Down-regulating TNF-α, IL-1β, and the number of inflammatory cells | [162] |
| Anti-inflammatory properties | Tetrandrine | PLLA | RAW 264.7, 20 mg; rat model | Down-regulating NO, TNF-a, IL-6, iNOS, and COX-2 | [163] |
| Anti-inflammatory properties | Ginsenoside Rb1 | MSCS/PCL | hDPSCs, 5% v/v; rabbit femoral defect model | Up-regulating IL-1RA and down-regulating IL-1β | [62] |
| Anti-inflammatory properties | Resveratrol | COLI/PAA | Chondrocytes, BMSCs, 0.5%; rabbit osteochondral defects model | Down-regulating IL-1β, MMP-13 and COX-2 | [137] |
| Anti-inflammatory properties | Resveratrol | PLGA microsphere | hMSCs, hTHP-1 monocytes, 25 µM | Up-regulating IL-10 and down-regulating TNF-α and IL-6 | [108] |
| Anti-inflammatory properties | Epigallocatechin gallate | Ti-6Al-4 V | hADSCs, Raw264.7, 0.1, 0.5, 1 mg/mL; rabbit tibias defect model | Up-regulating IL-10 and down-regulating IL-6 | [112] |
| Anti-inflammatory properties | Epigallocatechin gallate | Hyaluronic acid | Chondrocytes, 50 µM; mouse osteoarthritis model | Down-regulating IL-1β and TNF-α, | [139] |
| Anti-oxidant and anti-inflammatory properties | Epigallocatechin gallate | POSS | MC3T3-E1, 6 wt% | Down-regulating IL-6 | [114] |
| Anti-oxidant and anti-inflammatory properties | Epigallocatechin gallate | Gel sponges | UMR106, 0.07 mg; rat calvarial defects model | Down-regulating 4-HNE | [85] |
| Anti-oxidant properties | Resveratrol | PLA/OMMT | HASCs, 0.1 wt% | N/A | [110] |
| Anti-oxidant properties | Quercetin | CS/COL hydrogel | hPDLSC; 100 µM | N/A | [164] |
| Anti-oxidant properties | Epigallocatechin gallate | PLLA | ADSCs, Raw 264.7, 1 mg/mL; mouse calvarial defect | N/A | [113] |
Limitations, prospects, and conclusions
Although the efficacy of TCM in the treatment of bone regeneration and remodeling has been widely studied, the field is still in its infancy. First, the reliability of TCM is suspected. TCM is a unique Chinese health care system, covering a broad range of medical theories and practices. It has been used for maintaining health and disease treatment over 2000 years. In modern medicine, the use of western medicine has clear indications and contraindications, while TCM is usually applied based on experience. TCM has not be tested by modern scientific research methods, such as cohort studies, randomized controlled studies, and experimental studies. Thus, the safety and effectiveness of their clinical applications lack evaluation. Aristolochic acid has been used in China for hundreds and thousands of years; however, it has been considered as a factor in cancer and kidney damage [165–167]. Although scholars demonstrated that the combined use of berberine reduces the toxicity of aristolochic acid [168], there is still long before aristolochic acid is reapplied in humans for disease treatment. Areca catechu L. nut is a well-known traditional herbal medicine, which has been recorded in the pharmacopoeia of China. Areca catechu is known for the treatment of parasitic diseases, dyspepsia, anti-depressant, and anti-migraine effects [169, 170]. However, areca catechu is a group 1 carcinogen and related to oral disorders including submucous fibrosis, oral leukoplakia, and erythroplakia, oral lichenoid lesions, and oral cancer [171, 172].
Second, the mechanism of combining TCM with BTE to promote bone regeneration is in urgent need of further research. Various of TCM have demonstrated, at the cellular level, that they can promote the expression of osteogenic markers. Gavage or intraperitoneal injection in animal models also proved that TCM promotes new bone and blood vessel formation, inhibits osteoclastogenesis, and exhibits anti-inflammatory effects, thereby promoting the process of bone remodeling and regeneration. Schisandrin A, isolated from Schisandra chinensis (Turcz.) Baill, is a promising medicine for osteoporosis treatment by inhibiting RANKL-induced ROS via the overexpression of nuclear factor erythroid 2-related factor 2 (Nrf2), suppressing the differentiation of osteoclasts [173]. Gastrodin, extracted from Gastrodia elata Blume, could reduce IL-1β-induced apoptosis in chondrocytes and attenuate the release of inflammatory mediators IL-6 and TNF-α, thus ameliorating rat cartilage degeneration [174]. Morin, a flavonoid derived from old fustic and osage orange trees, stimulates the Wnt pathway by activating and translocating of β-catenin nuclei, thus promoting osteoblast development [175]. However, the efficiency of TCM in bone regeneration must be further verified, as they may not play the same role in BTE. Salvia miltiorrhiza belongs to the Lamiaceae family and is mainly applied to enhance blood circulation and for cardiovascular disease treatment [176, 177]. Because of its pharmacological properties, Salvia miltiorrhiza not only promotes bone formation by regulating ALP, OCN, OPG, and RANKL expression, but also by stimulating angiogenesis via upregulation of the expression of VEGF [177]. In the Salvia miltiorrhiza-coated Ti sample, little apatite was formed on the surface, which was not significantly different from the control group [178].
Third, most research was conducted on plant-derived phytochemicals, while the proteins and peptides, both plant- and animal-derived, have not been thoroughly researched due to large quality difference from batch to batch. Although the development of recombinant technology can solve this problem to a certain extent, few proteins and peptides have been investigated [179]. Ling Zhi-8, purified from Ganoderma lucidum, is an immunomodulatory protein consisting of 110 amino acid residues. Ling Zhi-8 is a promising anti-osteoporosis drug for both preventive and therapeutic effects, which can regulate RANK/RANKL/OPG signaling and inhibit the level of c-Fos and NFATc1, two key target genes of the osteoclast [180, 181]. In a standardized nasal bone defect, the polyurethane (PU)-based material was filled, and a higher bone volume was observed in the Ling Zhi-8-treated sample, although it was not as strong as BMP-2-treated sample [182]. Thus, the protein and peptide related research is promising. Not only proteins, but generally animal-derived TCMs were largely neglected due to slow and expensive research procedures, unstandardized material bases, and unclear active components [12]. Some of the animal-derived TCMs have been applied in BTE, such as Colla Cornus Cervi and Colla Plastri Testudinisis. Further, other animal-derived TCMs may also have potential in bone regeneration. Kangfuxin, extracted from Periplaneta americana, has been verified in the mechanism against osteoporosis, as it accelerates bone formation through stimulating osteoblasts and HUVECs activities, while decreasing bone absorption by inhibiting osteoclast activities [183]. The kangfuxin-coated alginate/carboxymethyl CS sponge exhibits excellent antibacterial, cytocompatibility, and rapid hemostasis effects, and stimulates wound healing [1]. Furthermore, there are even fewer studies on mineral-derived TCM in bone regeneration. Therefore, TCMs that we investigated are only the tip of the iceberg, and which of them may become the “artemisinin” in bone tissue engineering is subject to further research.
With the modernization of TCM and the development of analytical and detection techniques, TCM has gradually transformed from an experience-based to an evidence-based medicinal system. The combination of TCM with “-omics”, such as metabolomics [184], microbiome [185], proteomics [186], and herbgenomics [187], can elucidate the mechanism and molecular targets of TCM, and further promote the development of TCM in the direction of precision medicine. In summary, TCM is a promising therapeutic method both in bone regeneration and BTE. This review provides a reference for the research of TCM for the application in BTE.
Acknowledgements
Z.-R. Gao and Y.-Z. Feng contributed equally to this manuscript. This study was supported by the National Natural Science Foundation of China [Grants 81773339 and 81800788], the Science and Technology Department of Hunan Province, China [Grants 2017WK2041 and 2018SK52511], the fund for Xiangya Clinical Medicine Database of Central South University [Grant 2014-ZDYZ-1-16], and the Open Sharing Fund for the Large-scale Instruments and Equipment of Central South University.
Authors’ contributions
YG and YZF: Conceptualization, Supervision, Funding acquisition; ZRG, YQZ and JZ: Writing-Original Draft; ZRG and YG: Writing—Review & Editing; YHZ, QY and YC: Software; LT, SHZ, MAD: Supervision; YF, JH and ZYOY: Methodology. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng-Rong Gao and Yun-Zhi Feng contributed equally to this manuscript
References
- 1.He Y, Zhao W, Dong Z, Ji Y, Li M, Hao Y, Zhang D, Yuan C, Deng J, Zhao P, et al. A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. Int J Biol Macromol. 2021;167:182–192. doi: 10.1016/j.ijbiomac.2020.11.168. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Wang CZ, Hesse-Fong J, Lin JG, Yuan CS. Application of Chinese medicine in acute and critical medical conditions. Am J Chin Med. 2019;47(6):1223–1235. doi: 10.1142/S0192415X19500629. [DOI] [PubMed] [Google Scholar]
- 3.Li H. Advances in anti hepatic fibrotic therapy with traditional Chinese medicine herbal formula. J Ethnopharmacol. 2020;251:112442. doi: 10.1016/j.jep.2019.112442. [DOI] [PubMed] [Google Scholar]
- 4.Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 2017;69(24):2952–2966. doi: 10.1016/j.jacc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 5.Xue T. Synergy in traditional Chinese medicine. Lancet Oncol. 2016;17(2):e39. doi: 10.1016/S1470-2045(15)00557-4. [DOI] [PubMed] [Google Scholar]
- 6.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2):248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 7.Tu Y. Artemisinin—a gift from traditional Chinese medicine to the world (Nobel Lecture) Angew Chem Int Ed Engl. 2016;55(35):10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 8.Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, Lengfelder E, Dohner H, Burnett AK, Chen SJ, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133(15):1630–1643. doi: 10.1182/blood-2019-01-894980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KH, Morris-Natschke S, Qian K, Dong Y, Yang X, Zhou T, Belding E, Wu SF, Wada K, Akiyama T. Recent progress of research on herbal products used in traditional Chinese medicine: the herbs belonging to the divine Husbandman’s Herbal Foundation Canon (Shen Nong Ben Cao Jing) J Tradit Complement Med. 2012;2(1):6–26. doi: 10.1016/S2225-4110(16)30066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan K. Progress in traditional Chinese medicine. Trends Pharmacol Sci. 1995;16(6):182–187. doi: 10.1016/S0165-6147(00)89019-7. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Shen Y, Xu Y, Maqueda AS, Zheng J, Wu Q, Tam JP. A novel strategy for the discrimination of gelatinous Chinese medicines based on enzymatic digestion followed by nano-flow liquid chromatography in tandem with orbitrap mass spectrum detection. Int J Nanomed. 2015;10:4947–4955. doi: 10.2147/IJN.S82291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XM, Guo JL, Chen L, Ho PC. Application for proteomics analysis technology in studying animal-derived traditional Chinese medicine: a review. J Pharm Biomed Anal. 2020;191:113609. doi: 10.1016/j.jpba.2020.113609. [DOI] [PubMed] [Google Scholar]
- 13.Li JP, Liu Y, Guo JM, Shang EX, Zhu ZH, Zhu KY, Tang YP, Zhao BC, Tang ZS, Duan JA. A comprehensive strategy to evaluate compatible stability of Chinese medicine injection and infusion solutions based on chemical analysis and bioactivity assay. Front Pharmacol. 2017;8:833. doi: 10.3389/fphar.2017.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferron M, Boudiffa M, Arsenault M, Rached M, Pata M, Giroux S, Elfassihi L, Kisseleva M, Majerus PW, Rousseau F, et al. Inositol polyphosphate 4-phosphatase B as a regulator of bone mass in mice and humans. Cell Metab. 2011;14(4):466–477. doi: 10.1016/j.cmet.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233(4):2937–2948. doi: 10.1002/jcp.26042. [DOI] [PubMed] [Google Scholar]
- 16.Preethi Soundarya S, Haritha Menon A, Viji Chandran S, Selvamurugan N. Bone tissue engineering: scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int J Biol Macromol. 2018;119:1228–1239. doi: 10.1016/j.ijbiomac.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 17.McNeill EP, Zeitouni S, Pan S, Haskell A, Cesarek M, Tahan D, Clough BH, Krause U, Dobson LK, Garcia M, et al. Characterization of a pluripotent stem cell-derived matrix with powerful osteoregenerative capabilities. Nat Commun. 2020;11(1):3025. doi: 10.1038/s41467-020-16646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017;2(4):224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BaoLin G, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014;57(4):490–500. doi: 10.1007/s11426-014-5086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, Zhang X, Luo Y, Huang Y, Wu G. Slowly delivered icariin/allogeneic bone marrow-derived mesenchymal stem cells to promote the healing of calvarial critical-size bone defects. Stem Cells Int. 2016;2016:1416047. doi: 10.1155/2016/1416047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird NZ, Acri TM, Tingle K, Salem AK. Gene- and RNAi-activated scaffolds for bone tissue engineering: current progress and future directions. Adv Drug Deliv Rev. 2021;174:613–627. doi: 10.1016/j.addr.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Keefe RJ, Mao J. Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Eng Part B Rev. 2011;17(6):389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Xie J, Xiao X, Chen S, Wang Y. Development of nontoxic biodegradable polyurethanes based on polyhydroxyalkanoate and l-lysine diisocyanate with improved mechanical properties as new elastomers scaffolds. Polymers (Basel) 2019;11(12):1927. doi: 10.3390/polym11121927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y, Sun J, Zhao L, Zhang F, Liang XJ, Guo Y, Weir MD, Reynolds MA, Gu N, Xu HHK. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151–170. doi: 10.1016/j.biomaterials.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Tang G, Tan Z, Zeng W, Wang X, Shi C, Liu Y, He H, Chen R, Ye X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front Bioeng Biotechnol. 2020;8:587658. doi: 10.3389/fbioe.2020.587658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Ung CY, Li H, Kong CY, Wang JF, Chen YZ. Usefulness of traditionally defined herbal properties for distinguishing prescriptions of traditional Chinese medicine from non-prescription recipes. J Ethnopharmacol. 2007;109(1):21–28. doi: 10.1016/j.jep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Shi MM, Piao JH, Xu XL, Zhu L, Yang L, Lin FL, Chen J, Jiang JG. Chinese medicines with sedative-hypnotic effects and their active components. Sleep Med Rev. 2016;29:108–118. doi: 10.1016/j.smrv.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Wu T, Huang L. Therapeutic and delivery strategies of phytoconstituents for renal fibrosis. Adv Drug Deliv Rev. 2021;177:113911. doi: 10.1016/j.addr.2021.113911. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q, Jiang JG. Chemical metabolism of medicinal compounds from natural botanicals. Curr Med Chem. 2012;19(11):1682–1705. doi: 10.2174/092986712799945076. [DOI] [PubMed] [Google Scholar]
- 31.Shao H, Shen J, Wang M, Cui J, Wang Y, Zhu S, Zhang W, Yang H, Xu Y, Geng D. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials. 2015;60:92–99. doi: 10.1016/j.biomaterials.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Wang D, Yang D, Zhen W, Zhang J, Peng S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int. 2018;29(3):535–544. doi: 10.1007/s00198-017-4255-1. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Zhang SQ. Antiosteoporosis effects, pharmacokinetics, and drug delivery systems of icaritin: advances and prospects. Pharmaceuticals (Basel, Switzerland) 2022;15(4):397. doi: 10.3390/ph15040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z, Li T, Ma L, Chen W, Yu H, Abdul Q, Hou J, Tian B. Comparison of interaction between three similar chalconoids and alpha-lactalbumin: impact on structure and functionality of alpha-lactalbumin. Food Res Int. 2020;131:109006. doi: 10.1016/j.foodres.2020.109006. [DOI] [PubMed] [Google Scholar]
- 35.Santos CMM, Silva AMS. The antioxidant activity of prenylflavonoids. Molecules. 2020;25(3):696. doi: 10.3390/molecules25030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Liu J, Yang Y, Cao Q, Huo X, Ma S, Hu J, Pavalko FM, Liu Q. Hydroxy-safflower yellow A inhibits the TNFR1-mediated classical NF-kappaB pathway by inducing shedding of TNFR1. Phytother Res. 2016;30(5):790–796. doi: 10.1002/ptr.5579. [DOI] [PubMed] [Google Scholar]
- 37.Zhou D, Qu Z, Wang H, Su Y, Wang Y, Zhang W, Wang Z, Xu Q. The effect of hydroxy safflower yellow A on coronary heart disease through Bcl-2/Bax and PPAR-gamma. Exp Ther Med. 2018;15(1):520–526. doi: 10.3892/etm.2017.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi SY, Zhang Q, Liu CY, Xie H, Yue LF, Gao XM. Effects of hydroxy safflower yellow-A on tumor capillary angiogenesis in transplanted human gastric adenocarcinoma BGC-823 tumors in nude mice. J Tradit Chin Med. 2012;32(2):243–248. doi: 10.1016/S0254-6272(13)60019-9. [DOI] [PubMed] [Google Scholar]
- 39.Suh KS, Rhee SY, Kim YS, Lee YS, Choi EM. Xanthohumol modulates the expression of osteoclast-specific genes during osteoclastogenesis in RAW264.7 cells. Food Chem Toxicol. 2013;62:99–106. doi: 10.1016/j.fct.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 40.Jeong HM, Han EH, Jin YH, Choi YH, Lee KY, Jeong HG. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem Biophys Res Commun. 2011;409(1):82–89. doi: 10.1016/j.bbrc.2011.04.113. [DOI] [PubMed] [Google Scholar]
- 41.Xuan NT, Shumilina E, Gulbins E, Gu S, Gotz F, Lang F. Triggering of dendritic cell apoptosis by xanthohumol. Mol Nutr Food Res. 2010;54(Suppl 2):214–224. doi: 10.1002/mnfr.200900324. [DOI] [PubMed] [Google Scholar]
- 42.Bai L, Li X, He L, Zheng Y, Lu H, Li J, Zhong L, Tong R, Jiang Z, Shi J, et al. Antidiabetic potential of flavonoids from traditional Chinese medicine: a review. Am J Chin Med. 2019;47(5):933–957. doi: 10.1142/S0192415X19500496. [DOI] [PubMed] [Google Scholar]
- 43.Wang YS, Shen CY, Jiang JG. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res. 2019;150:104520. doi: 10.1016/j.phrs.2019.104520. [DOI] [PubMed] [Google Scholar]
- 44.Huang YF, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharmacol Res. 2020;158:104939. doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang ND, Han T, Huang BK, Rahman K, Jiang YP, Xu HT, Qin LP, Xin HL, Zhang QY, Li YM. Traditional Chinese medicine formulas for the treatment of osteoporosis: implication for antiosteoporotic drug discovery. J Ethnopharmacol. 2016;189:61–80. doi: 10.1016/j.jep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 46.Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, Zhao QW, Ma ZQ, Deng XY, Ma SP. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflamm. 2019;16(1):95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuli HS, Aggarwal V, Kaur J, Aggarwal D, Parashar G, Parashar NC, Tuorkey M, Kaur G, Savla R, Sak K, et al. Baicalein: a metabolite with promising antineoplastic activity. Life Sci. 2020;259:118183. doi: 10.1016/j.lfs.2020.118183. [DOI] [PubMed] [Google Scholar]
- 48.Bharti S, Rani N, Krishnamurthy B, Arya DS. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 2014;80(6):437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- 49.Zhao ZH, Ma XL, Zhao B, Tian P, Ma JX, Kang JY, Zhang Y, Guo Y, Sun L. Naringin-inlaid silk fibroin/hydroxyapatite scaffold enhances human umbilical cord-derived mesenchymal stem cell-based bone regeneration. Cell Prolif. 2021;54(7):e13043. doi: 10.1111/cpr.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed S, Khan H, Aschner M, Hasan MM, Hassan STS. Therapeutic potential of naringin in neurological disorders. Food Chem Toxicol. 2019;132:110646. doi: 10.1016/j.fct.2019.110646. [DOI] [PubMed] [Google Scholar]
- 51.Bai X, Yang P, Zhou Q, Cai B, Buist-Homan M, Cheng H, Jiang J, Shen D, Li L, Luo X, et al. The protective effect of the natural compound hesperetin against fulminant hepatitis in vivo and in vitro. Br J Pharmacol. 2017;174(1):41–56. doi: 10.1111/bph.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin K, Xu Y. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS ONE. 2015;10(6):e0129605. doi: 10.1371/journal.pone.0129605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu H, Sanabria-Cabrera J, Alvarez-Alvarez I, Robles-Diaz M, Stankeviciute S, Aithal GP, Bjornsson ES, Andrade RJ, Lucena MI. Prevention and management of idiosyncratic drug-induced liver injury: systematic review and meta-analysis of randomised clinical trials. Pharmacol Res. 2021;164:105404. doi: 10.1016/j.phrs.2020.105404. [DOI] [PubMed] [Google Scholar]
- 55.Soleimani V, Delghandi PS, Moallem SA, Karimi G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: an updated review. Phytother Res. 2019;33(6):1627–1638. doi: 10.1002/ptr.6361. [DOI] [PubMed] [Google Scholar]
- 56.Xiaoyan A, Jun Y, Min W, Haiyue Z, Li C, Kangde Y, Fanglian Y. Preparation of chitosan-gelatin scaffold containing tetrandrine-loaded nano-aggregates and its controlled release behavior. Int J Pharm. 2008;350(1–2):257–264. doi: 10.1016/j.ijpharm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Bhagya N, Chandrashekar KR. Tetrandrine—a molecule of wide bioactivity. Phytochemistry. 2016;125:5–13. doi: 10.1016/j.phytochem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Ma L, Yu Y, Liu H, Sun W, Lin Z, Liu C, Miao L. Berberine-releasing electrospun scaffold induces osteogenic differentiation of DPSCs and accelerates bone repair. Sci Rep. 2021;11(1):1027. doi: 10.1038/s41598-020-79734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xin BC, Wu QS, Jin S, Luo AH, Sun DG, Wang F. Berberine promotes osteogenic differentiation of human dental pulp stem cells through activating EGFR-MAPK-Runx2 pathways. Pathol Oncol Res. 2020;26(3):1677–1685. doi: 10.1007/s12253-019-00746-6. [DOI] [PubMed] [Google Scholar]
- 60.Cui Y, Xie J, Fu Y, Li C, Zheng L, Huang D, Zhou C, Sun J, Zhou X. Berberine mediates root remodeling in an immature tooth with apical periodontitis by regulating stem cells from apical papilla differentiation. Int J Oral Sci. 2020;12(1):18. doi: 10.1038/s41368-020-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Zhang X, Liu X, Zhang C, Shang W, Xue J, Chen R, Xing Y, Song D, Xu R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol. 2019;856:172418. doi: 10.1016/j.ejphar.2019.172418. [DOI] [PubMed] [Google Scholar]
- 62.Chen CY, Shie MY, Lee AK, Chou YT, Chiang C, Lin CP. 3D-printed ginsenoside Rb1-loaded mesoporous calcium silicate/calcium sulfate scaffolds for inflammation inhibition and bone regeneration. Biomedicines. 2021;9(8):907. doi: 10.3390/biomedicines9080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin R, Li T, Tian JX, Xi P, Liu RH. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit Rev Food Sci Nutr. 2018;58(4):568–574. doi: 10.1080/10408398.2016.1203755. [DOI] [PubMed] [Google Scholar]
- 64.Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. doi: 10.1016/j.biopha.2019.109767. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, Farag MA, Zhong Z, Zhang C, Yang Y, Wang S, Wang Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv Drug Deliv Rev. 2021;176:113870. doi: 10.1016/j.addr.2021.113870. [DOI] [PubMed] [Google Scholar]
- 66.Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58(9):1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 67.Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM. Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol Res. 2016;104:70–85. doi: 10.1016/j.phrs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 69.Yu YQ, Yang X, Wu XF, Fan YB. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. 2021;9:646554. doi: 10.3389/fbioe.2021.646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nussinovitch A, Gal A, Padula C, Santi P. Physical characterization of a new skin bioadhesive film. AAPS PharmSciTech. 2008;9(2):458–463. doi: 10.1208/s12249-008-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terzopoulou Z, Baciu D, Gounari E, Steriotis T, Charalambopoulou G, Tzetzis D, Bikiaris D. Composite membranes of poly(ε-caprolactone) with bisphosphonate-loaded bioactive glasses for potential bone tissue engineering applications. Molecules. 2019;24(17):3067. doi: 10.3390/molecules24173067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibraheem D, Elaissari A, Fessi H. Administration strategies for proteins and peptides. Int J Pharm. 2014;477(1–2):578–589. doi: 10.1016/j.ijpharm.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 73.Lutz R, Park J, Felszeghy E, Wiltfang J, Nkenke E, Schlegel KA. Bone regeneration after topical BMP-2-gene delivery in circumferential peri-implant bone defects. Clin Oral Implants Res. 2008;19(6):590–599. doi: 10.1111/j.1600-0501.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 74.Ryu WM, Kim SN, Min CH, Choy YB. Dry tablet formulation of PLGA nanoparticles with a preocular applicator for topical drug delivery to the eye. Pharmaceutics. 2019;11(12):651. doi: 10.3390/pharmaceutics11120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie XH, Wang XL, Zhang G, He YX, Wang XH, Liu Z, He K, Peng J, Leng Y, Qin L. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomed Mater (Bristol England) 2010;5(5):054109. doi: 10.1088/1748-6041/5/5/054109. [DOI] [PubMed] [Google Scholar]
- 76.Yin L, Wang K, Lv X, Sun R, Yang S, Yang Y, Liu Y, Liu J, Zhou J, Yu Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci Rep. 2017;7(1):8616. doi: 10.1038/s41598-017-07759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Zhang R, Tan X, Li B, Liu Y, Wang X. Synthesis and evaluation of BMMSC-seeded BMP-6/nHAG/GMS scaffolds for bone regeneration. Int J Med Sci. 2019;16(7):1007–1017. doi: 10.7150/ijms.31966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Zhao X, Pei D, Sun G, Li Y, Zhu C, Qiang C, Sun J, Shi J, Dong Y, et al. The promotion function of berberine for osteogenic differentiation of human periodontal ligament stem cells via ERK-FOS pathway mediated by EGFR. Sci Rep. 2018;8(1):2848. doi: 10.1038/s41598-018-21116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kazemi-Aghdam F, Jahed V, Dehghan-Niri M, Ganji F, Vasheghani-Farahani E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr Polym. 2021;269:118311. doi: 10.1016/j.carbpol.2021.118311. [DOI] [PubMed] [Google Scholar]
- 80.Chen J, Pan P, Zhang Y, Zhong S, Zhang Q. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair. Colloids Surf B Biointerfaces. 2015;134:401–407. doi: 10.1016/j.colsurfb.2015.06.072. [DOI] [PubMed] [Google Scholar]
- 81.Qiao T, Jiang S, Song P, Song X, Liu Q, Wang L, Chen X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloids Surf B Biointerfaces. 2016;146:221–227. doi: 10.1016/j.colsurfb.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Guo Z, Wu S, Li H, Li Q, Wu G, Zhou C. In vitro evaluation of electrospun PLGA/PLLA/PDLLA blend fibers loaded with naringin for guided bone regeneration. Dent Mater J. 2018;37(2):317–324. doi: 10.4012/dmj.2016-220. [DOI] [PubMed] [Google Scholar]
- 83.Xia L, Li Y, Zhou Z, Dai Y, Liu H, Liu H. Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion in vitro. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3545–3552. doi: 10.1016/j.msec.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 84.Fan D, Liu H, Zhang Z, Su M, Yuan Z, Lin Y, Yang S, Li W, Zhang X. Resveratrol and angiogenin-2 combined with PEGDA/TCS hydrogel for the targeted therapy of hypoxic bone defects via activation of the autophagy pathway. Front Pharmacol. 2021;12:618724. doi: 10.3389/fphar.2021.618724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang A, Honda Y, Li P, Tanaka T, Baba S. Integration of epigallocatechin gallate in gelatin sponges attenuates matrix metalloproteinase-dependent degradation and increases bone formation. Int J Mol Sci. 2019;20(23):6042. doi: 10.3390/ijms20236042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao H, Tang J, Zhou D, Weng Y, Qin W, Liu C, Lv S, Wang W, Zhao X. Electrospun icariin-loaded core-shell collagen, polycaprolactone, hydroxyapatite composite scaffolds for the repair of rabbit tibia bone defects. Int J Nanomed. 2020;15:3039–3056. doi: 10.2147/IJN.S238800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Lin X, Liu T, Deng L, Huang Y, Liu Y. Osteogenic enhancement between icariin and bone morphogenetic protein 2: a potential osteogenic compound for bone tissue engineering. Front Pharmacol. 2019;10:201. doi: 10.3389/fphar.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie XH, Wang XL, Zhang G, He YX, Leng Y, Tang TT, Pan X, Qin L. Biofabrication of a PLGA-TCP-based porous bioactive bone substitute with sustained release of icaritin. J Tissue Eng Regen Med. 2015;9(8):961–972. doi: 10.1002/term.1679. [DOI] [PubMed] [Google Scholar]
- 89.Xue D, Chen E, Zhang W, Gao X, Wang S, Zheng Q, Pan Z, Li H, Liu L. The role of hesperetin on osteogenesis of human mesenchymal stem cells and its function in bone regeneration. Oncotarget. 2017;8(13):21031–21043. doi: 10.18632/oncotarget.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai B, Zou Q, Zuo Y, Mei Q, Ma J, Lin L, Chen L, Li Y. Injectable gel constructs with regenerative and anti-infective dual effects based on assembled chitosan microspheres. ACS Appl Mater Interfaces. 2018;10(30):25099–25112. doi: 10.1021/acsami.8b06648. [DOI] [PubMed] [Google Scholar]
- 91.Gong M, Chi C, Ye J, Liao M, Xie W, Wu C, Shi R, Zhang L. Icariin-loaded electrospun PCL/gelatin nanofiber membrane as potential artificial periosteum. Colloids Surf B Biointerfaces. 2018;170:201–209. doi: 10.1016/j.colsurfb.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Lai Y, Cao H, Wang X, Chen S, Zhang M, Wang N, Yao Z, Dai Y, Xie X, Zhang P, et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, Guo Y, Li DX, Wang R, Fan HS, Xiao YM, Zhang L, Zhang XD. The effect of loading icariin on biocompatibility and bioactivity of porous beta-TCP ceramic. J Mater Sci Mater Med. 2011;22(2):371–379. doi: 10.1007/s10856-010-4198-y. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Cao L, Liu Y, Zheng A, Wu J, Jiang X, Ji P. Evaluation of synergistic osteogenesis between icariin and BMP2 through a micro/meso hierarchical porous delivery system. Int J Nanomed. 2017;12:7721–7735. doi: 10.2147/IJN.S141052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen SH, Wang XL, Xie XH, Zheng LZ, Yao D, Wang DP, Leng Y, Zhang G, Qin L. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: an in vitro efficacy study. Acta Biomater. 2012;8(8):3128–3137. doi: 10.1016/j.actbio.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 96.Deng Z, Chen J, Lin B, Li J, Wang H, Wang D, Pang L, Zeng X, Wang H, Zhang Y. A novel 3D printed bioactive scaffolds with enhanced osteogenic inspired by ancient Chinese medicine HYSA for bone repair. Exp Cell Res. 2020;394(2):112139. doi: 10.1016/j.yexcr.2020.112139. [DOI] [PubMed] [Google Scholar]
- 97.Tsuchiya S, Sugimoto K, Kamio H, Okabe K, Kuroda K, Okido M, Hibi H. Kaempferol-immobilized titanium dioxide promotes formation of new bone: effects of loading methods on bone marrow stromal cell differentiation in vivo and in vitro. Int J Nanomed. 2018;13:1665–1676. doi: 10.2147/IJN.S150786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vimalraj S, Saravanan S, Hariprabu G, Yuvashree R, Ajieth Kanna SK, Sujoy K, Anjali D. Kaempferol-zinc(II) complex synthesis and evaluation of bone formation using zebrafish model. Life Sci. 2020;256:117993. doi: 10.1016/j.lfs.2020.117993. [DOI] [PubMed] [Google Scholar]
- 99.Yang X, Almassri HNS, Zhang Q, Ma Y, Zhang D, Chen M, Wu X. Electrosprayed naringin-loaded microsphere/SAIB hybrid depots enhance bone formation in a mouse calvarial defect model. Drug Deliv. 2019;26(1):137–146. doi: 10.1080/10717544.2019.1568620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li CH, Wang JW, Ho MH, Shih JL, Hsiao SW, Thien DV. Immobilization of naringin onto chitosan substrates by using ozone activation. Colloids Surf B Biointerfaces. 2014;115:1–7. doi: 10.1016/j.colsurfb.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Song JE, Tian J, Kook YJ, Thangavelu M, Choi JH, Khang G. A BMSCs-laden quercetin/duck’s feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J Biomed Mater Res A. 2020;108(3):784–794. doi: 10.1002/jbm.a.36857. [DOI] [PubMed] [Google Scholar]
- 102.Chen S, Zhu L, Wen W, Lu L, Zhou C, Luo B. Fabrication and evaluation of 3D printed poly(l-lactide) scaffold functionalized with quercetin-polydopamine for bone tissue engineering. ACS Biomater Sci Eng. 2019;5(5):2506–2518. doi: 10.1021/acsbiomaterials.9b00254. [DOI] [PubMed] [Google Scholar]
- 103.Cordoba A, Satue M, Gomez-Florit M, Hierro-Oliva M, Petzold C, Lyngstadaas SP, Gonzalez-Martin ML, Monjo M, Ramis JM. Flavonoid-modified surfaces: multifunctional bioactive biomaterials with osteopromotive, anti-inflammatory, and anti-fibrotic potential. Adv Healthc Mater. 2015;4(4):540–549. doi: 10.1002/adhm.201400587. [DOI] [PubMed] [Google Scholar]
- 104.Song JE, Jeon YS, Tian J, Kim WK, Choi MJ, Carlomagno C, Khang G. Evaluation of silymarin/duck’s feet-derived collagen/hydroxyapatite sponges for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2019;97:347–55. doi: 10.1016/j.msec.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Khoobi MM, Naddaf H, Hoveizi E, Mohammadi T. Silymarin effect on experimental bone defect repair in rat following implantation of the electrospun PLA/carbon nanotubes scaffold associated with Wharton’s jelly mesenchymal stem cells. J Biomed Mater Res A. 2020;108(9):1944–1954. doi: 10.1002/jbm.a.36957. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Wang T, Li J, Cui X, Jiang M, Zhang M, Wang X, Zhang W, Liu Z. Bilayer membrane composed of mineralized collagen and chitosan cast film coated with berberine-loaded PCL/PVP electrospun nanofiber promotes bone regeneration. Front Bioeng Biotechnol. 2021;9:684335. doi: 10.3389/fbioe.2021.684335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo J, Zhu J, Wang L, Kang J, Wang X, Xiong J. Co-electrospun nano-/microfibrous composite scaffolds with structural and chemical gradients for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;119:111622. doi: 10.1016/j.msec.2020.111622. [DOI] [PubMed] [Google Scholar]
- 108.Rutledge KE, Cheng Q, Jabbarzadeh E. Modulation of inflammatory response and induction of bone formation based on combinatorial effects of resveratrol. J Nanomed Nanotechnol. 2016;7(1):350. doi: 10.4172/2157-7439.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei B, Wang W, Liu X, Xu C, Wang Y, Wang Z, Xu J, Guan J, Zhou P, Mao Y. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen Biomater. 2021;8(5):rbab044. doi: 10.1093/rb/rbab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karimi-Soflou R, Mohseni-Vadeghani E, Karkhaneh A. Controlled release of resveratrol from a composite nanofibrous scaffold: effect of resveratrol on antioxidant activity and osteogenic differentiation. J Biomed Mater Res A. 2021;110:21–30. doi: 10.1002/jbm.a.37262. [DOI] [PubMed] [Google Scholar]
- 111.Jain S, Krishna Meka SR, Chatterjee K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed Mater. 2016;11(5):055007. doi: 10.1088/1748-6041/11/5/055007. [DOI] [PubMed] [Google Scholar]
- 112.Lee S, Chang YY, Lee J, Madhurakkat Perikamana SK, Kim EM, Jung YH, Yun JH, Shin H. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater Sci. 2020;8(12):3404–3417. doi: 10.1039/D0BM00566E. [DOI] [PubMed] [Google Scholar]
- 113.Madhurakkat Perikamana SK, Lee SM, Lee J, Ahmad T, Lee MS, Yang HS, Shin H. Oxidative epigallocatechin gallate coating on polymeric substrates for bone tissue regeneration. Macromol Biosci. 2019;19(4):e1800392. doi: 10.1002/mabi.201800392. [DOI] [PubMed] [Google Scholar]
- 114.Jeong HG, Han YS, Jung KH, Kim YJ. Poly(vinylidene fluoride) composite nanofibers containing polyhedral oligomeric silsesquioxane(-)epigallocatechin gallate conjugate for bone tissue regeneration. Nanomaterials (Basel) 2019;9(2):184. doi: 10.3390/nano9020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kook YJ, Tian J, Jeon YS, Choi MJ, Song JE, Park CH, Reis RL, Khang G. Nature-derived epigallocatechin gallate/duck’s feet collagen/hydroxyapatite composite sponges for enhanced bone tissue regeneration. J Biomater Sci Polym Ed. 2018;29(7–9):984–996. doi: 10.1080/09205063.2017.1414480. [DOI] [PubMed] [Google Scholar]
- 116.Zhu B, Xu W, Liu J, Ding J, Chen X. Osteoinductive agents-incorporated three-dimensional biphasic polymer scaffold for synergistic bone regeneration. ACS Biomater Sci Eng. 2019;5(2):986–995. doi: 10.1021/acsbiomaterials.8b01371. [DOI] [PubMed] [Google Scholar]
- 117.Li M, Zhang C, Zhong Y, Zhao J. A novel approach to utilize icariin as icariin-derived ECM on small intestinal submucosa scaffold for bone repair. Ann Biomed Eng. 2017;45(11):2673–2682. doi: 10.1007/s10439-017-1900-y. [DOI] [PubMed] [Google Scholar]
- 118.Ge YW, Lu JW, Sun ZY, Liu ZQ, Zhou J, Ke QF, Mao YQ, Guo YP, Zhu ZA. Ursolic acid loaded-mesoporous bioglass/chitosan porous scaffolds as drug delivery system for bone regeneration. Nanomed Nanatechnol Biol Med. 2019;18:336–346. doi: 10.1016/j.nano.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 119.Wang XH, Guo YW, Tolba E, Kokkinopoulou M, Wiens M, Schroder HC, Muller WEG. Two-armed activation of bone mineral deposition by the flavones baicalin and baicalein, encapsulated in polyphosphate microparticles. Am J Chin Med. 2017;45(3):533–555. doi: 10.1142/S0192415X1750032X. [DOI] [PubMed] [Google Scholar]
- 120.Vimalraj S, Rajalakshmi S, Saravanan S, Raj Preeth D, R LAV, Shairam M, Chatterjee S. Synthesis and characterization of zinc-silibinin complexes: a potential bioactive compound with angiogenic, and antibacterial activity for bone tissue engineering. Colloids Surf B Biointerfaces. 2018;167:134–143. doi: 10.1016/j.colsurfb.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 121.Wang G, Zheng L, Zhao H, Miao J, Sun C, Ren N, Wang J, Liu H, Tao X. In vitro assessment of the differentiation potential of bone marrow-derived mesenchymal stem cells on genipin-chitosan conjugation scaffold with surface hydroxyapatite nanostructure for bone tissue engineering. Tissue Eng A. 2011;17(9–10):1341–1349. doi: 10.1089/ten.tea.2010.0497. [DOI] [PubMed] [Google Scholar]
- 122.Wang G, Qiu J, Zheng L, Ren N, Li J, Liu H, Miao J. Sustained delivery of BMP-2 enhanced osteoblastic differentiation of BMSCs based on surface hydroxyapatite nanostructure in chitosan-HAp scaffold. J Biomater Sci Polym Ed. 2014;25(16):1813–1827. doi: 10.1080/09205063.2014.951244. [DOI] [PubMed] [Google Scholar]
- 123.Lewandowska-Lancucka J, Gilarska A, Bula A, Horak W, Latkiewicz A, Nowakowska M. Genipin crosslinked bioactive collagen/chitosan/hyaluronic acid injectable hydrogels structurally amended via covalent attachment of surface-modified silica particles. Int J Biol Macromol. 2019;136:1196–1208. doi: 10.1016/j.ijbiomac.2019.06.184. [DOI] [PubMed] [Google Scholar]
- 124.Yang H, Liu WC, Liu X, Li Y, Lin C, Lin YM, Wang AN, Nguyen TT, Lin YC, Chung RJ. Study on proanthocyanidins crosslinked collagen membrane for guided bone tissue regeneration. J Appl Biomater Funct Mater. 2021;19:22808000211005379. doi: 10.1177/22808000211005379. [DOI] [PubMed] [Google Scholar]
- 125.Yuan Z, Wan Z, Wei P, Lu X, Mao J, Cai Q, Zhang X, Yang X. Dual-controlled release of icariin/Mg(2+) from biodegradable microspheres and their synergistic upregulation effect on bone regeneration. Adv Healthc Mater. 2020;9(11):e2000211. doi: 10.1002/adhm.202000211. [DOI] [PubMed] [Google Scholar]
- 126.Cordoba A, Monjo M, Hierro-Oliva M, Gonzalez-Martin ML, Ramis JM. Bioinspired quercitrin nanocoatings: a fluorescence-based method for their surface quantification, and their effect on stem cell adhesion and differentiation to the osteoblastic lineage. ACS Appl Mater Interfaces. 2015;7(30):16857–16864. doi: 10.1021/acsami.5b05044. [DOI] [PubMed] [Google Scholar]
- 127.Huang KH, Chen CY, Chang CY, Chen YW, Lin CP. The synergistic effects of quercetin-containing 3D-printed mesoporous calcium silicate/calcium sulfate/poly-epsilon-caprolactone scaffolds for the promotion of osteogenesis in mesenchymal stem cells. J Formos Med Assoc. 2021;120(8):1627–1634. doi: 10.1016/j.jfma.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 128.Gupta SK, Kumar R, Mishra NC. Influence of quercetin and nanohydroxyapatite modifications of decellularized goat-lung scaffold for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2017;71:919–928. doi: 10.1016/j.msec.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 129.Leena RS, Vairamani M, Selvamurugan N. Alginate/gelatin scaffolds incorporated with silibinin-loaded chitosan nanoparticles for bone formation in vitro. Colloids Surf B Biointerfaces. 2017;158:308–318. doi: 10.1016/j.colsurfb.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 130.Hara E, Honda Y, Suzuki O, Tanaka T, Matsumoto N. Epigallocatechin gallate-modified gelatins with different compositions alter the quality of regenerated bones. Int J Mol Sci. 2018;19(10):3232. doi: 10.3390/ijms19103232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rim YA, Nam Y, Ju JH. The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. Int J Mol Sci. 2020;21(7):2358. doi: 10.3390/ijms21072358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Batoon L, Millard SM, Wullschleger ME, Preda C, Wu AC, Kaur S, Tseng HW, Hume DA, Levesque JP, Raggatt LJ, et al. CD169(+) macrophages are critical for osteoblast maintenance and promote intramembranous and endochondral ossification during bone repair. Biomaterials. 2019;196:51–66. doi: 10.1016/j.biomaterials.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 133.Petersen A, Princ A, Korus G, Ellinghaus A, Leemhuis H, Herrera A, Klaumunzer A, Schreivogel S, Woloszyk A, Schmidt-Bleek K, et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat Commun. 2018;9(1):4430. doi: 10.1038/s41467-018-06504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang J, Liu Y, He L, Wang Q, Wang L, Yuan T, Xiao Y, Fan Y, Zhang X. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater. 2018;74:156–167. doi: 10.1016/j.actbio.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 135.Li D, Yuan T, Zhang X, Xiao Y, Wang R, Fan Y, Zhang X. Icariin: a potential promoting compound for cartilage tissue engineering. Osteoarthr Cartil. 2012;20(12):1647–1656. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 136.Wang Z, Li K, Sun H, Wang J, Fu Z, Liu M. Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in selfassembling peptide nanofiber hydrogel scaffolds. Mol Med Rep. 2018;17(6):8237–8243. doi: 10.3892/mmr.2018.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, Sun L, Zhang P, Song J, Liu W. An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater. 2014;10(12):4983–4995. doi: 10.1016/j.actbio.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 138.Yu F, Li M, Yuan Z, Rao F, Fang X, Jiang B, Wen Y, Zhang P. Mechanism research on a bioactive resveratrol- PLA-gelatin porous nano-scaffold in promoting the repair of cartilage defect. Int J Nanomed. 2018;13:7845–7858. doi: 10.2147/IJN.S181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin Y, Koh RH, Kim SH, Kim KM, Park GK, Hwang NS. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater Sci Eng C Mater Biol Appl. 2020;115:111096. doi: 10.1016/j.msec.2020.111096. [DOI] [PubMed] [Google Scholar]
- 140.Lu Z, Liu S, Le Y, Qin Z, He M, Xu F, Zhu Y, Zhao J, Mao C, Zheng L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. doi: 10.1016/j.biomaterials.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 141.Filipowska J, Tomaszewski K, Niedźwiedzki Ł, Walocha J, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291–302. doi: 10.1007/s10456-017-9541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kusumbe A, Ramasamy S, Adams R. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu Y, Cao L, Xia L, Wu Q, Wang J, Wang X, Xu L, Zhou Y, Xu Y, Jiang X. Evaluation of osteogenesis and angiogenesis of icariin in local controlled release and systemic delivery for calvarial defect in ovariectomized rats. Sci Rep. 2017;7(1):5077. doi: 10.1038/s41598-017-05392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jing X, Yin W, Tian H, Chen M, Yao X, Zhu W, Guo F, Ye Y. Icariin doped bioactive glasses seeded with rat adipose-derived stem cells to promote bone repair via enhanced osteogenic and angiogenic activities. Life Sci. 2018;202:52–60. doi: 10.1016/j.lfs.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 145.Shi GS, Li YY, Luo YP, Jin JF, Sun YX, Zheng LZ, Lai YX, Li L, Fu GH, Qin L, et al. Bioactive PLGA/tricalcium phosphate scaffolds incorporating phytomolecule icaritin developed for calvarial defect repair in rat model. J Orthop Transl. 2020;24:112–120. doi: 10.1016/j.jot.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang XL, Xie XH, Zhang G, Chen SH, Yao D, He K, Wang XH, Yao XS, Leng Y, Fung KP, et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J Orth Res. 2013;31(1):164–172. doi: 10.1002/jor.22188. [DOI] [PubMed] [Google Scholar]
- 147.Chung BH, Kim JD, Kim CK, Kim JH, Won MH, Lee HS, Dong MS, Ha KS, Kwon YG, Kim YM. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun. 2008;376(2):404–408. doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Chen SH, Lei M, Xie XH, Zheng LZ, Yao D, Wang XL, Li W, Zhao Z, Kong A, Xiao DM, et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013;9(5):6711–6722. doi: 10.1016/j.actbio.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 149.Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cordoba A, Manzanaro-Moreno N, Colom C, Ronold HJ, Lyngstadaas SP, Monjo M, Ramis JM. Quercitrin nanocoated implant surfaces reduce osteoclast activity in vitro and in vivo. Int J Mol Sci. 2018;19(11):3319. doi: 10.3390/ijms19113319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang C, Xiao F, Gu X, Zhai Z, Liu X, Wang W, Tang T, Wang Y, Zhu Z, Dai K, et al. Inhibitory effects of ursolic acid on osteoclastogenesis and titanium particle-induced osteolysis are mediated primarily via suppression of NF-kappaB signaling. Biochimie. 2015;111:107–118. doi: 10.1016/j.biochi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 153.Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T, Yoshizaki K, Fukumoto S, et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7(1):17696. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. 2018;36(4):1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 155.Lin PC, Chiou TW, Lin ZS, Huang KC, Lin YC, Huang PC, Syu WS, Harn HJ, Lin SZ. A proposed novel stem cell therapy protocol for liver cirrhosis. Cell Transpl. 2015;24(3):533–540. doi: 10.3727/096368915X687228. [DOI] [PubMed] [Google Scholar]
- 156.Qin L, Yao D, Zheng L, Liu WC, Liu Z, Lei M, Huang L, Xie X, Wang X, Chen Y, et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials. 2015;59:125–143. doi: 10.1016/j.biomaterials.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feng Q, Xu J, Zhang K, Yao H, Zheng N, Zheng L, Wang J, Wei K, Xiao X, Qin L, et al. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS Cent Sci. 2019;5(3):440–450. doi: 10.1021/acscentsci.8b00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li C, Luo T, Zheng Z, Murphy AR, Wang X, Kaplan DL. Curcumin-functionalized silk materials for enhancing adipogenic differentiation of bone marrow-derived human mesenchymal stem cells. Acta Biomater. 2015;11:222–232. doi: 10.1016/j.actbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bai J, Zhao J, Cui D, Wang F, Song Y, Cheng L, Gao K, Wang J, Li L, Li S, et al. Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-κB signaling pathway. Sci Rep. 2018;8(1):9173. doi: 10.1038/s41598-018-27217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Llopis-Grimalt MA, Arbos A, Gil-Mir M, Mosur A, Kulkarni P, Salito A, Ramis JM, Monjo M. Multifunctional properties of quercitrin-coated porous Ti-6Al-4V implants for orthopaedic applications assessed in vitro. J Clin Med. 2020;9(3):855. doi: 10.3390/jcm9030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guo YH, Zhao S, Du YX, Xing QJ, Chen BL, Yu CQ. Effects of ginsenoside Rg1-loaded alginate-chitosan microspheres on human bone marrow stromal cells. Biosci Rep. 2017;37(3):BSR20160566. doi: 10.1042/BSR20160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu X, Chen Y, Chen X, Su J, Huang C. Enhanced efficacy of baicalin-loaded TPGS polymeric micelles against periodontitis. Mater Sci Eng C Mater Biol Appl. 2019;101:387–395. doi: 10.1016/j.msec.2019.03.103. [DOI] [PubMed] [Google Scholar]
- 163.Wang QS, Cui YL, Gao LN, Guo Y, Li RX, Zhang XZ. Reduction of the pro-inflammatory response by tetrandrine-loading poly(l-lactic acid) films in vitro and in vivo. J Biomed Mater Res A. 2014;102(11):4098–4107. doi: 10.1002/jbm.a.35083. [DOI] [PubMed] [Google Scholar]
- 164.Arpornmaeklong P, Sareethammanuwat M, Apinyauppatham K, Boonyuen S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J Biomed Mater Res B Appl Biomater. 2021;109(10):1656–1670. doi: 10.1002/jbm.b.34823. [DOI] [PubMed] [Google Scholar]
- 165.Cyranoski D. China to roll back regulations for traditional medicine despite safety concerns. Nature. 2017;551(7682):552–553. doi: 10.1038/nature.2017.23038. [DOI] [PubMed] [Google Scholar]
- 166.Lu ZN, Luo Q, Zhao LN, Shi Y, Wang N, Wang L, Han ZG. The mutational features of aristolochic acid-induced mouse and human liver cancers. Hepatology. 2020;71(3):929–942. doi: 10.1002/hep.30863. [DOI] [PubMed] [Google Scholar]
- 167.Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, Suzuki Y, Thangaraju S, Ng CCY, Tan P, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med. 2017;9(412):eaan6446. doi: 10.1126/scitranslmed.aan6446. [DOI] [PubMed] [Google Scholar]
- 168.Wang P, Guo W, Huang G, Zhen J, Li Y, Li T, Zhao L, Yuan K, Tian X, Huang X, et al. Berberine-based heterogeneous linear supramolecules neutralized the acute nephrotoxicity of aristolochic acid by the self-assembly strategy. ACS Appl Mater Interfaces. 2021;13(28):32729–32742. doi: 10.1021/acsami.1c06968. [DOI] [PubMed] [Google Scholar]
- 169.Li L, Luo Z, Liu Y, Wang H, Liu A, Yu G, Li M, Yang R, Chen X, Zhu J, et al. Screening and identification of the metabolites in rat plasma and urine after oral administration of Areca catechu L. nut extract by ultra-high-pressure liquid chromatography coupled with linear ion trap-orbitrap tandem mass spectrometry. Molecules. 2017;22(6):1026. doi: 10.3390/molecules22061026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peng W, Liu YJ, Wu N, Sun T, He XY, Gao YX, Wu CJ. Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164:340–356. doi: 10.1016/j.jep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 171.Mehrtash H, Duncan K, Parascandola M, David A, Gritz ER, Gupta PC, Mehrotra R, Amer Nordin AS, Pearlman PC, Warnakulasuriya S, et al. Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 2017;18(12):e767–e775. doi: 10.1016/S1470-2045(17)30460-6. [DOI] [PubMed] [Google Scholar]
- 172.Lechner M, Breeze CE, Vaz F, Lund VJ, Kotecha B. Betel nut chewing in high-income countries-lack of awareness and regulation. Lancet Oncol. 2019;20(2):181–183. doi: 10.1016/S1470-2045(18)30911-2. [DOI] [PubMed] [Google Scholar]
- 173.Ni S, Qian Z, Yuan Y, Li D, Zhong Z, Ghorbani F, Zhang X, Zhang F, Zhang Z, Liu Z, et al. Schisandrin A restrains osteoclastogenesis by inhibiting reactive oxygen species and activating Nrf2 signalling. Cell Prolif. 2020;53(10):e12882. doi: 10.1111/cpr.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen J, Gu YT, Xie JJ, Wu CC, Xuan J, Guo WJ, Yan YZ, Chen L, Wu YS, Zhang XL, et al. Gastrodin reduces IL-1beta-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed Pharmacother. 2018;97:642–651. doi: 10.1016/j.biopha.2017.10.067. [DOI] [PubMed] [Google Scholar]
- 175.Wan J, Ma T, Jin Y, Qiu S. The effects of morin on bone regeneration to accelerate healing in bone defects in mice. Int J Immunopathol Pharmacol. 2020;34:2058738420962909. doi: 10.1177/2058738420962909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Keshavarzi Z, Shakeri F, Barreto GE, Bibak B, Sathyapalan T, Sahebkar A. Medicinal plants in traumatic brain injury: neuroprotective mechanisms revisited. BioFactors. 2019;45(4):517–535. doi: 10.1002/biof.1516. [DOI] [PubMed] [Google Scholar]
- 177.Yang Y, Chin A, Zhang L, Lu J, Wong RW. The role of traditional Chinese medicines in osteogenesis and angiogenesis. Phytother Res. 2014;28(1):1–8. doi: 10.1002/ptr.4959. [DOI] [PubMed] [Google Scholar]
- 178.Wu Z, Feng B, Weng J, Qu S, Wang J, Lu X. Biomimetic apatite coatings on titanium coprecipitated with cephradine and salviae miltlorrhizae. J Biomed Mater Res Part B Appl Biomater. 2008;84(2):486–492. doi: 10.1002/jbm.b.30895. [DOI] [PubMed] [Google Scholar]
- 179.Feng Y, Yin Z, Zhang D, Srivastava A, Ling C. Chinese medicine protein and peptide in gene and cell therapy. Curr Protein Pept Sci. 2019;20(3):251–264. doi: 10.2174/1389203719666180612082432. [DOI] [PubMed] [Google Scholar]
- 180.Yang Y, Yu T, Tang H, Ren Z, Li Q, Jia J, Chen H, Fu J, Ding S, Hao Q, et al. Ganoderma lucidum immune modulator protein rLZ-8 could prevent and reverse bone loss in glucocorticoids-induced osteoporosis rat model. Front Pharmacol. 2020;11:731. doi: 10.3389/fphar.2020.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang Y, Yang B. Anti-osteoporosis effect of Ganoderma (lingzhi) by inhibition of osteoclastogenesis. Adv Exp Med Biol. 2019;1182:263–269. doi: 10.1007/978-981-32-9421-9_11. [DOI] [PubMed] [Google Scholar]
- 182.Hsu HA, Wu CY, Chu JS, Lin LH, Lu CA, Ou KL. Effect of recombinant human bone morphogenetic protein-2 and Ling Zhi-8 on osteogenesis: a comparative study using a rabbit sinus model. J Oral Maxillofac Surg. 2014;72(9):1703-e1. doi: 10.1016/j.joms.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 183.Huang YF, Li LJ, Gao SQ, Chu Y, Niu J, Geng FN, Shen YM, Peng LH. Evidence based anti-osteoporosis effects of Periplaneta americana L. on osteoblasts, osteoclasts, vascular endothelial cells and bone marrow derived mesenchymal stem cells. BMC Complement Altern Med. 2017;17(1):413. doi: 10.1186/s12906-017-1917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu GS, Li HK, Zhang WD. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine. Chin J Nat Med. 2019;17(5):321–330. doi: 10.1016/S1875-5364(19)30037-8. [DOI] [PubMed] [Google Scholar]
- 185.Zhang R, Gao X, Bai H, Ning K. Traditional Chinese medicine and gut microbiome: their respective and concert effects on healthcare. Front Pharmacol. 2020;11:538. doi: 10.3389/fphar.2020.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Suo T, Wang H, Li Z. Application of proteomics in research on traditional Chinese medicine. Expert Rev Proteom. 2016;13(9):873–881. doi: 10.1080/14789450.2016.1220837. [DOI] [PubMed] [Google Scholar]
- 187.Xin T, Zhang Y, Pu X, Gao R, Xu Z, Song J. Trends in herbgenomics. Sci China Life Sci. 2019;62(3):288–308. doi: 10.1007/s11427-018-9352-7. [DOI] [PubMed] [Google Scholar]