To the editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with high mortality in kidney transplant recipients (KTRs).1 Unfortunately, they display a poor humoral immune response following coronavirus disease 2019 (COVID-19) mRNA vaccination.2 The use of anti–SARS-CoV-2 monoclonal antibodies was therefore proposed for preexposure prophylaxis in individuals who did not exhibit a significant antibody response following vaccination. Tixagevimab–cilgavimab (Evusheld; AstraZeneca) was found to be effective in preventing COVID-19 during Alpha and Delta waves.3 Because it retained a neutralizing activity against the Omicron variants BA.1 and BA.2, it was approved in many countries for preexposure prophylaxis of KTRs with a low anti–SARS-CoV-2 antibody response after vaccination.4 , 5 A recent study of Bertrand et al. revealed the potential clinical efficiency of tixagevimab–cilgavimab against Omicron in KTRs with weak or no response to vaccine.6 At the same time, Benotmane et al. reported serious Omicron infections despite prophylactic therapy using tixagevimab–cilgavimab.7 In light of these conflicting data, we report herein the impact of this preexposure prophylaxis on the incidence of symptomatic COVID-19; COVID-19–related hospitalizations, including intensive care unit hospitalizations; and death in a cohort of KTRs during the Omicron wave.
KTRs from Bordeaux University Hospital (France) were considered as nonresponders or low responders if they had an anti-spike antibody level of <7 binding antibody units/ml (threshold of detection) or between 7 and 264 binding antibody units/ml, respectively, after at least 3 doses of mRNA vaccines. All patients were to receive i.m. prophylactic injections of tixagevimab–cilgavimab (150 mg tixagevimab and 150 mg cilgavimab) between December 28, 2021, and February 28, 2022 (COVID-19 incidence of 779 of 100,000). This period corresponded to the peak of the Omicron wave observed on January 27, 2022 (COVID-19 incidence of 4021 of 100,000) in our region (https://www.santepubliquefrance.fr/). During this study period, BA.1 was the predominant variant until February 14, 2022, when the BA.2 variant became predominant. The last follow-up was on May 5, 2022. Diagnosis of COVID-19 was based on the reverse transcriptase–polymerase chain reaction of nasopharyngeal swabs, and genome sequencing was performed when suitable samples were available. All the data were recovered from our database (Réseau Aquitain de Néphrologie [R@N]: Commission Nationale de l'Informatique et des Libertés [CNIL] final agreement, decision 2009-413, number 1357154, July 2, 2009). Written informed consent was obtained from all patients.
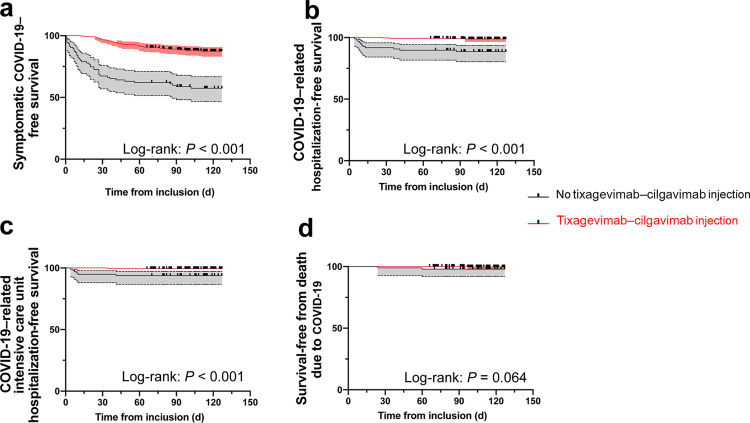
We identified 430 KTRs who failed to develop a protective humoral response after at least 3 doses of mRNA vaccine against SARS-CoV-2. Among them, 333 received prophylactic injections of tixagevimab–cilgavimab without obvious security concerns (no cardiovascular events or significant adverse events). A total of 97 patients did not receive tixagevimab–cilgavimab for the following reasons: refusal (n = 21 [4.8%]), recent cardiovascular events (which were a contraindication, according to the manufacturer’s recommendations; n = 10 [2.3%]), inability to plan injection in our outpatient clinic during this period (n = 51 [11.9%]), or other reasons (n = 15 [3.4%]). Table 1 presents the demographic characteristics of patients with and without tixagevimab–cilgavimab. Among the 333 KTRs who received tixagevimab–cilgavimab, 12.3% developed symptomatic COVID-19 compared with 43.3% among those who did not (hazard ratio [HR], 0.011; 95% confidence interval [CI], 0.063–0.198; P < 0.001; Figure 1a). SARS-CoV-2 sequencing was performed in 5 KTRs receiving tixagevimab–cilgavimab (BA.1, n = 3; BA.1.1, n = 1; and BA.2, n = 1). Hospitalization was required for 1.2% tixagevimab–cilgavimab KTRs in comparison with 11.3% KTRs without tixagevimab–cilgavimab (HR, 0.046; 95% CI, 0.013–0.158; P < 0.001; Figure 1b). Intensive care unit hospitalizations were required for 0.6% of KTRs with tixagevimab–cilgavimab and 6.2% without (HR, 0.045; 95% CI, 0.008–0.240; P < 0.001; Figure 1c). One KTR (0.3%) with tixagevimab–cilgavimab and 2 KTRs (2%) without died from COVID-19 acute respiratory distress syndrome (HR, 0.076; 95% CI, 0.005–1.161; P = 0.066; Figure 1d). Tixagevimab–cilgavimab viral neutralization activity was not conducted in this study.
Table 1.
Patient characteristics
| Characteristic | Tixagevimab–cilgavimab (n = 333) | No tixagevimab–cilgavimab (n = 97) |
|---|---|---|
| Age, yr | 60 ± 14.4 | 58.3 ± 14.3 |
| Time from kidney transplantation, mo | 92 ± 91.1 | 108 ± 58.2 |
| Body mass index, kg/m2 | 25.2 ± 5.3 | 25.4 ± 5.2 |
| Male sex | 204 (61.2) | 63 (64.9) |
| Immunosuppressive drugs at inclusion | ||
| Mycophenolic acid | 254 (76.2) | 84 (86.6) |
| Azathioprine | 10 (3) | 1 (1.03) |
| Corticosteroids | 256 (76.9) | 82 (84.5) |
| mTOR inhibitor | 43 (12.9) | 6 (6.2) |
| Tacrolimus | 248 (74.5) | 73 (75.2) |
| Ciclosporin | 44 (13.2) | 14 (14.4) |
| Belatacept | 25 (7.5) | 11 (11.3) |
| Weak responder/nonrespondera | 57 (17)/276 (83) | 9 (9)/88 (91) |
| Previous treatment with REGEN-CoV antibody combination | 137 (41.1) | 43 (44.3) |
| Serum creatinine, μmol/l | 167 ± 134 | 161 ± 55 |
| History of cardiovascular event | 25 (7.5) | 19 (19.5) |
| Cardiovascular event in the last 3 mo | 0 (0) | 10 (10.3) |
| Follow-up, d | 116 ± 14 | 117 ± 13 |
| Symptomatic COVID-19 | 41 (12.3) | 42 (43.3) |
| COVID-19–related hospitalization | 4 (1.2) | 11 (11.3) |
| COVID-19–related hospitalization in intensive care unit | 2 (0.6) | 6 (6.2) |
| COVID-19–related death | 1 (0.3) | 2 (2) |
BAU, binding antibody unit; COVID-19, coronavirus disease 2019; mTOR, mammalian target of rapamycin.
Data are given as mean ± SD or n (%).
weak responder: level of anti-spike antibody was between 7 and 264 BAU/ml.
Nonresponder: level of anti-spike antibody was <7 BAU/ml (threshold of detection);
Figure 1.
Survival-free from Omicron infection. (a) Symptomatic coronavirus disease 2019 (COVID-19)–free survival. (b) COVID-19–related hospitalization-free survival. (c) COVID-19–related intensive care unit hospitalization-free survival. (d) Survival-free from death due to COVID-19. Log-rank test was used to compare the 2 groups and was considered as significant when P < 0.05.
The first conclusion of this real-life study is that tixagevimab–cilgavimab use was associated with (i) a significantly lower risk of symptomatic COVID-19 and (ii) fewer COVID-19–related hospitalizations (including intensive care unit), compared with patients who did not receive this prophylactic treatment. Although this study is inherently retrospective and not randomized, it reports an effectiveness of the preexposure prophylaxis by tixagevimab–cilgavimab in KTRs, during an Omicron wave dominated by the BA.1 and BA.2 variants. However, symptomatic COVID-19 occurred in 12.3% of KTRs despite tixagevimab–cilgavimab injection, with 2 cases of serious infection and 1 death in a recent ABO-incompatible KTR treated for antibody-mediated rejection with antithymocyte globulins and plasma exchanges. These data suggest that 150 mg tixagevimab and 150 mg cilgavimab offers an incomplete protection. Consequently, the US Food and Drug Administration now recommends increasing the dose of tixagevimab–cilgavimab (https://www.fda.gov). The second lesson learned from this study is that COVID-19 preexposure prophylaxis in our center has not been implemented fast enough (11.9% of our KTRs did not obtain a tixagevimab–cilgavimab injection appointment during the incidence peak). The rate at which infection has spread has outstripped our limited human and organizational resources.
In conclusion, this retrospective real-life study supports (i) the relative effectiveness of tixagevimab–cilgavimab on COVID-19 infection caused by Omicron and (ii) the need to implement this type of prophylaxis to SARS-CoV-2 vaccination unresponsive patients more quickly.
Disclosure
All the authors declared no competing interests.
Data Statement
All relevant data are within the article.
Acknowledgments
The authors wish to thank the health care professionals of the University Hospital of Bordeaux who were involved in the care of the patients, and particularly Brigitte Bonpunt, Aurélie Seniuta, Laurence Pina, Véronique Bessac, and Charlotte Porredon. We also thank Audrey Macary, Audrey Montero, and Guillaume Rebillon for their help.
References
- 1.Caillard M.P.S., Anglicheau M.P.D., Matignon M.D.M., et al. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand D., Hamzaoui M., Lemée V., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin M.J., Ustianowski A., Wit S.D., et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takashita E., Kinoshita N., Yamayoshi S., et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand D., Laurent C., Lemée V., et al. Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 2022;102:440–442. doi: 10.1016/j.kint.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benotmane I., Velay A., Vargas G.G., et al. Pre-exposure prophylaxis with 300 mg Evusheld elicits limited neutralizing activity against the Omicron variant. Kidney Int. 2022;102:442–444. doi: 10.1016/j.kint.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]