Abstract
Background: To evaluate the potential impact of radiation time on radiation-induced lymphopenia (RIL) and subsequently recovery after stereotactic body radiation therapy (SBRT) and to examine the associations between radiation time and with patient outcomes in early-stage non-small cell lung cancer (NSCLC). Methods: Clinical and laboratory records of subjects consisted of 115 patients who had received SBRT for early-stage NSCLC. Clinical and laboratory records were retrospective reviewed to assess the changes in total lymphocyte counts (TLCs) following SBRT. Associations of TLCs kinetics with the clinical and treatment features, and outcomes were analyzed. Results: Most patients (100/115, 86.96%) experienced significantly decreased median TLCs following SBRT (1700 vs 1100 cells/µL; P < .001), and 52 patients (45.21%) met the criteria for lymphopenia. Six months after SBRT, 44 patients (38.26%) had recovered. A negative correlation between TLCs reduction and radiation time was observed (r = −0.381, P < .001). According to the receiver-operating characteristic curve analysis, the optimal cut-off value for radiation time to was 3950 s to predict lymphocyte count recovery (LR) following RIL was 3950 s (P < .001). Multivariate analyses demonstrated that radiation time was significantly associated with LR (odds ratio [OR], 0.113; 95% confidence interval [CI], 0.029-0.432; P = .001) but not TLCs reduction (P = .575). LR within 6 months after SBRT was associated with improved progression-free survival in patients without non-lymphopenia (P = .034), but had little effect in patients with lymphopenia (P = .405). Conclusion: A longer radiation time was associated with a lower rate of LR within 6 months after SBRT in patients with early-stage NSCLC. Given the association of severe and persistent RIL with survival in NSCLC, further study of the effect of radiation time on immune status is warranted.
Keywords: radiation-induced lymphopenia, lymphocyte count recovery, non-small cell lung cancer, radiotherapy, prognosis
Introduction
Lymphocytes are sensitive to ionizing radiation, and a reduction in peripheral total lymphocyte count (TLC) following radiotherapy (RT) is characterized by impaired host immune response to malignancy. 1 Numerous studies have demonstrated a link between radiation-induced lymphopenia (RIL) and poor prognosis in a wide variety of cancers, including gliomas, esophageal cancer, lung cancer, rectal cancer, pancreatic cancer, hepatocellular carcinoma, and cervical cancer.2–13 Moreover, higher TLCs are also associated with a high response rate and more durable treatment response in patients treated with immune checkpoint inhibitors.14–16 This observation further emphasizes the importance of preserving and maintaining circulating lymphocytes in cancer patients who receive the new therapeutic strategy that combines RT and immunotherapy.
Many risk factors for RIL have been described and include higher radiation dose, higher target volume, larger field size, more fraction number, and the use of photon RT (compared with proton RT).17–21 RIL most likely results from direct toxicity of radiation to circulating lymphocytes as these cells pass through the field of radiation.18,22 Thus, longer radiation time likely exposes more blood to the radiation, yielding more lymphocyte depletion; however, little is known about the impact of radiation time on RIL and the subsequent lymphocyte count recovery (LR). As a result, we sought to evaluate the potential impact of radiation time on RIL and subsequent LR after stereotactic body radiation therapy (SBRT) and to examine the association of these parameters with patient outcomes in early-stage non-small cell lung cancer (NSCLC).
Materials and Methods
Patient Population
The reporting of this study conforms to STROBE guidelines. 23 This study was approved by the X Hospital Ethics Committee (B2022-294). Written informed consent was obtained from each patient for the use of his or her clinical data in clinical studies. We retrospective analyzed our clinical database of early-stage NSCLC who received definitive SBRT between December 2014 and May 2018 at Zhongshan Hospital, Fudan University. The 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scans before SBRT in patients without pathological confirmation of disease were necessary. Patients who received SBRT with curative intent for early-stage NSCLC and had no history of another concomitant malignancy were selected for inclusion if there were available blood count values from within 10 days prior to commencement of SBRT, within 10 days immediately following completion of SBRT, and from at least 4 more dates within 6 months after SBRT. Those with a history of prior RT, other anti-cancer treatment, pre-SBRT TLCs < 1000 cells/µL, and other diseases that may affect TLCs (eg, severe infection or immunosuppression) were excluded.
Data Collection
The SBRT treatment plan and delivery method at our institution have been previously described.24,25 All patients were treated with SBRT using the Helical TomoTherapy (HT) Hi-Art treatment system (Accuray). Patient demographics, tumor details, treatment-related characteristics, and follow-up data were extracted from medical records. Patients with asthma, chronic obstructive pulmonary disease, or other lung diseases that cause the forced expiratory volume in 1 s (FEV1) to be <1.6 were considered to have underlying respiratory system disease. Dosimetric parameters and radiation time were extracted from the treatment plan system. Radiation time for the individual patient was defined as the length of time of circulating lymphocyte exposure to radiation, and this time was calculated by beam-on time per fraction multiplied by fraction number. To visualize the TLCs before and after SBRT, complete blood cell (CBC) count data, which included a pre-SBRT, a post-SBRT, and then a value at monthly intervals for 6 months, were assessed. For patients with missing CBC data at a time point of interest, the closest value to the desired date was used. We further examined the alteration of TLCs (ATLCs, post-SBRT-pre-SBRT TLCs) to evaluate the extent of reduction in TLC after SBRT. According to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, post-SBRT TLCs < 1000 cells/µL were considered to indicate lymphopenia, and post-SBRT TLCs ≥ 1000 cells/µL were non-lymphopenia (G0). Lymphopenia was further categorized into grade 1 (G1, <1000 cells/µL), grade 2 (G2, <800 cells/µL), grade 3 (G3, <500 cells/µL), and grade 4 (G4, <200 cells/µL). Considering the variations between different blood tests, only patients with post-SBRT TLC levels that were restored back to pre-SBRT levels at least twice during the first 6 months follow-up were considered to have recovered from RIL and were classified as the LR group.
Statistical Analyses
Data are presented as median (interquartile range [IQR]) for continuous data or number (proportion) for categorical variables. The TLCs change over time was assessed using Student's t-tests or Wilcoxon rank-sum tests, depending on the normality of the distribution. To illustrate CBC kinetics after SBRT, cell counts were assessed according to time after SBRT in months. Correlations between ATLCs and dose parameters and radiation time were analyzed using the Spearman correlation analysis. Univariable and multivariable linear regression analyses were performed to derive a model for prediction of ATLCs. Univariable and multivariable logistic regression analyses were performed to assess factors that are potentially associated with LR within 6 months after SBRT. Receiver-operating characteristic (ROC) curve analysis was used to achieve the thresholds with the maximum Youden index and determine the diagnostic accuracy of parameters for the identification of patients in the LR and no LR groups. Progression-free survival (PFS) was calculated from the date of SBRT completion to the date of disease progression or recurrence or to the last follow-up. Overall survival (OS) was determined until death from any cause or the last follow-up. Survival curves were generated using the Kaplan-Meier method, and comparisons were made using the log-rank test. Univariable and multivariable Cox proportional hazard regression analyses were used to determine risk factors for survival. Covariates with P ≤ .10 according to univariate analysis were incorporated in the multivariate model. All statistical tests were 2-sided, and a P-value <.05 indicated statistical significance. All statistical tests were performed using SPSS version 25 (SPSS).
Results
Patient Characteristics
A total of 115 patients with early-stage NSCLC met the selection criteria and were included in this study. The median number of blood tests per patient was 8 (IQR, 6-11). The median number of days for pre-SBRT counts was 2 days (IQR, 0-4) prior to commencement of SBRT, while that for post-SBRT counts was 3 days after SBRT completion (IQR, 2-5). The median age of all patients was 71 years (IQR, 64-78 years), and the median diameter of the primary tumor was 20.2 mm (IQR, 15.45-30.00 mm). Additional information about patient demographic, tumor, and treatment characteristics are listed in Table 1.
Table 1.
Baseline Patient, Tumor and Treatment Characteristics for All Patients (n = 115).
| Characteristic | n (%) or median (IQR) |
|---|---|
| Age at diagnosis (years) | 71 (64-78) |
| Sex | |
| Male | 73 (63.5%) |
| Female | 42 (36.5%) |
| ECOG performance status | |
| 0-1 | 80 (69.6%) |
| 2 | 35 (30.4%) |
| Smoking status | |
| Positive | 51 (44.3%) |
| Negative | 57 (49.6%) |
| Unknown | 7 (6.1%) |
| Underlying respiratory system disease | |
| Yes | 63 (54.8%) |
| No | 52 (45.2%) |
| Tumor diameter (mm) | 20.20 (15.45-30.00) |
| Tumor location | |
| Central | 33 (28.7%) |
| Peripheral | 82 (71.3%) |
| SUVmax | 6.00 (2.35-12.30) |
| Histology | |
| Adenocarcinoma | 69 (60.00%) |
| Squamous carcinoma | 22 (19.1%) |
| Unknown | 24 (20.9%) |
| Tumor volume (cm3) | |
| GTV | 9.71 (3.99-23.11) |
| PTV | 23.66 (11.92-48.62) |
| SBRT dose and fractionation | |
| 50 Gy in 5 fractions | 38 (33.0%) |
| 60 Gy in 10 fractions | 77 (67.0%) |
| Radiation time (seconds) | 3599 (2857-4876) |
| Key mean normal tissue doses (cGy) | |
| mean lung dose | 427.00 (313.00-600.00) |
| mean heart dose | 120.50 (23.25-409.75) |
| Radiation-induced lymphopenia | 63 (54.78%) |
| Lymphocytes recover to pre-SBRT level | 44 (38.26%) |
Abbreviations: IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
Lymphocyte Counts After SBRT
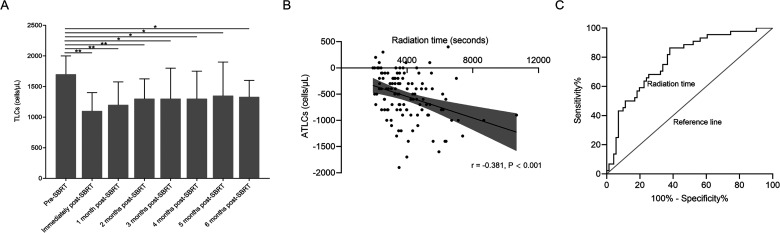
To understand the pattern of changes in CBC response to SBRT, CBC values were assessed with respect to time (Figure 1 and Supplemental Figure S1). Most patients (100/115, 86.96%) experienced significantly decreased median TLCs following SBRT (1700 vs 1100 cells/µL; P < .001). Median ATLCs after SBRT were −500 (IQR, −800 to −200) cells/µL. In total, 52 (45.21%) patients developed lymphopenia. Of these, 20 (17.39%) developed G1, 27 (23.48%) developed G2, and 5 (4.35%) developed G3 lymphopenia. No patient experienced G4 lymphopenia. Lymphocytes were restored from RIL at the 1st month follow-up (1100 vs 1200 cells/µL; P < .001) and then continued at the 2nd-month follow-up (1100 vs 1300 cells/µL; P < .001). Thereafter, no significant increase to restore the TLCs occurred in the following months. At 6 months of follow-up, the TLCs failed to recover to pre-SBRT levels in the whole cohort. Furthermore, when the distribution of TLCs in the 3rd month to the 6th month was compared to the 2nd month, no significant difference was found (P > .05) (Figure 1A).
Figure 1.
(A) Distribution of TLCs from baseline levels before SBRT to 6 months after SBRT in patients with early stage NSCLC. Note: Not all patients had TLCs data available at each time point. (B) Correlation between ATLCs and radiation time. (C) ROC curve analysis of radiation time for LR.
Abbreviations: TLC, total lymphocyte count; SBRT, stereotactic body radiation therapy; NSCLC, non-small cell lung cancer; ATLC, alteration of total lymphocyte count; ROC, receiver-operating characteristic; LR, lymphocyte count recovery.
In addition, 6 months after SBRT, 44 patients (38.26%) exhibited recovery, including 17 patients in the lymphopenia group and 27 patients in the non-lymphopenia group (P = .264). This result suggests that the ability to recover from RIL was not associated with post-SBRT TLCs. Based on the dynamic patterns of lymphocytes within the 6 months after SBRT, patients were divided into 4 groups: non-lymphopenia (G0) patients with LR (n = 27), non-lymphopenia (G0) patients with no LR (n = 36), lymphopenia (G1-3) patients with LR (n = 17), and lymphopenia (G1-3) patients with no LR (n = 35). As opposed to RIL, white blood cell (WBC), neutrophil, monocyte, and platelet counts were generally more stable because only 2 patients (1.74%) experienced G2 neutropenia after SBRT. Additionally, no marked changes in the distribution of these cells were observed during the 6 months of follow-up after SBRT (Supplemental Figure S1).
Association of Radiation Time and ATLCs After SBRT
Analysis revealed a negative correlation between radiation time and ATLCs (r = −0.381, P < .001; Figure 1). Spearman correlation tests described the relationship between the percentage of lung and heart receiving 5 to 50 Gy (in 5-Gy increments) and ATLCs (Supplemental Figure S2A). Correlation coefficients remained greatest in the lung V5 (r = −0.363, P < .001) and heart V15 (r = −0.260, P = .001). Given the strong collinearity among these dosimetric parameters, only the most relevant lung V5 (Vn, the percentage of organ volume receiving ≥Gy) and heart V15 were included in the final multivariate linear regression model. Associations between ATLCs and other related factors are shown in Table 2. In the univariate analysis, tumor diameter, location, pre-SBRT TLCs, gross tumor volume (GTV), planning tumor volume (PTV), lung V5, heart V15, and fractionation were all significant factors for ATLC (P < .05 for all comparisons). Multivariate linear regression analysis, however, identified higher pre-RT TLCs (P < .001), 60 Gy/10 fractions (compared with 50 Gy/5 fractions; P = .001), and larger PTV (P = .010) remained significantly associated with an increased risk of ATLCs, whereas radiation time did not (P = .575).
Table 2.
Univariate and Multivariate Liner Regression Associating Baseline Factors With ATLCs.
| Characteristic | Regression coefficient | 95% CI | P |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.414 | −6.470 to 9.299 | .723 |
| Sex (male vs female) | −103.327 | −274.322 to 67.669 | .234 |
| ECOG (0-1 vs 2) | 8.393 | −171.665 to 188.451 | .927 |
| Smoker (No vs others) | −44.374 | −209.880 to 121.133 | .596 |
| Underlying respiratory system disease (No vs others) | −57.295 | −223.423 to 108.832 | .496 |
| Tumor diameter (1 mm) | −9.045 | −16.423 to −1.668 | .017 |
| Tumor location (Peripheral vs central) | −199.446 | −378.800 to −20.092 | .030 |
| Pre-SBRT TLCs (1 cells/µL) | −0.484 | −0.593 to −0.376 | <.001 |
| GTV (1 cm3) | −3.904 | −7.001 to 0.807 | .014 |
| PTV (1 cm3) | −3.164 | −5.264 to −1.063 | .003 |
| Lung V5 (1%) | −17.154 | −25.873 to −8.435 | <.001 |
| Heart V15 (1%) | −9.389 | −17.657 to −1.120 | .026 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | −292.823 | −460.300 to −125.345 | .001 |
| Radiation time (seconds) | −0.067 | −0.109 to −0.025 | .002 |
| Multivariate analysis | |||
| Pre-SBRT TLCs (cells/µL) | −0.494 | −0.598 to −0.389 | <.001 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | −240.561 | −377.892 to −103.230 | .001 |
| PTV (1 cm3) | −8.404 | −14.712 to −2.096 | .010 |
Abbreviations: ATLC, alteration of total lymphocyte count; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SBRT, stereotactic body radiation therapy; GTV, gross tumor volume; PTV, planning target volume; Lung V5, the percentage of total lung volume receiving at least 5 Gy; Heart V15, the percentage of total heart volume receiving at least 15 Gy.
Impact of Radiation Time on LR After SBRT
ROC curve analysis was performed to determine the predictive value of radiation time on LR. The optimal cut-off value of radiation time was determined as 3950 s (P < .001), and the sensitivity, specificity, and accuracy of this prediction were 62.0%, 86.4%, and 78.0%, respectively. For patients with LR, median radiation time was significantly shorter than those without (3030.00 vs 4678.11 s; P < .001).
We further identified patient and treatment characteristics associated with LR within 6 months after SBRT (Table 3). According to univariate analyses, radiation time, tumor diameter, pre-SBRT TLCs, ATLCs, GTV, PTV, lung V50, and dose fractionation were all significantly associated with LR (P < .05 for all comparisons). Because lung and heart dose-volume histogram (DVH) parameters were all correlated with each other, only the optimal predictor of lung V50 was included in the multivariable logistic regression analysis (Supplemental Figure 2S). Multivariable logistic regression analysis identified underlying respiratory system disease (P = .016), ATLC (P < .001), lung V50 (P = .013), and radiation time (P = .001) as significant factors correlated with LR within 6 months after SBRT (Table 3). These results suggest that a longer radiation time is associated with a lower rate of LR following RIL (odds ratio [OR], 0.113; 95% confidence interval [CI], 0.029-0.432; P = .001).
Table 3.
Univariate and Multivariate Logistic Regression of Factors Associated With LR After SBRT.
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Univariate analysis | ||
| Age (<72 vs ≥72 years) | 0.547 (0.254 to 1.175) | .122 |
| Sex (female vs male) | 1.951 (0.865 to 4.402) | .107 |
| ECOG (0-1 vs 2) | 0.278 (0.639 to 1.435) | .278 |
| Smoker (No vs others) | 0.645 (0.337 to 1.232) | .184 |
| Underlying respiratory system disease (No vs others) | 0.475 (0.218 to 1.035) | .061 |
| Tumor diameter (<22.5 vs ≥22.5 mm) | 0.199 (0.084 to 0.476) | <.001 |
| Tumor location (Peripheral vs central) | 1.069 (0.467 to 2.449) | .874 |
| Pre-SBRT TLCs (<1600 vs ≥1600 cells/µL) | 0.201 (0.089 to 0.457) | <.001 |
| ATLCs (≥−350 vs <−350 cells/µL) | 0.042 (0.016 to 0.115) | <.001 |
| Post-SBRT TLCs (<1000 vs ≥1000 cells/µL) | 1.544 (0.719 to 3.317) | .265 |
| GTV (<8.2 vs ≥8.2 cm3) | 0.322 (0.147 to 0.702) | .004 |
| PTV (<32 vs ≥32 cm3) | 0.270 (0.116 to 0.629) | .002 |
| Lung V50 (<1 vs ≥1%) | 0.160 (0.069 to 0.372) | <.001 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | 0.245 (0.108 to 0.556) | .001 |
| Radiation time (<3950 vs ≥3950 s) | 0.097 (0.036 to 0.260) | <.001 |
| Multivariate analysis | ||
| Underlying respiratory system disease (No vs others) | 0.223 (0.066-0.760) | .016 |
| ATLCs (≥−350 vs <−350 cells/µL) | 0.045 (0.013-0.155) | <.001 |
| Lung V50 (<1 vs ≥1%) | 0.206 (0.059-0.714) | .013 |
| Radiation time (<3950 vs ≥3950 s) | 0.113 (0.029-0.432) | .001 |
Abbreviations: RL, lymphocyte recovery; SBRT, stereotactic body radiation therapy; OR, Odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; TLCs, total lymphocyte counts; ATLC, alteration of TLCs; GTV, gross tumor volume; PTV, planning target volume; Lung V50, the percentage of total lung volume receiving at least 50 Gy.
Outcome According to RIL and LR
The median follow-up period was 28 months (range, 5-68 months) for the entire cohort and 31 months (range, 5-68 months) for survivors. At the time of analysis, 37 patients (32.17%) had locoregional or distant progression, and 23 patients (20.00%) had died. The 2-year PFS and 2-year OS rates for the whole cohort were 72.3% and 81.3%, respectively.
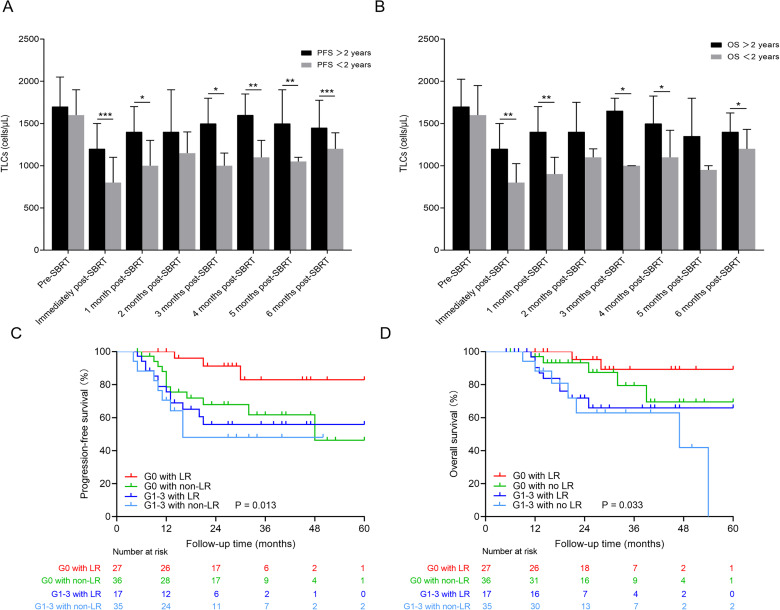
To assess the relationship between the lymphocyte kinetic and patient outcomes after SBRT, we stratified patients according to PFS and OS at 2 years after SBRT. As shown in Figure 2, patients with superior survival had higher median TLC values at most time points compared to those with shorter PFS and OS. Patients with G1-3 lymphopenia had inferior PFS and OS compared with those with G0 (P = .011 and P = .007, respectively). Specifically, the 2-year PFS rates were 53.49% for G1-3 lymphopenia and 86.21% for G0 (P < .001), while the 2-year OS rates were 65.85% for G1-3 and 92.73% for G0 (P = .010). No significant differences in PFS or OS were detected between patients with LR versus those with no LR (P = .270 and P = .755, respectively). In a subset of G0 patients (n = 63), patients with LR (n = 27) had superior PFS (P = .034) compared with those with no LR (n = 36). Regardless of whether patients experienced LR, in G1-3 lymphopenia patients (n = 52), the PFS and OS were similar between patients who had LR (n = 17) and those who did not (n = 35; P = .405 and P = .457, respectively; Figure 2).
Figure 2.
(A and B) Distribution of TLCs at each time stratified by survival (PFS > 2 years vs PFS < 2 years and OS > 2 years vs OS < 2 years). ***P < .001, **P < .01, and *P < .05. (C) PFS curves are shown for G0 lymphopenia patients with LR within 6 months after SBRT (red), G0 lymphopenia patients with no LR (green), G1-3 lymphopenia patients with LR (blue), and G1-3 lymphopenia patients with no LR (light blue). (D) OS curves for the groups of patients described for (C).
Abbreviations: TLC, total lymphocyte count; SBRT, stereotactic body radiation therapy; PFS, progression-free survival; OR, odds ratio; LR, lymphocyte count recovery.
Given the relatively short follow-up time and limited number of deaths, univariable and multivariable Cox proportional hazard regression analyses were performed only with PFS data (Table 4). By univariate analysis, tumor diameter (<22.5 mm), GTV (<8.2 cm3), PTV (<32 cm3), fractionation (50 Gy/5 fractions), post-SBRT TLCs (≥1000 cells/µL), and G0 status in patients with LR were all associated with improved PFS (P < .05 for all comparisons). According to multivariable analysis, when the dynamic pattern of lymphocytes was not accounted for as a covariate, post-SBRT levels were significantly associated with PFS (P��= .012); however, when the dynamic pattern of lymphocytes was added as a covariate, PFS was positively associated with lymphopenia patients with LR (P = .016), and the relationship with post-SBRT TLCs was no longer significant (P = .149).
Table 4.
Univariable and Multivariable Cox Proportional Hazard Regression Analyses of Factors Associated With PFS.
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Univariate analysis | ||
| Age (<72 vs ≥72 years) | 1.391 (0.725 to 2.669) | .321 |
| Sex (female vs male) | 1.513 (0.746 to 3.066) | .251 |
| ECOG (0-1 vs 2) | 1.170 (0.576 to 2.379) | .664 |
| Smoker (No vs others) | 1.159 (0.607 to 2.214) | .655 |
| Underlying respiratory system disease (No vs others) | 1.168 (0.608 to 2.242) | .642 |
| Tumor diameter (<22.5 vs ≥22.5 mm) | 2.228 (1.154 to 4.302) | .017 |
| Tumor location (Peripheral vs central) | 1.323 (0.624 to 2.805) | .465 |
| GTV (<8.2 vs ≥8.2 cm3) | 2.074 (1.024 to 4.200) | .043 |
| PTV (<32 vs ≥32 cm3) | 2.138 (1.115 to 4.101) | .022 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | 3.396 (1.413 to 8.160) | .006 |
| Pre-SBRT TLCs (<1600 vs ≥1600 cells/µL) | 0.709 (0.367 to 1.370) | .306 |
| Post-SBRT TLCs (lymphopenia vs ≥non-lymphopenia) | 0.440 (0.228 to 0.851) | .015 |
| LR (non-LR vs LR) | 0.683 (0.342 to 1.361) | .278 |
| Dynamic pattern of lymphocytes (G0 patients with LR vs others) | 4.591 (1.408 to 14.969) | .011 |
| Multivariate analysis without dynamic pattern of lymphocytes | ||
| Age (<72 vs ≥72 years) | 2.194 (1.111 to 4.336) | .024 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | 3.567 (1.456 to 8.741) | .005 |
| Post-SBRT TLCs (lymphopenia vs ≥non-lymphopenia) | 2.406 (1.213 to 4.772) | .012 |
| Multivariate analysis with dynamic pattern of lymphocytes | ||
| Age (<72 vs ≥72 years) | 2.101 (1.086 to 4.067) | .028 |
| Fractionation (50 Gy/5 fractions vs 60 Gy/10 fractions) | 2.941 (1.204 to 7.183) | .018 |
| Dynamic pattern of lymphocytes (G0 patients with LR vs others) | 4.415 (1.315 to 14.823) | .016 |
Abbreviations: PFS, progression-free survival; OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; PTV, planning target volume; SBRT, stereotactic body radiation therapy; TLCs, total lymphocyte counts; LR, lymphocyte counts recovery.
Discussion
In this study, we observed significant and durable clinical lymphocyte depletion following SBRT in patients with early-stage NSCLC, and most patients failed to restore their TLCs to pre-SBRT levels within 6 months after SBRT. Furthermore, we found that decreased values of TLCs varied with respect to different pre-SBRT TLC levels, RT fraction number, and PTV, whereas LR with 6 months after SBRT was associated with underlying respiratory system disease, ATLCs, lung V50, and radiation time. Finally, we found that LR within 6 months after SBRT was associated with improved survival in patients without lymphopenia but did not mitigate poor survival with lymphopenia. These findings suggest that shortening radiation time may promote recovery from lymphocyte depletion following SBRT and ultimately improve patient prognosis.
Currently, reports have indicated that the risk factors of RIL include a higher radiation dose, higher target volume, larger field size, and a greater number of fractions.3,16,19–21 Similarly, we also found that PTV and fraction number were inversely correlated with ATLCs. To date, however, few studies have focused on the impact of radiation time on TLC reduction and subsequently LR. Based on clinical observations, Yovino et al postulated a mathematical model for the estimation of the radiation dose on circulating lymphocytes during a course of external beam conventional fractionated RT (60 Gy in 30 fractions) for glioblastoma. These investigators found that shorter radiation time was associated with a lower dose of RT to the circulating lymphocytes at the first 10th fraction during conventional fractionated RT with 30 fractions, thereby reducing the risk and severity of RIL. 26 Here, we also found that ATLCs were inversely proportional to radiation time in actual patient data; however, in multivariate analysis for ATLCs, only pre-SBRT TLCs, fractionation, and PTV remained significantly associated, while radiation time did not (P = .575). There is a parallel relationship between PTV and radiation time. That is, an increase in the dose of radiation per fraction would shorten the radiation time to achieve the same biologically effective dose. The effect of radiation time on ATLCs was significant by univariate analysis, likely due to the underlying correlation between radiation time and PTV and dose fractionation. Interestingly, multivariate analysis for LR indicated that radiation time remained an independent predictor of ATLCs, while pre-SBRT TLCs, tumor diameter, GTV, PTV, and dose fractionation did not remain significantly associated. A longer radiation time was associated with a lower rate of LR following RIL (OR, 0.113; 95% CI, 0.029-0.432; P = .001). In clinical practice, however, we have traditionally ignored the radiation time in our development of RT plans. If our finding is validated in other cohorts, it would provide strong evidence for routine assessment of radiation time in the treatment plan, especially in the setting of preexisting lymphopenia. Combined with the analysis results above, these data support the application of a radiation regimen with few fractions due to the potential advantage for sparing RIL and maintaining TLCs after RT.
The absolute number of peripheral lymphocytes is essential for an efficient anti-tumor immune response. Several studies have demonstrated a strong link between severe and persistent RIL and poor clinical outcomes, including in early-stage NSCLC patients treated with SBRT.4,12 In our study, although no lymphopenia and G0 patients with LR exhibited superior PFS in univariate analysis, only G0 patients with LR along with age and dose fractionation emerged as significant prognostic variables in multivariate analysis. ATLCs likely reflect a transient response to SBRT, while LR is likely associated with a sustained response. Therefore, our data suggest that a combination of the degree of the TLC decline and the subsequent recovery of the TLCs within 6 months after SBRT is a better predictor of persistent anti-tumor immune function than the presence of lymphopenia alone. The fact that lymphopenia no longer predicts prognosis when the dynamic pattern of lymphocytes is factored in further lends support to the conclusion that lymphocytes kinetics following SBRT serve as a better predictor for prognosis in these patients. In addition, Deng et al reported that recovery from lymphopenia to (near) normal levels did not mitigate poor survival in esophageal cancer patients with severe RIL (G4) after chemoradiation therapy. 27 This result coincides with our finding that LR did not mitigate poor survival in NSCLC patients with lymphopenia. Although the extent of lymphocyte depletion after SBRT appears to have been compensated for in some patients, one aspect of effective immunity against the tumor remains impaired and is not recovered. This ineffective tumor immunity occurs primarily because the newly generated lymphocytes do not guarantee anti-tumor response. 28 This finding also suggests that recovered immune function exerts a crucial role in repressing the remaining tumor and delaying disease recurrence.
It is reasonably assumed that the degree and rapidity of decreased lymphocyte count would mostly depend on the volume of circulating blood exposed to a certain level of radiation. The estimated blood volume is the complexity of target volume size, the proportion of circulating blood volume covered by isodose line, radiation exposure time, velocity of blood flow, and so on. As shown in Supplemental Figure S3 of the isodose distribution of 4 representative early-stage lung cancer patients treated with SBRT, different isodose lines covered the heart (Supplemental Figure S3A) or great vessels (left pulmonary artery in Supplemental Figure S3B, aortic arch in Supplemental Figure S3C, and right pulmonary artery in Supplemental Figure S3D) in mediastinum. However, the velocity of blood flow in the heart, artery, and vein are different and changes from day to day. In addition, lymphocytes concentration in the blood changes from day to day and it also affects the degree and rapidity of decreased lymphocyte count after RT. It is difficult to estimate these data and blood volume even in a prospective analysis. Figure 2 showed the correlation between ATLCs and radiation time. In addition, multiple logistic regression analysis revealed that the contribution of radiation time to LR was still statistically significant after adjusting other confounding factors in our revised manuscript. These results suggested that the importance of radiation time on ATLCs after RT has long been neglected when we evaluated RT planning.
Our findings must be interpreted in light of the following limitations. First, the retrospective nature of this analysis is subject to all the limitations of post-hoc analyses. Second, we didn't perform the sample size/power analysis and included a relatively small sample size. Third, all patients in our study received SBRT using HT, which creates highly conformal plans at the target but usually results in a larger area of tissue receiving low doses of radiation and longer radiation times.29–31 Whether the long radiation time of HT increased lymphopenia risk and compromised the patient outcomes have prompted additional future investigations. Finally, our work still requires external validation to support our findings.
Conclusion
In conclusion, our study supports a strong link between radiation time and TLC kinetics following SBRT and their associated LR and survival outcomes in early-stage NSCLC. Our findings add to the evidence that supports the clinical importance of radiation time and fraction number for maintenance of lymphocytes after SBRT. These data require prospective validation and support further testing.
Supplemental Material
Supplemental material, sj-tif-1-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment
Supplemental material, sj-tif-2-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment
Supplemental material, sj-tif-3-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment
Abbreviations
- ATLCs
alteration of total lymphocyte counts
- CBC
complete blood cell
- CI
confidence interval
- DVH
dose-volume histogram
- FEV1
the forced expiratory volume in 1 s
- GTV
gross tumor volume
- HT
Helical TomoTherapy
- IQR
interquartile range
- LR
lymphocyte count recovery
- NSCLC
non-small cell lung cancer
- OR
odds ratio
- OS
overall survival
- PFS
progression-free survival
- PTV
planning tumor volume
- RIL
radiation-induced lymphopenia
- ROC
receiver-operating characteristic
- RT
radiotherapy
- SBRT
stereotactic body radiation therapy
- TLC
total lymphocyte count
- WBC
white blood cell
- 18F-FDG PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: The study was approved by the ethics board of Zhongshan Hospital, Fudan University (B2022-294).
ORCID iDs: Qianqian Zhao https://orcid.org/0000-0002-9323-9007
Zhaochong Zeng https://orcid.org/0000-0003-4330-3688
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Koukourakis MI, Giatromanolaki A. Lymphopenia and intratumoral lymphocytic balance in the era of cancer immuno-radiotherapy. Crit Rev Oncol Hematol. 2021;159:103226. doi: 10.1016/j.critrevonc.2021.103226. [DOI] [PubMed] [Google Scholar]
- 2.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571-576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084-1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13:1225-1231. doi: 10.6004/jnccn.2015.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild AT, Ye X, Ellsworth SG, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38:259-265. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho O, Oh YT, Chun M, Noh OK, Hoe JS, Kim H. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck. 2016;38(Suppl 1):E1061-E1067. doi: 10.1002/hed.24158 [DOI] [PubMed] [Google Scholar]
- 7.Cho O, Oh YT, Chun M, Noh OK, Lee HW. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol. 2016;37:971-978. doi: 10.1007/s13277-015-3888-y. [DOI] [PubMed] [Google Scholar]
- 8.Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127:329-335. doi: 10.1007/s11060-015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campian JL, Piotrowski AF, Ye X, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. 2017;135:343-351. doi: 10.1007/s11060-017-2580-z. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Xu X, Yue J, et al. Minimum absolute lymphocyte counts during radiation are associated with a worse prognosis in patients with unresectable hepatocellular carcinoma. Therap Adv Gastroenterol. 2017;10:231-241. doi: 10.1177/1756283X16685557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42-51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Maehata Y, Onishi H, Kuriyama K, et al. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int. 2013;2013:731346. doi: 10.1155/2013/731346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damen PJJ, Kroese TE, van Hillegersberg R, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2021;111(4):936-948. doi: 10.1016/j.ijrobp.2021.07.1695. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys. 2020;108(1):196-203. doi: 10.1016/j.ijrobp.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Patel RR, Verma V, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 2020;150:114-120. doi: 10.1016/j.radonc.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Pike LRG, Bang A, Mahal BA, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103:142-151. doi: 10.1016/j.ijrobp.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94:571-579. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3:512-519. doi: 10.1016/j.adro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther. 2018;4:23-32. doi: 10.14338/IJPT-17-00033.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rossum PSN, Deng W, Routman DM, et al. Prediction of severe lymphopenia during chemoradiation therapy for esophageal cancer: development and validation of a pretreatment nomogram. Pract Radiat Oncol. 2020;10(1):e16-e26. doi: 10.1016/j.prro.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q, Chen G, Ye L, et al. Treatment-duration is related to changes in peripheral lymphocyte counts during definitive radiotherapy for unresectable stage III NSCLC. Radiat Oncol. 2019;14:86. doi: 10.1186/s13014-019-1287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weeke E. The development of lymphopenia in uremic patients undergoing extracorporeal irradiation of the blood with portable beta units. Radiat Res. 1973;56(3):554-559. [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Internal Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 24.He J, Huang Y, Shi S, Hu Y, Zeng Z. Comparison of effects between central and peripheral stage I lung cancer using image-guided stereotactic body radiotherapy via helical tomotherapy. Technol Cancer Res Treat. 2015;14:701-707. doi: 10.1177/1533034615583206. [DOI] [PubMed] [Google Scholar]
- 25.Ye L, Shi S, Zeng Z, Huang Y, Hu Y, He J. Nomograms for predicting disease progression in patients of stage I non-small cell lung cancer treated with stereotactic body radiotherapy. Jpn J Clin Oncol. 2018;48:160-166. doi: 10.1093/jjco/hyx179. [DOI] [PubMed] [Google Scholar]
- 26.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140-144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng W, Xu C, Liu A, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019;133:9-15. doi: 10.1016/j.radonc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Guo Y, Wang L, et al. Recovery profiles of T-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int J Radiat Oncol Biol Phys. 2015;93:1118-1126. doi: 10.1016/j.ijrobp.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Xhaferllari I, El-Sherif O, Gaede S. Comprehensive dosimetric planning comparison for early-stage, non-small cell lung cancer with SABR: fixed-beam IMRT versus VMAT versus TomoTherapy. J Appl Clin Med Phys. 2016;17:329-340. doi: 10.1120/jacmp.v17i5.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q, Wang R, Zhu J, et al. Superiority of helical tomotherapy on liver sparing and dose escalation in hepatocellular carcinoma: a comparison study of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy. Onco Targets Ther. 2016;9:3807-3813. doi: 10.2147/OTT.S106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong CS, Ju SG, Ahn YC, et al. Normal lung sparing tomotherapy technique in stage III lung cancer. Radiat Oncol. 2017;12:167. doi: 10.1186/s13014-017-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment
Supplemental material, sj-tif-2-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment
Supplemental material, sj-tif-3-tct-10.1177_15330338221112287 for Shortened Radiation Time Promotes Recovery From Radiation-induced Lymphopenia in Early-Stage Non-small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy by Qianqian Zhao, Tingting Li, Shisuo Du, Jian He and Zhaochong Zeng in Technology in Cancer Research & Treatment