Abstract
Background
The systemic inflammatory response index (SIRI), a novel inflammation maker, has proven to be associated with prognostic outcomes in various diseases. However, few studies have been conducted assessing how SIRI may influence outcomes of patients on peritoneal dialysis (PD). Herein, we assessed the predictive value of SIRI on mortality all-cause mortality, including cardiovascular disease (CVD) in PD patients.
Methods
A total of 646 PD patients were enrolled in this study. PD patients received regular PD treatments at the Zhujiang Hospital from 1 January 2011 to 31 December 2018. SIRI values could be computed as follows: neutrophil count × monocyte count/lymphocyte count. Patients were divided into two groups according to the median level of SIRI. Cox regression analysis and Kaplan–Meier methods were applied to analyze the relationship between SIRI and mortality outcomes in PD patients.
Results
During the median 31-month follow-up period, 97 (15.0%) PD patients died from all-causes, and 47 (49.0%) died of CVD. Kaplan–Meier analyses revealed that a high SIRI corresponded to the high mortality of all-cause deaths, including CVD (both p < 0.001) in patients on PD. After adjusting for potential confounders, the higher SIRI level was significantly associated with an increased all-cause mortality (HR: 2.007, 95% CI: 1.304–3.088, p = 0.002) and cardiovascular mortality (HR: 2.847, 95% CI: 1.445–5.608, p = 0.002).
Conclusions
SIRI was a promising predictor of mortality in PD patients, with a higher SIRI corresponding to increased risk of mortality.
Keywords: Systemic inflammatory response index, microinflammation, peritoneal dialysis, all-cause mortality, cardiovascular disease
Introduction
Kidney failure has gradually become a major public health problem globally. Based on the 2019 Annual Data Report of US Renal Data System, the population of kidney failure patients reached 746,557 in 2017 [1]. Peritoneal dialysis (PD) is one of the most effective therapies for kidney failure patients, with an estimated 272,000 patients in the world currently undergoing PD treatment [2,3]. Although there are many advantages of PD treatment including ease of operation, it is reported that the 5-year cumulative survival for PD patients is only 60% in 2012 in Asia [4]. Specifically, cardiovascular disease (CVD)-associated deaths account for 40–60% of total deaths worldwide, and is the main cause of death in PD patients [5]. Therefore, the early identification of high-risk patients on PD is extremely important to reduce mortality.
Microinflammation, a well-known risk factor associated with PD treatment, plays an important role in promoting poor outcomes [6]. Oxidative stress, inflammatory cytokines, gut dysbiosis, metabolic acidosis, and vitamin D deficiency can aggravate and promote inflammation in patients with chronic kidney disease (CKD). Inflammation can exacerbate the occurrence of CVD, malnutrition, and anemia, which ultimately puts CKD patients at higher risk of death [7]. Previous studies have identified a strong relationship between microinflammation and cardiac valve calcification and ventricular hypertrophy that could increase CVD mortality in PD patients [8,9]. Malnutrition is another important factor affecting the poor prognosis of PD patients. The state of microinflammation can influence malnutrition by reducing patient appetites and exacerbating protein-energy wasting (PEW) [10]. The identification of inflammation mainly relies on measuring inflammatory markers, such as C-reactive protein (CRP), interleukin-6, and tumor necrosis factor-α, which play significant roles in promoting outcomes in PD patients. However, owing to the high price and inconvenience of these tests, inflammatory markers have not been widely used clinically. Therefore, exploring novel inflammation makers to predict the prognosis of PD patients require further exploration and identification.
Complete blood cell counts can be easily obtained and are routinely collected and tested in hospitalized patients. Previous research has shown that blood cell counts and associated ratios can predict poor prognosis in a variety of diseases [11–13]. The systemic inflammatory response index (SIRI) has recently been shown to reflect the microinflammation status, and is a predictive risk factor in the prognosis of malignant tumors [14–17]. However, there have been no reports to date regarding the association between SIRI and PD patient outcomes. For this reason, the goal of our study was to investigate the predictive value of SIRI and mortality in PD patients.
Methods
Participants
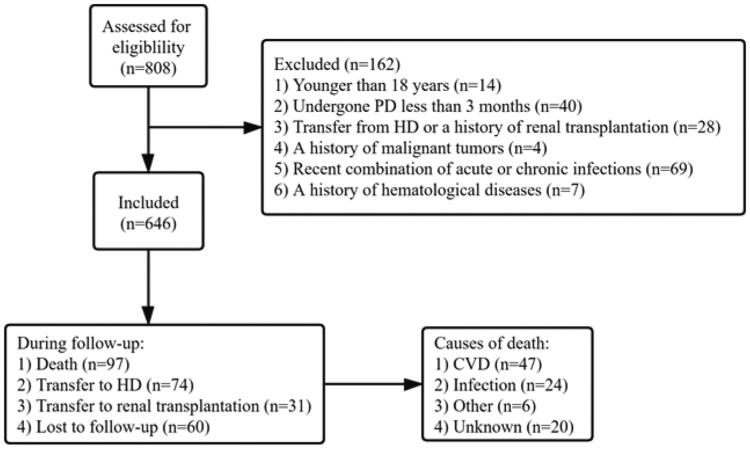
The retrospective cohort study was conducted in kidney failure patients who began PD treatment at Zhujiang Hospital from 1 January 2011 to 31 December 2018. Patients 18 years of age and older undergoing PD therapy for greater than 3 months were enrolled in our study. Exclusion criteria included: 1) patients transferred from hemodialysis (HD) or with a history of renal transplantation; 2) patients with acute or chronic infection, malignant tumors, hematological, or rheumatic diseases; and 3) patients who received immunosuppressive treatment regimens within 3 months of the study period (Figure 1).
Figure 1.
Study procedures, including how patients were selected and their outcomes. CVD: cardiovascular disease; PD: peritoneal dialysis; HD: hemodialysis.
Data collection
Demographic and laboratory data collected within 1–3 months of PD treatment were acquired and used as baseline data measurements. Demographic data included age, sex, cause of kidney failure, body mass index (BMI), CVD, hypertension, and diabetes mellitus status. Baseline laboratory data involved white blood cell count, neutrophil count, lymphocyte count, monocyte count, hemoglobin, red blood cell distribution width (RDW), serum urea nitrogen, serum creatinine, phosphorus, corrected serum calcium, cholesterol, triglyceride, serum albumin, and serum uric acid levels. The above data parameters were collected and analyzed at Zhujiang Hospital. SIRI values were computed as follows: neutrophil count × monocyte count/lymphocyte count [16]. The neutrophil-to-lymphocyte (NLR) ratio and monocyte-to-lymphocyte ratio were also calculated [12,13]. BMI was computed as weight/height2 (kg/m2)[18]. Kt/V was obtained by PD Adequest software version 2.0 (Baxter Healtheare Crop. Deerfield, IL, USA). The diagnostic criteria for diabetes were based on those laid out by the American Diabetes Association [19]. The diagnostic criteria for hypertension were determined by having two independent blood pressure readings ≥140/90 mmHg or by the use of antihypertensive drugs [20]. CVD diagnoses were considered for patients with one of the following conditions including cardiac arrhythmia, heart failure, coronary heart disease, cardiac arrest, stroke, or peripheral vascular disease [21].
Outcomes
The primary outcome in this study was all-cause death and the second outcome was CVD-related deaths. The end-point of the study was death, discontinued PD treatment or 31 December 2019. During follow up, the cause of death was recorded for respective patients. If the patient died in the hospital, the cause of death recorded on the death certificate was collected. If death occurred outside of the hospital, medical records were reviewed by medical professionals along with a detailed description of the death provided by patient family members to determine cause of death.
Statistical analysis
Patients were assigned to either the low SIRI group or high SIRI group based on the median SIRI values. For continuous variables, data were shown as the mean ± SD; in contrast, non-normal and categorical variable data were shown as the median value (interquartile range) and numbers (proportion). Appropriate statistical methods were used to compare two groups, including the independent-samples test, Mann–Whitney U-test, and Chi-squared test. Spearman correlation was applied to explore the relationship between SIRI and demographic and laboratory indexes. The survival curve of patients was plotted using standard Kaplan–Meier methods and analyzed by the log-rank test. COX regression analysis was used to analyze the hazard ratio and 95% CI. A significant variable by univariate analysis was further analyzed by multivariate analysis using forward stepwise regression for the independent prognostic value, with results shown in table 3/4. To evaluate the prognostic ability of SIRI, MLR, and NLR, the AUC was measured by plotting the ROC curve and verified by a Z test. All p values < 0.05 were considered to be statically significant. All data were analyzed using SPSS software version 25.0 (SPSS Inc., Chicago, IL) for Windows.
Results
A total of 646 PD patients were included in this study (Table 1). The average age of the patients was 50.05 ± 14.77, and 60.8% of them were male. The causes of kidney failure in the patient population were chronic glomerulonephritis (341, 52.8%), diabetic nephropathy (100, 15.5%), obstructive nephropathy (78, 12.1%), hypertension (70, 10.8%), and other causes (57, 8.8%).
Table 1.
Baseline characteristics of the study grouped by the SIRI.
| Variables | Total (N = 646) |
Low SIRI group (<1.28) (N = 320) |
High SIRI group (≥1.28) (N = 326) |
p | |
|---|---|---|---|---|---|
| Demographics | |||||
| No. of men/women | 393/253 | 180/140 | 213/113 | 0.018* | |
| Age (years) | 50.05 ± 14.77 | 47.63 ± 15.25 | 52.42 ± 13.90 | <0.001** | |
| BMI (kg/cm2) | 21.93 ± 2.59 | 21.92 ± 2.59 | 21.94 ± 2.57 | 0.830 | |
| Causes of ESRD (n, %) | |||||
| Chronic glomerulonephritis | 341 (52.8%) | 179 (55.9%) | 162 (49.7%) | – | |
| Diabetic nephropathy | 100 (15.5%) | 45 (14.1%) | 55 (16.9%) | – | |
| Hypertension nephropathy | 70 (10.8%) | 32 (10.0%) | 38 (11.7%) | – | |
| Obstructive nephropathy | 78 (12.1%) | 39 (12.2%) | 39 (12.0%) | – | |
| Others | 57 (8.8%) | 25 (7.8%) | 32 (9.8%) | – | |
| Complication (n, %) | |||||
| Cardiovascular disease | 203 (31.4%) | 90 (28.1%) | 113 (34.7%) | 0.074 | |
| Diabetes mellitus | 116 (25.7%) | 69 (21.6%) | 97 (29.8%) | 0.017* | |
| Hypertension | 618 (95.7%) | 304 (95%) | 314 (96.3%) | 0.410 | |
| Laboratory data | |||||
| SIRI | 1.28 (0.85–1.80) | 0.85 (0.63–1.06) | 1.79 (1.54–2.30) | <0.001** | |
| Neutrophil count (G/L) | 3.91 (3.05–5.08) | 3.14 (2.60–3.86) | 4.83 (3.94–5.72) | <0.001** | |
| Monocyte count (G/L) | 0.48 (0.38–0.62) | 0.40 (0.33–0.49) | 0.58 (0.46–0.71) | <0.001** | |
| Lymphocyte count (G/L) | 1.52 (1.21–1.92) | 1.62 (1.33–1.97) | 1.44 (1.14–1.89) | <0.001** | |
| NLR | 2.59 (1.96–2.40) | 2.00 (1.56–2.47) | 3.34 (2.70–3.97) | <0.001** | |
| MLR | 0.31 (0.24–0.42) | 0.25 (0.20–0.31) | 0.40 (0.32–0.49) | <0.001** | |
| White blood cell count (G/L) | 6.44 (5.28–7.82) | 5.54 (4.76–6.66) | 7.26 (6.18–8.72) | <0.001** | |
| Hemoglobin (g/L) | 103.51 ± 18.09 | 103.83 ± 18.78 | 103.20 ± 17.42 | 0.655 | |
| RDW (%) | 14.60 (13.60–16.03) | 14.40 (13.50–15.80) | 14.80 (13.70–16.20) | 0.023* | |
| Serum urea nitrogen (mmol/L) | 17.20 (13.78–20.92) | 17.30 (13.93–20.30) | 17.00 (13.48–22.00) | 0.559 | |
| Serum creatinine (μmol/L) | 712.80 (576.78–902.25) | 707.00 (587.00–907.75) | 714.50 (565.50–898.00) | 0.992 | |
| Corrected calcium (mmol/L) | 2.29 (2.19–2.40) | 2.30 (2.20–2.40) | 2.29 (2.17–2.42) | 0.867 | |
| Phosphorus (mmol/L) | 1.54 (1.28–1.81) | 1.54 (1.31–1.80) | 1.55 (1.27–1.86) | 0.740 | |
| Serum uric acid (μmol/L) | 407.00 (354.00–480.25) | 404.50 (359.25–468.75) | 409.00 (347.00–489.50) | 0.504 | |
| Triglyceride (mmol/L) | 1.29 (0.92–1.89) | 1.33 (0.92–1.82) | 1.29 (0.93–2.03) | 0.544 | |
| Cholesterol (mmol/L) | 4.30 (3.71–5.09) | 4.23 (3.69–5.03) | 4.37 (3.75–5.16) | 0.112 | |
| Serum albumin (g/L) | 31.87 ± 4.76 | 31.67 ± 4.57 | 32.05 ± 4.86 | 0.303 | |
| Total Kt/V | 2.13 (1.79–2.45) | 2.15 (1.78–2.48) | 2.12 (1.79–2.43) | 0.346 |
Bold values are when p is less than 0.05 or 0.01.
*p < 0.05; **p < 0.01.
SIRI: systemic inflammation response index; NLR: neutrophil count/monocyte count; MLR: monocyte count/lymphocyte count; RDW: red blood cell distribution width.
According to the median level of SIRI (1.28), PD patients were assigned to either a low SIRI group (n = 320) or high SIRI group (n = 326). At baseline, there was a higher percentage of males, diabetes-related kidney failure, increased levels of white blood cell, neutrophil, monocyte, and RDW counts among patients in the high SIRI group. Furthermore, patients in the high SIRI group were on average older and had lower lymphocyte counts than individuals in the low SIRI group. Additionally, NLR and MLR (all p < 0.05, Table 1) were higher in the high SIRI group. Spearman rank correlation analysis indicated that SIRI levels were negatively correlated with lymphocyte count, but positively correlated with age, white blood cell count, neutrophil, monocyte, RDW counts, and cholesterol levels (all p < 0.05, Table 2) in PD patients.
Table 2.
Correlation between baseline SIRI and clinical, laboratory parameters.
| SIRI | r | p |
|---|---|---|
| Age (years) | 0.207 | <0.001** |
| BMI (kg/m2) | −0.008 | 0.841 |
| White blood cell count (G/L) | 0.556 | <0.001** |
| Neutrophil count (G/L) | 0.708 | <0.001** |
| Monocyte count (G/L) | 0.659 | <0.001** |
| Lymphocyte count (G/L) | −0.207 | <0.001** |
| Hemoglobin (g/L) | 0.021 | 0.596 |
| RDW (%) | 0.125 | 0.001* |
| Serum urea nitrogen (mmol/L) | 0.031 | 0.431 |
| Serum creatinine (μmol/L) | 0.021 | 0.588 |
| Corrected calcium (mmol/L) | −0.030 | 0.949 |
| Phosphorus (mmol/L) | 0.002 | 0.960 |
| Serum uric acid (μmol/L) | 0.031 | 0.434 |
| Triglyceride (mmol/L) | 0.038 | 0.336 |
| Cholesterol (mmol/L) | 0.096 | 0.015* |
| Serum albumin (g/L) | 0.004 | 0.917 |
| Total Kt/V | −0.01 | 0.801 |
Bold values are when p is less than 0.05 or 0.01.
*p < 0.05; **p < 0.01.
RDW: red blood cell distribution width.
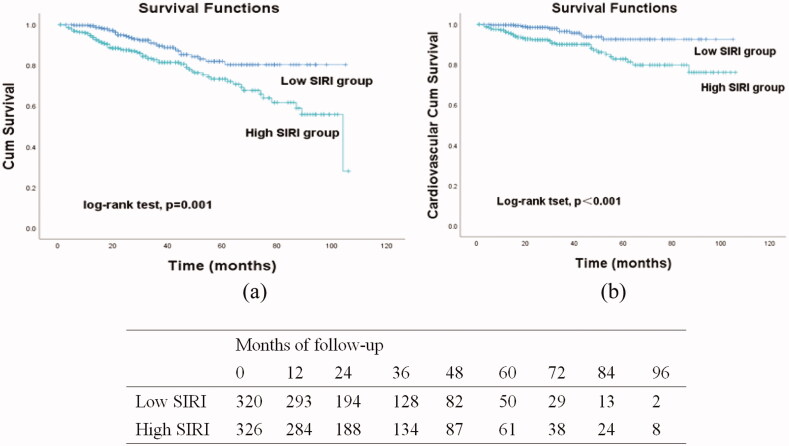
During the follow-up period, 165 patients discontinued the study due to initiating HD (74, 11.5%), receiving a kidney transplantation (31, 4.8%), or loss to follow up (60, 9.3%). A total of 97 (15.0%) deaths occurred, with 47 (49.0%) attributed to CVD (Figure 1). We observed statistically significant differences in all-cause mortality (log-rank = 11.58, p = 0.001) and CVD mortality (log-rank = 12.74, p < 0.001) between the two groups (Figure 2).
Figure 2.
Kaplan–Meier curves were used to analyze study endpoints that occurred during follow-up in patients grouped by the systemic inflammation response index (SIRI): overall survival (a) and cardiovascular event-free survival (b).
According to univariate Cox regression analysis, PD patients with a high SIRI level at baseline correlated with a high incidence of all-cause death (HR: 2.065, 95% CI: 1.347–3.166, p = 0.001). After adjustment of the data for potential confounders including CVD, diabetes, age, RDW, serum creatinine, serum albumin, high NLR levels, and high MLR levels, this association remained significant (HR: 2.007, 95% CI: 1.304–3.088, p = 0.002) (Table 3). Similarly, PD patients with high SIRI levels had a significantly increased risk of CVD mortality (HR: 3.201, 95% CI: 1.628–6.292, p = 0.001). After adjusting potential confounders including CVD, age, RDW, serum albumin, total Kt/V, high NLR levels, and high MLR levels, multivariate Cox regression analysis indicated that high SIRI levels were still associated with CVD mortality (HR: 2.847, 95% CI: 1.445–5.608, p = 0.002) (Table 4).
Table 3.
Univariate and multivariate Cox regression analyses of prognostic factors for all-cause mortality.
| Variable | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| SIRI (<1.28 vs. ≥1.28) | 2.065 | 1.347–3.166 | 0.001 | 2.007 | 1.304–3.088 | 0.002 |
| Sex (male = 1; female = 2) | 0.850 | 0.561–1.288 | 0.443 | |||
| Age (years) | 1.047 | 1.030–1.063 | <0.001 | 1.037 | 1.020–1.054 | <0.001 |
| BMI (kg/m2) | 1.038 | 0.955–1.129 | 0.376 | – | – | – |
| Cardiovascular disease (no = 0; yes = 1) | 1.644 | 1.094–2.471 | 0.017 | – | – | – |
| Diabetes mellitus (no = 0; yes = 1) | 2.217 | 1.468–3.348 | <0.001 | – | – | – |
| Hemoglobin (g/L) | 0.996 | 0.986–1.007 | 0.498 | – | – | – |
| RDW (%) | 1.093 | 1.004–1.189 | 0.039 | – | – | – |
| Serum creatinine (μmol/L) | 0.999 | 0.998–1.000 | 0.004 | – | – | – |
| Serum albumin (g/L) | 0.908 | 0.871–0.948 | <0.001 | 0.931 | 0.890–0.974 | 0.002 |
| Uric acid (μmol/L) | 0.999 | 0.997–1.001 | 0.494 | – | – | – |
| Cholesterol (mmol/L) | 1.051 | 0.888–1.244 | 0.565 | – | – | – |
| Triglyceride (mmol/L) | 1.079 | 0.925–1.259 | 0.334 | – | – | – |
| Total Kt/V | 0.748 | 0.501–1.117 | 0.156 | – | – | – |
| NLR (<2.59 vs. ≥2.59) | 1.546 | 1.028–2.325 | 0.037 | – | – | – |
| MLR (<0.31 vs. ≥0.31) | 2.314 | 1.484–3.068 | <0.001 | – | – | – |
SIRI: systemic inflammation response index; NLR: neutrophil count/monocyte count; MLR: monocyte count/lymphocyte count; RDW: red blood cell distribution width.
In univariate Cox regression analysis, variables with p < 0.1 will be included in multivariate Cox regression.
Table 4.
Univariate and multivariate Cox regression analyses of prognostic factors for CVD mortality.
| Variable | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| SIRI (<1.28 vs. ≥1.28) | 3.201 | 1.628–6.292 | 0.001 | 2.847 | 1.445–5.608 | 0.002 |
| Sex (male = 1; female = 2) | 0.629 | 0.337–1.176 | 0.147 | – | – | – |
| Age (years) | 1.048 | 1.025–1.072 | <0.001 | 1.044 | 1.021–1.068 | <0.001 |
| BMI (kg/m2) | 0.927 | 0.818–1.052 | 0.240 | – | – | – |
| Cardiovascular disease (no = 0; yes = 1) |
1.814 | 1.019–3.230 | 0.043 | – | – | – |
| Diabetes mellitus (no = 0; yes = 1) |
1.784 | 0.974–3.266 | 0.061 | – | – | – |
| Hemoglobin (g/L) | 1.002 | 0.987–1.018 | 0.776 | – | – | – |
| RDW (%) | 1.133 | 1.011–1.270 | 0.031 | – | – | – |
| Serum creatinine (μmol/L) | 0.999 | 0.998–1.000 | 0.076 | – | – | – |
| Serum albumin (g/L) | 0.920 | 0.865–0.978 | 0.007 | – | – | – |
| Uric acid (μmol/L) | 1.000 | 0.997–1.003 | 0.908 | – | – | – |
| Cholesterol (mmol/L) | 1.066 | 0.842–1.350 | 0.596 | – | – | – |
| Triglyceride (mmol/L) | 0.968 | 0.736–1.272 | 0.813 | – | – | – |
| Total Kt/V | 0.503 | 0.266–0.952 | 0.035 | – | – | – |
| NLR (<2.59 vs. ≥2.59) | 2.353 | 1.259–4.399 | 0.007 | – | – | – |
| MLR (<0.31 vs. ≥0.31) | 2.935 | 1.494–5.768 | 0.002 | – | – | – |
SIRI: systemic inflammation response index; NLR: neutrophil count/ monocyte count; MLR: monocyte count/lymphocyte count; RDW: red blood cell distribution width.
In univariate Cox regression analysis, variables with p < 0.1 will be included in multivariate Cox regression.
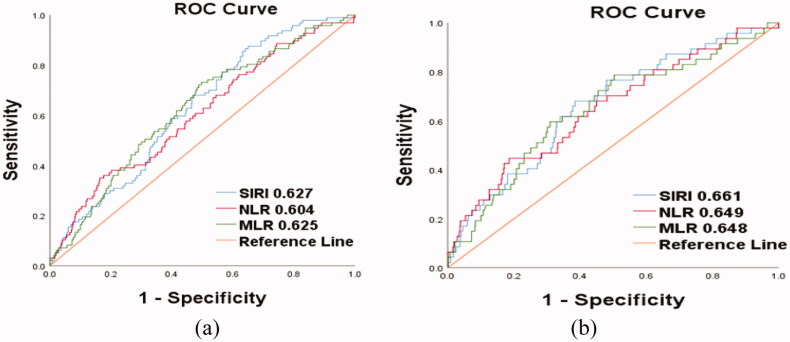
The ability of SIRI, NLR, and MLR to predict outcomes of PD patients was compared by measuring the AUC (Figure 3). SIRI showed a comparable power compared with the NLR and MLR for predicting all-cause and CVD-related deaths.
Figure 3.
ROC curves were drawn and the areas under the curves (AUC) were calculated to compare the predictive value of SIRI, NLR, and MLR in all-cause death and CVD death in PD patients.
Discussion
Microinflammation is universal and uncontrolled in patients undergoing PD. Chronic inflammation contributes to the uremic phenotype (such as CVD, PEW, and osteoporosis) in PD patients, and thus is a major contributor to increased all-cause and cardiovascular-related mortality [22]. SIRI is a novel marker of microinflammation, and to date, no studies have assessed whether SIRI levels could be associated with poor prognosis in PD patients. To the best of our knowledge, this is the first study to identify the relationship between SIRI and risk of mortality in patients on PD. Specifically, our data indicate that higher SIRI levels were predictive of an increased incidence of all-cause and CVD deaths in PD patients, an association that was still evident after proper adjustment of the data. Additionally, SIRI had a prognostic value comparable to the NLR and MLR.
First, by analyzing the data in the low and high SIRI groups, we found that patients in the high SIRI group were older on average than patients in the low SIRI group. The relationship between aging and inflammation has been well described in previous literature. Dysregulation and overactivation of inflammatory processes in the elderly results in thez persistence of chronic inflammatory conditions [23]. Furthermore, patients in the high SIRI group had a higher proportion of patients with diabetes, which is consistent with previous research. Inflammation contributes to the development of diabetes by causing insulin resistance, at the same time, the presence of hyperglycemia exacerbates inflammation [24]. Age and diabetes mellitus are traditional risk factors affecting the prognosis of PD patients. In 2014, a study in China determined that age is an independent risk factor for the prognosis of PD patients, which is consistent with our results [25]. In addition, previous research has shown that the survival rate of non-diabetic nephropathy patients with diabetes mellitus was decreased [26,27]. Diabetes mellitus as a complication of non-diabetic nephropathy has an important role in the prognosis of PD patients. However, in our study, there was not a statistically significant difference in diabetes incidence after multivariate Cox regression analysis, which could be due to the fact that this is a retrospective study with information bias. Finally, malnutrition is one of many long-term complications associated with PD, occurring in approximately 30–50% of patients [28]. Serum albumin, one of the nutritive indexes we assessed, was generally recognized as a protective factor in PD patients, with higher serum albumin levels correlated with a lower mortality rate, which we also observed in our study [29,30]. After adjusting for potential confounding factors, Cox regression analysis showed that serum albumin was negatively associated with mortality in PD patients.
We further explored the interaction between SIRI and other factors in our study using Spearman correlation analysis. The RDW has been observed to be a meaningful indicator of anemia and potential metabolic abnormalities including shortening of telomere length, inflammation, and oxidative stress [31]. In our study, we also observed a positive correlation between RDW and SIRI levels, with severe microinflammation correlating with increased severity of anemia in PD patients. Soohoo et al. found that RDW was linked to outcomes and hospitalizations in PD patients, with a higher RDW correlating with a higher risk of mortality and hospitalizations [32]. In our study, SIRI levels also were positively correlated with cholesterol. Increased cholesterol in PD patients could be due to both a loss of protein in the dialysate and stimulation of glucose in the PD solutions that thereby contribute to dyslipidemia in PD patients [33,34]. Previous work in the literature found that dyslipidemia was a major contributor to chronic intraperitoneal microinflammation [35], which is consistent with our findings that patients in the high SIRI group had higher total cholesterol levels (Table 2).
SIRI values were calculated by use of the following formula: neutrophil count × monocyte count/lymphocyte count. An increase in neutrophil and monocyte count and a decrease in lymphocyte count led to elevated SIRI levels. In previous studies, higher neutrophil and monocyte counts and lower lymphocyte counts have proven to be independent predictors of mortality risk in kidney failure patients [36–38]. In 2007, a 10-year prospective clinical study was conducted in the general population. The results showed that higher SIRI levels were linked to an increased risk of stroke and acute coronary syndrome, as well as all-cause mortality [39]. In addition, SIRI is associated with the prognosis of a variety of malignancies, such as cervical cancer [17]. In our study, we demonstrated that PD patients with a high SIRI level had a significantly increased risk for poor outcomes. Use of Cox proportional hazards models showed that high SIRI had significant predictive values for all-cause and CVD deaths. The risk of all-cause death and CVD death was 2.0 and 2.8 times higher in the high SIRI group than in the low SIRI group, respectively. The underlying mechanism by which SIRI affects the risk of all-cause death and CVD death in PD patients is unclear. SIRI is an indicator of inflammation. It integrates three immune pathways including neutrophils and monocytes that account for the persistent inflammatory response, and lymphocytes that account for immune regulation [40,41]. The higher the ratio, the greater the imbalance, and the more severe the inflammatory response. Based on our findings in this study, SIRI can be used as a risk stratification indicator for PD patients, as the early identification of microinflammation in PD patients along with a timely and powerful clinical intervention can effectively improve quality of life and prolong PD patient survival. Compared with a single indicator or a composite indicator of two factors, the composite indicator of three factors may be more stable and less susceptible to other factors, thus increasing the application value in predicting PD patients’ prognoses. Therefore, SIRI may be widely promoted and useful in clinical applications to monitor and assess mortality risk of patients on PD.
Although our data presented here is novel, there are several limitations of our study. First, our study is a retrospective, single-center study and may have potential bias. Second, we only measured patient’s baseline parameters; therefore, understanding how SIRI levels can change over time is extremely necessary and deserves further exploration. Finally, the study lacked a complete assessment of inflammatory markers, such as cytokines and high-sensitivity CRP. These markers should be included in further studies to further our understanding of the role inflammation and SIRI play in predicting PD patient prognosis.
Conclusion
In summary, our research presented here demonstrates that SIRI may be a useful index to indicate the prognosis of PD patients, with comparable power to the NLR and MLR.
Funding Statement
This work is supported by Traditional Chinese Medicine Bureau of Guangdong Province [No. 20201227].
Ethical approval
The Ethics Committee of Zhujiang Hospital approved this research (Ethics: 2022-KY-072-01), and this research was in adherence with the Declaration of Helsinki.
Author contributions
Jiaqi Li#: performed study, analyzed data, wrote article.
Yingxue Li#: performed study, wrote article.
Yaowei Zou: performed study, revised the manuscript.
Yaode Chen: performed study, collected data.
Lizhen He, Ying Wang, Jingxuan Zhou, Fangqi Xiao: collected data.
Hongxin Niu: designed study.
Lingli Lu: designed study, revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data set used in the study can be obtained from the corresponding author upon reasonable request.
References
- 1.Saran R, Robinson B, Abbott KC, et al. . US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1):A6–a7. [DOI] [PubMed] [Google Scholar]
- 2.Li PK, Chow KM, Van de Luijtgaarden MW, et al. . Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Devuyst O, Davies SJ, et al. . The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27(11):3238–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong VW, Li PK.. Peritoneal dialysis in Asia. Kidney Dis (Basel, Switzerland). 2015;1(3):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jegatheesan D, Cho Y, Johnson DW.. Clinical studies of interventions to mitigate cardiovascular risk in peritoneal dialysis patients. Semin Nephrol. 2018;38(3):277–290. [DOI] [PubMed] [Google Scholar]
- 6.Jovanovic DB, Stosović MD, Gojakovic BM, et al. . Inflammatory markers as mortality predictors in continuous ambulatory peritoneal dialysis patients. Renal Failure. 2015;37(2):230–236. [DOI] [PubMed] [Google Scholar]
- 7.Akchurin OM, Kaskel F.. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1-3):84–92. [DOI] [PubMed] [Google Scholar]
- 8.Wang AYM, Woo J, Wang M, et al. . Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2001;12(9):1927–1936. [DOI] [PubMed] [Google Scholar]
- 9.Wang AY. Consequences of chronic inflammation in peritoneal dialysis. Semin Nephrol. 2011;31(2):159–171. [DOI] [PubMed] [Google Scholar]
- 10.Wang AY, Sea MM, Tang N, et al. . Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol. 2004;15(12):3134–3143. [DOI] [PubMed] [Google Scholar]
- 11.Chen T, Yang M.. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int Immunopharmacol. 2020;78:106063. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Zhan X, Wang N, et al. . Monocyte/lymphocyte ratio and cardiovascular disease mortality in peritoneal dialysis patients. Mediat Inflamm. 2020;2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Lai X, Chen Q, ETNA, et al.. The relationship between neutrophil-to-lymphocyte ratio and the first occurrence of pneumonia in peritoneal dialysis patients. Clin Exp Nephrol. 2020;24(9):770–778. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Zhou Y, Xia S, et al. . Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. 2020;28(4):537–547. [DOI] [PubMed] [Google Scholar]
- 15.Valero C, Pardo L, Sansa A, et al. . Prognostic capacity of systemic inflammation response index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck. 2020;42(2):336–343. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco-Barcia V, Mondéjar Solís R, France T, et al. . A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20(2):254–264. [DOI] [PubMed] [Google Scholar]
- 17.Chao B, Ju X, Zhang L, et al. . A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front Oncol. 2020;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgen CS, Ängquist L, Appleyard M, et al. . Attitudes to and experiences with body weight control and changes in body weight in relation to all-cause mortality in the general population. PLoS One. 2019;14(8):e0220838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American diabetes A. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(1):S15–S33.33298413 [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, et al. . ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 21.Virani SS, Alonso A, Benjamin EJ, et al. . Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 22.Cobo G, Lindholm B, Stenvinkel P.. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(3):iii35–iii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgoni S, Kudryashova KS, Burka K, et al. . Targeting immune dysfunction in aging. Ageing Res Rev. 2021;70:101410. [DOI] [PubMed] [Google Scholar]
- 24.Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. . Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–444. [DOI] [PubMed] [Google Scholar]
- 25.Joshi U, Guo Q, Yi C, et al. . Clinical outcomes in elderly patients on chronic peritoneal dialysis: a retrospective study from a single center in China. Perit Dial Int. 2014;34(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei Y, Xiong Y, Zhang L, et al. . Comparison of Long-Term outcomes between peritoneal dialysis patients with diabetes as a primary renal disease or as a comorbid condition. PLoS One. 2015;10(5):e0126549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim WH, Johnson DW, Hawley C, et al. . Type 2 diabetes in patients with end-stage kidney disease: influence on cardiovascular disease-related mortality risk. Med J Aust. 2018;209(10):440–446. [DOI] [PubMed] [Google Scholar]
- 28.Kiebalo T, Holotka J, Habura I, et al. . Nutritional status in peritoneal dialysis: nutritional guidelines, adequacy and the management of malnutrition. Nutrients. 2020;12(6):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao N, Cheng BC, Yang HT, et al. . Time-varying serum albumin levels and all-cause mortality in prevalent peritoneal dialysis patients: a 5-year observational study. BMC Nephrol. 2019;20(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Han Q, Wang T, et al. . Serum albumin changes and mortality risk of peritoneal dialysis patients. Int Urol Nephrol. 2020;52(3):565–571. [DOI] [PubMed] [Google Scholar]
- 31.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. . Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. [DOI] [PubMed] [Google Scholar]
- 32.Soohoo M, Molnar MZ, Ujszaszi A, et al. . Red blood cell distribution width and mortality and hospitalizations in peritoneal dialysis patients. Nephrol Dial Transplant. 2019;34(12):2111–2118. [DOI] [PubMed] [Google Scholar]
- 33.Moradi H, Streja E, Vaziri ND.. ESRD-induced dyslipidemia-Should management of lipid disorders differ in dialysis patients? Semin Dial. 2018;31(4):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Q, Lin J, Li J, et al. . The effect of fluid overload on clinical outcome in Southern Chinese patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35(7):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepanova N, Driianska V, Savchenko S.. Dyslipidemia and intraperitoneal inflammation axis in peritoneal dialysis patients: a cross-sectional pilot study. Kidney Dis. 2020;6(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato A, Takita T, Furuhashi M, et al. . Blood monocyte count is a predictor of total and cardiovascular mortality in hemodialysis patients. Nephron Clin Pract. 2008;110(4):c235–43. [DOI] [PubMed] [Google Scholar]
- 37.Mitsopoulos E, Lysitska A, Zanos S, et al. . Normal white blood cell counts predict long-term mortality of hemodialysis patients. Int Urol Nephrol. 2020;52(4):783–790. [DOI] [PubMed] [Google Scholar]
- 38.Ateş K, Ateş A, Kutlay S, et al. . Total lymphocyte count in peripheral blood of peritoneal dialysis patients: relationship to clinical parameters and outcome. J Nephrol. 2004;17(2):246–252. [PubMed] [Google Scholar]
- 39.Jin Z, Wu Q, Chen S, et al. . The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and All-Cause mortality: a Ten-Year Follow-Up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fridman WH, Pagès F, Sautès-Fridman C, et al. . The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Wang K, Zheng H, et al. . Monocyte lymphocyte ratio predicts the new-onset of chronic kidney disease: a cohort study. Clin Chim Acta. 2020;503:181–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used in the study can be obtained from the corresponding author upon reasonable request.