SUMMARY
Background:
Non-alcoholic fatty liver disease (NAFLD) is found in inflammatory bowel disease (IBD). However, uncertainties remain on the magnitude of the association.
Aims:
To explore systematically the prevalence of, and risk factors for, NAFLD in IBD patients.
Methods:
We searched medical literature using Embase, PubMed, Web of Science, Scopus, and ProQuest, from inception to September 30, 2021. We included observational studies reporting the prevalence of NAFLD in ≥50 adult patients with IBD. Diagnosis of NAFLD could be based on imaging, histopathology, and/or hepatic steatosis index. Sex, age, body mass index (BMI), diabetes, hypertension, dyslipidemia, prior surgery (bowel resection), corticosteroids, biologics, and immunomodulators were assessed as potential risk factors for NAFLD.
Results:
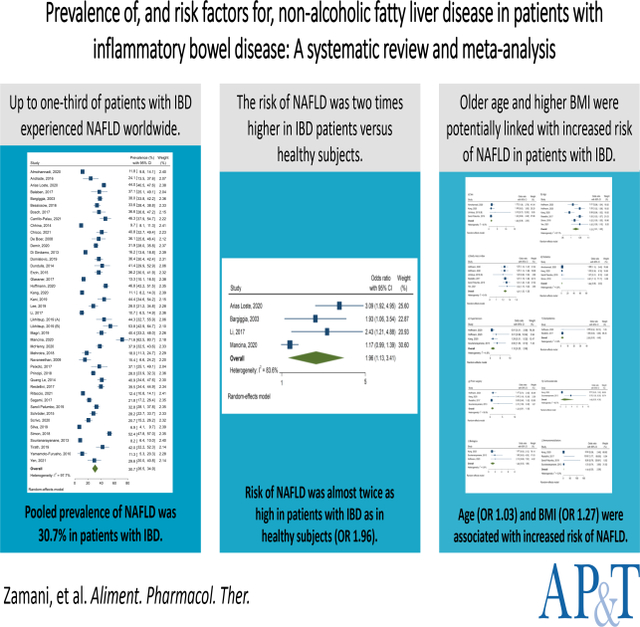
Of 1,893 citations, 44 eligible studies were finally included, comprising 14,947 subjects from 18 different countries. Pooled prevalence of NAFLD was 30.7% (95% confidence interval [CI] 26.5–34.9) in patients with IBD worldwide, which varied regionally. No significant difference was observed in odds ratio (OR) of NAFLD among Crohn’s disease (CD) patients compared with ulcerative colitis (UC) patients (1.16, 95% CI 0.93–1.44). Risk of NAFLD was almost twice as high in patients with IBD as in healthy subjects (OR 1.96, 95% CI 1.13–3.41). Age (adjusted OR 1.03, 95% CI 1.01–1.05) and BMI (adjusted OR 1.27, 95% CI 1.22–1.32) were statistically significantly associated with increased risk of NAFLD. The pooled prevalence of advanced liver fibrosis in IBD patients with NAFLD was 13.6% (95% CI 7.6–19.7) based on six studies.
Conclusion:
Up to one-third of patients with IBD experienced NAFLD worldwide. The risk of NAFLD was two times higher in IBD patients versus healthy subjects.
Graphical Abstract
INTRODUCTION
Inflammatory bowel diseases (IBD) are chronic diseases characterised by remitting and relapsing inflammation of the gastrointestinal tract, with negative effects on the patients’ social function and quality of life.1,2 Crohn’s disease (CD) and ulcerative colitis (UC) are the two main types of IBD that present specific characteristics, and their incidence and prevalence are globally increasing.3 These diseases frequently develop extraintestinal manifestations affecting different organs, such as hepatobiliary manifestations.4
Non-alcoholic fatty liver disease (NAFLD), a growing cause of chronic liver diseases (such as hepatic cirrhosis, and hepatocellular carcinoma), has been observed in IBD patients with various prevalence reported.5–7 It seems that there is a similarity between IBD and NAFLD in the epidemiology across geographic areas over time.8 However, the etiology of the association between these two diseases is unknown. Both diseases are multifactorial with environmental, genetic, immunologic, and pharmacological determinants.9,10
IBD and NAFLD are both associated with considerable healthcare expenditures, and their increasing prevalence would undoubtedly impose a growing economic burden.11,12 Moreover, IBD patients with concurrent NAFLD are potentially at a higher risk of liver abnormalities compared with those without, which can affect the clinical management of the patients with IBD.8,13 Comprehensive systematic analysis on the data related to coexistence magnitude of IBD and NAFLD would hopefully give a better insight into management strategies for these conditions. We have therefore performed a systematic review and meta-analysis on the prevalence of, and risk factors for, NAFLD in patients with IBD to examine these issues.
METHODS
Study protocol
The current systematic review and meta-analysis was presented according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guideline.14 The protocol of our study was documented online in the PROSPERO registry (CRD42021278106).
Information sources and search strategy
We searched the published literature using Embase, PubMed, Web of Science, Scopus, and ProQuest, from the inception to 30 September 2021, to identify the observational studies reporting the prevalence of NAFLD among IBD patients. No language restriction was applied. The related terms were searched in the Medical Subject Headings (MeSH) database, and finally, the following free text terms were selected as keywords: “inflammatory bowel disease” OR “inflammatory bowel diseases” OR “IBD” OR “Crohn disease” OR “Crohn’s disease” OR “ulcerative colitis” AND “NAFLD” OR “NAFL” OR “non-alcoholic fatty liver disease” OR “nonalcoholic fatty liver disease” OR “fatty liver disease” OR “fatty liver” OR “steatosis” OR “NASH” OR “steatohepatitis”. The search was limited to Title/Abstract. The full search strategy is provided in the Supplement. To identify additional relevant articles, we did a hand search of the reference lists of the related reviews and the retrieved papers.
Inclusion and exclusion criteria
To be eligible, individual studies had to report the prevalence of NAFLD in at least 50 unselected adult patients (aged ≥18 years old) with histologically or radiologically confirmed IBD. The diagnosis of NAFLD could be based on imaging (ultrasonography, computed tomography, magnetic resonance imaging, and/or transient elastography [FibroScan]), histopathology, and/or hepatic steatosis index (HSI). The exclusion criteria included: 1) Reviews, case reports, editorials, and letter to the editors; 2) Duplicate papers or assessing the same sample; 3) Studies did not exclude subjects with excessive alcohol consumption; 4) Studies included only subjects with a specific condition, such as morbid obesity; 5) Studies with less than 50 participants; 6) Surveys that recruited children; 7) Studies without extractable data; 8) Full-texts not being available.
Study selection and data extraction
Two reviewers (MZ and SA) independently evaluated the study suitability by screening the titles and abstracts of all references, and then full-texts of the potential papers, using the pre-designed eligibility forms. Any disagreements were resolved by consensus between the investigators or by the third author’s comment (RL). The degree of agreement was measured with a kappa statistic. Data were extracted from the retrieved articles onto a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, Washington) by two authors (MZ and SA) independently. Again, the discrepancies were resolved by consensus. The following data were finally collected: first author’s name, publication year, study location (country), diagnostic method of NAFLD, sample size, number of subjects by sex (if available), IBD subtype (CD or UC, if available), activity of IBD (active, or inactive, if available), CD location (ileal, colonic, ileocolonic, or upper gastrointestinal, if available), CD behavior (non-stricturing, non-penetrating, stricturing, or penetrating, if available), UC extent (proctitis, left-side, or extensive, if available), NAFLD severity (mild-to-moderate, or severe, if available), number of healthy controls (if available), risk factors (age, body mass index [BMI], diabetes, hypertension, dyslipidemia, prior surgery for IBD [bowel resection], corticosteroids, biologics, immunomodulators, or 5-aminosalicylic acid, if available), number of NAFLD in IBD patients, number of advanced liver fibrosis in IBD patients with NAFLD (if available), and diagnostic method of liver fibrosis. For the present study, control groups included healthy subjects without IBD who did not attend a hospital as outpatients. We grouped location of studies according to the World Health Organisation (WHO) Regions (Suppl Table 1).15 Google Translate was used for translation of non-English articles. With respect to duplicate publications, we chose the study with the most comprehensive details. Where the necessary information was unavailable, we emailed the corresponding authors.
Risk of bias assessment
In order to assess the risk of bias of the included studies, we used the checklist by Hoy et al.,16 which was designed for the prevalence studies. This tool has nine questions about target population, sampling frame, selection of sample, response rate by subjects, how to data collection, case definition, study instrument, same mode of data collection, and numerators and dominators for the parameters. There are two potential responses for each criterion, including “Yes” (score 0) or “No” (score 1). The total scores for each study would range from 0 to 9, and higher scores show higher risk of bias.
Study outcomes and statistical analysis
The proportion of patients with IBD with concomitant NAFLD in each study was combined to give a pooled prevalence rate of NAFLD using the Inverse Variance method (including a double arcsine transformation). The estimates were presented as percent and 95% confidence interval (CI). The I2 statistic was used to measure the heterogeneity between the studies, which ranges from 0% to 100% and is categorised as follows: 25% to 49% (low heterogeneity), 50% to 74% (moderate heterogeneity), and ≥75% (high heterogeneity).17 The chi-squared test with a p-value less than 0.10 was used to define a significant degree of heterogeneity.17 We performed subgroup analyses according to type of IBD (CD, or UC), geographical study location, publication date (2003–2010, 2011–2016, or 2017–2021), NAFLD severity, sex, diagnostic method of NAFLD, IBD activity, CD location or behavior, and UC extent. We classified NAFLD severity as mild-to-moderate (grades 1 and 2 on ultrasound, or controlled attenuation parameter [CAP] S1 and S2 on FibroScan) or severe (grade 3 on ultrasound, or CAP S3 on FibroScan). A p-value less than 0.05 indicated a significant difference between the subgroups. Regarding the risk factors mentioned earlier, we extracted odds ratios (ORs) with confounder adjustment, where applicable, and pooled them to obtain an overall estimate of adjusted OR (aOR). When a study presented information on the risk factors for CD and UC, we considered each report separately for analysis. Sex was also analysed as a risk factor. Pooling the data was conducted using a random-effects model to provide a more conservative estimate of the prevalence rate, and the odds, of NAFLD in different groups. The publication bias was assessed using Egger’s test where at least 10 studies were available.18 A p-value less than 0.05 was considered statistically significant for Egger’s test. The statistical analyses were conducted using STATA (StataCorp, College Station, TX).
RESULTS
Search results, study selection and characteristics
The search strategy initially recovered 1,893 citations. Of these, 92 articles appeared to be potentially pertinent to the study question and were retrieved for further evaluations. Finally, a total of 44 papers fulfilled the eligibility criteria (Figure 1), comprising 14,947 subjects from 18 different countries worldwide.5–7,13,19–58. There was an excellent agreement between the two investigators for the eligibility judgment (Kappa statistic = 0.85). All papers were published in English. A total of 19 studies were carried out in the European Region (seven in Italy, three in Turkey, two in Germany, two in Portugal, two in Spain, one in Croatia, one in Netherlands, one in Romania),5,13,19,21,23,26,28,30,32,35,36,43–46,48,52,56,58 18 studies in the Region of the Americas (13 in the USA, three in Canada, one in Mexico, and one in Brazil),6,7,22,24,25,27,29,34,37,39–41,47,49–51,54,55 four studies in the Western Pacific Region (China, Japan, Korea, and Taiwan each had an individual study).31,38,42,49, two studies in the South-East Asian Region (India),20,53 and one study in the Eastern Mediterranean Region (Qatar).57 Baseline characteristics, as well as risk of bias assessment, of the included studies are summarised in Suppl Table 2.
Figure 1.
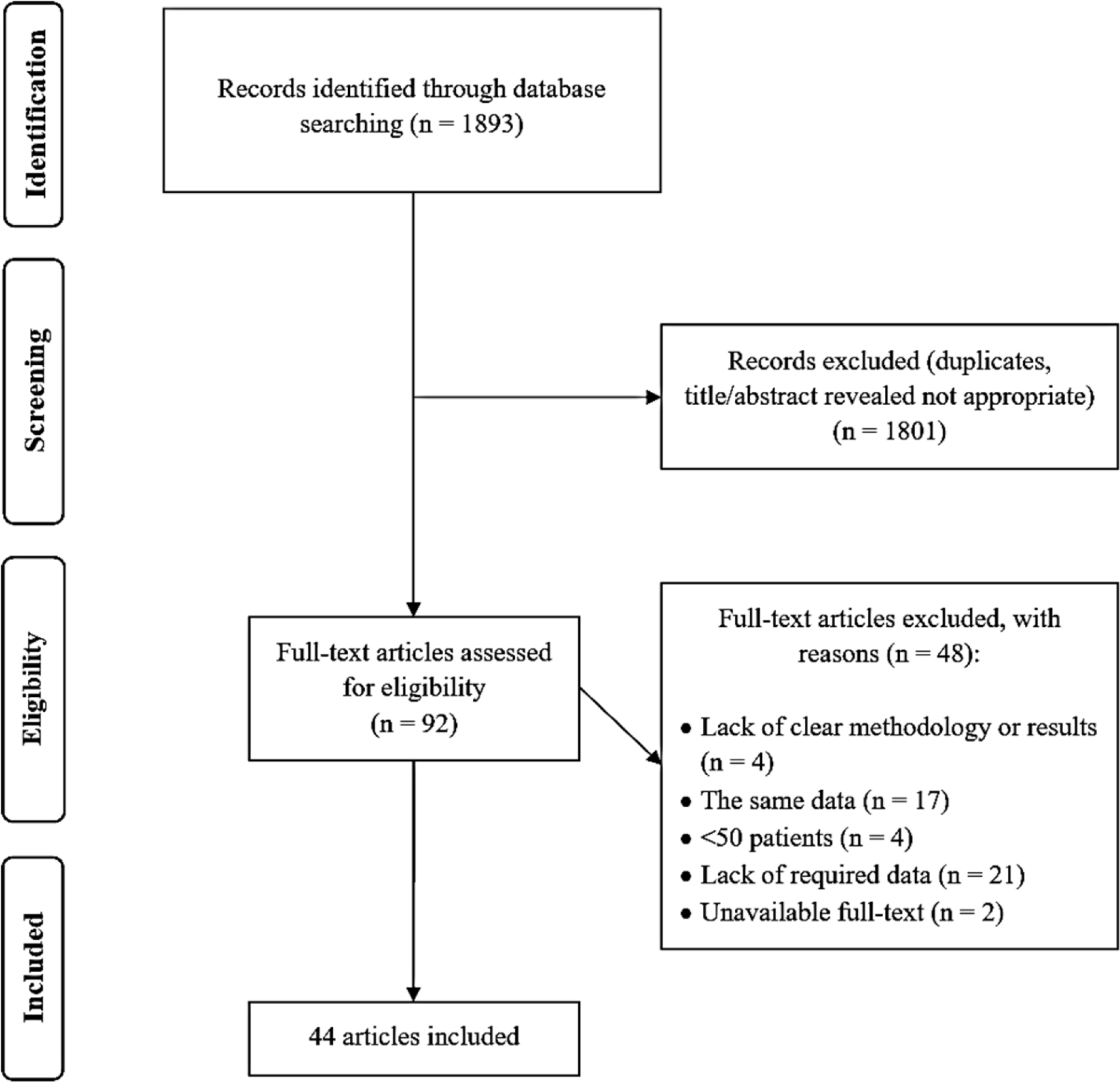
PRISMA flow diagram
Pooled prevalence of NAFLD in patients with IBD according to geographical study location and publication date
Based on 44 studies containing 14,947 patients, the global pooled prevalence of NAFLD was 30.7% (95% CI 26.5–34.9; I2=97.7%; p<0.001) in patients with IBD (Figure 2). According to WHO Regions, the highest prevalence of NAFLD in IBD patients was 36.9% (95% CI 31.2–42.6; I2= 95.1%; p<0.001) in the European Region, and the lowest was 11.8% (95% CI 9.7–13.9) in the Eastern Mediterranean Region (Table 1). The difference in the prevalence rates between different regions was significant (p<0.001). At country level, the highest prevalence of NAFLD in patients with IBD was 44.4% (95% CI 41.2–47.6; I2=0.0%; p=0.619) in Spain, and the lowest was 6.9% (95% CI 4.0–9.7) in Brazil (Suppl Table 3). Figure 3 denotes the prevalence of NAFLD among IBD patients across the world. The pooled prevalence in studies published during 2003–2010,19,23,41,50 2011–2016,7,13,22,24,27,28,47,48,56 and 2017–20215,6,20,21,25,26,29–40,42–46,49,51–55,57,58 was 25.2% (95% CI 10.2–40.2; I2=94.4%; p<0.001), 25.9% (95% CI 18.8–33.0; I2=97.4%; p<0.001), and 32.8% (95% CI 27.4–38.2; I2=97.8%; p<0.001), respectively, and the difference was not significant (p=0.258) (Table 1).
Figure 2.
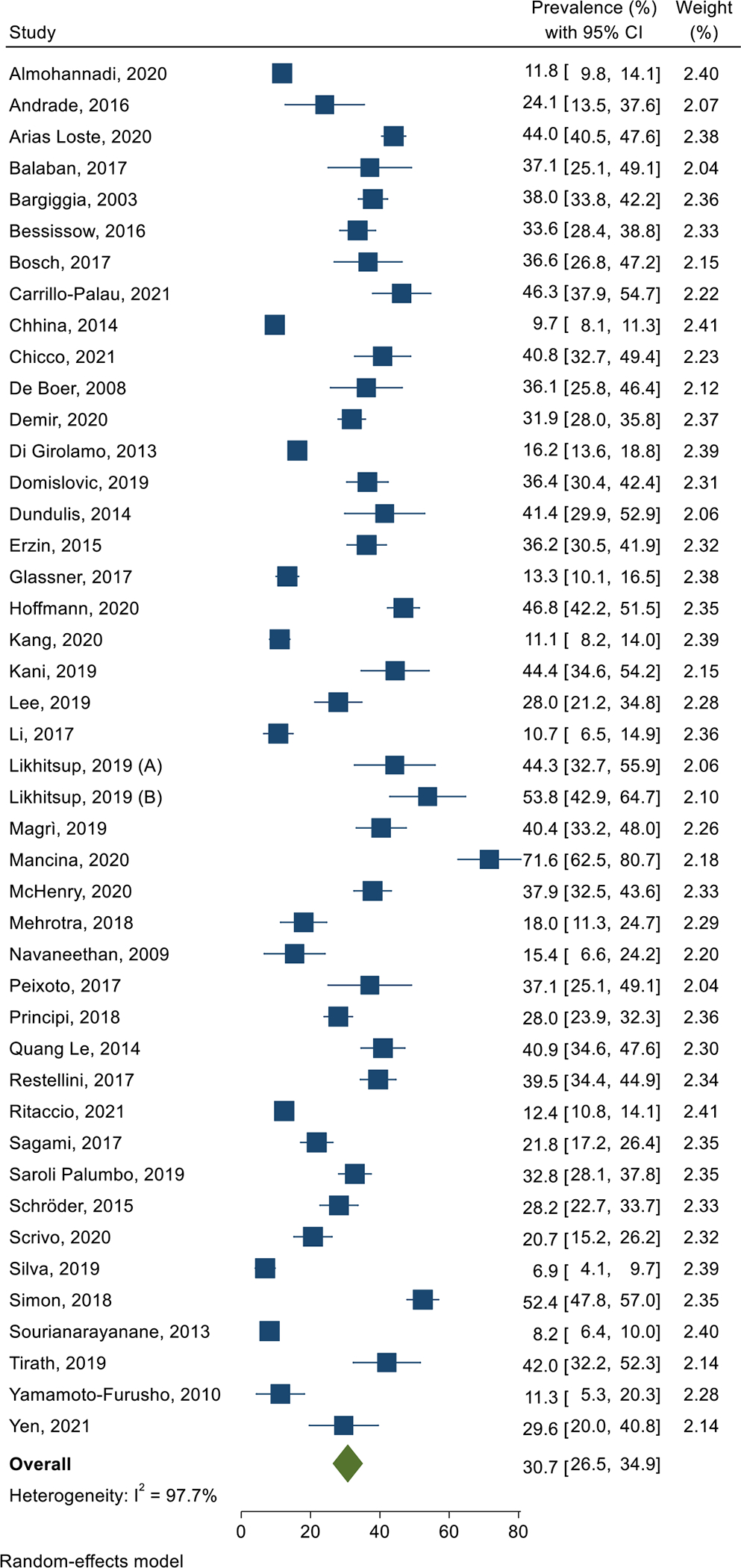
Forest plot of the pooled prevalence of non-alcoholic fatty liver disease in patients with inflammatory bowel disease
Table 1.
Pooled prevalence of non-alcoholic faatty liver disease (NAFLD) in patients with inflammatory bowel disease (IBD) according to sex, World Health Origanisation (WHO) regions, publication year, diagnostic method of NAFLD, and disease activity, localization and behavior
| Disease type | No. of studies | No. of IBD patients | Pooled prevalence of NAFLD, % (95% CI) | P-value for subgroups | I 2 | P-value for χ2 | |
|---|---|---|---|---|---|---|---|
| IBD | Overall | 44 | 14,947 | 30.7 (26.5–34.9) | 97.7% | <0.001 | |
| Sex | |||||||
| Men | 15 | 2,836 | 33.2 (25.5–40.9) | 0.040 | 96.0% | <0.001 | |
| Women | 15 | 2,698 | 27.0 (20.5–33.4) | 93.3% | <0.001 | ||
| WHO Region | |||||||
| Region of the Americas | 18 | 7,316 | 28.2 (22.2–34.3) | <0.001 | 98.0% | <0.001 | |
| South-East Asian Region | 2 | 228 | 29.7 (6.2–53.3) | 93.8% | <0.001 | ||
| European Region | 19 | 5,457 | 36.9 (31.2–42.6) | 95.1% | <0.001 | ||
| Eastern Mediterranean Region | 1 | 913 | 11.8 (9.7–13.9) | NA | NA | ||
| Western Pacific Region | 4 | 1,033 | 17.2 (10.3–24.1) | 88.7% | <0.001 | ||
| Publication year | |||||||
| 2003–2010 | 4 | 739 | 25.2 (10.2–40.2) | 0.258 | 94.4% | <0.001 | |
| 2011–2016 | 9 | 4,232 | 25.9 (18.8–33.0) | 97.4% | <0.001 | ||
| 2017–2021 | 31 | 9,976 | 32.8 (27.4–38.2) | 97.8% | <0.001 | ||
| Diagnostic method of NAFLD | |||||||
| Imaging | 35 | 11,239 | 30.5 (25.8–35.2) | <0.001 | 97.7% | <0.001 | |
| Histopathology | 4 | 1,025 | 36.2 (27.6–44.8) | 77.0% | 0.005 | ||
| HSI | 2 | 571 | 34.8 (30.9–38.7) | 0.0% | 0.494 | ||
| Imaging or histopathology | 1 | 1,672 | 12.4 (10.8–14.0) | NA | NA | ||
| Imaging or HSI | 2 | 440 | 30.8 (10.9–50.6) | 95.5% | <0.001 | ||
| Disease activity | |||||||
| Active | 6 | 690 | 34.5 (18.8–50.2) | 0.971 | 95.8% | <0.001 | |
| Remission | 6 | 1,276 | 34.2 (27.2–41.2) | 85.8% | <0.001 | ||
| CD | Overall | 25 | 5,223 | 34.4 (28.0–40.8) | 96.9% | <0.001 | |
| Sex | |||||||
| Men | 5 | 683 | 33.2 (16.5–50.0) | 0.736 | 96.5% | <0.001 | |
| Women | 5 | 470 | 29.7 (17.3–42.0) | 88.6% | <0.001 | ||
| WHO Region | |||||||
| Region of the Americas | 8 | 2,380 | 36.7 (23.6–49.8) | <0.001 | 98.1% | <0.001 | |
| South-East Asian Region | 1 | 100 | 42.0 (32.3–51.7) | NA | NA | ||
| European Region | 11 | 1,610 | 40.3 (34.4–46.2) | 82.2% | <0.001 | ||
| Eastern Mediterranean Region | 1 | 383 | 11.8 (8.5–15) | NA | NA | ||
| Western Pacific Region | 4 | 750 | 16.0 (7.3–24.7) | 90.6% | <0.001 | ||
| Publication year | |||||||
| 2003–2010 | 1 | 311 | 39.6 (34.1–45.0) | 0.420 | NA | NA | |
| 2011–2016 | 3 | 491 | 35.7 (29.5–42.0) | 52.6% | 0.121 | ||
| 2017–2021 | 21 | 4,421 | 33.9 (26.9–41.0) | 97.1% | <0.001 | ||
| Diagnostic method of NAFLD | |||||||
| Imaging | 21 | 3,903 | 34.1 (26.7–41.6) | <0.001 | 97.0% | <0.001 | |
| Histopathology | 1 | 39 | 61.5 (46.3–76.8) | NA | NA | ||
| HSI | 2 | 384 | 37.0 (32.2–41.8) | 0.0% | 0.872 | ||
| Imaging or histopathology | 1 | 897 | 14.6 (12.3–16.92) | NA | NA | ||
| Disease activity | |||||||
| Active | 5 | 341 | 40.0 (12.3–67.7) | 0.651 | 96.4% | <0.001 | |
| Remission | 5 | 856 | 33.4 (26.1–40.6) | 80.2% | 0.001 | ||
| Disease location | |||||||
| Ileal | 5 | 361 | 28.8 (19.1–38.5) | 0.527 | 76.4% | 0.002 | |
| Colonic | 5 | 236 | 18.4 (5.6–31.2) | 84.3% | <0.001 | ||
| Ileocolonic | 5 | 655 | 23.0 (11.0–35.0) | 93.2% | <0.001 | ||
| Upper gastrointestinal | 3 | 39 | 23.7 (10.2–37.2) | 0.0% | 0.983 | ||
| Disease behavior | |||||||
| Non-stricturing, non-penetrating | 4 | 409 | 24.3 (6.4–42.1) | 0.182 | 96.0% | <0.001 | |
| Stricturing | 4 | 267 | 30.0 (20.6–39.4) | 62.0% | 0.048 | ||
| Penetrating | 4 | 211 | 32.0 (15.3–48.6) | 85.5% | <0.001 | ||
| UC | Overall | 22 | 3,101 | 28.2 (22.0–34.3) | 95.1% | <0.001 | |
| Sex | |||||||
| Men | 2 | 120 | 32.9 (0.0–70.1) | 0.031 | 95.8% | <0.001 | |
| Women | 2 | 102 | 20.1 (0.0–52.4) | 95.9% | <0.001 | ||
| WHO Region | |||||||
| Region of the Americas | 7 | 992 | 19.3 (11.5–27.1) | <0.001 | 90.6% | <0.001 | |
| South-East Asian Region | 1 | 128 | 18.0 (11.3–24.6) | NA | NA | ||
| European Region | 10 | 1,168 | 40.0 (31.5–47.7) | 88.4% | <0.001 | ||
| Eastern Mediterranean Region | 1 | 530 | 11.9 (9.1–14.6) | NA | NA | ||
| Western Pacific Region | 3 | 283 | 17.2 (8.8–25.5) | 68.7% | 0.041 | ||
| Publication year | |||||||
| 2003–2010 | 2 | 265 | 25.7 (6.0–45.4) | 0.962 | 92.2% | <0.001 | |
| 2011–2016 | 3 | 353 | 28.1 (22.2–34.1) | 36.5% | 0.207 | ||
| 2017–2021 | 17 | 2,483 | 28.6 (21.4–35.8) | 95.7% | <0.001 | ||
| Diagnostic method of NAFLD | |||||||
| Imaging | 18 | 2,407 | 29.7 (22.3–37.0) | <0.001 | 95.1% | <0.001 | |
| Histopathology | 1 | 54 | 18.5 (8.2–28.9) | NA | NA | ||
| HSI | 2 | 187 | 30.6 (20.7–40.5) | 55.4% | 0.134 | ||
| Imaging or histopathology | 1 | 453 | 8.6 (6.0–11.2) | NA | NA | ||
| Disease activity | |||||||
| Active | 3 | 185 | 35.7 (13.9–57.5) | 0.865 | 87.0% | <0.001 | |
| Remission | 3 | 270 | 33.3 (16.4–50.2) | 89.4% | <0.001 | ||
| Disease extent | |||||||
| Proctitis | 2 | 190 | 21.5 (13.1–30.0) | 0.467 | 50.1% | 0.157 | |
| Left-side | 2 | 143 | 14.9 (4.7–25.2) | 64.3% | 0.094 | ||
| Extensive | 2 | 127 | 25.3 (9.8–40.7) | 76.9% | 0.038 |
CI=confidence interval. HIS=hepatic steatosis index. CD=Crohn’s disease. UC=ulcerative colitis
Figure 3.
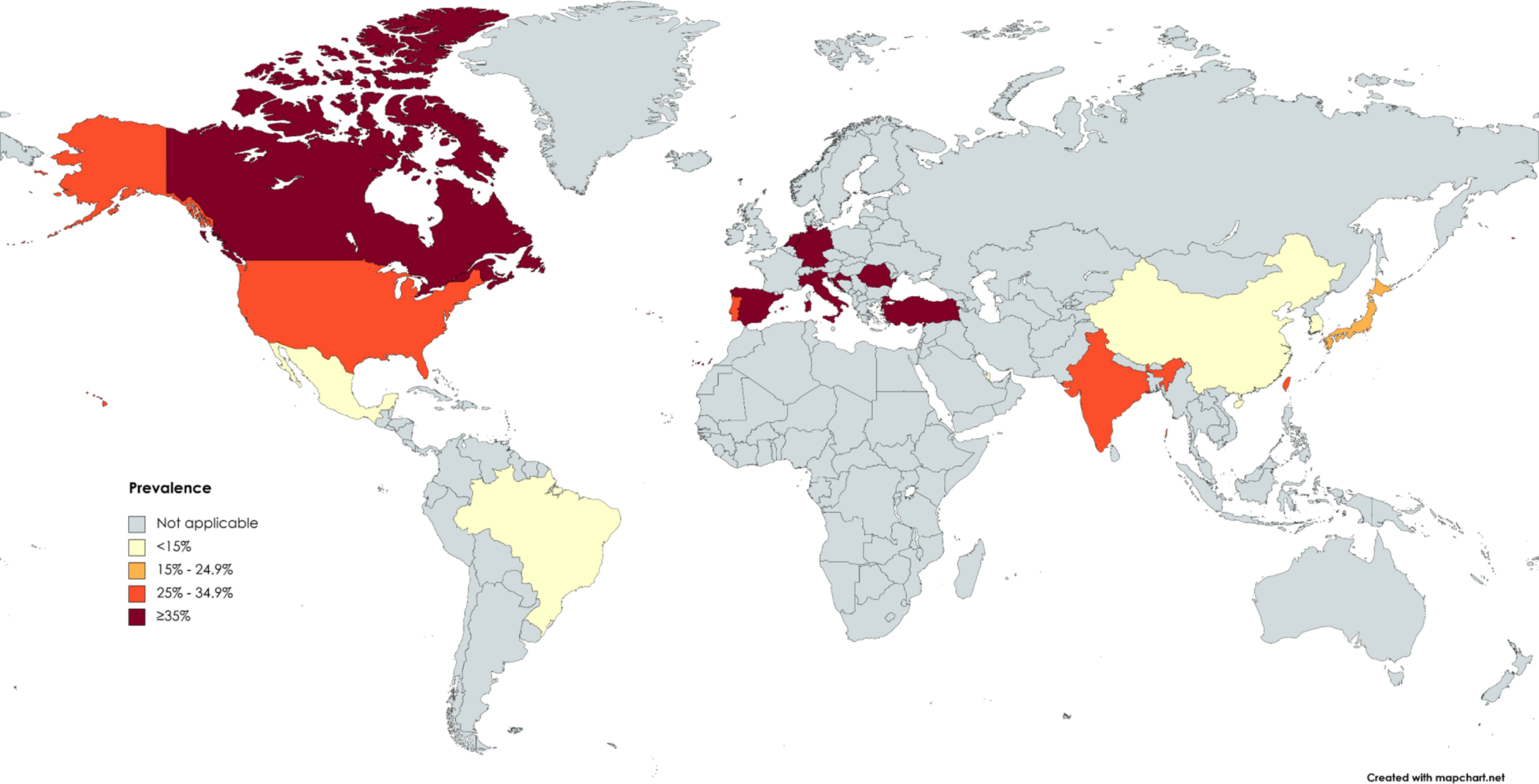
Prevalence of non-alcoholic fatty liver disease in patients with inflammatory bowel disease worldwide
Pooled prevalence of NAFLD in patients with IBD versus controls
There were four studies that compared the prevalence of NAFLD between IBD patients and healthy controls.23,33,36,43 Based on these studies that included 3,884 subjects, the pooled prevalence of NAFLD was higher in IBD patients compared with controls (40.8% [% 95 CI 21.2–60.3] versus 27.9% [% 95 CI 5.9–50.0]). The OR for NAFLD in patients with IBD compared with controls was 1.96 (95% CI 1.13–3.41), with high heterogeneity between the studies (I2=83.6%; p<0.001) (Suppl Figure 1).
Pooled prevalence of NAFLD in patients with IBD according to IBD subtype
The pooled prevalence of NAFLD was 34.4% (95% CI 28.0–40.8; I2=96.9%; p<0.001) in 25 studies containing 5,223 patients with CD,5,6,13,21,23–25,30–33,35–40,42,44,46,48,53–55,57 and 28.2% (95% CI 22.0–34.3; I2=95.1%; p<0.001) in 22 studies containing 3,101 patients with UC (Table 1 and Suppl Figure 2).5,6,13,20,23–25,30–33,35,36,39,42,44,46,48,50,54,55,57 The pooled OR for NAFLD in patients with CD versus patients with UC was 1.16 (95% CI 0.93–1.44) based on 20 studies, with moderate heterogeneity (I2=63.9%; p<0.001) but no publication bias (Egger’s test, p=0.65).5,6,13,23–25,30–33,35,36,39,42,44,46,48,54,55,57
Pooled prevalence of NAFLD in patients with IBD according to NAFLD severity
There were 11 studies reporting the prevalence of NAFLD in IBD patients according to NAFLD severity.5,19,21,23,26,28,30,35,39,44,57 The pooled prevalence of mild-to-moderate NAFLD was higher than severe NAFLD among patients with IBD (78.7% [% 95 CI 69.4–88.1] versus 21.3% [% 95 CI 11.9–30.6]). In patients with CD, the pooled prevalence of mild-to-moderate NAFLD was higher than severe NAFLD (69.7% [% 95 CI 64.4–74.9] versus 30.3% [% 95 CI 25.1–35.6]). Similarly, in patients with UC, a higher pooled prevalence of mild-to-moderate NAFLD was observed compared with severe NAFLD (75.9% [% 95 CI 68.4–83.5] versus 24.1% [% 95 CI 16.5–31.6]). The OR for severe NAFLD in patients with CD versus those with UC was 1.92 (95% CI 0.84–4.38) based on three studies, with moderate heterogeneity between the surveys (I2=61.6%; p=0.074)
Pooled prevalence of NAFLD in patients with IBD according to sex
There were 15 studies that reported the prevalence of NAFLD according to sex.5,6,13,24,30,31,33,35,37–39,42,48,53,54 Overall, the pooled prevalence was higher in men with IBD (33.2%, 95% CI 25.5–40.9; I2=96.0%; p<0.001) compared with women (27.0%, 95% CI 20.5–33.4; I2=93.3%; p<0.001) (Table 1). The aOR for NAFLD in men versus women with IBD was 1.50 (95% CI 0.96–2.05) according to four studies,31,39,54,57 without heterogeneity (I2=0.0%; p=0.856) (Figure 4a). Based on five studies on CD patients, again, the pooled prevalence of NAFLD was higher in men (33.2%, 95% CI 16.5–50.0; I2=96.5%; p<0.001) than in women (29.7%, 95% CI 16.5–50.0; I2=88.6%; p<0.001) (Table 1).30,33,37,38,53 The OR for NAFLD in men versus women with CD was 1.13 (95% CI 0.65–1.95), with moderate heterogeneity (I2=71.6%; p=0.007). Similarly, the pooled prevalence was higher in men with UC (32.9%, 95% CI 0.0–70.1; I2=95.8%; p<0.001) compared with women (20.1%, 95% CI 0.0–52.4; I2=95.9%; p<0.001) in two studies (Table 1).30,33 The OR in men versus women with UC was 1.97 (95% CI 1.06–3.67), with no heterogeneity between the studies (I2=0.0%; p=0.499).
Figure 4.
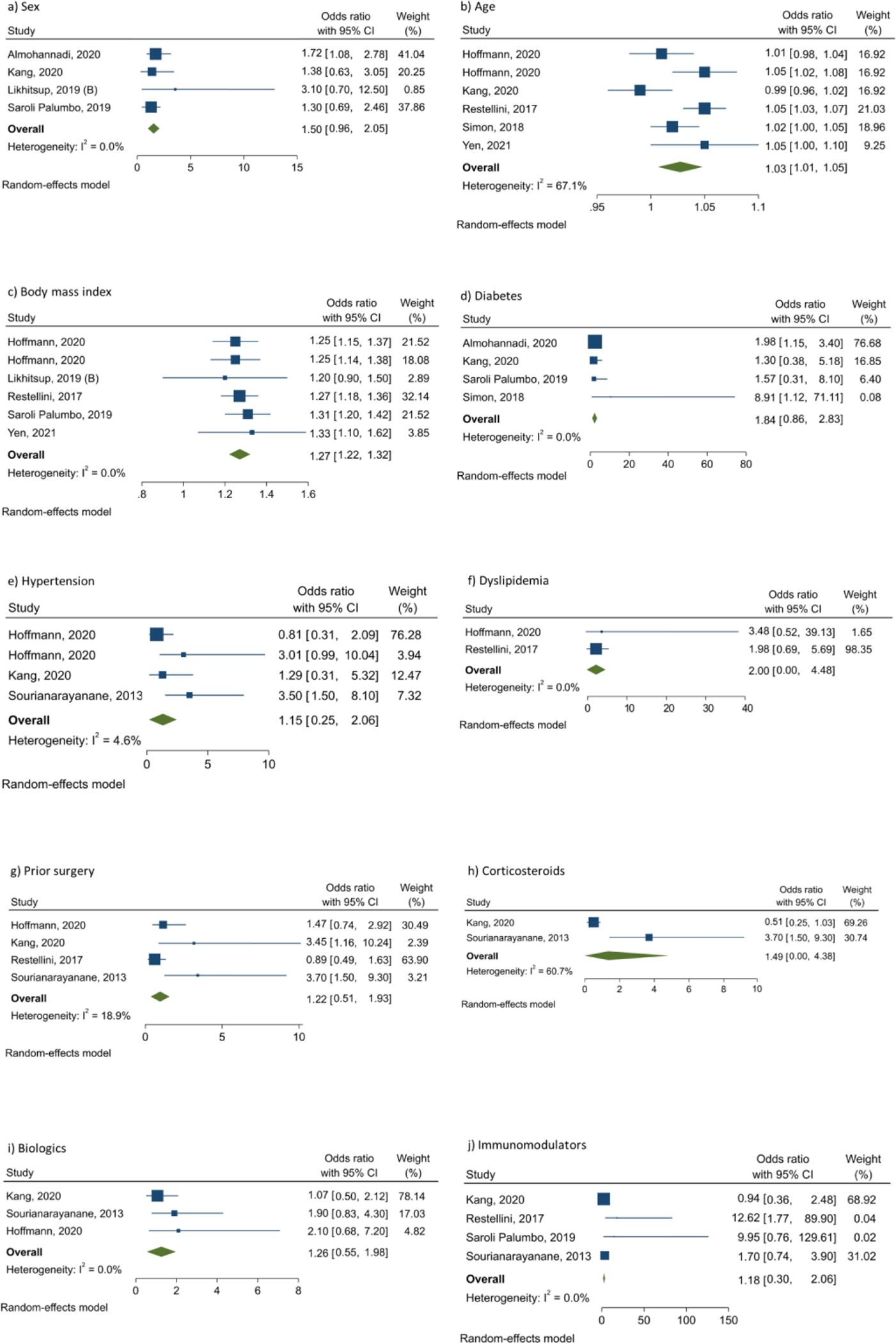
Factors potentially associated with risk of non-alcoholic fatty liver disease in patients with inflammatory bowel disease
Pooled prevalence of NAFLD in patients with IBD according to diagnostic method of NAFLD
For diagnosis of NAFLD in IBD patients, 35 studies used imaging techniques with a pooled prevalence of 30.5% (95% CI 25.8–35.2; I2=97.7%; p<0.001),5,7,13,20,21,23,26–42,44,45,47–51,53–55,57,58 four studies used histopathology with a pooled prevalence of 36.2% (95% CI 27.6–44.8; I2=77.0%; p=0.005),19,25,43,56 two studies used HSI with a pooled prevalence of 34.8% (95% CI 30.9–38.7; I2=0.0%; p=0.494),24,46 two studies used either imaging or HSI with a pooled prevalence of 30.8% (95% CI 10.9–50.6; I2=95.5%; p<0.001,22,52 and one study used either imaging or histopathology with a prevalence of 12.4% (95% CI 10.8–14.0).6 The difference in the prevalence rates between different diagnostic methods was significant (p<0.001). Prevalence of NAFLD in patients with CD and UC according to diagnostic method of NAFLD was separately represented in Table 1.
Pooled prevalence of NAFLD in patients with IBD according to disease activity
There were six studies that reported the prevalence of NAFLD according to IBD activity.5,24,30,35,37,38 The criteria used to stratify the patients according to the disease activity are summarised in Suppl Table 4. Overall, the pooled prevalence in subjects with active disease (34.5%, 95% CI 18.8–50.2; I2=95.8%; p<0.001) was very close to that in those with inactive disease (34.2%, 95% CI 27.2–41.2; I2=85.8%; p<0.001), and the difference was not statistically significant (p=0.971) (Table 1). The OR for NAFLD in active versus inactive disease was 0.95 (95% CI 0.51–1.78), with high heterogeneity (I2=87.4%; p<0.001).
Pooled prevalence of NAFLD in patients with IBD according to disease location, behavior or extent
Five papers reported the prevalence of NAFLD in patients with CD according to disease location, behavior, or extent.5,31,37,38,53 Concerning disease location in CD, the highest pooled prevalence related to ileal (28.8%, 95% CI 19.1–38.5; I2=76.4%; p=0.002), followed by upper gastrointestinal (23.7%, 95% CI 10.2–37.2; I2=0.0%; p=0.983), ileocolonic (23.0%, 95% CI 11.0–35.0; I2=93.2%; p<0.001), and colonic (18.4%, 95% CI 5.6–31.2; I2=84.3%; p<0.001), and the difference was not statistically significant (p=0.527) (Table 1). Regarding disease behavior in CD, the pooled prevalence was higher in penetrating (32.0%, 95% CI 15.3–48.6; I2=85.5%; p<0.001) and stricturing (30.0%, 95% CI 20.6–39.4; I2=62.0%; p=0.048) compared with non-stricturing/non-penetrating (24.3%, 95% CI 6.4–42.1; I2=96.0%; p<0.001), and the difference was not significant (p=0.182) (Table 1). About disease extent in UC, the pooled prevalence was highest in patients with extensive UC (25.3%, 95% CI 9.8–40.7; I2=76.9%; p=0.038), followed by those with proctitis (21.5%, 95% CI 13.1–30.0; I2=50.1%; p=0.157), and left-side UC (14.9%, 95% CI 4.7–25.2; I2=64.3%; p=0.094), and the difference was not significant (p=0.467) (Table 1).
Factors associated with risk of NAFLD in patients with IBD
Analysis of five studies showed that increase in age is associated with an increased risk of NAFLD in IBD patients (aOR 1.03, 95% CI 1.01–1.05),30,31,40,42,51 with moderate heterogeneity between the studies (I2=67.1%; p=0.010) (Figure 4b). Also, a direct association was found between BMI and odds of NAFLD in patients with IBD based on five surveys (aOR 1.27, 95% CI 1.22–1.32),30,39,42,51,54 without heterogeneity between the studies (I2=0.0%; p=0.949) (Figure 4c). There were four studies that evaluated the association between diabetes and NAFLD in IBD patients using aORs; in this regard, pooling the data showed that the aOR for NAFLD in diabetic compared with non-diabetic subjects was 1.84 (95% CI 0.86–2.83), with no heterogeneity between the studies (I2=0.0%; p=0.934) (Figure 4d). Regarding hypertension, analysis of three studies indicated that the pooled aOR in hypertensive compared with non-hypertensive subjects was 1.15 (95% CI 0.25–2.06; I2=4.6%; p=0.370) (Figure 4e).7,30,31 Based on two studies investigating the association between dyslipidemia and NAFLD in IBD patients, the pooled aOR for NAFLD in patients with dyslipidemia compared with those without was 2.00 (95% CI 0.00–4.48), with no heterogeneity between the studies (I2=0.0%; p=0.880) (Figure 4f).30,51 With respect to history of surgery for IBD (bowel resection), analysis of four studies indicated that the pooled aOR in patients with history of surgery compared with those without was 1.22 (95% CI 0.51–1.93; I2=18.9%; p=0.296) (Figure 4g).7,30,31,51 There were two studies investigating the association between corticosteroids use and NAFLD in IBD patients;7,31 in this regard, the pooled aOR in corticosteroids users compared with non-users was 1.49 (95% CI 0.00–4.38), with moderate heterogeneity between the studies (I2=60.7%; p=0.111) (Figure 4h). About biologics use, analysis of three studies showed that the pooled aOR in biologics users compared with non-users was 1.26 (95% CI 0.55–1.98), with no heterogeneity between the studies (I2=0.0%; p=0.610) (Figure 4i). Finally, regarding immunomodulators use, analysis of four studies indicated that the pooled aOR in immunomodulators users compared with non-users was 1.18 (95% CI 0.30–2.06), with no heterogeneity between the studies (I2=0.0%; p=0.815) (Figure 4j).
Prevalence of advanced liver fibrosis in IBD patients with NAFLD
A total of six studies reported the prevalence of advanced liver fibrosis in 1,012 IBD patients with NAFLD.6,24,37,39,43,45 The overall pooled prevalence was 13.6% (95% CI 7.6–19.7; I2=89.7%; p<0.001) (Figure 5). Out of six studies, two used invasive method (histopathology) for diagnosis of liver fibrosis with a pooled prevalence of 14.8% (95% CI 9.75–19.9; I2=48.4%; p=0.164),39,43 and four studies used non-invasive diagnostic methods (three used Fibrosis-4 index, and one used NAFLD fibrosis score) with a pooled prevalence of 13.1% (95% CI 4.2–21.9; I2=92.3%; p<0.001).6,24,37,45
Figure 5.
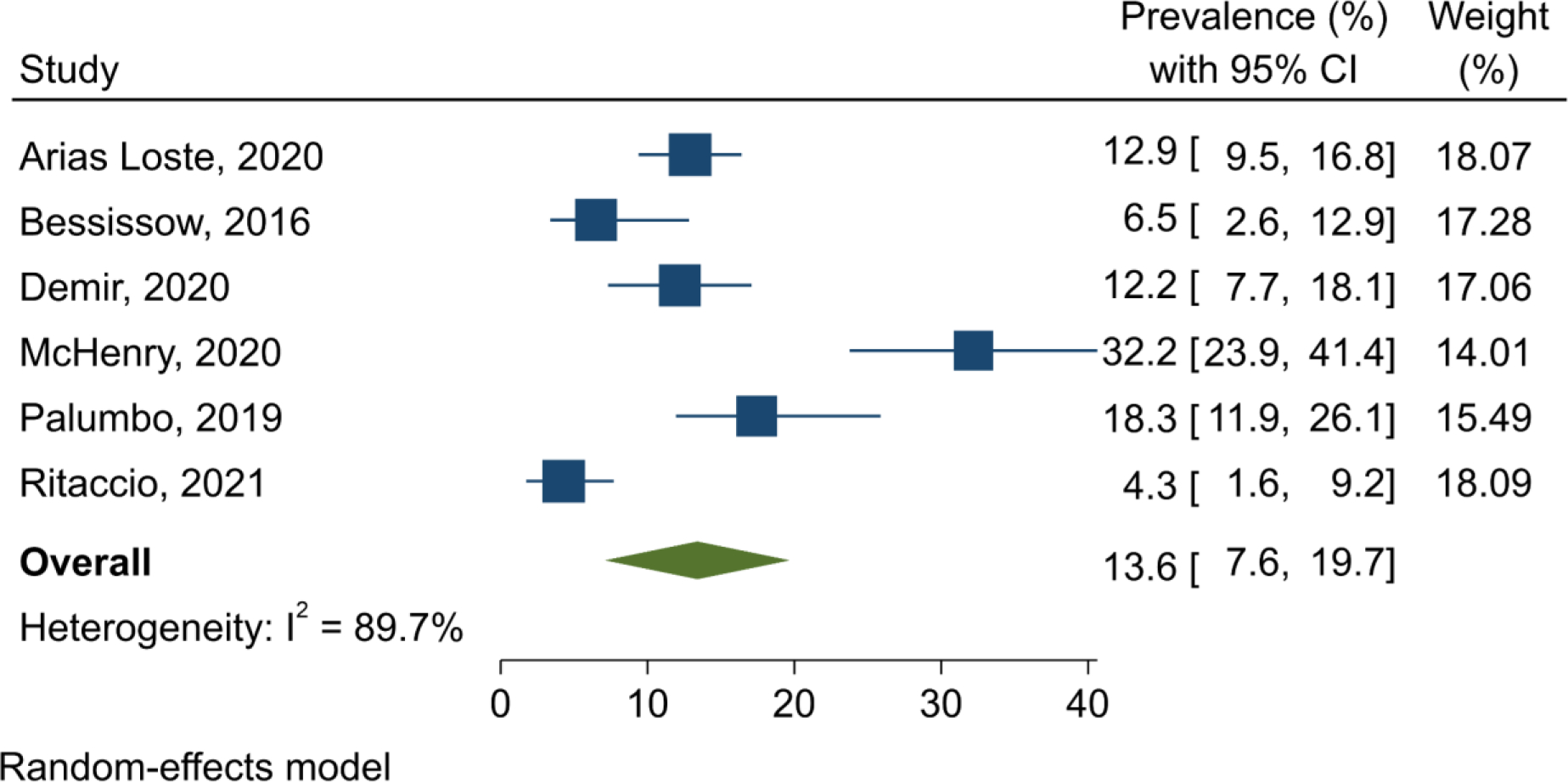
Forest plot of the pooled prevalence of advanced liver fibrosis in inflammatory bowel disease patients with non-alcoholic fatty liver disease
DISCUSSION
In this study, we systematically reviewed the available evidence on the prevalence of NAFLD in patients with IBD. We also attempted to investigate the potential association between 10 proposed factors with the risk of NAFLD in IBD patients. In this regard, we found 44 eligible studies out of thousands references. Our final analyses showed that the prevalence of NAFLD in patients with IBD was nearly 31% worldwide, which varies between different regions. The pooled prevalence estimate was highest in studies published during 2017–2021, and lowest in papers published during 2003–2010, but the difference was not statistically significant. Most of the studies (n=35) used imaging methods for diagnosis of NAFLD, with a pooled prevalence of about 31%. The overall prevalence of NAFLD was non-significantly higher in CD compared with UC (34% versus 28%). It was found that the risk of NAFLD is almost twice in patients with IBD versus healthy subjects. The prevalence of mild-to-moderate NAFLD was higher than severe NAFLD among IBD patients in all analyses. In patients with CD, the highest pooled prevalence of NAFLD pertained to penetrating pattern and ileal location disease. In patients with UC, extensive disease had the highest pooled prevalence of NAFLD. The prevalence of advanced liver fibrosis was approximately 14% in IBD patients with NAFLD.
According to the results of this study, although men with IBD had a higher prevalence of NAFLD compared with women with IBD, the pooled aOR was not statistically significant. Also, no significant difference was found between active and inactive disease in the prevalence of NAFLD. Among other potential factors, such as age, BMI, diabetes, hypertension, dyslipidemia, and prior surgery (bowel resection), only age and BMI were statistically significantly associated with increased risk of NAFLD in patients with IBD. Moreover, among the medications, such as corticosteroids, biologics, and immunomodulators, none of them was identified as significant risk factor for NAFLD in IBD patients.
For the current systematic review and meta-analysis, we attempted to perform a comprehensive systematic search of the literature in different databases with several keywords. We also carried out a recursive search using the bibliographies of the relevant articles. Eligibility assessment of the retrieved papers, and data extraction from the eligible studies, were done by two independent reviewers with consensus for discrepancies. The risk of bias assessment was performed for all studies. A random-effects model was used for pooling the obtained data in order to give more conservative estimates. Finally, we conducted extensive subgroup analyses to reduce the impact of heterogeneity on the study outcomes.
To the best of our knowledge, the present study is the first systematic review and meta-analysis that has comprehensively attempted to assess the prevalence of, and risk factors for, NAFLD in patients with IBD. There are a few meta-analyses previously published about this subject.8,59 In comparison with the most recent systematic review by Lin et al.,59 our study used more databases for primary search and included a greater number of studies (44 vs 27 studies) with larger total sample size (14,947 versus 7,640 subjects). Also, we conducted more subgroup analyses and provided more informative results, especially about the risk factors; for example, Lin et al.59 assessed only five risk factors, whereas we explored 10 potential risk factors with aORs. However, there are a few similarities between the two studies; for example, the overall prevalence of NAFLD among IBD patients was estimated very closely in both studies.59
As mentioned earlier, there was a variation between geographic regions in the prevalence of NAFLD in patients with IBD, that is, the prevalence was higher in developed countries compared with developing countries. A possible explanation could be related to the higher prevalence of metabolic conditions in western communities than developing communities.60,61 Of course, it is noteworthy that the number of papers included in the present study from developed countries was much greater than those from developing countries, and therefore, the results should be compared and interpreted with caution.
Our meta-analysis indicated no statistically significant difference in the odds of NAFLD between men and women with IBD, despite a higher prevalence of NAFLD seen in men than in women. Sex differences in NAFLD has been debatable in the literature. It has been stated that the prevalence of NAFLD in men is higher than in women in the younger ages; however, in the older ages (esp., after menopause), the prevalence would be higher in women, proposing a protective role for estrogen. Overall, the differences in the NAFLD occurrence between men and women could be attributed to differences in sex hormones, genetic factors, socio-cultural factors, and metabolic conditions.62,63
Analyses of the present study demonstrated notable results on the association between prevalence of NAFLD and medical history of the patients with IBD. In this regard, we found that diabetes, hypertension, dyslipidemia, and prior surgery for IBD (bowel resection) had non-significant associations with increased risk of NAFLD in IBD patients. The lack of significance was mainly due to small number of studies, leading to wide and overlapped 95% CIs. On the other hand, BMI was found to be directly associated with risk of NAFLD in patients with IBD. It seems that metabolic determinants are potentially associated with development of NAFLD in IBD patients. Of course, this association is seen among general population too;64 therefore, it might not be a specific finding among patients with IBD, especially that we found no studies comparing the development of NAFLD in IBD patients with metabolic conditions versus non-IBD controls with metabolic conditions. However, it is recommended to consider these comorbidities more carefully in clinical practice in order to prevent high-risk IBD patients from further outcomes.
There are inconsistent reports on the steatotic effect of drugs used for treatment of IBD on liver according to the literature.65,66 Among three therapeutic agent groups we evaluated (i.e., corticosteroids, biologics, and immunomodulators), none of them was statistically significantly associated with the risk of NAFLD in patients with IBD. Immunomodulators, such as methotrexate, azathioprine, and mercaptopurine, modify the overly immune response through decreasing the inflammation. It has been stated that patients under treatment of immunomodulators, especially methotrexate, may develop liver enzyme elevations, followed by hepatic fibrosis or cirrhosis with uncommon frequency.66 However, the hepatotoxicity mechanism of immunomodulators is still unclear.13 Recently, it has been observed in an experimental environment that methotrexate inhibits the mitochondrial nicotinamide adenine dinucleotide kinase in the hepatocytes and reduces the hepatocyte nicotinamide adenine dinucleotide phosphate levels, leading to accumulation of the triglyceride in the hepatocytes.67 A similar effect is declared for corticosteroids too, that is, lipogenesis and steatosis may be induced by corticosteroids in hepatocytes by various pathways.66 On the contrary, tumor necrosis factor (TNF)-alpha inhibitors, which are subset of biologic agents, have been reported to possibly prevent hepatic steatosis and steatohepatitis, mainly on the basis of in-vivo data.66,68 It has been reported that IBD patients with NAFLD under immunosuppressive treatment seem to be at a higher risk of hepatotoxicity; therefore, it might be better to avoid medications associated with hepatic steatosis (namely immunomodulators) in these patients.13 Altogether, the information on human studies is limited and new prospective trials should be designed to elucidate the role of the above-mentioned therapeutic drugs on the development of NAFLD in the patients with IBD.
Due to lack of available sources, we could not explicitly clarify whether the higher prevalence of NAFLD among IBD patients versus healthy controls relates to inner biologic mechanisms (such as systemic inflammation), another possible concurrent immune-mediated inflammatory disease, or factors specifically observed in IBD patients (e.g., drugs); therefore, new experimental and clinical studies should be carried out to reply these queries.
This study faced some limitations. First, high heterogeneity was seen between the studies in majority of the analyses, which was not explained in most of the subgroup analyses. It is not unexpected to observe substantial heterogeneities in the prevalence meta-analyses.69 Second, no available data exist from many countries around the world. According to the WHO regions, no eligible studies were found from the African region, and only one study was identified from the Eastern Mediterranean region. On the other hand, most of the included studies were from the European region and region of the Americans. This difference could be partly explained by that funding sources for research are more limited in many developing countries compared with developed countries, and hence, investigation of the NAFLD among patients with IBD as a research plan would be probably more difficult. Third, rather than looking at active versus inactive IBD, it would be more relevant to look at the disease pattern, i.e., chronic active versus relapsing-remitting, since ongoing systemic inflammation might contribute to metabolic syndrome, insulin resistance, and consequently NAFLD; however, we did not find extractable data in the studies about this subject, and therefore, we tried to assess the active phase versus remission phase. Fourth, a wide 95% CI was observed for several estimates, mainly owing to the small sample sizes included in those analyses.
In conclusion, the results of this systematic review and meta-analysis indicated that about one-third of the patients with IBD experienced NAFLD worldwide, with a prevalence of 34% in CD and 28% in UC. The prevalence varied across different countries and geographic regions. No significant difference was found between active and inactive disease in the NAFLD prevalence. The risk of NAFLD was two times higher in IBD patients versus healthy controls. Older age and higher BMI were potentially linked with increased risk of NAFLD in patients with IBD. Also, approximately one in seven IBD patients with NAFLD had advanced liver fibrosis. More studies are needed to be carried out on the epidemiology of NAFLD in IBD patients, especially in African and Asian countries, in order to reach more informative findings on the association between the two diseases. These results would be useful for further healthcare planning, and underline the importance of carefully screening and management of NAFLD in IBD patients by gastroenterologists in order to prevent the complications.
Supplementary Material
FUNDING STATEMENT
RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). SS is supported by NIDDK K23DK117058 and R03DK129631.
Footnotes
CONFLICT OF INTEREST DISCLOSURE STATEMENT
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
ETHICS COMMITTEE APPROVAL
Not required.
DATA SHARING STATEMENT
No additional data available.
REFERENCES
- 1.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359–370. [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 4.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Principi M, Iannone A, Losurdo G, et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis. 2018;24:1589–1596. [DOI] [PubMed] [Google Scholar]
- 6.Ritaccio G, Stoleru G, Abutaleb A, et al. Nonalcoholic Fatty Liver Disease Is Common in IBD Patients However Progression to Hepatic Fibrosis by Noninvasive Markers Is Rare. Dig Dis Sci. 2021;66:3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279–e285. [DOI] [PubMed] [Google Scholar]
- 8.Zou Z-Y, Shen B, Fan J-G. Systematic review with meta-analysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1764–1772. [DOI] [PubMed] [Google Scholar]
- 9.Ko J K, Auyeung K K. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–1096. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai W, Cagan A, He Z, Ananthakrishnan AN. A phenome-wide analysis of healthcare costs associated with inflammatory bowel diseases. Dig Dis Sci. 2021;66:760–767. [DOI] [PubMed] [Google Scholar]
- 12.Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68:2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schröder T, Schmidt KJ, Olsen V, et al. Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol. 2015;27:698–704. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.who.int/countries.
- 16.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 19.De Boer NK, Tuynman H, Bloemena E, et al. Histopathology of liver biopsies from a thiopurine-naive inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia. Scand J Gastroenterol. 2008;43:604–608. [DOI] [PubMed] [Google Scholar]
- 20.Mehrotra P, Mehrotra P. Non-alcoholic fatty liver disease in patients with Ulcerative colitis: a prospective Indian data. Hepatol Int. 2018;12:S455. [Google Scholar]
- 21.Peixoto A, Silva M, Morais R, et al. P417 Non-invasive assessment of liver fibrosis by transient elastography and AST to ALT ratio in patients with Crohn’s disease treated with methotrexate. J Crohns Colitis. 2017;11:S289. [Google Scholar]
- 22.Quang Le NH, Rollet KC, Afif W, Bitton A, Bessissow T, Sebastiani G. Mo1245 impact of inflammatory bowel disease activity on the incidence of non-alcoholic fatty liver disease: a 7-year longitudinal study. Gastroenterology. 2014;5:S-596. [Google Scholar]
- 23.Bargiggia S, Maconi G, Elli M, et al. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol. 2003;36:417–420. [DOI] [PubMed] [Google Scholar]
- 24.Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G. Incidence and predictors of nonalcoholic fatty liver disease by serum biomarkers in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1937–1944. [DOI] [PubMed] [Google Scholar]
- 25.Bosch DE, Yeh MM. Primary sclerosing cholangitis is protective against nonalcoholic fatty liver disease in inflammatory bowel disease. Hum Pathol. 2017;69:55–62. [DOI] [PubMed] [Google Scholar]
- 26.Carrillo-Palau M, Hernández-Camba A, Hernández Alvarez-Buylla N, et al. Insulin Resistance Is Not Increased in Inflammatory Bowel Disease Patients but Is Related to Non-Alcoholic Fatty Liver Disease. J Clin Med. 2021;10:3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhina S, Steadman K, Goel G, et al. Small Bowel Location, Stricturing Behavior, Vitamin D Receptor Polymorphism, and Metabolic Disease Susceptibility Genes Are Associated With NAFLD in Crohn’s Disease. Am J Gastroenterol. 2014;109:S504. [Google Scholar]
- 28.Di Girolamo M, Scancelli A, Bertani A, Sartini A, Merighi A, Villa E. P615 Ultrasonographic prevalence of liver steatosis in patients with inflammatory bowel disease in a single center. J Crohns Colitis. 2013:S258. [Google Scholar]
- 29.Glassner K, Malaty HM, Abraham BP. Epidemiology and risk factors of nonalcoholic fatty liver disease among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:998–1003. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann P, Jung V, Behnisch R, Gauss A. Prevalence and risk factors of nonalcoholic fatty liver disease in patients with inflammatory bowel diseases: A cross-sectional and longitudinal analysis. World J Gastroenterol. 2020;26:7367–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang MK, Kim KO, Kim MC, Park JG, Jang BI. Sarcopenia is a new risk factor of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Dig Dis. 2020;38:507–514. [DOI] [PubMed] [Google Scholar]
- 32.Kani HT, Deliktas İ, Yilmaz Y. Prevalence of fatty liver disease in patients with inflammatory bowel disease: a transient elastography study on the basis of a controlled attenuation parameter. Marmara Med J. 2019;32:68–70. [Google Scholar]
- 33.Li D, Lu C, Yu C. High incidence of non-alcoholic fatty liver disease in patients with Crohn’s disease but not ulcerative colitis. Int J Clin Exp Pathol. 2017;10:10633–10639. [PMC free article] [PubMed] [Google Scholar]
- 34.Likhitsup A, Dundulis J, Ansari S, et al. Prevalence of non-alcoholic fatty liver disease on computed tomography in patients with inflammatory bowel disease visiting an emergency department. Ann Gastroenterol. 2019;32:283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magrì S, Paduano D, Chicco F, et al. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: beyond the natural history. World J Gastroenterol. 2019;25:5676–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancina RM, De Bonis D, Pagnotta R, et al. Ulcerative colitis as an independent risk factor for hepatic steatosis. Gastroenterol Nurs. 2020;43:292–297. [DOI] [PubMed] [Google Scholar]
- 37.McHenry S, Tirath A, Tsai R, et al. Derivation and internal validation of a clinical prediction tool to predict nonalcoholic fatty liver disease in patients with Crohn’s disease. Inflamm Bowel Dis. 2020;26:1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagami S, Ueno Y, Tanaka S, et al. Significance of non-alcoholic fatty liver disease in Crohn’s disease: A retrospective cohort study. Hepatol Res. 2017;47:872–881. [DOI] [PubMed] [Google Scholar]
- 39.Saroli Palumbo C, Restellini S, Chao C-Y, et al. Screening for nonalcoholic fatty liver disease in inflammatory bowel diseases: a cohort study using transient elastography. Inflamm Bowel Dis. 2019;25:124–133. [DOI] [PubMed] [Google Scholar]
- 40.Simon TG, Van Der Sloot KW, Chin SB, et al. IRGM gene variants modify the relationship between visceral adipose tissue and NAFLD in patients with Crohn’s disease. Inflamm Bowel Dis. 2018;24:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto-Furusho JK, Sánchez-Osorio M, Uribe M. Prevalence and factors associated with the presence of abnormal function liver tests in patients with ulcerative colitis. Ann Hepatol. 2010;9:397–401. [PubMed] [Google Scholar]
- 42.Yen H-H, Su P-Y, Huang S-P, et al. Evaluation of non-alcoholic fatty liver disease in patients with inflammatory bowel disease using controlled attenuation parameter technology: A Taiwanese retrospective cohort study. PLoS One. 2021;16:e0252286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arias Loste M, García García M, Rodriguez-Duque J, et al. P175 Fatty liver disease as a complication of immune-mediated inflammatory diseases: prevalence and risk factors in inflammatory bowel disease. J Crohns Colitis. 2020;14:S225–S225. [Google Scholar]
- 44.Chicco F, Magrì S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demir M P783 Non-alcoholic fatty liver disease and advanced liver disease in patients with inflammatory bowel disease: prevalence and risk factors. J Crohns Colitis. 2020;14:S619–S620. [Google Scholar]
- 46.Domislovic V, Stromar IK, Premuzic M, et al. Retrospective study on incidence rates of NAFLD and advanced liver fibrosis in Crohn’s disease and ulcerative colitis. J Crohns Colitis. 2019;13:S496–S496. [Google Scholar]
- 47.Dundulis J, Helzberg J, Ansari S, El-Halawany H, Michelson R, Chhabra R. NAFLD Appears to Be the Most Common Liver Disease in IBD Patients; IBD is Likely a Risk Factor for NAFLD. Am J Gastroenterol. 2014;109:S147. [Google Scholar]
- 48.Erzin Y, Demir N, Cabuk C, Kantarci F, Hatemi I, Celik A. P565 The increased prevalence of non-alcoholic fatty liver disease in inflammatory bowel disease patients is not related to inflammatory load. J Crohns Colitis. 2015;9:S363–S363. [Google Scholar]
- 49.Lee A, Shah V, Nandi N. Sa1629 – The Risk Factors and Prevalence of Non-Alcoholic Fatty Liver Disease in Inflammatory Bowel Disease. Gastroenterology. 2019;156:S-1260–S-1261. [Google Scholar]
- 50.Navaneethan U, Remzi FH, Nutter B, Fazio VW, Shen B. Risk factors for abnormal liver function tests in patients with ileal pouch-anal anastomosis for underlying inflammatory bowel disease. Am J Gastroenterol. 2009;104:2467–2475. [DOI] [PubMed] [Google Scholar]
- 51.Restellini S, Palumbo CS, Chao C-y, Aruljothy A, Sebastiani G, Bessissow T. Screening for non-alcoholic fatty liver disease by transient elastography with controlled attenuation parameter in unselected patients with inflammatory bowel disease. Gastroenterology. 2017;152:S976. [Google Scholar]
- 52.Scrivo B, Celsa C, Busacca A, et al. P162 Prevalence of nafld (non alcoholic fatty liver disease) and fibrosis in inflammatory bowel disease: the impact of traditional risk factors, intestinal inflammation and genetic phenotype. J Crohns Colitis. 2020;14:S219–S220. [Google Scholar]
- 53.Tirath A, McHenry S, Ganesh G, et al. 783 Nonalcoholic Fatty Liver Disease in a South Asian Crohn’s Disease Population: External Validation of the St. Louis Steatosis Index. Am J Gastroenterol. 2019;114:S453–S454. [Google Scholar]
- 54.Likhitsup A, Dundulis J, Ansari S, et al. High prevalence of non-alcoholic fatty liver disease in patients with inflammatory bowel disease receiving anti-tumor necrosis factor therapy. Ann Gastroenterol. 2019;32:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva J, Brito BS, Silva INdN, et al. Frequency of hepatobiliary manifestations and concomitant liver disease in inflammatory bowel disease patients. Biomed Res Int. 2019;2019:7604939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrade P, Lopes S, Lopes J, Macedo G. P0275 A histological appraisal of hepatobiliary disorders in inflammatory bowel disease. United European Gastroenterol J. 2016;4:A252. [Google Scholar]
- 57.Almohannadi M, Chandra P, Varughese B, et al. Non-alcoholic fatty liver disease among patients with inflammatory bowel disease in Qatar: Prevalence and risk factors. J Crohns Colitis. 2020;14:S621–S621. [Google Scholar]
- 58.Balaban DV, Enache I, Macadon B, et al. P1670 Prevalence and quantitative assessment of liver steatosis in inflammatory bowel disease patients. United European Gastroenterol J. 2017;5:A735. [Google Scholar]
- 59.Lin A, Roth H, Anyane-Yeboa A, Rubin DT, Paul S. Prevalence of nonalcoholic fatty liver disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2021;27:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. [DOI] [PubMed] [Google Scholar]
- 62.Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34:1291–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yki-Järvinen H Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. [DOI] [PubMed] [Google Scholar]
- 65.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190. [DOI] [PubMed] [Google Scholar]
- 66.Chao C-Y, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: A review article. World J Gastroenterol. 2016;22:7727–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang K, Kim H, Fu Z, et al. Deficiency of the mitochondrial NAD kinase causes stress-induced hepatic steatosis in mice. Gastroenterology. 2018;154:224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopetuso LR, Mocci G, Marzo M, et al. Harmful Effects and Potential Benefits of Anti-Tumor Necrosis Factor (TNF)-α on the Liver. Int J Mol Sci. 2018;19:2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamani M, Derakhshan M, Zamani V, Shokri-Shirvani J. Editorial: the prevalence of Helicobacter pylori infection worldwide—knowns and unknowns. Authors’ reply. Aliment Pharmacol Ther. 2018;47:1331–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data available.


