Abstract
Background
Accurate diagnosis of canine distemper (CD), a highly contagious and acute viral disease, cannot be made solely based on clinical signs and haematological findings, but serological and molecular methods compatible with clinical signs are also required. The type of sample and method of tissue sampling are also very important. Sometimes in chronic cases, the canine distemper virus (CDV) may not be detected in blood and conjunctival specimens but can be detected in cerebrospinal fluid (CSF).
Objectives
The aim of this study was to evaluate and compare the suitability of CSF samples with whole blood and conjunctival samples in the detection of CDV.
Methods
The CDV was detected in CSF, whole blood and mucosal specimens in 20 dogs with obvious neurological with or without systemic signs congruous with CD by RT‐PCR and rapid immunochromatographic (IC) antigen test kit assays.
Results
Rapid kit results were positive for mucosal swabs in 10 cases (50%) and for CSF in 17 cases (85%); RT‐PCR results from whole blood were positive in 11 cases (55%) and from CSF in 16 cases (80%).
Conclusions
Our results revealed that dogs with neurological signs showing simultaneous or recent systemic symptoms, whole blood, CSF and mucosal swabs are suitable for the diagnosis of CDV by RT‐PCR and rapid IC antigen test kits, but dogs with neurological symptoms that are systematically asymptomatic or have had systemic signs for a long time, whole blood and mucosal swabs are not good samples while CSF is a good one.
Keywords: canine distemper virus, cerebrospinal fluid, mucosal specimens, rapid immunochromatographic antigen test kit, RT‐PCR, whole blood
Rapid kit results were positive for mucosal swabs in 10 cases (50%) and for CSF in 17 cases (85%); RT‐PCR results from whole blood were positive in 11 cases (55%) and CSF in 16 cases (80%). All mucosal swabs, whole blood and CSF are good samples for diagnosing CDV in a dog that has neurological signs with systemic symptoms of distemper, while the CSF is good sample in dogs that show only neurological signs of distemper without systemic symptoms.
1. INTRODUCTION
Canine distemper virus (CDV) is the cause of a highly contagious and lethal disease in several species of carnivores, including dogs (Zhao et al., 2010). It belongs to the family Paramyxoviridae and is a member of the genus Morbillivirus, which has an envelope, single‐negative‐stranded RNA (ssRNA) that encodes H (haemagglutinin) and F (fusion protein) glycoproteins (Beineke et al., 2009). Glycoprotein H acts as a key protein, because the distemper virus interacts with the SLAM receptor (which is located in lymphoid cells) through this protein and leads to immunosuppression (Martella et al., 2008; Ruiz‐Saenz et al., 2019). Like most of the other enveloped viruses, CDV is susceptible to an incompatible environment such as high temperature, sunlight or UV light and disinfectants; thus, the CD is more common in cold climates (Greene, 2012).
Canine distemper (CD) is mostly transmitted via oronasal aerosols (Greene, 2012) and may cause multisystemic clinical signs in affected dogs including prodromal (pyrexia, anorexia, lethargy), respiratory (pneumonia, dyspnoea and or tachypnoea, nasal and ocular purulent discharges), gastrointestinal (vomiting and diarrhoea), neurological (encephalitis, seizure, myoclonus, paraparesis or tetraparesis) and some miscellaneous disturbances (digital and nasal pads hyperkeratosis, keratitis, enamel hypoplasia and impetigo) (Greene, 2012; Martella et al., 2008). The manifestation of these clinical signs depends on the virus strain, environmental conditions as well as the age of the host and its immune status; nevertheless more than 50% of cases are probably subclinical (An et al., 2008; Fischer et al., 2013; Greene, 2012). Infection with this organism is more commonly described in unvaccinated puppies, and despite vaccination protocols, it remains a serious peril in unvaccinated or even in some vaccinated dog populations (Ek‐Kommonen et al., 1997; Xue et al., 2019). Dogs that are carrying CDV but showing no apparent signs of an illness can transmit it to susceptible dogs. Treatment is mostly unsuccessful; however, it should include appropriate fluid therapy and antibiotics as well as corticosteroid in some neurological forms. Unfortunately, the neurological form of the disease, even with treatment, is often irreversible, progressive and inexorable; in this case, euthanasia is proposed. Disease prevention by focusing on vaccination, reducing stressful factors, quarantining and maintaining a clean environment plays a key role in reducing the disease (Ettinger et al., 2017; Greene, 2012).
Rapid and precise diagnosis of CDV makes it easier to treat and conserve the infected dogs and also prevents further transmission to the vulnerable hosts. Therefore, it is important to choose a rapid and reliable diagnostic method. Variable signs of distemper and subclinical status of the disease in some cases is the main challenge of diagnosing the disease based on clinical and physical examination. Currently, routine laboratory and clinical findings are helpful for initial diagnosis but not sufficient for confirmation of the infection (Fischer et al., 2013; Saito et al., 2006). Recently, more sensitive and specific techniques such as reverse transcriptase PCR (RT‐PCR), real‐time PCR, enzyme‐linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), neutralisation antibody (NA) test and rapid immunochromatographic (IC) antigen test kits are available for rapid and definitive diagnosis of CDV (Athanasiou et al., 2018; Babalola et al., 2015; Di Francesco et al., 2012).
Antibody detection is often not beneficial in diagnosing the disease because previous vaccination and/or post‐infection (PI) status can interfere with the results. Consequently, diagnostic tests based on antigen or nucleic acid detection are more valuable and well‐timed (Elia et al., 2006). For this reason, rapid IC antigen test kit assay (fast, economical and user friendly) and RT‐PCR assay (high sensitive and specific) seem more applicable for diagnosis of CDV (Athanasiou et al., 2018; Fischer et al., 2013).
Some of the challenges in CDV detection are choosing the best tissue samples, early detection and reducing the cost of testing. In many dogs with distemper, especially in chronic cases and the neurological form of the disease, blood and mucus samples may not be helpful in diagnosing the disease, and other samples, such as cerebrospinal fluid or tissue samples, may be needed (Amude et al., 2006). Thus, the aim of this study was to detect CDV RNA in mucosal, whole blood and CSF samples to evaluate the suitability of these samples by RT‐PCR as a highly sensitive and specific assay and IC antigen test kit as a fast and cheap assay from dogs with neurological with or without systemic symptoms of distemper.
2. MATERIALS AND METHODS
2.1. Study conditions
This study was approved by ethics committee of Ferdowsi University of Mashhad, Iran (https://ethics.research.ac,ir/IR.UM.REC.1399.121). Twenty dogs with neurological signs of distemper with or without systemic signs with a mean age of 12.4 months (range 2.5–48), mostly unvaccinated (Chart 1) and 10 healthy dogs (as negative controls) were admitted to the Veterinary Teaching Hospital, Ferdowsi University of Mashhad, Iran were inserted into this study. A thorough history including recent vaccination, domicile and the date of symptoms onset was obtained from the owners and an entire physical examination was performed. The dogs’ individual data are listed in Table 1. Any endeavour was made to eleminate the diseases causing analogous clinical signs of CDV, including CPV, CAV type 1 and 2, lead poisoning, Bordetella bronchiseptica and salmonellosis by obtaining a detailed history, clinical signs and physical examination, disease progression, haematological evaluation and Rapid IC antigen test kits. Then, the owner's consent was obtained and the samples were collected by routine hospital procedures.
CHART 1.
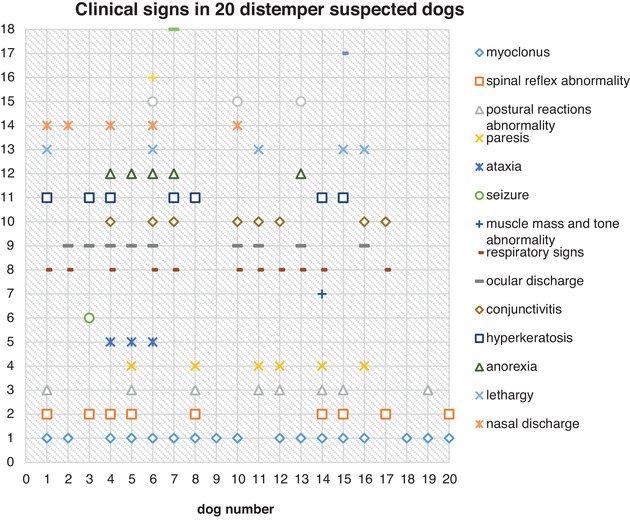
Clinical signs of twenty suspected dogs in this study
TABLE 1.
Age, sex, breed, vaccination status, season, origin, laboratory and clinical findings, outcome and detection of CDV by rapid test kits (from mucosal swabs and CSF) and RT‐PCR (from whole blood and CSF) of 20 suspected distemper dogs
| Clinical findings &signs onset to referral to the clinic (days) | IC assay | RT‐PCR assay | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Age (months) | Sex | Breed | Vaccination | Season | Origin | Systemic | Neurologic | Major laboratory findings | Mucosal | CSF | blood | CSF | Outcome |
| 1 | 3.5 | F | Doberman | No | fall | Private owner | Yes (7–14) | Yes (7–14) | Very severe neutrophilic leucocytosis, severe regenerative anaemia | + | + | + | + | Death after 2 d |
| 2 | 2.5 | F | Husky | No | winter | Private owner | Yes (17) | Yes (2) | Moderate degenerative anaemia, lymphopenia | + | + | + | + | Owner no answer |
| 3 | 3 | M | Mixed | No | winter | Shelter | Yes (2) | Yes (2) | Severe regenerative anaemia, moderate to severe lymphopenia | + | + | + | + | Death after 7 d |
| 4 | 6.5 | M | Sarabi | No | winter | Private owner | Yes (4) | Yes (4) | Mild to moderate regenerative anaemia, severe lymphopenia | + | + | + | + | Death after 10 d |
| 5 | 12 | M | Spitz | No | winter | Private owner | Yes (ND) | Yes (14) | Mild regenerative anaemia, moderate to severe lymphopenia | + | + | + | + | Death after 3 d |
| 6 | 4 | F | Spitz | No | summer | shelter | Yes (33) | Yes (7) | Moderate to severe regenerative anaemia, absolute lymphopenia | + | + | + | + | Death after 35 d |
| 7 | 7 | ND | mixed | ND | winter | stray | Yes (ND) | Yes (ND) | Moderate neutrophilic leucocytosis, severe lymphopenia | + | + | + | + | Death after 2 d |
| 8 | 24 | F | GSD | Yes | summer | Private owner | Yes (30) | Yes (5) | Mild regenerative anaemia, lymphopenia | – | + | + | + | Euthanasia after 14 d |
| 9 | 8 | F | Mixed | I | fall | Private owner | No | Yes (45) | Mild regenerative anaemia, lymphopenia | + | + | – | + | Death after 127 d |
| 10 | 4.5 | F | Mixed | No | winter | Shelter | Yes (5) | Yes (5) | Moderate regenerative anaemia, absolute lymphopenia (non‐count) | + | + | + | – | Sudden death after 6 d |
| 11 | 3 | ND | Mixed | ND | fall | Stray | Yes (ND) | Yes (ND) | ND | + | + | – | + | Euthanasia |
| 12 | 4.5 | M | Mixed | ND | winter | stray | Yes (ND) | Yes (ND) | ND | – | + | + | + | Euthanasia |
| 13 | 5 | F | Mixed | ND | summer | Stray | Yes (ND) | Yes (ND) | Moderate to severe regenerative anaemia, severe leucocytosis, neutrophilia, monocytosis, lymphocytosis | – | + | + | + | Death after 2 d |
| 14 | 36 | F | GSD | No | summer | Private owner | Yes (30) | Yes (30) | In range | – | + | – | + | Death after 19 d |
| 15 | 5 | M | Mixed | No | fall | Private owner | Yes (30) | Yes (30) | Mild degenerative anaemia | – | + | – | + | Live |
| 16 | 12 | M | Husky | I | fall | Private owner | Yes (7) | Yes (7) | Moderate degenerative anaemia, lymphopenia | – | + | – | + | Euthanasia after 163 d |
| 17 | 48 | M | Terrier | No | winter | shelter | Yes (60) | Yes (60) | Mild regenerative anaemia | – | + | – | – | Live |
| 18 | 8 | F | GSD | No | winter | shelter | No | Yes (2) | In range | – | – | – | + | Live |
| 19 | 4 | F | Mixed | I | spring | Private owner | No | Yes (10) | Moderate degenerative anaemia, mild lymphopenia | – | – | – | – | Live |
| 20 | 48 | M | Mixed | ND | spring | stray | No (ND) | Yes (ND) | In range | – | – | – | – | Live |
F: female; M: male; I: incomplete vaccination; ND: no data available; d: day.
Whole blood samples (approximately 4 ml) were collected into an EDTA‐treated tube from the cephalic or jugular vein; afterwards, animals were anesthetised with ketamine (10 mg/kg) and diazepam (1 mg/kg) intravenously, and CSF specimens (approximately 1 ml) were collected into 1.5 ml tubes without anticoagulant from cerebellomedullary cistern after disinfection with povidone‐iodine and 70% ethanol. All blood and CSF specimens were stored immediately after collection at –80°C before RT‐PCR analyses. Blood samples were checked for routine laboratory parameters and rapid IC antigen test kits (Anigen Rapid CDV Ag Test Kit, BioNote, Hwaseong, Korea) were used. For this purpose, a mucosal swab (combination of conjunctiva, pharynx and nose) was obtained and also 5 drops of the collected CSF were used according to the manufacturer's instruction.
2.2. RT‐PCR assay
2.2.1. RNA extraction
RNA was extracted from 2 ml of whole blood and 250 μl of CSF specimens by Blood RNA isolation kit (DENAzist, Mashhad, Iran) and RiboEx LS RNA isolation kit (GeneAll Biotechnology, Germany), respectively according to the manufacturer's instructions. DEPC‐treated water was used for negative controls. The RNA quality and quantity were analysed by NanoDrop spectrophotometer (Thermo Scientific NanoDrop) and electrophoresis in 1% agarose gel using loading buffers 6× (DENAzist, Mashhad, Iran) stained with DNA green viewer (Pars Tous Biotechnology, Mashhad, Iran), respectively. An ideal qualification range was obtained for each method. The total amount of RNA isolated varied between 20 and 1000 ng/ml (data are not shown).
2.2.2. cDNA synthesis
For whole blood samples, immediately after RNA extraction cDNA was made by cDNA synthesis kit (Pars Tous Biotechnology, Mashhad, Iran) containing RT buffer, 1 mM dnTP mixture, 8 mM MgCl2, Oligo d(t)16, random hexamer, thermostable H minus MMLV, RNAase inhibitor, stabiliser and 5 μl of extracted RNA. For CSF samples, cDNA was made by the AccuPower® CycleScript RT PreMix, lyophilised (Bioneer, South Korea) using 20 μl of CSF RNA according to the manufacturers’ instruction.
2.2.3. PCR method
Oligonucleotides primer pairs previously reported for CDV RNA detection [PP‐I, PP‐II and PP‐III, to amplify amplicons of 286, 259, and 899 bp length of the CDV nucleoprotein (NP) gene sequence, respectively, and one oligonucleotide primer pair for amplification of housekeeping (GAPDH) gene sequence] were used in this study (Frisk et al., 1999).
Ultrapure sterile water was used as a negative control and a proven sample by nucleotide sequencing was used as CDV positive control. The PCR optimised as follows: denaturation at 94°C for 1 min; 37 cycles of denaturation at 94°C for 1 min, annealing at 59°C for 2 min and extension at 72°C for 1 min; and the final extension at 72°C for 5 min. The PCR products were checked by electrophoresis in 1.5% 1× TBE buffer PH 8.4 agarose gel stained with DNA green viewer (Pars Tous Biotechnology, Mashhad, Iran) at 80 volts for 90 min and visualised under UV light (Figure 1). PCR products of one sample (dog no. 6 of Table 1) and all oligonucleotides primers excepted antisense of PP‐I and antisense of PP‐II were sequenced in Sanger sequencing protocol (Bioneer, South Korea). Nucleotide sequence alignment and sequence quality were analysed using SnapGene software version 3.2.1 without editing. Sequence resemblance was checked against sequences deposited in the NCBI GenBank using the BLAST software. The sequences determined in this study have been registered at the GenBank and their accession numbers are shown in the results.
FIGURE 1.
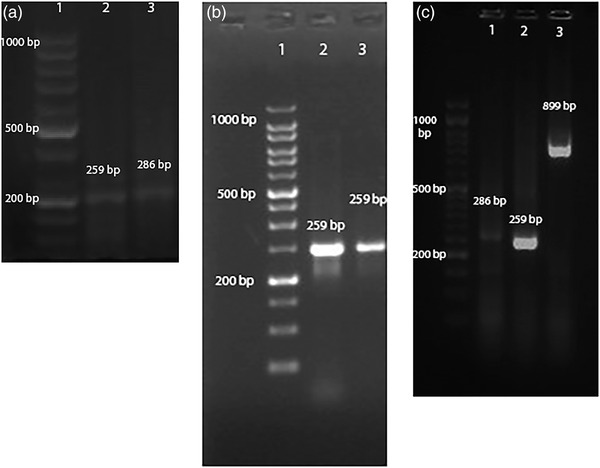
Detection of RT‐PCR products in CSF: Lane 1, ladder; Lane 2, PP‐II; Lane 3, PP‐I (a) and in whole blood: Lane 1, ladder; Lane 2 and Lane 3, PP‐II (b) and in whole blood: Lane 1, ladder; Lane 2, PP‐I; Lane 3, PP‐II; Lane 4, PP‐III (c)
2.3. Rapid immunochromatographic antigen test kits
Briefly, according to manufactures’ instructions (Anigen Rapid CDV Ag Test Kit, BioNote, Hwaseong, Korea), a combination of sterile swab was obtained from conjunctival, pharyngeal and nasal mucosa of each dog and after dipping the swab in the solvent and stirring it, 4–5 drops of this mixture (about 100 μl) were applied properly. For the CSF samples, after collecting the specimens under sterile conditions, 4–5 drops were tested directly without diluting the samples with commercial kits. The results were checked up to 5–10 min later, and if two purple bands (control and test) appeared, the results were considered positive (Figure 2).
FIGURE 2.
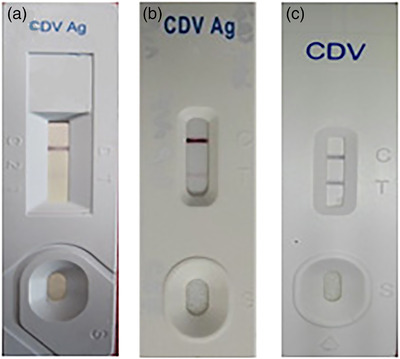
Rapid immunochromatographic antigen test kits; a negative result (a), an unsharpened test band in CSF (b) and a sharp test band from a mixture of conjunctival, pharyngeal, and nasal swabs (c)
2.3.1. Statistical analysis
By assuming that the RT‐PCR method represents a well‐characterised and highly specific method for detection of CDV RNA, the rapid IC antigen results were correlated with the results obtained with RT‐PCR assay. Therefore, sensitivity refers to the number of rapid IC antigen positive samples for the group of CDV‐positive dogs, whereas specificity expresses the number of rapid IC antigen‐negative samples for the group of animals that were negative for CDV by the RT‐PCR method. The agreement between the RT‐PCR and the IC was examined using kappa statistics.
3. RESULTS
3.1. Dogs’ characteristics
A sum of 20 dogs suspected to have distemper (test group) and 10 healthy dogs (control group) were used in this study (Table 1). The mean age of the dogs in the patient group was 12.4 months old (range 2.5–48 months) and the mean age of the dogs in the control group was 27.8 (range 9–60 months). Nine of the sick dogs were male and 9 were female and the sex of two of them was not recorded. Only one of the dogs had been fully vaccinated (at least 60 days before the appearance of the signs). Ten dogs were purebreds and 10 were mixed breeds. Fourteen dogs died or euthanized, five were recovered and the outcome of one was unknown (Table 1).
Among 18 dogs (dogs no. 1–18) whose distemper recognised at least by IC or RT‐PCR assays, 14 (77.7%) were referred in the cold season of the year (fall or winter) and 4 (22.3%) in summer (Table 1).
All dogs in the present study had neurological signs with or without systemic signs of CDV (Table 1). The most common neurological signs were myoclonus (17 of 20: 85%) and spinal reflex abnormalities (9 of 20: 45%) and the most common systemic signs were respiratory signs (11 of 20: 55%) and ocular discharges (9 of 20: 45%) (Chart 1). Out of 20 dogs, 3 dogs manifested neurological signs approximately 2–3 weeks after recovery from systemic signs, 3 dogs displayed neurologic signs without a history of systemic involvement, 8 dogs showed neurologic signs coincide via systemic illness and 6 dogs had an unknown history of systemic signs. Detail characteristics and clinical signs of these dogs are shown in Chart 1 and Table 1.
Haematological findings of infected dogs revealed that CBC values varied from normal range to neutrophilic leucocytosis, regenerative or degenerative anaemia, and mild‐to‐severe lymphopenia; however, regenerative anaemia and lymphopenia were the most common findings in infected dogs. It is noteworthy that all patients with lymphopenia and most dogs that had severe changes (increase or decrease) in CBC values died early after diagnosis (Table 1).
3.2. CDV detection by rapid immunochromatographic antigen test kits and RT‐PCR assays
Totally, in 18 out Of the 20 suspected dogs, distemper was confirmed by at least one of the rapid IC or RT‐PCR diagnostic methods. Rapid IC kit results were positive from mucosal swabs of 10 cases and CSF of 17 cases and RT‐PCR results were positive from whole blood of 11 cases and CSF of 16 cases. In the test group, RT‐PCR detected CDV RNA in 80% of CSF samples as 6 by PP‐I, 13 by PP‐II and 4 by PP‐III. In whole blood samples, CDV RNA was detected from 55% of samples as 7 by PP‐I, 10 by PP‐II and 4 by PP‐III (Table 2). The RT‐PCR results were negative for all 10 dogs in the control group.
TABLE 2.
Positive results of RT‐PCR by three primer pairs in 20 dogs with neurological signs of distemper
| RT‐PCR | Sample | GAPDH | PP‐I | PP‐II | PP‐III |
|---|---|---|---|---|---|
| CSF | 20 | 6 | 13 | 4 | |
| Whole blood | 20 | 7 | 10 | 4 | |
| sum | 40 | 13 | 23 | 8 |
Note: The most sensitive primer pairs were PP‐II, PP‐I and PP‐III, respectively.
Although most RT‐PCR results of whole blood samples revealed sharp bands, most results of CSF samples revealed unsharp bands (Figure 1).
3.3. Calculation of the sensitivity and specificity of IC assay compared with RT‐PCR assay
The RT‐PCR assay as one of the definitive diagnostic methods of CDV classified the results of the samples as valid positive or valid negative, and if the results of the rapid IC test kits assay disagreed with RT‐PCR, the results were classified as invalid positive or invalid negative. Therefore, the total sensitivity and specificity of rapid IC test kit assay for CSF samples were 93.8% and 50%, respectively (Table 3).
TABLE 3.
Calculation of sensitivity and specificity of the rapid IC kit assay compared to RT‐PCR assay in CSF specimens
| RT‐PCR results of CSF samples | Sum | |||
|---|---|---|---|---|
| + | – | |||
| Rapid IC test kit results of CSF samples | + | 15 | 2 | 17 |
| – | 1 | 2 | 3 | |
| Sum | 16 | 4 | 20 | |
Note: Per cent observed agreement = (15 + 2)/20 = 85%; sensitivity = 15/16 = 93.8%; specificity = 2/4 = 50%; Kappa = 0.48 (moderate).
3.4. Sequencing results
The sequencing results of all primers except antisense of PP‐I and antisense of PP‐II in comparison via other disposal sequences in NCBI GenBank disclosed the most nucleotide similarity level among the strains to canine distemper virus strain HL N (GenBank accession number EU489475.1) and canine morbillivirus strain PT61/Pt 2004 (GenBank accession number KX774415.1). The sequences identified in this study have been registered at the GenBank and the accession numbers are as follows: MZ707910 for PP‐I, MZ798146 for PP‐II and MZ802994S for PP‐III.
4. DISCUSSION
Canine distemper, an immunosuppressive viral disease, can entangle respiratory, gastrointestinal and central nervous systems (CNS) and cause clinical to subclinical symptoms. In this study, the most common clinical signs were myoclonus (85%), respiratory signs (55%), spinal reflex abnormalities (45%) and ocular discharges (45%). Although myoclonus is one of the most important neurological symptoms of distemper, it can be caused by other neurological disorders. Therefore, accurate diagnosis of distemper is significant for choosing treatment protocols (Amude et al., 2006). It is reported that canine distemper virus induces downregulation of GABAA, GABAB and GAT1 expression in brain tissue of dogs; thus one of the main causes of myoclonus in CDV infections may be the blockage of postsynaptic inhibition in neurons or a lack of metabolism of GABA (Çomakli et al., 2020). The distemper mortality rate was high in this study (75%), probably because all of the dogs had neurological manifestations, as some contents indicate that the nervous distemper is progressive and implacable (Ettinger et al., 2017; Greene, 2012).
In the current study, in agreement with Özkul et al. (2004), there was a relationship between distemper with vaccination and environment temperature. In the present study, 19 infected dogs had not been vaccinated or had incomplete vaccination history. However, one dog that had a full vaccination history developed systemic and nervous distemper. This suggests that distemper disease may also occur in a small number of vaccinated dogs; however, vaccination is one of the most effective ways to prevent the disease (Larson and Schultz, 2006; Zhao et al., 2010).
In the present study, 77.7% of infected dogs were referred in the cold seasons of the year. This indicates that inappropriate housing temperature may increase the incidence of CDV during the cold seasons. Like most other enveloped viruses, CDV is susceptible to heat, UV light and disinfectants. Therefore, it is predictable that the incidence of the disease is higher in fall and winter (Greene, 2012).
The dogs’ origin can be a risk factor because 50% of suspected dogs were referred from shelters or were stray dogs. This indicates that the CDV circulates among the dogs’ populations. So, disinfecting protocols, quarantining of suspected animals and also vaccination can control and reduce the CD outbreaks in kennels.
The ante‐mortem diagnosis of distemper is based on history, clinical signs and laboratory findings. Most CBC findings were included neutrophilia, regenerative anaemia and marked lymphopenia; nevertheless, this procedure yields limited information and several other diseases can cause these findings (Greene, 2012; Wang et al., 2017). Therefore, a definitive diagnosis can be determined with various techniques including serological, histopathological and molecular methods based on antibody, antigen or nucleic acid detection (Greene, 2012). Serological assays detecting antibodies (such as NA) can evaluate immunity against distemper, but are not practicable if the animal has been vaccinated or survived, because they may reveal false‐positive results (An et al., 2008; Jóźwik and Frymus, 2005). Today, the development of RT‐PCR facilitates the rapid and accurate recognition of infectious diseases from biological samples (Fischer et al., 2013), but it is a little costly. Currently, the rapid IC assays for diagnosing distemper disease are more attractive for clinicians since it is fast and cheap, ubiquitous and approximately authentic, and yet easy enough that it can be used even by owners at home (An et al., 2008).
In the present study, the sensitivity and specificity of the rapid IC assay relative to RT‐PCR were 93.8% and 50% for CSF samples, respectively. In another study, the sensitivity of this test was reported to be 100%. This high relatively sensitivity indicates that rapid IC test kits can be easily used in dogs suspected of having distemper disease for rapid and inexpensive diagnosis (An et al., 2008).
Some authors have been analysed different methods and samples for isolation and confirmation of CD in dogs. A similar study on serum, whole blood and CSF samples found that 82% of distemper positive cases were recognised by PP‐I, 53% by PP‐II and 41% by PP‐III (Frisk et al., 1999). In the present study, the order of primer pairs sensitivity was PP‐II, PP‐I and PP‐III. Variation in the population of dogs studied or differences in virus strains may cause these differences in results.
Our results indicate that in dogs with neurological signs of CDV with or without systemic involvement, the CSF samples are more sensitive than mucosal swabs or whole blood samples for CDV detection by both rapid IC and RT‐PCR assays. In other studies, the CDV was better detected in urine samples than serum and blood leucocytes (Amude et al., 2006; Elia et al., 2006; Saito et al., 2006).
Our results suggest that if a suspected dog recently manifests neurological with systemic signs compatible with CDV, all mucosal, whole blood and CSF samples may be good clinical specimens to detect CDV by rapid IC antigen test kits or RT‐PCR assays; and if a dog is referred only with neurological symptoms or the neurological symptoms are accompanied by chronic systemic signs of distemper, whole blood is not a good sample while CSF is a good one.
The CSF negative RT‐PCR result of dog no. 10 in the present study was probably false negative because its clinical and haematological findings and the results of all other diagnostic tests were more indicative of CDV infection (Table 1 and Chart 1). This may be due to the low levels of CDV RNA in the sample, improper sample handing or RNA extraction (Amude et al., 2006; Frisk et al., 1999). In the present study, we attempted to rule out the false‐negative results, since the samples were kept at –80°C immediately after collection and the extracted RNA was analysed as previously explained.
Although low viral loads of CDV in the CSF are adequate to cause neurological symptoms of the disease, detection of viral antigen by lower sensitive assays such as IC is more difficult than higher sensitive methods such as RT‐PCR and real‐time PCR. Our investigation revealed that although most of the rapid IC results were positive in CSF, most of them were less sharp than mucosal samples (Figure 2). Parallel results were obtained by RT‐PCR assay between CSF and whole blood samples (Figure 1).
As previously reported, the RT‐PCR assay is more sensitive and specific than IC assay because IC assay require high loads of viral antigen to make a sharp and conspicuous test band. So, this is one of the limitations of IC assays for the detection of CDV antigen, especially in the chronic phase of the disease or subclinically infected animals. In that case, RT‐PCR, real‐time PCR or nested PCR is recommended. Our curiosity in this study revealed that in some cases that the RT‐PCR results show a weak band, it is better to increase the cycle of the PCR program for more duplication of the virus gene, and also use a lower percentage of agarose gel (e.g. 1–1.5% instead of 2%) to visualise the PCR products because non‐sharped bands may be obscured by high agarose gel percentage and mislead the clinicians to a false‐negative result.
5. CONCLUSION
Distemper suppresses the immune system and causes a high mortality rate. Detailed history, complete physical examination, haematological findings and rapid IC antigen test kits can be very helpful for definitive diagnosis of distemper ante‐mortem. If the haematological findings reveal moderate‐to‐severe lymphopenia, the disease prognosis will be poor. For detecting of CDV in a dog that has recently manifested the neurological with systemic signs of distemper, all mucosal swabs, whole blood and CSF are good samples while dogs show only neurological signs of distemper or those with neurological signs with chronic systemic signs of the disease, whole blood and mucosal swabs are not good samples; however, the CSF is a suitable one.
DISCLOSURE OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualisation: Ali Asghar Sarchahi. Methodology: Ali Asghar Sarchahi, Mohammad Arbabi, Hadi Mohebalian. Writing‐original draft preparation: Mohammad Arbabi. Writing‐review and editing: Mohammad Arbabi, Ali Asghar Sarchahi, Hadi Mohebalian. Supervision: Ali Asghar Sarchahi.
ETHICS APPROVAL
This study was approved by the ethics committee of Ferdowsi University of Mashhad, Iran (https://ethics.research.ac.ir/IR.UM.REC.1399.121).
CONSENT TO PARTICIPATE
The treatment protocol was explained to the animal's owners and was started with their consent.
ACKNOWLEDGEMENTS
The authors would like to appreciate the research council of Ferdowsi University of Mashhad for financial support of this work in the form of Research Project No. 41658.
Sarchahi, A. A. , Arbabi, M. , & Mohebalian, H. (2022). Detection of canine distemper virus in cerebrospinal fluid, whole blood and mucosal specimens of dogs with distemper using RT‐PCR and immunochromatographic assays. Veterinary Medicine and Science, 8, 1390–1399. 10.1002/vms3.790
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- Amude, A. , Alfieri, A. , & Alfieri, A. (2006). Antemortem diagnosis of CDV infection by RT‐PCR in distemper dogs with neurological deficits without the typical clinical presentation. Veterinary Research Communications, 30, 679–687. [DOI] [PubMed] [Google Scholar]
- An, D. J. , Kim, T. Y. , Song, D. S. , Kang, B. K. , & Park, B. K. (2008). An immunochromatography assay for rapid antemortem diagnosis of dogs suspected to have canine distemper. Journal of Virological Methods, 147, 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou, L. V. , Kantere, M. C. , Kyriakis, C. S. , Pardali, D. , Moraitou, K. A. , & Polizopoulou, Z. S. (2018). Evaluation of a direct immunofluorescent assay and/or conjunctival cytology for detection of canine distemper virus antigen. Viral Immunology, 31, 272–275. [DOI] [PubMed] [Google Scholar]
- Babalola, E. T. , Olakunbi, O.‐O. S. , Omoshaba, E. O. , & Okonko, I. O. (2015). Seropositivity of canine distemper virus (cdv) in dogs presenting at Abeokuta, Nigeria. Public Health Research, 5, 109–119. [Google Scholar]
- Beineke, A. , Puff, C. , Seehusen, F. , & Baumgärtner, W. (2009). Pathogenesis and immunopathology of systemic and nervous canine distemper. Veterinary Immunology and Immunopathology, 127, 1–18. [DOI] [PubMed] [Google Scholar]
- Çomakli, S. , Özdemir, S. , & Değirmençay, Ş. (2020). Canine distemper virus induces downregulation of GABA A, GABA B, and GAT1 expression in brain tissue of dogs. Archives of Virology, 165, 1321–1331. [DOI] [PubMed] [Google Scholar]
- Di Francesco, C. E. , Di Francesco, D. , Di Martino, B. , Speranza, R. , Santori, D. , Boari, A. , & Marsilio, F. (2012). Detection by hemi‐nested reverse transcription polymerase chain reaction and genetic characterization of wild type strains of Canine distemper virus in suspected infected dogs. Journal of Veterinary Diagnostic Investigation, 24, 107–115. [DOI] [PubMed] [Google Scholar]
- Ek‐Kommonen, C. , Sihvonen, L. , Pekkanen, K. , Rikula, U. , & Nuotio, L. (1997). Outbreak off canine distemper in vaccinated dogs in Finland. The Veterinary Record, 141, 380–383. [DOI] [PubMed] [Google Scholar]
- Elia, G. , Decaro, N. , Martella, V. , Cirone, F. , Lucente, M. S. , Lorusso, E. , Di Trani, L. , & Buonavoglia, C. (2006). Detection of canine distemper virus in dogs by real‐time RT‐PCR. Journal of Virological Methods, 136, 171–176. [DOI] [PubMed] [Google Scholar]
- Ettinger, S. J. , Feldman, E. C. , & Côté, E. (2017). Textbook of veterinary internal medicine: Diseases of the dog and cat. Elsevier. [Google Scholar]
- Fischer, C. D. B. , Ikuta, N. , Canal, C. W. , Makiejczuk, A. , da Costa Allgayer, M. , Cardoso, C. H. , Lehmann, F. K. , Fonseca, A. S. K. , & Lunge, V. R. (2013). Detection and differentiation of field and vaccine strains of canine distemper virus using reverse transcription followed by nested real time PCR (RT‐nqPCR) and RFLP analysis. Journal of Virological Methods, 194, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk, A. , König, M. , Moritz, A. , & Baumgärtner, W. (1999). Detection of canine distemper virus nucleoprotein RNA by reverse transcription‐PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. Journal of Clinical Microbiology, 37, 3634–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, C. E. (2012). Infectious diseases of the dog and cat. Elsevier Saunders. [Google Scholar]
- Jóźwik, A. , & Frymus, T. (2005). Comparison of the immunofluorescence assay with RT‐PCR and nested PCR in the diagnosis of canine distemper. Veterinary Research Communications, 29, 347–359. [DOI] [PubMed] [Google Scholar]
- Larson, L. , & Schultz, R. (2006). Effect of vaccination with recombinant canine distemper virus vaccine immediately before exposure under shelter‐like conditions. Veterinary Therapeutics: Research in Applied Veterinary Medicine, 7, 113–118. [PubMed] [Google Scholar]
- Martella, V. , Elia, G. , & Buonavoglia, C. (2008). Canine distemper virus. The Veterinary Clinics of North America. Small Animal Practice, 38, 787–797. [DOI] [PubMed] [Google Scholar]
- Özkul, A. , Arda Sancak, A. , Güngör, E. , & Burgu, I. (2004). Determination and phylogenetic analysis of canine distemper virus in dogs with nervous symptoms in Turkey. Acta Veterinaria Hungarica, 52, 125–132. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Saenz, J. , Rendon Marin, S. , da Fontoura Budaszewski, R. , & Wageck Canal, C. (2019). Tropism and molecular pathogenesis of Canine Distemper Virus. Virology Journal, 16, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, T. , Alfieri, A. , Wosiacki, S. , Negrão, F. J. , Morais, H. S. A. , & Alfieri, A. F. (2006). Detection of canine distemper virus by reverse transcriptase‐polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Research in Veterinary Science, 80, 116–119. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, J. , Li, R. , Liu, L. , & Yuan, W. (2017). Rapid and sensitive detection of canine distemper virus by real‐time reverse transcription recombinase polymerase amplification. BMC Veterinary Research, 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, X. , Zhu, Y. , Yan, L. , Wong, G. , Sun, P. , Zheng, X. , & Xia, X. (2019). Antiviral efficacy of favipiravir against canine distemper virus infection in vitro. BMC Veterinary Research, 15, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J.‐J. , Yan, X.‐J. , Chai, X.‐L. , Martella, V. , Luo, G.‐L. , Zhang, H.‐L. , Gao, H. , Liu, Y.‐X. , Bai, X. , Zhang, L. , Chen, T. , Xu, L. , Zhao, C.‐F. , Wang, F.‐X. , Shao, X.‐Q. , Wu, W. , & Cheng, S.‐P. (2010). Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from breeding foxes, raccoon dogs and minks in China. Veterinary Microbiology, 140, 34–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


