Abstract
Objective
The number, unpredictability, and severity of seizures experienced by patients with Dravet syndrome (DS) negatively impact quality of life (QOL) for patients, caregivers, and families. Metrics are needed to assess whether patients with residual seizures have moved meaningfully toward seizure freedom after treatment with new antiseizure medications.
Methods
We evaluated the time required postrandomization for each patient to experience the same number of seizures experienced during baseline (i.e., time‐to‐nth seizure), using a post hoc time‐to‐event (TTE) analysis of data from two Phase 3 placebo‐controlled trials of adjunctive fenfluramine for DS (Study 1, N = 119; Study 2, N = 87). Patients aged 2–19 years were randomized to placebo or adjunctive fenfluramine (Study 1: .7 mg/kg/day or .2 mg/kg/day; Study 2: .4 mg/kg/day with stiripentol). Data were analyzed by Kaplan–Meier TTE curves and waterfall plots.
Results
The proportion of patients who never reached baseline seizure frequency was greater with fenfluramine than with placebo (Study 1: fenfluramine .7 mg/kg/day, 60%; fenfluramine .2 mg/kg/day, 31%; placebo, 13%; Study 2: fenfluramine .4 mg/kg/day, 58%; placebo, 2%). Median time‐to‐nth seizure was longer after fenfluramine than after placebo (Study 1: fenfluramine .7 mg/kg/day, 13 weeks; .2 mg/kg/day, 10 weeks; placebo, 7 weeks; Study 2: fenfluramine .4 mg/kg/day, 13 weeks; placebo, 5 weeks; p < .001). Longest duration of convulsive seizure‐free days was increased in active groups versus the placebo group (Study 1: fenfluramine .7 and .2 mg/kg/day, 25.0 and 15.0 days; placebo, 9.5 days [p = .0001; p = .0352]; Study 2: fenfluramine .4 mg/kg/day, 22.0 days; placebo, 13.0 days [p = .004]). The most common adverse events included decreased appetite, pyrexia, upper respiratory tract infection, diarrhea, and fatigue.
Significance
These data demonstrate that fenfluramine can significantly reduce day‐to‐day seizure burden in patients with DS, providing prolonged periods of convulsive seizure‐free days, which may help reduce the physical and emotional disease toll while improving health‐related QOL for patients and caregivers.
Keywords: Dravet syndrome, fenfluramine, seizure‐free days, time‐to‐event analysis
Key Points.
-
In two Phase 3 studies of patients aged 2–19 years with Dravet syndrome, fenfluramine:
-
o
Extended the time to reach the same number of seizures experienced during baseline; ~60% never reached baseline seizure count during treatment
-
o
Provided significantly more seizure‐free days and longer intervals of seizure freedom than placebo
-
o
Fenfluramine reduced day‐to‐day seizure burden in patients with Dravet syndrome, which may reduce the physical and emotional disease toll
Increasing TTE analysis may improve patient outcomes by reducing periods of cognitive loss and/or preventing loss of prior neurodevelopmental gains
1. INTRODUCTION
Patients with Dravet syndrome (DS), a developmental and epileptic encephalopathy with onset generally before 1 year of age, experience frequent, pharmacoresistant seizures that are associated with increased morbidity, mortality, and risk of sudden unexpected death in epilepsy. 1 , 2 , 3 , 4 Caregivers cite unpredictability and severity of seizure events as key concerns leading to high levels of anxiety and stress when caring for patients with DS. 2 , 5 In a pivotal Phase 3 clinical study (Study 1; NCT02682927, NCT02826863), patients treated with fenfluramine .7 mg/kg/day achieved a 62.3% greater reduction in mean monthly convulsive seizure frequency (MCSF) than was seen with placebo (p < .0001), and those treated with fenfluramine .2 mg/kg/day showed a 32.4% greater reduction in mean MCSF than occurred with placebo (p = .0209). 6 In a second pivotal Phase 3 clinical study (Study 2; NCT02926898), patients receiving stiripentol‐inclusive regimens with adjunctive fenfluramine (.4 mg/kg/day, dose adjusted for concomitant stiripentol) achieved a 54.0% greater percentage reduction in mean MCSF when compared to patients given placebo (p < .001). 7
Although a 50% or greater reduction in seizure frequency is useful as a regulatory endpoint for assessing treatment efficacy, 8 , 9 median percentage reduction in seizure frequency is a population‐based metric that does not fully convey whether individual patients with residual seizures have moved meaningfully toward seizure freedom, especially patients with high baseline seizure burden. 10 , 11 Time‐to‐nth seizure assesses how long it takes for each patient to reach the number of seizures typically experienced over the baseline period prior to randomization. It can provide an alternative way to differentiate among efficacious and nonefficacious treatments and provides information on change in seizure‐free days, which is typically reported per 28 days. 9 Time‐to‐event (TTE) analyses could potentially be adapted into regulatory endpoints to complement current metrics such as percentage change in seizure frequency and responder rates.
The objective of this investigation was to conduct an alternative, TTE post hoc analysis of data from two Phase 3 studies of fenfluramine for the treatment of seizures in DS. Time required postrandomization for each patient to experience the same number of seizures experienced during the baseline period (i.e., time‐to‐nth seizure) was examined, and numbers of convulsive seizure‐free days were compared across treatment groups.
2. MATERIALS AND METHODS
2.1. Study design
In Study 1, patients were observed for a 6‐week baseline period, then were randomized 1:1:1 to receive treatment with fenfluramine .7 mg/kg/day (maximum = 26 mg/day), fenfluramine .2 mg/kg/day, or placebo added to their current antiseizure medication (ASM) regimen for a 14‐week treatment period. Stiripentol was an exclusion criterion in Study 1. In Study 2, patients were observed for a 6‐week baseline period, then were randomized 1:1 to receive treatment with fenfluramine .4 mg/kg/day (maximum = 17 mg/day) added to their stiripentol‐inclusive regimen for a 15‐week treatment period. Doses were titrated during the first 2 weeks in Study 1 and during the first 3 weeks in Study 2, then were maintained for an additional 12 weeks in both studies. The CONSORT (Consolidated Standards of Reporting Trials) diagram delineating clinical trial design has been published as part of the primary articles. 6 , 7
2.2. Study participants and study drug
Patients with DS who were enrolled in Study 1 (N = 119, aged 2–18 years) or Study 2 (N = 87, aged 2–19 years) were included in this analysis. Fenfluramine was administered as fenfluramine HCl oral solution containing 2.2 mg/ml fenfluramine.
2.3. Study endpoints
In the TTE analysis, TTE was defined as time required during the treatment period to experience the same number of seizures (i.e., “nth” seizure) as had occurred during the 6‐week baseline period. Protocol‐specified secondary endpoints included number of convulsive seizure‐free days per 28 days and longest duration of convulsive seizure‐free days per 28 days.
2.4. Statistical analyses
Analyses were performed with the intent‐to‐treat population. Data were censored for patients who did not reach baseline seizure frequency by Week 14–15 or by the time of study discontinuation, or who had a substantial number (>10%) of missing diary days. Differences in Kaplan–Meier TTE curves between each fenfluramine treatment versus placebo were assessed using the log‐rank test. Differences in numbers of convulsive seizure‐free days between each fenfluramine treatment versus placebo were evaluated using an analysis of covariance model (α = .05). Differences in longest duration of convulsive seizure‐free days between each fenfluramine treatment versus placebo were evaluated using the Wilcoxon rank sum test.
3. RESULTS
3.1. Demographics and baseline characteristics
Demographics and baseline characteristics were comparable across treatment groups in both studies (Table 1) and have been published previously. 6 , 7 The median number of convulsive seizure‐free days at baseline ranged from 13.3 to 19.0 across studies and across treatment groups. The median number of convulsive seizures during the 6‐week baseline period ranged from 16.0 to 40.5. In Study 1, early discontinuation was recorded for six patients in the .7 mg/kg/day fenfluramine group, 0 patients in the .2 mg/kg/day fenfluramine group, and three patients in the placebo group. 6 Nineteen patients (48%) in the .7 mg/kg/day fenfluramine group, 12 patients (31%) in the .2 mg/kg/day fenfluramine group, and two patients (5%) in the placebo group were censored at last study day (≥89 days) upon transitioning into the long‐term open‐label extension study. In Study 2, early discontinuation was recorded for three patients in the placebo group and seven patients in the fenfluramine group. 7 Twenty‐one patients (49%) in the fenfluramine group and one patient in the placebo group (2%) were censored at last study day (≥100 days) upon transitioning into the long‐term open‐label extension study.
TABLE 1.
Patient demographics and baseline characteristics
| Characteristic | Study 1, N = 119 | Study 2, N = 87 | |||
|---|---|---|---|---|---|
| FFA .7 mg/kg/day | FFA .2 mg/kg/day | Placebo | FFA .4 mg/kg/day | Placebo | |
| n | 40 | 39 | 40 | 43 | 44 |
| Age, years | |||||
| Mean ± SD (median) | 8.8 ± 4.4 (8.5) | 9.0 ± 4.5 (8.0) | 9.2 ± 5.1 (8.5) | 8.8 ± 4.6 (9.0) | 9.4 ± 5.1 (9.0) |
| Minimum, maximum | 2, 18 | 2, 17 | 2, 18 | 2, 18 | 2, 19 |
| Age < 6 years, n (%) | 11 (28) | 9 (23) | 11 (28) | 12 (28) | 12 (27) |
| Males, n (%) | 21 (52) | 22 (56) | 21 (52) | 23 (53) | 27 (61) |
| Race, n (%) | |||||
| Caucasian | 34 (85) | 33 (85) | 31 (78) | 23 (53) | 29 (66) |
| Asian | 1 (3) | 2 (5) | 4 (10) | 2 (5) | 1 (2) |
| Other or not reported a | 5 (12) | 4 (10) | 5 (12) | 18 (42) | 14 (31) |
| Concomitant ASMs, n (%) | |||||
| Stiripentol | 0 | 0 | 0 | 43 (100) | 44 (100) |
| Valproate, all forms | 25 (62) | 24 (62) | 22 (55) | 38 (88) | 39 (89) |
| Clobazam | 24 (60) | 24 (62) | 22 (55) | 40 (93) | 42 (96) |
| Topiramate | 11 (28) | 10 (26) | 9 (22) | 14 (33) | 7 (16) |
| Levetiracetam | 4 (10) | 11 (28) | 11 (28) | 6 (14) | 5 (11) |
| Body weight, kg | |||||
| Mean ± SD | 31.8 ± 13.5 | 35.1 ± 19.6 | 31.7 ± 16.2 | 31.3 ± 14.9 | 36.2 ± 21.1 |
| BMI, kg/m2 | |||||
| Mean ± SD | 18.5 ± 3.5 | 19.3 ± 5.7 | 18.0 ± 3.8 | 17.3 ± 2.7 | 19.1 ± 4.9 |
| Median (minimum, maximum) number of seizures during 6‐week baseline period | 26.0 (7, 186) | 25.0 (7, 913) | 40.5 (5, 221) | 22.0 (4, 320) | 16.0 (4, 244) |
| Baseline convulsive seizure‐free days per 28 days | |||||
| Mean ± SD (median) | 15.2 ± 7.6 (17.6) | 16.4 ± 6.7 (18.7) | 13.3 ± 7.7 (13.3) | 18.0 ± 7.4 (20.0) | 19.0 ± 6.4 (21.0) |
| Minimum, maximum | 0, 24.0 | 0, 24.7 | 0, 24.7 | 0, 25 | 0, 26 |
Abbreviations: ASM, antiseizure medication; BMI, body mass index; FFA, fenfluramine.
Privacy laws in some regions preclude disclosure of certain personal information.
3.2. Time to event
A majority of patients treated with fenfluramine went through the entire treatment period without experiencing the same number of seizures that they had experienced during the 6‐week pretreatment baseline period on their current ASM regimen. Patients in all fenfluramine groups took significantly longer to reach baseline seizure count than those in the placebo group in both studies (Figure 1).
FIGURE 1.
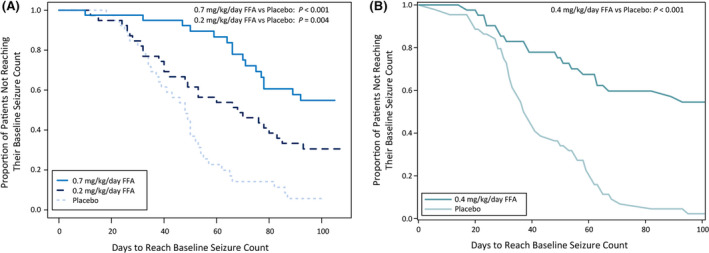
Kaplan–Meier analysis of days to reach baseline seizure count in patients receiving (A) fenfluramine (FFA) .7 mg/kg/day, FFA .2 mg/kg/day, or placebo in Study 1; or (B) FFA .4 mg/kg/day or placebo in Study 2. p values are versus placebo
In Study 1, median TTE was 13 weeks for fenfluramine .7 mg/kg/day (p < .001 compared to placebo), 10 weeks for fenfluramine .2 mg/kg/day, and 7 weeks for placebo (Figure 1). In the fenfluramine .7 mg/kg/day and .2 mg/kg/day groups, 60% and 31% of patients never reached baseline seizure count and were censored, compared with 13% in the placebo group (Figure 2).
FIGURE 2.
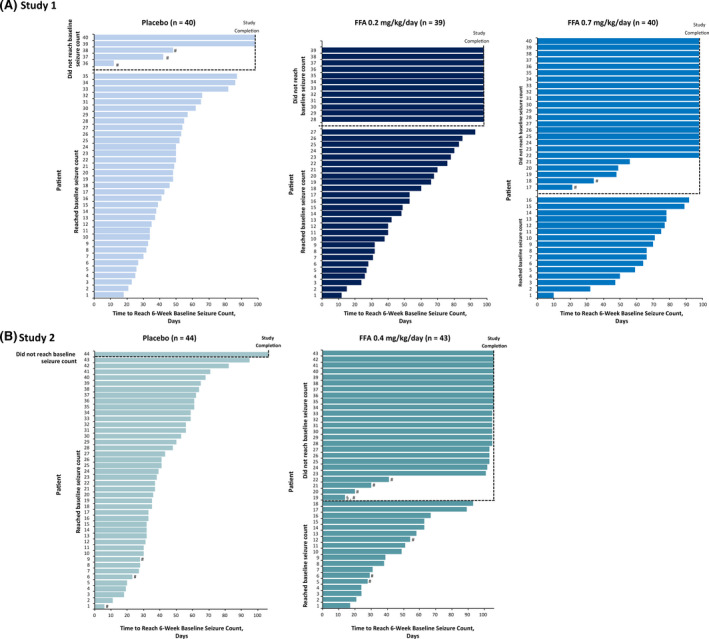
Individual patient time to reach baseline seizure count by treatment group. (A) Study 1, fenfluramine (FFA) .7 mg/kg/day, FFA .2 mg/kg/day, or placebo. (B) Study 2, FFA .4 mg/kg/day or placebo. #Patient did not complete the trial. §Data censored due to substantial number (>10%) of missing diary days
In Study 2, median TTE was 13 weeks in the fenfluramine group and 5 weeks in the placebo group (Figure 1). Baseline seizure count was never reached by 58% in the fenfluramine group versus 2% in the placebo group (Figure 2).
3.3. Convulsive seizure‐free intervals
Fenfluramine provided more convulsive seizure‐free days and longer intervals of seizure freedom per 28 days compared with placebo. In Study 1, during treatment, median numbers of convulsive seizure‐free days per 28 days postrandomization were 24.4, 20.9, and 15.1 days with fenfluramine .7 mg/kg/day, fenfluramine .2 mg/kg/day, and placebo, respectively (Figure 3), and 13–19 days at baseline (Table 1). On an annual basis, this would equate to an additional 121 and 76 convulsive seizure‐free days (3–4 months) with .7 mg/kg/day and .2 mg/kg/day fenfluramine, respectively, compared to placebo, plus current ASM regimen. The median longest duration of convulsive seizure‐free days during treatment and maintenance periods was 25.0, 15.0, and 9.5 days for fenfluramine .7 mg/kg/day, fenfluramine .2 mg/kg/day, and placebo, respectively (Table 2). The estimated median treatment difference in longest seizure‐free interval from placebo was 15.5 days and 4.5 days in the fenfluramine .7 mg/kg/day and .2 mg/kg/day groups, respectively (Table 2). Differences were statistically significant (p = .0001 for fenfluramine .7 mg/kg/day and p = .0352 for fenfluramine .2 mg/kg/day vs placebo).
FIGURE 3.
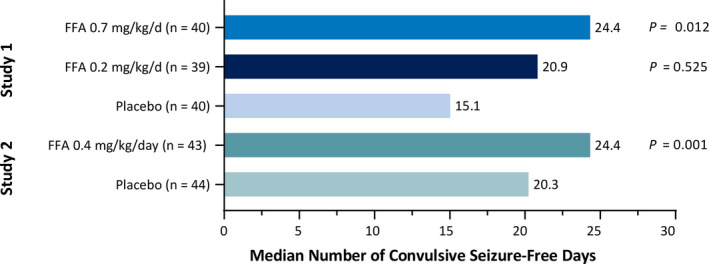
Number of convulsive seizure‐free days per 28 days by treatment group and study. p values are versus placebo by an analysis of covariance model, with treatment group and age group as factors. Median number of convulsive seizures is per 28 days. FFA, fenfluramine
TABLE 2.
Additional number of convulsive seizure‐free days and longest duration of convulsive seizure‐free days
| Study 1, N = 119 | Study 2, N = 87 | ||||
|---|---|---|---|---|---|
| FFA .7 mg/kg/day, n = 40 | FFA .2 mg/kg/day, n = 39 | Placebo, n = 40 | FFA .4 mg/kg/day, n = 43 | Placebo, n = 44 | |
| TTE analysis | |||||
| Patients who never reached baseline seizure count, n (%) | 24 (60) | 12 (31) | 5 (12) | 25 (58) | 1 (2) |
| Time to reach baseline seizure count from treatment start date, weeks, median | 13 | 10 | 7 | 13 | 5 |
| p a | <.001 | – | <.001 | – | |
| Longest duration of convulsive seizure‐free days | |||||
| Longest duration, days, median (range) | 25.0 (2–97) | 15.0 (3–106) | 9.5 (2–23) | 22.0 (3–105) | 13.0 (1–40) |
| Estimated median treatment difference from placebo, days (95% CI) | 15.5 (6–25) | 4.5 (0–9) | – | 8.5 (2.0–15.0) | – |
| p b | .0001 | .0352 | – | .004 | – |
| Number of convulsive seizure‐free days per 28 days, median (range) | 24.4 (1–28) | 20.9 (2–28) | 15.1 (1–26) | 24.4 (2–28) | 20.3 (0–26) |
| p b | .012 | .525 | – | .001 | – |
Abbreviations: CI, confidence interval; FFA, fenfluramine; TTE, time‐to‐event.
p values are versus placebo, calculated using log‐rank test.
p values are versus placebo, calculated using Wilcoxon rank sum test (95% CI).
In Study 2, the median number of convulsive seizure‐free days per 28 days postrandomization was 24.4 in the fenfluramine group compared with 20.3 days in the placebo group (p = .001; Figure 3) and 20–21 days at baseline (Table 1). On an annual basis, this difference between fenfluramine and placebo would equate to a median additional 54 convulsive seizure‐free days (approximately 2 months) compared with placebo + current ASM regimen. The longest duration of convulsive seizure‐free days was a median of 22.0 days in the fenfluramine group compared with 13.0 days in the placebo group (Table 2). The estimated median treatment difference in longest seizure‐free interval from placebo was 8.5 days in the fenfluramine group (p = .004; Table 2).
3.4. Seizure freedom
During the 14‐week Study 1 treatment period, seizure freedom was experienced by three (8%) patients each in the fenfluramine .7 mg/kg/day and .2 mg/kg/day groups and by 0 patients in the placebo group. Near seizure freedom (≤1 convulsive seizure) was reported in 10 (25%) patients in the .7 mg/kg/day group, in five (13%) patients in the .2 mg/kg/day group, and in 0 patients in the placebo group.
During the 15‐week Study 2 treatment period, seizure freedom was achieved by one (2%) patient in the active group compared with 0 in the placebo group. Near seizure freedom (≤1 convulsive seizure) was achieved by five patients (12%) in the fenfluramine group and by 0 in the placebo group.
3.5. Safety
In both studies, the most common adverse events occurring more frequently with fenfluramine than with placebo included decreased appetite, diarrhea, lethargy, nasopharyngitis, and pyrexia, as was previously published in the primary reports. No valvular heart disease or pulmonary arterial hypertension was observed in any patient at any time. 6 , 7
4. DISCUSSION
The day‐to‐day seizure burden in patients with DS can make it challenging for patients and caregivers/families to navigate their daily living routines (home and work). Change in group seizure frequency alone does not fully characterize how effective a treatment may be in moving patients who still have seizures (potentially due to high baseline seizure frequency) toward seizure freedom by providing longer gaps or convulsive seizure‐free “windows” between seizures, thereby alleviating some of the day‐to‐day burden. A TTE analytical approach to assessment of time‐to‐nth seizure provides a way to examine how long it takes a patient to experience the same number of seizures after treatment intervention as before the start of the new treatment, as another way of gauging effectiveness. In this post hoc analysis of primary randomized clinical trial data from two Phase 3 studies, fenfluramine extended TTE and provided significantly more convulsive seizure‐free days and longer intervals of seizure freedom than placebo. TTE analysis demonstrated statistical significance for both treatment groups compared with the placebo group in time to reach baseline MCSF, with 60% and 31% in the .7 mg/kg/day and .2 mg/kg/day fenfluramine groups, respectively, never reaching baseline seizure count in Study 1, and with 58% of patients in the fenfluramine .4 mg/kg/day group never reaching baseline seizure count in Study 2.
4.1. Seizure burden, convulsive seizure‐free days, and intervals between seizures
Results demonstrate that fenfluramine treatment provided longer windows of seizure freedom for most patients in this study (Figure 2). Near seizure freedom (≤1 seizure) was achieved by 18% of patients in the fenfluramine .7 mg/kg/day group in Study 1 and by 12% in Study 2, compared with 0% of patients in the placebo group in both studies. 6 These longer windows of seizure freedom afford several important therapeutic advantages for patients with DS and their families, including decreased day‐to‐day burden on patients, their caregivers, and the entire family unit. 12 Families report that patients with DS and other developmental and epileptic encephalopathies experience loss of cognitive abilities during periods of high seizure frequency. 12 , 13 Furthermore, longer windows of seizure freedom could improve patient outcomes by reducing periods of cognitive loss and/or preventing loss of previous neurodevelopmental gains. 14 Fenfluramine treatment improved executive function in patients with DS at 14 weeks 6 and at 1 year. 14 After 1 year of treatment, patients with clinically meaningful (≥50%) or profound (≥75%) reduction in seizure frequency from pretreatment baseline showed the most marked improvement in measures of executive function when compared with patients with <50% or <25% improvement, especially on measures of Cognitive Regulation Index and Emotional Regulation Index. 14 Taken together, these results suggest the potential for a positive impact on longer‐term outcomes when windows of time without central nervous system insult caused by seizure activity are increased. However, we acknowledge that the possible consequences of seizure‐free days for cognitive endpoints remain speculative until further research can evaluate the long‐term effects of observed improvements in executive function on day‐to‐day activities, such as going to school, and impact on the caregiver. These data suggest that other endpoints besides change in seizure frequency or percentage reduction (responder rates) might be considered as important indicators of clinical efficacy and could potentially be considered as endpoints for regulatory ASM clinical trials in the future. Use of the nth seizure as a primary endpoint, with patients ending the treatment period when they reach baseline seizure frequency, could reduce the study period and reduce the duration of exposure to ineffective treatments, thus reducing the duration of placebo exposure in patients with high seizure burden.
4.2. Potential impact on caregiver and family well‐being
Caregivers cite seizure unpredictability as the single most important concern impacting their own health and the well‐being of their family unit as a whole. 12 Inability to predict the timing and severity of seizures places the families of these patients in a perpetual state of fear, anxiety, and stress. 12 In a recent survey assessing how fenfluramine treatment affected quality of life for 59 patients with DS and their families, survey respondents reported that reduction in seizure frequency after fenfluramine led to significant improvement in metrics of well‐being, including feelings of stress, anxiety, depression, and being overwhelmed; sleep quality; stress levels experienced by siblings and the family as a whole; relationships among siblings and sibling mood; and the ability of the family to socialize with others. 15 Further research is needed to investigate whether the substantial improvement in convulsive seizure‐free intervals reported here would help reduce the physical and emotional toll of the unpredictability of the disease on caregivers and their families.
4.3. Limitations and future directions
This study has some limitations. The study is a post hoc analysis of randomized clinical studies for which TTE was not the primary endpoint. Data from patients who never reached their baseline seizure count were censored to ensure robustness of the statistical method; however, this practice could result in potential underestimation of TTE in those patients who discontinued the study early to enroll in the open‐label long‐term extension study.
Further research into the utility of TTE as a potential endpoint in clinical trials is needed. Depending on the sensitivity of such an endpoint to detect treatment differences between active and placebo groups, TTE could be considered by researchers and regulatory agencies as a primary or key secondary endpoint for randomized clinical trials, 9 and may be used to inform clinical decision‐making for health care professionals, patients, and caregivers. Further research is warranted to correlate metrics of health‐related quality of life in families with proportion of seizure‐free days or length of seizure‐free intervals, as described by Auvin and colleagues. 16
5. CONCLUSIONS
In this analysis, by using a TTE (i.e., time‐to‐nth seizure) analytical approach, we were able to further characterize the efficacy of fenfluramine in DS by demonstrating its ability to significantly extend the time it takes for patients to experience the same number of seizures—as they did on their current ASM regimen during the 6‐week baseline period. A majority of patients completed the entire treatment period without ever reaching their baseline seizure frequency. Moving patients in the direction of seizure freedom by extending “windows” of convulsive seizure‐free days between seizures may help to improve patient and caregiver quality of life by reducing the day‐to‐day burden, and may have a positive impact on longer term neurodevelopmental outcomes by reducing the time that patients experience regression or loss of abilities due to high seizure burden.
CONFLICT OF INTEREST
J.S. received research grants from Stoke, Marinus, Zogenix, and Biopharm; served as consultant/advisor for the Dravet Syndrome Foundation, Epygenix, Encoded, GW Pharma, Asceneuron, Longboard Pharmaceuticals, Knopp Biosciences, and Neurocrine; has stock options in Epygenix; served as a reviewer for the Epilepsy Study Consortium; and received travel support from Zogenix. N.S. received consulting fees from Zogenix; received research support from LivaNova and Biomarin; and served as a paid consultant for LivaNova. O.D. received research funding from Novartis, PTC Therapeutics, Zogenix, and GW Pharma; and holds equity interest in Rettco, Pairnomix, Tilray, Papa & Barkley, California Cannabis Enterprises, Tevard Biosciences, Regel Biosciences, Script Biosciences, Silver Spike Capital, and Silver Spike SPAC. S.A. received personal fees from Arvelle, Biocodex, GW Pharma, and Xenon; received personal fees and nonfinancial support from Biomarin, GW Pharma, and Nutricia; received personal fees/grants from Eisai and UCB Pharma for work as an investigator; and received research support from Zogenix. M.S.P. received research support from Stoke, Encoded, Marinus, Ovid, and Zogenix; and received honoraria (speakers' bureaus, advisory/consulting roles) from Greenwich Biosciences, Biomarin, Zogenix, Neurelis, Encoded, and Stoke. A.S. received personal fees or grants from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharma, Marinus Pharmaceuticals, UCB Pharma, and Zogenix; and received honoraria for consulting and serving on advisory boards from Zogenix. A.G.‐N. received personal fees or research grants from Arvelle Therapeutics, Bial, Biocodex, Eisai, Esteve, GW Pharma, PTC Therapeutics, Stoke, UCB, and Zogenix. D.D. served as a paid consultant for Zogenix. B.S.G. and A.R.G. serve as employees with ownership interest in Zogenix.
ACKNOWLEDGMENTS
The authors thank the patients who participated in studies NCT02682927, NCT02826863, and NCT02826898. This study was sponsored by Zogenix (Emeryville, CA, USA). Medical writing and editorial assistance were provided by Danielle Ippolito, PhD, CMPP, MWC, and Dolores Matthews, MEd, of PharmaWrite (Princeton, NJ, USA), and were funded by Zogenix.
Sullivan J, Specchio N, Devinsky O, Auvin S, Perry MS, Strzelczyk A, et al. Fenfluramine significantly reduces day‐to‐day seizure burden by increasing number of seizure‐free days and time between seizures in patients with Dravet syndrome: A time‐to‐event analysis. Epilepsia. 2022;63:130–138. 10.1111/epi.17106
REFERENCES
- 1. Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Jensen MP, Liljenquist KS, Bocell F, Gammaitoni AR, Aron CR, Galer BS, et al. Life impact of caregiving for severe childhood epilepsy: results of expert panels and caregiver focus groups. Epilepsy Behav. 2017;74:135–43. [DOI] [PubMed] [Google Scholar]
- 3. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gataullina S, Dulac O. From genotype to phenotype in Dravet disease. Seizure. 2017;44:58–64. [DOI] [PubMed] [Google Scholar]
- 5. Kroner BL, Ardini MA, Bumbut A, Gaillard WD. Parental perspectives of the impact of epilepsy and seizures on siblings of children with epilepsy. J Pediatr Health Care. 2018;32(4):348–55. [DOI] [PubMed] [Google Scholar]
- 6. Lagae L, Sullivan J, Knupp K, Laux L, Polster T, Nikanorova M, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2019;394(10216):2243–54. [DOI] [PubMed] [Google Scholar]
- 7. Nabbout R, Mistry A, Zuberi S, Villeneuve N, Gil‐Nagel A, Sanchez‐Carpintero R, et al. Fenfluramine for treatment‐resistant seizures in patients with Dravet syndrome receiving stiripentol‐inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77(3):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. 2010. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐clinical‐investigation‐medicinal‐products‐treatment‐epileptic‐disorders‐revision‐2_en.pdf. Accessed 12 Jun 2019.
- 9. French JA, Gil‐Nagel A, Malerba S, Kramer L, Kumar D, Bagiella E. Time to prerandomization monthly seizure count in perampanel trials: a novel epilepsy endpoint. Neurology. 2015;84(20):2014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perucca E. From clinical trials of antiepileptic drugs to treatment. Epilepsia Open. 2018;3(Suppl 2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018;208(5):226–33. [DOI] [PubMed] [Google Scholar]
- 12. Berg AT, Kaiser K, Dixon‐Salazar T, Elliot A, McNamara N, Meskis MA, et al. Seizure burden in severe early‐life epilepsy: perspectives from parents. Epilepsia Open. 2019;4(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villas N, Meskis MA, Goodliffe S. Dravet syndrome: characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017;74:81–6. [DOI] [PubMed] [Google Scholar]
- 14. Bishop KI, Isquith PK, Gioia GA, Gammaitoni AR, Farfel G, Galer BS, et al. Improved everyday executive functioning following profound reduction in seizure frequency with fenfluramine: analysis from a phase 3 long‐term extension study in children/young adults with Dravet syndrome. Epilepsy Behav. 2021;121:108024. [DOI] [PubMed] [Google Scholar]
- 15. Amtmann D, Salem R, Gammaitoni AR, Galer BS, Wilkie D, Jensen MP. Caregivers’ perspectives on the long‐term seizure‐ and non‐seizure‐related benefits of fenfluramine on patients with Dravet syndrome and their families. Presented at: American Academy of Neurology Virtual Meeting; April 17–22, 2021.
- 16. Auvin S, Damera V, Martin M, Holland R, Simontacchi K, Saich A. The impact of seizure frequency on quality of life in patients with Lennox‐Gastaut syndrome or Dravet syndrome. Epilepsy Behav. 2021;123:108239. [DOI] [PubMed] [Google Scholar]


