Abstract
Aims
To investigate to what extent multiple risk marker improvements confer lower risk of cardiovascular and kidney complications in a contemporary type 2 diabetes population.
Materials and methods
Post‐hoc analysis of the LEADER (n = 8638; median follow‐up 3.8 years) and SUSTAIN 6 (n = 3040; median follow‐up 2.1 years) cardiovascular outcome trials. Participants were those with baseline and year‐1 assessment of at least one of the parameters of interest; we pooled the liraglutide‐/semaglutide‐ and placebo‐treated groups and categorized them by number of risk markers with clinically relevant improvements after 1 year of study participation. We investigated risk of major adverse cardiovascular events (MACE), expanded MACE, cardiovascular death and nephropathy. Predefined clinically relevant changes: body weight loss ≥5%; reductions in: glycated haemoglobin ≥1%, systolic blood pressure ≥5 mmHg and low‐density lipoprotein cholesterol ≥0.5 mmol/L; estimated glomerular filtration rate change ≥0 ml/min/1.73 m2 and urinary albumin‐to‐creatinine ratio change ≥30% of baseline value. Cox regression analysed risk of outcomes adjusted for baseline risk marker levels and treatment group and stratified by trial.
Results
Participants with two, three, or four or more improved risk markers versus participants with no risk marker improvement had reduced risk of expanded MACE [hazard ratio (95% confidence interval) 0.80 (0.67‐0.96); 0.80 (0.66‐0.97); 0.82 (0.66‐1.02)], cardiovascular death [0.66 (0.45‐0.96), 0.67 (0.45‐0.99), 0.60 (0.38‐0.94)] and nephropathy [0.71 (0.52‐0.97), 0.48 (0.34‐0.68), 0.43 (0.29‐0.65)].
Conclusions
In persons with type 2 diabetes, improvements in ≥2 risk markers conferred cardiovascular risk reduction versus none or one improved risk marker. The nephropathy risk decreased with improvement in more risk markers. These findings stress the importance of multifactorial interventions targeting all risk markers.
Keywords: cardiovascular disease, diabetic nephropathy, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes is a complex disease with a high rate of both micro‐ and macrovascular complications and an increased risk of death compared with the general population. 1 When it comes to reducing this high risk of complications and death, evidence is centred around pharmacotherapies that target isolated risk markers, including glycated haemoglobin (HbA1c), low‐density lipoprotein‐cholesterol (LDL‐C) and blood pressure. 2 , 3 , 4 , 5
In the randomized Steno 2 study, multifactorial intervention for 8 years targeting several modifiable risk markers resulted in a median increase in long‐term survival of 7.9 years after 21 years of follow‐up and 50% reduced risk of cardiovascular disease as compared with standard of care. 6 Adding to the interest in multiple risk markers, a recent observational cohort study including all patients in the Swedish National Diabetes Register who had type 2 diabetes indicated that those with HbA1c, LDL‐C, albuminuria and blood pressure levels within the target range and non‐smokers had little or no excess risk of death, myocardial infarction or stroke, compared with the general population. 7
The extent to which improvements in multiple risk markers affect outcomes in type 2 diabetes has been sparsely investigated. Therefore, in two large cardiovascular outcome trials, we evaluated post hoc the importance of multiple risk marker improvement. We pooled the active treatment and placebo‐treated groups in all analyses to investigate, in a contemporary type 2 diabetes population and independent of specific treatments, to what degree clinically relevant improvement in multiple risk markers confers lower risk of micro‐ and macrovascular disease.
2. MATERIALS AND METHODS
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER, NCT01179048) and the Trial to Evaluate Cardiovascular and Other Long‐term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6, NCT01720446) trial designs and methods have been previously published in detail. 8 , 9 Briefly, both were multicentre, double‐blind, placebo‐controlled trials, including persons with type 2 diabetes and high cardiovascular risk (N = 9340 in LEADER and N = 3297 in SUSTAIN 6). Participants were randomly assigned to a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) or placebo, both added to the standard of care. In LEADER, liraglutide up to 1.8 mg subcutaneous once daily or matching placebo was given in a 1:1 ratio. In SUSTAIN 6, semaglutide 0.5 or 1.0 mg, or matching placebo, was given subcutaneous once weekly in a 1:1:1:1 ratio, pooled as semaglutide versus placebo for this analysis. The mean duration of follow‐up was 3.8 years in LEADER and 2.1 years in SUSTAIN 6.
Investigators in both trials were directed to treat all participants to the standard of care according to guidelines. Both studies were conducted according to the Declaration of Helsinki and approved by the relevant authorities.
2.1. Participants
This current post‐hoc analysis included participants from LEADER and SUSTAIN 6 who had baseline and year‐1 assessment of at least one of the six risk markers of interest: body weight, HbA1c, systolic blood pressure (SBP), LDL‐C, estimated glomerular filtration rate (eGFR) and urinary albumin‐to‐creatinine ratio (UACR).
LEADER and SUSTAIN 6 included persons with type 2 diabetes and an HbA1c level of 7.0% (53 mmol/mol) or above. Participants were eligible if they were drug‐naïve or treated with oral antihyperglycaemic agents or insulin. Key inclusion criteria were (a) age ≥50 years with cardiovascular morbidity (previous cardiovascular, cerebrovascular or peripheral vascular disease), or (b) age ≥60 years with one or more cardiovascular risk factors [persistent microalbuminuria (30‐299 mg/g) or proteinuria, hypertension and left ventricular hypertrophy by electrocardiogram or imaging, left ventricular systolic or diastolic dysfunction by imaging, or ankle/brachial index <0.9]. The published original trial reports contain the complete list of inclusion and exclusion criteria. 8 , 9
2.2. Risk markers
Six well‐established cardio‐renal risk markers that are easily accessible for many clinicians in the daily evaluation of patients were selected for evaluation, including body weight, HbA1c, SBP, LDL‐C, eGFR and UACR. We used SBP because it is the predominant risk factor among older adults and because the mean age of participants in this study was 64.3 years. 10 SBP and body weight were measured by standard methods at study sites. HbA1c, LDL‐C, serum creatinine and UACR were measured using routine methods in central laboratories (Dublin, Ireland; New York, USA; Tianjin, China; Singapore; Bangalore, India), and eGFR was calculated [Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation] at baseline and at regular intervals during the trials, including at week 52 (12 months) in LEADER and week 56 in SUSTAIN 6 (referred to as year 1). Urine albumin or creatinine measurements less than the lower limit of quantification (LLoQ, 3.0 mg/L) were imputed using a value of 0.5× LLoQ; measurements greater than the higher limit of quantification (HLoQ) were imputed using the HLoQ value in the calculation of UACR.
We defined clinically relevant risk marker improvement at year 1 from baseline as body weight loss ≥5%, HbA1c reduction ≥1%, SBP reduction ≥5 mmHg, LDL‐C reduction ≥0.5 mmol/L, eGFR change ≥0 ml/min/1.73 m2 and UACR change ≥30% of baseline value. Participants were categorized into five groups based on the number of risk marker improvements at year 1: Group G0 (reference): zero risk marker improvement; Group G1: one risk marker improvement; Group G2: two risk marker improvements; Group G3: three risk marker improvements; Group G4: four or more risk marker improvements.
2.3. Outcomes
We investigated the number of risk marker improvements at year 1 and the incidence of development of first outcome after year 1. To evaluate the association between risk marker improvement and outcome, outcomes developed in the first year of participation in both studies were excluded. For participants with an event in the first year, events developed after 1 year of participation were included. The four outcomes of interest were: (a) major adverse cardiovascular events (MACE), including cardiovascular death, non‐fatal myocardial infarction and non‐fatal stroke; (b) expanded MACE, including cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, revascularization (LEADER: coronary; SUSTAIN 6: coronary or peripheral) and hospitalization for unstable angina pectoris or heart failure; (c) cardiovascular death; and (d) a composite nephropathy endpoint defined as the new onset of macroalbuminuria or a doubling of serum creatinine level and an eGFR ≤45 ml/min/1.73 m2, or the need for continuous renal‐replacement therapy or death from renal disease.
2.4. Statistical analyses
Normally distributed data are presented as mean ± standard deviation, continuous‐scale skewed data (UACR) are presented as geometric mean (coefficient of variation) and median (interquartile range) and were log‐transformed for all analyses; categorical variables are presented as numbers and percentages.
A Cox regression model stratified by trial was used to analyse the time to first MACE, expanded MACE, cardiovascular death and the composite nephropathy endpoint from 1 year onwards according to risk marker categories, adjusted for randomized treatment (liraglutide/semaglutide vs. placebo) and continuous baseline levels of the risk markers.
Test for trend was evaluated in a Cox regression model stratified by trial, with number of improved risk markers as a continuous variable (hence the test for slope equals 0) and adjusted for randomized treatment and continuous baseline levels of the risk markers.
We also analysed the relative importance of the six risk marker improvements. Relative importance provides an estimate of how important each risk marker improvement is in terms of predicting each of the outcomes after year 1. 7 We calculated the relative importance as measured by the R2 values (explained relative risk in the Cox regression model) using an approach that has previously been described 11 ; we evaluated the R2 by using the full model as the Cox regression model for each endpoint with all six risk marker improvements (yes vs. no) adjusted for the continuous baseline levels of the six risk markers and treatment stratified by trial. A sensitivity analysis imputing missing values (single imputation) at year 1 for those participants with at least one measurement at year 1 was performed using the participant‐wise predicted values from a random slope model for each risk marker independently, with baseline value and treatment by a linear time interaction as fixed effects. After the imputation, the clinically relevant improvements were derived for each risk marker.
The statistical package used for these analyses was SAS, version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Study population
In total, 11 678 persons with type 2 diabetes were included in this study (N = 8638 in LEADER and N = 3040 in SUSTAIN 6).
The baseline characteristics of all participants and of the five groups (G0‐G4) based on number of improved risk markers are presented in Table 1. There was a tendency towards a higher number of women and higher baseline LDL‐C, SBP and UACR with an increasing number of improved risk markers. Separate baseline data for LEADER and SUSTAIN 6 are presented in Tables S1 and S2.
TABLE 1.
Baseline characteristics of the participants by risk marker improvement subgroups
| Baseline | G0 | G1 | G2 | G3 | G4 | Total |
|---|---|---|---|---|---|---|
| No. of participants, n (%) a | 1055 (9.0) | 3162 (27.1) | 3540 (30.3) | 2515 (21.5) | 1406 (12.0) | 11 678 (100) |
| Treated with liraglutide or semaglutide, n (%) | 322 (30.5) | 1202 (38.0) | 1727 (48.8) | 1550 (61.6) | 1059 (75.3) | 5860 (50.2) |
| Placebo, n (%) | 733 (69.5) | 1960 (62.0) | 1813 (51.2) | 965 (38.4) | 347 (24.7) | 5818 (49.8) |
| Age, years | 64.2 ± 7.4 | 64.3 ± 7.1 | 64.2 ± 7.2 | 64.4 ± 7.2 | 64.5 ± 7.2 | 64.3 ± 7.2 |
| Female, n (%) | 320 (30.3) | 1061 (33.6) | 1278 (36.1) | 991 (39.4) | 613 (43.6) | 4263 (36.5) |
| HbA1c, %d | 8.2 ± 1.2 | 8.5 ± 1.4 | 8.8 ± 1.6 | 8.8 ± 1.5 | 8.8 ± 1.4 | 8.7 ± 1.5 |
| HbA1c, mmol/mol | 66.0 ± 12.9 | 69.6 ± 15.5 | 72.5 ± 17.4 | 73.0 ± 16.7 | 72.8 ± 15.6 | 71.3 ± 16.3 |
| Body weight, kg d | 91.1 ± 20.7 | 91.6 ± 20.6 | 91.4 ± 20.6 | 92.4 ± 20.9 | 92.2 ± 21.5 | 91.7 ± 20.8 |
| Diabetes duration, years, median (IQR) | 11.5 (6.8‐16.9) | 11.8 (7.0‐17.7) | 11.6 (7.0‐17.1) | 11.7 (7.1‐17.2) | 12.4 (7.4‐18.3) | 11.8 (7.1‐17.5) |
| Current smoker, n (%) | 125 (11.8) | 413 (13.1) | 376 (10.6) | 301 (12.0) | 170 (12.1) | 1385 (11.9) |
| SBP, mmHg d | 131 ± 16 | 133 ± 17 | 136 ± 18 | 139 ± 18 | 141 ± 18 | 136 ± 17 |
| LDL‐C, mg/dl | 84.6 ± 32.5 | 84.3 ± 33.3 | 89.5 ± 35.5 | 92.6 ± 37.4 | 101.4 ± 41.6 | 89.8 ± 36.3 |
| LDL‐C, mmol/L d | 2.2 ± 0.8 | 2.2 ± 0.9 | 2.3 ± 0.9 | 2.4 ± 1.0 | 2.6 ± 1.1 | 2.3 ± 0.9 |
|
eGFR (CKD‐EPI), ml/min/1.73 m2 d |
81.4 ± 21.3 | 80.6 ± 21.6 | 80.4 ± 21.5 | 79.7 ± 22.0 | 79.2 ± 22.0 | 80.3 ± 21.7 |
| UACR, median (IQR) d | 11.9 (3.4‐64.8) | 12.5 (3.9‐57.5) | 14.6 (4.6‐63.1) | 17.2 (5.6‐69.9) | 22.3 (7.5‐96.0) | 15.3 (4.6‐67.5) |
| Established CVD, n (%) | 873 (82.7) | 2559 (80.9) | 2863 (80.9) | 2059 (81.9) | 1156 (82.2) | 9510 (81.4) |
| Presence of CVD risk factor, n (%) a , b | 182 (17.3) | 603 (19.1) | 677 (19.1) | 456 (18.1) | 250 (17.8) | 2168 (18.6) |
| Lipid‐lowering treatment, n (%) | 820 (77.7) | 2431 (76.9) | 2728 (77.1) | 1886 (75.0) | 1023 (72.8) | 8888 (76.1) |
| RAAS inhibition treatment, n (%) | 857 (81.2) | 2548 (80.6) | 2883 (81.4) | 2032 (80.8) | 1123 (79.9) | 9443 (80.9) |
| Metformin treatment, n (%) | 796 (75.5) | 2486 (78.6) | 2727 (77.0) | 1887 (75.0) | 1033 (73.5) | 8929 (76.5) |
| Insulin treatment, n (%) | 487 (46.2) | 1498 (47.4) | 1550 (43.8) | 1111 (44.2) | 631 (44.9) | 5277 (45.2) |
| SGLT‐2 inhibitor treatment, n (%) c | 1 (<0.1) | 0 (0.0) | 2 (<0.1) | 1 (<0.1) | 1 (<0.1) | 5 (<0.1) |
| Aspirin treatment, n (%) | 682 (64.6) | 2022 (63.9) | 2264 (64.0) | 1576 (62.7) | 868 (61.7) | 7412 (63.5) |
Note: Adapted from Zobel EH et al. The importance of addressing multiple risk markers in type 2 diabetes: results from the LEADER and SUSTAIN 6 trials.12. Abstract/FC 058 ©ERA‐EDTA GROUP. Reproduced by permission of Oxford University Press on behalf of the ERA‐EDTA. Table is not published under this article's licence and permission must be sought for any form of reuse.
Pooled data from the LEADER and SUSTAIN 6 trials. Data are presented as mean ± standard derivation, unless stated otherwise. Participants were categorized according to number of risk markers with an improvement at year 1 [none (group G0), one (G1), two (G2), three (G3) and four or more (G4)].
Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; IQR, interquartile range; LDL‐C, low‐density lipoprotein‐cholesterol; RAAS, renin‐angiotensin‐aldosterone system; SBP, systolic blood pressure; SGLT‐2, sodium‐glucose cotransporter‐2; UACR, urinary albumin‐to‐creatinine ratio.
Calculated as a percentage of the overall total (all other percentages were calculated out of the risk marker improvement subgroups).
Presence of CVD risk factor was defined as persistent microalbuminuria (30‐299 mg/g) or proteinuria, hypertension and left ventricular hypertrophy by electrocardiogram or imaging, left ventricular systolic or diastolic dysfunction by imaging, or ankle/brachial index <0.9.
SGLT‐2 inhibitors were not marketed before randomization in the LEADER trial, hence relatively few participants in the pooled population received this medication at baseline.
These parameters are risk markers that were evaluated in this post‐hoc analysis.
3.2. Risk marker improvement
The number of participants with one, two, three and four or more clinically relevant risk marker improvements at year 1 was 3162 (27.1%), 3540 (30.3%), 2515 (21.5%) and 1406 (12.0%), respectively (Table 1). There were 1055 (9.0%) participants without any clinically relevant risk marker improvements at year 1. In the LEADER trial, 138 (1.6%) participants died or discontinued the trial before the 1‐year visit and 564 (6.5%) participants were still in‐trial but missed the 1‐year assessment (or at baseline). In SUSTAIN 6, the corresponding numbers were 72 (2.4%) and 185 (6.1%) participants, respectively. For those participants with at least one assessment at year 1, almost 90% had all six assessments related to the risk markers at year 1.
3.3. Risk of outcomes
In the two studies combined, a total of 1111 (9.5%) participants experienced MACE, 1771 (15.2%) expanded MACE, 406 cardiovascular death (3.5%) and 621 (5.3%) developed the composite nephropathy endpoint after year 1 of study participation (Figure 1).
FIGURE 1.
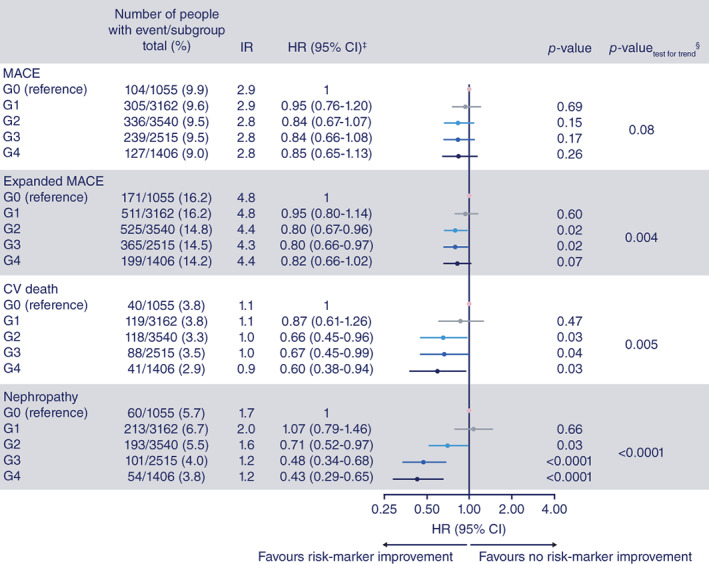
Outcomes according to number of risk marker improvements (adjusted by baseline variables) among persons with type 2 diabetes. From Zobel EH et al. The importance of addressing multiple risk markers in type 2 diabetes: results from the LEADER and SUSTAIN 6 trials.12 Abstract/FC 058 ©ERA‐EDTA GROUP. Reproduced by permission of Oxford University Press on behalf of the ERA‐EDTA. Figure is not published under this article's licence and permission must be sought for any form of reuse. HRs show risk for outcomes according to number of risk markers with a clinically relevant improvement among participants with type 2 diabetes. Post‐hoc analysis of data from the LEADER and SUSTAIN 6 trials included 11 678 persons with type 2 diabetes. Participants were categorized according to number of risk markers with an improvement at year 1 [none (group G0), one (G1), two (G2), three (G3) and four or more (G4)]. ‡Compared G1‐G4 with G0 (the reference group); §test for trend was evaluated in a Cox regression model with number of risk marker improvements as a continuous variable adjusted for treatment and baseline levels of the risk markers. CI, confidence interval; CV, cardiovascular; HR, hazard ratio; IR, incidence rate per 100 patient years of observation; MACE, major adverse cardiovascular events
The number of events, incidence rates and hazard ratios for all outcomes in participants with one, two, three, or four or more (G1‐G4) risk marker improvements at year 1, compared with participants without any risk marker improvements (G0), are presented in Figure 1, and separately for LEADER and SUSTAIN 6 participants in Figures S1 and S2. In a pooled analysis of LEADER and SUSTAIN 6, the hazard ratios for expanded MACE, cardiovascular death and the composite nephropathy endpoint were lower in the groups of participants with two, three or four or more risk marker improvements (G2‐G4), compared with the zero risk marker improvement group (G0: reference group). The hazard ratios for all four endpoints were comparable in the group of participants with one risk marker improvement (G1) and the group of participants with no risk marker improvements at year 1 (G0: reference group). There was a trend of decreased hazard ratios across the groups for MACE (p = .08), expanded MACE (p = .004), cardiovascular death (p = .005) and the composite nephropathy endpoint (p < .0001). Collapsing G0 with G1 versus collapsing G2, G3 and G4 and testing if these two groups were equal, we obtained the following p‐values for the endpoints: MACE (p = .048), expanded MACE (p = .0006), cardiovascular death (p = .0031) and the composite nephropathy endpoint (p < .0001).
We observed similar results in a separate analysis of LEADER and SUSTAIN 6 (Figures S1 and S2) and when the liraglutide/semaglutide and placebo groups were analysed separately (Figures S3 and S4). We also confirmed our findings in a sensitivity analysis with missing responses imputed (i.e. single imputation of missing risk markers at year 1; Figure S5), although results (trends) were slightly attenuated.
3.4. Relative importance of the individual risk marker improvement
Figure S6 shows the six risk marker improvements ranked in order of the highest to lowest R2 according to MACE, expanded MACE, cardiovascular death and the composite nephropathy endpoint. The R2 in this analysis could be interpreted in the same way as the coefficient of determination in the linear model. The R2 for all the improvements adjusted for baseline variables was modest and varied from 2% to 19% for first MACE and the composite nephropathy endpoint, respectively. For the individual improvements, HbA1c contributed most to the R2 for first MACE (56%) and expanded MACE (39%); improvement in eGFR contributed most to the R2 for cardiovascular death (47%) and improvement in UACR contributed most to the R2 for the composite nephropathy endpoint (58%).
3.5. Glucagon‐like peptide‐1 receptor agonist treatment
We observed an increase in the number of participants treated with GLP‐1RA versus placebo in the groups of participants with none, one, two, three, or four or more risk marker improvements as follows: 30.5% in G0, 38.0% in G1, 48.8% in G2, 61.6% in G3 and 75.3% in G4 (Table 1). Hence, 24.7% of the participants with four or more risk marker improvements were treated with placebo.
In two separate analyses, one including participants treated with semaglutide/liraglutide and one including participants treated with placebo, we observed similar results as in the pooled analysis (Figures S3 and S4).
In analyses without adjustment for randomized treatment, we confirmed our main findings (data not shown).
3.6. Sensitivity analysis on a modified nephropathy endpoint
We confirmed our findings for the composite nephropathy endpoint using a modified nephropathy endpoint defined as a doubling of serum creatinine level and an eGFR ≤45 ml/min/1.73 m2, or the need for continuous renal‐replacement therapy or death from renal disease. Participants with two, three, or four or more improved risk markers versus participants with no risk marker improvement had a reduced risk of the modified nephropathy endpoint: hazard ratio (95% confidence interval) 0.65 (0.39‐1.07), 0.43 (0.25‐0.74) or 0.32 (0.17‐0.61), respectively.
4. DISCUSSION
Our analyses of the two large cardiovascular outcome trials LEADER and SUSTAIN 6, including >11 000 persons with type 2 diabetes, show the association between the number of risk markers with a clinically relevant improvement within 1 year and the risk of developing micro‐ and macrovascular complications.
The risk markers evaluated were HbA1c, body weight, SBP, LDL‐C, UACR and eGFR. For the cardiovascular outcomes, a clinically relevant improvement at year 1 in two or more of these risk markers was associated with reduced cardiovascular risk, as compared with participants without a clinically relevant improvement in any of these risk markers. More specifically, risk reduction was more noticeable when two or more improvements were obtained as compared with only having no or one improvement. For the risk of developing the composite nephropathy endpoint, a clinically relevant improvement at year 1 in two or more risk markers resulted in lower risk. For this outcome, we observed a pronounced decrease in risk for each additional risk marker improved. Data were not strong enough to conclude that a stepwise association exists for cardiovascular risk reduction. Findings from separate analyses of LEADER and SUSTAIN 6 were similar to the findings from the pooled analysis.
Dedicated outcome trials to evaluate the importance of addressing multiple risk markers are laborious and expensive. Accordingly, while awaiting such trials to inform us on multiple risk marker improvement, we pursued insights from a post‐hoc analysis of two large, recent cardiovascular outcome trials. Our findings imply that, in a clinical setting, it is important to consider multiple factors. As such, our results are in line with findings from the randomized Steno 2 study. 6 , 13 , 14 The Steno 2 study (n = 160) combined lifestyle intervention targeting exercise, obesity, diet and smoking, as well as pharmacological therapy targeting glucose, lipids and blood pressure, including angiotensin‐converting enzyme inhibitors and aspirin, in persons with type 2 diabetes and microalbuminuria. 14 A 50% reduction in cardiovascular outcome of this multifactorial intervention was shown after 8 years 14 and a 20% reduction in risk of mortality after 13 years. 13 In addition, the benefit was sustained after 21 years of follow‐up, although all were treated similarly after the first 8 years. 6 , 15 , 16 , 17
The Japan Diabetes Optimal Integrated Treatment for three major risk factors of cardiovascular diseases (J‐DOIT3) study randomized 2542 persons with type 2 diabetes to receive conventional or intensive therapy for glucose, blood pressure and lipid control for a median of 8.5 years. 18 The study showed that intensified multifactorial intervention significantly reduced onset and progression of diabetic kidney disease, while the benefit on cardiovascular disease and mortality was less clear. 18 , 19
Epidemiological data support that the number of well‐controlled risk markers matters for the outcome. A recent cohort study including 271 174 persons with type 2 diabetes and 1 355 870 healthy controls followed up for a median of 5.7 years showed that the excess risk of cardiovascular disease seen in persons with type 2 diabetes, compared with the healthy controls, decreased stepwise for each risk marker within the target range at baseline. 7 The risk markers evaluated were HbA1c, LDL‐C, albuminuria, smoking and SBP, and thus overlap the risk markers we evaluated. 7
When we analysed the relative importance of the six risk markers in terms of R2 for each endpoint, HbA1c was the largest contributor for two of the four outcomes, followed by eGFR and UACR for one endpoint each. However, we did not observe that improvement in one risk marker constituted a major part of the explained relative risk, indicating that it was the multiple risk marker response that associated with improved outcome. There is extensive evidence that pharmacotherapies targeting only one of the six risk markers evaluated in this study reduce the risk of both micro‐ and macrovascular complications in type 2 diabetes. 3 , 4 , 20 , 21 , 22
The greater proportion of subjects treated with GLP‐1RAs in the groups with multiple improved risk markers indicates that treatment with GLP‐1RAs may improve these risk markers; however, 24.7% of the participants with four or more risk marker improvements were treated with placebo, showing how the risk markers in scope improved despite being in a placebo group.
Our study has several strengths, including the systematic, standardized measurement of risk markers and collection of outcomes and a large number of participants studied for a considerable length of time. One potential limitation is that two of the risk markers (UACR and eGFR) were also part of the renal outcome. However, we analysed improvement in the risk markers at year 1 and studied outcomes developed after year 1, and thereby mitigated the risk of reverse causation in the interpretation of the results.
This is a post‐hoc analysis with inherent risk of bias, hence the results should be interpreted with caution. We defined a clinically relevant improvement as a binary variable; on the one hand, this is an approach applicable to the clinic, but, on the other hand, the assessment could potentially be improved if we applied more advanced statistical models with the risk markers as continuous variables. The cut‐offs for clinically relevant risk marker improvement within 1 year were defined by the authors and agreed upon before any data analysis. Where possible, we tried to select previously used cut‐offs and, although chosen without data analysis, aiming at a potentially similar impact on outcome. We acknowledge that other cut‐offs could also be clinically relevant. For eGFR, a yearly reduction of approximately 1 ml/min/1.73 m2 could be expected from ageing, and it could be argued that a higher cut‐off value than the one chosen would better discriminate reduction in eGFR from real progression in kidney disease. However, we chose a cut‐off of 0, as eGFR was stable during 26 weeks of treatment with a GLP‐1RA in the PIONEER 5 study 23 and the LIRA‐RENAL study. 24 The mean level of kidney function in this cohort was in the normal range and, at these levels of renal function, the accuracy of the CKD‐EPI equation is limited and measures of eGFR based on cystatin C would potentially be more accurate.
The trial setting limits the generalizability of our findings. Because of the randomized study design, GLP‐1RA treatment was restricted to half of the population and improvement in the other half was because of initiation or intensification of other treatments or natural changes. Acknowledging that we pooled two randomized studies, our analysis was adjusted for treatment group (liraglutide/semaglutide and placebo), which also addresses the fact that more participants were on active treatment in the higher G classes (G0→G4). Therefore, our findings are not specifically related to the effects of GLP‐1RA treatment.
In conclusion, in a contemporary population with type 2 diabetes, improvements in two or more risk markers were associated with cardiovascular risk reduction, as compared with zero or one improved risk marker. This trend with increasing number of improvements and decreased risk was most noticeable for risk of nephropathy. Our findings stress the importance of multifactorial intervention in individuals with type 2 diabetes.
CONFLICT OF INTEREST
EHZ is now a Novo Nordisk A/S full‐time employee, but work related to this article was carried out when EHZ was employed full‐time by Steno Diabetes Center Copenhagen. BW, BJvS and SR are Novo Nordisk A/S full‐time employees and BW, BJvS, SR and TWH are Novo Nordisk shareholders. FP reports research grants from AstraZeneca and lecture fees from AstraZeneca, MSD, Janssen, Eli Lilly, Boehringer Ingelheim, Novo Nordisk A/S and Novartis, as well as being a consultant/advisory board member for AstraZeneca, Bayer, Amgen and MSD. PR has received honoraria from Steno Diabetes Center Copenhagen for advisory work, and for consultancy/education from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Merck, Mundipharma, Novo Nordisk, Vifor and Sanofi.
AUTHOR CONTRIBUTIONS
Emilie Hein Zobel, Tine W. Hansen, Frederik Persson, Bernt J. von Scholten, Benjamin Wolthers and Peter Rossing conceived and designed the research, analysed and interpreted the data; Søren Rasmussen performed the statistical analysis; Emilie Hein Zobel drafted the manuscript; Tine W. Hansen, Frederik Persson, Bernt J. von Scholten, Benjamin Wolthers and Peter Rossing critically revised the manuscript for key intellectual content; all authors approved the final version of the manuscript. Emilie Hein Zobel is the paper's guarantor, had full access to the data and is responsible for the integrity of the work as a whole.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14578.
Supporting information
Table S1 Baseline characteristics of the participants from the LEADER trial by risk marker improvement subgroups
Table S2 Baseline characteristics of the participants from the SUSTAIN 6 trial by risk marker improvement subgroups
Figure S1 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes in the LEADER trial
Figure S2 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes in the SUSTAIN 6 trial
Figure S3 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (liraglutide/semaglutide treatment only)
Figure S4 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (placebo treatment only)
Figure S5 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (with missing response data imputed)
Figure S6 Relative importance of risk markers for outcomes in the LEADER and SUSTAIN 6 trials
ACKNOWLEDGEMENTS
Data contained within this manuscript was accepted as an abstract at the European Renal Association ‐ European Dialysis and Transplant Association congress, 5‐8 June 2021. Medical writing support was provided by Brendan Deeney, BSc, and editorial assistance provided by Izabel James, MBBS, both of Ashfield MedComms, an Ashfield Health company, funded by Novo Nordisk A/S. Role of the sponsor: this study was supported by Novo Nordisk, which also had a role in the design, analysis and reporting of the trials.
Zobel EH, von Scholten BJ, Hansen TW, et al. The importance of addressing multiple risk markers in type 2 diabetes: Results from the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2022;24(2):281‐288. doi: 10.1111/dom.14578
Funding information This analysis was funded by Novo Nordisk A/S.
DATA AVAILABILITY STATEMENT
The subject level analysis data sets for the research presented in this publication are available from the corresponding author on reasonable request.
REFERENCES
- 1. Rawshani A, Rawshani A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;377:300‐301. [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703‐713. [PMC free article] [PubMed] [Google Scholar]
- 3. Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian simvastatin survival study (4S). Diabetes Care. 1997;20:614‐620. [DOI] [PubMed] [Google Scholar]
- 4. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685‐696. [DOI] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 6. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59:2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633‐644. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 10. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham heart study. Circulation. 2001;103:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 11. Heller G. A measure of explained risk in the proportional hazards model. Biostatistics. 2012;13:315‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zobel EH, von Scholten BJ, Hansen TW, et al. The importance of addressing multiple risk markers in type 2 diabetes: results from the LEADER and SUSTAIN 6 trials. Nephrol Dial Transplant. 2021;36(Suppl 1). 10.1093/ndt/gfab144.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580‐591. [DOI] [PubMed] [Google Scholar]
- 14. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383‐393. [DOI] [PubMed] [Google Scholar]
- 15. Oellgaard J, Gaede P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long‐term renal benefits. Kidney Int. 2017;91:982‐988. [DOI] [PubMed] [Google Scholar]
- 16. Oellgaard J, Gaede P, Rossing P, et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow‐up in the randomised Steno‐2 study. Diabetologia. 2018;61:1724‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaede P, Oellgaard J, Kruuse C, Rossing P, Parving HH, Pedersen O. Beneficial impact of intensified multifactorial intervention on risk of stroke: outcome of 21 years of follow‐up in the randomised Steno‐2 study. Diabetologia. 2019;62:1575‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ueki K, Sasako T, Okazaki Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99:256‐266. [DOI] [PubMed] [Google Scholar]
- 19. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951‐964. [DOI] [PubMed] [Google Scholar]
- 20. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373:1765‐1772. [DOI] [PubMed] [Google Scholar]
- 21. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861‐869. [DOI] [PubMed] [Google Scholar]
- 22. Jun M, Turin TC, Woodward M, et al. Assessing the validity of surrogate outcomes for ESRD: a meta‐analysis. J Am Soc Nephrol. 2015;26:2289‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515‐527. [DOI] [PubMed] [Google Scholar]
- 24. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA‐RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222‐230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of the participants from the LEADER trial by risk marker improvement subgroups
Table S2 Baseline characteristics of the participants from the SUSTAIN 6 trial by risk marker improvement subgroups
Figure S1 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes in the LEADER trial
Figure S2 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes in the SUSTAIN 6 trial
Figure S3 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (liraglutide/semaglutide treatment only)
Figure S4 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (placebo treatment only)
Figure S5 Outcomes according to number of risk marker improvements† among persons with type 2 diabetes (with missing response data imputed)
Figure S6 Relative importance of risk markers for outcomes in the LEADER and SUSTAIN 6 trials
Data Availability Statement
The subject level analysis data sets for the research presented in this publication are available from the corresponding author on reasonable request.


