Abstract
Background
Immune checkpoint inhibitors (ICIs) have shown significant improvements in patients with advanced non–small cell lung cancer (NSCLC). One of the major issues with ICIs is determining the optimal treatment duration.
Methods
This multicenter, retrospective study analyzed clinical outcomes in patients with NSCLC who completed 2 years of ICI therapy or were treated for more than 6 months and then discontinued ICIs without disease progression at 11 medical centers in Korea between August 2017 and December 2020.
Results
Ninety‐six patients who completed 2 years of ICIs were reviewed. The median durations of treatment and follow‐up were 24.0 and 33.9 months, respectively. The objective response rate (ORR) was 85.4%. The median progression‐free survival (PFS) and overall survival (OS) periods were not reached. After completion, the PFS and OS rates were 81.1% and 96.4%, respectively, at 12 months. Forty‐three patients were identified who discontinued ICIs without disease progression: 26 (60.5%) for adverse events and 17 (39.5%) for other causes. The median durations of treatment and follow‐up were 10.5 and 21.2 months, respectively. The ORR was 90.7%. The median PFS and OS periods were not reached. After discontinuation, the PFS and OS rates were 71.0% and 90.0%, respectively, at 12 months.
Conclusions
A significantly high proportion of patients who completed 2 years of ICI therapy continued to experience long‐term PFS. Even if ICIs are discontinued after 6 months in patients without disease progression, they may achieve a durable response and facilitate long‐term survival.
Lay Summary
The optimal treatment duration for immune checkpoint inhibitors (ICIs) remains to be determined.
This study reports the long‐term outcomes of patients with non–small cell lung cancer who completed 2 years of ICI therapy or achieved a durable response after the discontinuation of ICIs without disease progression in real‐world practice.
A significantly high proportion of patients who completed 2 years of ICIs continued to experience long‐term progression‐free survival.
In addition, even if ICIs are discontinued after 6 months in patients without disease progression, they may achieve a durable response and facilitate long‐term survival.
Keywords: discontinuation, duration of treatment, immune checkpoint inhibitor, immunotherapy, long‐term survival, non–small cell lung cancer
Short abstract
A significantly high proportion of patients who complete 2 years of immune checkpoint inhibitors (ICIs) continue to experience long‐term progression‐free survival. Even if ICIs are discontinued after 6 months in patients without disease progression, they may achieve a durable response and facilitate long‐term survival.
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD‐1) or programmed cell death ligand 1 (PD‐L1) have shown significant improvements in progression‐free survival (PFS), overall survival (OS), and objective response rates (ORRs) in comparison with platinum doublet chemotherapy in patients with advanced non–small cell lung cancer (NSCLC) without oncogenic driver mutations. 1 , 2 , 3
However, the optimal treatment duration of ICIs remains to be determined. Previous clinical trials have generally evaluated the use of ICIs for up to 2 years or longer in responsive patients. Importantly, some patients persistently respond over time even after discontinuation of this therapy, regardless of the reason for discontinuation. Several underlying hypotheses can be considered, including the idea that, from a pharmacodynamic point of view, the receptor occupancy of PD‐1 molecules on circulation T cells continues for several months, and an adaptive immune response through memory T cells within the tumor microenvironment has developed. 4
Recently, long‐term follow‐up of clinical outcomes with ICIs for metastatic NSCLC has been reported, and some proportion of patients experience long‐term OS benefits. 5 , 6 , 7 , 8 , 9 In the KEYNOTE‐010 trial, 79 of 690 patients completed 2 years of pembrolizumab therapy, and their PFS rates 1 and 2 years after completion were 72.5% (95% confidence interval [CI], 59.9%‐81.8%) and 57.7% (95% CI, 41.2%‐71.0%), respectively. 6 Also, in the KEYNOTE‐024 trial, 39 patients completed 2 years of pembrolizumab therapy, and the majority of the patients (82%) were still alive at the point of data cutoff at approximately 5 years. 8 In contrast with these results, in the exploratory end point from the CheckMate 153 trial, which was a prospective trial associated with the optimal duration of ICI treatment, patients who were assessed after a 1‐year fixed‐duration course of nivolumab in terms of either partial response (PR) or stable disease (SD) were randomized to observation or continuous nivolumab. This study showed that the continuous group achieved favored results in terms of PFS (24.7 vs 9.4 months; hazard ratio [HR], 0.56; 95% CI, 0.37‐0.84) and OS (not reached vs 28.8 months; HR, 0.62; 95% CI, 0.42‐0.92). These results suggest that treatment in patients who are responsive to ICIs should be continued for more than 1 year. 10 However, because of the small number of patients who completed the maximum number of cycles with nivolumab or pembrolizumab, the optimal treatment duration of ICIs still remains to be elucidated.
Recently, several retrospective studies have reported that patients who discontinue ICIs without disease progression show long‐lasting responses over time even after discontinuation of their therapy, regardless of the reason for the discontinuation, for several tumors. 11 , 12 , 13 , 14 , 15 , 16 , 17 However, there are limited real‐world data for long‐term outcomes in patients with NSCLC who have completed 2 years of ICI therapy or have achieved a durable response after discontinuation without disease progression.
On the basis of these issues, we investigated long‐term outcomes in patients with NSCLC who completed 2 years of ICI therapy or achieved a durable response after the discontinuation of ICIs without disease progression to obtain real‐world data.
Materials and Methods
Patients
This retrospective, multicenter study (including 11 tertiary referral centers) was conducted by the lung cancer committee of the Korean Cancer Study Group. Patients with histologically confirmed advanced and/or metastatic NSCLC who completed 2 years of ICI therapy (pembrolizumab, nivolumab, or atezolizumab) or were treated for more than 6 months and then discontinued ICIs without disease progression between August 2017 and December 2020 were identified, and their medical records were reviewed. The 2‐year treatment cutoff was attributed to the reimbursement policy in Korea. This study was approved by the institutional review board of each institution as required, and the need for informed consent was waived because of the retrospective nature of this study.
Treatment and Outcome
Patients received ICIs according to the label after Food and Drug Administration approval. The following clinicopathologic characteristics were collected for all patients: age, sex, Eastern Cooperative Oncology Group performance status, smoking history, histology, brain metastasis, epidermal growth factor receptor mutation status, anaplastic lymphoma kinase translocation status, PD‐L1 immunohistochemistry status, type of ICI, treatment line of ICI, treatment for adverse events (AEs), and causes of discontinuation of ICIs. All patients were evaluated radiologically for the clinical outcomes of ORR, PFS, and OS according to the Response Evaluation Criteria in Solid Tumors (version 1.1) through computed tomography or magnetic resonance imaging, and AEs were graded with the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.03) by the investigators.
Statistical Analyses
The cutoff date for data collection was December 31, 2020. Descriptive statistics were used to summarize patient and tumor characteristics. Survival analyses were performed with the Kaplan‐Meier method, and differences were analyzed with the log‐rank test. HRs and corresponding 95% CIs were calculated with the Cox proportional hazards model. PFS was calculated from the start of ICI treatment to the point of either disease progression or death from any cause. OS was calculated from the start of ICI therapy to the point of death from any cause. All P values were 2‐sided, and statistical significance was set at P < .05. Statistical analysis was performed with the Statistical Package for the Social Sciences software program (version 25; IBM Corporation, Armonk, New York).
Results
Patient Characteristics
We retrospectively reviewed 139 patients with advanced NSCLC (including 96 patients who completed 2 years of ICI therapy and 43 patients who were treated for more than 6 months and then discontinued ICIs without disease progression) from 11 tertiary referral centers in the Republic of Korea. ICI treatment was initiated between August 2017 and December 2020. The baseline characteristics of the patients at treatment initiation are summarized in Table 1.
TABLE 1.
Baseline Characteristics of Patients
| Patient Characteristic | Completed 2 y of ICIs (n = 96) | Discontinued ICIs Early (n = 43) |
|---|---|---|
| Age, median (range), y | 65 (38‐87) | 69.5 (43‐86) |
| Age ≥ 65 y, No. (%) | 49 (51.0) | 30 (69.8) |
| Sex, No. (%) | ||
| Male | 82 (85.4) | 40 (93.0) |
| Female | 14 (14.6) | 3 (7.0) |
| ECOG PS, No. (%) | ||
| 0 | 19 (19.8) | 12 (27.9) |
| 1 | 71 (74.0) | 28 (65.1) |
| ≥2 | 6 (6.2) | 3 (7.0) |
| Smoking history, No. (%) | ||
| Current or former | 69 (71.9) | 37 (86.0) |
| Never | 27 (28.1) | 5 (11.6) |
| Unknown | 0 (0.0) | 1 (2.3) |
| Histology, No. (%) | ||
| Squamous | 20 (20.8) | 12 (27.9) |
| Nonsquamous | 65 (67.7) | 24 (55.8) |
| Other | 9 (9.4) | 7 (16.3) |
| Unknown | 2 (2.1) | 0 (0.0) |
| Brain metastasis, No. (%) | 27 (28.1) | 8 (18.6) |
| EGFR mutation status, No. (%) | ||
| Mutant | 4 (4.2) | 2 (4.7) |
| Wild type | 92 (95.8) | 40 (93.0) |
| Unknown | 0 (0.0) | 1 (2.3) |
| ALK translocation present, No. (%) | ||
| Yes | 2 (2.1) | 0 (0.0) |
| No | 94 (97.9) | 41 (95.3) |
| Unknown | 0 (0.0) | 2 (4.3) |
| PD‐L1 status, No. (%) | ||
| ≥50% | 94 (97.9) | 38 (88.4) |
| 1%‐49% | 2 (2.1) | 3 (7.0) |
| <1% | 0 (0.0) | 2 (4.7) |
| Type of immune checkpoint inhibitor, No. (%) | ||
| Pembrolizumab | 61 (63.5) | 24 (55.8) |
| Nivolumab | 29 (30.2) | 14 (32.6) |
| Atezolizumab | 4 (4.2) | 3 (7.0) |
| Pembrolizumab + chemotherapy | 0 (0.0) | 2 (4.7) |
| Other | 2 (2.1) | 0 (0.0) |
| Lines of therapy for ICIs, No. (%) | ||
| 1 | 10 (10.4) | 6 (14.0) |
| 2 | 61 (63.5) | 30 (69.8) |
| ≥3 | 25 (26.1) | 7 (16.2) |
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; PD‐L1, programmed cell death ligand 1; PS, performance status.
For the 96 patients who completed 2 years of ICIs, the median age was 65 years (range, 38‐87 years). Sixty‐nine patients (71.9%) had a history of smoking, 90 patients (93.8%) had a good Eastern Cooperative Oncology Group performance status (defined as 0 or 1 point), 65 patients (67.7%) had nonsquamous cell carcinoma, 27 patients (28.1%) had brain metastasis, and most patients (97.9%) had high PD‐L1 expression (≥50%). ICIs were administered as a first‐line treatment to 10 patients (10.4%), as a second‐line treatment to 61 patients (63.5%), and as a later line of treatment to 25 patients (26.1%). Sixty‐one patients were treated with pembrolizumab, 29 patients were treated with nivolumab, 4 patients were treated with atezolizumab, and 2 patients were treated with other medications (1 patient with quavonlimab and 1 patient with pembrolizumab and quavonlimab).
Among the 43 patients who achieved a durable response but discontinued ICIs without disease progression, the median age was 69.5 years (range, 43‐86 years). Thirty‐seven patients (86.0%) had a history of smoking, 40 patients (93.0%) had a good Eastern Cooperative Oncology Group performance status (0 or 1 point), 24 patients (55.8%) had nonsquamous cell carcinoma, 8 patients (18.6%) had brain metastasis, and most patients (88.4%) had high PD‐L1 expression (≥50%). ICIs were administered as a first‐line treatment to 6 patients (14.0%), as a second‐line treatment to 30 patients (69.8%), and as a later line of treatment to 7 patients (16.2%). Twenty‐four patients were treated with pembrolizumab, 14 patients were treated with nivolumab, 3 patients were treated with atezolizumab, and 2 patients were treated with pembrolizumab plus cytotoxic chemotherapy.
Treatment Outcomes in Patients Who Completed 2 Years of ICI Therapy
The median duration of follow‐up after treatment initiation was 33.9 months (range, 24.2‐40.8 months). Among the 96 patients who completed 2 years of ICI therapy, 85.4% (82 of 96) experienced a response according to investigator assessments (Fig. 1A). The best‐response distribution was as follows: a complete response (CR) in 6 patients (6.3%), a PR in 76 patients (79.2%), and SD in 14 patients (14.6%).
Figure 1.
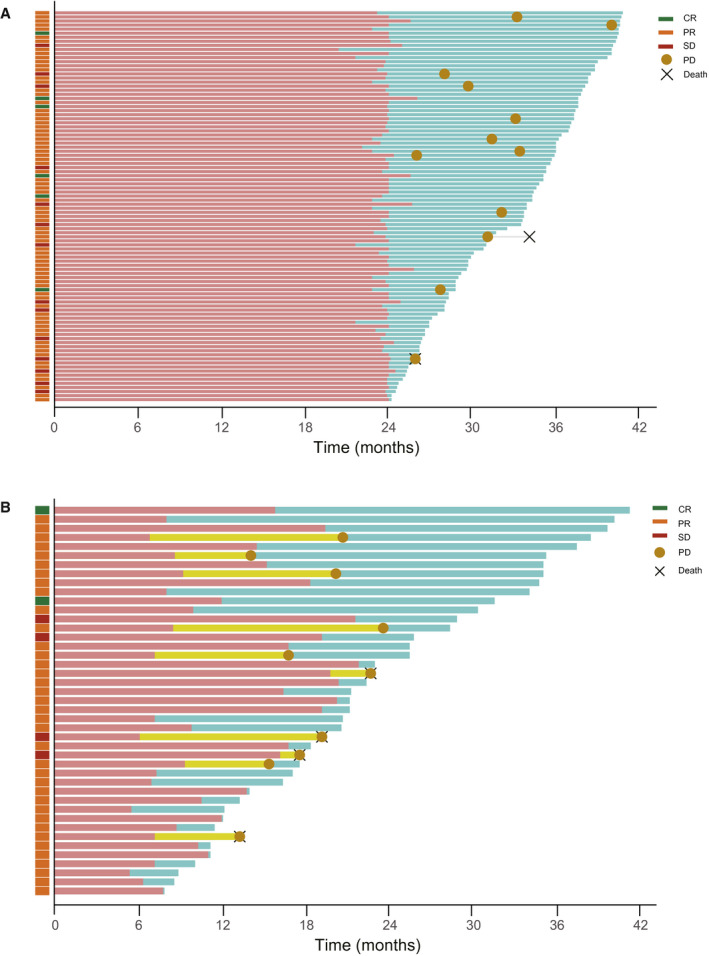
Swimmer plots indicating the duration of ICI treatment and follow‐up, progression‐free survival, and overall survival for patients who (A) completed 2 years of ICI therapy and (B) discontinued ICIs without disease progression. The light red bars indicate the duration of ICI treatment, the light teal bars indicate the follow‐up duration, and the light yellow bars indicate the time from the discontinuation of ICIs to disease progression. CR indicates complete response; ICI, immune checkpoint inhibitor; PD, progressive disease; PR, partial response; SD, stable disease.
The median OS was not reached, and the estimated OS rate at 12 months from the discontinuation of ICIs was 96.4% (Fig. 2A). At the data cutoff, 94 of the 96 patients (97.9%) were still alive. The median PFS was not reached, and the estimated PFS rate at 12 months from the discontinuation of ICIs was 81.1% (Fig. 2B). At the data cutoff, 84 of the 96 patients (87.5%) were still alive without disease progression. The median PFS according to the response was not reached for CR and PR and was not reached for SD. Patients obtaining an ORR had significantly longer PFS than those who achieved SD (P = .044; Fig. 2C). From discontinuation, the PFS and OS rates according to the response at 12 months were 83.7% and 97.4%, respectively, for CR and PR and 63.0% and 90.0%, respectively, for SD. The median PFS according to the line of treatment was not reached for first‐line therapy, second‐line therapy, or a later line of therapy (P = .407; Fig. 2D). There were no significant differences in PFS according to the line of treatment (P = .407), type of ICI (P = .465), or PD‐L1 status (P = .616). Also, there were no significant differences in OS according to the ORR (P = .076), line of treatment (P = .786), type of ICI (P = .954), or PD‐L1 status (P = .824).
Figure 2.
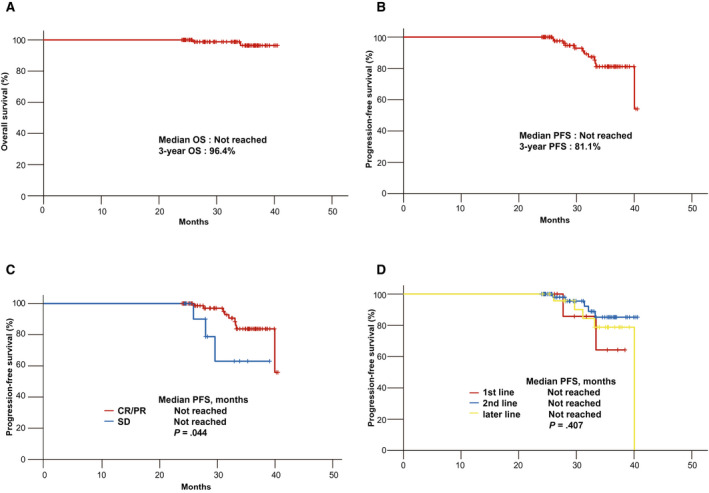
Kaplan‐Meier curves of a survival analysis of patients who completed 2 years of ICI therapy: (A) OS, (B) PFS, (C) PFS according to the response to treatment, and (D) PFS according to the line of treatment. CR indicates complete response; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Treatment Outcomes in Patients Who Discontinued ICIs Without Disease Progression
We analyzed 43 patients with NSCLC who were treated for more than 6 months but discontinued ICIs without disease progression. The most common causes of discontinuation were AEs (60.5%), which were followed by patient refusal (n = 6; 14.0%), follow‐up loss (n = 7; 16.3%), and financial burden (n = 4; 9.3%). The median treatment duration was 10.5 months, and the median follow‐up duration was 21.2 months. Overall, 90.7% of the patients (39 of 43) experienced a response, with a best‐response distribution of CR in 2 patients (4.7%), PR in 37 patients (86.0%), and SD in 4 patients (9.3%; Fig. 1B). Meanwhile, the best response during ICI treatment in patients who developed PD was CR in 0 patients (0 of 2; 0%), PR in 8 patients (8 of 37; 21.6%), and SD in 2 patients (2 of 4; 50%). Two patients achieving CR were treated with pembrolizumab as a second‐line treatment for 12 and 16 months, respectively, and experienced a durable response even after the discontinuation of ICIs without disease progression until the data cutoff at approximately 2 years (Table 2).
TABLE 2.
Treatment Duration and Outcomes According to the Best Response Among Patients Who Discontinued ICIs for a Cause Other Than Disease Progression
| Best Objective Response | No. of Patients | No. of Patients Diagnosed With PD | Follow‐Up Time From ICI Initiation, Median (Range), mo | Time on ICIs, Median (Range), mo | Follow‐Up Time After Discontinuing ICIs, Median (Range), mo |
|---|---|---|---|---|---|
| All | 43 (100) | 10 (23.3) | 21.2 (7.9‐41.3) | 10.5 (5.5‐22.2) | 7.2 (0‐32.1) |
| CR | 2 (4.7) | 0 (0.0) | 36.5 (31.6‐41.3) | 14.0 (12.0‐16.1) | 22.4 (19.6‐25.3) |
| PR | 37 (86.0) | 8 (21.6) | 21.2 (7.9‐40.2) | 10.0 (5.5‐22.2) | 6.6 (0‐32.1) |
| SD | 4 (9.3) | 2 (50.0) | 22.5 (17.6‐28.9) | 18.3 (6.2‐21.7) | 6.5 (1.1‐12.9) |
Abbreviations: CR, complete response; ICI, immune checkpoint inhibitor; PD, progressive disease; PR, partial response; SD, stable disease.
The median OS was not reached (Fig. 3A), and the estimated OS rate 12 months after the discontinuation of ICIs was 90.0%. At the data cutoff, 39 of the 43 patients (90.7%) were alive. The median PFS was not reached (Fig. 3B), and the estimated PFS rate 12 months after the discontinuation of ICIs was 71.0%. At the data cutoff, 33 of the 43 patients (76.7%) remained alive without progression. The median PFS according to the response to treatment was not reached for CR and PR and was not reached for SD (P = .499; Fig. 3C); moreover, the median OS according to the response to treatment was not reached for CR and PR and was not reached for SD (P = .019; Fig. 3D). The median PFS according to the cause of treatment discontinuation was not reached for AEs and was not reached for other causes (P = .124; Fig. 3E). The median PFS according to the duration of treatment was 20.7 months (95% CI, 15.7‐25.7 months) for more than 6 but less than 12 months, was not reached for more than 12 but less than 18 months, and was not reached for more than 18 but less than 24 months (P = .040; Fig. 3F). However, from the discontinuation of ICIs, the median PFS according to the response was not reached for CR and PR and was not reached for SD (P = .145; Fig. 4A); moreover, the median OS according to the response was not reached for CR and PR and was not reached for SD (P = .008; Fig. 4B). From the discontinuation of ICIs, the median PFS according to the cause of treatment discontinuation was not reached for toxicity and was not reached for other causes (P = .670; Fig. 4C); moreover, the median OS according to the cause of treatment discontinuation was not reached for toxicity and was not reached for other causes (P = .801; Fig. 4D). There were no significant differences in PFS according to the response to treatment (P = .499), cause of treatment discontinuation (P = .124), type of ICI (P = .701), line of treatment (P = .834), or PD‐L1 status (P = .594). Also, there was no significant difference in OS according to the line of treatment (P = .504), type of ICI (P = .814), PD‐L1 status (P = .145), or duration of treatment (P = .963).
Figure 3.
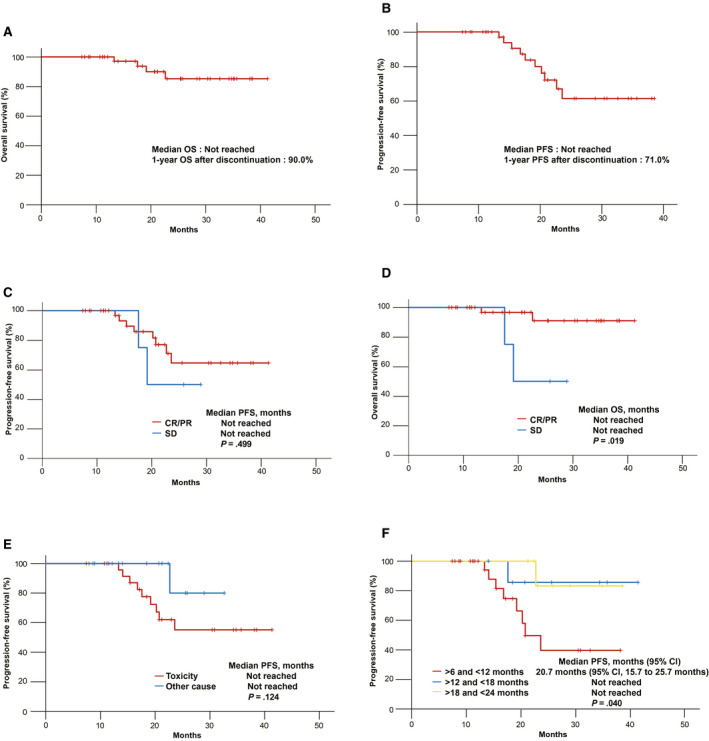
Kaplan‐Meier curves of a survival analysis of patients who discontinued ICIs without disease progression: (A) OS, (B) PFS, (C) PFS according to the response to treatment, (D) OS according to the response to treatment, (E) PFS according to the cause of treatment discontinuation, and (F) PFS according to the duration of treatment. CI indicates confidence interval; CR, complete response; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 4.
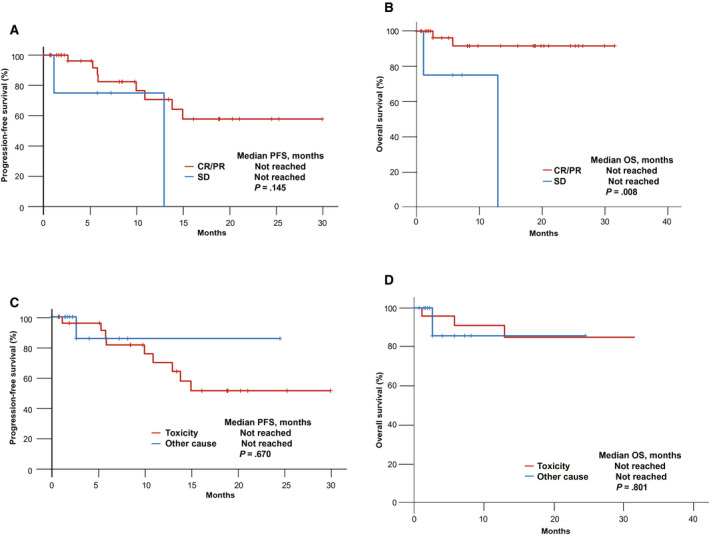
Kaplan‐Meier curves of a survival analysis from treatment discontinuation in patients who discontinued ICIs without disease progression: (A) PFS according to the response to treatment, (B) OS according to the response to treatment, (C) PFS according to the cause of treatment discontinuation, and (D) OS according to the cause of treatment discontinuation. CR indicates complete response; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Among the 26 patients who discontinued ICI treatment on account of AEs, treatment‐related grade 3 to 5 AEs occurred in 13 (50%), including 1 patient who died of pneumonitis; the recorded AEs included pneumonitis (n = 4), skin toxicity (n = 3), colitis (n = 1), hepatitis (n = 1), hypophysitis (n = 1), and others (n = 3). No new safety signals have been identified since prior results. Overall, 92.3% of the patients (24 of 26) experienced a response, and the best‐response distribution was CR in 2 patients (7.7%), PR in 22 patients (84.6%), and SD in 2 patients (7.7%); 30.8% of the patients (8 of 26) had AEs between 1 and 2 years.
Discussion
ICIs have demonstrated clinically meaningful improvements in patients with advanced NSCLC without oncogenic driver mutations. However, one of the major issues with ICIs involves determining the treatment duration. In general, oncologists prefer to continue ICIs until progression or toxicity occurs to maximize the efficacy of ICIs. However, whether prolonged ICI treatment leads to longer survival times is still unknown. Furthermore, high costs and inconvenience related to the long‐term use of ICIs remain major issues. Therefore, the principle of determining the treatment duration is the best balance of benefits and risks.
In this study, among 96 patients who completed 2 years of ICIs with long‐term follow‐up, the median PFS and OS were not reached, whereas after ICI discontinuation, the PFS and OS rates were 81.1% and 96.4% at 12 months; this indicated that a significantly high proportion of patients who completed 2 years of ICI treatment experienced long‐term PFS. In the KEYNOTE‐010 trial, the 1‐year PFS and OS rates after the completion of 2 years of pembrolizumab therapy were 72.5% (95% CI, 59.9%‐81.8%) and 98.7% (95% CI, 91.1%‐99.8%), respectively. Also, in the KEYNOTE‐024 trial, at the data cutoff, the majority of the patients (82%) who completed 2 years of pembrolizumab therapy remained alive approximately 5 years later. Because most of the patients who discontinued ICIs after 2 years of treatment experienced disease progression within the first 12 months, continuation of ICI treatment might be beneficial as previously observed in the CheckMate 153 study.
It is notable that, among 43 patients with NSCLC treated for more than 6 months with subsequent discontinuation of ICIs without disease progression, the median PFS and OS were not reached. From ICI discontinuation, the PFS and OS rates were 71.0% and 90.7%, respectively, at 12 months. When we consider that 90.7% of the patients achieved a response in this cohort, these results suggest that once the patients achieved a durable response at more than 6 months, a substantial number of them experienced long‐term PFS despite discontinuing ICIs. Intriguingly, 60.4% of the patients who discontinued ICIs experienced immune‐related AEs. Recently, a post hoc analysis of CheckMate 227 revealed that approximately half of the responders who had treatment‐related AEs leading to discontinuation maintained their responses for more than 3 years after treatment discontinuation. Similarly, in a post hoc analysis of CheckMate 9LA, it was found that 56% of responders who had a treatment‐related AE leading to discontinuation maintained their responses for more than 1 year after treatment discontinuation. 18 , 19
By subgroup analysis, we found that the risk of disease progression after the completion of 2 years of ICI therapy was significantly associated with ORR. The median PFS was significantly longer for CR and PR than SD (P = .044; Fig. 2C). This finding was also observed in a cohort of patients who discontinued treatment without disease progression. The median OS was significantly longer for CR and PR than SD (P = .019; Fig. 3D). Our results are consistent with previous pooled analyses of 4‐year survival rates with nivolumab and the KEYNOTE 001 trial, 5 , 7 which demonstrated that patients with CR and PR experienced long‐term survival. In addition, patients who were treated for less than 12 months had significantly shorter PFS (26.3 months; 95% CI, 20.9‐31.7 months) than those who were treated for more than 12 months (38.1 months; 95% CI, 33.9‐42.3 months; P = .011), and this was consistent with the CheckMate 153 results. Therefore, we suggest that more than 1 year of treatment in patients who are responsive to ICIs should be continued, especially if patients experience mild AEs. Also, we compared the characteristics of patients who had disease progression and patients who did not have disease progression. However, we did not find any significant difference between the 2 groups in baseline patients characteristics (Supporting Table 1). This study has several limitations. First, this was a retrospective study with a small sample size, which led to bias. Second, only Asian patients with NSCLC were analyzed in this study, and this limits its generalizability because of differences in molecular profiles and clinical features between Western and Eastern patients with NSCLC. Third, although immunotherapy monotherapy and immunotherapy plus chemotherapy received US Food and Drug Administration approval for the frontline treatment of advanced NSCLC in 2021, the majority of the patients in this study were treated at a second or later line because of the reimbursement policy in Korea; this limits its applicability to today's practice with a shifting standard of care. Nevertheless, to the best of our knowledge, this study is the largest data set to date to specifically address the outcomes of patients with advanced NSCLC who have completed 2 years of ICI therapy or have achieved a durable response but have discontinued ICIs without disease progression in real‐world practice.
In conclusion, in these real‐world data, we found that a significantly high proportion of patients who completed 2 years of ICIs continued to experience long‐term PFS. Even if ICIs are discontinued after 6 months in patients without disease progression, they may achieve a durable response and facilitate long‐term survival.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
Dong‐Wan Kim reports research funding from Alpha Biopharma, Amgen, AstraZeneca/Medimmune, Boehringer‐Ingelheim, Daiichi‐Sankyo, Hanmi, Janssen, Merus, Mirati Therapeutics, MSD, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, Chong Keun Dang, Bridge BioTherapeutics, and GSK and travel and accommodation support for advisory board meeting attendance from Amgen and Daiichi‐Sankyo. Youngjoo Lee reports participation on boards for Roche Korea and Yuhan Corporation. Hee Kyung Ahn reports payments or honoraria from Yuhan, BMS, MSD, AstraZeneca, Roche, Lilly, Pfizer, and Menarini. Myung‐Ju Ahn reports consulting fees from Alpha Pharmaceuticals and payments or honoraria from MSD, Merck, Lilly, AstraZeneca, Takeda, Yuhan, and Roche. The other authors made no disclosures.
Author Contributions
Hongsik Kim: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. Dong‐Wan Kim: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Miso Kim: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Youngjoo Lee: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Hee Kyung Ahn: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Jang Ho Cho: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Il Hwan Kim: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Yun‐Gyoo Lee: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Seong‐Hoon Shin: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Song Ee Park: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Jiyoon Jung: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Eun Joo Kang: Data curation, formal analysis, investigation, methodology, project administration, resources, supervision, and writing–review and editing. Myung‐Ju Ahn: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing.
Supporting information
Table S1
Kim H, Kim D‐W, Kim M, Lee Y, Ahn HK, Cho JH, Kim IH, Lee Y‐G, Shin S‐H, Park SE, Jung J, Kang EJ, Ahn M‐J. Long‐term outcomes in patients with advanced and/or metastatic non–small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: Multicenter, real‐world data (KCSG LU20‐11). Cancer.2022. 10.1002/cncr.33984
The first 2 authors contributed equally to this article.
References
- 1. Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non–small‐cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38:1608‐1632. [DOI] [PubMed] [Google Scholar]
- 2. Planchard D, Popat S, Kerr K, et al. Metastatic non–small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(suppl 4):iv192‐iv237. [DOI] [PubMed] [Google Scholar]
- 3. Remon J, Passiglia F, Ahn MJ, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15:914‐947. [DOI] [PubMed] [Google Scholar]
- 4. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single‐agent anti–programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167‐3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four‐year survival with nivolumab in patients with previously treated advanced non–small‐cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20:1395‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herbst RS, Garon EB, Kim DW, et al. Long‐term outcomes and retreatment among patients with previously treated, programmed death‐ligand 1 positive, advanced nonsmall‐cell lung cancer in the KEYNOTE‐010 study. J Clin Oncol. 2020;38:1580‐1590. [DOI] [PubMed] [Google Scholar]
- 7. Garon EB, Hellmann MD, Rizvi NA, et al. Five‐year overall survival for patients with advanced nonsmall‐cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE‐001 study. J Clin Oncol. 2019;37:2518‐2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Five‐year outcomes with pembrolizumab versus chemotherapy for metastatic non–small‐cell lung cancer with PD‐L1 tumor proportion score >/= 50. J Clin Oncol. 2021;39:2339‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nadal E, Massuti B, Domine M, Garcia‐Campelo R, Cobo M, Felip E. Immunotherapy with checkpoint inhibitors in non–small cell lung cancer: insights from long‐term survivors. Cancer Immunol Immunother. 2019;68:341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waterhouse DM, Garon EB, Chandler J, et al. Continuous versus 1‐year fixed‐duration nivolumab in previously treated advanced non–small‐cell lung cancer: CheckMate 153. J Clin Oncol. 2020;38:3863‐3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimura H, Araya T, Yoneda T, et al. Long‐lasting responses after discontinuation of nivolumab treatment for reasons other than tumor progression in patients with previously treated, advanced non–small cell lung cancer. Cancer Commun (Lond). 2019;39:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tachihara M, Negoro S, Inoue T, et al. Efficacy of anti–PD‐1/PD‐L1 antibodies after discontinuation due to adverse events in non–small cell lung cancer patients (HANSHIN 0316). BMC Cancer. 2018;18:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yilmaz M, Guven Mese S. Durable response after discontinuation of nivolumab therapy in the absence of disease progression or toxicity with two advanced NSCLC patients. J Oncol Pharm Pract. 2020;26:761‐767. [DOI] [PubMed] [Google Scholar]
- 14. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti–PD‐1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30:1154‐1161. [DOI] [PubMed] [Google Scholar]
- 16. Iivanainen S, Koivunen JP. Early PD‐1 therapy discontinuation in responding metastatic cancer patients. Oncology. 2019;96:125‐131. [DOI] [PubMed] [Google Scholar]
- 17. McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Ciuleanu TE, Cobo M, et al. First‐line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non–small cell lung cancer (NSCLC): two‐year update from CheckMate 9LA. J Clin Oncol. 2021;39:9000. [Google Scholar]
- 19. Paz‐Ares LG, Ciuleanu TE, Lee JS, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first‐line (1L) treatment for advanced non–small cell lung cancer (NSCLC): 4‐year update from CheckMate 227. J Clin Oncol. 2021;39:9016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


