Abstract
When a combination of hydrogen peroxide and hypochlorite was used to surface sterilize rice seeds, a 102- to 104-fold decrease in CFU was observed during the first 15 h after inoculation of the rice rhizosphere organism Burkholderia vietnamiensis TVV75. This artifact could not be eliminated simply by rinsing the seeds, even thoroughly, with sterile distilled water. When growth resumed, a significant increase in the frequency of rifampin- and nalidixic acid-resistant mutants in the population was observed compared to the control without seeds. This phenomenon was a specific effect of hypochlorite; it was not observed with hydrogen peroxide alone. It was also not observed when the effect of hypochlorite was counteracted by sodium thiosulfate. We hypothesized that the hypochlorite used for disinfection reacted with the rice seed surface, forming a chlorine cover which was not removed by rinsing and generated mutagenic chloramines. We studied a set of rifampin- and nalidixic acid-resistant mutants obtained after seed surface sterilization. The corresponding rpoB and gyrA genes were amplified and sequenced to characterize the induced mutations. The mutations in five of seven nalidixic acid-resistant mutants and all of the rifampin-resistant mutants studied were found to correspond to single amino acid substitutions. Hypochlorite surface sterilization can thus be a source of artifacts when the initial bacterial colonization of a plant is studied.
The use of hypochlorite salts for disinfection dates back to the mid-18th century. Since that time, chlorination has been the most widely used bactericidal treatment for conventional disinfection of municipal drinking water for prevention of epidemic diseases such as cholera and typhoid, and it is still the most widely used method for disinfecting water (18). Hypochlorite is also routinely used as a sanitizer for domestic uses, as well as in food-processing plants to remove surface contaminants which can alter food quality or lead to food-borne diseases (2, 3, 23, 29).
Hypochlorite is known to be a very effective to killer of bacteria; even micromolar concentrations are enough to reduce bacterial populations significantly (27). However, little is known about the exact mechanisms of bacterial killing by this sanitizer. When diluted in water, the hypochlorite salts used [NaOCl, Ca(OCl)2, LiOCl, and KOCl] lead to formation of HOCl, whose concentration is correlated with bactericidal activity (27). Bacterial killing by HOCl may be due at least in part to lethal DNA damage (13, 42). However, HOCl itself is so reactive that it is unlikely to penetrate cells and reach the DNA; rather, it seems that the bactericidal activity is due to formation of secondary products, as hypochlorous acid reacts avidly with a wide variety of subcellular compounds (membranes, proteins, etc.) (10, 18). In particular, HOCl reacts with NH4+ and organic amines to form highly toxic chloramines, which also are strong oxidizing and chlorinating compounds and could be the actual killing agents. These chloramines are very diffusible species that can enter cells through membranes and react with intracellular components, including DNA (10, 32, 33, 35).
In studies of plant-bacterium interactions, it is sometime necessary to use monoxenic models in which a surface-sterilized plant is associated with a bacterial strain. Oxidative agents like household bleach, which is composed of sodium hypochlorite, are the agents most commonly used for sterilization. Seeds are rinsed before bacterial inoculation. In this paradoxical situation the sanitizer is expected to be strong enough to kill all contaminants and mild enough to allow subsequent colonization by an inoculated microorganism.
In the course of a study of interactions between rice and the plant-growth-promoting bacterium Burkholderia vietnamiensis TVV75 (37), we addressed the question of whether seed surface sterilization by oxidative sanitizers is innocuous. We tested a routinely used treatment in which H2O2 and hypochlorite are combined and have both a bactericidal effect and a fungicidal effect but no effect on caryopse germination (29).
Effects were characterized on the basis of the behavior of inoculated cells at different levels, including the ability to grow (CFU counts), the physiological state of survivors (respiration), and the genotoxic effect (mutagenesis). We quantified two mutations, one conferring resistance to rifampin and one conferring resistance to nalidixic acid, and we studied the following classical targets of these mutations: for rifampin resistance, a short central conserved region of rpoB which codes for the RNA polymerase β subunit (7, 19, 34, 41); and for nalidixic acid resistance, a small region at the 5′ end of gyrA, the so-called quinolone resistance-determining region (QRDR) (this region is highly conserved in all known bacterial gyrases, and Nalr mutations affect amino acid residues equivalent to Ala-67 through Gln-106 encoded by the Escherichia coli gene [44]).
In this study, we found that using hypochlorite for rice seed disinfection actually strongly stresses the bacterial inoculum and that the surviving bacteria have an increased mutation burden.
MATERIALS AND METHODS
Reagents.
All of the chemicals used were analytical grade. Nalidixic acid, rifampin, and 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT) were purchased from Sigma-Aldrich Chemie GmbH, Steinheim, Germany. H2O2 was obtained from Prolabo, Fontenay sous Bois, France; sodium thiosulfate was obtained from Merck, Darmstadt, Germany; malachite green crystals were obtained from Réactifs RAZ, Clichy, France; and calcium hypochlorite (∼35% available Cl) was purchased from BDH Laboratory Supplies, Poole, England.
Rice seed disinfection.
To facilitate contact of bacteria with the disinfectant, rice seeds (cv. Cigalon) were dehusked before sterilization. Seeds (200 seeds per 100 ml) were immersed twice for 10 min in a 10% H2O2 solution and then for 1 h in a 1% filtered calcium hypochlorite solution, prepared extemporaneously, with agitation. After each immersion, the seeds were rinsed five times with demineralized sterile water (5 min per rinse). At the end of the disinfection process, the seeds were rinsed 10 times with demineralized sterile water. In one control the hypochlorite step was omitted. In another control a 2% sodium thiosulfate solution was used instead of five water rinses in the middle of the last rinse procedure in order to remove the chlorine cover left on the seed surface by hypochlorite disinfection (16). The rice seeds were then blotted on sterile Whatman filter paper sheets before they were placed into the wells of 12-well culture plates (10 seeds per well). There were also control treatments in which seeds were killed before disinfection by heating them in a dry oven at 100°C for 30 min.
Bacterial inoculation and mutant frequency measurement.
Bacteria were inoculated immediately into the 12-well culture plates. The type strain of B. vietnamiensis, TVV75 (= LMG 10929), was used. Cells were first cultivated in liquid Luria-Bertani (LB) medium for 18 h. A 10−2 dilution was then transferred to M9 liquid medium (24) supplemented with 0.1% yeast extract. After 48 h of cultivation (optical density at 600 nm, 1), cells were diluted in yeast extract-supplemented M9 medium to obtain a concentration of 106 CFU/ml. Aliquots (800 μl) were then inoculated into the wells of the culture plates containing disinfected rice seeds. Controls were prepared without seeds in the wells or with heat-killed rice seeds. For 2 days, bacterial growth was monitored by periodically counting the CFU on LB medium plates, and the frequency of resistance was measured by plating samples on LB medium plates supplemented with rifampin (50 μg/ml) or nalidixic acid (150 μg/ml).
Bacterial viability measurements.
The percentage of respiring cells was monitored with a microscope by using INT, a phenyltetrazolium chloride derivative which forms dense dark red crystals when it is reduced by metabolic electrons (45); 70 μl of a 0.2% INT solution was added to the contents of a well (700 μl of cell culture), and after 1 h of incubation in the dark, 14 μl of a 37% formaldehyde solution was added. After centrifugation, smears were prepared on slides and counterstained with a 0.05% malachite green solution. In all cases, cells were counted in five random fields with a microscope (magnification, ×1,200).
Competition assays.
The fitness of mutants obtained following hypochlorite treatment of seeds was evaluated by coculturing the mutants with the wild type. Strains were first cultivated in liquid LB medium for 18 h and then diluted (1/100) in modified M9 medium, as described above. After 48 h of cultivation (optical density at 600 nm, 1), cultures were diluted in modified M9 medium to obtain a concentration of 5 × 104 CFU/ml. In each well, 400 μl of a mutant and 400 μl of the wild-type strain were cocultured. Total bacterial growth was monitored by counting CFU on LB medium alone and CFU of the antibiotic-resistant strain on LB medium supplemented with rifampin (50 μg/ml) or nalidixic acid (150 μg/ml). Wild-type strain growth was then calculated by subtracting the number of CFU that developed on antibiotic-supplemented plates from the total number of CFU on LB medium plates.
DNA primers used for Rifr and Nalr mutant analysis.
Rifr and Nalr mutants were analyzed by amplifying the corresponding target genes, rpoB and gyrA, respectively. We aligned the gyrA and rpoB sequences available in the GenBank database (Table 1) by using the multiple-alignment ClustalW algorithm (36). In the conserved regions, primers F872 (GCAACCGCCGAGTACG) and F873 (CTGGCCTGACGTTGCAT) were designed to detect the short central fragment of rpoB, whereas F874 (CACCGGCGCGTACTGTA) and F875 (TGTGCGGCGGGATGTTG) were designed to amplify the N-termainal QRDR of gyrA. The DNA primers were designed by using the OLIGO software (31), and specificity was tested with GenBank data by using the BLASTn program (1). The expected DNA fragments corresponded to nucleotide positions 1346 to 2069 of the E. coli rpoB gene and nucleotide positions 133 to 556 of the E. coli gyrA gene. The oligonucleotide primers were synthesized by Eurogentec (Seraing, Belgium).
TABLE 1.
Bacterial DNA sequences used to design PCR primers to amplify the B. vietnamiensis gyrA and rpoB antibiotic target mutation areas
| Gene (partial sequence) | Bacterial species | GenBank accession no.a |
|---|---|---|
| gyrA | Neisseria gonorrhoeae | U08817 |
| Pseudomonas aeruginosa | L29417 | |
| Escherichia coli | AE000312 | |
| Klebsiella pneumoniae | X16817 | |
| Serratia marcescens | U56906 | |
| rpoB | Neisseria meningitidis | Z54353 |
| Pseudomonas putida | X15849 | |
| Escherichia coli | AE000472 | |
| Coxiella burnetti | U86688 | |
| Buchnera aphidicola | Z11913 |
PCR conditions.
We used colony lysates for PCR (9); one fresh colony was suspended in 100 μl of sterile ultrapure water and subjected to a hot-cold shock (5 min at 95°C and 5 min at −20°C twice). All PCR amplifications were performed in 100-μl (final volume) mixtures containing each primer at a concentration of 0.1 μM, each deoxynucleoside triphosphate at a concentration of 200 μM, 1.5 mM MgCl2, 1× Taq buffer, 2.5 U of Taq polymerase (Gibco BRL, Life Technologies, Paisley, Scotland), and 2 μl of bacterial lysate.
After denaturation for 3 min at 95°C, amplification with the primers was performed for 35 cycles of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C. One negative control containing water was included in each PCR experiment. The amplified DNA fragments from B. vietnamiensis TVV75 wild type and resistant mutants were purified from the agarose gel by using a Quiaquick gel extraction kit (Quiagen, Courtaboeuf, France) according to the manufacturer's instructions, before the double-stranded sequences were determined by Genome Express (Grenoble, France). The double-stranded sequences permitted us to determine that the nucleotide substitutions detected were not the result of Taq polymerase errors.
Purity control.
The identity of each Nalr or Rifr mutant with the parent strain was confirmed by repetitive extragenic palindromic PCR, as defined by De Bruijn (8; data not shown).
Nucleotide sequence accession numbers.
The GenBank/EMBL accession numbers for the partial sequences of the B. vietnamiensis LMG 10929 gyrA and rpoB genes are AJ251151 and AJ251152, respectively.
RESULTS AND DISCUSSION
Effect of seed disinfection on bacterial growth.
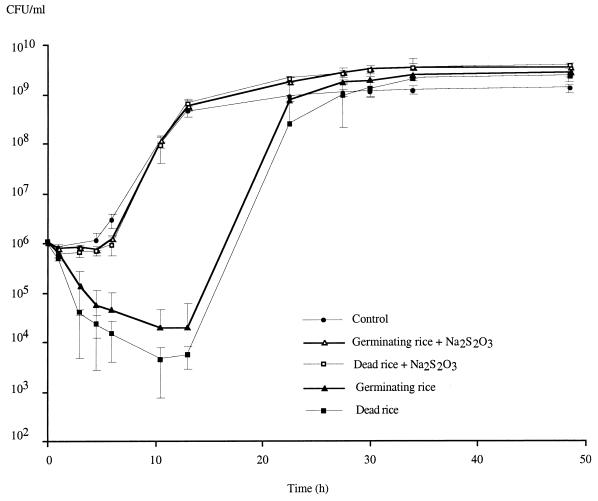
Monitoring of growth showed that within 10 h after inoculation, the number of CFU declined by 1 to 2 logs, whereas in the treatment without plants a 2- to 3-log increase was observed. Thus, a 4-log difference in the ability of B. vietnamiensis to form colonies on LB medium plates was observed compared to the control without rice (Fig. 1). This means that even though seeds were rinsed thoroughly, there were still toxic compounds present on their surfaces that killed bacteria or at least stressed them so much that they were unable to form colonies. After 10 h, bacterial growth resumed, more or less parallel to growth of the control, and it finally reached a comparable plateau. The surface sterilization protocol in which hypochlorite was used in combination with hydrogen peroxide thus had a significant effect on the growth of subsequently inoculated bacteria. In controls in which only hydrogen peroxide was used, the disinfection protocol had no effect on bacterial growth, showing that the use of hypochlorite salts, not the use of H2O2, was the reason for the observed deleterious effects (data not shown).
FIG. 1.
Effect of hypochlorite disinfection on growth of B. vietnamiensis TVV75 for controls without plants, dead rice surface sterilized with hypochlorite, germinating rice surface sterilized with hypochlorite, and corresponding thiosulfate treatments. Each value is the mean ± standard error based on four replicates.
In the control in which heat-killed rice was used, which was not rinsed with thiosulfate after disinfection, the decrease in the number of CFU was even greater than the decrease observed with germinating rice (Fig. 1). This result was probably due to increased formation of chloramine on dead rice surfaces because of the heat treatment, which could have rendered the rice tissues more accessible. However, another interpretation is that when not killed, the plant actively protected itself against reactive oxidative species produced by the sanitizer used, probably by synthesizing enzymes like peroxidases or superoxide dismutases, which could have protected bacteria as well.
Remnants of observed effects.
Formation of chloramines on rice seed surfaces could explain our inability to eliminate disinfection side effects in spite of the large number of rinses when hypochlorite was used. It actually seemed that some toxic compounds were covalently fixed on the seed surface instead of remaining diffusible compounds in the disinfecting solution. Formation of chloramine after reaction with bulky organic matter has been observed in different studies, and high ratios of HOCl to amino groups are known to favor this formation (23, 35). Lopes (23), for instance, noticed that most sanitizers, particularly hypochlorite, covalently react with the organic matter of fruits and vegetables. Gottardi and Nagl (16), in their studies of the effect of hypochlorite as a sanitizer in hospitals, also demonstrated that disinfectants containing active chlorine compounds like hypochlorite interact with skin surfaces, producing a so-called chlorine cover. This is a true chemical transformation (and not an absorption effect) of the protein matrix; covalent N-Cl bonds are formed due to substitution of protein N-H functions. Gottardi and Nagl demonstrated that the remnant oxidative power of the surface protein matrix can be mobilized inward by diffusion of low-molecular-weight components; N-Cl bonds are formed in amino acids, oligopeptides, or NH3, producing bactericidal diffusible chloramines (R-NHCl).
Our data suggest that similarly, a chlorine cover is formed during calcium hypochlorite disinfection of rice seeds; this chlorine cover cannot be eliminated easily by water rinses, and toxic chloramines are formed from the chlorine cover that are not concentrated enough to kill all bacteria but are active enough to stress the bacteria and decrease their ability to form colonies on LB medium plates. This hypothesis is supported by the observation that in the controls in which a sodium thiosulfate rinse was used, a reduction in bacterial growth was not observed (Fig. 1); thiosulfate can suppress the detrimental effects of hypochlorite on seed surfaces as well as it does on skin (16).
Physiological state of survivors.
As determined by microscopic examination of bacteria exposed to surface-sterilized seeds for 10 h, the vast majority of bacterial cells appeared to be dead. Monitoring of respiring cells via the INT reduction method revealed that approximately 18% of the intact cells contained formazan crystals, whereas the percentage was 90 to 98% for controls containing bacteria alone or bacteria in contact with seeds that had been rinsed with a sodium thiosulfate solution (data not shown). These results are reminiscent of those of Dukan et al. (12), who studied E. coli populations exposed to various HOCl stresses in nutrient-free buffer. Exposure to HOCl led to the formation of three subpopulations; most cells were dead, but there were still a few culturable bacteria (less than 0.01%) able to form colonies on solid medium, and about 10% that were unable to form colonies but still displayed respiratory or metabolic activity. The latter were called viable but nonculturable (VBNC) cells. Interestingly, some of these cells were able to reverse this state, provided that nutrients (especially a carbon source) were supplied. Recovery of culturability indicated that stress might induce an adaptive response that permits mildly injured cells to repair themselves or permits cells to bypass injury (10, 11, 13). These results are in good agreement with our data; i.e., three subpopulations (dead cells, VBNC cells, and survivors) were formed, and organic compounds supplied as exudates by the seeds permitted some subsequent growth. It is not clear, however, whether this growth can be attributed to survivors or to recovery of VBNC cells.
Mutagenic effects of disinfection and fitness of the mutants.
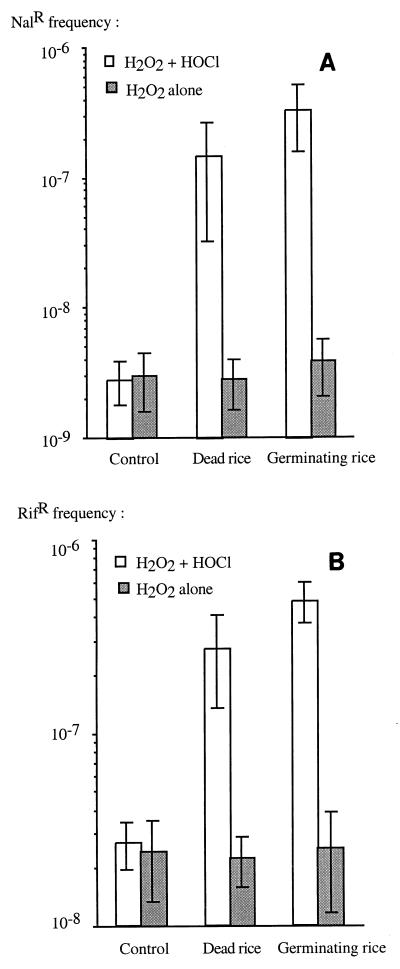
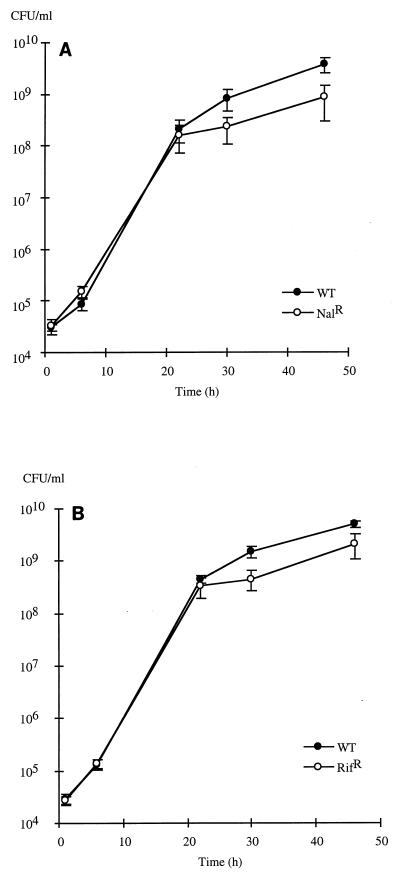
To further study the survivors, we tested them to determine antibiotic-resistant mutant frequencies once they had all reached the plateau phase. Controls in which only hydrogen peroxide was used in the disinfection protocol did not show any increase in the frequency of Rifr or Nalr mutants. Seed disinfection with both hydrogen peroxide and hypochlorite had a significant effect on variability in the survivors; a significant increase due to the presence of disinfected seeds (killed or alive) was observed when sodium thiosulfate was not used. The frequencies of antibiotic-resistant mutants were up to 20 times higher for rifampin resistance and up to 120 times higher for nalidixic acid resistance compared to the control without seeds (Fig. 2). These values might even be underestimates, as competition assays showed that most mutants had decreased fitness compared to the wild-type strain, which outnumbered them after the exponential growth phase (Fig. 3). The lower fitness of antibiotic-resistant strains than of the susceptible parent suggests that there is a physiological cost associated with the induced mutations, of which Rifr and Nalr are only the visible parts (4).
FIG. 2.
Effect of disinfection on the frequencies of nalidixic acid-resistant mutants (A) and rifampin-resistant mutants (B). Controls without plants and without hypochlorite were included. Each value is the mean ± standard error based on two independent experiments, each having four replicates.
FIG. 3.
Nal (A) and Rif (B) mutant competitiveness when organisms were challenged (50/50) with parental sensitive strain TVV75 in the absence of plants. Each curve shows the averages ± standard errors of values obtained with four different Nal and Rif mutants. WT, wild type.
Mutant analysis.
We checked whether hypochlorite-induced mutants were stable by growing them in the absence of antibiotics. All mutants retained their Nalr and Rifr phenotypes, suggesting that these resistance characteristics were due to stable bacterial genomic mutations. To verify that the mutations had occurred in the antibiotic target genes, we studied gyrA DNA gyrase) and rpoB (RNA polymerase) by PCR amplification and sequence analysis.
gyrA mutations in nalidixic acid-resistant mutants.
Primers F874 and F875 were used to PCR amplify the gyrA QRDR-containing region of B. vietnamiensis TVV75 and seven Nalr mutants. The deduced 141-amino-acid sequence of the GyrA fragment of B. vietnamiensis was closely related to the sequences of E. coli, Pseudomonas aeruginosa, and Neisseria meningitidis (82 to 83% identity and 92 to 93% similarity) (5, 22, 28). Compared to the wild type strain, five of seven mutants had a single base change that resulted in a single amino acid change (Table 2). Three of the amino acids were clustered at positions 82 to 87, whereas the fourth corresponded to amino acid 184 of the E. coli GyrA protein, which is not included in the QRDR. There were no differences between the sequence fragments of mutants N5 and N9 and the wild-type sequence.
TABLE 2.
DNA mutations found in gyrA amplified sequences of B. vietnamiensis TVV75 and nalidixic acid-resistant mutantsa
| Strain | Amino acid (codon) encoded by gyrA gene at position:
|
|||
|---|---|---|---|---|
| 82b | 83 | 87 | 184 | |
| TVV75 | Asp (GAT) | Thr (ACG) | Asp (GAC) | Arg (CGG) |
| N1 | —c | Lys (AAG) | — | — |
| N2 | — | — | Tyr (TAC) | — |
| N5 | — | — | — | — |
| N6 | Asn (AAT) | — | — | — |
| N7 | — | — | — | Pro (CCG) |
| N8 | — | Lys (AAG) | — | — |
| N9 | — | — | — | — |
No other nucleotide change was found in the 418-bp fragment.
The numbers are E. coli GyrA amino acid positions.
—, amino acid and codon are identical to those of strain TVV75.
Of the reported single-step mutations associated with quinolone resistance in bacteria, those that alter amino acids at positions 83 and 87 in the GyrA protein are the most frequent (9, 26, 30). The Thr-Lys change at position 83 found in B. vietnamiensis is similar to the Ser-Arg mutation at position 83 often found in quinolone-resistant mutants of the Enterobacteriaceae (39). The Asp-Tyr substitution at position 87 also has been observed in quinolone-resistant P. aeruginosa (22), Serratia marcescens (21), E. coli (17, 39), and Salmonella typhimurium (30).
Mutant N6 was characterized by a G-to-A transition at codon 82, leading to an Asp-to-Asn substitution. To our knowledge, only one mutation affecting codon 82 has been described previously (38), and this Asp-Gly substitution at position 82 alone could not confer nalidixic acid resistance; it had to be associated with a Gly-Asp mutation at position 81. In B. vietnamiensis, the mutated GyrA sequence contains a bulkier amino acid than Gly, so it is possible that this substitution is enough to confer nalidixic acid resistance. The last mutation found in the B. vietnamiensis gyrA fragments sequenced led to an Arg-Pro substitution at position 184, which is not in the QRDR. Moreover, no nucleotide substitution was found in the regions of strains N5 and N9 sequenced. Since only a small portion of the gyrA gene sequence of Burkholderia isolates has been examined, we cannot exclude the possibility that corresponding gyrA genes may have been mutated elsewhere. In addition, we cannot exclude the possibility that other target genes have been affected, as mutations affecting genes other than gyrA have been shown to confer fluoroquinolone resistance in some bacteria; these genes include genes encoding target protein GyrB of DNA gyrase and topoisomerase IV (14, 15, 17, 43).
rpoB mutations in rifampin-resistant mutants.
Primers F872 and F873 were designed to amplify and study the short central region of the rpoB gene in seven independent B. vietnamiensis Rifr clones. Alignment of the deduced protein sequence showed that the B. vietnamiensis RpoB fragment studied was very similar to the N. meningitidis fragment, exhibiting 83% identity and 92% similarity (28). This RpoB fragment was also related to those of E. coli and Pseudomonas putida, exhibiting 75 and 74% identity and 86 and 84% similarity with these polypeptides, respectively (5, 6).
Our sequence data confirmed that replacement of a limited number of amino acids in RpoB could result in rifampin resistance; the amplified fragment sequences revealed the presence of transition mutations in all mutants. Six of seven Rifr clones had a single nucleotide modification, and all of the modifications were clustered in a 62-bp region of the amplified fragments (Table 3). The other clone (R16) had two mutations in the same codon (CTG → CCA), which altered the Leu-533 codon to Pro. All of the mutations thus corresponded to a single amino acid change. Apart from these missense mutations in the region studied, the DNA sequences were identical to the sequence of the wild-type strain. All the mutations observed in B. vietnamiensis strains were previously associated with rifampin resistance in other bacteria (7, 20, 25, 34). In this study, the majority of mutations occurred in codon 526 (E. coli numbering), leading to a His-to-Tyr or a His-to-Arg change. Carter et al. (7) also found that mutations of the His residue at position 526 were predominant, suggesting that this residue plays a critical role in selection of antibiotic-resistant variants.
TABLE 3.
DNA mutations found in rpoB amplified sequences of B. vietnamiensis TVV75 and rifampin-resistant mutantsa
| Strain | Amino acid (codon) encoded by rpoB gene at position:
|
||||
|---|---|---|---|---|---|
| 513b | 516 | 526 | 531 | 533 | |
| TVV75 | Gln (CAG) | Asp (GAC) | His (CAC) | Ser (TCC) | Leu (CTG) |
| R1 | Arg (CGG) | —c | — | — | — |
| R2 | — | — | Tyr (TAC) | — | — |
| R3 | — | — | — | Phe (TTC) | — |
| R4 | — | — | Tyr (TAC) | — | — |
| R11 | — | Gly (GGC) | — | — | — |
| R15 | — | — | Arg (CGC) | — | — |
| R16 | — | — | — | — | Pro (CCA) |
No other nucleotide change was found in the 672-bp fragment.
The numbers are E. coli RpoB amino acid positions.
—, amino acid and codon are identical to those of strain TVV75.
The results of this study thus demonstrate that the use of hypochlorite as a seed surface disinfectant to prepare gnotobiotic models is a source of artifacts; not only does hypochlorite kill surface contaminants, but it also causes the death of most subsequently inoculated bacteria. Moreover, survivors exhibit increased mutagenesis.
Hydrogen peroxide is also known to produce secondary compounds with mutagenic effects (40). Nevertheless, we did not see such effects under our experimental conditions. We hypothesize that either hydrogen peroxide diffuses more readily into cells, where it is decomposed by catalase, or it is eliminated by washing. Even though the use of hydrogen peroxide does not result in all the artifacts caused by hypochlorite, unfortunately it cannot be used alone for seed disinfection because it is not efficient enough to eliminate all contaminants.
There are several possible ways to avoid the hypochlorite-induced biases. One possible way is to use products that neutralize chloramines in rinses, such as peptone (3), dithiothreitol (35), or ascorbic acid (42). In this study, we showed that using sodium thiosulfate to rinse disinfected seeds was very efficient, so we recommend use of this compound in seed disinfection protocols. Another way might be to use the new acid anionic sanitizers developed in the food industry (23).
ACKNOWLEDGMENT
We thank S. Dukan for comments on our work and critical reading of the manuscript.
REFERENCES
- 1.Altshul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Andrews S. Evaluation of surface disinfection procedures for enumarating fungi in foods: a collaborative study. Int J Food Microbiol. 1995;29:177–184. doi: 10.1016/0168-1605(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 3.Beuchat L R. Comparison of chemical treatments to kill Salmonella on alfalfa seeds destined for sprout production. Int J Food Microbiol. 1997;34:329–333. doi: 10.1016/s0168-1605(96)01202-0. [DOI] [PubMed] [Google Scholar]
- 4.Billington O J, McHugh T D, Gillespie S H. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:1866–1869. doi: 10.1128/aac.43.8.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Borodin A M, Danikovich A V, Allikmets R L, Rostapshov V M, Chemov I P, Azhikina T L, Monastyrskaya S, Sverdlov D. Nucleotide sequence of the rpoB gene coding for the beta-subunit of RNA polymerase in Pseudomonas putida. Dokl Biochem (Engl Transl Dokl Akad Nauk SSSR Ser Biokhim) 1988;302:1261–1265. [Google Scholar]
- 7.Carter P E, Abadi F J R, Yakubu D E, Pennington T H. Molecular characterization of rifampin-resistant Neisseria meningitidis. Antimicrob Agents Chemother. 1994;38:1256–1261. doi: 10.1128/aac.38.6.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dessus-Babus S, Bébéar C M, Charron A, Bébéar C, de Barbeyrac B. Sequencing of gyrase and topoisomerase IV quinolone-resistance-determining regions of Chlamydia trachomatis and characterization of quinolone-resistant mutants obtained in vitro. Antimicrob Agents Chemother. 1998;42:2474–2481. doi: 10.1128/aac.42.10.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukan S, Dadon S, Smulski D R, Belkin S. Hypochlorous acid activates the heat shock and soxRS systems of Escherichia coli. Appl Environ Microbiol. 1996;62:4003–4008. doi: 10.1128/aem.62.11.4003-4008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dukan S, Lévi Y, Touati D. Recovery of culturability of an HOCl-stressed population of Escherichia coli after incubation in phosphate buffer: resuscitation or regrowth? Appl Environ Microbiol. 1997;63:4204–4209. doi: 10.1128/aem.63.11.4204-4209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dukan S, Belkin S, Touati D. Reactive oxygen species are partially involved in the bactericidal action of hypochlorous acid. Arch Biochem Biophys. 1999;367:311–316. doi: 10.1006/abbi.1999.1265. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottardi W, Nagl M. Which conditions promote a remnant (persistent) bactericidal activity of chlorine covers? Zentralbl Hyg Umweltmed. 1998;201:325–335. [PubMed] [Google Scholar]
- 17.Heisig P. Gentic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Agency for Research on Cancer. Hypochlorite salts. IARC (Int Agency Res Cancer) Monogr Eval Carcinog Risk Chem Hum. 1991;52:159–176. [PMC free article] [PubMed] [Google Scholar]
- 19.Jin D J, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 20.Kapur V, Li L-L, Iordanescu S, Hamrick M R, Wanger A, Kreiswirth B N, Musser J M. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase β subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol. 1994;32:1095–1098. doi: 10.1128/jcm.32.4.1095-1098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J H, Cho E H, Kim K S, Kim H Y, Kim Y M. Cloning and nucleotide sequence of the DNA gyrase gyrA gene from Serratia marcescens and characterization of mutations in gyrA of quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1998;42:190–193. doi: 10.1128/aac.42.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kureishi A, Diver J M, Beckthold B, Schollaardt T, Bryan L E. Cloning and nucleotide sequence of Pseudomonas aeruginosa DNA gyrase gyrA gene from strain PAO1 and quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1994;38:1944–1952. doi: 10.1128/aac.38.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes J A. Susceptibility of antibiotic-resistant and antibiotic-sensitive foodborne pathogens to acid anionic sanitizers. J Food Prot. 1998;61:1390–1395. doi: 10.4315/0362-028x-61.10.1390. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 25.Matsiota-Bernard P, Vrioni G, Marinis E. Characterization of rpoB mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Greece. J Clin Microbiol. 1998;36:20–23. doi: 10.1128/jcm.36.1.20-23.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouneimné H, Robert J, Jarlier V, Cambau E. Type II topoisomerase mutations in ciprofloxacin-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:62–66. doi: 10.1128/aac.43.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawara S, Goto T, Nara M, Ozawa Y, Hotta K, Arata Y. Spectroscopic characterization and the pH dependence of bactericidal activity of the aqueous chlorine solution. Anal Sci. 1998;14:691–698. [Google Scholar]
- 28.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 29.Piemas V, Guiraud J P. Disinfection of rice seeds prior to sprouting. J Food Sci. 1997;62:611–615. [Google Scholar]
- 30.Reyna F, Huesca M, Gonzalez V, Fuchs L Y. Salmonella typhimurium gyrA mutations associated with fluoroquinolone resistance. Antimicrob Agents Chemother. 1995;39:1621–1623. doi: 10.1128/aac.39.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rychlik W, Rhoads R E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989;17:8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih K L, Lederberg J. Effects of chloramine on Bacillus subtilis deoxyribonucleic acid. J Bacteriol. 1976;125:934–945. doi: 10.1128/jb.125.3.934-945.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih K L, Lederberg J. Chloramine mutagenesis in Bacillus subtilis. Science. 1976;192:1141–1143. doi: 10.1126/science.818709. [DOI] [PubMed] [Google Scholar]
- 34.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 35.Thomas E L, Jefferson M M, Bennett J J, Learn D B. Mutagenic activity of chloramines. Mutat Res. 1987;188:35–43. doi: 10.1016/0165-1218(87)90112-1. [DOI] [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trân Van V, Berge O, Ngô Kê S, Balandreau J, Heulin T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil. 2000;218:273–284. [Google Scholar]
- 38.Truong Q C, Nguyen Van J-C, Shlaes D, Gutmann L, Moreau N J. A novel, double mutation in DNA gyrase A of Escherichia coli conferring resistance to quinolone antibiotics. Antimicrob Agents Chemother. 1997;41:85–90. doi: 10.1128/aac.41.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weigel L M, Steward C D, Tenover F C. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob Agents Chemother. 1998;42:2661–2667. doi: 10.1128/aac.42.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weitzman S A, Stossel T P. Mutations caused by human phagocytes. Science. 1981;212:546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- 41.Williams D L, Spring L, Collins L, Miller L P, Heifets L B, Gangadharam P R J, Gillis T P. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–1857. doi: 10.1128/aac.42.7.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wlodkowski T J, Rosenkranz H S. Mutagenicity of sodium hypochlorite for Salmonella typhimurium. Mutat Res. 1975;31:39–42. doi: 10.1016/0165-1161(75)90061-8. [DOI] [PubMed] [Google Scholar]
- 43.Yamagishi J, Yoshida H, Yamayoshi M, Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986;204:367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann R, Iturriaga R, Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978;36:926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]