Abstract
Objective
The neuromodulatory effects of focused ultrasound (FUS) have been demonstrated in animal epilepsy models; however, the safety and efficacy of FUS in humans with epilepsy have not been well established. Patients with drug‐resistant epilepsy (DRE) undergoing stereo‐electroencephalography (SEEG) provide an opportunity to investigate the neuromodulatory effects of FUS in humans.
Methods
Patients with DRE undergoing SEEG for localization of the seizure onset zone (SOZ) were prospectively enrolled. FUS was delivered to the SOZ using a neuronavigation‐guided FUS system (ceiling spatial‐peak temporal‐average intensity level = 2.8 W/cm2, duty cycle = 30%, modulating duration = 10 min). Simultaneous SEEG recordings were obtained during sonication and for 3 days after treatment. Seizures, interictal epileptiform discharges, and adverse events after FUS were monitored.
Results
Six patients met the eligibility criteria and completed FUS treatment. A decrease in seizure frequency was observed in two patients within the 3‐day follow‐up; however, one patient presented an increase in the frequency of subclinical seizures. Posttreatment magnetic resonance imaging revealed neither lesion nor brain edema. Significant changes in spectral power of SEEG were noted at the targeted electrodes during FUS treatment. One patient reported subjective scalp heating during FUS, and one patient developed transient naming and memory impairment that resolved within 3 weeks after FUS.
Significance
FUS can be safely delivered to the SOZ of patients with DRE, resulting in significant changes in spectral power of SEEG. A larger sample cohort and pursuing optimal sonication parameters will be required to elucidate the neuromodulatory effects of FUS when used for seizure control.
Keywords: drug‐resistant epilepsy, focused ultrasound, low‐intensity, neuromodulation, stereo‐electroencephalography
Key Points.
SEEG provides a chance to investigate the neuromodulatory effects of FUS in humans
FUS can be delivered to seizure onset brain substrate without significant adverse events
FUS resulted in significant changes in spectral power of intracranial EEG
1. INTRODUCTION
Epilepsy is a disease characterized by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition. 1 Surgical intervention has been shown to confer improvements in seizure‐free outcomes and quality of life for patients with drug‐resistant epilepsy (DRE). 2 , 3 Surgical interventions for DRE include resection, disconnection, and neuromodulation. In cases where the seizure foci are amenable to surgical resection, seizure freedom is achieved in 44%–80% of patients with seizures localized to the temporal lobe, and in 15%–65% of those with extratemporal lobe epilepsy. 4 In many cases deemed unsuitable for surgical resection, disconnection provides relief from seizures via disruption of the epileptic network and isolation of seizure foci. 5 Neuromodulation is an alternative treatment option in cases for which surgical resection or disconnection are not viable options. Current neuromodulation therapies for DRE include deep‐brain stimulation (DBS), vagus nerve stimulation (VNS), and responsive neurostimulation. They were proved to be effective in reducing seizure frequency by 50%–60% in long‐term treatment. 6 Current neuromodulation devices also necessitate surgery for the implantation of electrodes, pulse generators, and battery replacements, thereby exposing patients to perioperative risks.
The ability of focused ultrasound (FUS) to noninvasively ablate the epileptogenic focus and modulate brain circuits or neuronal activities across a broad range of acoustic parameters (intensity, duty cycle, pulse repetition frequency, and pulse duration) has made it a promising investigational therapy for epilepsy. 7 , 8 , 9 , 10 , 11 In contrast to the neuroablative effects of high‐intensity FUS, low‐intensity pulsed FUS produces neuromodulatory effects and demonstrates suppressive effects on the frequency of epileptic signal bursts in electroencephalographic (EEG) readings in a number of animal studies. 11 , 12 In a preclinical study conducted in 2020, we demonstrated the neuromodulatory effects of low‐intensity pulsed FUS treatment, which effectively suppressed pentylenetetrazol (PTZ)‐induced abnormal bursts in rats. 13 The degree to which these neuromodulatory effects are generalizable to humans remains unknown, and the safety of low‐intensity pulsed FUS when administered to patients with DRE has yet to be determined. The primary objectives of this Phase 1, open‐label, uncontrolled trial were to investigate the safety of transcranial FUS in patients with DRE and to explore its neuromodulatory effects on stereo‐EEG (SEEG) recordings.
2. MATERIALS AND METHODS
2.1. Patients
Between June 2018 and October 2020, we screened adult patients suffering from focal DRE who underwent SEEG implantation and recording for consideration of surgical resection. DRE was defined as recurrent seizures despite trials of at least two full‐dose antiseizure medications. Exclusion criteria included the following: (1) concurrent active psychiatric mood disorders, coexisting medical conditions, active or history of substance abuse, currently pregnant or breast‐feeding; (2) metallic implants including pacemaker, VNS, or DBS; (3) scalp infection, coagulopathy, or significant bleeding after SEEG implantation; and (4) <20 mm distance between inner table of skull and the epileptogenic focus (i.e., the target of FUS). 14
This study was approved by the institutional review board of Taipei Veterans General Hospital (2018‐07‐002A), and registered at ClinicalTrials.gov (NCT03860298). All patients provided written informed consent before joining the study. This study was funded by the NaviFUS Corporation, with involvement limited to technical support. Although this was an industry‐sponsored study, the principal investigator and coinvestigators maintained full authority over all aspects of the study, including the study design, patient enrollment, patient outcome assessment, and data management.
2.2. SEEG implantation
All of the patients underwent surgical implantation (via stereotaxy) of intracranial depth electrodes (ADtech, 6–16 contacts; multicontacts, interval = 3–10 mm, diameter = .86 mm). 15 , 16 The trajectory, target, and number of implanted electrodes were guided by a hypothesized epileptogenic network derived from clinical data and the results of video‐EEG, magnetic resonance imaging (MRI), 17 F‐fluorodeoxyglucose positron emission tomography, and neuropsychological testing. SEEG and scalp EEG were simultaneously recorded on a Nicolet system (Quantum, Natus Medical), with a maximum number of 128 recording channels. Data were digitized at a sampling rate of 1024 Hz with 16‐bit resolution. Patients were simultaneously monitored via video surveillance to validate the electroclinical features of the seizures. The treatment workflow is illustrated in Figure 1A.
FIGURE 1.
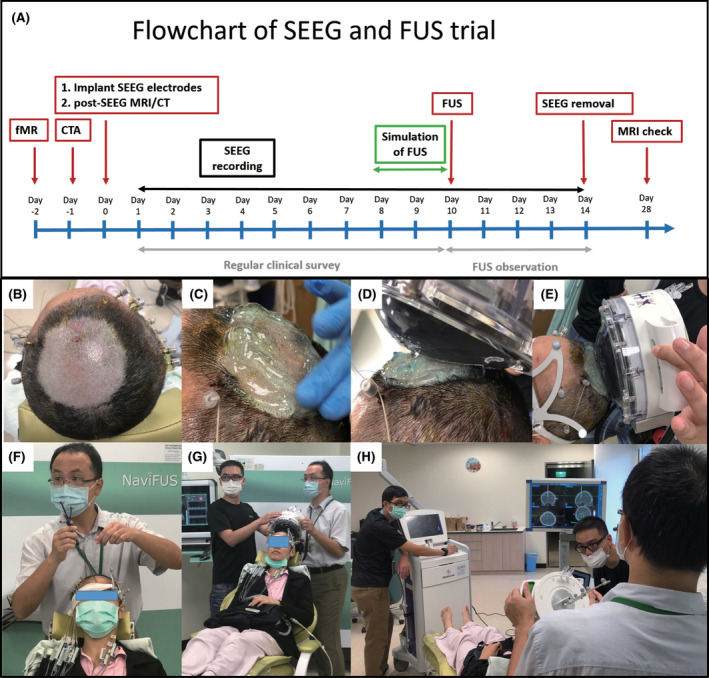
(A) Treatment workflow as a sequence of radiological evaluation, stereo‐electroencephalographic (SEEG) implantation and recording, and focused ultrasound (FUS) treatment. (B–H) Steps covered during FUS trial: (B) shaving head, (C) applying gel, (D, E) application of transducer with water bag, (F, G) registration of neuronavigation, and (H) sonication. (In this trial, the principal investigator [H.‐Y.Y.] administered and explained the consent to the patient. Patients, the neurosurgeon [C.‐C.L.], and his coworkers [H.Y.M. and H.H.C.], who were showed in this figure, agreed to have their image published in this article. All have seen the photo, image, text, and other material relating to them.) CT, computed tomography; CTA, computed tomographic angiography; MRI, magnetic resonance imaging
2.3. FUS system
The FUS system in the current study was a noninvasive transcranial FUS delivery platform, comprising multichannel hemispheric phased array ultrasound generating units designed to deliver focused energy to intracranial targets under guidance from a neuronavigation tracking system (NaviFUS Corporation; Figure 1F–H). The system is compatible with the StealthStation S7 neuronavigation system (Medtronic), which includes a fixed supplementary phantom probe by which to establish reference coordinates. Following registration, the treatment plan can be displayed on the neuronavigation system in real time throughout the treatment process.
2.4. Treatment target, planning, and delivery
Enrollment in this study was limited to patients for whom a habitual seizure onset zone (SOZ) could be conclusively established. The SOZ was identified based on spontaneous seizures (at least three times). FUS targets were determined by epileptologists (H.‐Y.Y., C.‐C.C., and C.‐C.L.). The regions presenting the earliest changes in SEEG during habitual seizures were considered treatment targets. All sonications targeted SOZs, and no attempt was made to disconnect SOZs from connections to deep seizure networks. Prior to sonication, the overall treatment plans (including the simulated trajectory, focal beam distortions, and attenuations) were reviewed by the treatment team. Treatment was performed under burst‐tone nonthermal low‐intensity FUS sonication conditions (the ceiling level of transcranial spatial‐peak temporal‐average intensity is requested to be <2.8 W/cm2, pulse repetition frequency = 100 Hz, burst length/focus exposure = 3 ms, duty cycle = 30%). Total exposure time was 10 min. The employed clinically approved device provides build‐in treatment planning tools to consider the acoustic energy loss based on calvarium cortical‐marrow porosity information obtained from computed tomographic images (transcranial penetration was estimated to range from 20.9% to 24.0% in acoustic pressure). The exact output level was then scaled to deliver the designated exposure level. The single steering focus was cylindrical, with the −3‐dB dimension estimated at roughly 20 mm along the long axis and 2 mm along the short axis. Discrepancies in the steering position of the transcranial focal beam were estimated at <1.5 mm, after controlling for a steering incident angle of <15° (Figure S1). The distance from the focal beam depth to scalp ranged from 43 to 60 mm, maintaining a minimal distance of 20 mm between the inner table of the skull and the treatment target.
Throughout the procedure, the patient was maintained in a sitting position with the head shaved (Figure 1B), and ultrasound gel was used to ensure acoustic coupling between the scalp and transducer (Figure 1C). Under the real‐time guidance of the neuronavigation system, the transducer, over any smooth portion of the scalp, was able to be directed at the sonication target (Figure 1D–H). The patients remained awake throughout the entire procedure, which allowed them to report any discomfort associated with the treatment. The trial protocol stipulated that the procedure would be halted in cases of discomfort, clinical seizure, or significant change in SEEG recording. In the event that a treatment session was stopped prior to completion, the trial protocol permitted a second session 1 h later as long as no clinical contraindications were observed. Simultaneous SEEG recordings were obtained throughout each treatment session. Note that due to the limited number of channels provided by the portable recording device (maximum of 64 channels, sampling rate of 512 Hz; LTM, Natus Medical Incorporated), preference was given to electrode contacts covering the epileptogenic network (Figure S2). Following completion of the FUS procedure, the patients returned to the epilepsy monitoring unit for an additional 3 days, during which SEEG recording was continued (full electrode contact recording, ranging between 60 and 110 channels). SEEG electrodes were explanted at 3 days post‐FUS.
2.5. Assessment of safety and efficacy
Changes in SEEG signals were monitored in real time throughout the FUS sessions. Alterations in vital signs, clinical and neurological status, subclinical seizures, and clinical seizures were monitored as well. The monitored vital signs included blood pressure, heart rate, respiratory rate, and body temperature. Physical and neurological examinations were performed by the treatment team immediately after the session and at 1 day, 2 days, and 3 days postsonication. Brain MRI scans were obtained at the 14th day posttreatment to identify instances of unwanted lesioning due to treatment. Relevant neurological symptoms (including seizures) were monitored for at least 14 days after FUS. Adverse events were defined as those specifically associated with FUS treatment.
Assessments of FUS treatment effects were based on clinical seizure frequency and SEEG recordings. The frequency of clinical seizures and interictal epileptiform discharges (IEDs) within 24 h prior to treatment were compared to those within 72 h after FUS. IEDs were identified by obtaining 3‐min SEEG recordings at intervals of 30 min. Only IEDs localized to the SOZ were quantified. The short‐term effects of FUS treatment on SEEG signals were recorded during peritreatment periods, including 10‐min intervals before (T1 period), during (T2 period), and after (T3 period) sonication. All SEEG data were preprocessed and analyzed offline using the NicoletOne built‐in trend analysis package (Natus), and Brainstorm. 18 The filtered SEEG recordings were subsequently divided into consecutive epochs of 3 s in off‐line mode. From each patient, 200 filtered epochs for each period were extracted for spectral analysis, illustrating the oscillatory dynamics of underlying neuronal activity. Spectral density analysis of short‐term changes in SEEG was performed using the fast Fourier transform. Power values of each epoch in each period were obtained from four frequency bandwidths, including delta (≥.5 Hz, <4 Hz), theta (≥4 Hz, <8 Hz), alpha (≥8 Hz, <13 Hz), and beta (≥13 Hz, <30 Hz). The Mann–Whitney U test was used to examine changes between the T2 and T1 periods, and between the T3 and T1 periods. A p value < .05 was considered statistically significant.
3. RESULTS
3.1. Patients
The median age of the six patients in this study was 31.5 years (range = 26–42 years) at time of FUS, with a median duration of epilepsy of 12 years (range = 3–21 years). Table 1 details the baseline demographics and characteristics of the study cohort. Four of the six patients were male (67%). The median number of antiseizure medications used was five (range = 3–5). Seizure frequency ranged between two events per month to three events per day.
TABLE 1.
Patient demographics and characteristics
| Patient # | Sex | Age at onset/SEEG | ASMs, n | Aura | Seizure characteristics | Seizure frequency, n | Seizure duration | Radiological evidence [MRI] | Seizure onset zone [defined on SEEG] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 21/27 | 5 | Diplopia | Staring, increased eye blinking, and swallowing | 3–4/month | 1–2 min | Negative, prior left amygdalectomy | Left posterior temporal lobe |
| 2 | Male | 17/26 | 5 | None | Staring, automatism in both hands, impaired awareness, bil. TC | 2–5/month | 10 s | Negative | Left medial part of superior frontal gyrus |
| 3 | Male | 20/40 | 5 | None | Staring, leg movement, right hand stereotypes, and impaired awareness ± bil. TC | 5–10/month | 10 s | Right frontal encephalomalacia, prior insular lesionectomy | Right frontal operculum and anterior limiting sulcus of insula |
| 4 | Male | 7/42 | 3 | None | Staring, vocalization, pursing of lips, arm posturing, ± facial twitching, impaired awareness | 2–3/month | 1–2 min | Negative | Left anterior temporal lobe including amygdala and hippocampus (habitual seizures); right mesial temporal lobe (subclinical seizures) |
| 5 | Female | 3/29 | 5 | General malaise | Neck flexion, shoulder and arm raising, aware/unaware | 2–3/day | 5–20 s | Negative, prior right frontotemporal corticectomy | Right frontal operculum |
| 6 | Female | 4/34 | 5 | None | Pouting, motionless, staring and leaning forward with both hands in flexion posture, and impaired awareness | 2–3/day | 5–10 s | Negative | Left anterior cingulate gyrus |
Abbreviations: ASM, antiseizure medication; bil. TC, bilateral tonic–clonic; MRI, magnetic resonance imaging; SEEG, stereo‐electroencephalography.
3.2. Radiological and pathological changes after treatment
Figure 2A presents MRIs obtained before SEEG implantation, after SEEG implantation, during FUS planning, and after FUS treatment (Patient 2). None of the post‐FUS T1/T2‐weighted images obtained from the six patients revealed radiological changes related to FUS treatment (Figure S3). There was no evidence of contrast leakage in T1‐weighted images from the four patients who received gadolinium contrast injection during post‐FUS MRIs, thereby indicating the integrity of the blood–brain barrier. Histological specimens from Patient 2, who underwent left frontal corticectomy at 2 months after FUS, presented indications of gliosis without any other cortical or subcortical changes attributable to FUS treatment. Normal cortical lamination was seen, and no focal edema was observed in cerebral white matter (Figure 2B).
FIGURE 2.
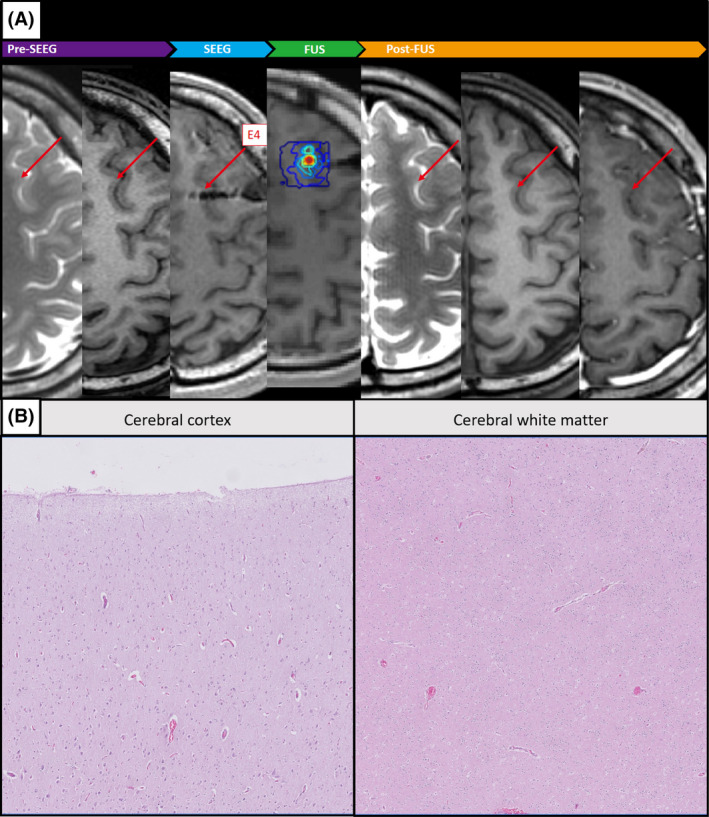
Magnetic resonance imaging from Patient 2. (A) Prior to stereo‐electroencephalography (SEEG) implantation (T2‐weighted imaging [T2WI] and T1WI), after SEEG implantation (T1WI), focused ultrasound (FUS) treatment plan (T1WI), and post‐FUS treatment (T2WI, T1WI, and T1WI + contrast). (B) Histological specimen from subsequent surgical resection showing that the cortex and subcortical structures were unaffected by FUS treatment
3.3. Seizure frequency
After FUS, the electroclinical features of the seizures were characterized using simultaneous video and SEEG recordings. Figure 3A and Table S1 detail the frequency of seizures before and after treatment. Seizures were recorded in three patients (Patients 2, 5, and 6) within 72 h after FUS. Following FUS, two of these patients (Patients 5 and 6) presented a decrease in seizure frequency. Although one of these patients (Patient 2) presented an increase in seizure frequency, the additional seizures were subclinical.
FIGURE 3.
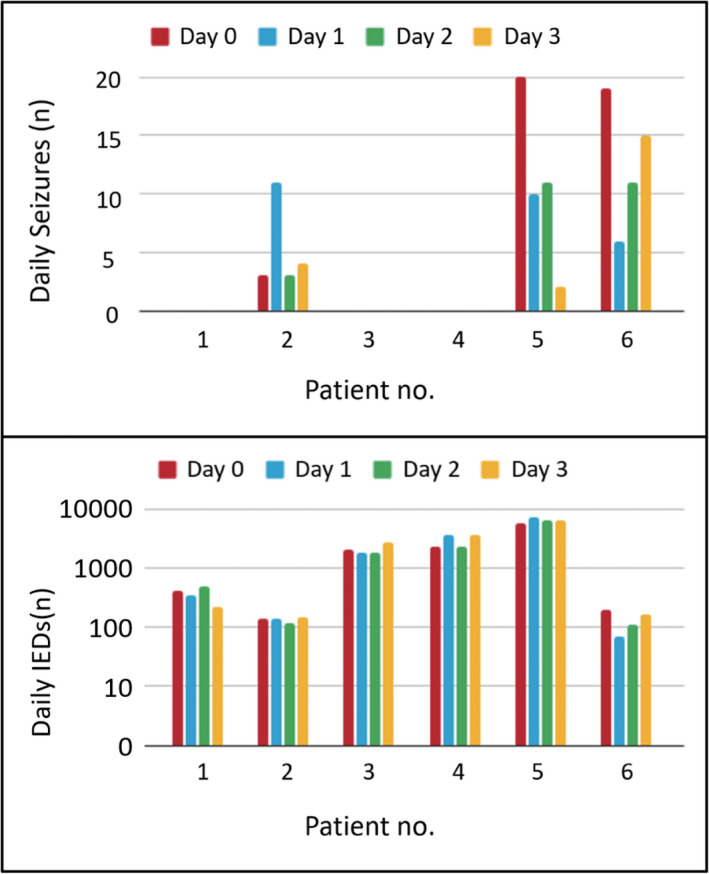
Seizure and interictal epileptiform discharge (IED) frequencies. (A) Seizure frequencies from the six patients within 24 h prior to treatment and within 72 h after treatment. (B) IED frequencies from the six patients within 24 h prior to treatment and within 72 h after treatment
3.4. Interictal epileptiform discharges
Figure 3B and Table S1 detail the frequency of IEDs among patients before and after treatment. Baseline IEDs varied between 138 and 5620 instances per day. Four of the patients presented a decrease in IED frequencies within the 72 h after treatment, whereas two patients (Patients 4 and 5) presented an increase. The patterns of these changes were not consistent among the six patients. Note that SEEG data were visually inspected for signal contamination (i.e., artifacts and noise associated with bad electrodes), and artifacts associated with sonication were filtered out (Figure S4).
3.5. Adverse events
Adverse events associated with FUS occurred in two patients. The treatment was halted for Patient 2 after 260 s of sonication due to uncomfortable scalp heating. Subsequent treatment, repeated after 1 h, was completed without complications. The distance between scalp surface and target alone may not account for the heating, as this distance was the largest among the cohort (60 mm). Patient 5 experienced impairment in naming and memory between Days 4 and 11 after FUS (i.e., 1 day after SEEG explantation). This patient demonstrated no other focal neurological deficits, and neuroimaging demonstrated no evidence of complications. Scalp EEG readings provided no evidence of continuous slowing or nonconvulsive seizures. Naming impairment was conditionally attributable to a functional neurologic disorder. These symptoms appeared to have no impact on daily life and completely resolved within 3 weeks. The exact etiology of these symptoms was unclear, although FUS could not be excluded as a potential cause.
3.6. FUS and neural activity
SEEG waveforms obtained during FUS (T1, T2, and T3 periods) from electrode contacts in the target area (SOZ) and nontarget area (>3 cm from the SOZ) were used to characterize the spatial distribution of FUS effects on neural activity. During the T3 period, decreases in spectral power were observed in all frequency bands of SEEG signals from the target electrodes; however, no significant changes were observed in nontarget electrodes (see Patient 2 in Figure 4A). Spectral analysis of signals from the target electrode revealed decreased amplitudes in the delta to beta bands, indicating that sonication for a period of 10 min was sufficient to suppress neural activity (Figure 4B). The SEEG spectral power changes during the T3 period showed that the modulation effect was more prominent in the target area compared to the nontarget area.
FIGURE 4.
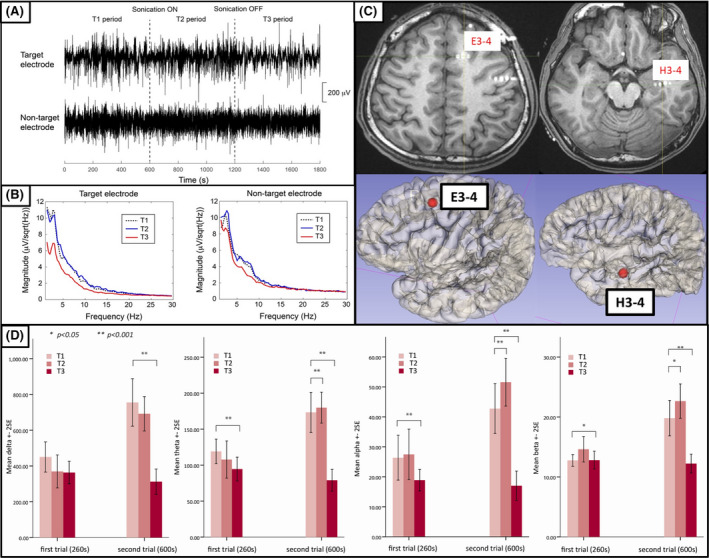
(A) Stereo‐electroencephalographic waveforms from target electrodes (contacts E3–4) and nontarget electrodes (contacts H3–4) of Patient 2 during T1, T2, and T3 periods. (B) Spectral power during each recording period (T1–T3) covering 1–30 Hz from target and nontarget electrodes. (C) Localization of target electrodes (E3–4) and nontarget electrodes (H3–4) on magnetic resonance imaging and three‐dimensional representation. (D) Power of alpha, beta, theta, and delta waves of target electrode (E3–4) during T1, T2, and T3 periods. The left column in each histogram shows the first trial (260 s), and the right column shows the second trial (600 s)
Table 2 lists the changes in SEEG spectral power in various frequency bands from target electrodes. Most of the patients (n = 5) presented increases in SEEG spectral power at least in one of the four frequency bands during sonication, followed by decreases in power after sonication (n = 3). After sonication, Patients 2 and 4 presented significant decreases in power from the delta to beta bands (p < .01). Nonetheless, we were unable to establish a correlation between significant short‐duration changes in SEEG spectral power and subsequent seizure control, based on the frequency of seizures.
TABLE 2.
Changes in power of SEEG waveform, adverse events, and seizure recurrence
| Patient # | Target (electrode)/depth from scalp surface | Electrode contacts during treatment, n | T2 SEEG band power [compared to T1] | T3 SEEG band power [compared to T1] | Adverse events | Time to seizure recurrence | Subsequent surgical intervention (months after FUS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recorded | Non‐recorded | All | δ | θ | α | β | δ | θ | α | β | |||||
| 1 | Left fusiform gyrus (F2)/52 mm | 53 | 7 | 60 | – | – | ↑* | – | – | ↓** | – | – | None | 30 days (auras); 5 months (habitual) | RFTC at fusiform gyrus (8) |
| 2 | Left premotor gyrus (E4)/60 mm | 62 | 30 | 92 | – | ↑** | ↑** | ↑* | ↓** | ↓** | ↓** | ↓** | Heating of scalp, headache | 14 days | Left frontal corticectomy (3) |
| 3 | Right frontal operculum (E'3–4)/48 mm | 60 | 24 | 84 | – | ↑* | – | – | – | – | – | – | mild headache | 8 days | RFTC for right frontal operculum (2) |
| 4 | Left body of hippocampus (J1–2)/58 mm | 54 | 54 | 108 | – | – | – | – | ↓** | ↓** | ↓** | ↓** | None | 21 days | Left ATL suggested, patient hesitated |
| 5 | Right superior border of insula (F'2)/43 mm | 58 | 26 | 84 | – | ↑* | – | – | – | – | – | ↑** | Naming and memory impairments | 6 hours | RFTC to left insula (5) |
| 6 | Left anterior cingulate gyrus (B1)/54 mm | 58 | 52 | 110 | – | ↑** | ↑** | ↑* | – | – | – | – | mild headache | 4 hours | Left medial frontal RFTC (1.5 m) |
Abbreviations: –, no change; ↑, increase;↓, decrease; ATL, anterior temporal lobectomy; FUS, focused ultrasound; RFTC, radiofrequency thermocoagulation; SEEG, stereo‐electroencephalographic; T1, 10 min prior to treatment; T2, during sonication; T3, 10 min after treatment.
*p < .05, **p < .01.
The patient who underwent two treatment sessions (Patient 2) provided two SEEG datasets. Analysis of the SEEG recordings revealed dose‐dependent effects of treatment (Figure 4D). In the first trial (260 s), there was a slight decrease in the spectral power of the delta band, and significant decreases in spectral powers of theta, alpha, and beta bands after sonication. In the second trial (600 s), the decreases in spectral power were more dramatic between during (T2 period) and after (T3 period) sonication.
4. DISCUSSION
4.1. Application of FUS in DRE patients
In this pilot study, FUS could be safely delivered to the target SOZ in six patients with DRE, and no patients had increased clinical seizures or suffered severe treatment‐emergent adverse events. Although decrements in seizure frequency were seen in only two patients after treatment, the FUS did induce SEEG spectral power changes at the target.
The use of FUS for the treatment of epilepsy has recently attracted widespread attention. High‐intensity FUS (i.e., lesioning effects) 17 , 19 , 20 and low‐intensity FUS (i.e., modulatory effects) 21 have both undergone limited clinical trials. Clinical trials at the University of Virginia and Nicklaus Children's Hospital (Miami, Florida) are using high‐intensity FUS to induce thermal ablation of proposed seizure foci. The targeted regions include tumors or abnormally functioning tissues located deep in the brain, and abnormal brain tissue located in the subcortical regions near the surface of the brain. At the Ohio State University, their goal is to stop the propagation of the seizures by ablating the anterior thalamic nucleus, a critical node involved in the generalization of seizures, which proved to be a good target in previous DBS studies. 22 Our study, similar to work conducted at Brigham Women's Hospital 21 and the University of California, Los Angeles (in press), investigated the neuromodulatory effects of low‐intensity FUS in the treatment of DRE. Researchers hypothesize that the delivery of low‐energy (low‐intensity) pulsed ultrasound to the epileptogenic foci within the brain can alter the neuronal pathways involved in seizures. Table 3 lists previous and ongoing studies on the use of FUS in the treatment of epilepsy.
TABLE 3.
Series or trials of FUS for treatment of epilepsy using low‐intensity FUS
| Series/trial | N | Age, years | Type of FUS device | FUS protocol | Target and Tx goal | SOZ localization | EEG monitor | EEG change | Seizure frequency decrement | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Author, year (site) | ||||||||||
| Yamaguchi, 2020 (Japan) 20 | 1 | 26 | MRgFUS (Insightect, ExAblate) | Six sonications at 50–53°C were applied to five target sites | Hypothalamic hamartoma, lesioning | Via image | No | No spikes post‐FUS | Yes, seizure‐free | Nausea and vomiting |
| Abe, 2020 (Japan) 17 | 1 | 36 | MRgFUS (Insightect, ExAblate) | Repetitive, low power, 10–20 s, 42–44°C | MTS, lesioning | Via image | No | N/A | Yes, seizure‐free for 1 year | Dizziness |
| Parker, 2020 (New York, USA) 19 | 2 | – | MRgFUS (Insightect, ExAblate) | N/A | MTS, lesioning | Via image | No | N/A | N/A | No |
| Brinker, 2020 (BWH, USA) 21 | 1 | 26 | PLIFUS | ISPTA: .5–2.25 W/cm2, duty cycle: 36%−50%, 7‐second interstimulations lasting 140s | MTS, modulation | Via image | Yes, scalp EEG | N/A | N/A | No |
| Current study (Taiwan) | 6 | 26–42 | LIFUP (NaviFUS system) | ISPTA: 2.8 W/cm2, .75 MI, duty cycle: 30%, 600 s | SOZ proved via SEEG, modulation | Via SEEG | Yes, SEEG | Yes | 33%, n = 2/6 | Heat and FND, n = 2/6 |
| Clinical trial [recruiting] | ||||||||||
| Stanford, Mayo Clinic, UVA (USA) | 20 | – | MRgFUS (Insightect, ExAblate) | N/A | SOZ proved via noninvasive study, lesioning | Via image | No | – | – | – |
| Ohio State University (USA) | 10 | – | MRgFUS (Insightect, ExAblate model) | N/A | ANT, lesioning | Via image | No | – | – | – |
| BWH (USA) | 10 | – | PLIFUS | ISPTA: .5–2.25 W/cm2, duty cycle: 36%−50%, interstimulation interval: 7 s, 140 s | MTS, modulation | Via image | Yes, scalp EEG | – | – | – |
| UCLA (USA) | 12 | – | LIFUP (BX pulsar 1002) | ISPTA: .2–.3 W/cm2, duty cycle: 5%, 60 s | Temporal lobe, modulation | Via image | Yes, Scalp EEG | – | – | – |
| MGH (USA) | 3 | – | LIFUP (BX pulsar 1002) | ISPTA: .2–.3 W/cm2, duty cycle: 5%, 60 s | Temporal lobe, modulation | Via image | No | – | – | – |
Abbreviations: ANT, anterior nucleus of thalamus; BWH, Brigham and Women's Hospital; EEG, electroencephalographic; FND, functional neurologic disorder; FUS, focused ultrasound; ISPTA, spatial‐peak temporal‐average intensity; LIFUP, low‐intensity focused ultrasound pulsation; MGH, Massachusetts General Hospital; MI, mechanical index; MRgFUS, magnetic resonance‐guided focused ultrasound; MTS, mesial temporal sclerosis; N/A, not available/applicable; PLIFUS, pulsed low‐intensity focused ultrasound; SOZ, seizure onset zone; Tx, treatment; UCLA, University of California, Los Angeles; UVA, University of Virginia.
4.2. Effects of FUS on EEG spectral power
Animal studies have demonstrated that low‐intensity FUS can be used to modulate brain circuitry and neuronal activity. 11 , 13 In these studies on kainate‐ or PTZ‐treated rats, FUS was shown to suppress the number of epileptic signal bursts in EEG recordings. 11 , 13 In the study by Min et al., the investigators reported suppression of epileptic signal bursts in PTZ‐treated rats. 11 FUS‐mediated reductions in epileptic EEG activity were most obvious in the theta band. 23 , 24 , 25 In a recently published preclinical study, 13 we reported that FUS treatment for a period of 600 s with .75–1.5 mechanical index (MI) and 8%–30% duty cycle was effective in suppressing abnormal bursts in PTZ‐treated rats. The FUS treatment reduced the number of spikes in the abnormal bursts from 45 to 5.4 (p < .001). Unlike animal models with status epilepticus and continuous epileptiform spikes, most patients with epilepsy present focal seizures with ictal and interictal phases. The suppression of epileptic activity in animals is not easily replicated in humans. In this human SEEG study, changes in spectral power were observed during and after sonication. Our results demonstrated that low‐intensity FUS (.75 MI, 30% duty cycle, 600 s) can be safely delivered into the human brain without serious adverse events.
In the current study, we incidentally observed a potential dose–effect relationship in a patient who underwent sonication twice for different durations. The magnitude of the reduction in waveform amplitude was greater following the completion of the longer session, which suggests a relationship between sonication duration and treatment effects (Figure 4D). These findings are in line with those reported by Min et al. 11 They also reported that a second sonication session had more pronounced suppressive effects; however, it must be noted that potential additive effects from the first treatment attempt cannot be excluded.
Knowledge of the means by which FUS mediates cellular discharge could facilitate the establishment of effective FUS parameters. 11 Most recent studies on the use of ultrasound for neuromodulation have been performed under relatively low pressure (<.6 MPa at focus), low frequencies (sub‐MHz), and short pulse durations (≤300 ms), based on the assumption that mechanical effects are the main driver behind the modulation effects. 26 , 27 , 28 , 29 In one study of Xenopus oocyte, 27 mechanical waves induced by FUS appeared to cause membrane stretching, resulting in the opening of various ion channels (e.g., mechanosensitive ion channels). Among the mechanical forces on cellular membranes, it appears that oscillations and acoustic radiation force (ARF) were the main drivers, based on the observation that cavitation‐related brain tissue damage is rare in situations involving pressure of <40 MPa. 30 The treatment protocol in the current study involves pressure values that fall far below the threshold for disruption of the blood–brain barrier (BBB) or the formation of microbubbles. 31 Without disruption of the BBB, changes in membrane capacitance caused by oscillations in FUS‐induced pressure may in turn alter the membrane potential, as manifest in the observed impacts on EEG spectral power without changes in MRI. Steady pressure exerted by ARF on the target throughout the FUS session may stretch cell membranes to such an extent that conformation states of ion channels or other active molecules associated with the membrane are affected. 32 , 33
Therefore, the mechanisms of the neuromodulation observed in our study are better explained by the mechanical effect than the thermal, cavitation, or ablation effect. To seek optimal parameters may be necessary in future studies to achieve durable neuromodulatory effects (e.g., increase in MI, or multiple rounds).
4.3. FUS for SEEG patients: Technical considerations
This study enrolled patients who underwent SEEG for consideration of subsequent surgical intervention. Obvious changes in SEEG spectral power indicated that sonication could potentially have immediate effects. Furthermore, SEEG sampling is more accurate when applied to humans than animals. SEEG makes it possible to approach specific anatomical structures (e.g., hippocampus, amygdale, cingulate, cortical sulci) to observe a range of modulation effects. Despite the risk of SEEG electrodes affecting the trajectory of sonication, this method still appears to be the ideal approach to real‐time monitoring. The very superficial and very deep areas of the brain may limit the use of FUS, due to heating of the dura and skull. Of note, the vibration noise of ultrasound may happen occasionally via depth electrodes, 13 not only at the target but also in the contacts around the target. Electrodes may cause the refraction or diffraction of sound, and it needs to be investigated with simultaneous SEEG signals during sonication in our future study.
4.4. Forced normalization phenomenon after FUS
Patient 5 experienced naming and memory impairment for 8 days from Days 4 to 11 after sonication of the non‐language‐dominant frontal operculum. Given that disturbance of expressive language would be unlikely with sonication of the nondominant hemisphere, we considered the patient's postoperative symptoms consistent with the forced normalization phenomenon. This phenomenon is characterized by the emergence of psychiatric disturbances following the establishment of seizure control or reduction in epileptiform activity on EEG in a patient with previously uncontrolled epilepsy. 34 Seizure reduction was significant in Patient 5, which decreased from 20 attacks/day (baseline) to 10 (Day 1), 11 (Day 2), and 2 (Day 3), coinciding with the emergence of the functional neurological disorders. These symptoms could not be explained by neuroimaging results. The mechanism of forced normalization remains unclear. Hypothesized mechanisms include alteration in the inhibitory mechanisms of the limbic system, antagonism between seizures and psychosis, ongoing status epilepticus in the limbic system, and propagation of epileptiform discharges along unusual pathways. 35 The phenomenon is more common in patients with intellectual disability and DRE, and after neuromodulation (e.g., VNS for patients with Lennox–Gastaut syndrome). 36 , 37 , 38
4.5. Study limitations
This was a Phase 1 pilot study; therefore, sham treatment was not employed, and the number of patients was limited. There are also inherent limitations to any study using SEEG, such as the limited number of contacts in the epileptic network leading to sampling error when the network is scaled up. As the primary purpose of the study was to demonstrate that this low‐intensity FUS system can be safely used in humans based on our prior preclinical work, conclusions regarding its efficacy in producing desirable and durable clinical effects cannot be drawn. These effects may require sonication at higher powers, which is under investigation in our ongoing clinical trial.
5. CONCLUSIONS
Our results demonstrated that low‐intensity FUS can be safely delivered to the target area in patients with DRE without significant adverse events. The changes in neural activities without structural lesion suggested a neuromodulation effect. The results of this Phase 1 pilot study provide evidence supporting further efforts to assess the efficacy of FUS for epilepsy. A larger patient cohort and a wider range of sonication parameters will be required to gain meaningful insight into the use of FUS for the treatment of epilepsy.
CONFLICT OF INTEREST
H.‐L.L. has provided technical consulting service for NaviFUS Corporation, and he currently holds patents relating to biomedical ultrasound. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
H.‐Y.Y., C.‐C.L., C.‐C.C., F.‐J.H., and H.‐L.L. contributed to the study design. H.‐Y.Y., C.‐C.L., C.‐C.C., and Y.‐H.C. recruited patients and collected data. F.‐J.H., Y.‐H.C., and S.‐J.P. performed statistical analysis. Y.‐H.C. collected the image and EEG data. C.‐C.L., H.‐Y.Y., C.‐C.C., and F.‐J.H. wrote the manuscript. H.‐L.L. and C.‐J.C. critically reviewed the article. All authors interpreted the data, reviewed the manuscript, and approved the final version.
ETHICS APPROVAL
This study was approved by the institutional review board of Taipei Veterans General Hospital (2018–07‐002A).
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of the Brain Research Center, National Yang Ming Chiao Tung University, through the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project of the Ministry of Education of Taiwan. We are grateful to our research assistant, Li‐Chun Wang, for data recording and transcription.
Lee C‐C, Chou C‐C, Hsiao F‐J, Chen Y‐H, Lin C‐F, Chen C‐J, et al. Pilot study of focused ultrasound for drug‐resistant epilepsy. Epilepsia. 2022;63:162–175. doi: 10.1111/epi.17105
Funding information
This study was funded by NaviFUS Corporation; this involved only technique support, not study design, patient enrollment, patient outcome analysis, or interpretation of data. Although this is an industry‐sponsored study, the principal investigator and coinvestigators had full power to handle the study without any interference from company, including report writing and the decision to submit the paper for publication. The study was also supported in part by the Taiwan Ministry of Science and Technology (107‐2314‐B‐075‐059‐MY3 and 109‐2314‐B‐075‐053) and National Health Research Institutes (NHRI‐EX109‐10905NI, NHRI‐EX110‐11006NC). The support was mainly in personnel, the clinical database, and supplies related to SEEG.
REFERENCES
- 1. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. [DOI] [PubMed] [Google Scholar]
- 2. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group . A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med. 2001;345(5):311–8. [DOI] [PubMed] [Google Scholar]
- 3. Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, et al. Surgery for drug‐resistant epilepsy in children. N Engl J Med. 2017;377(17):1639–47. [DOI] [PubMed] [Google Scholar]
- 4. Tellez‐Zenteno JF, Dhar R, Wiebe S. Long‐term seizure outcomes following epilepsy surgery: a systematic review and meta‐analysis. Brain. 2005;128(5):1188–98. [DOI] [PubMed] [Google Scholar]
- 5. Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46(Suppl 1):30–1. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Otaibi FA, Hamani C, Lozano AM. Neuromodulation in epilepsy. Neurosurgery. 2011;69(4):957–79. [DOI] [PubMed] [Google Scholar]
- 7. Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127(3289):83–4. [DOI] [PubMed] [Google Scholar]
- 8. Gavrilov LR, Tsirulnikov EM, Davies IA. Application of focused ultrasound for the stimulation of neural structures. Ultrasound Med Biol. 1996;22(2):179–92. [DOI] [PubMed] [Google Scholar]
- 9. Harvey EN. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am J Physiol Legacy Content. 1929;91(1):284–90. [Google Scholar]
- 10. Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17(2):322–9. [DOI] [PubMed] [Google Scholar]
- 11. Min B‐K, Bystritsky A, Jung K‐I, Fischer K, Zhang Y, Maeng L‐S, et al. Focused ultrasound‐mediated suppression of chemically‐induced acute epileptic EEG activity. BMC Neurosci. 2011;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hakimova H, Kim S, Chu K, Lee SK, Jeong B, Jeon D. Ultrasound stimulation inhibits recurrent seizures and improves behavioral outcome in an experimental model of mesial temporal lobe epilepsy. Epilepsy Behav. 2015;49:26–32. [DOI] [PubMed] [Google Scholar]
- 13. Chen SG, Tsai CH, Lin CJ, Lee CC, Yu HY, Hsieh TH, et al. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. 2020;13:35‐46. [DOI] [PubMed] [Google Scholar]
- 14. Odéen H, de Bever J, Almquist S, Farrer A, Todd N, Payne A, et al. Treatment envelope evaluation in transcranial magnetic resonance‐guided focused ultrasound utilizing 3D MR thermometry. J Ther Ultrasound. 2014;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talairach J, Bancaud J, Szikla G, Bonis A, Geier S, Vedrenne C. New approach to the neurosurgery of epilepsy. Stereotaxic methodology and therapeutic results. 1. Introduction and history [in French]. Neurochirurgie. 1974;20(Suppl 1):1–240. [PubMed] [Google Scholar]
- 16. Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57(4):706–18. [PubMed] [Google Scholar]
- 17. Abe K, Yamaguchi T, Hori H, Sumi M, Horisawa S, Taira T, et al. Magnetic resonance‐guided focused ultrasound for mesial temporal lobe epilepsy: a case report. BMC Neurol. 2020;20(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user‐friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parker WE, Weidman EK, Chazen JL, Niogi SN, Uribe‐Cardenas R, Kaplitt MG, et al. Magnetic resonance–guided focused ultrasound for ablation of mesial temporal epilepsy circuits: modeling and theoretical feasibility of a novel noninvasive approach. J Neurosurg. 2020;133(1):63–70. [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi T, Hori T, Hori H, Takasaki M, Abe K, Taira T, et al. Magnetic resonance–guided focused ultrasound ablation of hypothalamic hamartoma as a disconnection surgery: a case report. Acta Neurochir. 2020;162(10):2513–7. [DOI] [PubMed] [Google Scholar]
- 21. Brinker ST, Preiswerk F, White PJ, Mariano TY, McDannold NJ, Bubrick EJ. Focused ultrasound platform for investigating therapeutic neuromodulation across the human hippocampus. Ultrasound Med Biol. 2020;46(5):1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R , et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. [DOI] [PubMed] [Google Scholar]
- 23. Kubova H, Mockova M, Mares P. Midazolam suppresses spike‐and‐wave rhythm accompanying three different models of epileptic seizures. Physiol Res. 1999;48:491–500. [PubMed] [Google Scholar]
- 24. Zhou YD, Lee S, Jin Z, Wright M, Smith SE, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konopacki J, Golebiewski H, Eckersdorf B, Kowalczyk T, Bocian R. In vitro recorded theta‐like activity in the limbic cortex: comparison with spontaneous theta and epileptiform discharges. Acta Neurobiol Exp. 2000;60:67–85. [DOI] [PubMed] [Google Scholar]
- 26. di Biase L, Falato E, Di Lazzaro V. Transcranial focused ultrasound (tFUS) and transcranial unfocused ultrasound (tUS) neuromodulation. From theoretical principles to stimulation practices. Front Neurol. 2019;10:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J. Ultrasound modulates ion channel currents. Sci Rep. 2016;6(1):24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tyler WJ. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 2011;17:25–36. [DOI] [PubMed] [Google Scholar]
- 29. Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low‐intensity, low‐frequency ultrasound. PLoS One. 2008;3(10):e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–48. [DOI] [PubMed] [Google Scholar]
- 31. Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48(4):279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duck FA, Baker AC, Starritt HC. Ultrasound in medicine. Boca Raton, FL: CRC Press; 1978. [Google Scholar]
- 33. Kubanek J, Shukla P, Das A, Baccus SA, Goodman MB. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J Neurosci. 2018;38(12):3081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landolt H. Some clinical EEG correlations in epileptic psychoses (twilight states). Electroencephalogr Clin Neurophysiol. 1953;5:121. [Google Scholar]
- 35. Wolf P. Acute behavioral symptomatology at disappearance of epileptiform EEG abnormality. Paradoxical or "forced" normalization. Adv Neurol. 1991;55:127–42. [PubMed] [Google Scholar]
- 36. Gatzonis SD, Stamboulis E, Siafakas , Angelopoulos E, Georgaculias N, Sigounas E, et al. Acute psychosis and EEG normalisation after vagus nerve stimulation. J Neurol Neurosurg Psychiatry. 2000;69(2):278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumer D, Davies K, Alexander A, Morgan S. Major psychiatric disorders subsequent to treating epilepsy by vagus nerve stimulation. Epilepsy Behav. 2001;2(5):466–72. [DOI] [PubMed] [Google Scholar]
- 38. Lee S, Denton A, Ladino LD, Waterhouse K, Vitali A, Tellez‐Zenteno JF. Forced normalization after turning off vagus nerve stimulation in Lennox–Gastaut syndrome. Epilepsy Behav Case Rep. 2019;11:81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1


