Summary
Multiple myeloma (MM) is characterized by malignant plasma cell infiltration of the bone marrow. In extramedullary multiple myeloma (EMD), a subclone of these cells migrates out of the bone marrow. Out of 4 985 MM patients diagnosed between 2005 and 2017 in the Czech Republic, we analyzed 234 secondary EMD patients to clarify risk factors of secondary EMD development. We found younger age [<65 years; odds ratio (OR) 4·38, 95% confidence interval (CI): 2·46–7·80, P < 0·0001], high lactate dehydrogenase (LDH) levels (>5 μkat/l; OR 2·07, 95% CI: 1·51–2·84, P < 0·0001), extensive osteolytic activity (OR 2·21, 95% CI: 1·54–3·15, P < 0·001), and immunoglobulin A (IgA; OR 1·53, 95% CI: 1·11–2·11, P = 0·009) or the non‐secretory type of MM (OR 2·83; 95% CI: 1·32–6·04, P = 0·007) at the time of MM diagnosis to be the main risk factors for secondary EMD development. Newly diagnosed MM (NDMM) patients with subsequent EMD had inferior median progression‐free (PFS) and overall (OS) survival when compared to NDMM patients without future EMD [mPFS: 13·8 months (95% CI: 11·4–16·3) vs 18·8 months (95% CI: 17·7–19·9), P = 0·006; mOS: 26·7 months (95% CI: 18·1–35·4) vs 58·7 months (95% CI: 54·8–62·6), P < 0·001]. We found that NDMM patients with specific risk factors associated with secondary EMD development have a more aggressive disease course before secondary EMD develops.
Keywords: multiple myeloma, extramedullary disease, prognostic factors
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy. It accounts for 1·7% of all cancers and 10% of all haematological malignancies. 1 Average incidence in Europe is 5/100 000. 2 , 3 In the last 20 years, novel drugs [proteasome inhibitors (PIs), immunomodulatory drugs (IMIDs), monoclonal antibodies, etc.] have significantly prolonged survival of newly diagnosed MM (NDMM) as well as relapsed/refractory MM (RRMM) patients. 1 , 4
Better imaging techniques [computed tomography (CT), positron emission tomography (PET), PET‐CT or magnetic resonance imaging (MRI)] show higher detection rates of so‐called extramedullary myeloma (EMD). 5 , 6 , 7 , 8 , 9 In EMD, a subclone of plasma cells (PCs) migrates out of the bone marrow (BM) infiltrating soft tissues. 10 Changes in adhesion as well as secondary genetic changes in this subclone have been described, including TP53 mutations, translocation t(4;14), deletion del(13), etc. 6 , 11 , 12 However, causes of EMD have not been clarified.
EMD is classified as primary EMD (found at the time of MM diagnosis) and secondary EMD (at the time of MM relapse); clinical behaviour of primary and secondary EMD is markedly different. 7 , 11 While prognosis of primary EMD versus NDMM without EMD is similar, 13 , 14 secondary EMD is associated with a poor prognosis. 6 We previously showed that in secondary EMD patients, the worst prognosis was observed in soft‐tissue EMD (EMD‐S), when PCs completely lose their dependence on the BM microenvironment, infiltrating soft tissues. On the other hand, extramedullary lesions arising from bone (EMD‐B) have relatively better prognosis. 7
According to unsatisfactory treatment outcomes, there is a clinical need to diagnose patients with high risk of secondary EMD development as early as possible. Unfortunately, there is a lack of evidence about clinical features of the patients before secondary EMD develops. Therefore, we analyzed disease course before EMD appearance in a real‐life group of secondary EMD patients.
Methods
Clinical characteristics of patients
This real‐life retrospective study was carried out at haematological centres in the Czech Republic between 2005 and 2017. All MM patients’ data were recorded in the Registry of Monoclonal Gammopathies (RMG) of the Czech Myeloma Group. All participants provided written informed consent approved by institutional Ethics boards in accordance with the latest Helsinki declaration.
In total, 4 985 MM patients were evaluated; 543 EMD patients (10·9%) were found. Out of this number, 309 primary EMD and 234 secondary EMD patients were identified. As reference, 2 092 MM patients with no EMD involvement during the entire follow‐up period were included — we excluded living patients with shorter follow‐up than five years.
Secondary EMD was found in 111 patients at first relapse, in 61 at second and 62 at third or higher relapse. Median follow‐up of secondary EMD patients from the time of MM diagnosis was 3·8 years. In secondary EMD patients, 61·1% (143/234) of patients had EMD‐B, 30·3% (71/234) had EMD‐S, and 8·6% (20/234) of patients were missing data. Only first occurrences of EMD were evaluated.
Before secondary EMD diagnosis, 19·2% of patients were treated with PIs, 22·6% with IMIDs, and 41·5% of patients with both PIs and IMIDs; 16·7% of patients were treated with conventional chemotherapy without novel drugs, and 54·2% of patients underwent autologous stem cell transplant (ASCT). No obligatory diagnostic protocol was used in this study. Diagnostic methods and clinical evaluations at the time of secondary EMD diagnosis were used in a real‐life setting corresponding to patients’ symptoms and actual clinical availability of diagnostics.
Diagnostics of secondary EMD lesions
In secondary EMD patients, EMD lesions were detected in 119 patients by skeletal survey, in 41 patients by MRI, in 21 patients by CT and in 12 by PET/CT. EMD involvement was also diagnosed by other methods, i.e. scintigraphy, ultrasonography, endoscopy or clinical evaluation. Findings of EMD lesions were confirmed by surgical sampling and histology evaluation, when clinically needed and safe for the patient.
Cytogenetics
Interphase fluorescent in situ hybridization (I‐FISH) analysis was performed on separated PCs as previously described 12 at the time of MM diagnosis.
Statistics
Data were described by absolute and relative frequencies of categorical variables. Logistic regression analysis was used to assess the association of baseline characteristics at MM diagnosis with EMD occurrence in relapse. Differences in overall (OS) and progression‐free (PFS) survival between patients with future EMD and RRMM group not evolving EMD according to line of therapy was computed by the Kaplan–Meier method and statistical significance of differences in survival among subgroups was assessed using the log‐rank test. The same methodology was used for identification of secondary EMD as prognostic factor of survival in RRMM patients. Treatment response was assessed according to the current International Myeloma Working Group (IMWG) criteria. 15 Independence of secondary EMD as a prognostic survival factor was verified in a multivariable Cox proportional hazard model in context of other well‐known prognostic factors. All statistical tests were performed at a significance level of α = 0·05 (all tests two‐sided). Analysis was performed in the SPSS software (release 2017: IBM SPSS Statistics for Windows, Version 25·0·0·1; IBM Corp. Armonk, NY, USA) and R version 4·0·1. (www.r‐project.org).
Results
Clinical features associated with secondary EMD development
Clinical characteristics at the time of MM diagnosis of both secondary EMD patients as well as reference MM patients are summarized in Table I. We compared these groups and identified associations between clinical, laboratory and cytogenetic features at MM diagnosis and risk for subsequent development of EMD (Fig 1).
Fig 1.
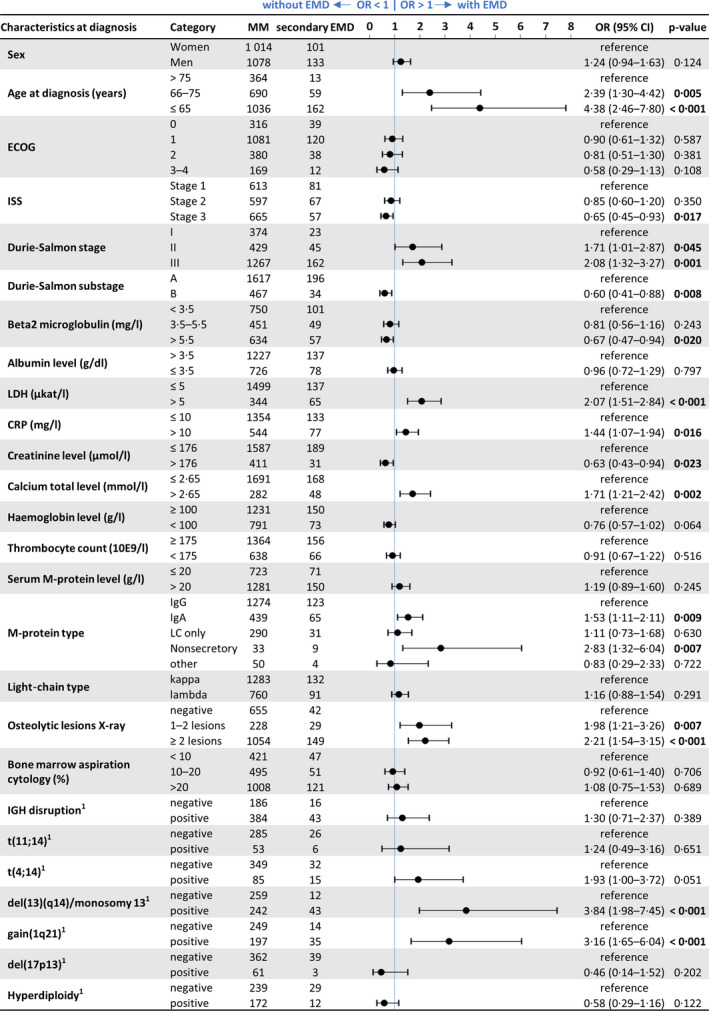
Clinical features measured at diagnosis in multiple myeloma (MM) and secondary extramedullary multiple myeloma (EMD) patients. CI, confidence interval; CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; IGH, immunoglobulin heavy chain; ISS, International Staging System; LDH, lactate dehydrogenase; OR, overall response. [Colour figure can be viewed at wileyonlinelibrary.com]
In younger NDMM patients (<65 years), there was a significantly higher risk of secondary EMD development [odds ratio (OR) 4·38; 95% confidence interval (CI): 2·46–7·80, P < 0·0001). Moreover, NDMM patients who developed secondary EMD had significantly higher LDH levels (>5 μkat/l; OR 2·07, 95% CI: 1·51–2·84, P < 0·0001), more than two osteolytic lesions diagnosed by skeletal survey (OR 2·21, 95% CI: 1·54–3·15, P < 0·001), hypercalcaemia (>2·65 mmol/l; OR 1·71, 95% CI: 1·21–2·42, P = 0·002) and IgA M‐protein type (OR 1·53, 95% CI: 1·11–2·11, P = 0·009) or the non‐secretory type of MM (OR 2·83, 95% CI: 1·32–6·04, P = 0·007).
In NDMM patients who subsequently developed secondary EMD, there were significant differences in the presence of del(13)(q14) (48·3% vs 78·2%, P < 0·001) and gain (1q21) (44·2% vs 71·4%, P < 0·001). While other aberrations were analyzed, they were not statistically significant. Detailed results of I‐FISH analysis are shown in Table SI.
Survival of MM patients before secondary EMD development
We compared survival intervals of MM patients with secondary EMD involvement in the next relapse/progression with patients without any EMD involvement in the future. We analyzed survival from the start of the first line of treatment according to the state of EMD in the second line based on a condition that both groups had to initiate the second line of treatment. Patients who developed EMD in the third or higher lines were not included in this calculation. We proceeded analogously for survival from the second and third lines of therapy (Fig 2).
Fig 2.
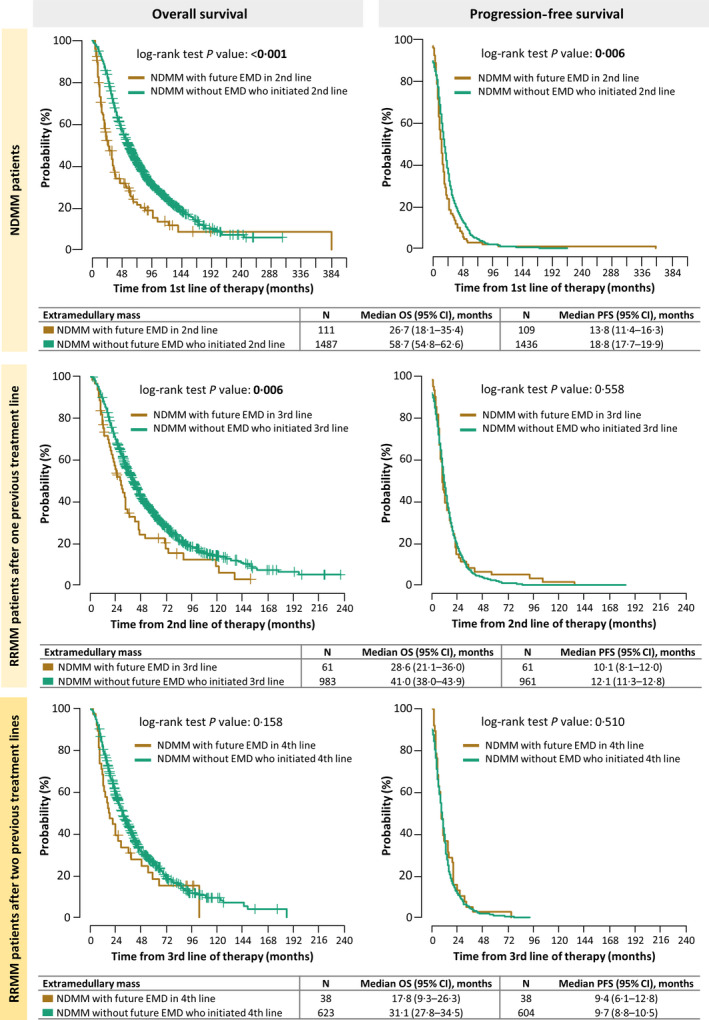
Effect of future secondary extramedullary multiple myeloma (EMD) development on progression‐free (PFS) and overall (OS) survival in separate treatment lines. CI, confidence interval; NDMM, newly diagnosed multiple myeloma; RRMM, as relapsed/refractory multiple myeloma. [Colour figure can be viewed at wileyonlinelibrary.com]
NDMM patients. NDMM patients who subsequently developed EMD had significantly shorter median PFS, when compared to NDMM patients without future EMD involvement (13·8 months, 95% CI: 11·4–16·3 vs 18·8 months, 95% CI: 17·7–19·9; P = 0·006). Median OS was significantly shorter in NDMM patients who subsequently developed secondary EMD when compared to NDMM patients without any future EMD involvement (26·7 months, 95% CI: 18·1–35·4 vs 58·7 months, 95% CI: 54·8–62·6; P < 0·001).
RRMM patients after one previous treatment line. RRMM patients who developed EMD in the next disease progression and RRMM patients without future EMD involvement had comparable median PFS (10·1 months, 95% CI: 8·1–12·0 vs 12·1 months, 95% CI: 11·3–12·8; P = 0·558). Median OS was significantly shorter in RRMM patients who developed EMD when compared to RRMM without any future EMD involvement (28·6 months, 95% CI: 21·1–36·0 vs 41·0 months, 95% CI: 38·0–43·9; P = 0·006).
RRMM patients after two previous treatment lines. RRMM patients after two previous treatment lines who developed EMD in the next disease progression and RMMM patients without future EMD involvement had comparable median PFS (9·4 months, 95% CI: 6·1–12·8 vs 9·7 months, 95% CI: 8·8–10·5; P = 0·510). While there was trend toward worse OS between RRMM patients who developed EMD and RRMM patients who did not (17·8 months, 95% CI: 9·3–26·3 vs 31·1 months, 95% CI: 27·8–34·5; P = 0·158), these results were not statistically significant.
Survival after the secondary EMD development
Multivariate analysis showed secondary EMD as an independent risk factor for PFS [hazard ratio (HR) 1·39, 95% CI: 1·06–1·81, P = 0·016] and OS (HR 1·61, 95% CI: 1·20–2·15, P = 0·001) of RRMM patients. From the time of secondary EMD diagnosis, median PFS was 4·7 months (95% CI: 3·5‐5·8) and median OS was 8·6 months (95% CI: 6·3‐11·0).
Discussion
As a result of remarkable progress in the treatment of MM, it is slowly turning into a chronic disease. Due to the widespread use of new drugs, better treatment results are achieved even at MM relapse. In case of relapsed or even refractory MM patients, the disease may become stabilized several times. 1 , 4 , 16 , 17 , 18 , 19
At the same time, extramedullary myeloma is still a challenge even in the era of new drugs. Especially, secondary EMD is a hard‐to‐treat entity associated with poor prognosis. 7 , 13 Standard widespread treatment protocols for RRMM patients based on bortezomib, lenalidomide or pomalidomide do not significantly improve prognosis of secondary EMD. 20 , 21 , 22 , 23 , 24 There are only limited data for the second‐generation PI carfilzomib in this patient population. Case reports and analyses of small cohorts showed unsatisfactory results, far worse than in non‐EMD patients. 25 , 26 , 27 , 28 Similarly, ixazomib did not show significant improvement of secondary EMD prognosis in real‐life analysis. 29 Anti‐CD38 antibodies (daratumumab, isatuximab) have not overcome poor prognosis of secondary EMD, possibly due to low expression of the CD38 surface antigen in EMD PCs. 30 , 31 A much lower response rate was also shown in a subset of EMD patients treated with the anti‐BCMA (B cell maturation antigen) drug conjugate belantamab. 32 Preliminary results of the peptide–drug conjugate melphalan flufenamide (melflufen) in a heavily pretreated cohort of secondary EMD patients seem promising, but longer follow‐up is necessary. 33 In the current era of immunotherapy, promising results were achieved in several trials of BCMA‐targeted CAR‐T cells. The CAR‐T cells had impressive results even in secondary EMD‐S patients, resulting in longer remissions in some trials. 34 , 35 , 36
Unfortunately, there are no current protocols for secondary EMD treatment.
For better understanding of development of secondary EMD, we identified 234 secondary EMD among almost 5 000 MM patients diagnosed in Czech haematologic centres from 2005 to 2017. To the best of our knowledge, this dataset is the largest in the world so far. With respect to our retrospective cohort and relatively low number of patients pretreated by both IMIDs and PIs, as a recently most frequent induction treatment regimen, 32 , 37 , 38 , 39 , 40 we found similar numbers of EMD patients as in recently published large clinical trials focusing on (PI + IMIDs)‐exposed patients. 41 , 42 These results show PI and IMIDs do not induce EMD; we indirectly confirmed that fact in a much larger cohort of patients. 22 Moreover, similar numbers of EMD patients were found in previous analyses of heavily pretreated patients 21 , 22 when compared to recent clinical trials, focusing on triple or penta‐refractory patients. 43 , 44 , 45 , 46 From those findings, we suppose that modern treatment regimens including a new generation of IMIDs, PI and monoclonal antibodies do not induce more EMD. Comparing length of previous treatment in different analyses and incidence of EMD, time of disease duration seems to play an important role in EMD development. 21 Thus, we focused on patient‐ and disease‐related factors as the most important aspects in EMD development.
In our analysis, younger age, extensive bone disease (numerous osteolytic lesions and hypercalcaemia), higher LDH and IgA or a non‐secretory type of MM at the time of MM diagnosis were significantly associated with further development of secondary EMD. In similar studies, comparable results for younger age, type of monoclonal immunoglobulin and extensive bone disease were found. 21 In another analysis, hypercalcaemia was also confirmed as a risk factor for secondary EMD development. 22
We found an increased presence of del(13)/monosomy13 and gain (1q21) at the time of MM diagnosis in patients with future secondary EMD. These results are in concordance with our previous results that showed increased presence of both aberrations at the time of EMD diagnosis both in primary and secondary EMD patients. 47 In other analyses, del (17p) was found to be the most frequent cytogenetic aberration in EMD patients, both in BM and in the extramedullary tumour site. 48 , 49 , 50 We did not observe a higher incidence of del (17p) or other tested aberrations in patients with future EMD development. Possibly, subsequent acquisition of del(17p) in the disease course, influencing secondary EMD development, occurs. 51 , 52 However, with respect to the low number of analyzed EMD samples in our study, more robust cytogenetic analyses need to be performed.
In our study, NDMM patients before EMD development had significantly inferior median PFS when compared to NDMM patients who never developed EMD. We presume that NDMM patients who progress to EMD early have a specific and more aggressive disease from the onset of MM. A similar situation was described in NDMM patients with high‐risk cytogenetics when the disease course was impaired from the beginning. 53 , 54 While EMD pathogenesis has not been clarified yet, there may be a hidden molecular mechanism affecting disease course before EMD is revealed. Thus, these patients subsequently manifest with extramedullary involvement, leading to further disease escalation and early death.
Surprisingly, more advanced RRMM patients who developed EMD in the further disease course had comparable treatment outcomes as reference RRMM patients who never developed EMD. However, like in NDMM patients with EMD progression, their prognosis dramatically changed with EMD development. 6
These clinical observations may be explained by more sudden changes in the MM clone leading to EMD involvement in advanced MM patients. 55 As previously described, treatment‐related factors do not seem to be involved in EMD development. 21 Another explanation may be a sudden loss of balance between different subclones leading to expansion of an aggressive clone, independent of the BM environment. 56 Unfortunately, mechanisms leading to these changes in secondary EMD patients remain unknown. Regardless of the time of the secondary EMD development, patients` outcomes remain poor. 6
A clear limitation of our analysis was the low number of highly sensitivity diagnostic methods such as PET/CT or whole‐body MRI. These limitations arise from the retrospective character of this study and time of our data collection during which access to these diagnostic methods dramatically changed. 9 , 57 , 58 , 59 In our study, the most frequent diagnostic method was X‐ray, what clearly could lead to bias from undetected small EMD‐B or asymptomatic EMD‐S lesions. 9 On the other hand, incidence of secondary EMD in our cohort was comparable to that in the longitudinal real‐life study (1971–2007) published by Varettoni et al. 6 In a newer study, only 3% of secondary EMD were found threenyears after MM diagnosis. 23 A similar incidence of secondary EMD (3–7%) within five years from the diagnosis was found by the Arkansas group while using PET/CT in diagnostic work‐up. 11 Taken together, according to recent recommendations, PET/CT or whole‐body MRI clearly reveals more EMD patients due to their well‐defined sensitivity. 9 , 57 In our study, the approach based on combining basic diagnostic methods according to patient`s symptoms had acceptable results.
Despite the absence of clearly defined data from clinical trials, an aggressive treatment approach to EMD patients is generally accepted. 60 , 61 Using high‐sensitive diagnostic methods, describing clinical features of MM patients at high risk of EMD development may lead to closer follow‐up. We believe that a future focus on the pre‐EMD period may lead to improvement of the poor prognosis of these patients. More clinical and molecular analyses should be performed to identify the optimal treatment approach.
Conclusion
We found that MM patients with future EMD development show specific features (younger age, extensive bone disease, IgA, or non‐secretory type of MM) which are present already at the time of MM diagnosis. In NDMM patients, secondary EMD developed shortly after MM diagnosis, showing the aggressive disease pattern from the beginning. In more pre‐treated MM patients, disease course before secondary EMD development was similar to that in other MM patients. After a yet unknown event, EMD occurs probably as a terminal event in MM evolution. Regardless of time, when PCs lose their dependence on the BM microenvironment, there is an absolute turnover of the disease course, leading to early death. We confirmed secondary EMD development as a strong independent negative prognostic factor in MM.
Authors contribution
MS, SS and LP designed the study. PK, JR, IS, JS, PP, AJ, TJ, VS, VM and RH performed research. MS, SS, LP and JM co‐wrote the paper. LP, LB and JJ analyzed data. All authors critically reviewed and approved the manuscript.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the University Hospital Brno, Czech Republic (2016).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of interest
The authors declare to have no conflicts of interest. The funders had no role in the design of the investigation, in the collection, analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the results”.
Supporting information
Table SI. Cytogenetic aberrations at the time of MM diagnosis (MM versus secondary EMD patients).
Acknowledgements
This work was supported by grant AZV 17‐29343A. The authors would like to thank all the patients and their caregivers who participated in this study, and all data managers from participating centres in the Czech Republic.
References
- 1. Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos M‐V, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:nrdp201746. [DOI] [PubMed] [Google Scholar]
- 2. Malúšková D, Svobodová I, Kučerová M, Brožová L, Mužík J, Jarkovský J, et al. Epidemiology of multiple myeloma in the Czech Republic. Klin Onkol. 2017;30(Suppl 2):35–42. [DOI] [PubMed] [Google Scholar]
- 3. Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–34. [DOI] [PubMed] [Google Scholar]
- 4. Morgan GJ, Rasche L. Haematological cancer: where are we now with the treatment of multiple myeloma? Nat Rev Clin Oncol. 2017;14(8):461–2. [DOI] [PubMed] [Google Scholar]
- 5. Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R. Extramedullary disease in multiple myeloma ‐ controversies and future directions. Blood Rev. 2019;36:32–9. [DOI] [PubMed] [Google Scholar]
- 6. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21(2):325–30. [DOI] [PubMed] [Google Scholar]
- 7. Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft‐tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone‐related extramedullary relapse. Haematologica. 2014;99(2):360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18‐F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up‐front autologous transplantation. Blood. 2011;118(23):5989–95. [DOI] [PubMed] [Google Scholar]
- 9. Minarik J, Hrbek J, Pika T, Novak M, Bacovsky J, Heman M, et al. X‐ray in multiple myeloma ‐ not a golden standard any more: case series. J Bone Marrow Res. 2014;02. [Google Scholar]
- 10. Menu E, Asosingh K, Indraccolo S, Raeve HD, Riet IV, Valckenborgh EV, et al. The involvement of stromal derived factor 1alpha in homing and progression of multiple myeloma in the 5TMM model. Haematologica. 2006;91(5):605–12. [PubMed] [Google Scholar]
- 11. Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over‐represented in high‐risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besse L, Sedlarikova L, Greslikova H, Kupska R, Almasi M, Penka M, et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur J Haematol. 2016;97(1):93–100. [DOI] [PubMed] [Google Scholar]
- 13. Beksac M, Seval GC, Kanellias N, Coriu D, Rosiñol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. 2020;105(1):201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montefusco V, Gay F, Spada S, De Paoli L, Di Raimondo F, Ribolla R, et al. Outcome of paraosseous extra‐medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica. 2020;105(1):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M‐V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos MA, Oriol A, Nahi H, San‐Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375(14):1319–31. [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med. 2018;379(19):1811–22. [DOI] [PubMed] [Google Scholar]
- 18. Richardson PG, Attal M, Rajkumar SV, San Miguel J, Beksac M, Spicka I, et al. A phase III randomized, open label, multicenter study comparing isatuximab, pomalidomide, and low‐dose dexamethasone versus pomalidomide and low‐dose dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2019;37(Suppl 15):8004. [Google Scholar]
- 19. Chari A, Martinez‐Lopez J, Mateos M‐V, Bladé J, Benboubker L, Oriol A, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019;134(5):421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laura R, Cibeira MT, Uriburu C, Yantorno S, Salamero O, Blade J, et al. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur J Haematol. 2006;76(5):405–8. [DOI] [PubMed] [Google Scholar]
- 21. Mangiacavalli S, Pompa A, Ferretti V, Klersy C, Cocito F, Varettoni M, et al. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann Hematol. 2017;96(1):73–80. [DOI] [PubMed] [Google Scholar]
- 22. Varga C, Xie W, Laubach J, Ghobrial IM, O'Donnell EK, Weinstock M, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide–bortezomib combinations. Br J Haematol. 2015;169(6):843–50. Available from: http://www.readcube.com/articles/ 10.1111/bjh.13382 [DOI] [PubMed] [Google Scholar]
- 23. Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25(6):906–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiménez‐Segura R, Granell M, Gironella M, Abella E, García‐Guiñón A, Oriol A, et al. Pomalidomide‐dexamethasone for treatment of soft‐tissue plasmacytomas in patients with relapsed/refractory multiple myeloma. Eur J Haematol. 2019;102(5):389–94. [DOI] [PubMed] [Google Scholar]
- 25. Zhou X, Flüchter P, Nickel K, Meckel K, Messerschmidt J, Böckle D, et al. Carfilzomib based treatment strategies in the management of relapsed/refractory multiple myeloma with extramedullary disease. Cancers. 2020;12(4):1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Español I, Romera M, Gutiérrez‐Meca MD, García MDC, Tejedor A, Martínez A, et al. Carfilzomib and dexamethasone for extramedullary myeloma with pleuropericardial involvement. Clin Case Rep. 2017;5(8):1258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atalay F. The use of carfilzomib to treat extramedullary plasmacytoma and review of the literature [Internet]. 2020. [cited 2021 Sep 27]. Available from: https://www.jahjournal.org/article.asp?issn=1658‐5127;year=2020;volume=11;issue=2;spage=74;epage=76;aulast=Atalay
- 28. Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728–34. [DOI] [PubMed] [Google Scholar]
- 29. Minarik J, Pika T, Radocha J, Jungova A, Straub J, Jelinek T, et al. Survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (Rd) in relapsed and refractory multiple myeloma patients in routine clinical practice. BMC Cancer. 2021;21(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jelinek T, Sevcikova T, Zihala D, Popkova T, Kapustova V, Broskevicova L, et al. Limited efficacy of daratumumab in multiple myeloma with extramedullary disease. Leukemia. 2021;1–4. Online ahead of print. 10.1038/s41375-021-01343-w [DOI] [PubMed] [Google Scholar]
- 31. Beksac M, Richardson PG, Unal A, Corradini P, DeLimpasi S, Gulbas Z, et al. Isatuximab Plus Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma and Soft‐Tissue Plasmacytomas: Icaria‐MM Subgroup Analysis. In ASH; 2020. [cited 2021 Sep 27]. Available from: https://ash.confex.com/ash/2020/webprogram/Paper134727.html
- 32. Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Longer term outcomes with single‐agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13‐month follow‐up from the pivotal DREAMM‐2 study. Cancer [Internet]. 2021. [cited 2021 Sep 27];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/ 10.1002/cncr.33809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson PG, Mateos M‐V, Oriol A, Larocca A, Cavo M, Rodríguez‐Otero P, et al. MM‐186: HORIZON (OP‐106): Melflufen Plus Dexamethasone in 55 Patients with Relapsed/Refractory Multiple Myeloma (RRMM) with Extramedullary Disease (EMD): subgroup analysis. Clin Lymphoma Myeloma Leuk. 2021;21:S427. [Google Scholar]
- 34. Wang DI, Wang J, Hu G, Wang W, Xiao YI, Cai H, et al. A phase 1 study of a novel fully human BCMA‐targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood. 2021;137(21):2890–901. [DOI] [PubMed] [Google Scholar]
- 35. Raje NS, Siegel DS, Jagannath S, Lonial S, Munshi NC, Moreau P, et al. Idecabtagene Vicleucel (ide‐cel, bb2121) in relapsed and refractory multiple myeloma: analyses of high‐risk subgroups in the KarMMa study. Blood. 2020;136(Suppl 1):37–8. [Google Scholar]
- 36. Deng H, Liu M, Yuan T, Zhang H, Cui R, Li J, et al. Efficacy of humanized anti‐BCMA CAR T cell therapy in relapsed/refractory multiple myeloma patients with and without extramedullary disease. Front Immunol. 2021;12:3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gay F, Cerrato C, Petrucci MT, Zambello R, Gamberi B, Ballanti S, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(Suppl 15):8002. [Google Scholar]
- 38. Cavo M, Pantani L, Pezzi A, Petrucci MT, Patriarca F, Di Raimondo F, et al. Bortezomib‐thalidomide‐dexamethasone (VTD) is superior to bortezomib‐cyclophosphamide‐dexamethasone (VCD) as induction therapy prior to autologous stem cell transplantation in multiple myeloma. Leukemia. 2015;29(12):2429–31. [DOI] [PubMed] [Google Scholar]
- 39. Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013‐04 trial. Blood. 2016;127(21):2569–74. [DOI] [PubMed] [Google Scholar]
- 40. Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG S0777): a randomised, open‐label, phase 3 trial. Lancet. 2017;389(10068):519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(6):781–94. [DOI] [PubMed] [Google Scholar]
- 42. Attal M, Richardson PG, Rajkumar SV, San‐Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low‐dose dexamethasone versus pomalidomide and low‐dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA‐MM): a randomised, multicentre, open‐label, phase 3 study. Lancet. 2019;394(10214):2096–107. [DOI] [PubMed] [Google Scholar]
- 43. Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM‐2): a two‐arm, randomised, open‐label, phase 2 study. Lancet Oncol. 2020;21(2):207–21. [DOI] [PubMed] [Google Scholar]
- 44. Richardson PG, Bringhen S, Voorhees P, Plesner T, Mellqvist U‐H, Reeves B, et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O‐12‐M1): a multicentre, international, open‐label, phase 1–2 study. Lancet Haematol. 2020;7(5):e395–407. [DOI] [PubMed] [Google Scholar]
- 45. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B‐cell maturation antigen‐directed chimeric antigen receptor T‐cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE‐1): a phase 1b/2 open‐label study. Lancet. 2021;398(10297):314–24. [DOI] [PubMed] [Google Scholar]
- 47. Besse L, Sedlarikova L, Kryukov F, Nekvindova J, Radova L, Slaby O, et al. Circulating serum microRNA‐130a as a novel putative marker of extramedullary myeloma. PLoS One. 2015;10(9):e0137294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Billecke L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol. 2013;161(1):87–94. [DOI] [PubMed] [Google Scholar]
- 49. Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118(6):1574–84. [DOI] [PubMed] [Google Scholar]
- 50. Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, et al. Features of extramedullary disease of multiple myeloma: high frequency of P53 deletion and poor survival: a retrospective single‐center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15(5):286–91. [DOI] [PubMed] [Google Scholar]
- 51. López‐Anglada L, Gutiérrez NC, García JL, Mateos MV, Flores T, San Miguel JF. P53 deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur J Haematol. 2010;84(4):359–61. [DOI] [PubMed] [Google Scholar]
- 52. Hebraud B, Magrangeas F, Cleynen A, Lauwers‐Cances V, Chretien M‐L, Hulin C, et al. Role of additional chromosomal changes in the prognostic value of t(4;14) and del(17p) in multiple myeloma: the IFM experience. Blood. 2015;125(13):2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book. 2016;36:e418–23. [DOI] [PubMed] [Google Scholar]
- 54. Sonneveld P, Avet‐Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neuse CJ, Lomas OC, Schliemann C, Shen YJ, Manier S, Bustoros M, et al. Genome instability in multiple myeloma. Leukemia. 2020;34(11):2887–97. [DOI] [PubMed] [Google Scholar]
- 56. Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zamagni E, Tacchetti P, Cavo M. Imaging in multiple myeloma: How? When? Blood. 2019;133(7):644–51. [DOI] [PubMed] [Google Scholar]
- 58. Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–56. [DOI] [PubMed] [Google Scholar]
- 59. Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of 18F‐FDG–PET/CT according to deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39(2):116–25. [DOI] [PubMed] [Google Scholar]
- 60. Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127(8):971–6. [DOI] [PubMed] [Google Scholar]
- 61. Gagelmann N, Eikema D‐J, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high‐risk cytogenetics: a study from the chronic malignancies working party of the European society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25(11):2134–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Cytogenetic aberrations at the time of MM diagnosis (MM versus secondary EMD patients).


