Summary
Increasing numbers of elderly (≥65 years) patients are listed for kidney transplantation. This study compares the survival outcome between living (LDK), regularly allocated (ETKAS), and Eurotransplant Senior Program (ESP) donor kidneys in elderly recipients. This is a single‐center retrospective cohort study of elderly kidney transplant recipients transplanted between 2005 and 2017. Primary outcome measures were nondeath‐censored graft, death‐censored graft, and patient survival. In total, 348 patients were transplanted, 109 recipients (31.3%) received an LDK, 100 (28.7%) an ETKAS, and 139 (40%) an ESP kidney. 62.5% were male, and median age was 68 years. LDK recipients had significantly better 5‐year nondeath‐censored graft survival compared with ETKAS and ESP (resp. 71.0% vs. 66.1% vs. 55.6%, P = 0.047). Death‐censored graft survival after 1 year was significantly better in LDK recipients (99.1%) (ETKAS 90.8%; ESP 87.7%, P < 0.001). After 5 years, the difference remained significant (P < 0.001) with little additional graft loss (97.7% vs. 88.1% vs. 85.6). There was no significant difference in patient survival after 5 years (71.7% vs. 67.4% vs 61.9%, P = 0.480). In elderly recipients, the patient survival benefits of an LDK are limited, but there is decreased death‐censored graft loss for LDK recipients. Nevertheless, graft survival in ETKAS and ESP remains satisfactory.
Keywords: deceased donor, elderly patients, graft survival, kidney transplantation, living donor, patient survival
Increasing numbers of elderly (≥65 years) patients are listed for kidney transplantation. In elderly recipients the patient survival benefits of a living donor kidney are limited, but there is decreased death‐censored graft loss for these recipients. Nevertheless, graft survival for recipients with a deceased donor remains satisfactory.

Introduction
An increasing number of elderly patients over 65 years with end‐stage kidney disease (ESKD) are potential candidates for kidney transplantation. The percentage of elderly transplanted patients in the Netherlands increased from 22.8% in 2005 to 39.8% in 2017 [1, 2]. Previously, in patients with ESKD, living donor kidney (LDK) transplantation has been associated with superior graft and patient survival as compared with deceased donor kidney transplantation [3, 4]. Consequently, living donor kidney transplantation has become standard of care and is also increasingly performed in elderly patients.
Besides living donation and the regular (ETKAS) program for kidney allocation within Eurotransplant (donors <65 years), elderly patients with ESKD can also be listed for the Eurotransplant Senior Program (ESP). In the ESP allocation system, elderly recipients (≥65 years) receive kidneys from elderly deceased donors (≥65 years) without HLA matching, targeting a short cold ischemia time. Waiting time for a deceased donor kidney within ESP is shorter than in ETKAS [5, 6]. With these three transplant modalities available, it is important for elderly patients and care‐providers to understand the strengths and limitations of these different transplantation modalities to make an informed choice.
In view of the limited life expectancy of elderly transplant recipients, it is reasonable to question whether putting living donors at risk is justified by the potential health gains for the recipient as compared with a deceased donor kidney transplantation.
Currently, limited published data are available on the outcome of elderly recipients of a living donor kidney (LDK) compared with elderly recipients of a deceased donor kidney from regular or older donors. Thus far, no study has directly compared the outcome of these three modalities of kidney transplantation in elderly, and available studies are mostly outdated and therefore might not be completely representative of the current outcome of kidney transplantation [5, 7, 8, 9, 10, 11].
To support elderly patients, potential living donors, and care professionals in choosing the most appropriate transplant modality, we aimed to compare actual patient and transplant survival in elderly recipients of a LDK, ETKAS, and ESP donor kidney, respectively.
Methods
Study subjects
Between January 1, 2005 and August 30, 2017, 351 consecutive elderly patients, defined as ≥65 years at time of transplantation, received either an LDK, an ETKAS, or an ESP kidney transplant in our center. Donors within ETKAS are ≤65 years old, donors within ESP are ≥ 65 years, and living donors can be of all ages. Two patients were excluded because they received a multiorgan transplant, and one because age at transplantation could not be verified, resulting in a total of 348 patients eligible for analyses. If patients received a second kidney transplant within the follow‐up period, they were censored at the time of that transplantation. In total, 348 patients were included, and these patients were followed up until August 30, 2018, until death, or until censoring (Fig. 1).
Figure 1.
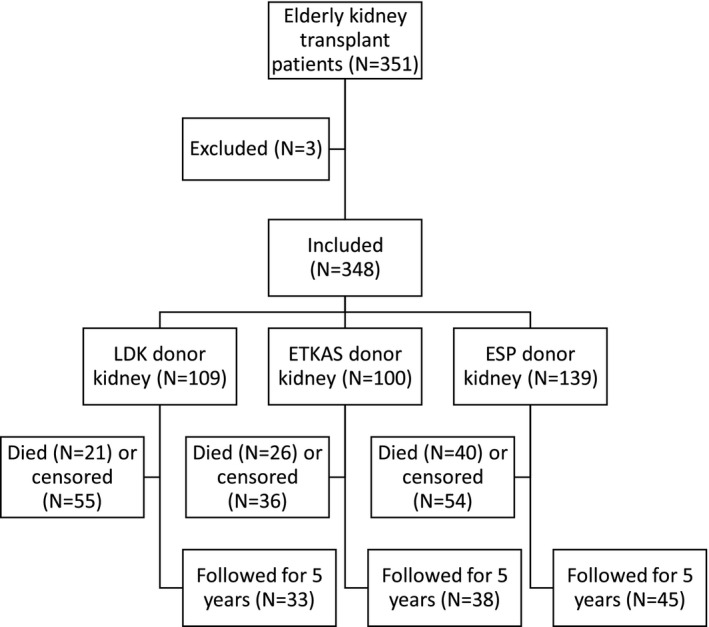
Study inclusion flow chart. LDK: Living donor kidney; ETKAS: Deceased donor allocated within Eurotransplant kidney allocation system; ESP: Deceased donor allocated within the Eurotransplant Senior Program.
This study was conducted according to the guidelines in the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism and was approved by the institutional review board of the UMCG in May 2019 (Research Register number: 201900325).
Study design
This study is a single‐center retrospective cohort study of elderly kidney transplant recipients. All patients receiving a kidney transplant in our center are included in the local kidney transplant registry. This database includes baseline characteristics from the donor and the recipient (Table 1). The recipients’ comorbidities were obtained from the institutional electronic patient record. The comorbidities were indexed according to the Charlson Comorbidity Index (CCI) [12], and patients were categorized in 3 groups according to the CCI score (i.e., 2, 3–4, and ≥5 points). Of note, as the presence of kidney disease is awarded with 2 points according to the CCI, the group of patients with 2 points represents patients without comorbidities.
Table 1.
Baseline characteristics.
| Characteristics | Population | LDK | ETKAS | ESP | P‐value LDK vs. ETKAS vs. ESP | P‐value LDK vs. ETKAS | P‐value LDK vs. ESP | P‐value ETKAS vs. ESP |
|---|---|---|---|---|---|---|---|---|
| N (%) | 348 (100%) | 109 (31.3%) | 100 (28.7%) | 139 (40.0%) | ||||
| Donor age, years | 65.0 [53.0–68.0] | 64.0 [47.5–68.0] | 52.0 [43.0–58.0] | 68.0 [66.0–70.0] | <0.001* | <0.001* | <0.001* | <0.001* |
| Donor sex, male (%) | 171 (49.1%) | 45 (41.3%) | 56 (56.0%) | 70 (50.4%) | 0.097 | 0.033* | 0.155 | 0.389 |
| Deceased donor type DBD (%) | – | – | 53 (53.0%) | 79 (56.8%) | – | – | – | 0.556 |
| Donor cause of death (%) | – | – | – | – | – | 0.522 | ||
| Trauma | – | – | 17 (17.0%) | 19 (13.7%) | ||||
| Cerebrovascular accident or intracranial hemorrhage | – | – | 46 (46.0%) | 72 (51.8%) | ||||
| Circulational | – | – | 1 (1.0%) | 0 (0.0%) | ||||
| Not otherwise specified | – | – | ||||||
| Cold ischemia time, hours | 10 [2–14] | 2 [2–2] | 13.5 [10–17] | 12 [10–15] | <0.001* | <0.001* | <0.001* | 0.062 |
| Total HLA mismatches | 3 [2–5] | 3 [2–4] | 2 [1–3] | 4.5 [3–5] | <0.001* | <0.001* | <0.001* | <0.001* |
| PRA at transplantation (%) | 0.224 | 0.252 | 0.464 | 0.111 | ||||
| 0 | 317 (91.1%) | 101 (92.7%) | 86 (86.0%) | 130 (93.5%) | ||||
| ≤5% | 16 (4.6%) | 3 (2.8%) | 7 (7.0%) | 6 (4.3%) | ||||
| >5% | 15 (4.3%) | 5 (4.6%) | 7 (7.0%) | 3 (2.2%) | ||||
| First transplant | 338 (97.1%) | 107 (98.2%) | 92 (92%) | 139 (100%) | 0.001* | 0.037* | 0.109 | 0.001* |
| Recipient sex, male (%) | 217 (62.5%) | 62 (56.9%) | 64 (64.0%) | 91 (65.9%) | 0.323 | 0.293 | 0.145 | 0.756 |
| Recipient age, years | 68 [66–70] | 68 [66–70] | 68 [66–70] | 68 [66–71] | 0.798 | 0.792 | 0.690 | 0.511 |
| BMI | 26.9 ± 4.0 | 29.9 ± 4.1 | 26.6 ± 3.6 | 27.1 ± 4.3 | 0.577 | 0.549 | 0.641 | 0.277 |
| Primary diagnosis | 0.017* | 0.203 | 0.251 | 0.449 | ||||
| Unknown | 94 (27.2%) | 20 (18.5%) | 31 (31.0%) | 43 (31.2%) | ||||
| Glomerulonephritis | 59 (17.1%) | 17 (15.7%) | 16 (16.0%) | 26 (18.8%) | ||||
| Interstitial nephritis | 19 (5.5%) | 6 (5.6%) | 9 (9.0%) | 4 (2.9%) | ||||
| Cystic kidney disease | 49 (14.2%) | 18 (16.7%) | 11 (11.0%) | 20 (14.5%) | ||||
| Other congenital and hereditary kidney disease | 2 (0.6%) | 1 (0.9%) | 1 (1.0%) | 0 (0.0%) | ||||
| Renal vascular disease | 70 (20.2%) | 26 (24.1%) | 16 (16.0%) | 28 (20.3%) | ||||
| Diabetes Mellitus | 35 (10.1%) | 10 (9.3%) | 13 (13.0%) | 12 (8.7%) | ||||
| Other multisystem diseases | 11 (3.2%) | 6 (5.6%) | 2 (2.0%) | 3 (2.2%) | ||||
| Other | 7 (2.0%) | 4 (3.7%) | 1 (1.0%) | 2 (1.4%) | ||||
| Preemptive transplant | 94 (27%) | 65 (59.6%) | 11 (11.0%) | 18 (12.9%) | <0.001* | <0.001* | <0.001* | 0.649 |
| Dialysis vintage, years † | 2.8 [1.8–4.2] | 1.5 [0.6–2.1] | 3.6 [1.8–5.1] | 2.9 [2.2–4.2] | <0.001* | <0.001* | <0.001* | 0.134 |
| <Median ‡ | 127 (36.5%) | 38 (34.9%) | 33 (33.0%) | 56 (40.3%) | ||||
| CCI | 0.642 | 0.461 | 0.519 | 0.626 | ||||
| 2 | 159 (45.7%) | 55 (50.5%) | 44 (44.0%) | 60 (43.2%) | ||||
| 3–4 | 150 (43.1%) | 42 (38.5%) | 47 (47.0%) | 61 (43.9%) | ||||
| 5+ | 39 (11.2%) | 12 (11.0%) | 9 (9.0%) | 18 (12.9%) |
Statistically significant difference.
Excluding preemptive transplantation.
n (%) on dialysis shorter than population median dialysis duration (2.8 years).
The primary outcomes of this study were 1‐ and 5‐year nondeath‐censored graft survival, 1‐ and 5‐year death‐censored graft survival, and 1‐ and 5‐year patient survival. In this manuscript, nondeath‐censored graft survival will be referred to as graft survival. Secondary outcome measures were 1‐year renal function, assessed using the creatinine based CKD‐EPI equation to determine eGFR, primary nonfunction (PNF), delayed graft function (DGF), defined as the need for dialysis in the first week after transplantation, treated rejection in the first year after transplantation and 24 hour urinary creatinine excretion at 1 year post‐transplantation as a measure for muscle mass [13, 14].
For those on dialysis at 1 year post‐transplantation, eGFR were missing, and an eGFR of 10 ml/min/1.73 m2 was used for analyses. There was no additional imputation in case of missing values. Dialysis vintage was categorized into three categories: First, preemptive transplantation, meaning no dialysis; second, dialysis vintage shorter than the population median dialysis duration; and third, dialysis vintage longer than the median dialysis duration for that donor type. Median dialysis time was determined from recipients who were on dialysis, excluding those transplanted preemptively (Table 1).
Analysis of data
Two‐sample t‐tests and Mann‐Whitney U‐tests or one‐way ANOVA and Kruskal‐Wallis tests were used to compare groups of continuous variables. For categorical variables, Χ2‐tests were performed.
Graft‐, death‐censored graft, and patient survival were analyzed using Kaplan‐Meier curves and survival tables. In order to correct for potential confounders, Cox regression was performed for the following potential confounders: recipient age, recipient sex, BMI, CCI, dialysis vintage and year of transplantation. Interaction of the donor type with age, sex, BMI, dialysis vintage, and CCI score was tested with LDK used as a reference. Hazard ratios are given for patient mortality and graft loss with LDK as the reference group.
Continuous normally distributed variables are reported as mean±SD, continuous non‐normally distributed variables as median [IQR], categorical variables as n (%). A P‐value below 0.05 was considered significant. All data were analyzed using IBM SPSS Statistics for Windows, version 23.0.0.3 (IBM Corp. Armonk, NY. Released 2015).
Results
Out of the 348 participants, 109 (31.3%) received an LDK, 100 (28.7%) an ETKAS, and 139 (40.0%) an ESP donor kidney. Characteristics of donors and recipients are shown in Table 1. The median recipient age was 68 years [66–70], 217 (62.5%) were male, and 130 participants (37.4%) had no comorbidities. 97.1% were first transplants. There were no significant differences between the groups in sex‐distribution, age, and comorbidities at baseline (see Table 1).
There were no significant differences in panel reactive antibody (PRA) levels at transplantation (Table 1). However, there were, as expected, significantly more HLA‐mismatches in the ESP group (4.5 [3–5]) compared with the ETKAS (2 [1–3]) and LDK (3 [2–4]) groups, (P < 0.001) and significantly more HLA‐mismatches in the LDK group compared with the ETKAS group (P < 0.001). In the LDK group, 59.6% of patients were transplanted preemptively, compared with 11% in the ETKAS and 12.9% in the ESP group (P < 0.001). At transplantation, the LDK group had a significantly shorter median dialysis vintage (1.5 years [0.7–2.1]) compared with the ETKAS (3.6 years [1.8–5.1]) and the ESP (2.9 [2.2–4.2]) groups (P < 0.001).
There were no significant differences between the groups in immunosuppression, with 342 (98,3%) patients receiving a corticosteroid (P = 0.942), followed by 335 (96.3%) patients receiving mycophenolate mofetil (P = 0.552). 222 (63.8%) patients received Tacrolimus (P = 0.119) and 126 (36.2%) Cyclosporine A (P = 0.119).
Graft survival
The 1‐year graft survival was 95.4% in the LDK group, 82% in the ETKAS group, and 85.6% in the ESP group (P = 0.008). At 5 years, graft survival was 71%, 66%, and 55.6% (P = 0.047), respectively. The difference between LDK and ETKAS was significant at 1 year (P = 0.002), but not at 5 years (P = 0.083). The differences between ETKAS and ESP were not significant at 1 year (P = 0.469) or 5 years (P = 0.512), but the differences between LDK and ESP were significant at both 1 year (P = 0.010) and 5 years (P = 0.014) (Fig. 2).
Figure 2.
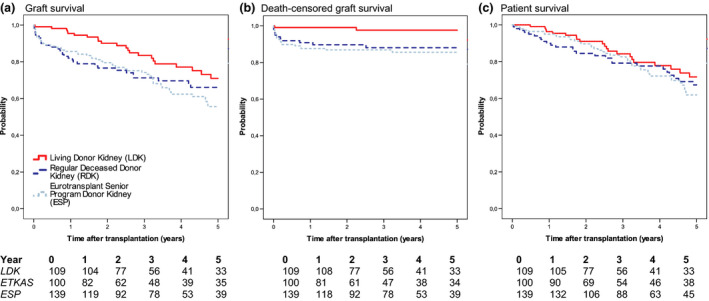
Survival curves with patients at risk. (a) Graft survival (P = 0.047); (b) Death‐censored graft survival (P = 0.005); (c) Patient survival (P = 0.480).
After Cox regression analysis with adjustment for recipient age, recipient sex, BMI, CCI, dialysis vintage, and year of transplantation, the difference in graft survival at 1 year differed significantly between LDK and ETKAS (HR for graft loss: 3.89 [1.31–11.48], P = 0.014) and LDK and ESP (3.13 [1.08–9.08], P = 0.036). However, at 5 years, after correction for the same confounders, the difference in graft survival was no longer significantly different between LDK and ETKAS (1.48 [0.79–2.78], P = 0.227) or between LDK and ESP (1.69 [0.95–3.03], P = 0.076) (Table 2).
Table 2.
Cox regression analysis.
| Unadjusted model | Adjusted model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LDK | ETKAS | ESP | ETKAS | ESP | |||||
| HR [95% CI] | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P | |
| 1 Year | |||||||||
| Graft loss | 1 (Reference) | 4.26 [1.58–11.49] | 0.004* | 3.37 [1.26–8.97] | 0.015* | 3.89 [1.31–11.48] | 0.014* | 3.13 [1.08–9.08] | 0.036* |
| Death‐censored graft loss | 1 (Reference) | 10.48 [1.33–82.70] | 0.026* | 14.08 [1.87–105.79] | 0.010* | 12.08 [1.43–102.30] | 0.022* | 14.71 [1.82–118.78] | 0.012* |
| Patient mortality | 1 (Reference) | 2.86 [0.90–9.11] | 0.076 | 1.40 [0.41–4.80] | 0.589 | 1.82 [0.49–6.83] | 0.373 | 0.78 [0.19–3.12] | 0.721 |
| 5 Year | |||||||||
| Graft loss | 1 (Reference) | 1.61 [0.93–2.79] | 0.091 | 1.86 [1.13–3.08] | 0.015* | 1.48 [0.79–2.78] | 0.227 | 1.69 [0.95–3.03] | 0.076 |
| Death–censored graft loss | 1 (Reference) | 6.46 [1.43–29.16] | 0.015* | 7.84 [1.83–33.66] | 0.006* | 7.36 [1.50–36.15] | 0.014* | 8.12 [1.74–37.91] | 0.008* |
| Patient mortality | 1 (Reference) | 1.30 [0.73–2.32] | 0.366 | 1.38 [0.81–2.33] | 0.237 | 1.11 [0.56–2.18] | 0.765 | 1.12 [0.60–2.08] | 0.720 |
Statistically significant difference. The following variables were taken into account: recipient age, recipient sex, BMI, CCI, dialysis vintage, and year of transplantation.
Death‐censored graft survival
Death‐censored graft survival at one year was 99.1% in the LDK group. In comparison, the ETKAS and ESP groups had a significantly lower death‐censored graft survival at one year (90.8%, P = 0.006 and 87.7%, P = 0.001). The difference remained stable and significant at 5 years, with approximately 2% additional graft loss in all groups (LDK 97.7% vs ETKAS 88.1%, P = 0.005; LDK vs ESP 85.6%, P = 0.001) (Fig. 2). ETKAS and ESP did not differ significantly at 1 year (P = 0.471) or 5 years (P = 0.608).
Death‐censored graft survival at one year after transplantation remained significantly higher in the LDK group after adjustment for recipient age, recipient sex, BMI, CCI, dialysis vintage, and year of transplantation (Table 2).
Patient survival
The 1‐year patient survival of the overall study population was 95% with no significant difference between the groups with 96.3% in the LDK group, 90% in the ETKAS group, and 95% in the ESP group ETKAS, and ESP did not differ significantly (P = 0.122). At 5 years, patient survival was numerically the highest in the LDK group with 71.2%, 67.4% in the ETKAS group, and 61.9% in the ESP group (P = 0.48) (Fig. 2).
After Cox regression analysis, adjusted recipient age, recipient sex, BMI, CCI, dialysis vintage, year of transplantation, and donor type did not significantly influence the patient survival at 5 years post‐transplantation (HR for mortality: ETKAS 1.11 [0.56–2.18], P = 0.765; 1.12 [0.60–2.08], P = 0.720) (Table 2).
There were no differences in outcome in graft‐, death‐censored graft, or patient survival between recipients of an LDK with a donor younger than 65 years compared with a donor of 65 years or older. There were also no differences between a donor kidney after cardiac (DCD) or brain death (DBD) in either the ETKAS or the ESP groups, respectively (data not shown).
Secondary outcome measures
Kidney function (eGFR) at one year did not differ significantly between the LDK (56.0 [48.0–69.0] ml/min/1.73 m2) and ETKAS (57.0 [39.0–72.0] ml/min/1.73 m2) groups (P = 0.459) but was significantly lower in the ESP group (38.5 [27.0–49.0] ml/min/1.73 m2). There was a significant difference between ESP and LDK (P < 0.001) and ESP and ETKAS (P < 0.001), but not between LDK and ETKAS (P = 0.459) (Fig. 3). Values were missing from a total of 91 patients, 27 (25%) from the LDK group, 29 (29%) from the ETKAS group, and 35 (25%) from the ESP group.
Figure 3.
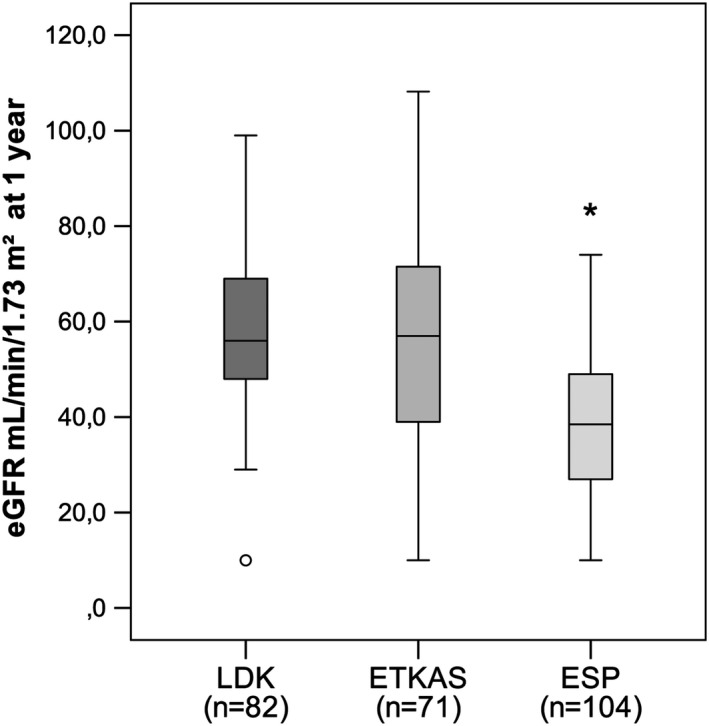
eGFR per group. LDK: Living donor kidney; ETKAS: Deceased donor allocated within Eurotransplant kidney allocation system; and ESP: Deceased donor allocated within the Eurotransplant Senior Program.
DGF did not occur (0.0%) in the LDK group but occurred equally (P = 0.524) in the ETKAS (23%) and ESP (26.6%) group. In the LDK group, PNF occurred in 0.9% of cases (1 patient), in the ETKAS group in 4% (4 patients), and in the ESP group in 4.3% (4 patients). This difference was not significantly different (P = 0.269).
The incidence of treated rejection in the first year did not differ significantly between the groups (LDK 14.1% vs ETKAS 7.9% vs ESP 8.9%, P = 0.352).
Discussion
In this study, we did not find significant differences in patient survival in elderly recipients of an LDK, ETKAS, and ESP kidney allograft at one year and five years after transplantation. Survival rates in this population were around 94% at one year and 65% at five years. However, in terms of graft and death‐censored graft survival, we found superior survival in the LDK group. Recipients of a living donor kidney had only 2.3% death‐censored graft loss at 5 years, compared with 11.9% in the ETKAS group and 14.4% in the ESP group.
The main benefits of transplantation with a living donor in elderly recipients appear to be twofold. First, a living donor provides the possibility to aim for a preemptive transplantation. Second, living donor transplantation is associated with a 10% advantage in graft survival within the first year post‐transplant compared with the deceased donor groups. We did not find a difference in graft survival between ETKAS or ESP recipients. Although median time on dialysis was shorter for a recipient of an ESP kidney (1.9 years) than for an ETKAS kidney (2.6 years), this difference was not statistically significant. The relatively small difference in waiting time is influenced by the fact that patients are generally listed for both the ESP and the EKTAS program. This leads to relatively early transplants in elderly recipients within the regular EKTAS allocation as this competes with the waiting time‐based ESP allocation. It is important to note that transplant function of ESP kidneys at 1 year post‐transplantation was significantly worse than that of a living or ETKAS‐allocated transplant.
The first aspect of our study encompassed the comparison of living donors and regular (EKTAS) allocation. Two previous studies compared survival differences between similar groups. In a United Network of Organ Sharing (UNOS) cohort of 258 kidney transplant recipients aged 75 and older, Macrae et al [7] showed about 20% higher patient survival at 5 years post‐transplantation for living compared with deceased donor kidney recipients. They also found substantial life prolongation for both living and deceased donor kidney recipients, compared with patients remaining on the waiting list. However, compared with this historic cohort with older patients, the 5‐year patient survival in our study was 10% better in the living donor group and 20% better in the deceased donor group. A 2017 study by Thiessen et al [9] compared elderly living and deceased donor recipients. In contrast to our study, they demonstrated a patient survival benefit in favor of younger donors, but again overall patient survival in our cohort was higher. Moreover, Thiessen et al. found superior outcomes in deceased donor kidney recipients transplanted from 2005 onwards. Cohort studies have also found a progressive improvement of graft and patient survival of kidney transplant recipients over sequential decades [15, 16].
The second aspect of our study encompassed the comparison of ESP and ETKAS allocation. Two previous studies, by Fabrizii et al [10] and Frei et al [5], have compared ETKAS and ESP allocation, both in patients transplanted between 1999 and 2002.
Fabrizii et al [10] also found no significant differences in patient and graft survival between ESP and ETKAS recipients aged 65 and older.
In contrast, Frei et al [5] compared ESP recipients with ETKAS recipients aged 60–64 with all‐age donors and found a significant difference in favor of ETKAS recipients in patient, graft, and death‐censored graft survival, at 1 and 5 years post‐transplantation. However, in this study, the recipients of an ETKAS allocated kidney were younger than those in the ESP group.
Reduced patient survival was also found in a recent study by Peters‐Sengers et al [11] in recipients ≥65 years receiving an ESP kidney compared with a deceased donor kidney younger than 65. However, this study did not find a difference in graft survival at 5 years between younger and older donors. Again, the observed difference in patient survival compared with our study might be caused by the earlier inclusion period with poorer overall results in their study.
In our study, the difference in patient survival of elderly transplant recipients depending on donor type has disappeared. We hypothesize that with current transplant practice, a threshold of minimum graft quality has been achieved, and further improvement does not result in an equal concurrent improvement of patient survival in elderly recipients in whom the remaining life span after transplantation is limited by age‐related comorbidity, even with the highest quality kidney transplant. In line with this, graft loss from one year after transplantation onwards is almost completely determined by patient’s death, and death‐censored graft loss is a rare event.
Thereby, it could be hypothesized that the causes of death in elderly are determined by pretransplant conditions and the occurrence of post‐transplant complications, especially infections.
For now, with the increasing number of elderly patients waiting for transplantation, our data are a valuable addition to the knowledge base guiding decisions in the transplantation of the elderly. However, this study also has an important limitation, as it is a single‐center observational study, with a limited number of patients and therefore a limited power. Moreover, we did not compare survival of transplanted patients with survival of elderly patients in dialysis.
This is important given that transplantation with a living donor has the additional advantage of a shorter waiting time compared with transplantation with a deceased donor. In our population, around 60% of LDK recipients were transplanted preemptively, compared with 12% in the ETKAS and ESP groups. This shorter waiting time might lower the risk of graft failure and patient mortality compared with the relatively high dialysis mortality for waitlisted patients. A reduction in waiting time might also improve the quality of life, as the quality of life is higher for transplant recipients compared with those on dialysis [17, 18, 19, 20, 21, 22]. As LDK are, on average, transplanted earlier in the disease process, often preemptively, the increased graft survival could be partly caused by lead time bias (23). However, despite the presence of lead time bias, the benefit of LDK remains a potentially better and earlier improvement of quality of life, despite the comparable patient survival after deceased kidney transplantation.
We conclude that there are differences in outcome between the different donor types; however, in our opinion, all three donor types show acceptable results in the elderly population. After the first year, death‐censored graft loss is a rare event in all donor types. Patient survival was not significantly better after living donation in our elderly recipients than in ESP and ETKAS, with a difference of less than ten percent at five years. Which transplantation modality is best suited for the individual elderly recipient has to be decided with the individual patient, in the context of his medical situation, preferences, and the availability of a potential living donor. Results of this study can help in making the optimal informed decision.
Authorship
Erzsi Tegzess, BSc, was involved in research design, acquiring the data, data analysis, and interpretation and writing the manuscript. Antonio W. Gomes Neto, MD, was involved in acquiring the data, data analysis, and reviewing the manuscript. Robert A. Pol, MD, PhD, was involved in reviewing the manuscript. Silke E. de Boer, MD, was involved in reviewing the manuscript. Hessel Peters‐Sengers, PhD, was involved in research design and reviewing the manuscript. Jan‐Stephan F. Sanders, MD, PhD, was involved in data analysis and interpretation and writing the manuscript. Stefan P. Berger MD, PhD, was involved in research design, data analysis, and interpretation and writing the manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
None.
References
- 1. van Leiden HA, Heemskerk MBA, de Buijzer E, Smand CE, Busato CMJ, Haase‐Kromwijk BJJM. Nederlandse Transplantatie Stichting Jaarverslag 2005.
- 2. de Groot S, Haase‐Kromwijk B, Heemskerk M, et al. Nederlandse Transplantatie Stichting Jaarverslag 2017.
- 3. Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995; 333: 333. [DOI] [PubMed] [Google Scholar]
- 4. Roodnat JI, van Riemsdijk IC, Mulder PGH, et al. The superior results of living‐donor renal transplantation are not completely caused by selection or short cold ischemia time: a single‐center, multivariate analysis. Transplantation 2003; 75: 2014. [DOI] [PubMed] [Google Scholar]
- 5. Frei U, Noeldeke J, Machold‐Fabrizii V, et al. Prospective age‐matching in elderly kidney transplant recipients–a 5‐year analysis of the Eurotransplant Senior Program. Am J Transplant 2008; 8: 50. [DOI] [PubMed] [Google Scholar]
- 6. Wachttijd niertransplantatie per bloedgroep . Available at: https://www.transplantatiestichting.nl/page/wachttijd‐niertransplantatie‐per‐bloedgroep. Accessed February 22, 2020.
- 7. Macrae J, Friedman AL, Friedman EA, Eggers P. Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States. Int Urol Nephrol 2005; 37: 641. [DOI] [PubMed] [Google Scholar]
- 8. Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation 2007; 83: 1069. [DOI] [PubMed] [Google Scholar]
- 9. Thiessen C, Wang J, Skrip L, Yoo PS. Patient and graft survival among sexagenarian and septuagenarian renal transplant recipients and donors: the context for older recipients. Prog Transplant 2017; 27: 257. [DOI] [PubMed] [Google Scholar]
- 10. Fabrizii V, Kovarik J, Bodingbauer M, Kramar R, Hörl WH, Winkelmayer WC. Long‐term patient and graft survival in the eurotransplant senior program: a single‐center experience. Transplantation 2005; 80: 582. [DOI] [PubMed] [Google Scholar]
- 11. Peters‐Sengers H, Berger SP, Heemskerk MB, et al. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J Am Soc Nephrol 2017; 28: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373. [DOI] [PubMed] [Google Scholar]
- 13. Stam SP, Eisenga MF, Gomes‐Neto AW, et al. Muscle mass determined from urinary creatinine excretion rate, and muscle performance in renal transplant recipients. J Cachexia Sarcopenia Muscle 2019; 10: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24‐hour urinary creatinine method. Am J Clin Nutr 1983; 37: 478. [DOI] [PubMed] [Google Scholar]
- 15. Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation 2007; 84: 611. [DOI] [PubMed] [Google Scholar]
- 16. Zhu D, Everly MJ. Deceased donor kidney transplantation in the United States from 1988 to 2011: an analysis of the OPTN/UNOS registry. Clin Transpl 2012; 5: 1‐12. [PubMed] [Google Scholar]
- 17. Feiten en cijfers . Available at: https://www.nierstichting.nl/leven‐met‐een‐nierziekte/feiten‐en‐cijfers/. Accessed February 23, 2020.
- 18. Zazzeroni L, Pasquinelli G, Nanni E, Cremonini V, Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta‐analysis. Kidney Blood Press Res 2017; 42: 717. [DOI] [PubMed] [Google Scholar]
- 19. Tamura Y, Urawa A, Watanabe S, et al. Mood status and quality of life in kidney recipients after transplantation. Transplant Proc 2018; 50: 2521. [DOI] [PubMed] [Google Scholar]
- 20. Czyżewski L, Sańko‐Resmer J, Wyzgał J, Kurowski A. Assessment of health‐related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis. Ann Transplant 2014; 19: 576. [DOI] [PubMed] [Google Scholar]
- 21. Dudley C, Harden P. Renal association clinical practice guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract 2011; 118(Suppl 1): c209. [DOI] [PubMed] [Google Scholar]
- 22. European Renal Best Practice Transplantation Guideline, Development Group . ERBP guideline on the management and evaluation of the kidney donor and recipient. Nephrol Dialysis Transplant 2013; 28(Suppl 2): ii1 [DOI] [PubMed] [Google Scholar]
- 23. Laging M, Kal‐van Gestel JA, Weimar W, Roodnat JI. Living donor kidney transplantation should be promoted among “Elderly” patients. Transplant Direct 2019; 5: e496. [DOI] [PMC free article] [PubMed] [Google Scholar]


