Abstract
Inherited metabolic disorders (IMDs) are a heterogeneous group of rare disorders characterized by disruption of metabolic pathways. To date, data on incidence and prevalence of IMDs are limited. Taking advantage of a functioning network within the Austrian metabolic group, our registry research aimed to update the data of the “Registry for Inherited Metabolic Disorders” started between 1985 and 1995 with retrospectively retrieved data on patients with IMDs according to the Society for the Study of Inborn Errors of Metabolism International Classification of Diseases 11 (SSIEM ICD11) catalogue. Included in this retrospective register were 2631 patients with an IMD according to the SSIEM ICD11 Classification, who were treated in Austria. Thus, a prevalence of 1.8/10 000 for 2020 and a median minimal birth prevalence of 16.9/100 000 (range 0.7/100 000‐113/100 000) were calculated for the period 1921 to February 2021. We detected a male predominance (m:f = 1.2:1) and a mean age of currently alive patients of 17.6 years (range 5.16 months‐100 years). Most common diagnoses were phenylketonuria (17.7%), classical galactosaemia (6.6%), and biotinidase deficiency (4.2%). The most common diagnosis categories were disorders of amino acid and peptide metabolism (819/2631; 31.1%), disorders of energy metabolism (396/2631; 15.1%), and lysosomal disorders (395/2631; 15.0%). In addition to its epidemiological relevance, the “Registry for Inherited Metabolic Disorders” is an important tool for enhancing an exchange between care providers. Moreover, by pooling expertise it prospectively improves patient treatment, similar to pediatric oncology protocols. A substantial requirement for ful filling this goal is to regularly update the registry and provide nationwide coverage with inclusion of all medical specialties.
Keywords: gender distribution, inborn errors of metabolism, inherited metabolic disorders, minimal birth prevalence, minimal prevalence, registry study
Abbreviations
- ICD 11
International Classification of Diseases 11
- IMD
inherited metabolic disorders
- PKU
phenylketonuria
- SSIEM
Society for the Study of Inborn Errors of Metabolism
1. INTRODUCTION
Since the first description of the first four inherited metabolic disorders (IMDs) by Garrod in the early 1920s, 1 knowledge about the natural history, diagnostic, and therapeutic options for these disorders has increased greatly. IMDs are a heterogeneous group of rare disorders (prevalence <1/2000) caused by disrupted metabolic pathways responsible for either breakdown, storage, or synthesis of carbohydrates, fatty acids, or proteins. 2 The global birth prevalence is estimated to be 43.4 to 58.4 per 100 000 live births. 3 Even if most IMDs follow autosomal recessive inheritance, a few are X‐linked or autosomal dominantly inherited. 4
In order to classify all IMDs, the SSIEM‐ICD11 Classification with 15 main disease groups and 612 single diagnoses was developed and intended for the new ICD11, but never implemented (see Supporting Information S1 “SSIEM‐ICD11 classification” and Figure 1). 5
FIGURE 1.
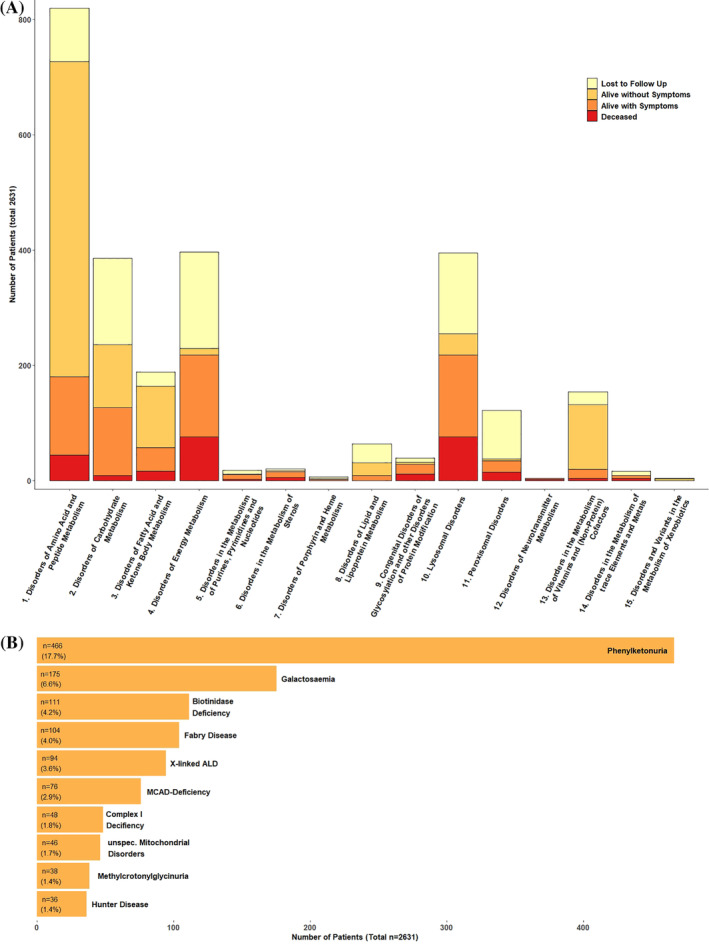
(A) Frequency and outcome of IMDs in Austria according to the 15 main categories of the ICD 11 SSIEM Classification. The x‐axis depicts category number, y‐axis number of affected patients in the outcome categories: unknown, alive—asymptomatic, alive—symptomatic, deceased. (B) The ten most frequent diagnoses for the 2631 study patients with IMDs in Austria according to the ICD 11 SSIEM Classification. For more detailed information, see Supporting Information S2 “Characteristics of the 10 most common IMDs in Austria”
If detected and treated early, an increasing number of IMDs have good prognosis. 4 Although IMDs can manifest with clinical symptoms at any age, 25% of all affected patients show first clinical symptoms in the newborn period. 4 , 6 Thus, the implementation of newborn screening programs is important. In Austria, newborn screening for IMDs was introduced in 1966, and currently detects 25 different IMDs. 7 Casual therapies—some relatively easy (dietary treatments) and some very expensive (enzyme replacement therapies)—are available for a growing number of IMDs. One of the main fundamentals for creating a national policy for the care of such patients is knowledge about the incidence/prevalence of these disorders. While estimates have been calculated for the incidence of various IMD groups, 8 , 9 , 10 , 11 , 12 to our knowledge there is no compilation of the real minimal birth prevalence in a nation.
The first report of the Austrian “Registry for Inherited Metabolic Disorders” covered the period 1985 to 1995. The registry was started as an initiative of the “Austrian Metabolic Group,” which is part of the Austrian Society of Paediatric and Adolescent Medicine (Österreichische Gesellschaft für Kinder‐ und Jugendheilkunde) and was initially administered by Susanne Fang‐Kircher. Data collection between 2000 and 2013 was either not complete or absent. 13 Since 2014, the registry has been administered by Daniela Karall and Sabine Scholl‐Bürgi. With this study, we aimed to improve the registry by updating patient data. Approximately 100 years after Garrod initially described the first four IMDs it seems to be an appropriate time to undertake this effort.
Conceived as a central reporting system and national database, the Austrian registry of IMDs not only plays an epidemiological role by recording minimal birth prevalence, minimal prevalence, and national distribution of patients with IMDs, but also aims to facilitate an exchange between medical specialists, that is, of new diagnostic and therapeutic options, between treatment centers. Furthermore, it can be used as an important tool to keep track of patients and their clinical status, facilitate long‐term analysis, or adjust the diagnosis. In addition, a national database can help establish contact between patients and their families in order for them to exchange their experiences and therefore be a substantial help in coping with the disease. For these aspects, the registry structure warrants safekeeping of patient's data and privacy.
2. METHODS
2.1. Inclusion criteria
All patients with an IMD according to the SSIEM‐ICD11 Classification (see Supporting Information S1 “SSIEM‐ICD11 classification” and Figure 1), who received treatment or were diagnosed in Austria, were included in this retrospective survey. We excluded patients whose minimal dataset consisting of birth date and diagnosis was not complete.
2.2. Data collection
To obtain the maximal number of patients and achieve a national coverage that is as comprehensive and complete as possible, all 169 Austrian departments for Pediatrics, Neurology, and Internal Medicine were regularly every 6 to 12 months invited to register every patient with an IMD, who was or is currently being treated at their center. In 2020, 66/169 centers responded and of those 29 centers were involved in treatment of patients with IMDs including the four major pediatrics centers Medical University Hospital of Vienna, Salzburg University Hospital of the Paracelsus Medical Private University, Medical University Hospital of Graz, and Medical University Hospital of Innsbruck. The last reporting period began in March 2020 and ended in February 2021. Patients from earlier reporting periods also were included in the registry, but if not updated in 2020, the outcome was changed to “lost to follow up.”
The dataset was pseudonymized and included birth date, gender, ethnicity, consanguinity, diagnosis, date of diagnosis, diagnostic setting, and clinical outcome. Minimal dataset consisted of birth date and diagnosis.
Diagnostic setting covers the categories: (a) newborn screening, (b) selective screening, and (c) family screening. Clinical outcome was divided into the following four categories: (a) alive—without symptoms, (b) alive—with symptoms, (c) dead, (d) lost to follow‐up.
Gender distribution was evaluated. X‐linked mode of inheritance 14 was evaluated separately.
Finally, as an example, phenylketonuria was evaluated for minimal prevalence, minimal birth prevalence, age at diagnosis and outcome.
2.3. Calculation of minimal birth prevalence and prevalence for 2020
Using the birth date of every included patient, the minimal birth prevalence per year from 1921 to 2020 was calculated by dividing the total of all patients born with inherited errors of metabolism per year by all live births per year. Data source for the number of live births per year was Statistics Austria. 15
To evaluate the minimal prevalence of IMDs in Austria for 2020, the sum of all patients who are still alive was divided by the Austrian population in 2020. Data source for the population in Austria in 2020 was Statistics Austria. 15
2.4. Statistical analysis
For analysis of data the opensource R software environment for statistical analysis (R version 4.0.2 [June 22, 2020]) and RStudio (Version 1.4.615) was used (R Core Team, 2020). For the creation of figures the R package “ggplot2” was used (Wickham, 2016).
3. RESULTS
3.1. Establishing a nation‐wide registry for IMDs
This retrospective registry study included 2631 patients with IMDs. Of the 2774 initially reported patients 143 did not meet the inclusion criteria and were excluded. Currently, 1627/2631 (61.8%) are alive. Thus, by using the Austrian population statistics for 2020, a minimal prevalence of 1.8/10 000 patients with IMDs for 2020 was calculated. 15
3.2. Diagnoses and respective outcome
We detected 247 different diagnoses according to the SSIEM‐ICD11 classification. The three most common categories were disorders of amino acid and peptide metabolism (819/2631; 31.1%), disorders of energy metabolism (396/2631; 15.1%) and lysosomal disorders (395/2631; 15%) (see Figure 1). Of all the study patients 17.7% had phenylketonuria (466/2631), 6.6% had a classical galactosaemia (175/2631), and 4.2% had a biotinidase deficiency (111/2631) (see Figure 2, Table 1, and Supporting Information). Data on diagnosis setting were available for 1988/2631 (75.6%) patients. For 48.9% of all study patients (972/1988) newborn screening, for 43.7% (868/1988) selective screening and for 7.4% (148/1988) family screening detected their diagnosis (see Table 1 and Supporting Information S2 “Characteristics of the 10 most common IMDs in Austria”).
FIGURE 2.

Minimal yearly birth prevalence of IMDs per 100 000 live births in Austria between 1921 and 2021. The dotted line marks the introduction of newborn screening in Austria. Data on live births per year were retrieved from Statistics Austria. 15 The x‐axis indicates the year, y‐axis number of patients with IMD born in the respective year. For more detailed information, see Supporting Information “Birth prevalence of the 10 most common IMD's in Austria”
TABLE 1.
Characteristics of the study cohort
| All IMDs | Phenylketonuria | |||
|---|---|---|---|---|
| Number | Percent % | Number | Percent % | |
| Total number of patients | 2631 | 100 | 466/2631 | 17.7 |
| Patients alive (2020) | 1627/2631 | 61.8 | 434/466 | 93.1 |
| Minimal prevalence (2020) | 1.82/10 000 | — | 0.09/10 000 | — |
| Birth prevalence (median) | 16.9/100 000 | — | 8.9/100 000 | — |
| Patient age (median) | 17.6 | — | 19.8 | — |
| Age at diagnosis (median) | 1.92 months | — | 11 days | — |
| Gender | 2624/2631 | 99.7 | 466/466 | 100 |
| m:f ratio | 1.2:1 | — | 1.2:1 | — |
| Male | 1452/2624 | 55.3 | 252/466 | 54.1 |
| Female | 1172/2624 | 44.7 | 214/466 | 45.9 |
| Unknown | 7/2631 | 0.27 | 0/466 | 0 |
| Diagnosis setting | 1988/2631 | 75.6 | 421/433 | 97.2 |
| Newborn screening | 972/1988 | 48.9 | 433/466 | 92.9 |
| Selective screening | 868/1988 | 43.7 | 10/466 | 2.2 |
| Family screening | 148/1988 | 7.4 | 2/466 | 0.4 |
| Unknown | 643/2631 | 24.4 | 21/466 | 4.5 |
| Outcome | 1890/2631 | 71.8 | 435/466 | 93.4 |
| Alive—with symptoms | 667/1890 | 35.3 | 15/435 | 3.5 |
| Alive—without symptoms | 960/1890 | 50.8 | 419/435 | 96.3 |
| Deceased | 263/1890 | 13.9 | 1/435 | 0.2 |
| Lost to follow‐up | 741/2631 | 28.2 | 31/466 | 6.7 |
Note: For more detailed information see Supporting Information S2 “Characteristics of the 10 most common IMDs in Austria.”
Information on clinical outcome was available for 1890/2631 (71.8%) patients; 86.1% (1627/1890) are alive, 35.3% (667/1890) with and 50.8% (960/1890) without symptoms. Unfortunately, 10% (263/ 2631) have died (see Figure 1 and Table 1).
3.3. Minimal birth prevalence and age of patients
Despite the fact that not all patients with IMDs have been included in the registry, we estimated a median minimal birth prevalence of IMDs in Austria of 16.9/100 000 from 1921 to 2021. The lowest birth prevalence was 0.7/100 000 in 1921 and the highest birth prevalence was 113/100 000 in 2010 (see Figure 3 and Supporting Information S3 “Birth prevalence of the ten most common IMDs in Austria between 1921 and 2021”).
FIGURE 3.
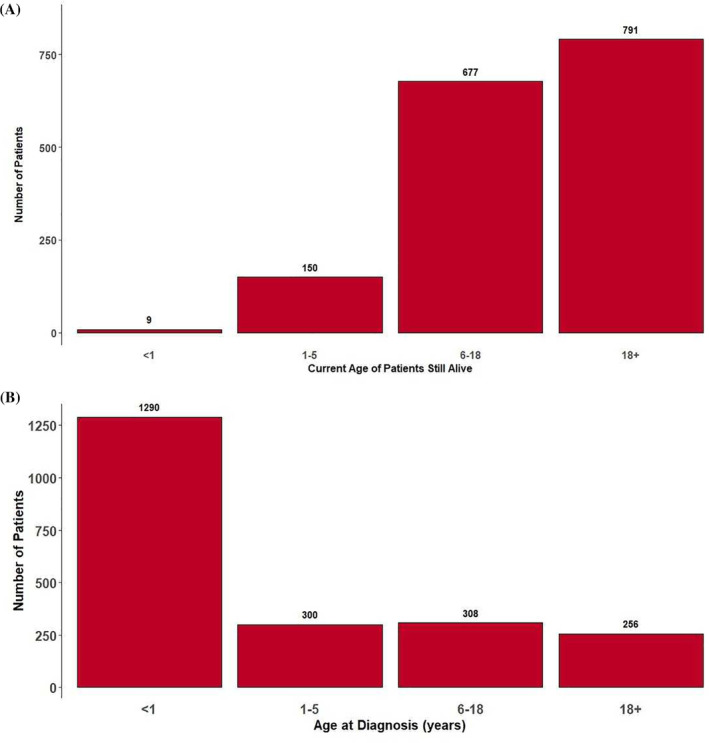
(A) Age structure of all patients still alive in Austria in 2020 (1627/2631). Most patients (48.6% [791/1627]) were adults (>18 years, range 18‐100); 41.6% (677/1627) were between 6 and 18 years; 9.2% (150/1627) were between 1 and 5; and 0.6% (9/1627) were between 0 and 12 months. (B) Age structure at date of diagnosis (2155/2631). Date of diagnosis is known for 81.9% (2155/2631) of the cohort. Median age at diagnosis was 1.92 months (range 32th week of pregnancy—74.2 years). Two patients were diagnosed prenatally (alkaptonuria, transcobalamin II deficiency). Most patients (59.9% [1290/2155]) were diagnosed in the first year of life, namely between 1 and 5 years of age in 13.9% (300/2155), between 6 and 18 years in 14.3% (308/2155), and over 18 years in 11.9% (257/2155)
In the group of the 1627 patients currently still alive, median age is 17.6 (range 5.16 months‐100 years). The 100‐year‐old is a male patient with diagnosis of Fabry disease (see Table 1 and supplemental material).
Most patients (48.6% [791/1627]) were adults (>18 years, range 18‐100); 41.6% (677/1627) were between 6 and 18 years of age; 9.2% (150/1627) were between 1 and 5; and 0.6% (9/1627) were between 0 and 12 months of age (see Fig.).
Of the 2631 study patients, 263 (10.0%) are deceased. For 170/263 (64.6%) patients, information about the date of death is available. Median age at death was 2.12 (range 0‐84.3) years.
The date of diagnosis is known for 81.9% (2154/2631) of the cohort. Median age at diagnosis was 1.92 months (range 32 weeks of pregnancy‐74.2 years). The 74.2‐year‐old patient had a mitochondrial disorder. The first diagnosis of an IMD in Austria was Gaucher disease, diagnosed in 1948. Two patients were diagnosed prenatally (alkaptonuria, transcobalamin II deficiency). Most patients (59.9% [1290/2155]) were diagnosed within the first year of life, namely between 1 and 5 years of age in 13.9% (300/2155), between 6 and 18 years in 14.3% (308/2155), and over 18 years in 11.9% (256/2155) (see Figure 3).
3.4. Gender differences
Data on gender were available from 99.7% of all study patients (2624/2631). With 1452 male (55.3%) and 1172 female (44.7%) patients, there was a male predominance (ratio m:f = 1.24:1). For further analysis, the 295/2631 patients (196 male, 99 female) with an X‐linked inherited diagnosis were excluded. Even then, male predominance persisted with 1256/2329 male (53.9%) and 1073/2329 female (46.1%) patients (ratio m:f = 1.17:1) (see Table 1, Figure 4 and Supporting Information S4 “Gender distribution of the 15 main categories of the SSIEM‐ICD11‐classification”).
FIGURE 4.
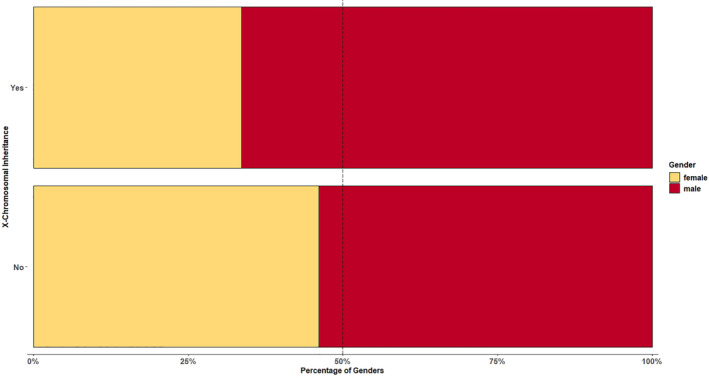
There was a male predominance in our cohort (m:f = 1.24:1).). For further analysis, the 295/2631 patients (196 male, 99 female) with an X‐linked inherited diagnosis were excluded. Even then, male predominance persisted with a ratio m:f = 1.17:1. For more detailed information, see Supporting Information S4 “Gender distribution of the 15 main categories of the SSIEM‐ICD11‐classification”
3.5. Medical centers and ethnicities
Information on ethnicity was available for 1877/2631 of the study patients, comprising 77 different ethnicities. The five most common ones were Austria (1214/1877; 64.7%), Turkey (250/1877; 13.3%), Italy (56/1877, 3%), Serbia (36/1877; 1.9%), and Germany (36/1877; 1.9%).
Data on consanguinity were available for 219/2631 of the study patients, namely positive for 95/219 and negative for 124/219.
All nine Austrian states are involved in providing therapy to patients with IMDs. Of the 2631 study patients, 43.1% (1135/2631) were cared for in Vienna, 23.1% (609/2631) in Tyrol, 13.9% (367/2631) in Styria, 11.6% (304/2631) in Salzburg, 2.9% (77/2631) in Upper Austria, 2.7% (72/2631) in Vorarlberg, 1.4% (38/2631) in Lower Austria, 1% (27/2631) in Carinthia, and 0.08% (2/2631) in Burgenland.
Data on medical specialty/discipline are available for 2555/2631 of the study patients. Most patients (86.0% [2197/2555]) with IMDs received treatment or were diagnosed at departments of pediatrics, 8.2% (210/2555) neurology, 4.9% (124/2555) internal medicine, and 0.9% (24/2555) at departments of other medical specialties (eg, dermatology; see Figure 5).
FIGURE 5.
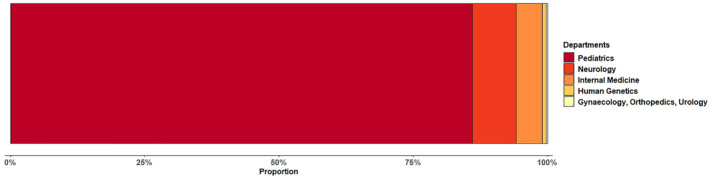
Distribution of treating disciplines. Most patients (86.0% [2197/2555]) with IMDs received treatment at departments of pediatrics, 8.2% (210/2555) neurology, 4.9% (124/2555) internal medicine, and 0.9% (24/2555) at departments of other medical specialties (eg, dermatology)
Median age of patients currently alive and cared by pediatricians (n = 1378) is 16.19 years; 594/1378 (43%) patients were older than 18 years and 784/1378 were younger than 18 years.
3.6. Phenylketonuria (PKU)
In the Austrian IMD register, 17% of all patients are reported to have phenylketonuria (classical PKU, hyperphenylalaninemia, or atypical PKU) (466/2631). The global PKU prevalence is estimated to be 1:23 930 newborns. 16 In Europe, prevalence of PKU varies from 1:4000 (Italy) to 1:112 000 (Finland). 16 The first patient with phenylketonuria in our cohort was born in 1964. In our study the median minimal birth prevalence for phenylketonuria since 1964 was 8.9/100 000 (range 0‐27.5/100 000) live births—the minimal prevalence for PKU in 2020 was 0.48/10000. Of the 466 patients with PKU, median age was 19.8 years (range 0.44‐56.4 years). Median age at diagnosis was 11 days. Most (97.3% [433/466]) of the patients with PKU were diagnosed through newborn screening. For phenylketonuria, sex distribution was male‐predominant with 252 (54.1%) male and 214 female (45.9%) patients. Outcome was available for 435 patients. Of them, 15/435 (3.5%) live with, 419/435 (96.3%) without symptoms, and 1/435 (0.2%) patient has deceased (see Table 1 and Supporting Information “Characteristics of the 10 most common IMDs in Austria”).
4. DISCUSSION
The registry provides an unique data collection of IMDs in Austria in an effort to improve general understanding of IMDs and patient's treatment. With 247 recorded different disorders (40.4% of the diagnoses listed in the SSIEM‐ICD11 classification) our study documents the broad diversity of IMDs in terms of diagnosis, age and outcome in Austria.
Taking advantage of the fact that Austria is a relatively small country with four historically established major pediatric centers specialized in IMDs (Medical University Hospital of Vienna, Salzburg University Hospital of the Paracelsus Medical Private University, Medical University Hospital of Graz, and Medical University Hospital of Innsbruck) and about 100 physicians involved in the care of patients with IMDs, we aimed to establish a real minimal birth prevalence for our country and to complete and update the data in the Austrian IMD Registry. 13
Median minimal birth prevalence of IMDs in Austria in the last hundred years was calculated to be 16.9 per 100 000 live births (0.7‐113). There were 0.7 registered cases of IMDs per 100 000 live births in 1921, and the birth prevalence increased up to 113/100 000 by 2010. Hence, by 2010 the birth prevalence in Austria is higher than the estimated global (50.9/100 000 live births) and Italian birth prevalence (26.9/100 000 live births). 3 , 9 The observed higher birth prevalence and increase of course has to be attributed to enhanced diagnostic methods like implementation of newborn screening programs (starting in 1966) and for example, tandem mass spectrometry (started in 2002) or new genetic methods. 6 Historically, patients were seen by their local physician. Presumably, several disorders went undiagnosed and some patients, who were diagnosed but not treatable, remained in the primary care setting. Over the years, most patients with IMDs have been diagnosed and/or treated at pediatric departments, even though 50% are adults.
From the 2631 study patients, age at diagnosis ranges from 32nd week of pregnancy to 74.2 years. The age range at diagnosis indicates that some patients with IMDs have not yet been diagnosed and therefore the minimal birth prevalence after 2010 is lower than before 2010.
Newborn screening for IMDs was introduced in Austria in 1966 and since 2002 up to 30 IMDs have been screened for. Newborn screening comprises 48.9% of diagnoses in our cohort. We assume, this could be a reason that at present 59% of all patients with IMDs in Austria are alive and asymptomatic under their therapeutic regimens as 25% of all IMDs manifest in newborn period. 16 Although most disorders are diagnosed in the first year of life, the median age of our cohort was 17.6 years. Hence half (48.6%) of the patients are adults. Nevertheless, 86% of all patients are managed at pediatric departments. Inclusion of neurologists, internists and other specialties in the training and treatment of patients with IMDs is necessary in order to ensure care of the adult group and also a timely transition of patients with IMDs diagnosed in childhood when reaching juvenile and adult age.
In line with previous literature, our study cohort shows a male predominance with a ratio m:f = 1.2:1 for IMDs, even after excluding X‐linked inherited diseases. 9 Further research is needed to investigate this sex difference. Comparing with data of Austria statistic, there was a male predominance in the Austria population between 2010 and 2020. 15
The registry makes it possible to analyze individual IMD groups and individual disorders, thus helping understand their epidemiology and clinical course (see Table 2). As an example, we chose to analyze the ten most common disorders more closely (see Supporting Information S2 “Characteristics of the 10 most common IMDs in Austria”).
There are several limitations to this registry study. As data collection is retrospective it relies on reports of treating physicians. Of the total cohort of 2631 patients with IMDs, 28.2% (= 741/2631) were recorded as lost to follow‐up. However, this is comparable with other registry studies. 9 Therefore, as only the minimal prevalence can be estimated, it is likely that the actual prevalence of IMDs in Austria is higher than 1.8/10 000. Further, the data set is still not complete, as we are aware that some patients are not reported to the registry because of being undiagnosed or treated abroad.
Finally, since IMDs are a heterogeneous group of mostly (very) rare disorders, every single experience and exchange between care providers is essential for improving patient care. Thus, the registry could play an essential role in providing a national and international exchange between medical centers and evaluating the care of patients over a longer period of time. It also allows patients with IMDs to come into contact with each other, exchange their experiences and learn about clinical trials being conducted.
5. CONCLUSION
Beside its epidemiological value, the Austrian Registry for IMDs also permits an exchange between care providers and patients. A total of 247 diagnoses are recorded in our cohort and illustrate the heterogeneity of IMDs.
Also when X‐linked inherited IMDs were excluded we recorded a male predominance of IMDs in our cohort (m:f = 1.2:1). Further studies are needed to investigate this distribution.
Thanks to improved treatment and early diagnosis most of our patients are now adults. Thus, in future, medical care needs to also be provided by other disciplines such as Internal Medicine and Neurology.
An essential requirement for a functioning registry is that it be updated on a regular basis by the members of the Austrian Register Group.
CONFLICT OF INTEREST
The authors have no conflicts of interests to declare.
AUTHOR CONTRIBUTIONS
Gabriele Ramoser and Federica Caferri were involved in project design, data collection and evaluation and manuscript preparation. Bernhard Radlinger performed data analysis and interpretation and manuscript preparation. Gabriele Ramoser, Federica Caferri, Bernhard Radlinger, Sabine Scholl‐Bürgi, and Daniela Karall were involved in project design, data collection, discussed the data, and reviewed the manuscript.
ETHICS STATEMENT
The approval of the Ethics Committee of the Medical University of Innsbruck and Vienna for this study is available.
Supporting information
Supporting Information S1 SSIEM‐ICD11 classification.
Supporting Information S2 Characteristics of the 10 most common IMDs in Austria.
Supporting Information S3 Birth prevalence of the 10 most common IMDs in Austria between 1921 and 2021.
Supporting Information S4 Details of gender distribution of the 15 main categories
Appendix R. AUSTRIAN IMD REGISTRY GROUP (AUTHORS IN ALPHABETICAL ORDER)
Albrecht U, Medical University of Innsbruck, Clinic for Pediatric I, Inherited Metabolic Disorders, Anichstrasse 35, 6020 Innsbruck, Austria; patient care
Bauer A, MD, University Hospital Innsbruck, Clinic for Internal Medicine III, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Baumgartner M, MD, Hospital Barmherzige Schwestern Linz, Clinic for Pediatrics, Seilerstätte 4, 4010 Linz, Austria; patient care
Bernar B, Medical University of Innsbruck, Clinic for Pediatric I, Inherited Metabolic Disorders, Anichstrasse 35, 6020 Innsbruck, Austria; patient care
Bernert G, MD, Clinic Favoriten, Department of Pediatrics, Kundratstrasse 3, 1100 Vienna, Austria; patient care
Birnbacher R, MD, Hospital Villach, Clinic for Pediatrics, Nikolaigasse 43, 9500 Villach, Austria; patient care
Blassnig‐Ezeh A, MD, Hospital Feldkirch, Clinical for Pediatrics, Carinagasse 47, 6800 Feldkirch, Austria; patient care
Bodamer O, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria, patient care
Bonfig W, MD, Hospital Wels‐Grieskirchen, Clinic for Pediatrics, Grieskirchner Straße 42, 4600 Wels, Austria; patient care and data collection (up to 50 patients)
Bösch S, MD MSc; Department of Neurology, Medical University Innsbruck, Anichstrasse 35, A‐6020 Innsbruck, Austria, patient care
Bruckner R, MD, Hospital Oberwart, Clinic for Pediatrics, Dornburggasse 80, 7400 Oberwart, Austria; patient care
Bruckner T, MD, Hospital Mödling, Clinic for Internal Medicine, Sr.M.‐Restituta‐Gasse 12, 2340 Mödling, Austria; patient care
Brunner‐Krainz M, MD, University Hospital Graz, Clinic for Pediatrics, Auenbruggerplatz 1, 8036 Graz; patient care and data collection (above 100 patients)
Demmelbauer N, MD, Hospital Ried, Clinic for Internal Medicine, Schlossberg 1, 4910 Ried im Innkreis, Austria; patient care
Dilch A, MD, Gottfried von Preyer Hospital, Clinic for Pediatrics, Schrankenberggasse 31, 1100 Vienna, Austria; patient care
Eitelberger G, MD, Hospital Wels‐Grieskirchen, Clinic for Pediatrics, Grieskirchner Straße 42, 4600 Wels, Austria; patient care
Emhofer J, Hospital Steyr, Clinic for Pediatrics, Sierninger Straße 170, 4400 Steyr, Austria; patient care
Farid G, MD, Hospital Steyr, Clinic for Pediatrics, Sierninger Straße 170, 4400 Steyr, Austria; patient care and data collection (up to 50 patients)
Follmer D, MD, Hospital Elisabethinen Linz, Clinic for Internal Medicine, Fadlingerstraße 1, 4020 Linz, Austria; patient care
Furthner D, MD, Hospital Vöcklabruck, Dr. Wilhelm‐Bock‐Straße 1, 4840 Vöcklabruck, Austria, patient care
Geiger H, MD, Hospital of Dornbirn, Clinical for Pediatrics, Lustenauer Straße 4, 6850 Dornbirn, Austria; patient care
Greber‐Platzer S, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care, responsible for newborn screening
Gremel K, MD, Speising Hospital Vienna, Clinic for Orthopedics, Speisinger Straße 109, 1130 Vienna, Austria; patient care
Grillenberger A, MD, University Hospital Linz, Clinic for Pediatrics, Krankenhausstraße 7a, 4020 Linz, Austria; patient care
Haberlandt E, Hospital of Dornbirn, Clinical for Pediatrics, Lustenauer Straße 4, 6850 Dornbirn, Austria; patient care and data collection (up to 50 patients)
Harm F, MD, Hospital St. Pölten, Clinic for Internal Medicine, Dunant‐Platz 1, 3100 St. Pölten, Austria, patient care
Hauser E, MD, Hospital Mödling, Clinic for Internal Medicine, Sr.M.‐Restituta‐Gasse 12, 2340 Mödling, Austria; patient care and data collection (up to 50 patients)
Herbst S, MD, University Hospital Innsbruck, Clinic for Pediatrics, Anichstraße 35, 6020 Innsbruck, Austria; data collection from 2014 (above 100 patients)
Hofer J, MD, Hospital Barmherzige Brüder Linz, Clinic for Neurology, Seilerstätte 2, 4021 Linz, Austria; patient care and data collection (up to 50 patients)
Höfle G, MD, Hospital Hohenems, Clinic for internal medicine, Bahnhofstraße 31, 6845 Hohenems, Austria; patient care and data collection (up to 50 patients)
Högler W, MD, Johannes Kepler University Linz, Department of Pediatrics and Adolescent Medicine, Krankenhausstraße 26‐30, 4020 Linz, Austria; patient care and data collection (up to 50 patients)
Huemer M, MD, Hospital Bregenz, Clinic for Pediatrics, Carl‐Pedenz‐Straße 2, 6900 Bregenz, Austria; patient care and data collection (50‐100 patients)
Hufgard‐Leitner M, MD, University Hospital Vienna, Clinic for Internal Medicine III, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care and data collection (50‐100 patients)
Ilencikova D, MD, University Hospital Linz, Clinic for Genetics, Krankenhausstraße 7a, 4020 Linz, Austria; patient care
Jahnel J, MD, Hospital Klagenfurt, Clinic for Pediatrics, Feschnigstraße 11, 9020 Klagenfurt, Austria; patient care
Jarz‐Lackner H, MD, Hospital Elisabethinen Linz, Clinic for Pediatrics, Fadlingerstraße 1, 4020 Linz, Austria; patient care
Kerbl R, MD, Department of Pediatrics and Adolescent Medicine, General Hospital Hochsteiermark/Leoben, Vordernberger Straße 42, 8700 Leoben, Austria; patient care and data collection (up to 50 patients)
Kiechl S, MD, University Hospital Innsbruck, Department of Neurology, Medical University of Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Kircher SG (Fang‐Kircher), MD, PhD, Center of Pathobiochemistry and Genetics, Medical University of Vienna, initial and further data collection (above 100 patients)
Klingelmair G, MD, University Hospital Innsbruck, Clinic for Urology, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Koch, J, MD, Medical University Salzburg, Clinic for Pediatrics, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Kollar H, MD, Clinic Penzing Vienna, Baumgartner Höhe 1, 1140 Vienna, Austria; data collection
Konstantopoulou V, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; medical lead of newborn screening, patient care and data collection (above 100 patients)
Kurnik P, MD, Hospital Klagenfurt, Clinic for Pediatrics, Feschnigstraße 11, 9020 Klagenfurt, Austria; patient care
Lagler F, MD, Medical University Salzburg, Clinic for Pediatrics, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Lanz S, MD, University Hospital Graz, Clinic for Pediatrics, Auenbruggerplatz 1, 8036 Graz, patient care and data collection (above 100 patients)
Lechner W, MD, Hochsteiermark Clinic, Clinic for Internal Medicine, Tragösserstrasse 18 600 Bruck an der Mur, Austria; patient care
Leitner M, MD, University Hospital Vienna, Clinic for Internal Medicine III, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Löscher W, MD PhD, Department of Neurolog Medical University Innsbruck Anichstasse 356 020 Innsbruck Austria; patient care and data collection (50‐100 patients)
Mayr JA, Medical University Salzburg, Clinic for Pediatrics, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Manner H, MD, Clinic for Orthopedics, Speisinger Straße 109, 1130 Vienna, Austria; patient care
Mäser M, MD, Hospital Feldkirch, Clinical for Pediatrics, Carinagasse 47, 6800 Feldkirch, Austria; patient care
Meister B, MD, University Hospital Innsbruck, Clinic for Pediatrics, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Möslinger D, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care and data collection (above 100 patients)
Müller‐Sacherer T, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Oberndorfer S, MD, Hospital St. Pölten, Clinic for Neurology, Dunant‐Platz 1, 3100 St. Pölten, Austria; patient care
Peitsch I, MD, University Hospital Vienna, Clinic for Internal Medicine III, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care and data collection (50‐100 patients)
Peter S, MD, St Anna Children's Hospital Vienna, Clinic for ENT, Kinderspitalgasse 6, 1090 Vienna, Austria; patient care
Petzer A, MD, Hospital Elisabethinen Linz, Clinic for Internal Medicine, Fadlingerstraße 1, 4020 Linz, Austria; patient care
Pilshofer V, MD, Hospital Elisabethinen Linz, Clinic for Pediatrics, Fadlingerstraße 1, 4020 Linz, Austria; patient care
Plecko B, MD, University Hospital Graz, Clinic for Pediatrics, Auenbruggerplatz 1, 8036 Graz; patient care and data collection (above 100 patients)
Pölzl G, MD, University Hospital Innsbruck, Clinic for Internal Medicine III, Center for Cardiac Diseases, Anichstraße 35, 6020 Innsbruck, Austria; patient care and data collection (up to 50 patients)
Puttinger R, MD, Medical university Salzburg, Clinic for Genetics and Pediatrics, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Rechberger E, MD, Hospital Ried, Clinic for Internal Medicine, Schlossberg 1, 4910 Ried im Innkreis, Austria; patient care
Rittinger V, MD, Medical University Salzburg, Clinic for Pediatrics, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Salzer H, MD, Hospital Tulln, Clinic for Pediatrics, Alter Ziegelweg 10, 3430 Tulln an der Donau, Austria; patient care
Schabasser P, MD, Hospital Mistelbach, Clinic for Pediatrics, Liechtensteinstraße 67, 2130 Mistelbach, Austria; patient care
Scherer T, MD, Medical University of Vienna, Department of Medicine III, Division of Endocrinology and Metabolism, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care and data collection (50‐100 patients)
Scheibenreiter S, MD, University Hospital Vienna, Clinic for Pedaitrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Simma B, MD, Carinagasse 47, Clinic Feldkirch, A‐6807 Feldkirch; patient care
Spenger J, MD, Medical University Salzburg, Clinic for Pediatrics, inherited metabolic disorders, Müllner Hauptstraße 48, 5020 Salzburg, Austria, patient care and data collection (above 100 patients)
Sperl W, MD, Medical university Salzburg, Clinic for Pediatrics, inherited metabolic disorders, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care
Stadler C, MD, Hospital Klagenfurt, Clinic for Neurology, Feschnigstraße 11, 9020 Klagenfurt, Austria; patient care and data collection (up to 50 patients)
Stangl R, MD, Hospital St. Pölten, Clinic for Pediatrics, Dunant‐Platz 1, 3100 St. Pölten, Austria; patient care
Steiner E, MD, Hospital Wels‐Grieskirchen, Clinic for Pediatrics, Grieskirchner Straße 42, 4600 Wels, Austria; patient care
Stelzl W, MD, Hospital Feldkirch, Clinical for Pediatrics, Carinagasse 47, 6800 Feldkirch, Austria; patient care
Sterniste W, MD, Donaustadt Clinic Vienna, Langobarden Straße 122, 1220 Vienna; patient care
Stockner H, MD, University Hospital Innsbruck, Clinic for Neurology, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Stögmann, W, MD, Gottfried von Preyer Hospital, Clinic for Pediatrics, Schrankenberggasse 31, 1100 Vienna, Austria; patient care
Stöckler‐Ipsiroglu S, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Stulnig T, MD, University Hospital Vienna, Clinic for Internal Medicine III, Währinger Gürtel 18‐20, 1090 Vienna, Austria; Third Department of Medicine and Karl Landsteiner Institute for Metabolic Diseases and Nephrology, Clinic Hietzing, Vienna Health Care Group, 1130 Vienna, Austria; patient care and data collection (50‐100 patients)
Sunder‐Plassmann G, MD, Medical University of Vienna, Department of Medicine III, Division of Nephrology and Dialysis, Austria; patient care and data collection (50‐100 patients)
Timischl R, MD, Hospital Steyr, Clinic for Neurology, Sierninger Straße 170, 4400 Steyr, Austria; patient care
Tiefenthaler M, MD, University Hospital Innsbruck, Clinic for Internal Medicine IV, Anichstraße 35, 6020 Innsbruck, Austria; patient care
Tscharre A, MD, Hospital Feldkirch, Clinical for Pediatrics, Carinagasse 47, 6800 Feldkirch, Austria; patient care
Voigtländer T, MD, University Hospital Vienna, Clinic for Neurology, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Voitl P, MD, University Hospital Vienna, Clinic for Pediatrics, Währinger Gürtel 18‐20, 1090 Vienna, Austria; patient care
Wagentristl HP, MD, Hospital Eisenstadt, Clinic for Pediatrics, Johannes von Gott‐Platz 1, 7000 Eisenstadt, Austria; patient care and data collection (up to 50 patients)
Wallner M, MD, Hospital Wels‐Grieskirchen, Clinic for Internal Medicine, Grieskirchner Straße 42, 4600 Wels, Austria; patient care and data collection (up to 50 patients)
Weber J, MD, Hospital Klagenfurt, Clinic for Neurology, Feschnigstraße 11, 9020 Klagenfurt, Austria; patient care and data collection (up to 50 patients)
Weis D. Department of Medical Genetics, Kepler University Hospital Med Campus IV, Krankenhausstraße 26‐30, 4020 Linz, Austria; patient care
Werner P, MD, Hospital Rankweil, Clinic for Neurology, Valdunastraße 16, 6830 Rankweil, Austria; patient care and data collection (up to 50 patients)
Wimmer A, MD, Hospital Ried, Clinic for Pediatrics, Schlossberg 1, 4910 Ried im Innkreis, Austria; patient care and data collection (up to 50 patients)
Wintergerst U, MD, Hospital Braunau, Clinic for Pediatrics, Ringstraße 60, 5280 Braunau am Inn, Austria; patient care and data collection (up to 50 patients)
Wortmann Saskia B., MD, PhD, University Children's Hospital Salzburg, Parcelsus Medical University, Müllner Hauptstraße 48, 5020 Salzburg, Austria; patient care and data collection (above 100 patients)
Wukovits P, MD, St Anna Children's Hospital Vienna, Clinic for Pediatrics, Kinderspitalgasse 6, 1090 Vienna, Austria; patient care
Zarits P, MD, Hospital Barmherzige Brüder Eisenstadt, Johannes von Gott Platz 1, 7000 Eisentstadt; patient care
Zdenek J, MD, Hospital Zwettl, Clinic for Pediatrics, Propstei 5, 3910 Zwettl, Austria; patient care
Zidar A, MD, Hospital St. Pölten, Clinic for Neurology, Dunant‐Platz 1, 3100 St. Pölten, Austria; patient care
Zodl H, MD, Hospital Wiener Neustadt, Clinic for Internal Medicine, Corvinusring 3‐5, 2700 Wiener Neustadt, Austria; patient care
Zöggeler T, Medical University of Innsbruck, Clinic for Pediatric I, Inherited Metabolic Disorders, Anichstrasse 35, 6020 Innsbruck, Austria; patient care
Zschocke J, MD, University Hospital Innsbruck, Clinic for Genetics, Anichstraße 35, 6020 Innsbruck, Austria; classification of IMD (“SSIEM ICD‐11”)
Zwiauer K, MD, Hospital St. Pölten, Clinic for Pediatrics, Dunant‐Platz 1, 3100 St. Pölten, Austria; patient care
Ramoser G, Caferri F, Radlinger B, et al. 100 years of inherited metabolic disorders in Austria—A national registry of minimal birth prevalence, diagnosis, and clinical outcome of inborn errors of metabolism in Austria between 1921 and 2021. J Inherit Metab Dis. 2022;45(2):144‐156. doi: 10.1002/jimd.12442
Gabriele Ramoser and Federica Caferri are contributed equally; Sabine Scholl‐Bürgi and Daniela Karall shared senior authorship.
The members of “Austrian IMD Registry Group” are given in Appendix.
Communicating Editor: Matthias Baumgartner
Contributor Information
Gabriele Ramoser, Email: gabriele.ramoser@tirol-kliniken.at.
Federica Caferri, Email: federica.caferri@tirol-kliniken.at.
Sabine Scholl‐Bürgi, Email: sabine.scholl-buergi@tirol-kliniken.at.
Daniela Karall, Email: daniela.karall@i-med.ac.at.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Piro A, Tagarelli G, Lagonia P, Quattrone A, Tagarelli A. Archibald Edward Garrod and alcoptonuria: “Inborn errors of metabolism” revisited. Genet Med. 2010;12(8):475‐476. [DOI] [PubMed] [Google Scholar]
- 2. Jeanmonod R, Asuka E, Jeanmonod D. Inborn errors of metabolism. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK459183/#_NBK459183_pubdet_ [PubMed] [Google Scholar]
- 3. Waters D, Adeloye D, Woolham D, Wastnedge E, Patel S, Rudam I. Global birth prevalence and mortality from inborn errors of metabolism: a systematic analysis of the evidence. J Glob Health. 2018;8(2):021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pelin TK, Nur A. Inborn errors of immunity and metabolic disorders: ancient understanding, diagnosis and treatment approaches. J. Paediatr Endocrinol Metab. 2021;34(3):277‐294. [DOI] [PubMed] [Google Scholar]
- 5. Society for the Studies of Inborn Errors of Metabolism [Internet] . SSIEM Classification of Inborn Errors of Metabolism. https://www.ssiem.org/images/centralstore/resources/SSIEMClassificationIEM2011.pdf; 2011.
- 6. Ezgu F. Inborn errors of metabolism. Adv Clin Chem. 2016;73:195‐250. [DOI] [PubMed] [Google Scholar]
- 7. Kasper DC, Ratschmann R, Metz TF, et al. The National Austrian Newborn Screening Program—Eight years experience with mass spectrometry. Past, present, and future goals. Wien Klin Wochenschr. 2010;122(21‐22):607‐613. [DOI] [PubMed] [Google Scholar]
- 8. Hillert A, Anikster Y, Belanger‐Quintana A, et al. The genetic landscape and epidemiology of phenylketonuria. Am J Hum Genet. 2020;107(2):234‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dionisi‐Vici C, Rizzo C, Burlina AB, et al. Inborn errors of metabolism in the Italian pediatric population: A national retrospective survey. J Pediatr. 2002;140(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 10. Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126(8):1905‐1912. [DOI] [PubMed] [Google Scholar]
- 11. Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia 1969‐1996. Pediatrics. 2000;105(1):10. [DOI] [PubMed] [Google Scholar]
- 12. Summar ML, Koelker S, Freedenberg D, et al. The European Registry and Network for Intoxication Type Metabolic Diseases (E‐IMD), and The Members of the Urea Cycle Disorders Consortium (UCDC). The incidence of urea cycle disorders. Mol Genet Metab. 2013;110:179‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang‐Kircher S. Österreichisches Stoffwechselregister. Wien Klin Wochenschr. 1997;109:89‐92. [PubMed] [Google Scholar]
- 14. Ferreira CR, Van Karnebeek CDM, Vockley J, Blau N. A proposed nosology of inborn errors of metabolism. Genet Med. 2019;21(1):102‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Statistic Austria [Internet] . Geburten und Sterbefälle. https://www.statistik.at/web_de/presse/122656.html; 2019.
- 16. El Hattab AW. Inborn errors of metabolism. Clin Perinatol. 2015;42(2):413‐439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1 SSIEM‐ICD11 classification.
Supporting Information S2 Characteristics of the 10 most common IMDs in Austria.
Supporting Information S3 Birth prevalence of the 10 most common IMDs in Austria between 1921 and 2021.
Supporting Information S4 Details of gender distribution of the 15 main categories
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


