Abstract
Background
Oral finasteride is a well‐established treatment for men with androgenetic alopecia (AGA), but long‐term therapy is not always acceptable to patients. A topical finasteride formulation has been developed to minimize systemic exposure by acting specifically on hair follicles.
Objectives
To evaluate the efficacy and safety of topical finasteride compared with placebo, and to analyse systemic exposure and overall benefit compared with oral finasteride.
Methods
This randomized, double‐blind, double dummy, parallel‐group, 24‐week study was conducted in adult male outpatients with AGA at 45 sites in Europe. Efficacy and safety were evaluated. Finasteride, testosterone and dihydrotestosterone (DHT) concentrations were measured.
Results
Of 458 randomized patients, 323 completed the study and 446 were evaluated for safety. Change from baseline in target area hair count (TAHC) at week 24 (primary efficacy endpoint) was significantly greater with topical finasteride than placebo (adjusted mean change 20.2 vs. 6.7 hairs; P < 0.001), and numerically similar between topical and oral finasteride. Statistically significant differences favouring topical finasteride over placebo were observed for change from baseline in TAHC at week 12 and investigator‐assessed change from baseline in patient hair growth/loss at week 24. Incidence and type of adverse events, and cause of discontinuation, did not differ meaningfully between topical finasteride and placebo. No serious adverse events were treatment related. As maximum plasma finasteride concentrations were >100 times lower, and reduction from baseline in mean serum DHT concentration was lower (34.5 vs. 55.6%), with topical vs. oral finasteride, there is less likelihood of systemic adverse reactions of a sexual nature related to a decrease in DHT with topical finasteride.
Conclusion
Topical finasteride significantly improves hair count compared to placebo and is well tolerated. Its effect is similar to that of oral finasteride, but with markedly lower systemic exposure and less impact on serum DHT concentrations.
Introduction
Androgenetic alopecia (AGA), or male pattern baldness, is a genetically determined disorder caused by susceptibility of hair follicles to androgenic miniaturization, occurring most commonly at the scalp vertex. 1 The condition affects more than half of men below 50 years of age. 2 Even clinically imperceptible hair loss has been correlated with decreased quality of life. 3 Men predisposed to AGA have an increased rate of conversion of testosterone to dihydrotestosterone (DHT) within the hair follicle, a process involving type II 5α‐reductase. 4 Finasteride, a synthetic anti‐androgen that inhibits type II 5α‐reductase, was approved initially in tablet form for the treatment of male AGA. 5
The clinical efficacy of oral finasteride for treating AGA is well established. Long‐term studies of oral finasteride 1 mg in men with AGA demonstrated slower progression of hair loss or enhanced hair growth, compared with baseline and/or placebo, as early as 3 months after starting the treatment and extending for 5–10 years. 6 , 7 , 8 , 9 , 10 Although oral finasteride is generally well tolerated, 5 in some patients 5α‐reductase inhibition is associated with sexual adverse effects (erectile dysfunction, ejaculation problems, decreased libido), 11 and increased risk of depression, 12 prompting health authorities in some countries to include warnings in the product labelling. 13 Topical administration of finasteride offers the potential to reduce systemic effects related to its mechanism of action by preferentially inhibiting 5‐α reductase in the scalp, as has been suggested in recent years. 14
Preliminary results regarding topical application of finasteride for treatment of AGA were promising. In a testosterone‐induced alopecia albino mouse model, higher follicular density and anagen:telogen ratios were observed in the group treated with topical finasteride 2% solution. 15 In humans, topical finasteride application for the treatment of AGA was first explored by Mazarella and colleagues in a study involving 52 patients (28 males) with AGA. Beginning from 6 months, a progressive and significant decrease in the rate of hair loss was observed in the topical finasteride vs. placebo group, with no significant changes in plasma levels of total testosterone, free testosterone and DHT between treatment groups. 16 Phase I‐II studies of finasteride 0.25% topical solution in male volunteers demonstrated that a once daily application of up to 200 µL (4 sprays of 50 µL, each delivering 0.144 mg/spray to non‐overlapping areas) exerted a maximal effect on scalp DHT concentrations with less reduction of serum DHT compared with higher doses. 17 , 18
The current phase III study aimed to evaluate the efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) of topical finasteride (0.25% solution applied once daily in a volume between 50 µL and 200 µL) compared with placebo and to assess the overall patient benefit of topical finasteride relative to oral finasteride at 24 weeks.
Materials and methods
Patients and methods
This was a multicentre, randomized, double‐blind, double‐dummy, parallel‐group, placebo‐controlled study in men with AGA (Clinicaltrials.gov identifier: NCT03004469). 19
Eligible patients were male outpatients aged 18–40 years with mild to moderate vertex male pattern hair loss according to a modified Norwood/Hamilton classification scale (III vertex, IV or V). 20 Main exclusion criteria were: abrasion or abnormalities to the scalp; hair transplant or hair weaving; clinically relevant abnormal laboratory values; hypersensitivity or allergy; recent history of local infections of the head; history of infertility or difficulty fathering children; history of relevant significant disease; active seborrheic dermatitis; history of varicocele; concurrent use of corticosteroids, anabolic steroids or over‐the‐counter ‘hair restorers’; use of drugs with anti‐androgenic properties within 6 months; treatment in the past year with minoxidil, zidovudine, cyclosporine, diazoxide, phenytoin, systemic interferon, psoralens, streptomycin, penicillamine, benoxaprofen, tamoxifen, phenothiazines, cytotoxic agents, finasteride or dutasteride; and light or laser treatment of the scalp within the last 3 months.
The experimental drug was topical finasteride 0.25% w/w (concentration of 2.275 mg/mL) delivered using a spray applicator with a plastic cone that prevents product dispersion in the air (Fig. 1). Each actuation delivers 50 µL of solution, equivalent to 0.114 mg of finasteride. The topical placebo comparator contained the same excipients as the experimental solution (ethanol, propylene glycol, hydroxypropyl chitosan and purified water) and was identical in appearance to and indistinguishable from active treatment. Oral finasteride 1 mg was the reference drug for systemic exposure and was provided as film‐coated tablets (Propecia®, Merck, Kenilworth, NJ, USA) over‐encapsulated to maintain study blinding. The matching oral placebo was an inert powder‐filled capsule indistinguishable from the oral reference drug.
Fig. 1.

Finasteride 0.25% spray applicator and mode of administration on the scalp.
Using a computer‐generated list, eligible patients were randomly allocated in a 2 : 2 : 1 ratio to one of three treatment arms: topical finasteride and oral placebo (‘topical finasteride’ arm), topical placebo and oral placebo (‘placebo’ arm), or topical placebo and oral finasteride (‘oral finasteride’ arm).
All patients applied topical spray each morning to a dry scalp, at the dose recommended by the study doctor (1–4 sprays, or 50–200 µL of solution depending on the extent of hair loss). The first spray was applied over a target 1 cm2 circular area in the scalp vertex, marked by a small dot tattoo to identify it for repeated efficacy assessments. Additional sprays (2–4), if recommended by the study doctor, were applied to cover the rest of the baldness area. The topical spray solution was to be left in place for at least 6–8 h, then washed off with shampoo.
Baseline assessments were performed at screening (week –2). Patients were randomized on Day 1 and treated for 24 weeks. Assessments and study procedures were performed at weeks 4, 8, 12 and 24. A follow‐up visit took place at week 28. In the event of early termination, patients were followed up at the early termination visit.
Patients maintained a diary of daily compliance. Bottles and blister packs of study medications were returned and assessed by investigators for compliance.
Efficacy assessment
At screening, standardized colour global photographs of the scalp vertex were taken with the head in a stereotactic positioning device to confirm the Hamilton Norwood inclusion criterion. For patients fulfilling inclusion and exclusion criteria at the baseline visit, investigators selected a circular 1 cm2 area in the anterior leading edge of the thinning area on the vertex as the target area. Hairs in this area were clipped to approximately 1 mm in length and a small dot tattoo was placed in the centre of the circle of clipped hairs to allow for subsequent accurate identification. Macrophotographs were taken of this target area at baseline, weeks 12 and 24 using an established validated technique as a basis for counting hairs on the digital images. 21
The primary efficacy variable was the change from baseline in target area hair count (TAHC) at week 24 as assessed on the macrophotographs. Main secondary efficacy variables were change from baseline in TAHC at week 12; change from baseline in target area hair width (TAHW) at week 24; patient‐assessed scores on the Male Hair Growth Questionnaire (MHGQ; 7 items regarding treatment efficacy and satisfaction with appearance) 22 at Week 24; investigator‐assessed change from baseline in patient hair growth/loss at the vertex (on a 7‐point scale from −3 = greatly decreased to +3 = greatly increased) at week 24; and blinded‐assessor evaluation of change from baseline in patient hair growth/loss at the vertex (on a 7‐point scale from −3 = greatly decreased to +3 = greatly increased) at week 24. Study investigators examined patients’ scalps directly at follow‐up visits. The blinded assessor (an experienced independent dermatologist) was limited to making assessments on global photographs with reference to the baseline photograph.
Safety assessment
Treatment emergent adverse events (TEAEs) were recorded. Investigators assessed their severity, seriousness and causal relationship to study treatment. Patients were monitored by physical examination, vital signs and body weight. Routine haematology, blood chemistry and urinalysis were conducted. Investigators used the Severity Score for Skin Irritation scale 23 to assess local tolerability at the application site from week 4 to Week 28. Patients completed the self‐administered Sexual Dysfunction Questionnaire (International Index of Erectile Function, version 2) 24 at each visit from weeks 4 to 28.
PK and PD assessment
The plasma PK profiles of finasteride following topical application and oral administration, and any impact of treatment on serum DHT concentrations (PD), were assessed by analysing blood samples collected at randomization and at weeks 4, 8, 12, 24 and 28 (post‐treatment). Samples were taken at approximately 1 h (+30 min) post‐dose when maximum finasteride plasma concentrations after oral administration were expected to be reached. Finasteride and DHT analyses were performed according to the ‘OECD Principles of Good Laboratory Practices’ for testing of chemicals using previously fully validated bioanalytical methods. 25 Finasteride concentrations in plasma, and DHT concentrations in serum, were determined using supported‐liquid extraction followed by analysis using high performance liquid chromatography followed by tandem mass spectrometric detection. Lower limits of quantification were established at 4 pg/mL and 2 ng/dL for plasma finasteride and serum DHT, respectively.
Statistical analysis
A total of 450 patients were to be randomized in a 2 : 2 : 1 allocation ratio, allowing for 20% attrition. A group sample size of 144 patients in the topical finasteride arm and placebo arm was calculated to achieve 99% power to detect the superiority of topical finasteride vs. placebo for the primary endpoint, with a significance level (alpha) of 0.05 using a two‐sided two‐sample t‐test. A group sample size of approximately 72 patients in the oral finasteride arm was calculated to achieve a power close to one to reject the null hypothesis of an equal decrease in serum DHT between oral finasteride and topical finasteride with an alpha of 0.05 using a two‐sided two‐sample equal‐variance t‐test.
The intention‐to‐treat (ITT) population was defined in the study protocol as all patients with valid measurements for the primary efficacy variable at baseline and on treatment. The per protocol (PP) population was defined as all patients in the ITT population who did not take prohibited medications and who completed the study without any major protocol violations. The safety population consisted of all randomized patients who received at least one application of study drug. The modified ITT (mITT) population was defined a posteriori, as all randomized patients who received at least one application of study drug, and was therefore equivalent to the safety population.
The main efficacy analyses were based on the ITT population. Analyses performed on the PP population were considered supportive. Post hoc sensitivity analyses for efficacy were performed on the ITT population for endpoints dependent on the baseline macrophotograph (i.e. TAHC, TAHW), and on the mITT population for endpoints not dependent on the baseline hair count (i.e. investigator and blinded‐assessor assessments of patient hair growth/loss change; MHGQ). Post hoc analyses applied a multiple imputation with jump‐to‐reference approach, assuming the same result as that observed in the placebo group, to handle missing data from patients who withdrew early or did not have a valid macrophotograph. This conservative method assumes that a patient with missing values follows a profile equivalent to that of a patient in the reference group which, in this case, was the placebo group.
The primary efficacy variable, change from baseline in TAHC at week 24, was analysed using the SAS PROC MIXED procedure and summarized descriptively. Data were fitted by a mixed linear model for repeated measures with treatment, centre, visit and treatment‐by‐visit interaction as fixed effects, and baseline hair count as a covariate. Correlation between two repeated measurements (over the post‐baseline visits) was modelled. Maximum likelihood estimates of the treatment mean difference were computed at week 24 together with associated two‐sided 95% confidence intervals (CI) calculated using the Newton‐Raphson algorithm implemented in the SAS Mixed Procedure. A two‐sided test P‐value ≤0.05 was considered statistically significant. The same statistical approach was used for secondary efficacy variables. All computations were performed using the SAS® version 9.4 statistical software package (SAS Institute Inc., Cary, NC, USA).
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and local laws and regulations of participating countries. The study protocol, patient information sheet and informed consent form were reviewed and approved by independent ethics committees. All patients provided written informed consent prior to participation. Procedures were enacted to ensure patient confidentiality and data protection.
Results
Participating sites and patient populations
Patients were enrolled at 45 sites in five countries: Belgium (4 sites), Germany (18 sites), Spain (8 sites), Hungary (6 sites) and Russian Federation (9 sites). The study took place between August 2016 and March 2018.
Patient disposition is shown in Fig. 2. A total of 458 patients were randomized to treatment (189 to topical finasteride, 184 to placebo, 85 to oral finasteride) and 323 patients completed the study. Percentages of patients not completing the study, and reasons for discontinuation, were similar among treatment groups. Overall, 250 patients (54.6%) had evaluable hair count measurements from the macrophotographs both at baseline and on treatment and formed the ITT population. A total of 446 patients formed the safety (and mITT) population.
Fig. 2.
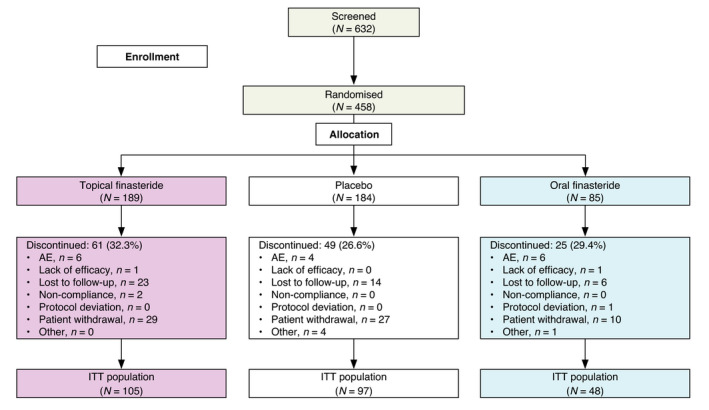
Patient disposition. AE, adverse event; ITT, intention to treat.
Patient demographics and baseline characteristics
Demographic details and baseline hair loss pattern in the ITT population are shown in Table 1. Mean age was approximately 32 years and about half of patients in each group had type III vertex pattern hair loss. Mean TAHC at baseline was similar among treatment groups: 201.0 ± 67.6 hairs for topical finasteride, 204.8 ± 67.2 hairs for placebo and 201.9 ± 72.9 hairs for oral finasteride. Mean TAHW at baseline was also similar, with values of 44.4, 46.3 and 46.0 µm, respectively. Treatment groups were broadly similar with respect to other demographic variables (including alcohol and tobacco use) and baseline characteristics.
Table 1.
Demographics and baseline characteristics (ITT population)
| Characteristic | Topical finasteride (N = 105) | Placebo (N = 97) | Oral finasteride (N = 48) |
|---|---|---|---|
| Age (years), mean (SD) | 32.5 (5.4) | 31.8 (4.9) | 32.3 (5.5) |
| Weight (kg), mean (SD) | 83.6 (17.5) | 84.3 (14.6) | 83.3 (12.4) |
| Caucasian, n (%) | 103 (98.1) | 96 (99.0) | 46 (95.8) |
| Male pattern baldness†, n (%) | |||
| Type III vertex | 50 (47.6) | 45 (46.4) | 26 (54.2) |
| Type IV | 27 (25.7) | 31 (32.0) | 14 (29.2) |
| Type V | 28 (26.7) | 20 (20.6) | 8 (16.7) |
| Other | 0 (0.0) | 1 (1.0) | 0 (0.0) |
ITT, intention to treat; SD, standard deviation.
According to the Norwood/Hamilton Scale.
Efficacy analyses
At week 24, the adjusted mean change from baseline in TAHC was significantly greater with topical finasteride than with placebo (20.2 vs. 6.7 hairs; P < 0.001), and was numerically similar to that with oral finasteride (21.1 hairs; Fig. 3). The post hoc sensitivity analysis produced robust results with the same conclusion: the adjusted mean change from baseline in TAHC at week 24 was significantly greater with topical finasteride than placebo (16.3 vs. 6.3 hairs; p = 0.012) and numerically similar to that with oral finasteride (18.7 hairs; Fig. 4).
Fig. 3.
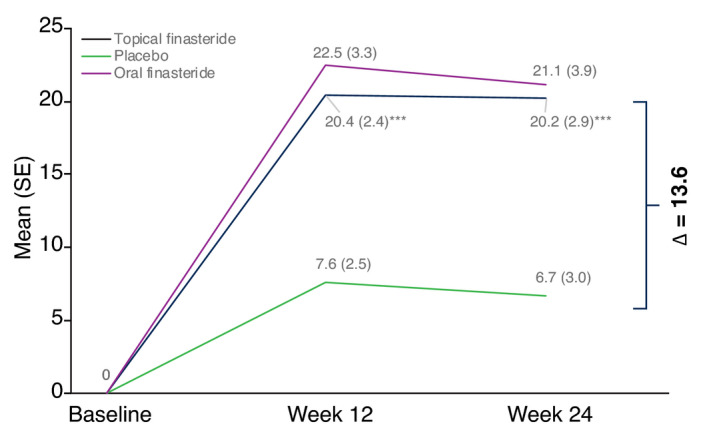
Adjusted mean change from baseline in target area hair count in the vertex at week 12 and at week 24 (primary efficacy endpoint) in the intention to treat population. *** P < 0.001 vs. placebo. SE, standard error.
Fig. 4.
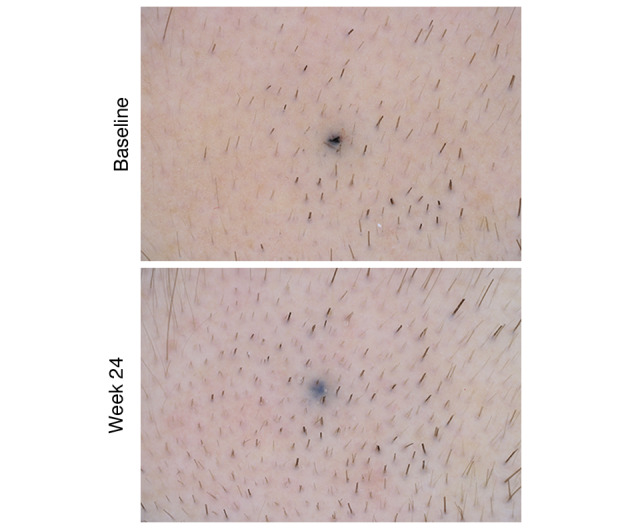
Baseline and week 24 macrophotograph of a patient treated with finasteride 0.25% topical solution who was rated as showing marked improvement (Canfield Scientific, Inc., Parsippany, NJ, USA).
At week 12, a statistically significant increase from baseline in TAHC relative to placebo was observed with topical finasteride (Fig. 3). Changes from baseline in other secondary efficacy outcomes in the ITT population are summarized in Table 2. The adjusted mean change from baseline to week 24 in TAHW indicated negligible changes with any study treatment. At week 24, the patient‐assessed MHGQ score for the item ‘overall assessment’ was similar across treatment groups. The investigator‐assessed adjusted mean change from baseline to week 24 in patient hair growth/loss at the vertex was statistically significantly greater with topical finasteride than placebo (0.8 vs. 0.3, P < 0.001) and was numerically similar to that with oral finasteride (0.7). Blinded‐assessor evaluation of patient hair growth/loss at the vertex indicated no to minimal change from baseline in any group at week 24.
Table 2.
Changes from baseline in secondary efficacy variables (ITT population)
| Variable | Topical finasteride (N = 105) | Placebo (N = 97) | Oral finasteride (N = 48) |
|---|---|---|---|
| Hair width, μm [adjusted mean (SE)] | |||
| Week 24 | −0.81 (0.35) | −1.53 (0.37) | 0.72 (0.47) |
| Self‐administered MHGQ overall score† [adjusted mean (SE)] | |||
| Week 24 | 2.8 (0.75) | 3.0 (0.95) | 2.9 (0.89) |
| Investigator‐assessed change in patient hair growth/loss‡ [adjusted mean (SE)] | |||
| Week 24 | 0.8 (0.09)* | 0.3 (0.09) | 0.7 (0.12) |
| Blinded‐assessor change in patient hair growth/loss‡ [adjusted mean (SE)] | |||
| Week 24 | 0.2 (0.09) | 0.1 (0.09) | 0.3 (0.12) |
ITT, intention to treat; MHGQ, Male Hair Growth Questionnaire; SE, standard error.
P < 0.001 vs. placebo.
Assessed on a 5‐point scale from 1 = very satisfied to 5 = very dissatisfied.
Assessed on a 7‐point scale from −3 = greatly decreased to +3 = greatly increased.
To assist in interpreting the clinical relevance of the findings, a post hoc sensitivity analysis was performed on the mITT/safety population. For MHGQ and investigator and blinded‐assessor evaluations of the change in patient hair growth/loss, results were dichotomized into ‘responder’ and ‘non‐responder’ categories, where response for each item was defined as showing any degree of improvement. The percentage of responders with respect to investigator assessment, blinded‐assessor assessment and three items of the MHGQ were significantly greater with topical finasteride than placebo (Table 3).
Table 3.
Percentage of responders for secondary endpoints at week 24: post hoc sensitivity analysis (safety population)
| Responders a | Topical finasteride (N = 181) | Placebo (N = 181) | Oral finasteride (N = 84) | P‐value (topical finasteride vs. placebo) |
|---|---|---|---|---|
| Investigator assessment (%) | 42.0 | 27.6 | 35.7 | <0.005 |
| Blinded‐assessor assessment (%) | 26.0 | 16.0 | 28.6 | 0.02 |
| MHGQ – patient assessment (%) | ||||
| Smaller bald spot | 29.3 | 21.0 | 31.0 | 0.069 |
| Hair appearance | 40.9 | 28.7 | 36.9 | 0.015 |
| Hair growth | 39.8 | 32.0 | 31.0 | 0.12 |
| Slow down hair loss | 41.4 | 31.5 | 40.5 | <0.05 |
| Satisfaction – front hair line | 21.6 | 11.1 | 17.9 | 0.007 |
| Satisfaction – head top | 26.0 | 19.3 | 23.8 | 0.13 |
| Overall change | 26.5 | 19.9 | 25.0 | 0.13 |
MHGQ, Male Hair Growth Questionnaire.
Response for each parameter was defined as showing any degree of improvement.
Safety assessment
The percentage of patients with TEAEs in the topical finasteride group was similar to that in the placebo group and lower than that in the oral finasteride group (Table 4). Most TEAEs (96.9%) were mild or moderate in intensity. Ten patients reported treatment‐emergent serious AEs of which none was considered by investigators to be related to study medication.
Table 4.
Frequency of treatment emergent adverse events (TEAEs) in the safety population
| Topical finasteride (N = 181) | Placebo (N = 181) | Oral finasteride (N = 84) | Total (N = 446) | |
|---|---|---|---|---|
| Patients with TEAEs, n (%) | 75 (41.4) | 76 (42.0) | 41 (48.8) | 192 (43.0) |
| Mild | 59 (32.6) | 60 (33.1) | 33 (39.3) | 152 (34.1) |
| Moderate | 32 (17.7) | 31 (17.1) | 19 (22.6) | 82 (18.4) |
| Severe† | 4 (2.2) | 3 (1.7) | 2 (2.4) | 9 (2.0) |
| Patients with TEAEs leading to study discontinuation, n (%) | 5 (2.8) | 4 (2.2) | 6 (7.1) | 15 (3.4) |
| Patients with treatment‐related TEAEs‡, n (%) | 18 (9.9) | 12 (6.6) | 10 (11.9) | 40 (9.0) |
| Patients with treatment‐related TEAEs leading to study discontinuation | 4 (2.2) | 2 (1.1) | 2 (2.4) | 8 (1.8) |
| Patients with treatment‐emergent serious AEs | 4 (2.2) | 5 (2.8) | 1 (1.2) | 10 (2.2) |
| Patients with treatment‐related serious AEs | 0 | 0 | 0 | 0 |
Cases of unknown intensity were assumed to be severe.
Includes AEs considered related or possibly related to study drug and AEs with unknown or missing relationship to study drug.
Incidences of treatment‐related TEAEs were 9.9%, 6.6% and 11.9% in the topical finasteride, placebo and oral finasteride groups, respectively. Withdrawal rates due to treatment‐related TEAEs were 2.8% and 2.2% in the topical finasteride and placebo groups, respectively, and 7.1% in the topical finasteride group. Treatment‐related TEAEs reported by ≥3 patients in any of the topical finasteride, placebo or oral finasteride groups were: pruritus (2.2% vs. 0.6% vs. 1.2% of patients, respectively), erythema (2.2%, 0%, and 0%, respectively), and loss of or reduction in libido (0.6%, 2.8% and 4.8%, respectively).
There were no clinically meaningful changes from baseline, or differences among treatment groups, in mean values for haematology, serum chemistry and urinalysis results.
Incidence rates of skin irritation as evaluated using the Severity Score for Skin Irritation scale were <1% in all treatment groups.
There were no significant differences between topical finasteride and placebo in mean scores for any item on the Sexual Dysfunction Questionnaire at week 12 or 24. Mean scores for all items were similar between topical finasteride and oral finasteride at weeks 12 and 24.
Treatment‐related sexual adverse events (sexual dysfunction, erectile dysfunction, libido decreased, loss of libido) were reported in 2.8% vs. 3.3% vs. 4.8% of patients treated with topical finasteride, placebo, or oral finasteride. Discontinuations due to treatment‐related sexual adverse events were reported in 0% vs. 1.1% vs. 2.4% of patients, respectively.
PK and PD analyses
Mean ± SD maximum plasma finasteride concentrations were 36.5 ± 45.9 pg/mL with topical finasteride and 7166 ± 12 744 pg/mL with oral finasteride at week 12; and were 48.0 ± 87.2 pg/mL and 5029 ± 4182 pg/mL, respectively, at week 24.
Mean serum DHT concentrations in the placebo group remained unaffected during the study (range: 38.5–39.8 ng/dL). Mean serum DHT concentrations at week 24 were 34.6% lower than at baseline in the topical finasteride group (25.75 vs. 39.32 ng/dL, respectively), and were 55.6% lower than at baseline in the oral finasteride group (15.75 vs. 35.50 ng/dL, respectively). The adjusted mean difference in the change from baseline in serum DHT concentrations was statistically significant between topical finasteride and placebo (P < 0.05), and between topical finasteride and oral finasteride (P < 0.05), at each of weeks 4, 8, 12 and 24 (Fig. 5).
Fig. 5.
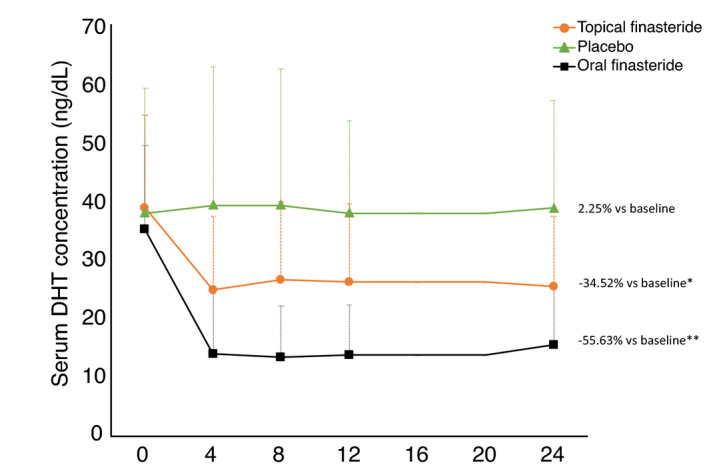
Mean serum dihydrotestosterone (DHT) concentrations during treatment for 24 weeks with topical finasteride, placebo, or oral finasteride. The difference in mean values from baseline to week 24 is shown at the end of each line. * P < 0.05 vs. placebo and oral finasteride at all time points; ** P < 0.05 vs. placebo at all time points. Note: error bars indicate standard deviation.
The lower impact of topical finasteride on DHT levels was not accompanied by any shift from normal to high plasma testosterone concentrations for patients in this group; this was also the case for the placebo group. In the oral finasteride group, this shift occurred in four (6.7%) patients.
Discussion
Although oral finasteride has proven effective in treating AGA, the occurrence of adverse effects has been a point of concern. Since the 1990s, interest had shifted towards topical application of finasteride to improve the risk‐to‐benefit ratio, as summarized in a recent systematic review. 14 To test the hypothesis, this 24‐week phase III study used a robust design, incorporating placebo and active control arms and a double dummy technique to maintain blinding.
A statistically significant greater improvement in hair count at the scalp vertex was demonstrated with topical finasteride over placebo, with an effect evident at 12 weeks. The efficacy of topical finasteride was numerically similar to that of oral finasteride. Of interest was that the increase in hair count was not accompanied by any appreciable change in hair width compared with baseline values in any treatment group (Table 2). As such, hair width may not be of utility as an endpoint to monitor treatment effect. At study end, the change in patient hair growth/loss at the vertex significantly favoured topical finasteride vs. placebo when assessed by the investigator, and was numerically similar between topical and oral finasteride. Small discrepancies, ranging from 0.2 to 0.6 on the 7 point scale, were observed between assessments made by the investigator and blinded evaluator which may be due to the way in which these assessments were generated. Investigators were able to examine the patient more closely at follow‐up visits with the option to touch the hair and scalp and part the hair. In contrast, the blind assessor had access only to two‐dimensional photographs which could be compared with the baseline image. Although the investigators’ assessment was more favourable, there is no suggestion that investigators were biased towards a more positive opinion of treatment success with topical finasteride, since assessments were made under blinded conditions. With regard to patients’ own perception of treatment success, as reflected in responses to the MHGQ, even in this relatively short‐term study over 24 weeks, it is interesting that patients reported statistically significant improvement in three of the six items on the questionnaire. Post hoc sensitivity analyses demonstrated the consistency and robustness of the main analysis.
While there was a trend towards more treatment‐related skin and application site reactions (e.g. pruritus, erythema) with topical finasteride than with placebo or oral finasteride, events that occurred were mainly mild or moderate in intensity. Incidences of skin irritation as assessed by the Severity Score for Skin Irritation scale were low (<1%) in all treatment groups.
Some patients treated with oral finasteride may experience adverse effects potentially related to the circulating plasma concentration of drug required to achieve effective concentrations at the scalp. There is ongoing debate whether, in some cases, use of oral finasteride 1 mg/day to treat male pattern hair loss may be associated with irreversible sexual dysfunction and severe depression. 26 , 27 , 28 , 29 As demonstrated in this study, maximum mean plasma finasteride concentrations were more than 100‐fold lower with the topical vs. oral formulation, and the impact on serum DHT concentrations after 24 weeks’ treatment was statistically significantly lower with topical vs. oral finasteride (reductions of 34.6% and 55.6%, respectively). While this does not exclude the possibility of systemic adverse events related to decreased DHT in both groups, the probability is lower with topical than oral finasteride. A trend was evident for fewer treatment‐related sexual adverse events, and associated treatment discontinuations, in the topical vs. oral finasteride group.
European evidence‐based guidelines recommend topical minoxidil and oral finasteride for AGA and suggest low‐level laser light therapy as an ancillary therapy. 30 Although follicular unit transplantation (FUT) and non‐surgical methods such as platelet‐rich plasma (PRP) 31 and microneedling with PRP 32 have been developed, current European guidelines suggest FUT only in combination with oral finasteride as a treatment option for AGA. 30 Stem cell procedures are in development for patients resistant to other therapies. 33 The availability of topical finasteride provides an additional treatment option that is effective and generally well tolerated.
The main finding of the study was that the change from baseline in hair count was significantly greater with topical finasteride than placebo, and similar to that observed with oral finasteride. This result was achieved with markedly lower systemic exposure to finasteride and less impact on serum DHT concentrations compared with oral finasteride. Topical finasteride was well tolerated and had a safety profile not meaningfully different from that of placebo. As such, topical finasteride appears to be a useful option for treatment of AGA in men. Further studies would be useful to demonstrate the long‐term efficacy of topical finasteride. Understanding the reasons for the relatively high number of treatment discontinuations and negligible changes from baseline to end of treatment in certain subjective measures such as the patient‐assessed MHGQ score for ‘overall assessment’ and blinded‐assessor evaluation of patient hair growth/loss may assist in designing future studies.
Supporting information
Supplementary Material
Acknowledgements
Writing assistance was provided by Rob Furlong and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain) with funding from Almirall S.A. (Barcelona, Spain). The authors thank the principal investigators at all participating sites: Sofie De Schepper, Universitair Ziekenhuis Gent, Gent, Belgium. Barbara Boone, Universitair Ziekenhuis Gent, Gent, Belgium. Jan Gutermuth, Universitair Ziekenhuis Brussel, Brussels, Belgium. Véronique Del Marmol, Hôpital Erasme, Brussels, Belgium. Jean‐Jacques Stene, Saint‐Pierre University Hospital, Brussels, Belgium. Bertrand Richert, Saint‐Pierre University Hospital, Brussels, Belgium. Thomas Dirschka, Centroderm GmbH, Wuppertal, Germany. Rodrigo da Mota, Medical Skin Center – Dr. Hilton & Partner, Düsseldorf, Germany. Rolf Hoffmann, Rolf Hoffmann Privatpraxis für Dermatologie, Freiburg, Germany. Peter Weisenseel, SCIderm GmbH, Hamburg, Germany. Nina Magnolo, Universitätsklinikum Münster Zentralklinikum, Münster, Germany. Athanasios Tsianakas, Universitätsklinikum Münster Zentralklinikum, Münster, Germany. Charlotte von Engelhardt, Klinische Forschung Schwerin GmbH, Schwerin, Germany. Peter Heymer, Klinische Forschung Dresden GmbH, Dresden, Germany. Georg Popp, Licca Clinical Research Institute, Augsburg, Germany. Andrei Khariouzov, Klinische Forschung Berlin‐Mitte GmbH, Berlin, Germany. Irmgard Marten, Klinische Forschung Hannover‐Mitte, Hannover, Germany. Andreas Klare, Klinische Forschung Hamburg GmbH, Hamburg, Germany. Julia Chevts, Klinische Forschung Karlsruhe, Karlsruhe, Germany. Klaus Hoffmann, St. Josef‐Hospital im Katholischen Klinikum Bochum, Bochum, Germany. Beatrice Gerlach, Praxis für Dermatologie und Venerologie, Dresden, Germany. Nina Otberg, Haut‐ & Lasercentrum, Potsdam, Germany. Tobias Fischer, Universitätsklinikum Schleswig‐Holstein, Luebeck, Germany. Roland Aschoff, Universitätsklinikum Carl Gustav Carus, Dresden, Germany. Noemi Bakos, Allergo‐Derm Kft, Szolnok, Hungary. Eva Remenyik, Debreceni Egyetem Klinikai Központ, Debrecen, Hungary. Anita Furesz, Clinexpert Gyogycentrum, Budapest, Hungary. Enikő Telegdy, Vas Megyei Markusovszky Kórház Nonprofit Zrt, Szombathely, Hungary. Rita Rez, Evolúció Központ, Sátoraljaújhely, Hungary. Péter Holló, Óbudai Egészségügyi Centrum, Budapest, Hungary. Oleg Ziganshin, SBHI “Chelyabinsk Regional Clinical Skin and Venereal Diseases Dispensary”, Chelyabinsk, Russian Federation. Yuri Perlamutrov, Moscow State University of Medicine and Dentistry, Moscow, Russian Federation. Marina Tarasova, State Organization of the Ryazan Region “Regional Clinical Skin and Venereal Diseases Dispensary”, Ryazan, Russian Federation. Olga Mikerina, Sanavita, Saint‐Petersburg, Russian Federation. Alexey Kubanov, Russian Medical Academy for Postgraduate Education, Moscow, Russian Federation. Konstantin Raznatovsky, North‐Western State Medical University, Saint‐Petersburg, Russian Federation. Vadim Temnikov, Rostov Regional Clinic for Skin and Venereal, Bataysk, Russian Federation. Vladimir Yakusevich, Clinical Hospital of Emergency Medical Care, Yaroslavl, Russian Federation. Alexander Solomatin, Centre of the Ministry of Economic Development of Russia, Moscow, Russian Federation. Pablo Luis Ortiz Romero, Hospital Universitario 12 de Octubre, Madrid, Spain. Susana Puig, Hospital Clínic de Barcelona, Barcelona, Spain. Isabel Betlloch Mas, Hospital General Universitario de Alicante, Alicante, Spain. Javier Sánchez Pérez, Hospital Universitario de la Princesa, Madrid, Spain. Jesús Gardeazabal García, Hospital de Cruces, Barakaldo, Spain. Agustin España, Clínica Universidad de Navarra, Pamplona, Spain. Juan Alberto Ruano Ruiz, Hospital Universitario Reina Sofía, Córdoba, Spain. Eduardo López Bran, Hospital Clínico San Carlos, Madrid, Spain. Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement. [Correction added on 22 May 2022, after first online publication: CRUI‐CARE funding statement has been added.]
Clinical Study Report Code: PM1541; EudraCT No. 2015‐002877‐40
Conflict of interest
BM Piraccini has received honoraria for advisory boards or grants for clinical studies from Giuliani, ISDIN, Legacy Healthcare, Pierre Fabre‐Dermo‐Cosmétique and Pfizer. U Blume‐Peytavi has received honoraria for advisory boards or grants for clinical studies from Bayer, Follicum, Johnson & Johnson, Neuroderm, Pierre Fabre Dermo‐Cosmétique, Pfizer, Polichem, Regeneron and Sanofi Regeneron. JM Jansat, M Falqués, R Otero, ML Tamarit, J Galván and E Massana are full‐time employees of Almirall S.A. F Scarci was a full‐time employee of Almirall S.A. at the time this clinical trial was completed. V Tebbs was previously a full‐time employee of Almiral Hermal GmbH and provided medical consultancy advice for Almirall S.A. in respect of this study.
Funding sources
Polichem S.A, 50 Val Fleuri, 1526 Luxembourg. Almirall S.A, Laureà Miró, 408‐410, 08980 Sant Feliu de Llobregat, Barcelona, Spain
References
- 1. Whiting DA. Male pattern hair loss: current understanding. Int J Dermatol 1998; 37: 561–566. [DOI] [PubMed] [Google Scholar]
- 2. Batrinos ML. The endocrinology of baldness. Hormones 2014; 13: 197–212. [DOI] [PubMed] [Google Scholar]
- 3. Han SH, Byun JW, Lee WS et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicentre study. Ann Dermatol 2012; 24: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bingham KD, Shaw DA. The metabolism of testosterone by human male scalp skin. J Endocrinol 1973; 57: 111–121. [DOI] [PubMed] [Google Scholar]
- 5. McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs 1999; 57: 111–126. [DOI] [PubMed] [Google Scholar]
- 6. Finasteride Male Pattern Hair Loss Study Group . Long‐term (5‐year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol 2002; 12: 38–49. [PubMed] [Google Scholar]
- 7. Kaufman KD, Rotonda J, Shah AK, Meehan AG. Long‐term treatment with finasteride 1 mg decreases the likelihood of developing further visible hair loss in men with androgenetic alopecia (male pattern hair loss). Eur J Dermatol 2008; 18: 400–406. [DOI] [PubMed] [Google Scholar]
- 8. Rossi A, Cantisani C, Scarnò M, Trucchia A, Fortuna MC, Calvieri S. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10‐year follow‐up. Dermatol Ther 2011; 24: 455–461. [DOI] [PubMed] [Google Scholar]
- 9. Yanagisawa M, Fujimaki H, Takeda A, Nemoto M, Sugimoto T, Sato A. Long‐term (10‐year) efficacy of finasteride in 523 Japanese men with androgenetic alopecia. Clin Res Trials 2019; 5: 1–5. [Google Scholar]
- 10. Shin JW, Chung EH, Kim MB, Kim TO, Kim WI, Huh CH. Evaluation of long‐term efficacy of finasteride in Korean men with androgenetic alopecia using the basic and specific classification system. J Dermatol 2019; 46: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coskuner ER, Ozkan B, Culha MG. Sexual problems of men with androgenic alopecia treated with 5‐alpha reductase inhibitors. Sex Med Rev 2019; 7: 277–282. [DOI] [PubMed] [Google Scholar]
- 12. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α‐reductase inhibitors. JAMA Intern Med 2017; 177: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5‐alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol 2016; 9: 56–62. [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SW, Juhasz M, Mobasher P, Ekelem C, Mesinkovska NA. A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J Drugs Dermatol 2018; 17: 457–463. [PMC free article] [PubMed] [Google Scholar]
- 15. Noubarani M, Rostamkhani H, Erfan M et al. Effect of adiantum capillus veneris linn on an animal model of testosterone‐induced hair loss. Iran J Pharm Red 2014; 13(Suppl): 113–118. [PMC free article] [PubMed] [Google Scholar]
- 16. Mazzarella GF, Loconsole GF, Cammisa GA, Mastrolonardo GM, Vena G. Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16‐month therapy course. J Dermatol Treat 1997; 8: 189–192. [Google Scholar]
- 17. Caserini M, Radicioni M, Leuratti C, Annoni O, Palmieri R. A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. Int J Clin Pharmacol Ther 2014; 52: 842–849. [DOI] [PubMed] [Google Scholar]
- 18. Caserini M, Radicioni M, Leuratti C, Terragni E, Iorizzo M, Palmieri R. Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. Int J Clin Pharmacol Ther 2016; 54: 19–27. [DOI] [PubMed] [Google Scholar]
- 19. U.S. National Library of Medicine . ClinicalTrials.gov. A multicentre, randomized, double‐blind, parallel‐group, controlled study, to assess the efficacy and safety of P‐3074 cutaneous spray, solution, in the treatment of male pattern baldness. https://clinicaltrials.gov/ct2/show/NCT03004469
- 20. Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975; 68: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 21. Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin 1996; 14: 713–721. [DOI] [PubMed] [Google Scholar]
- 22. Barber BL, Kauffman KD, Kozloff RC, Girman CJ, Guess HA. A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatol Treat 1998; 9: 181–186. [Google Scholar]
- 23. Berger RS, Bowman JP. A reappraisal of the 21‐day cumulative irritation test in man. J Toxicol. Cut Ocular Toxicol 1982; 1: 109–115. [Google Scholar]
- 24. Bayraktar Z, Atun AI. Despite some comprehension problems the International Index of Erectile Function is a reliable questionnaire in erectile dysfunction. Urol Int 2012; 88: 170–176. [DOI] [PubMed] [Google Scholar]
- 25. OECD . Series on Principles of Good Laboratory Practice and Compliance Monitoring. Available from: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=en. Accessed 22 January 2021.
- 26. Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med 2011; 8: 1747–1753. [DOI] [PubMed] [Google Scholar]
- 27. Singh MK, Avram M. Persistent sexual dysfunction and depression in finasteride users for male pattern hair loss: a serious concern or red herring? J Clin Aesthet Dermatol 2014; 7: 51–55. [PMC free article] [PubMed] [Google Scholar]
- 28. Haber RS, Gupta AK, Epstein E, Carviel JL, Foley KA. Finasteride for androgenetic alopecia is not associated with sexual dysfunction: a survey‐based, single‐centre, controlled study. J Eur Acad Dermatol Venereol 2019; 33: 1393–1397. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen DD, Marchese M, Cone EB et al. Investigation of suicidality and psychological adverse events in patients treated with finasteride. JAMA Dermatol 2021; 157: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanti V, Messenger A, Dobos G et al. Evidence‐based (S3) guideline for the treatment of androgenetic alopecia in women and in men ‐ short version. J Eur Acad Dermatol Venereol 2018; 32: 11–22. [DOI] [PubMed] [Google Scholar]
- 31. Rinaldi S, Bussa M, Mascaro A. Update on the treatment of androgenetic alopecia. Eur Rev Med Pharmacol Sci 2016; 20: 54–58. [PubMed] [Google Scholar]
- 32. Shah KB, Shah AN, Solanki RB, Raval RC. A comparative study of microneedling with platelet‐rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology 2017; 9: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elmaadawi IH, Mohamed BM, Ibrahim ZAS et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat 2018; 29: 431–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


