ABSTRACT
Osteoporosis is a systemic skeletal disease characterized by low bone mass and bone structural deterioration that may result in fragility fractures. Use of bone imaging modalities to accurately predict fragility fractures is always an important issue, yet the current gold standard of dual‐energy X‐ray absorptiometry (DXA) for diagnosis of osteoporosis cannot fully satisfy this purpose. The latest high‐resolution peripheral quantitative computed tomography (HR‐pQCT) is a three‐dimensional (3D) imaging device to measure not only volumetric bone density, but also the bone microarchitecture in a noninvasive manner that may provide a better fracture prediction power. This systematic review and meta‐analysis was designed to investigate which HR‐pQCT parameters at the distal radius and/or distal tibia could best predict fragility fractures. A systematic literature search was conducted in Embase, PubMed, and Web of Science with relevant keywords by two independent reviewers. Original clinical studies using HR‐pQCT to predict fragility fractures with available full text in English were included. Information was extracted from the included studies for further review. In total, 25 articles were included for the systematic review, and 16 articles for meta‐analysis. HR‐pQCT was shown to significantly predict incident fractures and/or major osteoporotic fractures (MOFs). Of all the HR‐pQCT parameters, our meta‐analysis revealed that cortical volumetric bone mineral density (Ct.vBMD), trabecular thickness (Tb.Th), and stiffness were better predictors. Meanwhile, HR‐pQCT parameters indicated better performance in predicting MOFs than incident fractures. Between the two standard measurement sites of HR‐pQCT, the non‐weight‐bearing distal radius was a more preferable site than distal tibia for fracture prediction. Furthermore, most of the included studies were white‐based, whereas very few studies were from Asia or South America. These regions should build up their densitometric databases and conduct related prediction studies. It is expected that HR‐pQCT can be used widely for the diagnosis of osteoporosis and prediction of future fragility fractures. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: HR‐pQCT, FRAGILITY FRACTURE, OSTEOPOROSIS, SYSTEMATIC REVIEW, META‐ANALYSIS
Introduction
With the aging population, age‐associated diseases have become a major concern worldwide. Osteoporosis is a skeletal disease characterized by low bone mass and bone structural deterioration leading to increased risk of fracture.( 1 ) Osteoporosis has no symptoms until an individual encounters a fracture. Currently, the dual energy X‐ray absorptiometry (DXA) is the gold‐standard imaging tool in assessing bone mineral density (BMD) and fracture risk.( 2 ) DXA can measure an integral BMD (including cortical and trabecular BMD) and provide spatial distribution of the bone mass. With the World Health Organization (WHO) definition, a T‐score derived from DXA is clinically useful in identifying patients with osteoporosis (T‐score of −2.5 or below).( 3 ) Previous prospective studies showed that the risk of fracture is inversely related to the areal BMD (aBMD) measured by DXA.( 4 , 5 , 6 ) Despite this, many fragility fractures are found in osteopenic patients who have relatively high aBMD.( 7 ) aBMD values overlap substantially between patients with and without fractures. The fracture prediction power of DXA is therefore suboptimal.
According to the National Institutes of Health (NIH), major parameters for defining osteoporosis is based on both bone mass and structure that are the key determinants of bone strength.( 8 ) Recent advances in bone imaging permit the assessment of bone microstructure in vivo using high‐resolution peripheral quantitative computed tomography (HR‐pQCT). HR‐pQCT is a noninvasive three‐dimensional (3D) imaging technique that quantitatively measures volumetric BMD (vBMD) at the peripheral skeletal sites (distal radius and distal tibia). It provides accurate evaluation of the volumetric bone density in a 3D manner within cortical and trabecular compartments, independent of the bone size. vBMD is of intriguing potential to be used for early detection of osteoporosis and incorporation into the algorithm of fracture prediction. Therefore, HR‐pQCT is of good potential for clinical detection of osteoporosis and an updated guideline of standardizing HR‐pQCT imaging techniques has recently been published.( 9 )
HR‐pQCT has shown a good ability in predicting fracture risk, as reported in a number of studies using HR‐pQCT.( 10 , 11 , 12 , 13 , 14 ) However, these studies reported successful predictions with different and inconsistent HR‐pQCT parameters, such as total vBMD (Tt.BMD), trabecular vBMD (Tb.BMD), cortical thickness (Ct.Th), and trabecular thickness (Tb.Th) in the Os des Femmes de Lyon (OFELY) study( 10 ); Tt.BMD, Tb.BMD, trabecular number (Tb.N,) and trabecular separation (Tb.Sp) in the Canadian Multicentre Osteoporosis Study (CaMos) study( 11 ); Tb.N, Tb.Sp, and connectivity density (Conn.D) in Structure of Aging Men's Bones (STRAMBO) study.( 13 ) HR‐pQCT can generate up to 18 geometric, microstructural, and biomechanical (derived by finite element analysis) parameters. These parameters carry different meanings and implications in various applications, yet they may be difficult for users to understand their underlying impact. It is therefore useful to understand these parameters and compare their strength in fracture risk prediction. The objective of this systematic review and meta‐analysis is to investigate which HR‐pQCT bone quality parameters at distal radius and/or distal tibia can best predict fragility fractures in various ethnic populations.
Materials and Methods
Search strategy
A literature search was performed in Embase, PubMed, and Web of Science databases. The keywords “HR‐pQCT OR high resolution peripheral quantitative computed tomography OR XtremeCT” AND “fracture*” were used to search in all fields. Last access to these databases was on January 31, 2021. The Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines were followed.
Search criteria
Inclusion criteria were as follows: (i) clinical studies, (ii) subjects aged ≥50 years or postmenopausal, (iii) fragility fractures, ie, those resulting from falling from standing height.( 15 ) Exclusion criteria were as follows: (i) non‐English articles, (ii) review, conference abstract, case report, protocol article, (iii) involving diseases affecting bone metabolism, (iv) nonstandard HR‐pQCT parameters or evaluation, (v) precision or validation or machine learning studies, and (vi) fractures of skull, face, fingers, and toes.
Selection of studies
Study selection was conducted by two independent reviewers. Duplicates were removed. Irrelevant papers were screened out through the titles and abstracts based on inclusion and exclusion criteria. Full text of relevant articles was retrieved and reviewed for the eligibility. Disagreements were resolved by discussion and consensus.
Data extraction
The following information was extracted by reviewers: study design, ethnicity, sex, age, sample size, trauma degree, fragility fracture site, fracture prediction results (odds ratio [OR], hazard ratio [HR]), model of HR‐pQCT, site of measurement, and data of HR‐pQCT–related parameters.
Assessment of quality of included studies
Two authors independently conducted quality assessment of the selected studies. Disagreements were settled by discussion. The methodological quality was assessed using the Newcastle‐Ottawa Scale validated for observational studies, for which separate tools are developed for cohort and case control studies.( 16 ) The form assigns a maximum of four points for selection, two points for comparability, and three points for exposure or outcomes.
Data analysis
A meta‐analysis on peripheral bone microarchitecture parameters and estimated bone strength in patients with fragility fracture was performed by computing risk ratios (RRs) and 95% confidence intervals (CIs) using a fixed‐effects model. Quantitative analyses were performed on a time‐to‐event basis and were confined to data derived from the period of follow‐up. Data analysis was carried out based on (i) bone microarchitecture parameters and estimated bone strength and (ii) study design (by cohort studies or case–control studies), followed by an overall effect by combining the outcomes from both study types. Data heterogeneity was represented using chi‐square test and heterogeneity (I 2) estimate. Test for overall effect was performed using Z‐statistics. Subgroup analysis was tested for the study types (ie, cohort versus case control studies). Publication bias was analyzed using Egger's test. Publication bias was indicated when p < 0.05. The Egger's test was carried out by Meta‐Essentials (Erasmus Research Institute of Management [ERIM], Rotterdam, Netherlands) and the corresponding funnel plots were prepared by RevMan software (version 5.4; The Cochrane Collaboration, London, UK). RevMan was used for all analyses and production of plots. The inverse variance (IV) method was used to weight the study effect size. Test of contrast comparing the effect sizes of the three best performance HR‐pQCT parameters were carried out using Cohen's d, which was derived through the effect size calculations for t test in IBM SPSS version 27 (IBM Corp, Armonk, NY, USA). In brief, Cohen's d point estimates of ~0.2 are regarded as small effects, values ~0.5 as medium‐sized effects, and those ≥0.8 as large effects.( 17 )
Results
Search results
In the search, 1941 papers were identified from various databases. After removing duplicates, 695 papers remained, from which 544 papers were excluded based on the selection criteria after screening. The full texts of 151 papers were retrieved for further assessment of eligibility; 126 papers were further excluded, because these studies did not involve fracture discrimination or prediction. Hence, 25 papers were finally included in this systematic review including 18 case–control studies( 10 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ) and seven cohort studies.( 11 , 12 , 13 , 14 , 35 , 36 , 37 ) Out of 25 papers, only 16 papers were included for meta‐analysis, in which there were 11 case–control studies( 10 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ) and five cohort studies.( 11 , 12 , 13 , 35 , 36 ) Figure 1 summarizes the selection process of the included papers.
Fig 1.
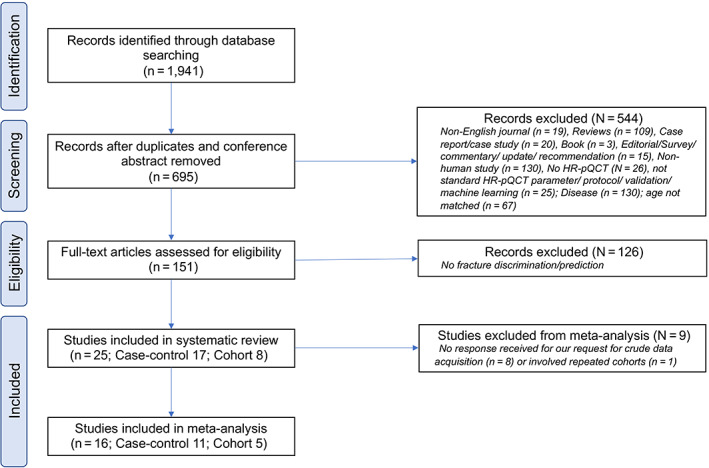
Flowchart showing the selection process of literature search.
Characteristics of the papers
Among 25 included papers, the sample size ranged from 48 to 1794 participants, whereas one study involved up to 7254 participants because it grouped the participants from eight cohorts for analysis.( 14 ) Some studies used the participants from the same cohort for different analyses; eg, five studies from OFELY (France),( 10 , 18 , 19 , 20 , 35 ) four studies from MrOS (The Osteoporotic Fractures in Men Study, USA or Sweden),( 12 , 24 , 32 , 37 ) two studies from STRAMBO (France),( 13 , 22 ) two studies from CaMOS (Canada),( 11 , 23 ) one study from Hertfordshire Cohort Study (HCS, UK),( 25 ) one study from Geneva Retirees Cohort (GERICO, Switzerland), (36 ) and one study from Global Longitudinal Study of Osteoporosis in Women (GLOW, UK).( 34 ) One study assessed eight cohorts (Framingham, Mayo Clinic, Qualité Osseuse Lyon Orléans [QUALYOR, France], STRAMBO, OFELY, GERICO, CaMos, and MrOS).( 14 )
The mean age of the participants in the 25 studies was 65 to 85 years. Nine studies analyzed the prediction for incident fractures,( 11 , 14 , 20 , 21 , 22 , 23 , 25 , 32 , 34 ) eight studies for both incident and major osteoporotic fractures,( 10 , 12 , 13 , 24 , 30 , 35 , 36 , 37 ) five studies for vertebral fractures only,( 19 , 28 , 29 , 31 , 33 ) two studies for hip fractures only,( 26 , 27 ) and one for wrist fracture only.( 18 ) Table 1 summarizes the included 25 studies.
Table 1.
Summary of the Included Studies in the Systematic Review
| Study design | Included studies | Cohort | Ethnicity | Follow‐up | Sex/age | Sample size | Trauma degree | Fracture site | Fracture prediction |
|---|---|---|---|---|---|---|---|---|---|
| Cohort a | Biver and colleagues( 36 ) | GERICO study | Swiss | 5.0 ± 1.8 years |
Women Age: 65.0 ± 1.5 years |
Women: 740 | Low‐trauma only (n = 85); 47% had prior low trauma fracture after age 20 years |
Incident fractures – exclude fingers, toes, skull, face Subgroup analysis in major osteoporotic fracture |
HR |
| Cohort a | Burt and colleagues( 11 ) | CaMOS Cohort | Canadian | 5.0 ± 0.5 years |
Women Age: 68.4 ± 7.0 years |
Women: 149 | Fragility fracture (n = 22); 45% had previous fracture | Incident fractures ‐ exclude fingers, toes, nose | OR |
| Cohort | Ohlsson and colleagues( 37 ) | MrOS Sweden Cohort | Swedish | 5.3 ± 2.0 years |
Men Age: 80.2 ± 3.5 years |
Men: 456 | Fragility fracture (n = 71) |
Incident fractures exclude skull, face Subgroup analysis in major osteoporotic fracture |
HR; no crude data |
| Cohort a | Langsetmo and colleagues( 12 ) | MrOS US, at year 14 visit | American |
Mean: 1.7 years (IQR 1.4–2.3, max. 2.9) |
Men Age: 84.4 ± 4.2 years |
Men: 1794 | Regardless of site and trauma (n = 127); 33% had prior fracture after age 50 years |
All incident fractures Subgroup analysis in major osteoporotic fracture |
HR |
| Cohort | Samelson and colleagues( 14 ) | 8 Cohorts | Multiple (American, Canadian, European) | 4.63 ± 2.41 years (range 0.01–11.04) |
Men Age: 67 ± 9 years Women Age: 72 ± 9 years |
Men: 2486 Women: 4768 |
Regardless of site and trauma (n = 765); 23% had previous fracture | All incident fractures | HR (repeated cohorts) |
| Cohort a | Sornay‐Rendu and colleagues( 35 ) | OFELY study at Year 14 visit | French | 9.4 years (IQ:1.0) |
Women Age: 68 ± 9.0 years |
Women: 589 | Low‐trauma only (n = 135); 32% had prior low trauma fracture after age 40 years |
Incident fractures exclude head, toes, fingers Subgroup analysis in major osteoporotic fracture |
HR |
| Cohort a | Szulc and colleagues( 13 ) | STRAMBO study | French | 8 years (IQR: 6.0–8.0) |
Men Age: 72.5 years |
Men: 819 | Fragility fracture (n = 105); 23% had previous fracture |
Incident fractures – exclude skull, face, hand, fingers, toes Subgroup analysis in major osteoporotic fracture |
HR |
| Case control a | Boutroy and colleagues( 18 ) | OFELY cohort at Year 13 visit | French | N/A |
Women Age: 73.4 ± 6.0 years |
Case: 33 Control: 33 |
Low‐trauma only | Wrist fracture only | OR |
| Case control | Boutroy and colleagues( 30 ) | Multicenter analysis | Multiple (American, European, Asian, Argentines) | N/A |
Women Age: 66 ± 8 years |
Case: 470 Control: 909 |
Low‐trauma only |
All incident fractures Subgroup analysis in major osteoporotic fracture |
OR; no crude data |
| Case control a | Edwards and colleagues( 25 ) | HCS cohort‐UK at follow‐up | British | N/A |
Men Age: 75.8 ± 2.4 years Women Age: 76.4 ± 2.5 years |
Case: 44(M); 48(F) Control: 133(M); 111(F) |
No information | All incident fractures | OR |
| Case control | Fink and colleagues( 32 ) | MrOS US, at Year 14 visit | American | N/A |
Men Age: 84.4 ± 4.2 years |
Case: 344 Control: 1450 |
Regardless of site and trauma | All incident fractures | OR; no crude data |
| Case control | Johansson and colleagues( 31 ) | Gothenburg Swedish Women Cohort | Swedish | N/A |
Women Age: 77 ± 1.5 years |
Case: 277 Control: 750 |
By VFA | Vertebral fracture only | OR; no crude data |
| Case control | Litwic and colleagues( 34 ) | GLOW study at Year 5 | British | N/A |
Women Age: 70.6 ± 5.4 years |
Case: 63 Control: 258 |
No information | All incident fractures | Cluster analysis; no crude data |
| Case control | Melton and colleagues( 28 ) | Mayo Clinic | American | N/A |
Women Age: 74.8 ± 7.9 years |
Case:40 Control:40 |
Minimal or moderate trauma | Vertebral fracture only | OR; no crude data |
| Case control | Melton and colleagues( 29 ) | Mayo Clinic | American | N/A |
Women Age: 69.6 ± 9.9 years |
Case:193 Control: 90 |
Minimal or moderate trauma | Vertebral fracture only | OR; no crude data |
| Case control a | Sornay‐Rendu and colleagues( 10 ) | OFELY study at Year 13 visit | French | N/A |
Women Age: 73.7 ± 8.0 years |
Case: 101 Control: 101 |
Low‐trauma only |
Incident fracture exclude head, toes, fingers Subgroup analysis in wrist fracture |
OR |
| Case control a | Sornay‐Rendu and colleagues( 19 ) |
Case: 30% from OFELY; 70% new admitted Control: OFELY study at Year 14 visit |
French | N/A |
Women Age: 74 ± 9 years |
Case: 100 Control: 362 |
Low‐trauma only | Vertebral fracture only | OR |
| Case control a | Stein and colleagues( 21 ) | American | N/A |
Women Age: 68 ± 7 years |
Case: 68 Control:101 |
Low‐trauma only | All incident fractures | OR | |
| Case control a | Sundh and colleagues( 24 ) | MrOS‐Sweden Gothenburg cohort at Year 5 | Swedish | N/A |
Men Age: 80.2 ± 3.5 years |
Case: 87 Control: 369 |
No information |
Incident fracture exclude hand, finger, foot, toe, skull Subgroup analysis in peripheral fracture, osteoporotic fracture, multiple fractures |
OR |
| Case control a | Sundh and colleagues( 27 ) | Gothenburg Swedish Women Cohort | Swedish | N/A |
Women Age: 77.7 ± 1.6 years |
Case: 46 Control: 361 |
No information | Hip fracture only | OR |
| Case control | Torres and colleagues( 33 ) | Brazilian | N/A |
Men Age: 79.0 ± 4.1 years Women Age: 79.6 ± 4.1 years |
Case: 28(M); 75(F) Control: 72(M); 101(F) |
By VFA | Vertebral fracture only | OR; no crude data | |
| Case control a | Vilayphiou and colleagues( 20 ) | OFELY cohort at Year 13 visit | French | N/A |
Women Age: 73.7 ± 8.1 years |
Case: 101 Control: 101 |
Low‐trauma only | Incident fracture‐ exclude head, toes, fingers | OR |
| Case control a | Vilayphiou and colleagues( 22 ) | STRABMO cohort | French | N/A |
Men Age: 71 ± 10 years |
Case:185 Control: 185 |
Low‐trauma only | Incident fracture‐ exclude head, toes, fingers | OR |
| Case control a | Wong and colleagues( 23 ) | CaMOS cohort | Canadian | N/A |
Women Age: 75 ± 9 years |
Case: 40 Control: 57 |
Low‐trauma only | Incident fracture exclude skull, fingers, toes | OR |
| Case control a | Zhu and colleagues( 26 ) | Asian | N/A |
Women Age: 79.2 ± 5.5 years |
Case: 24 Control: 24 |
Low‐trauma only | Hip fracture only | OR |
HR = hazard ratio; OR = odds ratio; VFA = vertebral fracture assessment.
Paper included in meta‐analysis.
Quality of selected studies
Tables 2 and 3 summarizes the quality of the 25 studies using the Newcastle‐Ottawa Quality Scale. All studies were of good quality and suitable for quantitative analysis.
Table 2.
Quality Assessment Using Newcastle‐Ottawa Scale for the Case Control Studies
| Selection | Comparability | Exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cross‐sectional studies | Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for important factor or additional factor | Ascertainment of exposure | Same method of ascertainment for cases and controls | Nonresponse rate | Total score |
| Boutroy and colleagues( 18 ) | * | * | * | ** | * | * | 7 | ||
| Edwards and colleagues( 25 ) | * | * | ** | * | * | 6 | |||
| Sornay‐Rendu and colleagues( 10 ) | * | * | * | ** | * | * | * | 8 | |
| Sornay‐Rendu and colleagues( 19 ) | * | * | * | * | * | * | 6 | ||
| Stein and colleagues( 21 ) | * | * | * | * | ** | * | * | 8 | |
| Sundh and colleagues( 24 ) | * | * | * | ** | * | * | 7 | ||
| Sundh and colleagues( 27 ) | * | * | * | ** | * | * | 7 | ||
| Vilayphiou and colleagues( 20 ) | * | * | * | ** | * | * | 7 | ||
| Vilayphiou and colleagues( 22 ) | * | * | * | ** | * | * | * | 8 | |
| Wong and colleagues( 23 ) | * | * | * | * | * | * | 6 | ||
| Zhu and colleagues( 26 ) | * | * |
* |
** | * | * | * | 8 | |
* Each star represents 1 score.
Table 3.
Quality Assessment Using Newcastle‐Ottawa Scale for the Cohort Studies
| Selection | Comparability | Exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Control for important factor or additional factor | Ascertainment of outcome | Was follow‐up long enough for outcome to occur | Adequacy of follow‐up of cohorts | Total score |
| Biver and colleagues( 36 ) | * | * | * | * | ** | * | * | * | 9 |
| Burt and colleagues( 11 ) | * | * | * | * | ** | * | * | * | 9 |
| Langsetmo and colleagues( 12 ) | * | * | * | ** | * | * | * | 8 | |
| Sornay‐Rendu and colleagues( 35 ) | * | * | * | ** | * | * | * | 8 | |
| Szulc and colleagues( 13 ) | * | * | * | ** | * | * | * | 8 | |
* Each star represents 1 score.
Study design
Among 25 included studies, 18 papers were case–control studies( 10 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ) and seven were cohort studies.( 11 , 12 , 13 , 14 , 35 , 36 , 37 ) In the case–control studies, the fracture participants mostly had low or moderate trauma, whereas four studies did not specify the trauma degree of participants.( 24 , 25 , 27 , 34 ) In the cohort studies, the fracture participants were also of low trauma and 23% to 47% of participants had previous fractures (Table 1).
Gender and ethnicity
Sixteen of 25 studies included women only,( 10 , 11 , 18 , 19 , 20 , 21 , 23 , 26 , 27 , 28 , 29 , 30 , 31 , 34 , 35 , 36 ) six studies men only,( 12 , 13 , 22 , 24 , 32 , 37 ) and three studies both genders.( 14 , 25 , 33 ) For ethnicity, the 25 studies included participants as follows: seven French,( 10 , 13 , 18 , 19 , 20 , 22 , 35 ) five American,( 12 , 21 , 28 , 29 , 32 ) four Swedish,( 24 , 27 , 31 , 37 ) two Canadian,( 11 , 23 ) two British,( 25 , 34 ) two multiple,( 14 , 30 ) one Swiss,( 36 ) one Asian,( 26 ) and one Brazilian.( 33 ) In summary, seven were from North America, one from South America, 14 from Europe, one from Asia, and two from multiple countries (Table 1).
Follow‐up period
In the seven cohort studies included, the mean follow‐up period ranged from 1.7 to 9.4 years. There were two studies with mean follow‐up time of less than 5 years( 12 , 14 ) and three studies with up to around 5 years.( 11 , 36 , 37 ) Two studies followed the patients up to a mean period of 8 years( 13 ) and 9.4 years,( 35 ) respectively.
HR‐pQCT machine
Almost all (22/25) studies used Scanco HR‐pQCT (XtremeCT version I, XT I; SCANCO Medical AG, Brüttisellen, Switzerland) machines. Two studies used Scanco XtremeCT version II (XT II) model( 12 , 32 ); one study used a Scanco prototype machine.( 28 ) Among the 16 studies in meta‐analysis, all of them used XT I, except a cohort study by Langsetmo and colleagues( 12 ) using XT II (Table 4). According to Scanco information (http://www.scanco.ch/), XT I and XT II are compatible but their major differences include resolution (82 μm in XT I versus 61 μm in XT II), scanning time (3 minutes in XT I versus 2 minutes in XT II). For the prototype machine, the spatial resolution was at 165 μm.( 38 ) In Melton and colleagues' study( 28 ) using a prototype machine, only microstructural parameters were reported, whereas volumetric density and related finite element analysis were measured by a single‐energy QCT and a third‐party software (ON Diagnostics, Berkeley, CA, USA), respectively.
Table 4.
Summary of HR‐pQCT Details of the Included Studies
| Study design | Included studies | Model of HR‐pQCT | Site(s) | Volumetric density parameter (mg HA/cm3) | Bone geometry (mm2) | Trabecular microarchitecture | Cortical structural parameters | μFEA | μFEA inputs |
|---|---|---|---|---|---|---|---|---|---|
| Cohort a | Biver and colleagues( 36 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) Ct.Po (%) |
Stiffness (N/mm) Est. failure load (N) Modulus (N/mm2) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Cohort a | Burt and colleagues( 11 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) |
Est. failure load (N) [Numerics88] |
Elastic modulus of 6.829 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Cohort | Ohlsson and colleagues( 37 ) | XtremeCT I | Tibia | Ct.BMD | Ct.Ar |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct Pm (mm) Ct.Po (%) |
Stiffness (N/mm) Est. failure load (N) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Cohort a | Langsetmo and colleagues( 12 ) | XtremeCT II | Tibia, diaphyseal tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) |
Ct.Th (mm) Ct.Po (%) |
Est. failure load (N) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 7.5% critical volume |
| Cohort | Samelson and colleagues( 14 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Ct.Ar/Tt.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) |
Est. failure load (N) [Scanco IPL] |
Elastic modulus of 6.829 GPa; Axial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Cohort a | Sornay‐Rendu and colleagues( 35 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Sp.SD (mm) Conn.D SMI |
Ct.Th (mm) Ct.Po (%) |
Stiffness (N/mm) Est. failure load (N) [Scanco IPL] |
Elastic modulus of 20 GPa (cortical bone) and 17.5 GPa (trabecular bone); Axial compression; Yield criterion 0.35% critical strain, 2% critical volume |
| Cohort a | Szulc and colleagues( 13 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
— |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) Conn.D |
Stiffness (N/mm) Est. failure load (N) [Scanco IPL] |
Elastic modulus of 20GPa (cortical bone) and 17.5GPa (trabecular bone); Axial compression; Yield criterion 0.35% critical strain, 2% critical volume | |
| Case control a | Boutroy and colleagues( 18 ) | XtremeCT I | Radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) |
Stiffness (N/mm) Est. failure load (N) % load trab distal % load trab proximal Tb Von Mises stress Ct Von Mises stress [Scanco IPL] |
Elastic modulus of 20 GPa (cortical bone) and 17.5 GPa (trabecular bone); Axial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Case control | Boutroy and colleagues( 30 ) | XtremeCT I | Tibia, radius |
Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) | — | — |
| Case control a | Edwards and colleagues( 25 ) | XtremeCT I | Radius |
Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) |
— | — |
| Case control | Fink and colleagues( 32 ) | XtremeCT II | Tibia, diaphyseal tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) |
Ct.Th (mm) Ct.Po (%) |
Est. failure load (N) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 1% critical volume |
| Case control | Johansson and colleagues( 31 ) | XtremeCT I | Tibia, 14% tibia, radius, 14% radius |
Tt.BMD Ct.BMD |
Ct.Ar |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) |
— | — |
| Case Control | Litwic and colleagues( 34 ) | XtremeCT I | Tibia, radius |
Tb.BMD Ct.BMD |
Tt.Ar Tb.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) |
— | — |
| Case control | Melton and colleagues( 28 ) | XtremeCT prototype | Radius | — | — |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
— | — | — |
| Case control | Melton and colleagues( 29 ) | XtremeCT I | Radius |
Tb.BMD Ct.BMD |
Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) Conn.D |
Ct.Th (mm) | — | — |
| Case control a | Sornay‐Rendu and colleagues( 10 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
— |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) | — | — |
| Case control a | Sornay‐Rendu and colleagues( 19 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
— |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) | — | — |
| Case control a | Stein and colleagues( 21 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) |
Stiffness (N/mm) [Scanco IPL] |
Elastic modulus of 15 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Case control a | Sundh and colleagues( 24 ) | XtremeCT I | Tibia | Ct.BMD | Ct.Ar |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) Ct.Po Dm (mm) |
— | — |
| Case control a | Sundh and colleagues( 27 ) | XtremeCT I | Tibia, 14% tibia | Ct.BMD | Ct.Ar |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) |
Ct.Th (mm) Ct.Po (%) |
— | — |
| Case control | Torres and colleagues( 33 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
— |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) Ct.Po (%) Ct.Po Dm (mm) |
Stiffness (N/mm) Est. failure load (N) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
| Case control a | Vilayphiou and colleagues( 20 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD |
Tt.Ar |
Tb.N (mm−1) Tb.Sp.SD (mm) |
Ct.Th (mm) |
Stiffness (N/mm) Est. failure load (N) % load trab distal % load trab proximal Tb Von Mises stress Ct Von Mises stress [Scanco IPL] |
Elastic modulus of 20 GPa (cortical bone) and 17.5 GPa (trabecular bone); Axial compression; Yield criterion 0.35% critical strain, 2% critical volume |
| Case control a | Vilayphiou and colleagues( 22 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar |
Tb.N (mm−1) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) |
Stiffness (N/mm) Est. failure load (N) % load trab distal % load trab proximal Tb Von Mises stress Ct Von Mises stress [Scanco IPL] |
Elastic modulus of 20 GPa (cortical bone) and 17.5 GPa (trabecular bone); Axial compression; Yield criterion 0.35% critical strain, 2% critical volume |
| Case control a | Wong and colleagues( 23 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
— |
BV/TV(%) Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) |
Ct.Th (mm) | — | — |
| Case control a | Zhu and colleagues( 26 ) | XtremeCT I | Tibia, radius |
Tt.BMD Tb.BMD Ct.BMD |
Tt.Ar Ct.Ar |
Tb.N (mm−1) Tb.Th (mm) Tb.Sp (mm) Tb.Sp.SD (mm) |
Ct.Th (mm) Ct.Po (%) |
Stiffness (N/mm) Est. failure load (N) [Scanco IPL] |
Elastic modulus of 10 GPa; Uniaxial compression; Yield criterion 0.7% critical strain, 2% critical volume |
Paper included in meta‐analysis.
Due to the differences in spatial resolution, the image processing method is also different between XTI and XTII machines. Higher spatial resolution in XTII allows direct measurement of trabecular microarchitecture (bone volume/total volume [BV/TV], Tb.Th, Tb.Sp) and cortical thickness (Ct.Th), whereas a derived (indirect) morphological method of evaluation was used in XTI. The image analysis methods for measuring bone area, vBMD and Tb.N are the same in both generations of HR‐pQCT machine.( 9 , 39 , 40 ) Fully automated threshold‐based approach for cortical porosity (Ct.Po) evaluation, as one of the standard parameters, was used in XTII. In XTI, extended cortical analysis by semiautomated contouring process for Ct.Po evaluation (threshold‐based approach) was required.( 9 )
Scanning position
Most of the studies (18/25) measured both distal tibia and distal radius using HR‐pQCT; three measured distal tibia only( 24 , 27 , 37 ) and four measured distal radius only.( 18 , 25 , 28 , 29 ) All of them used standard protocol (fixed offset scanning) to measure the extremities, whereas four studies also measured diaphyseal region in addition to the standard sites( 12 , 27 , 31 , 32 ) (Table 4). Regarding the standard scanning protocol, the total scanning region was 9.02, 10.2, and 10 mm (in length) in XT I, XT II, and prototype machine, respectively. For XT I, the first CT slice was 9.5 mm (for radius) and 22.5 mm (for tibia) proximal to the reference line (endplate). For XT II, the first CT slice was 9.0 mm (for radius) and 22.0 mm (for tibia) proximal to the reference line (endplate).( 9 ) For the prototype HR‐pQCT machine, the first CT slice was 6.0 mm proximal to the reference line (endplate) for distal radius only.( 38 )
Finite element analysis
In the 25 included studies, all of them measured volumetric density, geometric and/or structural parameters, whereas only 14 studies reported estimated bone strength generated by micro‐finite element analysis (μFEA).( 11 , 12 , 13 , 14 , 18 , 20 , 21 , 22 , 26 , 32 , 33 , 35 , 36 , 37 ) Of the 14 studies, almost all of them used Scanco default Image Processing Language (IPL) for μFEA analysis, except one using a third‐party finite element software (FAIM, version 6; Numerics88 Solutions, Calgary, AB, Canada).( 11 ) μFEA model inputs are summarized in Table 4.
Meta‐analysis
In the meta‐analysis, only studies with crude OR or HR results available or provided by the authors are included for analysis. Nine of 25 studies were excluded from the analysis, because no response was received for our request for crude data acquisition( 28 , 29 , 30 , 31 , 32 , 33 , 34 , 37 ) or they involved repeated cohorts.( 14 ) Although a few studies adopted the patients from the same cohort, the 16 included studies should have no overlapping of database, because they involved different fracture types, patient groups, or ethnicities. In the meta‐analysis, the included studies have two types of study design: cohort and case–control. A total of 56 analyses were performed in the meta‐analysis to analyze 14 HR‐pQCT parameters, covering vBMD, cortical and trabecular microarchitecture, and FEA parameters, involving two scanning sites (distal radius and distal tibia) for predicting both incident fracture and major osteoporotic fracture (MOF).
There were 16 studies included in the meta‐analysis (five cohort studies and 11 case control studies). The total number of subjects for 16 studies was 6904 (female, n = 3288; male, n = 3616). The total number of incident fracture was 1392, in which 709 was MOF. Publication bias was not present in all parameters (all p > 0.05), except Ct.Th at both distal radius (p = 0.02), tibia (p = 0.01), and BV/TV at distal tibia (p = 0.04) (Supplementary Table S1). Therefore, the risk of bias from the selected publications was minimal.
For selection of the best performance HR‐pQCT parameters in predicting incident fracture and MOF, the meta‐analysis results should meet the following criteria: (i) risk ratio or effect size >1.0 (except cortical porosity and trabecular separation <1.0), (ii) I 2 estimate <65%, (iii) generalizability in predicting both incident fractures and MOFs, and (iv) their successful prediction at both distal radius and tibia.
The following highlights the meta‐analysis results with effect size over 1.0 (except cortical porosity and trabecular separation <1.0) with low‐to‐moderate heterogeneity (I 2 < 65%) in predicting fractures. The parameters not meeting these criteria are summarized in Supplementary Table S2.
Meta‐analysis of incident fracture prediction
vBMD
Cortical vBMD measured at both radius and tibia could significantly predict incident fracture (radius: RR = 1.50, 95% CI = 1.39–1.61, I 2 = 18%, p < 0.01; tibia: RR = 1.64, 95% CI = 1.52–1.76, I 2 = 43%, p < 0.01) (Fig. 2A,B ). Trabecular vBMD measured at radius only showed significant prediction (RR = 1.81, 95% CI = 1.68–1.96, I 2 = 60%, p < 0.01) (Supplementary Fig. S1A ).
Fig 2.
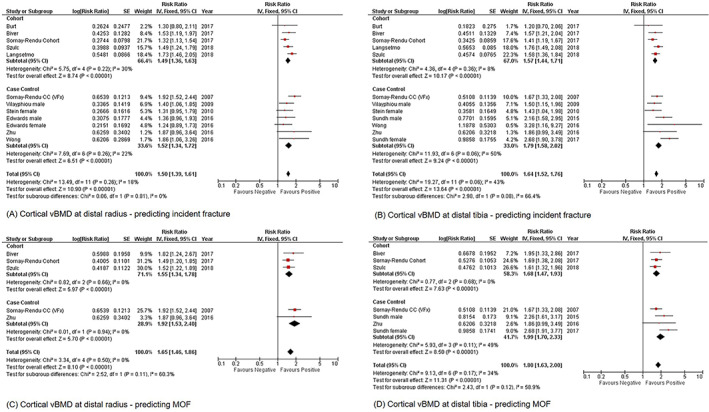
Forest plot of Ct.vBMD measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, and (D) distal tibia; MOF. Ct.vBMD = cortical volumetric bone mineral density; MOF = major osteoporotic fracture.
Cortical parameters
Ct.Th measured at radius only could significantly predict incident fracture (RR = 1.64, 95% CI = 1.51–1.78, I 2 = 42%, p < 0.01) (Supplementary Fig. S2A ).
Trabecular bone microarchitecture parameters
Tb.N measured at both radius and tibia could predict incident fracture significantly (radius: RR = 1.68, 95% CI = 1.56–1.82, I 2 = 62%, p < 0.01; tibia: RR = 1.39, 95% CI = 1.29–1.49, I 2 = 61%, p < 0.01) (Supplementary Fig. S3A,B ). Tb.Th measured at both radius and tibia could predict incident fracture significantly (radius: RR = 1.37, 95% CI = 1.25–1.49, I 2 = 0%, p < 0.01; tibia: RR = 1.30, 95% CI = 1.19–1.42, I 2 = 15%, p < 0.01) (Fig. 3A,B ). Tb.Sp measured at radius only could predict incident fracture significantly (RR = 0.63, 95% CI = 0.59–0.68, I 2 = 60%, p < 0.01) (Supplementary Fig. S4).
Fig 3.
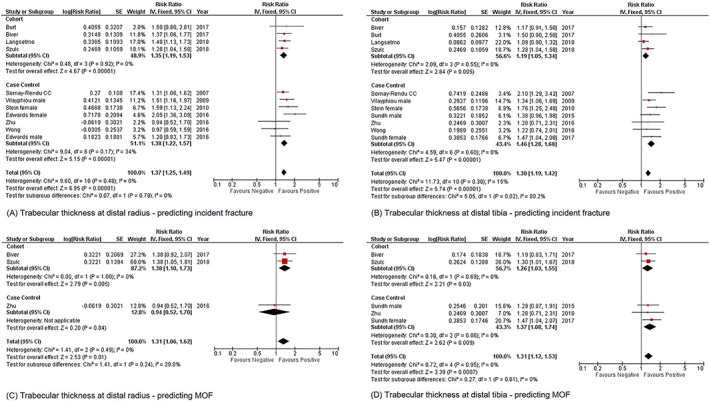
Forest plot of Tb.Th measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, and (D) distal tibia; MOF. MOF = major osteoporotic fracture; Tb.Th = trabecular thickness.
Fig 4.
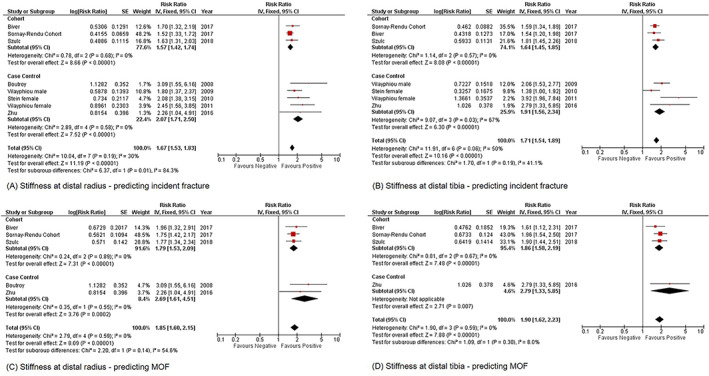
Forest plot of stiffness measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF. MOF = major osteoporotic fracture.
Finite element analysis parameters
Stiffness measured at both radius and tibia could predict incident fracture significantly (radius: RR = 1.67, 95% CI = 1.53–1.83, I 2 = 30%, p < 0.01; tibia: RR = 1.71, 95% CI = 1.54–1.89, I 2 = 50%, p < 0.01) (Fig. 4A,B ). Estimated failure load measured at both radius and tibia could predict incident fracture significantly (radius: RR = 1.72, 95% CI = 1.57–1.88, I 2 = 44%, p < 0.01; tibia: RR = 1.76, 95% CI = 1.60–1.93, I 2 = 0%, p < 0.01) (Supplementary Fig. Supplementary Fig. S5 A,B).
Meta‐analysis of MOF prediction
vBMD
Cortical vBMD measured at radius and tibia could significantly predict MOF (radius: RR = 1.65, 95% CI = 1.46–1.86, I 2 = 0%, p < 0.01; tibia: RR = 1.80, 95% CI = 1.63–2.00, I 2 = 34%, p < 0.01) (Fig. 2C,D ). Trabecular vBMD measured at radius only showed significant prediction (RR = 2.24, 95% CI = 1.95–2.57, I 2 = 42%, p < 0.01) (Supplementary Fig. S1 C).
Cortical parameters
Ct.Th measured at radius only could significantly predict MOF (RR = 1.77, 95% CI = 1.55–2.00, I 2 = 62%, p < 0.01) (Supplementary Fig. S2 C). Only cortical porosity measured at tibia could predict MOF significantly (RR = 0.59, 95% CI = 0.51–0.68, I 2 = 57%, p < 0.01) (Supplementary Fig. Supplementary Fig. S6).
Trabecular bone microarchitecture parameters
Tb.N measured at radius only could predict MOF significantly (RR = 2.10, 95% CI = 1.86–2.37, I 2 = 50%, p < 0.01) (Supplementary Fig. S3C ). Tb.Th measured at both radius and tibia could predict MOF significantly (radius: RR = 1.31, 95% CI = 1.06–1.62, I 2 = 0%, p < 0.01; tibia: RR = 1.31, 95% CI = 1.12–1.53, I 2 = 0%, p = 0.01) (Fig. 3C,D ).
Finite element analysis parameters
Stiffness measured at both radius and tibia could predict MOF significantly (radius: RR = 1.85, 95% CI = 1.60–2.15, I 2 = 0%, p < 0.01; tibia: RR = 1.90, 95% CI = 1.62–2.23, I 2 = 0%, p < 0.01) (Fig. 4C,D ). Estimated failure load measured at tibia could predict MOF significantly (RR = 2.07, 95% CI = 1.80–2.38, I 2 = 31%, p < 0.01) (Supplementary Fig. Supplementary Fig. S5D ).
Meta‐analysis in predicting incident fracture and MOF by DXA
Among the 16 studies included in meta‐analysis, there were six studies (one cohort and five case control) involving both HR‐pQCT parameters and DXA areal BMD (aBMD) in predicting incident fracture or MOF. Due to limited number of studies, we grouped the aBMD data of all DXA measuring sites (lumbar spine, proximal femur and distal radius) together to perform the meta‐analysis. Results showed that aBMD could predict incident fracture and MOF significantly (incident fracture: RR = 1.30, 95% CI = 1.28–1.32, p < 0.01; MOF: RR = 1.53, 95% CI = 1.41–1.65, p < 0.01). However, there were significant heterogeneity from the dataset (incident fracture: I 2 = 89%, p < 0.01; MOF: I 2 = 93%, p < 0.01) (Supplementary Table S3).
Effect sizes of the three best performance HR‐pQCT parameters
Cohen's d was used to compare the effect size among cortical vBMD, Tb.Th, and stiffness. All d values were equal to or larger than 0.8, which means that large effects (not overlapping) were observed (Supplementary Table S4).
Discussion
This review aimed to investigate which HR‐pQCT parameters at the distal radius and/or distal tibia can best predict fragility fractures. All the included papers showed positive prediction of HR‐pQCT for incident fractures and/or MOFs, in which almost all of the HR‐pQCT parameters showed significant prediction. Among all the parameters, the meta‐analysis showed that cortical vBMD, Tb.Th, and stiffness were best prediction parameters of HR‐pQCT, in terms of their effect size, heterogeneity, and their generalizability in predicting incident fractures and MOFs at both measurement sites. The three factors measured at distal radius and distal tibia could successfully predict both incident fractures and MOFs with the effect size up to 1.90, after screening out the analyses with high heterogeneity (I 2 > 65%).
Of the three outstanding predictors, stiffness is a HR‐pQCT parameter of estimated bone strength. This indicates that estimated bone strength can predict fragility fractures well, whereas vBMD or microarchitecture parameters are other important ones. On the other hand, geometric parameters, eg, total area, cortical area, trabecular area, show less predictive power. Almost all the included studies with FEA consistently used the default Image Processing Language (IPL) of HR‐pQCT for analysis, except one using third‐party FEA software.( 11 ) The possible reason why estimated bone strength shows a good prediction power is that μFEA analysis has included geometric, microarchitecture, and vBMD data altogether to estimate mechanical parameters: stiffness and estimated failure load.( 41 ) Therefore, estimated bone strength is relatively more all‐round and advanced than other individual geometric, structural or vBMD parameter.
The other two outstanding predictors are cortical vBMD and Tb.Th. Because trabecular bone is metabolically more active than cortical bone, trabecular bone loss usually occurs early in osteoporosis. The decrease in trabecular bone is caused by thinning of the trabeculae and especially in early postmenopausal women,( 42 ) by disruption of the trabecular microstructure and loss of trabecular elements. This will eventually reduce the trabecular number and increase the trabecular separation in later phase. Hence, trabecular thinning is an early phenomenon of osteoporosis, thus Tb.Th is a sensitive parameter. During trabecular bone loss, cortical bone will share more loading. With the progression of osteoporosis, cortical bone will deteriorate to be more porous and cortical trabecularization will be resulted,( 43 ) leading to a great loss of cortical bone mass (yet Ct.vBMD may increase, because the zone formally included in the cortical compartment will be included in the trabecular compartment instead), whereas fracture risk will also be sharply increased. This may explain why cortical vBMD is a sensitive HR‐pQCT parameter for fragility fracture prediction.
Between incident fracture and MOF prediction, most of the HR‐pQCT parameters performed better in MOF. A few parameters showed the effect size >2.0 in MOF prediction, such as Tb.vBMD, Tb.N measured at distal radius, and estimated failure load measured at distal tibia, whereas none of them demonstrated the effect size >2.0 in incident fracture prediction. These results revealed that HR‐pQCT parameters might reflect the mechanical competence or bone quality of three MOF sites (wrist, spine, and hip) better than other regions. A study by Liu and colleagues( 44 ) substantiated that the prediction of stiffness of proximal femur and vertebrae by HR‐pQCT parameters were comparable with direct measurements of proximal femur and lumbar spine by DXA and central QCT, respectively. Hence, HR‐pQCT‐based images and FEA of distal radius and tibia were good indicators of mechanical properties of the lumbar spine and proximal femur.( 44 )
HR‐pQCT has two standard measurement sites: distal radius and distal tibia. Our review showed that more parameters measured at the distal radius could significantly predict both incident fractures and MOFs than those at the distal tibia. The distal radius is therefore a more preferable measurement site for fracture prediction. The distal radius has been reported to be an ideal site for screening primary osteoporotic distal radius fracture.( 45 ) Miyamura and colleagues( 45 ) showed that fracture group had significantly lower BMD and T‐score of ultradistal, mid‐distal, and one‐third distal forearm than control group; DXA measurements exhibited high correlation with vBMD measured by computed tomography (r = 0.83–0.92). Because distal radius of nondominant side is a non‐weight‐bearing region, osteoporosis developed in this region cannot be offset by most osteogenic weight‐bearing activities, thus providing a sensitive site for osteoporosis detection. On the other hand, distal tibia, as a weight‐bearing site, will be influenced by most daily activities and cannot predict fragility fractures as good as the distal radius.
Cortical porosity (Ct.Po) is a unique parameter provided by HR‐pQCT that no other imaging system can measure. This is defined as the average fraction of void volumes within the cortical bone volume.( 46 ) Ct.Po has been reported to have prominent increase at the fifth decade and reach a plateau before the sixth decade,( 47 ) which may be an important determinant of fracture risk. In our meta‐analysis, the results revealed that Ct.Po measured at distal tibia could predict MOF significantly. Hung and colleagues( 47 ) also substantiated that Ct.Po at the distal tibia increased from around 2.5% to 7.5% from the age of 50 to 60 years, which should weaken the cortical strength substantially, thus supporting the MOF prediction power of Ct.Po. However, more studies should be conducted to validate its ability of prediction, because many analyses on Ct.Po in our review showed high data heterogeneity, leading to difficult interpretation.
DXA is currently the gold standard tool in assessing BMD and predicting fracture risk. Our findings support that bone quality measured by HR‐pQCT could predict both incident fracture and major osteoporotic fracture. However, based on the results of this meta‐analysis, we cannot conclude whether HR‐pQCT parameters predict fractures better than DXA. The major reason is that significant heterogeneity (I 2 = 89–93) was found in the analysis of DXA in predicting fractures, which is not suitable for direct comparison with HR‐pQCT. A large cohort prospective consortium study by Samelson and colleagues( 14 ) have shown that HR‐pQCT parameters could predict incident fractures independently of femoral neck aBMD and Fracture Risk Assessment Tool (FRAX) score. Moreover, the predictive model (area under the receiver operating characteristic curve) involving HR‐pQCT parameters plus aBMD or FRAX score was significantly improved, as compared with aBMD or FRAX score alone. Further review is suggested to compare the predictive ability between HR‐pQCT and DXA in predicting incident fractures.
This systematic review indicated that most of the studies (21/25) included were from North America and Europe, whereas very few were from South America, Asia, or multi‐countries. This means the fragility fracture prediction by HR‐pQCT is mainly white‐based, yet these analyses may not be directly applicable to Asians. Ethnic differences in BMD has been well reported; eg, total hip and spine BMD were 4% to 5% lower among Chinese women than US whites.( 48 ) Vertebral fracture rate of Asians was higher than that of whites but lower hip fracture rate, resulting in a high vertebral‐to‐hip fracture ratio.( 49 ) Also, a report demonstrated that Chinese women had cortical and trabecular microstructural differences from white women.( 50 ) This is therefore highly recommended to establish their countries' bone databases of HR‐pQCT and conduct related fracture prediction studies. Also, there is a lack of robust data from Africa and among Africa descent, which will be an area of future research.
There are some limitations in this study. In the included cohort studies, many of them involved participants with previous fractures, while those in case–control studies were mostly first‐time fracture patients. Also, case–control studies were retrospective in nature. These might cause substantial data heterogeneity among the studies.
In conclusion, our study showed that HR‐pQCT could predict fragility fractures well and many parameters were of good prediction power to predict fragility fractures or incident fractures. Of all the HR‐pQCT parameters, stiffness, cortical vBMD, and Tb.Th were the outstanding predictor parameters. Also, of the two standard measurement sites by the HR‐pQCT of distal radius and tibia, distal radius was shown to be a more preferable measurement site for fracture prediction. To date, most of the studies were white‐based; Asian and African regions should build up their own densitometric databases and conduct related prediction studies, and Asians have been reported to exhibit different bone structure from whites. It is expected that HR‐pQCT can ultimately predict and prevent fragility fractures in the future, if applied widely in the community.
Disclosures
All authors state that they have no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4449.
Supporting information
Supplementary Fig. S1. Forest plot of trabecular vBMD measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S2. Forest plot of cortical thickness (Ct.Th) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S3. Forest plot of trabecular number (Tb.N) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S4. Forest plot of trabecular separation (Tb.Sp) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S5. Forest plot of estimated failure load measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S6. Forest plot of cortical porosity (Ct.Po) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Table S1. Egger's test results of publication bias
Supplementary Table S2. Summary table of all meta‐analysis data and heterogeneity test results for HR‐pQCT parameters
Supplementary Table S3. Meta‐analysis of DXA in predicting incident fracture and MOF
Supplementary Table S4. Determining the effect size overlapping among cortical vBMD, Tb.Th and stiffness in different fracture sites using Cohen's d
Acknowledgments
This study was supported by Group Research Scheme (Ref: 3110146) and Project Impact Enhancement Fund (Ref: PIEF/U3/01) of The Chinese University of Hong Kong.
Author's roles: Wing‐Hoi Cheung: conceptualization; investigation; methodology; supervision; writing‐original draft; writing‐review and editing; grant acquisition. Vivian Wing‐Yin Hung: conceptualization; data analysis; investigation; methodology; validation; writing‐review and editing. Ka‐Yee Cheuk: conceptualization; data analysis; methodology; validation. Wai‐Wang Chau: data analysis; methodology; validation. Kelvin Kam‐Fai Tsoi: data analysis; validation; writing‐review and editing. Ronald Man‐Yeung Wong: conceptualization; writing‐review and editing. Simon Kwoon‐Ho Chow: conceptualization; writing‐review and editing. Tsz‐Ping Lam: conceptualization; investigation; supervision; writing‐review and editing. Patrick Shu‐Hang Yung: conceptualization; investigation; supervision. Sheung‐Wai Law: conceptualization; investigation; supervision; writing‐review and editing. Qin Ling: conceptualization; investigation; supervision; writing‐review and editing.
†Wing‐Hoi Cheung and Vivian Wing‐Yin Hung are co‐first authors.
Contributor Information
Sheung‐Wai Law, Email: lawsw@cuhk.edu.hk.
Ling Qin, Email: lingqin@cuhk.edu.hk.
DATA AVAILABILITY STATEMENT
Data available on request from the authors
References
- 1. Report of a WHO Study Group . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1‐129. [PubMed] [Google Scholar]
- 2. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195‐202. [DOI] [PubMed] [Google Scholar]
- 3. Kanis JA, Adami S. Bone loss in the elderly. Osteoporos Int. 1994;4(suppl 1):59‐65. [DOI] [PubMed] [Google Scholar]
- 4. Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long‐term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947‐1954. [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Johnell O, Oden A, Dawson A, de Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12(12):989‐995. [DOI] [PubMed] [Google Scholar]
- 6. Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185‐1194. [DOI] [PubMed] [Google Scholar]
- 7. Sornay‐Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20(10):1813‐1819. [DOI] [PubMed] [Google Scholar]
- 8. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy . Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785‐795. [DOI] [PubMed] [Google Scholar]
- 9. Whittier DE, Boyd SK, Burghardt AJ, et al. Guidelines for the assessment of bone density and microarchitecture in vivo using high‐resolution peripheral quantitative computed tomography. Osteoporos Int. 2020;31(9):1607‐1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sornay‐Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22(3):425‐433. [DOI] [PubMed] [Google Scholar]
- 11. Burt LA, Manske SL, Hanley DA, Boyd SK. Lower bone density, impaired microarchitecture, and strength predict future fragility fracture in postmenopausal women: 5‐year follow‐up of the Calgary CaMos cohort. J Bone Miner Res. 2018;33(4):589‐597. [DOI] [PubMed] [Google Scholar]
- 12. Langsetmo L, Peters KW, Burghardt AJ, et al. Volumetric bone mineral density and failure load of distal limbs predict incident clinical fracture independent HR‐pQCT BMD and failure load predicts incident clinical fracture of FRAX and clinical risk factors among older men. J Bone Miner Res. 2018;33(7):1302‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szulc P, Boutroy S, Chapurlat R. Prediction of fractures in men using Bone microarchitectural parameters assessed by high‐resolution peripheral quantitative computed tomography‐the prospective STRAMBO study. J Bone Miner Res. 2018;33(8):1470‐1479. [DOI] [PubMed] [Google Scholar]
- 14. Samelson EJ, Broe KE, Xu H, et al. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol. 2019;7(1):34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence: Clinical Guidelines . Osteoporosis: Assessing the Risk of Fragility Fracture. London: National Institute for Health and Care Excellence: Clinical Guidelines; 2017. [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 17. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. xxi, p 567. [Google Scholar]
- 18. Boutroy S, van Rietbergen B, Sornay‐Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR‐pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392‐399. [DOI] [PubMed] [Google Scholar]
- 19. Sornay‐Rendu E, Cabrera‐Bravo JL, Boutroy S, Munoz F, Delmas PD. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res. 2009;24(4):737‐743. [DOI] [PubMed] [Google Scholar]
- 20. Vilayphiou N, Boutroy S, Sornay‐Rendu E, et al. Finite element analysis performed on radius and tibia HR‐pQCT images and fragility fractures at all sites in postmenopausal women. Bone. 2010;46(4):1030‐1037. [DOI] [PubMed] [Google Scholar]
- 21. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25(12):2572‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vilayphiou N, Boutroy S, Szulc P, et al. Finite element analysis performed on radius and tibia HR‐pQCT images and fragility fractures at all sites in men. J Bone Miner Res. 2011;26(5):965‐973. [DOI] [PubMed] [Google Scholar]
- 23. Wong AK, Beattie KA, Min KK, et al. A trimodality comparison of volumetric bone imaging technologies. Part III: SD, SEE, LSC association with fragility fractures. J Clin Densitom. 2015;18(3):408‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sundh D, Mellstrom D, Nilsson M, Karlsson M, Ohlsson C, Lorentzon M. Increased cortical porosity in older men with fracture. J Bone Miner Res. 2015;30(9):1692‐1700. [DOI] [PubMed] [Google Scholar]
- 25. Edwards MH, Robinson DE, Ward KA, et al. Cluster analysis of bone microarchitecture from high resolution peripheral quantitative computed tomography demonstrates two separate phenotypes associated with high fracture risk in men and women. Bone. 2016;88:131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu TY, Hung VW, Cheung WH, Cheng JC, Qin L, Leung KS. Value of measuring Bone microarchitecture in fracture discrimination in older women with recent hip fracture: a case‐control study with HR‐pQCT. Sci Rep. 2016;6:34185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sundh D, Nilsson AG, Nilsson M, Johansson L, Mellstrom D, Lorentzon M. Increased cortical porosity in women with hip fracture. J Intern Med. 2017;281(5):496‐506. [DOI] [PubMed] [Google Scholar]
- 28. Melton LJ 3rd, Riggs BL, Keaveny TM, et al. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007;22(12):1885‐1892. [DOI] [PubMed] [Google Scholar]
- 29. Melton LJ 3rd, Riggs BL, Keaveny TM, et al. Relation of vertebral deformities to bone density, structure, and strength. J Bone Miner Res. 2010;25(9):1922‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boutroy S, Khosla S, Sornay‐Rendu E, et al. Microarchitecture and peripheral BMD are impaired in postmenopausal white women with fracture independently of total hip T‐score: an international multicenter study. J Bone Miner Res. 2016;31(6):1158‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansson L, Sundh D, Zoulakis M, et al. The prevalence of vertebral fractures is associated with reduced hip Bone density and inferior peripheral appendicular volumetric bone density and structure in older women. J Bone Miner Res. 2018;33(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 32. Fink HA, Langsetmo L, Vo TN, et al. Association of high‐resolution peripheral quantitative computed tomography (HR‐pQCT) bone microarchitectural parameters with previous clinical fracture in older men: the Osteoporotic Fractures In Men (MrOS) Study. Bone. 2018;113:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torres GHF, Guzman LFE, Alvarenga JC, Lopes NHM, Pereira RMR. Association of moderate/severe vertebral fractures with reduced trabecular volumetric bone density in older women and reduced areal femoral neck bone density in older men from the community: a cross‐sectional study (SPAH). Maturitas. 2019;120:61‐67. [DOI] [PubMed] [Google Scholar]
- 34. Litwic AE, Westbury LD, Robinson DE, Ward KA, Cooper C, Dennison EM. Bone phenotype assessed by HRpQCT and associations with fracture risk in the GLOW study. Calcif Tissue Int. 2018;102(1):14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sornay‐Rendu E, Boutroy S, Duboeuf F, Chapurlat RD. Bone microarchitecture assessed by HR‐pQCT as predictor of fracture risk in postmenopausal women: the OFELY study. J Bone Miner Res. 2017;32(6):1243‐1251. [DOI] [PubMed] [Google Scholar]
- 36. Biver E, Durosier‐Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S. Evaluation of radius microstructure and areal Bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res. 2018;33(2):328‐337. [DOI] [PubMed] [Google Scholar]
- 37. Ohlsson C, Sundh D, Wallerek A, et al. Cortical bone area predicts incident fractures independently of areal bone mineral density in older men. J Clin Endocrinol Metab. 2017;102(2):516‐524. [DOI] [PubMed] [Google Scholar]
- 38. Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D‐QCT of the human forearm. Technol Health Care. 1998;6(5‐6):329‐337. [PubMed] [Google Scholar]
- 39. Mikolajewicz N, Bishop N, Burghardt AJ, et al. HR‐pQCT measures of Bone microarchitecture predict fracture: systematic review and meta‐analysis. J Bone Miner Res. 2020;35(3):446‐459. [DOI] [PubMed] [Google Scholar]
- 40. Manske SL, Davison EM, Burt LA, Raymond DA, Boyd SK. The estimation of second‐generation HR‐pQCT from first‐generation HR‐pQCT using in vivo cross‐calibration. J Bone Miner Res. 2017;32(7):1514‐1524. [DOI] [PubMed] [Google Scholar]
- 41. Engelke K, van Rietbergen B, Zysset P. FEA to measure bone strength: a review. Clin Rev Bone Miner Metab. 2016;14(1):26‐37. [Google Scholar]
- 42. Chen HY, Zhou XR, Fujita H, Onozuka M, Kubo KY. Age‐related changes in trabecular and cortical Bone microstructure. Int J Endocrinol. 2013;2013:213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR‐pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25(10):2229‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyamura S, Kuriyama K, Ebina K, et al. Utility of distal forearm DXA as a screening tool for primary osteoporotic fragility fractures of the distal radius: a case‐control study. JB JS Open Access. 2020;5(1):e0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bjornerem A. The clinical contribution of cortical porosity to fragility fractures. Bonekey Rep. 2016;5:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hung VW, Zhu TY, Cheung WH, et al. Age‐related differences in volumetric bone mineral density, microarchitecture, and bone strength of distal radius and tibia in Chinese women: a high‐resolution pQCT reference database study. Osteoporos Int. 2015;26(6):1691‐1703. [DOI] [PubMed] [Google Scholar]
- 48. Nam HS, Kweon SS, Choi JS, et al. Racial/ethnic differences in bone mineral density among older women. J Bone Miner Metab. 2013;31(2):190‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bow CH, Cheung E, Cheung CL, et al. Ethnic difference of clinical vertebral fracture risk. Osteoporos Int. 2012;23(3):879‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sum M, Zhu TY, Zhou B, et al. Chinese women in both the United States and Hong Kong have cortical microstructural advantages and more trabecular plates compared with white women. JBMR Plus. 2019;3(4):e10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Forest plot of trabecular vBMD measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S2. Forest plot of cortical thickness (Ct.Th) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S3. Forest plot of trabecular number (Tb.N) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S4. Forest plot of trabecular separation (Tb.Sp) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S5. Forest plot of estimated failure load measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Fig. S6. Forest plot of cortical porosity (Ct.Po) measured at distal radius and distal tibia for predicting incident fracture and MOF, (A) distal radius; incident fracture, (B) distal tibia; incident fracture, (C) distal radius; MOF, (D) distal tibia; MOF.
Supplementary Table S1. Egger's test results of publication bias
Supplementary Table S2. Summary table of all meta‐analysis data and heterogeneity test results for HR‐pQCT parameters
Supplementary Table S3. Meta‐analysis of DXA in predicting incident fracture and MOF
Supplementary Table S4. Determining the effect size overlapping among cortical vBMD, Tb.Th and stiffness in different fracture sites using Cohen's d
Data Availability Statement
Data available on request from the authors


