Summary
Background
Dupilumab is an antibody against interleukin‐4 receptor α, used in the treatment of atopic dermatitis (AD).
Objectives
To evaluate the efficacy and safety of dupilumab in adult Chinese patients with moderate‐to‐severe AD.
Methods
In this randomized, double‐blind, placebo‐controlled, parallel‐group, phase III study, conducted between December 2018 and February 2020, patients with AD received dupilumab (300 mg) or placebo once every 2 weeks for 16 weeks, and were followed up for 12 weeks. The primary efficacy endpoint was the proportion of patients with both an Investigator’s Global Assessment score of 0–1 and a reduction from baseline of ≥ 2 points at week 16.
Results
Overall, 165 patients (mean age 30·6 years; 71·5% male patients) were randomized; 82 patients were randomized to dupilumab and 83 patients were randomized to placebo. At week 16, 26·8% of patients in the dupilumab group and 4·8% of patients in the placebo group achieved the primary endpoint [difference 22·0%, 95% confidence interval (CI) 11·37–32·65; P < 0·001]. Compared with placebo, higher proportions of patients in the dupilumab group achieved ≥ 75% reduction in the Eczema Area and Severity Index score (57·3% vs. 14·5%; difference 42·9%, 95% CI 29·75–55·97; P < 0·001) and had ≥ 3‐point (52·4% vs. 9·6%; difference 42·8%, 95% CI 30·26–55·34; P < 0·001) and ≥ 4‐point (39·0% vs. 4·8%; difference 34·2%, 95% CI 22·69–45·72; P < 0·001) reductions in weekly average daily peak daily pruritus numerical rating scale scores. The incidence of treatment‐emergent adverse events during the treatment period was similar in the two groups. The incidence of conjunctivitis, allergic conjunctivitis and injection site reaction was higher in the dupilumab group than in the placebo group.
Conclusions
In adult Chinese patients, dupilumab was effective in improving the signs and symptoms of AD and demonstrated a favourable safety profile.
Short abstract
What is already known about this topic?
Two randomized, placebo‐controlled phase III trials (SOLO 1 and SOLO 2) have demonstrated that dupilumab is effective and safe when used in the treatment of atopic dermatitis in adults.
However, very few Chinese participants were included and, therefore, the efficacy and safety of dupilumab in this population is unclear.
What does this study add?
The findings of this randomized, placebo‐controlled phase III trial show that dupilumab is effective in improving the signs and symptoms of atopic dermatitis in adult Chinese patients and has a favourable safety profile in this population.
Plain language summary available online
Atopic dermatitis (AD), particularly moderate‐to‐severe AD, imposes a heavy burden on patients, 1 especially in the presence of associated atopic comorbidities such as asthma and food allergies. 2 , 3 Moreover, AD has a daily impact on patients’ health‐related quality of life, 1 , 2 including sleep disturbances, functional impairment, limited activities of daily living, 2 and depression and anxiety. 4 While mild AD can be treated with topical therapies, treatment of moderate‐to‐severe AD often requires the use of systemic therapies, including systemic corticosteroids and immunosuppressants such as ciclosporin and methotrexate. 5 However, many of these therapies are associated with unacceptable adverse events (AEs) and cannot be used for prolonged periods. 5 Therefore, there is an unmet need for safe and well‐tolerated therapies for moderate‐to‐severe AD.
Dupilumab is a recombinant human monoclonal IgG4 antibody against the α subunit of the interleukin (IL)‐4 receptor. 6 , 7 As this is a shared subunit of the IL‐4 and IL‐13 receptors, dupilumab is able to inhibit the effects of both IL‐4 and IL‐13 signalling. 6 , 7 In 2017, dupilumab was approved in the USA for the treatment of adult patients with moderate‐to‐severe AD whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable. 7 Dupilumab was also approved in the European Union in 2017 and in China in 2020 for the treatment of adults with moderate‐to‐severe AD. 6
Two randomized, placebo‐controlled phase III studies (SOLO 1 and SOLO 2) established the efficacy of dupilumab monotherapy in the treatment of adult AD, including in Asian patients from Japan and Korea. 8 However, very few Chinese patients were included in either SOLO 1 or SOLO 2, 8 and the efficacy and safety of dupilumab in the Chinese population remain unclear. Therefore, this study was conducted to evaluate the efficacy and safety of dupilumab monotherapy in adult Chinese patients with moderate‐to‐severe AD.
Patients and methods
This was a randomized, double‐blind, placebo‐controlled, parallel‐group, phase III study (ClinicalTrials.gov registration: NCT03912259), which was conducted in 27 hospitals in China. To participate in this study, patients were required to be aged ≥ 18 years old, have moderate‐to‐severe AD (diagnosed according to the American Academy of Dermatology criteria) 9 for ≥ 3 years which could not be adequately controlled with topical medications or for which topical treatment was inadvisable, have an Eczema Area and Severity Index (EASI) score ≥ 16, an Investigator’s Global Assessment (IGA) score ≥ 3 and ≥ 10% of body surface area (BSA) affected by AD at the screening and baseline visits, in addition to a numerical rating scale (NRS) average weekly score for maximum itch intensity of ≥ 4 at baseline. A complete list of inclusion and exclusion criteria is provided in Table S1 (see Supporting Information).
The study consisted of a 16‐week treatment period, with study visits at 0, 2, 4, 8, 12 and 16 weeks, and a 12‐week follow‐up period. Patients were randomized at a 1 : 1 ratio to receive subcutaneous dupilumab 300 mg or matching placebo, administered once every 2 weeks at the study site. Randomization was stratified by baseline disease severity (IGA 3 vs. IGA 4). On day 1, patients were administered either a 600‐mg loading dose of dupilumab or a double dose of placebo, depending on their treatment allocation. All patients were required to use moisturizers (emollients) at least twice daily for at least seven consecutive days immediately preceding randomization and throughout the study. Treatment with any of the following was prohibited during the study: live (attenuated) vaccines, immunomodulating biologics, topical corticosteroids or calcineurin inhibitors, systemic corticosteroids or nonsteroidal immunomodulating or immunosuppressive drugs, investigational drugs or any other drugs that could interfere with the study, in addition to initiation or uptitration of antigen‐specific immunotherapy. However, if medically necessary (i.e. to control intolerable AD symptoms), rescue treatment for AD with otherwise prohibited medications or procedures could be used, which was left to the discretion of the investigators. Those who received topical rescue medications continued to receive study treatment, whereas study treatment was discontinued immediately in those who received systemic rescue medication (i.e. corticosteroids or nonsteroidal systemic immunosuppressive/immunomodulating drugs). Study medication could be resumed if this was deemed appropriate by the investigator and the sponsor, but no sooner than five half‐lives after the last dose of systemic rescue medication.
Randomization was conducted according to a centralized randomization scheme using interactive response technology. The sponsor provided a centrally generated randomized treatment number list to the investigator, who was responsible for enrolling the study participants and assigning them to their randomized treatment groups. The study medication (dupilumab or placebo) was provided in identically matched 2‐mL prefilled syringes, labelled according to the randomized treatment number list. In order to maintain blinding, randomization codes were not accessible to study participants, investigators or study site personnel, except in the event of an AE when knowledge of the study medication was required to treat the patient. Assay results for IgE and thymus and activation‐regulated chemokine (TARC) were not accessible to study participants, investigators or study site personnel, as these data had the potential for unblinding.
Endpoints
The primary efficacy endpoint was the proportion of patients with both an IGA score (on a 5‐point scale) of 0 or 1 and a reduction of ≥ 2 points in their IGA score from baseline at week 16. This endpoint was chosen for consistency with SOLO 1 and SOLO 2. 8
Secondary efficacy endpoints were the proportion of patients with EASI 50, EASI 75 and EASI 90 at week 16 (reduction of ≥ 50%, ≥ 75% and ≥ 90% in the EASI score, respectively); absolute and percentage changes in EASI score; the proportion of patients with ≥ 3‐point and ≥ 4‐point reduction in the weekly average of peak daily pruritus NRS scores; absolute and percentage change in the weekly average of peak daily pruritus NRS scores; change in percentage BSA of AD involvement; change in Dermatology Life Quality Index (DLQI); change in Patient‐Oriented Eczema Measure (POEM); and absolute and percent change in EuroQol‐5 Dimension (EQ‐5D) questionnaire score from baseline to week 16, in addition to the percentage change in the weekly average of peak daily pruritus NRS scores from baseline to week 2. In addition, the proportion of patients achieving both IGA 0–1 and a reduction of ≥ 2 points from baseline at week 16 was analysed in subgroups according to demographics, baseline disease severity, previous use of systemic medication and atopic/allergic disease history.
Safety endpoints included the incidence of treatment‐emergent AEs (TEAEs) and serious AEs (SAEs). A TEAE was defined as any untoward medical occurrence that took place during the period from the first administration of study treatment to the end of the study, regardless of its causal relationship to treatment. TEAEs were coded using the Medical Dictionary for Regulatory Activities version 22·1. In addition, haematology, blood chemistry, urinalysis, vital signs, electrocardiographic assessments and serum markers of type 2 inflammation, including TARC and IgE, were evaluated.
Statistics
Assuming that 37% of patients in the dupilumab group and 12% of patients in the placebo group would achieve the primary efficacy endpoint, a sample of 160 patients (80 patients in the dupilumab and 80 patients in the placebo group) would provide 94% power to detect the difference between the groups with a continuity‐corrected χ2‐test and a two‐sided significance level of 0·05.
Efficacy analyses were conducted in the intention‐to‐treat (ITT) population, which included all randomized patients (treatment group defined as ‘randomization assigned’), using the Cochran–Mantel–Haenszel test adjusted by baseline disease severity (moderate or severe). If the between‐group difference in the proportion of patients who had achieved the primary endpoint was significant at the 0·05 level, then secondary efficacy endpoints were evaluated following a prespecified hierarchical testing procedure (Table S2; see Supporting Information) for multiplicity adjustment in order to control family‐wise type I error.
In the primary analysis, patients who used rescue therapy were considered to be nonresponders from that time. If a patient withdrew from the study, this patient was counted as a nonresponder for endpoints after withdrawal, and patients with missing values at week 16 were also counted as nonresponders at week 16. A sensitivity analysis was conducted using observed response values regardless of rescue therapy use; all patients with missing values in this analysis continued to be counted as nonresponders.
Descriptive safety analyses were conducted in the safety population, which comprised all patients included in the ITT population who received study treatment.
Statistical calculations were performed using nQuery Advisor 7·0 software (Statistical Solutions Ltd, Cork, Ireland).
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines for Good Clinical Practice, and all applicable laws, rules and regulations. All study participants provided written informed consent before any study procedures were conducted.
Results
Patients
The study was initiated in December 2018 and was completed as planned in February 2020. Overall, 165 patients were randomized to study treatment; 82 patients were randomized to dupilumab and 83 patients to placebo (Figure 1). All randomized patients received study treatment and were included in the analysis.
Figure 1.
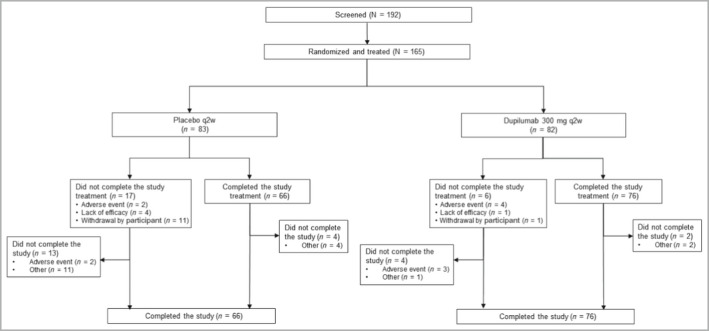
Distribution of patients throughout the study. q2w, every 2 weeks.
Patient characteristics at baseline were balanced between the treatment groups (Table 1 and Tables S3 and S4; see Supporting Information). The mean age of patients was 30·6 years, and most patients were male (n = 118, 71·5%). Baseline disease characteristics for all AD assessments were similar between treatment groups and consistent with moderate‐to‐severe AD defined by the protocol. In total, 43·6% of patients had moderate AD (IGA 3) and 56·4% had severe AD (IGA 4) (Table 1). The majority of patients (n = 164, 99·4%) had inadequate response to topical corticosteroid treatment, and topical therapies were inadvisable in 26·7% (n = 44) of patients. Many patients had comorbid atopic conditions other than AD, including allergic rhinitis (n = 89, 53·9%), asthma (n = 36, 21·8%) and allergic conjunctivitis (n = 17, 10·3%) (Table S4).
Table 1.
Demographic and disease characteristics at baseline
| Placebo (N = 83) | Dupilumab (N = 82) | Total (N = 165) | |
|---|---|---|---|
| Age, years | 26·0 (21·0–35·0) | 28·0 (24·0–35·0) | 28·0 (22·0–35·0) |
| Male sex | 60 (72·3) | 58 (70·7) | 118 (71·5) |
| Duration of AD,a years | 12·0 (7·0–20·0) | 13·0 (10·0–22·0) | 13·0 (9·0–21·0) |
| BSA affected by AD, % | 55·0 (40·1–71·0) | 55·0 (35·0–73·5) | 55·0 (39·5–73·0) |
| EASI score | 31·0 (24·5–39·8) | 30·3 (22·8–42·9) | 30·8 (23·3–40·7) |
| IGA score of 4 | 46 (55) | 47 (57) | 93 (56·4) |
| Weekly average of peak daily pruritus NRS scoresb | 8·0 (6·8–9·0) | 8·0 (6·8–8·9) | 8·0 (6·8–9·0) |
| POEM score | 24·0 (20·0–27·0) | 25·0 (21·0–27·0) | 25·0 (20·0–27·0) |
| DLQI score | 18·0 (14·0–23·0) | 18·5 (12·0–22·0) | 18·0 (13·0–23·0) |
AD, atopic dermatitis; BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; IQR, interquartile range; NRS, numerical rating scale; POEM, Patient‐Oriented Eczema Measure. aAge at chronic AD diagnosis was calculated as year of chronic AD diagnosis (year of birth + 1). bObtained over the 7‐day period prior to the baseline visit. Data are presented as n (%) or median (IQR).
The proportion of patients who completed study treatment was higher in the dupilumab group (n = 76, 93%) than in the placebo group (n = 66, 80%). The most common reasons for treatment discontinuation were withdrawal by patient (dupilumab: n = 1, 1·2%; placebo: n = 11, 13·3%), TEAE (dupilumab: n = 4, 4·9%; placebo: n = 2, 2·4%) and lack of efficacy (dupilumab: n = 1, 1·2%; placebo: n = 4, 4·8%).
During the treatment period, the use of rescue medications was less common in the dupilumab group than in the placebo group. This was true for any (19·5% vs. 50·6%), topical (19·5% vs. 50·6%) and systemic (2·4% vs. 4·8%) rescue medications. The most common rescue medication was tacrolimus, which was used in 13·4% of patients in the dupilumab group and 43·4% of patients in the placebo group. Topical corticosteroids were used in 15·9% of patients in the dupilumab group and in 39·8% of patients in the placebo group. Systemic corticosteroids were used in 1·2% and 2·4% of patients, respectively.
Efficacy
Efficacy results are shown in Figures 2, 3, 4, 5, Figure S1 (see Supporting Information) and in Tables S5 and S6 (see Supporting Information). At week 16, the proportion of patients who achieved an IGA score of 0 or 1 and a reduction in the IGA score of ≥ 2 points from baseline (primary efficacy endpoint) was significantly higher in the dupilumab group (n = 22, 26·8%) than in the placebo group (n = 4, 4·8%) (Table S5, Figure 2). The difference between the treatment arms was 22·0% [95% confidence interval (CI) 11·37–32·65; P < 0·001]. In the sensitivity analysis using all observed values regardless of rescue medication use, with patients with missing values counted as nonresponders, the proportion of patients who achieved the primary endpoint remained significantly higher in the dupilumab group (n = 25, 30·5%) than in the placebo group (n = 4, 4·8%; between‐group difference 25·7%, 95% CI 14·69–36·65; P < 0·001). In prespecified subgroup analyses of the proportion of patients achieving both IGA 0–1 and a reduction of ≥ 2 points from baseline at week 16 according to demographics, baseline disease severity, previous use of systemic medication and atopic/allergic disease history, dupilumab increased the proportion of patients with IGA 0 or 1 and a reduction from baseline of ≥ 2 points at week 16 across the majority of subgroups (Figure S1).
Figure 2.
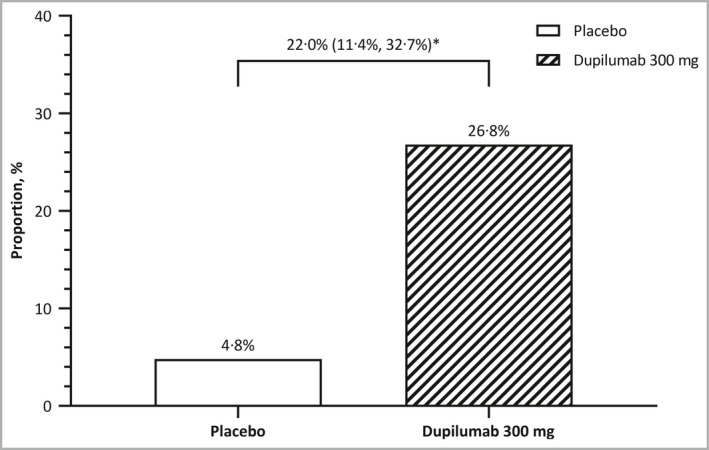
Proportion of patients who had an Investigator’s Global Assessment (IGA) score of 0 or 1 and achieved a reduction of ≥ 2 points in their IGA score from baseline at week 16 (primary endpoint). *P < 0·001 for difference (95% confidence interval).
Figure 3.
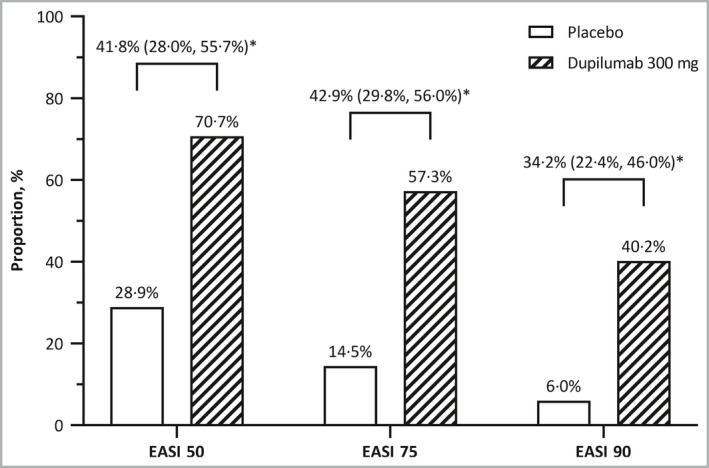
Proportions of patients who achieved Eczema Area and Severity Index (EASI) 50, EASI 75 and EASI 90 at week 16. *P < 0·001 for difference (95% confidence interval).
Figure 4.
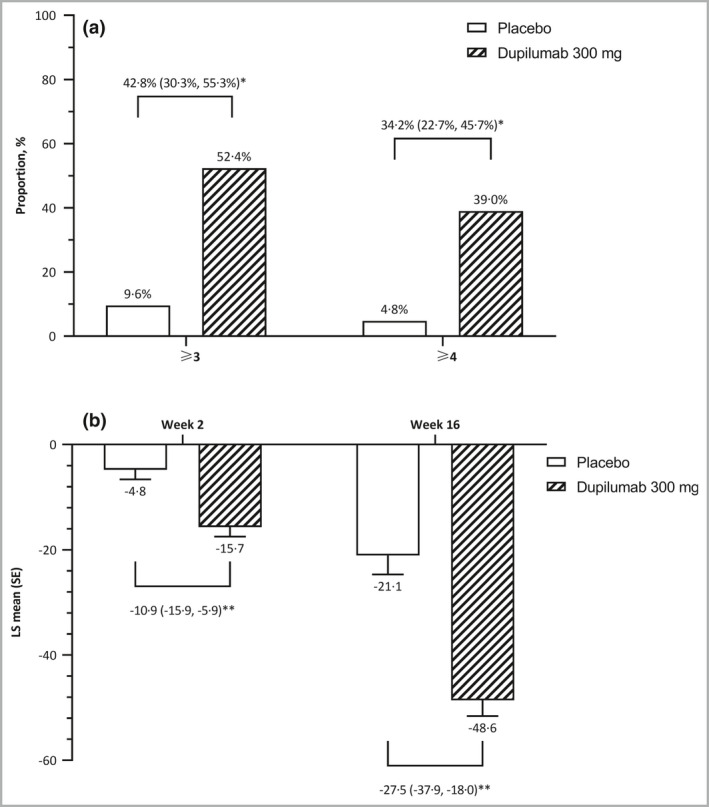
Change in weekly average peak pruritus NRS score at week 16. (a) Proportions of patients who had ≥ 3‐point and ≥ 4‐point reduction in weekly average peak pruritus NRS score from baseline. (b) Percentage change in weekly average of peak daily pruritus NRS score from baseline. CI, confidence interval; LS, least squares; NRS, numerical rating scale; SE standard error. *P < 0·001 for difference (95% CI). **P < 0·001 for LS mean difference (95% CI).
Figure 5.
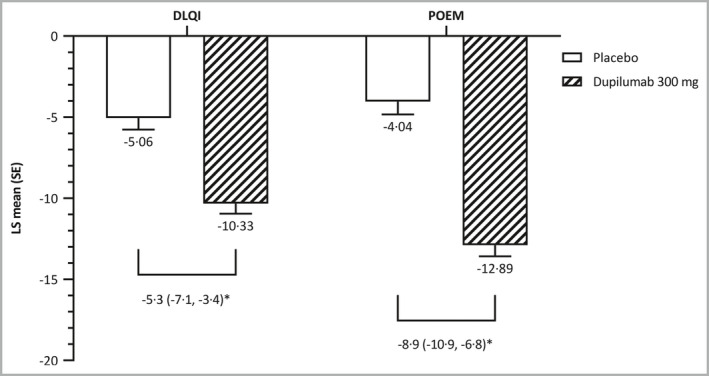
Patient‐reported outcomes. DLQI, Dermatology Life Quality Index; LS, least squares; POEM, Patient‐Oriented Eczema Measure; SE, standard error. *P < 0·001 for LS mean difference (95% confidence interval).
With multiplicity control, dupilumab was superior to placebo with regard to all secondary efficacy endpoints in the predefined hierarchy (P < 0·001 for all comparisons) (Table S5). Compared with placebo, the proportion of patients who achieved EASI 75 at week 16 was significantly higher with dupilumab (57·3% vs. 14·5%; difference 42·9%, 95% CI 29·75–55·97) (Figure 3 and Table S5). Dupilumab was also superior to placebo at week 16 in terms of EASI 50 (70·7% vs. 28·9%; difference 41·8%; 95% CI 27·96–55·68) and EASI 90 (40·2% vs. 6·0%; difference 34·2%; 95% CI 22·44–46·00). Indeed, achievement of EASI 75, EASI 50 and EASI 90 was greater with dupilumab than with placebo at all assessment timepoints beginning at week 2. Dupilumab was also superior to placebo with respect to the mean percentage change in EASI score and mean change in percentage BSA affected by AD from baseline to week 16 (Table S5).
Dupilumab was significantly more effective than placebo in improving measures of pruritus. At week 16, significantly higher proportions of patients in the dupilumab group than in the placebo group had ≥ 3‐point (52·4% vs 9·6%; difference 42·8%; 95% CI 30·26–55·34) and ≥ 4‐point (39·0% vs 4·8%; difference 34·2%; 95% CI 22·69–45·72) reductions in the weekly average of peak daily pruritus NRS scores (P < 0·001 for both endpoints) (Table S5 and Figure 4a). Furthermore, the proportions of patients who had ≥ 3‐point and ≥ 4‐point reductions in the weekly average of peak daily pruritus NRS scores were greater with dupilumab than with placebo at all assessment timepoints beginning at week 2. Dupilumab was associated with significantly greater absolute change in peak pruritus NRS score at week 16 and percentage changes in mean peak pruritus NRS score at week 2 and week 16 (Table S5 and Figure 4b).
In addition, dupilumab was significantly more effective than placebo in improving patient‐reported outcomes. At week 16, patients who received dupilumab had significantly greater reductions in DLQI and POEM scores from baseline than did patients who received placebo (Figure 5), and patients who received dupilumab also had significantly greater reductions in the EQ‐5D index utility score (Table S5). Mean scores for each of the patient‐reported outcomes (EASI, NRS, POEM and DLQI) at baseline and week 16 are shown in Table S6.
Safety
During the treatment period, the incidence of TEAEs was similar in the dupilumab (n = 63, 77%) and the placebo groups (n = 67, 81%) (Table 2). Most TEAEs were mild (dupilumab: n = 44, 54%; placebo: n = 49, 59%) or moderate (dupilumab: n = 14, 17%; placebo: n = 12, 15%) in intensity. Severe TEAEs occurred in five (6%) patients in the dupilumab group and six (7%) patients in the placebo group. The incidence of treatment‐emergent SAEs was lower in the dupilumab group (n = 1, 1%) than in the placebo group (n = 3, 4%).
Table 2.
Treatment‐emergent adverse events (TEAEs) reported by ≥ 5% of patients in either treatment group during the treatment period
| Primary system organ class | TEAEs | |
|---|---|---|
| Preferred term, n (%) | Placebo (N = 83) | Dupilumab (N = 82) |
| Any | 67 (81) | 63 (77) |
| Infections and infestations | 21 (25) | 28 (34) |
| Upper respiratory tract infection | 6 (7) | 9 (11) |
| Conjunctivitis | 4 (5) | 8 (10) |
| Eye disorders | 4 (5) | 9 (11) |
| Conjunctivitis allergic | 1 (1) | 7 (9) |
| Skin and subcutaneous tissue disorders | 40 (48) | 26 (31) |
| Atopic dermatitis | 38 (46) | 20 (24) |
| General disorders and administration site conditions | 5 (6) | 9 (11) |
| Injection site reaction a | 2 (2) | 7 (9) |
| Investigations | 16 (19) | 17 (21) |
| Proteinuria | 7 (8) | 7 (9) |
| Blood uric acid levels increased | 5 (6) | 1 (1) |
Includes injection site mass, injection site pain, or injection site swelling.
The most common TEAEs that occurred during the treatment period (incidence ≥ 5% in either treatment group) by preferred term were AD, upper respiratory tract infection, conjunctivitis, allergic conjunctivitis, injection site reaction, proteinuria and increased blood uric acid levels (Table 2). Of these, upper respiratory tract infections, conjunctivitis, allergic conjunctivitis and injection site reactions occurred more frequently in patients who received dupilumab than in those who received placebo, whereas AD and increased blood uric acid levels were more common in patients who received placebo.
Three patients in the placebo group experienced SAEs; one patient (1%) had an anaphylactic reaction, one had AD and one had a connective tissue disorder. One patient in the dupilumab group experienced an SAE (lung adenocarcinoma). This event was considered by the study investigator as ‘unlikely related’ to dupilumab treatment. Four patients (5%) in the dupilumab group and two patients (2%) in the placebo group discontinued study treatment owing to TEAEs. No deaths occurred during the study.
No meaningful changes in vital signs, physical examination, or electrocardiographic assessments occurred in either treatment group during the treatment period. There were no meaningful changes in laboratory safety parameters. Dupilumab showed an acceptable laboratory safety profile.
Discussion
The results of this randomized, double‐blind, placebo‐controlled, phase III study show that dupilumab is effective in improving the signs and symptoms of moderate‐to‐severe AD in adult Chinese patients. Compared with placebo, dupilumab significantly reduced the overall severity of the disease, reduced the extent and severity of lesions, reduced itching and improved quality of life. Dupilumab also had an acceptable safety profile.
The efficacy and safety profiles of dupilumab in Chinese patients are comparable with those reported in non‐Chinese Asian patients and in the overall population in the randomized, placebo‐controlled phase III SOLO 1 and SOLO 2 monotherapy studies, despite the fact that patients in the present study were younger on average and had higher baseline disease severity, as shown by the proportion of patients with an IGA score of 4 (56·4% vs. 47–49%). 8
Prespecified subgroup analyses of the primary endpoint demonstrated that treatment with dupilumab increased the proportion of patients with an IGA score of 0 or 1 and led to a reduction in IGA score from baseline of ≥ 2 points at week 16 across the majority of subgroups. However, large‐scale cohort studies may be helpful in gaining an accurate understanding of the profile of Chinese patients who respond best to dupilumab treatment. In addition, evaluating other parameters, such as inflammatory biomarkers, may help to better identify dupilumab responders. Previous studies have shown that increased eosinophil count and elevated lactate dehydrogenase levels, in addition to increases in serum IgE levels while receiving dupilumab treatment, may serve as markers of poor response. 10 , 11 A prospective, observational study designed to evaluate the association between the effectiveness of dupilumab and changes in a number of biomarkers in Japanese patients with AD is currently under way. 12
Previous studies have shown that there are a number of differences between Asian patients and patients of other ethnicities in terms of the clinical presentation, genetic predisposition and pathophysiology of AD. 13 , 14 However, an analysis of data pooled from the SOLO 1 and SOLO 2 studies, in addition to the 52‐week, randomized, placebo‐controlled CHRONOS study showed that the efficacy of dupilumab in Asian patients was comparable with its efficacy in the overall patient population 15 ; similarly, its efficacy in Japanese patients was consistent with that in the overall patient population in a pooled analysis of the 16‐week phase IIb AD‐1021 study, and SOLO 1 and CHRONOS studies. 16 In addition, dupilumab was effective in the treatment of AD in two retrospective real‐world studies, one of which was conducted in Korean patients and another in Japanese patients. 11 , 17 Collectively, these findings demonstrate that the inhibition of IL‐4 and IL‐13 signalling by dupilumab results in significant clinical improvement in Asian patients with AD, supporting the crucial role of IL‐4 and IL‐13 in the pathogenesis of this disease.
This study had several limitations. Firstly, it consisted of a 16‐week treatment period and a 12‐week follow‐up period, for a total observation time of 28 weeks, which may not be sufficient to confirm the long‐term effectiveness and safety of dupilumab in Chinese patients. However, the long‐term safety and effectiveness of dupilumab have been assessed in the international CHRONOS and LIBERTY AD OLE study, 18 , 19 providing evidence that it can be used as a continuous long‐term treatment for AD. Secondly, this study recruited Chinese patients with moderate‐to‐severe AD whose condition was not controlled with topical medications or for whom such medications were inadvisable, in accordance with the current approved indication for dupilumab. Therefore, the results may not be generalizable to other populations of Chinese patients with AD who have different disease or treatment characteristics.
In conclusion, this study shows that treatment with dupilumab significantly improves the signs and symptoms of AD in adult Chinese patients and is well tolerated in this patient population.
Author Contribution
Yan ZHAO: Data curation (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Liming WU: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Qianjin Lu: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Xinghua Gao: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Xiaohong ZHU: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Xu Yao: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Lin‐feng Li: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Wei LI: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Yangfeng Ding: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Zhiqiang Song: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). ling ling liu: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Ningning DANG: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Chun‐Lei Zhang: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Xiaoming Liu: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Jun Gu: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Jinyan Wang: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Songmei Geng: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Quanzhong LIU: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Yifeng Guo: Data curation (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Lichee DONG: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (lead); Project administration (lead); Supervision (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Huijuan SU: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Funding acquisition (equal); Investigation (equal); Methodology (equal); Software (lead); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Lili BAI: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). John T. O'Malley: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Junxiang LUO: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Elizabeth Laws: Conceptualization (equal); Funding acquisition (lead); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Leda Mannent: Conceptualization (equal); Investigation (equal); Validation (equal); Writing‐review & editing (equal). Marcella Ruddy: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Nikhil Amin: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Ashish Bansal: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Takayuki Ota: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Min WANG: Validation (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Jianzhong Zhang: Conceptualization (lead); Data curation (equal); Investigation (lead); Methodology (lead); Project administration (lead); Supervision (lead); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Table S1 Key recruitment criteria.
Table S2 Hierarchical testing procedure.
Table S3 Medical history (≥ 10% of patients in either treatment group) by primary system organ class and preferred term.
Table S4 History of atopic/allergic conditions at baseline.
Table S5 Efficacy analysis.
Table S6 Eczema Area and Severity Index (EASI), peak daily pruritus numerical rating scale (NRS), Patient‐Oriented Eczema Measure (POEM) and Dermatology Life Quality Index (DLQI) scores at baseline and week 16. Week 16 data are for all observed values regardless of the use of recue medication.
Figure S1 Forest plot of the subgroup analysis of patients who achieved the primary efficacy endpoint [Investigator’s Global Assessment (IGA) 0–1 and ≥ 2‐point reduction in IGA from baseline at week 16].
Acknowledgments
We would like to thank Georgii Filatov of Springer Healthcare Communications, who provided the medical writing support for this manuscript. This medical writing assistance was funded by Sanofi. The authors individually and collectively are responsible for all content and editorial decisions, and received no payment from Sanofi directly or indirectly (through a third party) related to the development/presentation of this publication. We would like to thank Dong Lv, Hong Ren, Xiumin Yang, Danqi Deng, Shanshan Li, Xiuping Han, Zhenshu Biao and Min Zheng who contributed to patient enrolment.
Funding sources This study was funded by Sanofi and Regeneron. The study sponsors participated in the study design; collection, analysis and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication. Medical writing and editorial support were funded by Sanofi.
Conflicts of interest L.D., H.S., L.B., J.T.O’M., J.L., E.L., L.M. and M.W. are employees of Sanofi. M.R., N.A., A.B. and T.O. are employees of Regeneron.
Plain language summary available online
References
- 1. Chiesa Fuxench ZC, Block JK, Boguniewicz M et al. Atopic dermatitis in America study: a cross‐sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol 2019; 139:583–90. [DOI] [PubMed] [Google Scholar]
- 2. Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol 2019; 123:144–51. [DOI] [PubMed] [Google Scholar]
- 3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011; 242:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalgard FJ, Gieler U, Tomas‐Aragones L et al. The psychological burden of skin diseases: a cross‐sectional multicenter study among dermatological out‐patients in 13 European countries. J Invest Dermatol 2015; 135:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Megna M, Napolitano M, Patruno C et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb) 2017; 7:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Dupixent: EPAR – [product information]. Available at https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent (last accessed 8 September 2021).
- 7. US Food and Drug Administration . Dupixent [prescribing information]. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761055s020lbl.pdf (last accessed 8 September 2021).
- 8. Simpson EL, Bieber T, Guttman‐Yassky E et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 9. Eichenfield LF, Tom WL, Chamlin SL et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang TH, Chen YC, Lin SY et al. Treatment of atopic dermatitis with dupilumab in Taiwan: dynamic changes of IgE levels as a potential response biomarker. Eur J Dermatol 2019; 29:658–9. [DOI] [PubMed] [Google Scholar]
- 11. Jang DH, Heo SJ, Jung HJ et al. Retrospective study of dupilumab treatment for moderate to severe atopic dermatitis in Korea: efficacy and safety of dupilumab in real‐world practice. J Clin Med 2020; 9:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakahara T, Izuhara K, Onozuka D et al. Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: a study protocol. Medicine (Baltimore) 2020; 99:e22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups‐Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol 2018; 27:340–57. [DOI] [PubMed] [Google Scholar]
- 14. Brunner PM, Guttman‐Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol 2019; 122:449–55. [DOI] [PubMed] [Google Scholar]
- 15. Alexis AF, Rendon M, Silverberg JI et al. Efficacy of dupilumab in different racial subgroups of adults with moderate‐to‐severe atopic dermatitis in three randomized, placebo‐controlled phase 3 trials. J Drugs Dermatol 2019; 18:804–13. [PubMed] [Google Scholar]
- 16. Katoh N, Kataoka Y, Saeki H et al. Efficacy and safety of dupilumab in Japanese adults with moderate‐to‐severe atopic dermatitis: a subanalysis of three clinical trials. Br J Dermatol 2020; 183:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uchida H, Kamata M, Kato A et al. One‐year real‐world clinical effectiveness, safety, and laboratory safety of dupilumab in Japanese adult patients with atopic dermatitis: a single‐center retrospective study. J Am Acad Dermatol 2021; 84:547–50. [DOI] [PubMed] [Google Scholar]
- 18. Blauvelt A, de Bruin‐Weller M, Gooderham M et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 19. Beck LA, Thaci D, Deleuran M et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open‐label study of adults with moderate‐to‐severe atopic dermatitis. Am J Clin Dermatol 2020; 21:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Key recruitment criteria.
Table S2 Hierarchical testing procedure.
Table S3 Medical history (≥ 10% of patients in either treatment group) by primary system organ class and preferred term.
Table S4 History of atopic/allergic conditions at baseline.
Table S5 Efficacy analysis.
Table S6 Eczema Area and Severity Index (EASI), peak daily pruritus numerical rating scale (NRS), Patient‐Oriented Eczema Measure (POEM) and Dermatology Life Quality Index (DLQI) scores at baseline and week 16. Week 16 data are for all observed values regardless of the use of recue medication.
Figure S1 Forest plot of the subgroup analysis of patients who achieved the primary efficacy endpoint [Investigator’s Global Assessment (IGA) 0–1 and ≥ 2‐point reduction in IGA from baseline at week 16].


