Abstract
Background
The lack of an agreed international minimum approach to measuring cannabis use hinders the integration of multidisciplinary evidence on the psychosocial, neurocognitive, clinical and public health consequences of cannabis use.
Methods
A group of 25 international expert cannabis researchers convened to discuss a multidisciplinary framework for minimum standards to measure cannabis use globally in diverse settings.
Results
The expert‐based consensus agreed upon a three‐layered hierarchical framework. Each layer—universal measures, detailed self‐report and biological measures—reflected different research priorities and minimum standards, costs and ease of implementation. Additional work is needed to develop valid and precise assessments.
Conclusions
Consistent use of the proposed framework across research, public health, clinical practice and medical settings would facilitate harmonisation of international evidence on cannabis consumption, related harms and approaches to their mitigation.
Keywords: assessment, cannabis, dose, measurement, standardisation, iCanntoolkit
INTRODUCTION
The legal status of cannabis is rapidly changing and producing a proliferation of new products that vary in potency, formulation, chemical composition, and methods of administration. This presents significant global challenges in measuring cannabis use [1]. There are currently no agreed‐upon minimum standards for quantifying cannabis use or cannabis dosage across research, clinical, public health and medical settings.
In this article, the term ‘cannabis use’ refers to the use of cannabis products containing delta‐9‐tetrahydrocannabinol (THC). Cannabis use is inherently difficult to measure [2] because of variations in properties of commonly available cannabis products: potency, chemovars, types (e.g. herbal cannabis, hashish and concentrates) and modes of administration (e.g. bongs, joints, blunts, pipes, edibles, rigs, oils and vaporizers). Patterns of cannabis use also vary between individuals, local customs, regional availability and jurisdictions. Universal standardised tools for measuring cannabis exposure are required to better integrate the evidence on the antecedents, correlates and consequences of use.
The clearest harms arise from high‐frequency use of cannabis products with high levels of THC [3, 4, 5, 6]. Surveys that ask only about frequency of cannabis use are unable to account for quantity of use, which may vary widely. In some countries, the majority of cannabis is consumed by the minority of daily or near‐daily users of cannabis [7]. We need better data on their patterns of cannabis use.
THC content also varies between cannabis products at least as much as the alcohol content of beer, wine and spirits. The average THC concentration of flower products has increased substantially in multiple countries (can exceed 20% THC) [8] and concentrates often have substantially higher THC concentration than even high‐potency flower (often 60%–80% THC) [9]. THC is only one of many psychoactive cannabinoids in the cannabis plant, but is most associated with abuse liability. There are now cannabis products that predominantly contain very little THC or other cannabinoids (e.g. cannabigerol [CBG] and cannabinol [CBN]), as well as synthetic cannabinoids with varying chemical composition, for which abuse liability and long‐term adverse health effects are unknown. For the present paper, we confine our attention to cannabis products containing THC (alone or in combination with other chemical compounds present in cannabis or synthetic cannabinoids). In the future, new tools may need to be developed to adequately measure use of different cannabinoids (e.g. cannabidiol [CBD]‐based products and synthetic cannabinoid receptor agonists).
In most jurisdictions, cannabis remains illegal, thus cannabis products rarely come with informative labels. Even in regulated cannabis markets, licensed producers' labels are not always accurate, so consumers may not be able to accurately describe what they have consumed.
Currently, we have no universal, agreed upon minimum standards to measure cannabis consumption in different populations (e.g. general and clinical populations). This issue prevents the integration of the international and multidisciplinary evidence on the psychosocial, neurocognitive, clinical, public health correlates of cannabis use [10]. It also limits our understanding of the psychosocial harms related to cannabis use and efforts to mitigate them. A set of agreed upon minimum standards is required to monitor and compare levels of exposure to cannabis. Such a set of minimum standards has been called for by researchers in the field [1, 11, 12] to enable the timely integration of data on cannabis use across international settings and multiple disciplines.
EXPERT CONSENSUS FRAMEWORK ON MINIMUM STANDARDS FOR QUANTIFYING CANNABIS USE
Here, we outline an expert consensus‐based framework on minimum standards for quantifying cannabis use. This framework is supported by 25 international multidisciplinary cannabis researchers. To achieve consensus we used a Delphi methodology. First, experts were invited to represent a wide range of world regions (North and South America, Europe and Australasia), from multiple disciplines (epidemiologists, academics, clinical psychologists, psychopharmacologists, pharmacologists, neuroscientists and economists), and from countries that vary with respect to legal status of cannabis. Experts were invited to present relevant evidence on (i) standardised measures of cannabis use; (ii) measuring cannabis use in illegal markets; and (iii) measuring cannabis use in legal markets. Each presentation was followed by questions and discussion. Next, a moderated discussion was used to build consensus on what should be included in an international cannabis toolkit. Following the meeting, the process of consensus continued with drafting of the manuscript by a subset of experts, and consequent edits and revisions from all experts, until a consensus was agreed upon.
THE INTERNATIONAL CANNABIS TOOLKIT
The framework of the International Cannabis Toolkit (iCannToolkit) (Figure 1) consists of a three‐layered hierarchical pyramid (universal, detailed self‐report and biological measures) in which each layer reflects different levels of measurement, costs and ease of implementation. Supporting information outlines the items of the iCannToolkit for ease of use.
FIGURE 1.
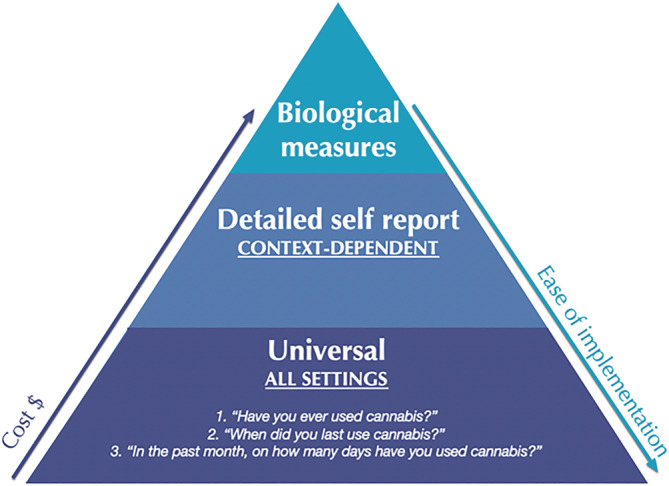
Expert consensus‐based framework on minimum standards for quantifying cannabis use. From the base up, the measurements increase in cost and their ease of implementation diminishes
Base layer: universal measures
At the base of the pyramid, are a core set of universal measures to quantify self‐reported cannabis use globally in research, clinical practice, public health and medicinal settings.
Expert‐recommended universal measures
They consist of three key items:
-
1
‘Have you ever used cannabis?’
(Record: yes, no)
This item is a brief screening of lifetime cannabis use. It will help discontinue administration of the iCannToolkit, should participants respond that they have never used cannabis.
-
2
‘When did you last use cannabis?’
(Record hours, days, months or years as appropriate)
This item is aimed at establishing whether an individual has recently used or the duration of abstinence. Recent use of cannabis is associated with acute intoxication and related psychomotor and cognitive alterations and, in some people, with psychotogenic and anxiogenic effects. The duration of acute intoxication effects differ if cannabis is consumed via inhalation (ranging between 10 minutes and 1–3 hours, potentially persisting for up to 8 hours) or orally (ranging between 30 minutes and 4–6 hours, potentially persisting up to 24 hours) [13].
Abstinence can be associated with stress, withdrawal and strong cravings in frequent users. Withdrawal symptoms typically onset between 2 and 3 days, peak after 2 to 6 days and last for 4 to 14 days [14]. This time course is consistent with the reversal of cannabinoid receptor downregulation in PET imaging studies [15].
-
3
‘In the past month, on how many days have you used cannabis?’
(Record n days/month from: never, 0 to 30)
This item is a brief assessment of frequency of cannabis use. It is similar to items with established validity and reliability in quantifying frequency of alcohol use, for example, item 1 of the Alcohol Use Disorder Identification Test [16]: ‘How often do you have a drink containing alcohol?’ with answers including, never; monthly or less; 2 to 4 times a month; 2 to 3 times a week; 4 or more times a week. This item enables a full range of cannabis use frequencies to be reported by the individual, allowing a more fine grained understanding of cannabis exposure, such as how the number of days of cannabis use are associated with problematic use [17].
In line with international monitoring recommendations from the EMCDDA, we classify ‘daily or near‐daily use’ as 20 + days per month (i.e. daily or near daily use) [18]. Use on 20 + days a month is strongly indicative of high risk use because ~32% of daily users meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, (DSM‐5) criteria for cannabis use disorder (CUD) [19]. When this universal item is used, ratings of frequent use (20 + days per month) warrant a more detailed assessment of their cannabis use (setting‐specific measures) and associated health effects.
Methodological considerations and recommended settings for universal measures
The universal use of these two core items will enable rapid and consistent data on cannabis use. It is recommended that item 1 and item 2 are used to understand both frequency and recency of use in surveys, epidemiological, clinical, cognitive and neuroimaging studies and clinical practice. For example, this can distinguish a frequent user who has achieved recent abstinence from a frequent user who continues to use or has recently relapsed. However, item 2 may be used alone in hospital emergency settings where the priority is to ascertain the presence of recent cannabis exposure to determine if symptoms may be because of cannabis‐related intoxication. The use of universal measures in isolation (i.e. without the items of the additional layers of the framework) would provide a somewhat superficial characterization of cannabis exposure. Thus, using the universal measures alone may be less suitable to characterize cannabis use patterns over time in samples with a history of prolonged and chronic use, CUDs and people in treatment for cannabis use.
Mid‐layer: setting‐specific measures
The second layer of the pyramid measures use in specific settings to quantify additional aspects of cannabis use. Setting‐specific measures should be used in addition to the universal items.
Expert‐recommended setting‐specific measures
Setting‐specific measures vary widely as a function of the distinct goals in assessing cannabis use in different contexts (e.g. research, clinical practice, hospitals and schools), the legal status of cannabis in the context of interest and the resources that are available for measurement (e.g. respondents' time, funding, research personnel and training opportunities). Therefore, the choice of setting‐specific measures is determined by the specific reasons why these tools are administered.
We recommend tools that can quantify detailed aspects of cannabis exposure across multiple settings. The Timeline Follow‐Back (TLFB) methodology is a reliable and valid tool to confirm recent cannabis use level (e.g. how often and how much cannabis is used over a set period of time) [20, 21, 22]. The TLFB can be used with varying timeframes, offering flexibility according to the context and question at hand. For example, using the TLFB for the following short‐term periods can provide useful contextual information: past 1 or 2 days to confirm recent use in intervention and epidemiological studies; and past week during CUD treatment or a clinical trial with regular (e.g. weekly) assessments. Such timeframes may be feasible for surveys for small intervention studies or large‐scale epidemiological studies and for longitudinal/cross‐sectional observational studies on general community samples where cannabis use is highly prevalent and may be measured in relation to other tools to track cannabis use‐related adverse psychosocial outcomes. The TLFB can also be used in relation to longer time frames: past month to confirm a detailed history of current levels of cannabis use, past 3 months or past year to confirm a longer history of use in a longitudinal research study [22]. These longer timeframes may prove useful in clinical treatment services, clinical practice, school counselling services and judiciary settings, to decide if a more comprehensive assessment of problems related to cannabis use is needed.
The TLFB can provide a more reliable assessment of frequency of use according to the timeframe specified than a brief item on frequency of use. Furthermore, an additional key item that can be extracted from the TLFB is how much cannabis is used in a typical day when the person is using cannabis. One method of assessing how much cannabis is used is by assessing the quantity based on the cannabis product type (e.g. flower in grams, liquids or tinctures in mL, edibles in mg THC).
Some people who use cannabis may not be able to measure quantity of use in grams [23], so in some cases it may not be possible to estimate standard doses of THC. In these instances, we recommend quantification based on the metric that is most familiar for them (e.g. number of joints, bongs, gummy bears and hits) [23]. Interpretation of these data can be informed by future ecological studies, such as the dose of THC in a standard joint in the location of the study [24, 25].
TLFB information on the amount of cannabis used in a typical consumption day can capture individual variability in cannabis dosage. It is one of the few measures of exposure for studies that do not have the technical and financial capacity to use biological measures. Therefore, cannabis dosage estimated using the TLFB can help differentiate intra‐individual changes in cannabis dosage over time. Quantitation of daily use is also an important outcome metric in clinical studies for individuals whose treatment goal may be a reduction in use rather than abstinence [26].
The TLFB can collect information on cannabis other than the frequency and amount used. This will vary according to context and may include, but is not limited to, co‐administration of cannabis with alcohol and also co‐administration with tobacco, which is particularly prevalent in Europe [23] and is crucial for understanding the health effects of cannabis use.
More comprehensive assessments of cannabis exposure that require 30 + minutes for their administration can be used in settings where there is time for more extended testing. These include semi‐structured interviews that detailed lifetime cannabis use history, based on the TLFB. For example, the Cannabis Use History, Semi‐Structured Interview [27], provides comprehensive information on cannabis use history, such as: age of first use onset, age of regular use onset, periods of abstinence and their duration, variation over time in the level of cannabis dosage and how often cannabis has been used, thereby allowing to measure cumulative and/or average dosage and frequency over the lifetime and/or over selected periods of time (e.g. past year). These tools provide a comprehensive characterisation of the amount and frequency of cannabis use where there is sufficient time and resources for training and administration. Alternatively, shorter measures include estimates of frequency and potency of cannabis use from the Cannabis Experiences Questionnaire [28], which have demonstrated associations with mental health outcomes such as psychosis [5, 6]. Setting‐specific measures could differ considerably as a function of context‐related needs and therefore, hinder direct integration of their outcome measures.
Top layer: biological measures
Biological measures of cannabinoid consumption are at the top of the pyramid because they represent the objective gold standard by quantifying levels of distinct cannabinoids in biological matrices.
Expert‐recommended biological measures
The expert group recommends the use of biological measures of cannabinoids that are robustly associated with clinical outcomes relevant to problematic use (e.g. sustained abstinence, withdrawal and CUD severity), including the quantity of THC‐COOH in urine [17].
First, the presence of THC‐COOH in urine and of THC in saliva are relatively easy and inexpensive to measure using qualitative testing kits (e.g. testing strips). However, these tests typically report a binary outcome—cannabinoid present/absence—based on predefined cut‐offs (typically 50 ng/mL for THC‐COOH in urine). Qualitative tests cannot measure the level of THC or its metabolites. These measures are recommended for clinical treatment services and the judicial system as ways of confirming cannabis consumption (note that it may take a heavy user anywhere from 2 to 8 weeks for an initial negative test after quitting) in cannabis users trying to quit, or can identify individuals as having recently used cannabis. They can also be used in hospital emergency settings to assess whether symptoms may be related to THC intake.
Second, the quantitative level of THC‐COOH in urine can be obtained to corroborate self‐reported continuous abstinence [29]. It also predicts withdrawal symptoms [30], CUD severity, tolerance to the acute effects of cannabis [17] and level of cognitive impairment [31]. The level of THC‐COOH in urine can also be used to confirm regular cannabis use status in chronic users, where residual circulating levels of cannabinoids from chronic use can be detected up to 4 weeks after last use [32]. Therefore, the level of THC‐COOH in urine is recommended for use in experimental, neuroimaging and cognitive studies as a way of corroborating current cannabis use or abstinence. It can also be used to examine the quantity of THC exposure such as in clinical trials of CUD [33]. It cannot sensitively measure the duration of abstinence because of the long half‐life of THC and individual differences in metabolism. Its major limitations are that it requires access and costs of laboratory facilities and analyses, it requires multiple samples and creatinine normalisation for reliable testing and does not offer an immediate test result, unlike rapid, qualitative testing. Therefore, it is particularly well suited to research settings where detailed assessment of cannabis exposure is required. Often, drug testing programs will integrate the use of both qualitative and quantitative urine THC‐COOH testing.
Third, the quantitative level of THC in saliva is a proxy of recent cannabis exposure that has been associated with cognitive function and driving performance [34]. Limitations of this measure include uncertainty of time of last exposure and risk of contamination of the buccal area (e.g. food). It may be used in experimental, neuroimaging and cognitive studies investigating how recent cannabis use affects acute drug effects, brain function and cognition.
Fourth, THC, THC‐COOH or 11‐OH‐THC in plasma provide precise and reliable quantitation of THC consumption and metabolism. Measuring these involves taking a blood sample and quantifying THC or its metabolites either in that sample, or using a centrifuge to extract plasma for analysis. As a more invasive measure, it may reduce compliance levels. It is recommended for use in settings where rigorous checking of THC and other cannabinoid levels is required. THC in plasma can be used to confirm recent exposure to THC in experimental studies with acute cannabinoid administration. Interpretation of results showing THC levels in blood can be more complicated in sober chronic users and people exposed to environmental cannabis smoke.
Last, the level of THC and other chemicals (e.g. cannabinoids such as CBD, cannabichromene [CBC], tetrahydrocannabivarin [THCV], CBG or terpenoids such as pinene, myrcene and limonene) can be measured in cannabis products supplied by users to determine what they have been exposed to [35, 36]. Such information is useful for correlating the presence and concentration of THC and other chemical entities with outcomes of interest such as product abuse liability, risk for CUD or for specific adverse events, and broader public health‐related outcomes. When combined with information on the quantity of grams consumed, this type of testing provides a more precise assessment of standard THC units [2].
The use of biological measures of cannabinoids, other than testing kit strips, is often not viable because they are expensive and/or not easy to implement. Biological measures of cannabinoids require: the purchase of equipment to collect samples and conduct toxicological analyses, specialised/secure facilities and equipment for storage (e.g. lockable freezers), courier and laboratory analyses, individuals trained to collect and process samples as well as licenses required for measuring chemical compounds in cannabis itself.
AREAS OF FUTURE WORK
The measurement of cannabis use will need to be refined. First, challenges need to be addressed in quantifying cannabis use, as forms of cannabis use are increasingly diversifying. Specifically, there is a need for validation of improved TLFB procedures to quantify cannabis dosage. Limitations of TLFB quantities at present (e.g. grams or number of joints) do not capture the wide range of cannabis products available in all world regions (e.g. cannabis edibles, drinks, concentrates or vaping liquids).
In the future, visual aids might be used to identify specific cannabis products and their weight or volume along with the dose of THC they contain. To aid validation of this approach, we have included an item to facilitate research in the iCannToolkit (see sample item 4 in Supporting Information). By combining information on product weight and potency (which can be difficult to self‐report) [37], a standardised dose of THC can also be estimated (5 mg = 1 standard THC unit) [2] using the iCannToolkit, in keeping with recommendations by the National Institute of Drug Abuse and other research institutes (e.g. National Cancer Institute, https://www.drugabuse.gov/about-nida/noras-blog/2021/05/establishing-5mg-thc-standard-unit-research). A benefit of recording standardised doses of THC is that they can be applied to all cannabis products (including edible products and drinks), which cannot be estimated in terms of grams of cannabis or other methods such as the number of joints consumed [2].
Second, the universal measures may benefit from a graduated frequency approach to increase feasibility for national surveys. Third, from an epidemiological perspective, a denominator of identifying whether an individual was ever a daily/near daily user may aid estimates of quit ratios, and long‐term indicators of uptake. Further research will inform cut‐offs of frequency of use (e.g. 20 + days as recommended to European Monitoring Centre for Drugs and Drug Addiction [EMCDDA]) that are more‐fine grained and evidence‐based according to risk of CUD or other outcomes.
Third, the iCannToolkit measures cannabis exposure across multiple settings (e.g. research, clinical practice, public health and medicinal). Its administration is not sufficient for diagnosing CUD or for measuring the consequences associated with cannabis exposure. Therefore, the identification of risky use could be achieved by the concurrent use of the iCannToolkit and other measures of cannabis use related risks—including physical problems (e.g. poor respiratory function), mental health problems (e.g. psychosis, anxiety and depression) and risky behaviours (e.g. driving or operating machinery while intoxicated).
Finally, in jurisdictions where cannabis use is legal, new opportunities (and challenges) may arise and become important for cannabis use quantification. These may include a greater standardisation of products, improved product labelling requirements with respect to dose, proactive market monitoring by producers or an independent body and more detailed population‐level monitoring (e.g. waste water testing, regulators' seed‐to‐sale market tracking data and cannabis loyalty‐card data akin to monitoring eating habits by accessing grocery store loyalty card data sets).
CONCLUSIONS
In conclusion, the expert group provides a consensus‐based framework with guidance and structure with the aim of harmonising methodology for the quantification of cannabis use, while permitting flexibility according to context, cost and ease of implementation. The iCannToolkit items can be used in a variety of settings to measure cannabis exposure and related consequences. The use of this framework has the potential to strengthen the integration of international evidence in ways that could have multiple benefits: identifying how much cannabis use constitutes ‘risky use’, informing the development of guidelines for harm reduction, educating consumers about the consequences of their cannabis use and enabling clinicians to monitor treatment outcome and relapse in disorders that may be caused or treated by cannabis‐based products. Although additional work is required to better refine these assessments and to determine whether and how to integrate alternative products (e.g. those containing chemicals other than THC or that use a novel method of administration), this can be accomplished within the proposed framework.
DECLARATION OF INTERESTS
A.G. received funding from Novartis for work outside this area (a phase III cocaine trial). A.E. has received speaker honorarium from GW Pharmaceuticals. A.W. is the founder of the Global Drug Survey. C.H. became a full‐time employee of GW Pharmaceuticals after the consensus meeting. D.H. has served as a paid expert witness on behalf of public health authorities in Canada in response to legal challenges from the cannabis industry. H.L.P. has received honoraria and travel grants from Janssen and Lundbeck. J.B. has received unrestricted research funding to study smoking cessation from companies who manufacture smoking cessation medications (Pfizer and J&J). R.V. receives consulting fees for Canopy Health Innovations and Syqe Medical, and is on the Scientific Advisory Board for MyMD Pharmaceuticals and Artiam Bio. H.V.C. has consulted for Janssen. V.L., H.V.C, W.H., T.P.F., E.W., T.G., W.L., A.C.C., J.P.C., R.L.P., M.v.L., K.P., P.G., M.A.E., S.H.G., J.M. and C.M. have no competing interests to declare.
AUTHOR CONTRIBUTIONS
Valentina Lorenzetti: Conceptualization; funding acquisition; methodology; project administration; resources. Chandni Hindocha: Conceptualization; formal analysis; methodology; project administration. Kat Petrilli: Methodology. Paul Griffiths: Formal analysis; funding acquisition; methodology; resources. Jamie Brown: Formal analysis; methodology. Álvaro Castillo‐Carniglia: Formal analysis; methodology. Jonathan Caulkins: Formal analysis; methodology. Amir Englund: Formal analysis; methodology. Mahamoud El Sohly: Formal analysis; methodology. Suzanne Gage: Formal analysis; methodology. Teodora Groshkova: Formal analysis; methodology. Antoni Gual: Formal analysis; methodology. David Hammond: Formal analysis; methodology. Will Lawn: Formal analysis; methodology. Hugo López‐Pelayo: Formal analysis; methodology. Jakob Manthey: Formal analysis; methodology. Claire Mokrysz: Formal analysis; methodology. Rosalie Liccardo Pacula: Formal analysis; methodology. Margriet van Laar: Formal analysis; methodology. Ryan Vandrey: Formal analysis; methodology. Elle Wadsworth: Formal analysis; methodology. Adam Winstock: Formal analysis; methodology. Wayne Hall: Conceptualization; formal analysis; methodology. H Valerie Curran: Conceptualization; formal analysis; methodology. Tom Freeman: Conceptualization; funding acquisition; methodology; project administration; resources.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
The authors received funding from the Society for the Study of Addiction, European Monitoring Centre for Drugs and Drug Addiction. H.L.P. works under the CERCA Programme/Generalitat de Catalunya and receives funding from the Spanish Ministry of Science, Innovation and Universities, Instituto de Salud Carlos III through a ‘Juan Rodes’ contract (JR19/00025).
Lorenzetti V, Hindocha C, Petrilli K, Griffiths P, Brown J, Castillo‐Carniglia Á, et al. The International Cannabis Toolkit (iCannToolkit): a multidisciplinary expert consensus on minimum standards for measuring cannabis use. Addiction. 2022;117:1510–1517. 10.1111/add.15702
Funding information The European Monitoring Centre for Drugs and Drug Addiction; Instituto de Salud Carlos III; Ministry of Science, Innovation and Universities; Generalitat de Catalunya; Society for the Study of Addiction
REFERENCES
- 1. Solowij N, Lorenzetti V, Yücel M. Effects of cannabis use on human behavior: A call for standardization of cannabis use metrics. JAMA Psychiat. 2016;73:995–6. [DOI] [PubMed] [Google Scholar]
- 2. Freeman TP, Lorenzetti V. ‘Standard THC units’: A proposal to standardize dose across all cannabis products and methods of administration. Addiction. 2019;115:1207–1216. [DOI] [PubMed] [Google Scholar]
- 3. Freeman T, Winstock A. Examining the profile of high‐potency cannabis and its association with severity of cannabis dependence. Psychol Med. 2015;45:3181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hines LA, Freeman TP, Gage SH, Zammit S, Hickman M, Cannon M, et al. Association of high‐potency cannabis use with mental health and substance use in adolescence. JAMA Psychiat. 2020;77:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in South London with first‐episode psychosis attributable to use of high potency cannabis: A case‐control study. Lancet Psychiatry. 2015;2:233–8. [DOI] [PubMed] [Google Scholar]
- 6. Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer‐Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU‐GEI): A multicentre case‐control study. The Lancet Psychiat. 2019;6:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan GC, Hall W. Estimation of the proportion of population cannabis consumption in Australia that is accounted for by daily users using Monte Carlo simulation. Addiction. 2020;115:1182–6. [DOI] [PubMed] [Google Scholar]
- 8. Freeman TP, Craft S, Wilson J, Stylianou S, ElSohly M, Di Forti M, et al. Changes in delta‐9‐tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: Systematic review and meta‐analysis. Addiction. 2020;116:1000–1010. [DOI] [PubMed] [Google Scholar]
- 9. Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G. Variation in cannabis potency and prices in a newly legal market: Evidence from 30 million cannabis sales in Washington state. Addiction. 2017;112:2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Temple EC, Brown RF, Hine DW. The 'grass ceiling': Limitations in the literature hinder our understanding of cannabis use and its consequences. Addiction. 2011;106:238–244. [DOI] [PubMed] [Google Scholar]
- 11. Hoch E, Lorenzetti V. Mapping and mitigating the health risks of legalizing recreational cannabis use: A call for synergy between research and policy. World Psychiatry. 2020;19:189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzetti V, Solowij N, Yücel M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. 2016;79:e17–31. [DOI] [PubMed] [Google Scholar]
- 13. Grabenauer M. Differences in Cannabis Impairment and its Measurement Due to Route of Administration. 2020. [Google Scholar]
- 14. Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. [DOI] [PubMed] [Google Scholar]
- 15. D'Souza DC, Cortes‐Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimag. 2016;1:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohn MJ, Babor TF, Kranzler HR. The alcohol use disorders identification test (AUDIT): Validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–32. [DOI] [PubMed] [Google Scholar]
- 17. Curran HV, Hindocha C, Morgan CJ, Shaban N, Das RK, Freeman TP. Which biological and self‐report measures of cannabis use predict cannabis dependency and acute psychotic‐like effects? Psychol Med. 2019;49:1574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . Characteristics of frequent and high‐risk cannabis users. 2015.
- 19. Santaella‐Tenorio J, Levy NS, Segura LE, Mauro PM, Martins SS. Cannabis use disorder among people using cannabis daily/almost daily in the United States, 2002–2016. Drug Alcohol Depend. 2019;205:107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sobell LC, Sobell MB. Timeline Follow‐Back: A technique for assessing self‐reported alcohol consumption. Totowa NJ: Humana Press; 1992. [Google Scholar]
- 21. Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow‐back for self‐reported use of cannabis and other illicit substances—Systematic review and meta‐analysis. Addict Behav. 2012;37:225–33. [DOI] [PubMed] [Google Scholar]
- 22. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. [DOI] [PubMed] [Google Scholar]
- 23. Hindocha CFTP, Ferris JA, Lynskey MT, Winstock AR, Hindocha C, Freeman TP, et al. No smoke without tobacco: A global overview of cannabis and tobacco routes of administration and their association with intention to quit. Front Psych. 2016;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casajuana C, López‐Pelayo H, Balcells MM, Miquel L, Teixidó L, Colom J, et al. Working on a standard joint unit: A pilot test. Adicciones. 2017a;29:227–232. [DOI] [PubMed] [Google Scholar]
- 25. Casajuana KC, Balcells‐Olivero MM, López‐Pelayo H, Miquel L, Teixidó L, Colom J, et al. The standard joint unit. Drug Alcohol Depend. 2017b;176:109–16. [DOI] [PubMed] [Google Scholar]
- 26. American Psychiatric Association . DSM‐V Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth Edition. 2013.
- 27. Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: An updated review of the literature. Curr Pharm des. 2014;20:2138–67. [DOI] [PubMed] [Google Scholar]
- 28. Barkus EJ, Stirling J, Hopkins RS, Lewis S. Cannabis‐induced psychosis‐like experiences are associated with high schizotypy. Psychopathology. 2006;39:175–8. [DOI] [PubMed] [Google Scholar]
- 29. Schuster R, Hoeppner S, Evins A, Gilman J. Early Onset Marijuana Use Is Associated With Learning Inefficiencies. 2016. [DOI] [PMC free article] [PubMed]
- 30. Claus BB, Specka M, McAnally H, Scherbaum N, Schifano F, Bonnet U. Is the urine cannabinoid level measured via a commercial point‐of‐care Semiquantitative immunoassay a cannabis withdrawal syndrome severity predictor? Front Psych. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long‐term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S‐H, Huestis MA. Urinary elimination of 11‐nor‐9‐carboxy‐delta9‐tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loflin MJ, Kiluk BD, Huestis MA, Aklin WM, Budney AJ, Carroll KM, et al. The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda. Drug Alcohol Depend. 2020;107993. 212:107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramaekers JG, Moeller M, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9‐THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend. 2006;85:114–22. [DOI] [PubMed] [Google Scholar]
- 35. Freeman TP, Morgan CJ, Hindocha C, Schafer G, Das RK, Curran HV. Just say ‘know’: How do cannabinoid concentrations influence users' estimates of cannabis potency and the amount they roll in joints? Addiction. 2014;109:1686–94. [DOI] [PubMed] [Google Scholar]
- 36. van der Pol P, Liebregts N, Brunt T, van Amsterdam J, de Graaf R, Korf DJ, et al. Cross‐sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: An ecological study. Addiction. 2014;109:1101–9. [DOI] [PubMed] [Google Scholar]
- 37. Hammond D, Goodman S. Knowledge of Tetrahydrocannabinol and Cannabidiol Levels Among Cannabis Consumers in the United States and Canada, Cannabis and Cannabinoid Research. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


