Abstract
Ehlers‐Danlos syndromes (EDS) are a group of inherited connective tissue disorders. Patients with EDS exhibit distinct pathologies of the teeth and the oral cavity. Here, we summarize the current knowledge in the various EDS types, in particular regarding severe changes in oral health‐related quality of life, the differential emergence of periodontitis, characteristic yet highly cumbersome dental manifestations, apparent anomalies of oral soft tissues, and relevant issues related to dental implantology. Resolution of remaining open questions will primarily rely on the standardization of diagnostic criteria. Clinical centers that specialize on this rare pathology need to apply congruent approaches for exact characterization of clinical features in conjunction with genetic validation that should be reached without exception in all patients and relevant family members.
Keywords: Ehlers‐Danlos, hypermobility, periodontitis, pulp stones, root anomalies
1. INTRODUCTION
A healthy mouth facilitates painless feeding, eases chewing, and enables articulated speech. It thus contributes largely to personal well‐being and socializing, yet only when being devoid of discomfort or embarrassment. Oral diseases and conditions such as dental caries, periodontitis, and malocclusion can cause pain and may ultimately lead to loss of oral function. In severe cases, happiness and social well‐being are greatly compromised (Tuchtenhagen, Ortiz, Ardenghi, & Antunes, 2021; Yoon et al., 2013).
Individuals diagnosed with one of the Ehlers‐Danlos syndromes (EDS) often report reduced oral health‐related quality of life due to physical pain, psychologic discomfort, and other disabling concerns (Berglund & Bjorck, 2012; Hanisch et al., 2020). Many individuals with EDS are seen by a number of different healthcare professionals including geneticists, physicians, and allied health professionals, but enquiry about and monitoring of oral health problems is not typically a part of assessment, the firm belief that this is the sole duty of the dentist (Jacobson et al., 2020). Regrettably, many dentists—even when specialized or working at university centers—lack knowledge of individual genetic diseases manifesting in the oral and maxillofacial region (Kuhne, Kleinheinz, Jackowski, Koppe, & Hanisch, 2020). Also, it is difficult to distinguish specific EDS‐associated oral manifestations from common pathologies such as plaque‐induced caries and general periodontitis.
The aim of this review is to summarize the current knowledge on EDS‐specific oral manifestations. It further aims to inform nondental and dental health care providers seeing EDS patients of previous findings and open questions in the field.
2. THE EHLERS‐DANLOS SYNDROMES
The EDS are a group of inherited connective tissue disorders characterized by joint hypermobility, skin hyperextensibility, and variable tissue fragility. The current EDS classification distinguishes 13 different EDS types with different clinical manifestations and pathogenetic mechanisms (Malfait et al., 2017). Most EDS types except hypermobile EDS (hEDs) are inherited as monogenic traits, mostly caused by mutations in genes that encode distinct types of collagen or collagen‐modifying enzymes.
2.1. Collagen and the biology of dental tissues
Enamel forms the outer layer of the tooth crown. It is first formed from an epithelium, which then mineralizes, thus consisting in normal cases of approximately 96% hydroxyapatite crystals (Figure 1). It contains low amounts of Collagen type I and VII, which notably are concentrated close to the dentino‐enamel junction. Collagen type VII is believed to provide a strong bonding between enamel and dentine (McGuire, Walker, Mousa, Wang, & Gorski, 2014). Dentine forms the bulk of the tooth and is composed of 70% hydroxyapatite crystals embedded in a three‐dimensional collagenous network. It is primarily made up of Type I collagen containing traces of Type III and V collagen and is further associated with noncollagenous proteins and proteoglycans. In stark contrast to that, the dental pulp exhibits only loose connective tissue, and it is highly innervated. Type I, III, and V collagen represent 56%, 41%, and 2% of the total collagen, respectively (Tsuzaki, Yamauchi, & Mechanic, 1990).
FIGURE 1.
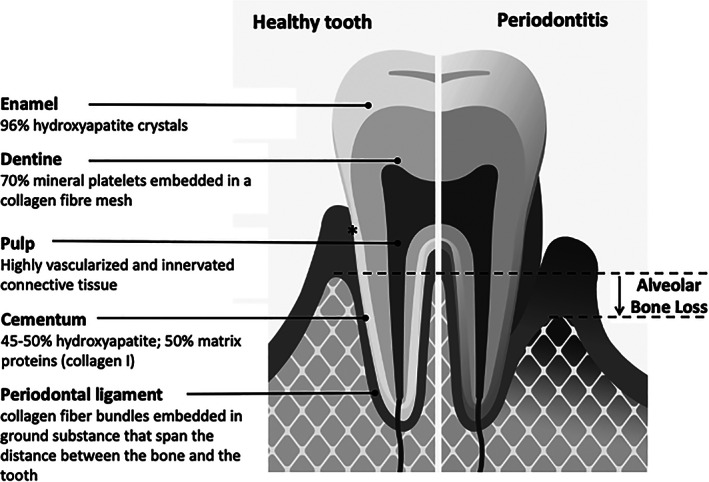
Dental health and periodontal disease. The tooth is composed of enamel, dentine, cementum, and the pulp. In healthy individuals, the alveolar bone ends approximately 1 mm beyond the cemento‐enamel junction (marked by an asterisk). Periodontitis is characterized by progressive destruction of the tooth‐supporting tissues (alveolar bone, periodontal ligament, root cementum, and gingival attachment)
The periodontal ligament is a highly specialized connective tissue spanning the distance between the alveolar bone and the tooth root. It is made up of collagen fiber bundles embedded in ground substance, predominantly spinning together Collagen types I, III, and XII (Nanci, 2008). The root cementum tightly links the collagen‐fiber bundles to the root. Root cementum consists of approximately 50% inorganic hydroxyapatite and an organic matrix that is mainly composed of Collagen type I with traces of Type III and XII and noncollageneous proteins including several proteoglycans. The side of the gingiva that faces the tooth provides the epithelial and connective tissue attachment. It thereby seals the oral cavity from the underlying structures.
2.2. Periodontitis is a specific feature of periodontal EDS but not of other EDS types
Periodontitis is an inflammatory disease of the tooth‐supporting tissues (Figure 1). Thereby, alveolar bone, periodontal ligament, gingiva, and root cementum are continuously destroyed, ultimately leading to edentulism. Periodontitis is a highly prevalent disease, with an estimated prevalence in the general population of up to 83%, depending on age and severity (Demmer, Papapanou, Jacobs, & Desvarieux, 2010). Of note, severe periodontitis is found in 8% of adults aged 35–45 years; the prevalence increasing to 20% in people aged 65–75 years (Jordan et al., 2014).
There are still unanswered questions in the field. A particularly important one is whether individuals with any of the distinct types of EDS exhibit a higher risk of periodontal disease (Abel & Carrasco, 2006; Jepsen et al., 2018). Currently, there is no reliable evidence for an increased periodontitis risk associated with EDS, with the exception of periodontal EDS (pEDS) (Kapferer‐Seebacher, Lundberg, Malfait, & Zschocke, 2017). In this EDS type, periodontitis is not only a defining feature but also shows an early onset and rapid progression in adolescents and young adults. This has been well documented in almost all affected individuals (Kapferer‐Seebacher et al., 2016; Malfait et al., 2017). Onset of periodontal destruction was reported at a mean age of 14 years (Kapferer‐Seebacher et al., 2016). Without periodontal treatment, complete tooth loss occurred at a mean age of 20 years in previously identified individuals (range 14–48 years) (Kapferer‐Seebacher et al., 2017).
Two recent pedigree analyses confirm that severe early onset periodontitis is a distinguishing feature between pEDS and classical EDS (cEDS) or vascular EDS (vEDS). In one case, a child with cEDS displayed periodontal bone loss in the primary dentition and a gingival phenotype specific for pEDS (Stock et al., 2021). The genetic analysis elucidated two pathogenic mutations, a COL5A1 mutation causative of cEDS together with a de novo C1R mutation causative of pEDS. The same was observed in a case of vEDS accompanied by severe periodontitis when two pathogenic variants were detected in COL3A1 (causative of vEDS) and C1R, respectively (Wu et al., 2018). These cases illustrate that clinical studies in larger cohorts are needed to better understand the still unclear spectra of symptoms, and the importance of genetic validation to provide clear evidence regarding the clinical manifestations of different EDS types. To date, with the exception of the cases above, not a single individual with genetically validated vEDS or cEDS with severe early periodontitis has been reported. Two independent prospective case–control studies with a total of 23 vEDS patients actually revealed a decreased prevalence of periodontitis in vEDS (De Coster, Martens, & De Paepe, 2005; Ferré et al., 2012).
Another recent case report of an individual with a phenotypic overlap of periodontal and vEDS also demonstrates the importance of taking a stringent dental history (Pitney & Pitney, 2020). The affected individual reported severe dental problems with bleeding gums, gingival recession, and early loss of teeth. Tooth loss, however, was due to plaque‐induced caries and dental fractures, and there was no evidence of periodontitis. In this case, subsequent genetic analysis unambiguously confirmed the diagnosis of vEDS.
2.3. There is limited evidence for relevant dental anomalies in different EDS types
Various dental problems including an increased risk of dental caries and tooth fractures are claimed to be associated with EDS. However, these statements are mostly based on single‐case reports or personal recollections of affected individuals. Hence, the true prevalence and clinical relevance are unknown. A recent study investigated oro‐dental manifestations of hypermobile EDS (hEDS) compared to healthy controls (Honore, Lauridsen, & Sonnesen, 2019). The researchers found no statistically significant differences between hEDS and healthy controls with regard to the prevalence of dental caries, tooth fractures, number of teeth, enamel defects, mucosal abnormalities, crown or root morphology, or pulp calcifications. However, they found significantly higher plaque indices in hypermobile EDS, which may subsequently cause an increased caries prevalence.
Two case–control studies evaluated oral manifestations in a total of 23 individuals with vEDS and 96 healthy controls (De Coster et al., 2005; Ferré et al., 2012). Most frequent dental abnormalities observed in vEDS were pulp shape modifications (75%) and root abnormalities such as root fusion (50%) or excessive root length (69%) (Ferré et al., 2012), all of representing biological variation rather than pathology. There were no significant differences with regard to tooth number or other dental manifestations such as the prevalence of caries or the number of tooth fractures.
Classical EDS, however, may be associated with abnormal dentinogenesis that results in localized root anomalies. Shortened or bulbous roots appear to be specific features of this condition (Hakki, Aktas, Alanay, Avunduk, & Hakki, 2017; Pope et al., 1992), and subsequent loosening of the teeth may be misinterpreted as localized periodontitis. Calcification of the pulp, that is, pulp stones or obliteration is another common finding in cEDS (Kapferer‐Seebacher, Schnabl, Zschocke, & Pope, 2020) (Figure 2). This is of limited clinical relevance as it does not usually cause pulp disease or individual symptoms (Sener, Cobankara, & Akgunlu, 2009). However, pulp calcification may complicate root canal treatments as it may block access to canal orifices and alter the internal anatomy (Goga, Chandler, & Oginni, 2008).
FIGURE 2.
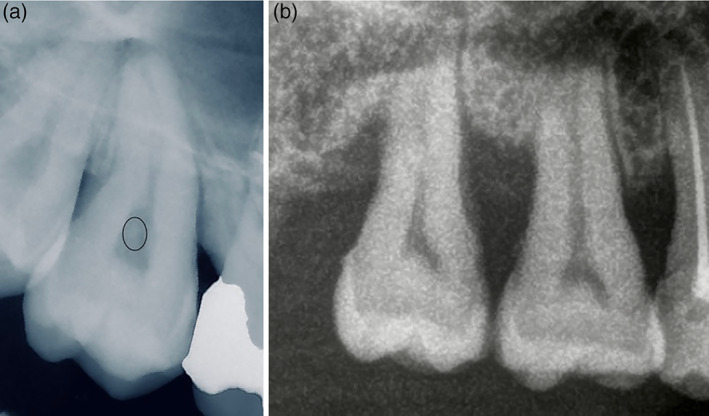
Dental abnormalities. (a) Pulp stones are calcifications of the pulp (marked with a circle) and usually do not cause pulp disease or subjective symptoms. (b) Maxillary molars usually have three roots. Molars presenting with root fusion have only one root
Dentinogenesis imperfecta (DI) has been reported in osteogenesis imperfecta/EDS overlap syndrome and was also described in single individuals with arthrochalasia (aEDS) and spondylodysplastic EDS (spEDS) (Budsamongkol et al., 2019; Nicholls et al., 2000; Shi et al., 2015; Van Damme et al., 2018). Affected children presented clinically with abraded teeth exposing dentin and pulp due to reduced hardness and elasticity of enamel and dentin (Budsamongkol et al., 2019). Dental radiographs exhibit large pulp caves, thin enamel with reduced radiopacity, and curved roots. DI represents the dental manifestation of osteogenesis imperfecta, that is, collagen 1 deficiency/defects, and considering the clinical overlaps, it is not surprising that DI is also observed in EDS types linked to specific mutations in the collagen 1 genes.
Crown malformations and tooth discolorations pointing to enamel irregularities were reported in single individuals with kyphoscoliotic (kEDS), dermatosparactic (dEDS), and aEDS (Arun, Nalbantgil, & Sayınsu, 2006; Malfait et al., 2004; Ooshima, Abe, Kohno, Izumitani, & Sobue, 1990). Tooth rotation or transposition and abnormalities in tooth number reported in single individuals of various EDS types may, however, be a coincidental finding (Kapferer‐Seebacher et al., 2020).
2.4. Some EDS types have specific oral soft tissue manifestations
One of the earliest reports on oral soft tissue aberrations in 11 patients with EDS highlighted gingival fragility and high translucency (Barabas, 1967). Nearly 50 years later, a particular gingival phenotype with generalized thinness and translucency of the gingiva was described for vEDS (Ferré et al., 2012). Lack of attached gingiva leading to severe gingival recession and tissue fragility has been identified as a pathognomonic feature of pEDS (Kapferer‐Seebacher et al., 2021) (Figure 3).
FIGURE 3.
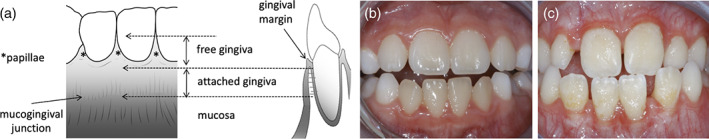
Specific gingival phenotype with periodontal Ehlers‐Danlos syndrome. (a,b) The gingiva and the mucosa are the lining epithelia of the oral cavity. The gingiva is keratinized and thicker than the mucosa to provide mechanical protection. It is subdivided into the nonattached free gingiva, which includes the gingival margin and the papillae, and the attached gingiva. The attached gingiva is tightly and unmovable bound to the periosteum via Collagen type I fibers. The border between attached gingiva and mucosa constitutes the mucogingival junction. In vivo, it is sometimes visible as a white line. The oral mucosa is only loosely connected to the periosteum, it is moveable, thin, more fragile, translucent and blood vessels are visible. (c) Lack of attached gingiva, which is pathognomonic for periodontal EDS, is recognized by thin and translucent gums with increased vascular visibility and leading to increased gum fragility
Severe and generalized gingival hyperplasia has been described in children with dEDS. In these cases, gingival enlargement was nodular, fragile, and inflamed (De Coster, Malfait, Martens, & De Paepe, 2003; Malfait et al., 2004). Interestingly, gingival descriptions for other EDS types are missing. However, easy wounding with oral appliances, tearing of the gingiva while suturing and incidental injury occurring during dental treatment have been reported without specification for a specific EDS type (Abel & Carrasco, 2006; Mitakides & Tinkle, 2017).
Absence or hypoplasia of lingual and/or inferior labial frenula has been documented in 90% of EDS cases compared to 1.8% in controls and appears to be a characteristic marker of at least classical and hypermobile EDS (De Felice, Toti, Di Maggio, Parrini, & Bagnoli, 2001; Savasta et al., 2019).
2.5. Local anesthetics seem to be less effective
The adequacy of pain prevention during dental procedures through application of local anesthetics has been evaluated in two studies (Hakim, Grahame, Norris, & Hopper, 2005; Schubart et al., 2019). In the latter and larger study, EDS patients reported nearly three times more often a nonresponse of local anesthetics compared to unaffected individuals, suggesting that local anesthetics are less effective during dental procedures. This finding was supported by a subsequent questionnaire‐based analysis among patients with hEDS showing lower efficacy of dental anesthetics (Honore et al., 2019). The agent with the highest reported success rate in EDS patients was articaine (Schubart et al., 2019), but there is currently no evidence that would allow recommendations for or against particular drugs. In addition, observations in one type of EDS cannot be assumed to apply to others.
2.6. Dental implants are problematic in periodontal EDS
There are no clinically controlled studies on dental implants in EDS. A case series on dental implant success in five female patients with hEDS or cEDS revealed the absence of implant pathology in all individuals after 12–12 years (Jensen & Storhaug, 2012). There is no evidence that would point toward reduced implant success in most EDS types, with the important exception of pEDS. Severe and rapidly progressing peri‐implant disease was shown in a case series of three individuals with pEDS, including a case with explantation as early as 5 years after implantation (Rinner et al., 2018). Clinical studies on implant success and failure in different EDS types are needed.
2.7. Oral health‐related quality of life is reduced in people with EDS patients
In a recent study, physical oral health was clinically determined in a series of patients with EDS. In parallel, the patients provided self‐reports on quality of life (QOL) related to oral health (Oelerich, Kleinheinz, Reissmann, Koppe, & Hanisch, 2020). The results showed that the objectively measured oral health of participants with EDS often appeared good even if the participants reported low oral health‐related QOL. Important factors that contributed to reduced self‐reported oral health‐related QOL were physical pain in the mouth area and oral dysfunction—mainly due to malocclusion caused by skeletal and/or dentoalevolar dysgnathia, temporomandibular joint disorder, anxiety, and a feeling of insecurity with respect to teeth, dentures, or mouth (Berglund & Bjorck, 2012; Hanisch et al., 2020). There is a paucity of studies on individual QOL regarding oral health in individuals with EDS and most other rare diseases.
2.8. Available evidence is limited
For several EDS types, systematic clinical oral investigations are missing or incomplete. In most studies, the number of investigated individuals is small, not least because EDS in general is rare, and types and subtypes do not come as large local clusters. Also, EDS may be undiagnosed in many individuals, and often a clinical diagnosis is genetically unconfirmed. Exact molecular data are essential as different types and locations in the same gene may have very different functional effects and clinical consequences. This is clearly illustrated, for example, with collagen 1 mutations that can cause a wide range of phenotypes. Single‐case reports on oral manifestations lacking exact clinical/genetic diagnosis of specific EDS types and without proof of specific pathogenesis are not only useless but more than that, they strengthen myths and unsettle patients and dentists.
3. CONCLUSION
In view of the rarity of most types of EDS, and their diverse oral pathologies and oral health‐related conditions, only multicenter studies will allow the field to move forward. Studies with cross‐sectional or longitudinal design with sufficiently large numbers of individuals with genetically defined EDS types will need to build on systematic dental examination and radiographic analysis in order to provide standards for oral health‐related conditions in EDS, as EDS comprises a range of different clinical features and pathogenetic mechanisms. With the exception of well‐defined hEDS based on internationally accepted clinical criteria (Malfait et al., 2017), case reports/series of other types of EDS should always include description of the genetic variant(s) of the individual(s) being described.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by a grant from the EDS Society (Grant Number 2019.02.PRO05).
Lepperdinger, U. , Zschocke, J. , & Kapferer‐Seebacher, I. (2021). Oral manifestations of Ehlers‐Danlos syndromes. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 187C:520–526. 10.1002/ajmg.c.31941
This article is an extension of either a plenary guest speaker lecture or an abstract presented at the EDS ECHO Conference on the Ehlers Danlos Syndromes held October 2–3, 2020. The Editors‐in‐Chief of this journal affirm that this article was evaluated editorially and rigorously edited by the expert Guest Editors for this issue, Dr. Hakim, Dr. Francomano and Dr. Tinkle, and was not anonymously peer reviewed.
Funding information EDS Society, Grant/Award Number: 2019.02.PRO05
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abel, M. D. , & Carrasco, L. R. (2006). Ehlers‐Danlos syndrome: Classifications, oral manifestations, and dental considerations. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 102(5), 582–590. 10.1016/j.tripleo.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Arun, T. , Nalbantgil, D. , & Sayınsu, K. (2006). Orthodontic treatment protocol of Ehlers‐Danlos syndrome type VI. The Angle Orthodontist, 76(1), 7. [DOI] [PubMed] [Google Scholar]
- Barabas, G. M. (1967). The oral manifestations of Ehlers‐Danlos syndrome. (Master of dental surgery). University of Manchester
- Berglund, B. , & Bjorck, E. (2012). Women with Ehlers‐Danlos syndrome experience low oral health‐related quality of life. Journal of Orofacial Pain, 26(4), 307–314. [PubMed] [Google Scholar]
- Budsamongkol, T. , Intarak, N. , Theerapanon, T. , Yodsanga, S. , Porntaveetus, T. , & Shotelersuk, V. (2019). A novel mutation in COL1A2 leads to osteogenesis imperfecta/Ehlers‐Danlos overlap syndrome with brachydactyly. Genes & Diseases, 6(2), 138–146. 10.1016/j.gendis.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster, P. J. , Malfait, F. , Martens, L. C. , & De Paepe, A. (2003). Unusual oral findings in dermatosparaxis (Ehlers‐Danlos syndrome type VIIC). Journal of Oral Pathology & Medicine, 32(9), 568–570. [DOI] [PubMed] [Google Scholar]
- De Coster, P. J. , Martens, L. C. , & De Paepe, A. (2005). Oral health in prevalent types of Ehlers‐Danlos syndromes. Journal of Oral Pathology & Medicine, 34(5), 298–307. 10.1111/j.1600-0714.2004.00300.x [DOI] [PubMed] [Google Scholar]
- De Felice, C. , Toti, P. , Di Maggio, G. , Parrini, S. , & Bagnoli, F. (2001). Absence of the inferior labial and lingual frenula in Ehlers‐Danlos syndrome. Lancet, 357(9267), 1500–1502. 10.1016/S0140-6736(00)04661-4 [DOI] [PubMed] [Google Scholar]
- Demmer, R. T. , Papapanou, P. N. , Jacobs, D. R., Jr. , & Desvarieux, M. (2010). Evaluating clinical periodontal measures as surrogates for bacterial exposure: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). BMC Medical Research Methodology, 10, 2. 10.1186/1471-2288-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré, F. C. , Frank, M. , Gogly, B. , Golmard, L. , Naveau, A. , Chérifi, H. , … Fournier, B. P. J. (2012). Oral phenotype and scoring of vascular Ehlers‐Danlos syndrome: A case‐control study. British Medical Journal Open, 2(2), e000705. 10.1136/bmjopen-2011-000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga, R. , Chandler, N. P. , & Oginni, A. O. (2008). Pulp stones: A review. International Endodontic Journal, 41(6), 457–468. 10.1111/j.1365-2591.2008.01374.x [DOI] [PubMed] [Google Scholar]
- Hakim, A. J. , Grahame, R. , Norris, P. , & Hopper, C. (2005). Local anaesthetic failure in joint hypermobility syndrome. Journal of the Royal Society of Medicine, 98, 84–85. 10.1258/jrsm.98.2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakki, S. S. , Aktas, D. , Alanay, Y. , Avunduk, M. C. , & Hakki, E. E. (2017). Dental findings and mutational analysis of a case with Ehlers‐Danlos syndrome. Journal of Dentistry and Oral Biology, 2(15), 5. [Google Scholar]
- Hanisch, M. , Blanck‐Lubarsch, M. , Bohner, L. , Suwelack, D. , Kleinheinz, J. , & Koppe, J. (2020). Oral conditions and oral health‐related quality of life of people with Ehlers‐Danlos syndromes (EDS): A questionnaire‐based cross‐sectional study. Medicina (Kaunas, Lithuania), 56(9), 448. 10.3390/medicina56090448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore, M. B. , Lauridsen, E. F. , & Sonnesen, L. (2019). Oro‐dental characteristics in patients with hypermobile Ehlers‐Danlos syndrome compared to a healthy control group. Journal of Oral Rehabilitation, 46(11), 1055–1064. 10.1111/joor.12838 [DOI] [PubMed] [Google Scholar]
- Jacobson, E. , Gressett Hall, M. , Strain, P. , Alfath, Z. , Gair, A. , Truong, U. , … Crespo, E. (2020). Oral health for physicians: A course for medical students. Pediatrics, 146, 2. 10.1542/peds.146.1_MeetingAbstract.394-a [DOI] [Google Scholar]
- Jensen, J. L. , & Storhaug, K. (2012). Dental implants in patients with Ehlers‐Danlos syndrome: A case series study. International Journal of Prosthodontics, 25(1), 60–62. [PubMed] [Google Scholar]
- Jepsen, S. , Caton, J. G. , Albandar, J. M. , Bissada, N. F. , Bouchard, P. , Cortellini, P. , … Yamazaki, K. (2018). Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and Peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S219–S229. 10.1111/jcpe.12951 [DOI] [PubMed] [Google Scholar]
- Jordan, R. A. , Bodechtel, C. , Hertrampf, K. , Hoffmann, T. , Kocher, T. , Nitschke, I. , … Group, D. V. S. I . (2014). The fifth German Oral health study (Funfte deutsche Mundgesundheitsstudie, DMS V) ‐ rationale, design, and methods. BMC Oral Health, 14, 161. 10.1186/1472-6831-14-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapferer‐Seebacher, I. , Lundberg, P. , Malfait, F. , & Zschocke, J. (2017). Periodontal manifestations of Ehlers‐Danlos syndromes: A systematic review. Journal of Clinical Periodontology, 44(11), 1088–1100. 10.1111/jcpe.12807 [DOI] [PubMed] [Google Scholar]
- Kapferer‐Seebacher, I. , Oakley‐Hannibal, E. , Lepperdinger, U. , Johnson, D. , Ghali, N. , Brady, A. F. , … van Dijk, F. S. (2021). Prospective clinical investigations of children with periodontal Ehlers‐Danlos syndrome identify generalized lack of attached gingiva as a pathognomonic feature. Genetics in Medicine, 23(2), 316–322. 10.1038/s41436-020-00985-y [DOI] [PubMed] [Google Scholar]
- Kapferer‐Seebacher, I. , Pepin, M. , Werner, R. , Aitman, T. J. , Nordgren, A. , Stoiber, H. , … Wilflingseder, D. (2016). Periodontal Ehlers‐Danlos syndrome is caused by mutations in C1R and C1S, which encode subcomponents C1r and C1s of complement. American Journal of Human Genetics, 99(5), 1005–1014. 10.1016/j.ajhg.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapferer‐Seebacher, I. , Schnabl, D. , Zschocke, J. , & Pope, F. M. (2020). Dental manifestations of Ehlers‐Danlos syndromes: A systematic review. Acta Dermato‐Venereologica, 100, 152–160. 10.2340/00015555-3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne, A. , Kleinheinz, J. , Jackowski, J. , Koppe, J. , & Hanisch, M. (2020). Study to investigate the knowledge of rare diseases among dentists, orthodontists, periodontists, oral surgeons and craniomaxillofacial surgeons. International Journal of Environmental Research and Public Health, 18(1), 139. 10.3390/ijerph18010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait, F. , de Coster, P. , Hausser, I. , van Essen, A. J. , Franck, P. , Colige, A. , … de Paepe, A. (2004). The natural history, including orofacial features of three patients with Ehlers‐Danlos syndrome, dermatosparaxis type (EDS type VIIC). American Journal of Medical Genetics. Part A, 131(1), 18–28. 10.1002/ajmg.a.30299 [DOI] [PubMed] [Google Scholar]
- Malfait, F. , Francomano, C. , Byers, P. , Belmont, J. , Berglund, B. , Black, J. , … Tinkle, B. (2017). The 2017 international classification of the Ehlers‐Danlos syndromes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 175(1), 8–26. 10.1002/ajmg.c.31552 [DOI] [PubMed] [Google Scholar]
- McGuire, J. D. , Walker, M. P. , Mousa, A. , Wang, Y. , & Gorski, J. P. (2014). Type VII collagen is enriched in the enamel organic matrix associated with the dentin‐enamel junction of mature human teeth. Bone, 63, 29–35. 10.1016/j.bone.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitakides, J. , & Tinkle, B. T. (2017). Oral and mandibular manifestations in the Ehlers‐Danlos syndromes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 175(1), 220–225. 10.1002/ajmg.c.31541 [DOI] [PubMed] [Google Scholar]
- Nanci, A. (2008). Chapter 1: Structure of the oral tissues. In Ten Cate's histology (7th ed.). St.Louis: Mosby Elsvier. [Google Scholar]
- Nicholls, A. C. , Sher, J. L. , Wright, M. J. , Oley, C. , Mueller, R. F. , & Pope, F. M. (2000). Clinical phenotypes and molecular characterisation of three patients with Ehlers‐Danlos syndrome type VII. Journal of Medical Genetics, 37(11), E33–E333. 10.1136/jmg.37.11.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelerich, O. , Kleinheinz, J. , Reissmann, D. R. , Koppe, J. , & Hanisch, M. (2020). Correlation between Oral health‐related quality of life and objectively measured oral health in people with Ehlers‐Danlos syndromes. International Journal of Environmental Research and Public Health, 17(21), 8243. 10.3390/ijerph17218243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooshima, T. , Abe, K. , Kohno, H. , Izumitani, A. , & Sobue, S. (1990). Oral manifestations of Ehlers‐Danlos syndrome type VII: Histological examination of a primary tooth. Pediatric Dentistry, 12(2), 102–106. [PubMed] [Google Scholar]
- Pitney, T. , & Pitney, M. (2020). Phenotypic mimicry between periodontal and vascular Ehlers‐Danlos variants resolved by molecular genetic testing. International Journal of Dermatology, 59(11), e387–e388. 10.1111/ijd.15118 [DOI] [PubMed] [Google Scholar]
- Pope, F. M. , Komorowska, A. , Lee, K. W. , Speight, P. , Zorawska, H. , Ranta, H. , … MacKenzie, J. L. (1992). Ehlers Danlos syndrome type I with novel dental features. Journal of Oral Pathology & Medicine, 21(9), 418–421. [DOI] [PubMed] [Google Scholar]
- Rinner, A. , Zschocke, J. , Schossig, A. , Grobner, R. , Strobl, H. , & Kapferer‐Seebacher, I. (2018). High risk of peri‐implant disease in periodontal Ehlers‐Danlos syndrome. A case series. Clinical Oral Implants Research, 29(11), 1101–1106. 10.1111/clr.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savasta, S. , Bassanese, F. , Hruby, C. , Foiadelli, T. , Siri, B. , Gori, V. , … Marseglia, G. L. (2019). Absence of lingual frenulum in children with Ehlers‐Danlos syndrome: A retrospective study of forty cases and literature review of a twenty years long debate. Minerva Pediatrica, 73(3), 230–235. 10.23736/S0026-4946.19.05530-0 [DOI] [PubMed] [Google Scholar]
- Schubart, J. R. , Schaefer, E. , Janicki, P. , Adhikary, S. D. , Schilling, A. , Hakim, A. J. , … Raj, S. R. (2019). Resistance to local anesthesia in people with the Ehlers‐Danlos syndromes presenting for dental surgery. Journal of Dental Anesthesia and Pain Medicine, 19(5), 261–270. 10.17245/jdapm.2019.19.5.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener, S. , Cobankara, F. K. , & Akgunlu, F. (2009). Calcifications of the pulp chamber: Prevalence and implicated factors. Clinical Oral Investigations, 13(2), 209–215. 10.1007/s00784-008-0212-x [DOI] [PubMed] [Google Scholar]
- Shi, X. , Lu, Y. , Wang, Y. , Zhang, Y. A. , Teng, Y. , Han, W. , … Han, J. (2015). Heterozygous mutation of c.3521C>T in COL1A1 may cause mild osteogenesis imperfecta/Ehlers‐Danlos syndrome in a Chinese family. Intractable Rare Dis Res, 4(1), 49–53. 10.5582/irdr.2014.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, F. , Hanisch, M. , Lechner, S. , Biskup, S. , Bohring, A. , Zschocke, J. , & Kapferer‐Seebacher, I. (2021). Prepubertal periodontitis in a patient with combined classical and periodontal Ehlers‐Danlos syndrome. Biomolecules, 11(2), 149. 10.3390/biom11020149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki, M. , Yamauchi, M. , & Mechanic, G. L. (1990). Bovine dental pulp collagens: Characterization of types III and V collagen. Archives of Oral Biology, 35(3), 195–200. [DOI] [PubMed] [Google Scholar]
- Tuchtenhagen, S. , Ortiz, F. R. , Ardenghi, T. M. , & Antunes, J. L. F. (2021). Oral health and happiness in adolescents: A cohort study. Community Dentistry and Oral Epidemiology, 49(2), 176–185. 10.1111/cdoe.12589 [DOI] [PubMed] [Google Scholar]
- van Damme, T. , Pang, X. , Guillemyn, B. , Gulberti, S. , Syx, D. , de Rycke, R. , … Malfait, F. (2018). Biallelic B3GALT6 mutations cause spondylodysplastic Ehlers‐Danlos syndrome. Human Molecular Genetics, 27(20), 3475–3487. 10.1093/hmg/ddy234 [DOI] [PubMed] [Google Scholar]
- Wu, J. , Yang, J. , Zhao, J. , Wu, J. R. , Zhang, X. , Leung, W. K. , & Sun, W. B. (2018). A Chinese family with periodontal Ehlers‐Danlos syndrome associated with missense mutation in the C1R gene. Journal of Clinical Periodontology, 45(11), 1311–1318. 10.1111/jcpe.12988 [DOI] [PubMed] [Google Scholar]
- Yoon, H. S. , Kim, H. Y. , Patton, L. L. , Chun, J. H. , Bae, K. H. , & Lee, M. O. (2013). Happiness, subjective and objective oral health status, and oral health behaviors among Korean elders. Community Dentistry and Oral Epidemiology, 41(5), 459–465. 10.1111/cdoe.12041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


