Abstract
The use of Kluyveromyces phaffii DBVPG 6076 killer toxin against apiculate wine yeasts has been investigated. The killer toxin of K. phaffii DBVPG 6076 showed extensive anti-Hanseniaspora activity against strains isolated from grape samples. The proteinaceous killer toxin was found to be active in the pH range of 3 to 5 and at temperatures lower than 40°C. These biochemical properties would allow the use of K. phaffii killer toxin in wine making. Fungicidal or fungistatic effects depend on the toxin concentration. Toxin concentrations present in the supernatant during optimal conditions of production (14.3 arbitrary units) exerted a fungicidal effect on a sensitive strain of Hanseniaspora uvarum. At subcritical concentrations (fungistatic effect) the saturation kinetics observed with the increased ratio of killer toxin to H. uvarum cells suggest the presence of a toxin receptor. The inhibitory activity exerted by the killer toxin present in grape juice was comparable to that of sulfur dioxide. The findings presented suggest that the K. phaffii DBVPG 6076 killer toxin has potential as a biopreservative agent in wine making.
Since Bevan and Makower (3) discovered the killer phenomenon in strains of Saccharomyces cerevisiae, several other yeast species have been found to produce a toxic proteinaceous factor that kills sensitive strains (17, 20, 21, 30, 31). Killer strains of S. cerevisiae show an antiyeast spectrum restricted to sensitive Saccharomyces strains except for a report on the killing of Torulopsis glabrata (37). Unlike the genus Saccharomyces a wide spectrum of intergeneric activity was reported for killer toxins from other genera such as Pichia (13, 14, 25), Hansenula (1, 18), Williopsis (34), and Kluyveromyces (22, 37).
Several potential applications for the killer phenomenon have been suggested since it was determined and studied. In fermentation industries, the killer character can be used to combat wild, contaminating Saccharomyces strains (33, 36). In the food industry, killer yeasts have been proposed to control spoilage yeasts in the preservation of food (16). In the medical field, killer yeasts have been used in the biotyping of pathogenic yeasts (15, 18), and the killer toxins of Pichia anomala (25, 26) and Williopsis mrakii (34) have been proposed as antimycotic agents.
In wine making, killer yeasts belonging to S. cerevisiae are currently used to initiate wine fermentation to improve the process of wine making and wine quality (28, 33). However, the main limit of the killer toxin of S. cerevisiae wine yeast (K2 type) resides in its narrow antiyeast spectrum which, being restricted to sensitive Saccharomyces strains, does not affect wild yeasts, such as those belonging to the genera Hanseniaspora/Kloeckera, Pichia, and Saccharomycodes.
Several ecological studies (9, 11, 12, 23) have clearly demonstrated that apiculate yeasts (Hanseniaspora/Kloeckera) predominate on grape surfaces and in freshly pressed juice. The control of the growth of apiculate yeasts in a nonsterile environment such as grape must is generally carried out by sulfur dioxide. However, several institutions, such as the World Health Organization and the European Economic Community, have highlighted the need to reduce the use of this antimicrobial agent in food products because of its toxicity. In this context, the use of a killer toxin as a control agent for apiculate yeasts in the prefermentative stage and during the fermentation of grape must has to be encouraged in order to reduce or eliminate the use of SO2. The findings of this study indicate that the killer toxin produced by Kluyveromyces phaffii DBVPG 6076 may be used as a biological agent against apiculate yeasts, which are usually present in freshly pressed juice and during the first stage of alcoholic fermentation.
MATERIALS AND METHODS
Cultures and media.
The strains used in the present study were from the Industrial Yeasts Collection of the University of Perugia (DBVPG): K. phaffii DBVPG 6076, a killer yeast; S. cerevisiae 6500 (NCYC 1006; National Collection of Yeast Cultures, Norwich, England), a sensitive strain; S. cerevisiae DBVPG 6664 (commercial strain Prise de Mousse; Red Star, Milwaukee, Wis.), a selected starter culture resistant to the DBVPG 6076 toxin; and Hanseniaspora uvarum DBVPG 3037. Strains isolated from grape berries from various vineyards of the Umbria region, classified as Hanseniaspora spp. by the methods of Kurtzman and Fell (10), were used to evaluate the frequency of the killer activity of DBVPG 6076. All yeast strains were subcultured at 6-month intervals on malt agar and maintained at 6°C. DBVPG 6076 was grown in yeast extract-peptone-dextrose (YPD) medium. The composition of YPD buffered at pH 4.5 (0.1 M citrate-phosphate buffer) was as follows (per liter): Bacto yeast extract, 10 g; Bacto Peptone, 10 g; and glucose, 50 g. Grape juice obtained from Grechetto grapes (pH 3.29; sugar, 209 g liter−1) was used for trials in natural medium.
Assessment of DBVPG 6076 killer toxin (Kpkt) activity.
The frequency of killer toxin activity was evaluated by streak plate agar diffusion assay (21). Approximately 105 cells ml−1 (final concentration) of the strain to be tested for sensitivity to the killer toxin were uniformly suspended in 20 ml of Bacto malt agar (Difco Laboratories, Detroit, Mich.) buffered at pH 4.5 (0.1 M citrate-phosphate buffer), maintained at 45°C in a water bath, and poured immediately into sterile petri dishes. DBVPG 6076 was streaked on the agar surface, and the plates were incubated at 20°C for 72 h. Killer activity was evident as a clear zone of inhibition surrounding the streak.
Toxin preparations were assayed by the well test method of Somers and Bevan (29). Toxin samples were filter sterilized though 0.45-μm-pore-size membrane filters (Millipore Corp., Bedford, Mass.). Seventy microliters of toxin sample was placed in wells (7-mm diameter) cut in the malt agar medium buffered at pH 4.5 (0.1 M citrate-phosphate buffer) (Difco). Malt agar plates had been previously seeded with a sensitive indicator strain. The killing activity of each sample was measured and defined as the mean zone of inhibition of replicate wells after incubation for 72 h at 20°C.
Kpkt production.
DBVPG 6076 was grown in YPD broth in a 2-liter flask with 1 liter of working volume for 72 h at 25°C on a rotary shaker (150 rpm−1). After centrifugation (3,000 × g for 10 min at 4°C), the supernatant was filtered though 0.45-μm-pore-size membrane filters (Millipore). Ice-cold ethanol was added to the filtrate to a final volume of 50% (vol/vol). After 2 h at −18°C, the resulting precipitate was collected by centrifugation (10,000 × g for 30 min) and resuspended in a reduced volume of 10 mM citrate-phosphate buffer (pH 4.5). For further concentration, Amicon YM10 (10-kDa-cutoff membrane) (Pharmacia, Uppsala, Sweden) was used.
Measurement of Kpkt activity.
Under the experimental conditions used, a linear relationship was observed between the logarithm of killer toxin concentration and the diameter of the inhibition zone assayed by the well test method. One arbitrary unit (AU) is defined as the toxin concentration in the supernatant that caused a clear zone of 8.0 mm (actual diameter, 15 mm, minus diameter of the 7-mm well), using S. cerevisiae DBVPG 6500 as a sensitive indicator strain and 70 μl of sample.
Effects of proteolitic enzymes and temperature stability.
Five hundred microliters of concentrated killer toxin was mixed with 125 μl of type IV papain solution (10 mg ml−1, 10 to 15 U mg−1). The toxin and protease solution was added to 4.5 ml of YPD broth inoculated with a sensitive H. uvarum DBVPG 3037 strain (initial inoculum, 106 cells ml−1). After an incubation period of 20 h at 20°C, the effects of the proteolytic enzyme were evaluated by the well test assay and a viable-cell count. Similar procedures were carried out in order to evaluate the temperature stability of the killer toxin. Samples of killer toxin were incubated at 20, 25, 30, 35, 40, 45, or 50°C for 2 h, mixed with YPD broth, and then inoculated with a sensitive H. uvarum DBVPG 3037 strain (initial inoculum, 106 cells ml−1). Killer toxin was also subjected to heat treatment (100°C for 10 min). Killer activity was determined by the well test assay and a viable-cell count after incubation for 24 h at 20°C.
pH stability.
In order to determine the pH range of activity, samples of killer toxin were tested by the well test assay using malt agar plates buffered at pH values of 2.8, 3.0, 3.5, 4.0, 4.5, 5.0, and 6.0.
Fungistatic and fungicidal effects.
Experiments were carried out in 300-ml Erlenmeyer flasks containing 100 ml of YPD broth buffered at pH 4.5 at 20°C. A 48-h preculture of H. uvarum DBVPG 3037, also grown at 20°C in the same medium as the test, was inoculated to obtain an initial count of 105 cells ml−1. Different toxin concentrations were added, and cell growth was evaluated by measuring optical density at 580 nm (OD580) using a DU 640 spectrophotometer (Beckman, Fullerton, Calif.) or by counting viable cells on plates. A control test (without toxin) was included in all assays.
Growth rate reduction assay.
The reduction of the growth rate was carried out according to the procedures of Sawant et al. (25), evaluating toxin concentrations lower than that which caused fungicidal activity. Cells of H. uvarum DBVPG 3037 in log phase were inoculated at an initial OD580 of 0.1 in 300-ml Erlenmeyer flasks containing 100 ml of YPD broth and incubated at 25°C in a rotary shaker (150 rpm). Killer toxin was added to the medium at the following concentrations (AU): 0.14, 0.26, 0.71, 1.43, 2.86, 5.72, 11.44, and 14.3. Optical densities were evaluated at 0 h and at 1-h intervals up to 9 h. Growth rates were determined by linear regression analyses of optical densities from 4 to 9 h of growth.
Activity of Kpkt in grape juice.
Trials in grape juice were carried out in 300-ml Erlenmeyer flasks containing 100 ml of pasteurized grape juice (100°C for 10 min). Concentrated Kpkt was added, and the procedure was standardized to provide the following final concentrations (AU ml−1): 5.14, 7.15, and 14.3. Sulfur dioxide at 37.5 (free SO2, 3.20 ml liter−1), 75.0 (free SO2, 6.08 ml liter−1), and 150 (free SO2, 10.28 ml liter−1) ml liter−1 was added 24 h before the inoculation in order to obtain the binding equilibrium. Precultures of H. uvarum DBVPG 3037 were grown at 20°C in the same medium as the test for 48 h and inoculated in order to obtain an initial count of 105 cells ml−1. Experiments were carried out in static conditions at 20°C by fitting each flask with a glass valve containing sulfuric acid, which allowed only CO2 to escape the system (5). An inoculum of S. cerevisiae DBVPG 6664 starter culture (5 × 106 cells ml−1) was added 48 h after the beginning of fermentation. S. cerevisiae was preincubated by the procedures used for the inoculum of H. uvarum DBVPG 3037. Trial fermentations with pure cultures of S. cerevisiae DBVPG 6664 and H. uvarum DBVPG 3037 were also included as control tests. The progress of fermentation was monitored by the amount of weight lost due to the carbon dioxide evolved. When the weight of the apparatus became constant, the fermentation was considered to be finished and samples were collected by filtration (0.45-μm-pore-size membrane; Millipore) for chemical analysis. Volatile acidity (expressed as grams of acetic acid per liter) was quantified by steam distillation according to official analytical procedures (6). Acetaldehyde and ethyl acetate were detected by gas-liquid chromatographic analysis as described by Bertuccioli (2).
RESULTS
Killer activity of K. phaffii on apiculate wine yeasts.
The evaluation of the spectrum of activity of DBVPG 6076 is the first step toward the practical application of this killer toxin in the control of apiculate yeasts in wine making. Thus, 298 strains of apiculate yeasts belonging to Hanseniaspora spp., isolated from 52 different grapes and sampled in the course of four years, were tested for their sensitivity to the K. phaffii DBVPG 6076 killer strain. Interestingly, 94.9% of the strains of apiculate wine yeasts were sensitive to the killer activity of K. phaffii (Table 1).
TABLE 1.
Frequency of killer activity of K. phaffii DBVPG 6076 among Hanseniaspora spp. strains isolated from grape berries from different vineyards of the Umbria region
| Yr of isolation | No. of sensitive strains | No. of resistant strains | No. of samplesa | Sensitive strains (%) |
|---|---|---|---|---|
| 1994 | 80 | 1 | 20 | 98.8 |
| 1995 | 90 | 1 | 12 | 98.9 |
| 1996 | 98 | 14 | 12 | 87.5 |
| 1997 | 30 | 0 | 8 | 100.0 |
| Total | 298 | 16 | 52 | 94.9 |
Number of bunches from which the apiculate strains were isolated.
Characterization of Kpkt.
Studies were carried out in order to elucidate the biochemical properties of Kpkt, particularly in relation to its possible use in wine making. The toxin was concentrated 10-fold by ethanol precipitation and this or more-concentrated preparations were used for successive characterization of the killer factor. The activity of Kpkt in the supernatant and after precipitation in ethanol are shown in Table 2.
TABLE 2.
Killer activity of Kpkt in the supernatant and after ethanol precipitationa
| Activity measured | Toxin concn factor | AU ml−1 | Killer activity against:
|
|
|---|---|---|---|---|
| S. cerevisiae (diam [mm]) | H. uvarum (diam [mm]) | |||
| In the supernatant | 1 | 14.3 | 8.0 | 4.0 |
| After ethanol precipitation | 10 | 143.3 | 16.0 | 8.0 |
Mean of eight different experiments. The variation was less than 5%.
Since the killer toxins known so far are proteins or glycoproteins, the effects of protease treatment on the killer factor of K. phaffii were assayed. Papain, which breaks sulfide bonds, inactivated the killer toxin (Table 3).
TABLE 3.
| Time (h) | CFU (106 ml−1)
|
||
|---|---|---|---|
| Control
|
Killer toxin and papain | ||
| No killer toxin | Killer toxin | ||
| 0 | 0.3 | 0.2 | 0.4 |
| 24 | 58.0 | 0.2 | 18.0 |
Toxin concentration, 14.3 AU ml−1.
The diameters of the zones of inhibition (measured in the well test assay) for S. cerevisiae were as follows: without killer toxin, 0.0 mm; with killer toxin, 8.5 mm; and with killer toxin and papain, 0.0 mm. The diameters of the zones of inhibition (measured in the well test assay) for H. uvarum were as follows: without killer toxin, 0.0 mm; with killer toxin, 4.5 mm; and with killer toxin and papain, 0.0 mm.
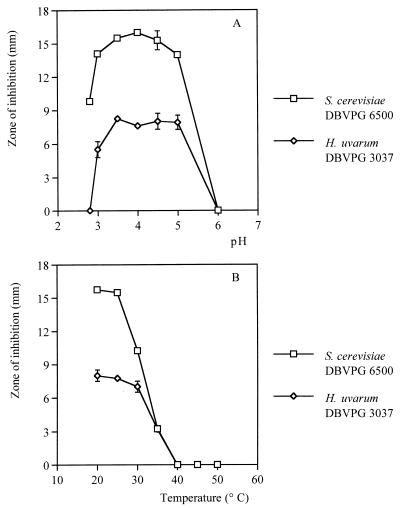
Further characterization of the DBVPG 6076 toxin was carried out by evaluating the effects of pH and temperature on the activity of the killer factor. Results reported in Fig. 1A show that Kpkt was active in the pH range of 3.0 to 5.0, but a 30% reduction in activity was observed at pH 3.0 against the sensitive H. uvarum strain. The killer activity observed for both sensitive strains was completely lost at pH 6. At pH 2.8 the toxin showed reduced activity against S. cerevisiae, whereas no activity was observed against H. uvarum. Regarding the influence of temperature, the killer activity was stable up to 25°C (Fig. 1B), whereas higher temperatures caused a progressive decrease of the activity of Kpkt. No activity was found after 2 h of incubation at 40°C. Heat treatment (100°C for 10 min) caused loss of activity. These results were confirmed by a viable-cell count assay (data not shown).
FIG. 1.
Effects of pH (A) and temperature (B) on the activity of Kpkt. pH effects were evaluated by the well test assay. Malt agar was buffered from pH 2.8 to 6.0 10 mM citrate-phosphate buffer. The toxin concentration was 14.3 AU ml−1. After 2 h of incubation at various temperatures, the killer activity was evaluated by the well test assay (pH 4.5). The toxin concentration was 14.3 AU ml−1. Data are given as means ± standard deviations of at least duplicate experiments.
Mode of action of Kpkt against H. uvarum DBVPG 3037.
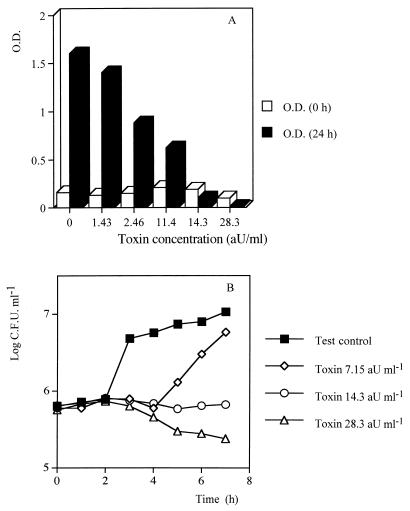
The effects of Kpkt on H. uvarum DBVPG 3037 growth were tested in liquid media at different concentrations of the killer factor. The growth of H. uvarum (assayed by measuring the OD580 after 24 h of incubation at 20°C) showed a progressive reduction with increased toxin concentrations. Interestingly, at the toxin concentration corresponding to 14.3 AU in the supernatant (Fig. 2A), no growth was observed. In another trial, the evaluation of the viable cells during the first 8 h of growth confirmed that the critical concentration necessary for a fungicidal effect was 14.3 AU (Fig. 2B). Lower concentrations appeared fungistatic for H. uvarum, extending lag phase for 2 h before growth resumed.
FIG. 2.
Fungistatic and fungicidial activity of Kpkt evaluated by OD unit (A) and log CFU ml−1 (B). Cells were grown in YPD broth buffered at pH 4.5. The initial inoculum contained 105 H. uvarum cells ml−1. Each sampling point represents the mean of duplicate experiments. The variation was less than 10%.
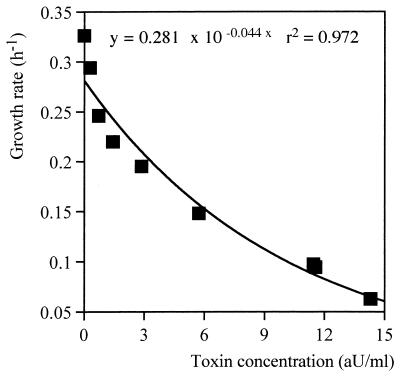
In order to assess the modality of action of Kpkt, the growth rate reduction assay (25) was carried out. The plotting of increased concentrations of the killer toxin against the growth rate (Fig. 3) showed an exponential relationship with typical saturation kinetics.
FIG. 3.
Effects of increasing concentrations of Kpkt on the growth rate of H. uvarum DBVPG 3037. Procedures are described in Materials and Methods. Each sampling point represents the mean of duplicate experiments. The variation was less than 10%.
Activity of Kpkt in grape juice.
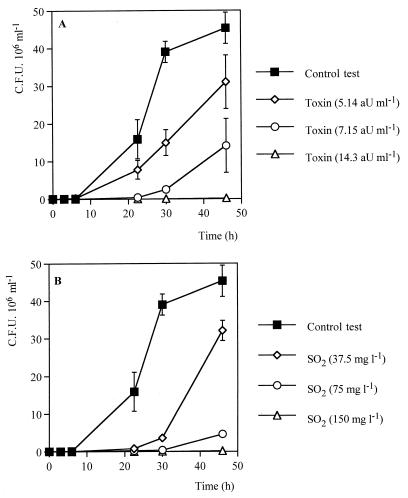
In order to verify the potential of DBVPG 6076 as a biological antiyeast agent in wine making, DBVPG 6076 toxin activity was assayed in natural grape juice. Moreover, the activities of Kpkt and SO2, the chemical antiseptic agent universally used in winemaking, were compared. Figure 4 shows the development of viable H. uvarum cells in the presence of different concentrations of Kpkt and SO2 during the first 48 h of growth at the stage of fermentation when apiculate yeasts naturally dominate the process. The positive control (inoculum of H. uvarum without antimicrobial agents) showed 45 × 106 cells ml−1 after 48 h of fermentation. As expected, the presence of toxin caused reduced growth of H. uvarum (Fig. 4A). A toxin concentration of 14.3 AU ml−1 resulted in a complete inhibition of the apiculate strain after 48 h of incubation. Absence of growth was obtained with 7.15 AU of toxin ml−1 within 24 h. After this time, reduced growth was exhibited by an apiculate yeast showing approximately 10 × 106 cells ml−1 after 48 h. Lower concentrations (5.14 AU ml−1) caused only reduced growth of H. uvarum without a prolonged lag phase. The effects of sulfur dioxide on the growth of H. uvarum were similar to those of the DBVPG 6076 toxin (Fig. 4B). After 48 h of incubation, no growth was exhibited in the presence of 150 mg of SO2 liter−1, whereas 37.5 and 75 mg of this antiseptic agent liter−1 caused lag phases of 24 and 30 h, respectively, before growth resumed.
FIG. 4.
Comparison of Kpkt (A) and SO2 (B) activities against H. uvarum DBVPG 3037 in natural grape juice. The initial inoculum of H. uvarum was 105 cells per ml. The grape juice had a pH of 3.29 and a sugar concentration of 209 g liter−1. Data are given as means ± standard deviations of at least duplicate experiments. The presence of toxin was relieved during the time of fermentation (data not shown).
The evaluation of some fermentation products obtained after the inoculation of the S. cerevisiae starter culture (Table 4) showed that the control of H. uvarum is accompanied by a progressive reduction of ethyl acetate. Amounts of ethyl acetate similar to those produced by the S. cerevisiae control test were exhibited by the trials containing 14.3 aU of toxin ml−1 and 150 mg of SO2 liter−1. Increased amounts of ethyl acetate were caused by lower concentrations of two antiseptic agents without reaching the taste threshold (150 mg liter−1) (8). No relevant differences were found among the trials for volatile acidity, whereas the trials with SO2 showed a small increase of acetaldehyde compared to the trial tests.
TABLE 4.
Influence of Kpkt and SO2 activity on some fermentation products of wines
| Organism(s) | Volatile acidity (g liter−1) | Acetaldehyde (mg liter−1) | Ethyl acetate (mg liter−1) |
|---|---|---|---|
| H. uvarum | 0.47 | 21.0 | 307 |
| S. cerevisiae | 0.33 | 29.5 | 43 |
| H. uvarum and S. cerevisiae | 0.42 | 12.3 | 150 |
| H. uvarum, S. cerevisiae, and toxin (5.14 AU) | 0.36 | 15.4 | 138 |
| H. uvarum, S. cerevisiae, and toxin (7.15 AU) | 0.36 | 21.0 | 128 |
| H. uvarum, S. cerevisiae, and toxin (14.3 AU) | 0.34 | 15.2 | 45 |
| H. uvarum, S. cerevisiae, and SO2 (37.5 mg liter−1) | 0.36 | 24.4 | 130 |
| H. uvarum, S. cerevisiae, and SO2 (75 mg liter−1) | 0.32 | 27.0 | 104 |
| H. uvarum, S. cerevisiae, and SO2 (150 mg liter−1) | 0.38 | 36.0 | 38 |
DISCUSSION
The use of S. cerevisiae killer yeasts in vinification prevents stuck fermentations caused by wild killer yeasts (33). However, antiyeast activity restricted to sensitive Saccharomyces strains does not permit the control of wild non-Saccharomyces, particularly apiculate yeasts which are present in freshly pressed grape juice. K. phaffii exhibits killer activity against apiculate yeasts (Kloeckera apiculata/H. uvarum) (22) and other spoilage yeasts (16). In order to assess the potential use of DBVPG-6076 in wine making we verified the following features: (i) the diffusion of killer activity among apiculate wine yeasts, (ii) the killer toxin activity at the conditions used in vinification, and (iii) the antiseptic effect compared with that of a chemical preservative agent used in wine making (SO2).
Results showed that Kpkt exhibits widespread killer activity against apiculate wine yeasts. Evidence from protease treatments suggests that Kpkt is a protein with sulfide bonds like several other killer factors (4, 35), since papain has been shown to destroy the toxin and its activity. Like most toxins (28), Kpkt is unstable at both high temperatures and high pH values. In contrast to the toxin of Kluyveromyces lactis (32,35), Kpkt has a low pH range of activity (up to pH 3.0). These findings are in agreement with the use of Kpkt at the majority of pHs and temperatures in wine making conditions.
The characterization of Kpkt activity against H. uvarum indicates that fungistatic or fungicidal effects depend on the toxin concentration. A toxin concentration of 14.3 aU ml−1 exerted zymocidal activity against H. uvarum DBVPG 3037. At subcritical concentrations of toxin (fungistatic effect), the saturation kinetics observed with an increased ratio of Kpkt to H. uvarum cells suggest the presence of a toxin receptor, probably on the cell wall. Cell wall receptors are known to mediate toxin action in the killer systems of several yeast species (7, 19, 24, 27).
The activity of Kpkt in grape juice is comparable to that of sulfur dioxide. The toxin concentration normally present in the supernatant (14.3 AU ml−1) is capable of controlling apiculate yeasts for 48 h at cell densities generally found in grape juice at the beginning of wine fermentation (9). The inhibition exerted by Kpkt on apiculate fermentation activity is reflected by the decrease in by-products such as ethyl acetate, the main compound responsible for the vinegary odor in wines, and acetaldehyde (linked to SO2 addition), an undesirable compound due to its capacity to combine with SO2, which is added for the preservation of wine. In conclusion, Kpkt, the sole toxin known to exhibit killer activity against apiculate yeasts, has great potential as a biopreservative agent in wine making and can profitably be substituted for SO2 at the prefermentation stage. Moreover, this killer toxin could be also used in the food industry since broad-spectrum activity against spoilage yeasts was shown (16).
Further studies are in progress to acquire additional information on the biochemical properties of Kpkt, contributing to the understanding and development of a novel biopreservative agent to combat wild microflora in winemaking.
ACKNOWLEDGMENTS
We thank Fausto Maccarelli for technical assistance and two anonymous reviewers for helpful comments on the manuscript.
This work was supported in part by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST) and the University of Ancona (progetto di Ateneo).
REFERENCES
- 1.Ashida S, Shimazaki S T, Kitano K, Hara S. New killer toxin of Hansenula mrakii. Agric Biol Chem. 1983;47:2953–2955. [Google Scholar]
- 2.Bertuccioli M. Determinazione gas-cromatografica diretta di alcuni composti volatili nei vini. Vini d'Italia. 1982;24:149–156. [Google Scholar]
- 3.Bevan E A, Makower M. The physiological basis of the killer character in yeast. In: Geerts S J, editor. Genetics today, XIth International Congress on Genetics vol. 1. Oxford, England: Pergamon Press; 1963. pp. 202–203. [Google Scholar]
- 4.Chen W-B, Han Y F, Jong S-C, Chang S-C. Isolation, purification, and characterization of a killer protein from Schwanniomyces occidentalis. Appl Environ Microbiol. 2000;66:5348–5352. doi: 10.1128/aem.66.12.5348-5352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciani M, Rosini G. Definizione dell'indice di moltiplicazione della CO2 nella valutazione, per via ponderale della capacita alcoligena di un lievito. Ann Fac Agrar Univ Stud Perugia. 1987;41:753–762. [Google Scholar]
- 6.European Economic Community. Methods of community analyses of wines. Regulation no. 2676/90 of the Commission, 17 September 1990. Brussels, Belgium: European Economic Community; 1990. [Google Scholar]
- 7.Hutchins K, Bussey H. Cell wall receptor for yeast killer toxin: involvement of (1 → 6) β-d-glucan. J Bacteriol. 1983;154:161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson R S. Sensory perception and wine assessment. In: Jackson R S, editor. Wine science. San Diego, Calif: Academic Press, Inc.; 1994. p. 447. [Google Scholar]
- 9.Kunkee R E, Goswell R W. Table wines. In: Rose A H, editor. Alcoholic beverages. London, England: Academic Press; 1977. pp. 315–386. [Google Scholar]
- 10.Kurtzman C P, Fell J W, editors. The yeasts, a taxonomic study. 4th ed. Amsterdam, The Netherlands: Elsevier Science B. V.; 1998. [Google Scholar]
- 11.Martini A. Origin and domestication of the wine yeast Saccharomyces cerevisiae. J Wine Res. 1993;4:165–176. [Google Scholar]
- 12.Martini A, Ciani M, Scorzetti G. Direct enumeration and isolation of wine yeasts from grape surfaces. Am J Enol Vitic. 1996;47:435–440. [Google Scholar]
- 13.Middelbeek E J, Hermans J M H, Stumm C. Production, purification and properties of a Pichia kluyveri killer toxin. Antonie Leeuwenhoek. 1979;45:437–450. doi: 10.1007/BF00443282. [DOI] [PubMed] [Google Scholar]
- 14.Middelbeek E J, van de Laar H H A M, Hermans J M H, Stumm C, Vogels G D. Physiological conditions affecting the sensitivity of Saccharomyces cerevisiae to a Pichia kluyveri killer toxin and energy requirement for toxin action. Antonie Leeuwenhoek. 1980;46:483–497. doi: 10.1007/BF00395829. [DOI] [PubMed] [Google Scholar]
- 15.Morace G, Manzara S, Dettori G, Fanti F, Campana L, Polonelli L, Chezzi C. Biotyping of bacterial isolates using the killer system. Eur J Epidemiol. 1989;5:85–90. doi: 10.1007/BF00144830. [DOI] [PubMed] [Google Scholar]
- 16.Palpacelli V, Ciani M, Rosini G. Activity of different “killer” yeasts on strains of yeast species undesiderable in food industry. FEMS Microbiol Lett. 1991;84:75–78. doi: 10.1016/0378-1097(91)90398-t. [DOI] [PubMed] [Google Scholar]
- 17.Phillskirk G, Young T W. The occurence of killer character in yeasts of various genera. Antonie Leeuwenhoek. 1975;41:147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- 18.Polonelli L, Archibusacci C, Sestito M, Morace G. Killer system: a simple method for differentiating Candida albicans strains. J Clin Microbiol. 1983;17:774–780. doi: 10.1128/jcm.17.5.774-780.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radler F, Schmitt M J, Meyer B. Killer toxin of Hanseniaspora uvarum. Arch Microbiol. 1990;154:175–178. doi: 10.1007/BF00423329. [DOI] [PubMed] [Google Scholar]
- 20.Rosini G. Interaction between killer strains of Hansenula anomala var. anomala and Saccharomyces cerevisiae yeast species. Can J Microbiol. 1985;31:300–302. [Google Scholar]
- 21.Rosini G. The occurence of killer characters in yeasts. Can J Microbiol. 1983;29:1462–1464. doi: 10.1139/m83-224. [DOI] [PubMed] [Google Scholar]
- 22.Rosini G, Cantini M. Killer character in Kluyveromyces yeasts: activity on Kloeckera apiculata. FEMS Microbiol Lett. 1987;44:81–84. [Google Scholar]
- 23.Rosini G, Federici F, Martini A. Yeast flora of grape berries during ripening. Microb Ecol. 1982;8:83–89. doi: 10.1007/BF02011464. [DOI] [PubMed] [Google Scholar]
- 24.Santos A, Marquina D, Leal J A, Peinado J M. (1 → 6)-β-d-Glucan as cell wall receptor for Pichia membranifaciens killer toxin. Appl Environ Microbiol. 2000;66:1809–1813. doi: 10.1128/aem.66.5.1809-1813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawant A D, Abdelal A T, Ahearn D G. Anti-Candida albicans activity of Pichia anomala as determined by a growth rate reduction assay. Appl Environ Microbiol. 1988;54:1099–1103. doi: 10.1128/aem.54.5.1099-1103.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawant A D, Abdelal A T, Ahearn D G. Purification and characterization of the anti-Candida toxin of Pichia anomala WC 65. Antimicrob Agents Chemother. 1989;33:48–52. doi: 10.1128/aac.33.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt M, Radler F. Molecular structure of the cell wall receptor for killer toxin KT28 in Saccharomyces cerevisiae. J Bacteriol. 1988;170:2192–2196. doi: 10.1128/jb.170.5.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu K. Killer yeasts. In: Fleet G H, editor. Wine microbiology and biotechnology. Chur, Switzerland: Harwood Academic; 1993. pp. 243–264. [Google Scholar]
- 29.Somers J M, Bevan E A. The inheritance of the killer character in yeast. Genet Res Commun. 1969;13:71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- 30.Starmer W T, Ganter P F, Aberdeen V, Lachance M A, Phaff H J. The ecological role of killer yeasts in natural communities of yeasts. Can J Microbiol. 1987;33:783–796. doi: 10.1139/m87-134. [DOI] [PubMed] [Google Scholar]
- 31.Stumm C, Hermans J M H, Middelbeek E J, Croes A F, De Vries G J M L. Killer-sensitive relationships in yeasts from natural habitats. Antonie Leeuwenhoek. 1977;43:125–128. doi: 10.1007/BF00395667. [DOI] [PubMed] [Google Scholar]
- 32.Sugisaki Y, Gunge N, Sakaguchi K, Kamasaki M, Tamura G. Characterization of novel killer toxin encoded by a double-stranded linear DNA plasmid of Kluyveromyces lactis. Eur J Biochem. 1984;141:241–245. doi: 10.1111/j.1432-1033.1984.tb08183.x. [DOI] [PubMed] [Google Scholar]
- 33.van Vuuren H J J, Jacobs C J. Killer yeasts in the wine industry: a review. Am J Enol Vitic. 1992;43:119–128. [Google Scholar]
- 34.Walker G M, McLeod A H, Hodgson V J. Interactions between killer yeasts and pathogenic fungi. FEMS Microbiol Lett. 1995;127:213–222. doi: 10.1111/j.1574-6968.1995.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C, Whittaker P A. Factors affecting activity and stability of the Kluyveromyces lactis killer toxin. Appl Environ Microbiol. 1989;55:695–699. doi: 10.1128/aem.55.3.695-699.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young T W. Killer yeasts. In: Rose A H, Harrison J S, editors. The yeasts. 2nd ed. Vol. 2. London, England: Academic Press; 1987. pp. 131–164. [Google Scholar]
- 37.Young T W, Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek. 1978;44:59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]