Abstract
Objective
To investigate whether first‐trimester maternal haemodynamic adaptation impacts placental, embryonic and fetal development as well as birth outcomes in pregnancies with and without placenta‐related complications.
Design
Prospective observational cohort.
Setting
A Dutch tertiary hospital.
Population
Two hundred and fourteen ongoing pregnancies.
Methods
At 7, 9 and 11 weeks of gestation, we assessed maternal haemodynamic adaptation (mean arterial blood pressure [MAP], uterine artery [UtA] blood flow) and placental development (placental volume [PV], uteroplacental vascular volume [uPVV]) using three‐dimensional power Doppler ultrasound volumes, and embryonic development (crown–rump length, embryonic volume). At 22 and 32 weeks of gestation, fetal development was assessed by estimated fetal weight. Birth outcomes (birthweight, placental weight) were extracted from medical records. Linear mixed modelling and linear regression analyses were applied.
Main outcome measures
Birthweight centile and placental weight.
Results
In placenta‐related complications (n= 55, 25.7%), reduced haemodynamic adaptation, i.e. higher UtA pulsatility index (PI) and resistance index (RI) trajectories, was associated with smaller increase in PV (β = −0.559, 95% CI −0.841 to −0.278, P< 0.001; β = −0.579, 95% CI −0.878 to −0.280, P< 0.001) and uPVV trajectories (UtA PI: β = −0.301, 95% CI −0.578 to −0.023, P= 0.034). At birth, reduced haemodynamic adaptation was associated with lower placental weight (UtA PI: β = −0.502, 95% CI −0.922 to −0.082, P= 0.022; UtA RI: β = −0.435, 95% CI −0.839 to −0.032, P= 0.036). In pregnancies without placenta‐related complications, higher MAP trajectories were positively associated with birthweight centile (β = 0.398, 95% CI 0.049–0.748, P= 0.025).
Conclusions
Reduced first‐trimester maternal haemodynamic adaptation impacts both placental size and vascularisation and birthweight centile, in particular in pregnancies with placenta‐related complications.
Tweetable abstract
Reduced first‐trimester maternal haemodynamic adaptation to pregnancy impairs early placental development.
Keywords: 3D ultrasound, birth outcomes, placenta‐related complications, uterine artery blood flow
Introduction
Placental development in the first trimester of pregnancy is key to the success of pregnancy and health during the life course. 1 Insufficient spiral artery remodelling and impaired maternal haemodynamic adaptation of the uteroplacental vascular network during this early period in pregnancy can result in hypoperfusion of the uteroplacental circulation, involved in the causative pathways of placenta‐related complications that present from mid‐gestation onwards. 2 , 3 , 4 Placenta‐related complications include pregnancy‐induced hypertension, pre‐eclampsia, fetal growth restriction (FGR), preterm birth and babies born small‐for‐gestational age (SGA). 5 , 6 , 7 , 8 , 9 , 10 , 11
In clinical practice, maternal haemodynamic adaptation is assessed by mean arterial blood pressure (MAP), determined by cardiac output and systemic vascular resistance, and uterine artery (UtA) blood flow. 3 From the first‐trimester onwards, a physiological course of haemodynamic adaptation translates into a decrease in MAP (up to mid‐gestation) and resistance to UtA blood flow. Using Doppler ultrasound, UtA blood flow can be measured by the pulsatility index (PI) and resistance index (RI). 12 Persistent high resistance to UtA blood flow in the second‐ and third‐trimester is positively associated with placenta‐related complications. 12 , 13 From this background, we postulate that impaired first‐trimester maternal haemodynamic adaptation also increases the risk of adverse early and late placental and embryonic and fetal development.
Previously introduced techniques, such as three‐dimensional (3D) ultrasonography combined with Virtual Organ Computer‐aided AnaLysis (VOCAL)™, enable the assessment of placental volume (PV). 14 A smaller first‐trimester PV is associated with higher second‐trimester UtA resistance, as well as placenta‐related complications. 15 , 16 When Virtual Reality (VR) is combined with 3D power Doppler ultrasonography, uteroplacental vascularisation volumes (uPVV) can be measured in a reproducible and accurate manner, using depth perception and offering 3D interaction by creating a hologram from the 3D ultrasound data set. 17 , 18 , 19 The uPVV measurements were performed on a 3D VR desktop system, allowing for more precise and detailed evaluation of placental structures because of the option of image enlargement, image rotation and the actual use of all 3D dimensions. 19 As such, uPVV in the first trimester can be considered a marker representing not only maternal haemodynamic adaptation to pregnancy but also placental development. Here we consider PV and uPVV as surrogate outcomes of placental development only. 20
A longitudinal 2‐weekly assessment of maternal haemodynamic adaptation to pregnancy as early as from 7 weeks onwards is unique. Moreover, the assessment of associations with placental, embryonic and fetal development this early using 3D Power Doppler ultrasound combined with VOCAL and VR has not been performed previously. Therefore, the overall aim of this study is to investigate associations between the extent of early first‐trimester maternal haemodynamic adaptation and early placental development, i.e. PV, uPVV and embryonic and fetal development, i.e. crown–rump length (CRL), embryonic volume (EV), estimated fetal weight (EFW), and birth outcomes, i.e. birthweight, placental weight.
Methods
Study design
This study was part of the Virtual placenta study (registration number Dutch Trial Register: NTR6854), embedded in the ongoing prospective Rotterdam Periconception Cohort (Predict study). 21 Women were eligible with a singleton pregnancy less than 10 weeks of gestation. Between January 2017 and March 2018, a total of 241 participants were enrolled. Women were excluded from further analysis in the event of a miscarriage, conception after oocyte donation or withdrawal. At enrolment, participants filled out a questionnaire on general characteristics, medical (and obstetric) history and lifestyle behaviours. Measurements of height and weight were standardised to calculate body mass index (BMI) at the first study visit. Geographic origin was categorised as Dutch, Western and Non‐Western. 22 Educational level was categorised as low, middle or high according to the classification of Statistics Netherlands. 23
Visits were scheduled at 7, 9, 11, 22 and 32 weeks of gestation. During all visits, blood pressure in mmHg was assessed by a nurse or physician in a sitting position, on the right arm, using a manual blood‐pressure cuff and stethoscope to measure systolic and diastolic blood pressure. The MAP in mmHg was calculated from the systolic blood pressure + 2*diastolic blood pressure, divided by 3.
Gestational age (GA) in spontaneous pregnancies was based on the first day of the last menstrual period in regular cycles defined as a cycle duration between 25 and 35 days. For pregnancies conceived after in vitro fertilisation (IVF) with or without intracytoplasmic sperm injection (ICSI), GA was calculated from the oocyte pick‐up day plus 14 days. In pregnancies from cryopreserved embryos, GA was defined as the transfer date plus 19 days. GA was based on CRL when a difference of more than 6 days existed between the GA based on last menstrual period and the GA based on CRL, or in the case of an irregular menstrual cycle or unknown last menstrual period. 17
Ultrasound
At 7, 9 and 11 weeks of gestation, a transvaginal 3D power Doppler ultrasound volume was obtained to visualise the vascularisation of the whole gestational sac including the placenta. At 22 and 32 weeks of gestation transabdominal ultrasound was performed to estimate fetal growth by parameters needed to calculate EFW using the Hadlock formula (encompassing the biparietal diameter, head circumference, abdominal circumference and femur length). 24 At all study visits, pulsed wave Doppler ultrasound was used in a two‐dimensional plane to assess UtA blood flow by determination of the bilateral UtA PI and UtA RI. 25
Ultrasound scans were performed by trained sonographers using a Voluson Expert E8 or E10 ultrasound system with a transvaginal 6–12 MHz transducer (GE, Zipf, Austria) with standard power Doppler settings as described previously (power Doppler gain ‘−8.0’, pulse repetition frequency ‘0.6 kHz’, wall motion filter ‘low1’, quality ‘high’). 17 During 3D scanning, participants were asked to hold their breath for approximately 30 seconds to minimise breathing artefacts. To increase the chances of obtaining a complete and high‐quality volume, a minimum of two uterine volumes was recorded at a perpendicular angle (90°) to each other. Total ultrasound scanning time was always limited to 30 minutes per visit according to international safety guidelines. 26 , 27
Offline 3D measurements
Image quality was scored on a four‐point scale ranging between zero and three, based on the presence of artefacts (due to fetal movements causing blurring of the volume or acoustic shadowing), the ability to distinguish between myometrial and trophoblastic tissue, and completeness of the placenta. For sufficient quality, a score between zero and two was assigned. An incomplete or bad‐quality volume received a score of three and was excluded.
Placental volume was measured offline using VOCAL by two trained observers according to the following protocol: to measure PV, the circumference of the placenta and embryonic cavity was traced in two‐dimensional planes with a 15‐degree rotation step, resulting in a total of 12 placental slices from which a 3D volume was reconstructed. The placental tissue was distinguished from its surroundings by the difference in echogenicity between trophoblast and myometrium. Placental tracing resulted in a total pregnancy volume including the gestational sac. Next, the gestational sac volume was calculated by tracing the gestational sac contours in a similar way to the placenta. The PV in cubic centimetres was then calculated by subtraction of the embryonic cavity volume from the gestational sac volume. 15
The uPVV was measured using a VR desktop system with the V‐Scope volume rendering application. The VR desktop consists of a 3D monitor, a tracking system for a pointing device, a pair of stereoscopic glasses and a six degrees‐of‐freedom mouse for 3D volume manipulation. The protocol for uPVV measurements has been shown to be reproducible within and between observers. 17 In short, uPVV was measured by removal of the power Doppler signal of the embryonic or fetal structures. Then, the difference in echogenicity between the myometrium and placenta was used to erase the power Doppler signal of blood vessels up to the myometrial–placental tissue interface. 17
The CRL was measured three times using a length‐measuring tool in VR, placing the calipers in a straight line from the crown to caudal rump. By rotating the hologram, a correct position of the line in the midsagittal plane was verified. The average was used for analysis. Embryonic volume (EV) was measured once based on grey values by a semi‐automated volume‐measuring application. The technique and reliability of CRL and EV measurements have been validated previously. 28 , 29
To determine the relative vascularisation degree of the placental bed, the uPVV/PV ratio was calculated.
Definitions of maternal and fetal placenta‐related complications
The occurrence of maternal and fetal placenta‐related complications was retrieved from medical records. Pregnancy‐induced hypertension was defined as a systolic blood pressure above 140 mmHg or diastolic blood pressure above 90 mmHg after 20 weeks of gestation without signs of hypertension before pregnancy or presence of proteinuria. 30 Pre‐eclampsia was defined as hypertension with systolic blood pressure above 140 mmHg or diastolic blood pressure above 90 mmHg after 20 weeks of gestation and presence of more than 300 mg proteinuria in a 24‐hour period. 30 , 31 A fetal abdominal circumference and/or EFW below the tenth centile according to Hadlock curves or a more than twenty‐centile decrease on the growth curve, compared with previous measurements with at least a time span of 2 weeks, was defined as FGR. 32 , 33 A GA at birth below 37 weeks was defined as preterm birth and SGA was defined as birthweight below the tenth centile on growth curves specific for GA at birth, parity and fetal gender. 30 , 34
Statistical analysis
Inherent to the explorative nature of our study we did not perform a power calculation. Moreover, no reference values for the placental measurements in VR are available, making it impossible to determine the estimates of expected mean differences needed for power calculation.
Baseline characteristics of the study population were presented as medians with interquartile range or number with percentage. First, to establish normal distributions, data were transformed using a natural log transformation or square root for non‐volumetric parameters (MAP, UtA PI, UtA RI and CRL) and cubic root for volumetric parameters (i.e. PV, uPVV, uPVV/PV ratio and EV).
This was followed by a two‐step approach to analyse the associations between the haemodynamic and placental parameters. We applied linear mixed models using the individual trajectories of longitudinal measurements (i.e. trajectories of MAP, UtA PI, UtA RI, PV, uPVV, uPVV/PV ratio, CRL, EV and EFW as response variable), with GA as independent variable. In these models we allowed for subject‐specific intercepts and slopes (the random effects). Where possible, both the standardised random intercepts and slopes were extracted and used as summaries representing first‐trimester haemodynamic adaptation (i.e. MAP, UtA PI and UtA RI). In the next analysis the estimates of first‐trimester haemodynamic adaptation were used as covariates in linear regression analyses. Here we used the random effects of the mixed effects models for the trajectories of first‐trimester placental development and the trajectories of first‐trimester embryonic growth, trajectories of second‐ and third‐trimester EFW, GA at birth, birthweight centile and placental weight as outcomes. Analyses were stratified for pregnancies with and without placenta‐related complications. The associations were assessed in a crude model (data not shown). Thereafter, a second model was adjusted for maternal age, parity, conception mode, smoking, fetal gender, BMI and preconception initiation of folic acid supplement use based on the characteristics of the study population and literature. As a last step, sensitivity analyses were performed in strictly dated pregnancies only.
All analyses were performed using SPSS software (version 25.0; IBM, Armonk, NY, USA) and RStudio Statistics (version 3.5.0, 2018). Values of P less than 0.05 were considered statistically significant.
Results
A total of 214 ongoing pregnancies out of 241 pregnancies were included in the analysis. Twenty‐two women were excluded as the result of miscarriage, four women were excluded because of oocyte donation and one woman withdrew from the study (Figure S1). A total of 466 3D ultrasound data sets were available for further analysis (respectively 101 data sets were available at 7 weeks of gestation, 182 data sets at 9 weeks of gestation and 183 data sets at 11 weeks of gestation), of which 328 (70.4%) were usable for VOCAL measurements (respectively 88 data sets were available at 7 weeks of gestation, 138 data sets at 9 weeks of gestation and 102 data sets at 11 weeks of gestation) and 346 (74.2%) for VR measurements (respectively 82 data sets were available at 7 weeks of gestation, 151 data sets at 9 weeks of gestation and 113 data sets at 11 weeks of gestation). 33
Table 1 shows the study population’s baseline characteristics, stratified for pregnancies with and without placenta‐related complications. Placenta‐related complicated pregnancies (n= 55, 25.7%) showed a shorter GA at birth (median 38+0 weeks versus 39+2 weeks, P< 0.001), lower birthweight (median 2735 g versus 3415 g, P< 0.001) and lower placental weight at birth (median 353 g versus 459 g, P< 0.001) compared with the uncomplicated pregnancies (n= 159, 74.3%).
Table 1.
Baseline characteristics of the Virtual Placenta study population
| Characteristic |
All pregnancies (n= 214) |
Placenta‐related complications (n= 55) |
No placenta‐related complications (n= 159) |
P value* |
|---|---|---|---|---|
| Maternal | ||||
| Age, years | 32.1 [29.0; 35.5]** | 30.6 [29.0; 34.3]** | 32.5 [29.1; 35.8]** | 0.101 |
| Nulliparous | 115 (53.7%) | 28 (50.9%) | 87 (54.7%) | 0.625 |
| Mode of conception, IVF/ICSI | 87 (40.7%) | 17 (30.9%) | 70 (44.0%) | 0.088 |
| GA at first visit, days | 55 [51; 65]** | 59 [51; 65]** | 55 [51; 65]** | 0.917 |
| Geographic origin | ||||
| Dutch | 165 (78.9%) | 38 (73.1%) | 127 (80.9%) | 0.453 |
| Western other | 6 (2.9%) | 1 (1.9%) | 5 (3.2%) | |
| Non‐Western | 38 (18.2%) | 13 (25.0%) | 25 (15.9%) | |
| Educational level | ||||
| Low | 18 (8.6%) | 7 (13.5%) | 11 (6.9%) | 0.262 |
| Intermediate | 69 (32.9%) | 14 (26.9%) | 55 (34.6%) | |
| High | 123 (58.6%) | 31 (59.6%) | 92 (57.9%) | |
| BMI measured at first visit in first trimester (kg/m2) | 24.9 [22.2; 28.4]** | 24.8 [22.2; 27.5]** | 24.0 [21.4; 27.0]** | 0.235 |
| Folic acid supplement use, yes | 210 (98.1%) | 54 (98.2%) | 156 (98.1%) | 0.974 |
| Preconception initiation | 175 (81.8%) | 40 (72.7%) | 135 (84.9%) | 0.131 |
| Alcohol consumption, yes | 57 (26.6%) | 10 (18.2%) | 47 (29.6%) | 0.100 |
| Smoking, yes | 28 (13.1%) | 10 (18.2%) | 18 (11.3%) | 0.193 |
| Birth outcomes | ||||
| Major congenital anomalies | 8 (3.7%) | 0 (0%) | 8 (5.0%) | 0.090 |
| GA at birth, weeks | 39+1 [38+2; 40+0]** | 38+0 [36+1; 39+1]** | 39+2 [38+4; 40+2]** | <0.001* |
| Birthweight, grams | 3305 [2930; 3565]** | 2735 [2329; 2931]** | 3415 [3175; 3685]** | <0.001* |
| Placental weight, grams | 435 [350; 517]** | 353 [301; 444]** | 459 [407; 567]** | <0.001* |
| Fetal gender, boys | 106 (49.5%) | 30 (54.5%) | 76 (49.7%) | 0.535 |
| Placenta‐related complications*** | 55 (25.7%) | 55 (100%) | NA | NA |
| PIH | 9 (4.2%) | 9 (16.4%) | NA | NA |
| Pre‐eclampsia | 7 (3.3%) | 7 (12.7%) | NA | NA |
| FGR | 15 (7.0%) | 15 (27.3%) | NA | NA |
| Preterm birth**** | 23 (10.7%) | 23 (41.8%) | NA | NA |
| SGA | 18 (8.4%) | 18 (32.7%) | NA | NA |
FGR, fetal growth restriction, defined as abdominal circumference and/or estimated fetal weight (EFW) below the tenth centile; GA, gestational age or a more than twenty‐centile decrease on the growth curve, compared with previous measurements with a time span of at least 2 weeks; NA, not applicable; PIH, pregnancy‐induced hypertension; SGA, small‐for‐gestational age, defined as birth weight below the tenth percentile.
P value tested between pregnancies with and without placenta‐related complications, significance at P< 0.05.
Expressed as median [interquartile range, p25; p75].
Overlapping diagnoses.
Iatrogenic and spontaneous preterm births.
In placenta‐related complicated pregnancies, UtA PI and UtA RI values at 11 weeks of gestation were higher compared with uncomplicated pregnancies (median PI: 2.03 versus 1.74, P= 0.047; median RI: 0.79 versus 0.75, P= 0.021). In addition, in placenta‐related complicated pregnancies PV (cm3) and uPVV (cm3) values were lower at 9 weeks of gestation (median PV: 39.51 versus 45.50, P= 0.028; median uPVV: 5.49 versus 9.27, P= 0.018). Furthermore, in these pregnancies PV was lower at 11 weeks of gestation (median: 79.13 versus 95.46, P= 0.002) (Figure 1; Table S1).
Figure 1.
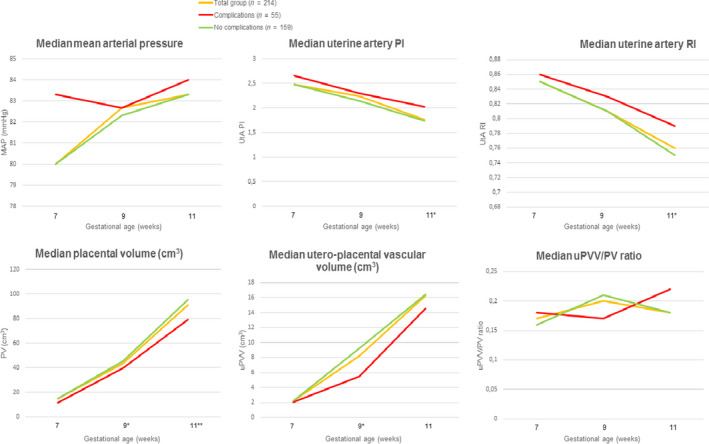
First‐trimester maternal haemodynamic parameters and placental parameters, stratified for pregnancies with and without placenta‐related complications.
First‐trimester maternal haemodynamic adaptation and first‐trimester trajectories of placental development
No significant associations were established between first‐trimester maternal haemodynamic adaptation, assessed by MAP trajectories, and placental development (Table 2).
Table 2.
Associations between reduced first‐trimester maternal haemodynamic adaptation and trajectories of placental development, stratified for pregnancies with and without placenta‐related complications
|
First‐trimester PV trajectory () Random intercept |
First‐trimester uPVV trajectory () Random intercept |
First‐trimester uPVV/PV ratio trajectory Random intercept |
First‐trimester uPVV/PV ratio trajectory Random slope |
|
|---|---|---|---|---|
| Pregnancies with placenta‐related complications (n = 55) | ||||
| Higher first‐trimester trajectory of MAP | ||||
| Random intercept (β), 95% CI | 0.154 (−0.772; 1.080) | 0.036 (−0.796; 0.868) | −0.191 (−0.923; 0.540) | 0.210 (−0.484; 0.903) |
| Random slope (β), 95% CI | −0.343 (−1.262; 0.577) | −0.189 (−0.992; 0.614) | 0.201 (−0.517; 0.920) | −0.236 (−0.917; 0.445) |
| Higher 1st trimester trajectory of UtA PI | ||||
| Random intercept (β), 95% CI | −0.559 (−0.841;−0.278)** | −0.301 (−0.578;−0.023)* | −0.134 (−0.391; 0.122) | −0.034 (−0.282; 0.212) |
| Random slope (β), 95% CI | NA | NA | NA | NA |
| Higher first‐trimester trajectory of UtA RI | ||||
| Random intercept (β), 95% CI | −0.579 (−0.878;−0.280)** | −0.255 (−0.553; 0.043) | −0.086 (−0.361; 0.190) | −0.084 (−0.347; 0.178) |
| Random slope (β), 95% CI | NA | NA | NA | NA |
| Pregnancies without placenta‐related complications (n = 159) | ||||
| Higher first‐trimester trajectory of MAP | ||||
| Random intercept (β), 95% CI | −0.166 (−0.552; 0.220) | −0.229 (−0.617; 0.180) | −0.198 (−0.615; 0.220) | −0.133 (−0.557; 0.291) |
| Random slope (β), 95% CI | 0.230 (−0.161; 0.620) | 0.203 (−0.197; 0.602) | 0.112 (−0.313; 0.537) | 0.134 (−0.297; 0.566) |
| Higher first‐trimester trajectory of UtA PI | ||||
| Random intercept (β), 95% CI | −0.090 (−0.260; 0.080) | −0.303 (−0.470;−0.136)** | −0.214 (−0.397; 0.031) | 0.179 (−0.006; 0.365) |
| Random slope (β), 95% CI | NA | NA | NA | NA |
| Higher first‐trimester trajectory of UtA RI | ||||
| Random intercept (β), 95% CI | −0.057 (−0.228; 0.114) | −0.316 (−0.483;−0.150)** | −0.244 (−0.428;−0.059)* | 0.239 (0.053; 0.425)* |
| Random slope (β), 95% CI | NA | NA | NA | NA |
Fully adjusted model for maternal age, parity, conception mode, body mass index, smoking, preconception initiation of folic acid supplement use and fetal gender. Random slope not available for first‐trimester UtA PI, UtA RI, PV and uPVV.
Significance at P< 0.05.
Significance at P< 0.01.
In placenta‐related complicated pregnancies, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the UtA PI trajectory, was negatively associated with PV trajectories (random intercept adjusted β = −0.559, 95% CI −0.841 to −0.278, P< 0.001) and uPVV trajectories (random intercept adjusted β = −0.301, 95% CI −0.578 to −0.023, P= 0.034). Reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the UtA RI trajectory, was negatively associated with PV trajectories (random intercept adjusted β = −0.579, 95% CI −0.878 to −0.280, P< 0.001) (Table 2).
In pregnancies without placenta‐related complications, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the UtA PI and UtA RI trajectories was negatively associated with uPVV trajectories (PI: random intercept adjusted β = −0.303, 95% CI −0.470 to −0.136, P< 0.001, RI: random intercept adjusted β = −0.316, 95% CI −0.483 to −0.150, P< 0.001). Moreover, negative associations were observed for trajectories of the uPVV/PV ratio (random intercept adjusted β = −0.244, 95% CI −0.428 to −0.059, P= 0.010; random slope adjusted β = 0.239, 95% CI 0.053–0.425, P= 0.012) (Table 2).
First‐trimester maternal haemodynamic adaptation and trajectories of embryonic and fetal development
No significant associations were identified between maternal haemodynamic adaptation and embryonic and fetal development (Table S2 and S3) in pregnancies with or without placenta‐related complications.
First‐trimester maternal haemodynamic adaptation and birth outcomes
In placenta‐related complicated pregnancies, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the UtA PI and UtA RI trajectories, was only associated with a lower placental weight (UtA PI random intercept adjusted β = −0.502, 95% CI −0.922 to −0.082, P= 0.022; UtA RI random intercept adjusted β = −0.435, 95% CI −0.839 to −0.032, P= 0.036) (Table 3). No significant associations were revealed between maternal haemodynamic adaptation reflected by MAP and birth outcomes (GA at birth, birthweight centile and placental weight).
Table 3.
Associations between reduced first‐trimester maternal haemodynamic adaptation and birth outcomes, stratified for pregnancies with and without placenta‐related complications
| GA at birth (days) | Birthweight centile (p) | Placental weight (grams) | |
|---|---|---|---|
| Pregnancies with placenta‐related complications (n = 55) | |||
| Higher first‐trimester trajectory of MAP | |||
| Random intercept (β), 95% CI | −0.048 (−1.246; 1.149) | −0.066 (−0.799; 0.668) | −0.900 (−2.302; 0.501) |
| Random slope (β), 95% CI | 0.066 (−1.091; 1.224) | 0.193 (−0.517; 0.903) | 0.754 (−0.571; 2.079) |
| Higher first‐trimester trajectory of UtA PI | |||
| Random intercept (β), 95% CI | −0.018 (−0.434; 0.398) | −0.166 (−0.442; 0.090) | −0.502 (−0.922;−0.082)* |
| Random slope (β), 95% CI | NA | NA | NA |
| Higher first‐trimester trajectory of UtA RI | |||
| Random intercept (β), 95% CI | 0.035 (−0.408; 0.479) | −0.234 (−0.503; 0.036) | −0.435 (−0.839;−0.032)* |
| Random slope (β), 95% CI | NA | NA | NA |
| Pregnancies without placenta‐related complications (n = 159) | |||
| Higher first‐trimester trajectory of MAP | |||
| Random intercept (β), 95% CI | 0.148 (−0.035; 0.330) | 0.398 (0.049; 0.748)* | 0.095 (−0.585; 0.771) |
| Random slope (β), 95% CI | −0.174 (−0.362; 0.014) | −0.342 (−0.701; 0.018) | 0.072 (−0.655; 0.799) |
| Higher first‐trimester trajectory of UtA PI | |||
| Random intercept (β), 95% CI | −0.092 (−0.171;−0.012)* | −0.126 (−0.280; 0.028) | −0.147 (−0.433; 0.140) |
| Random slope (β), 95% CI | NA | NA | NA |
| Higher first‐trimester trajectory of UtA RI | |||
| Random intercept (β), 95% CI | −0.110 (−0.187;−0.033)** | −0.107 (−0.258; 0.044) | −0.017 (−0.296; 0.263) |
| Random slope (β), 95% CI | NA | NA | NA |
Fully adjusted model for maternal age, parity, conception mode, body mass index, smoking, preconception initiation of folic acid supplement use and fetal gender. Random slope not available for first‐trimester UtA PI and UtA RI.
Significance at P < 0.05.
Significance at P < 0.01.
In pregnancies without placenta‐related complications, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the MAP trajectories, was associated with a higher birthweight centile (adjusted β = 0.398, 95% CI 0.049–0.748, P= 0.026). Furthermore, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in the UtA PI and UtA RI trajectories, was associated with a lower GA at birth (PI: random intercept adjusted β = −0.092, 95% CI −0.171 to −0.012, P= 0.024; RI random intercept adjusted β = −0.110, 95% CI −0.187 to −0.033, P= 0.005) (Table 3).
Discussion
Main findings
We demonstrated that in particular in placenta‐related complicated pregnancies, reduced first‐trimester maternal haemodynamic adaptation, assessed by higher MAP and UtA blood flow resistance, is associated with a smaller increase in first‐trimester placental development, i.e. PV and uPVV. No significant associations were identified between maternal haemodynamic adaptation and embryonic and fetal development. At birth, reduced first‐trimester maternal haemodynamic adaptation to pregnancy, as assessed by higher UtA blood flow resistance, is associated with lower placental weight in pregnancies with placenta‐related complications. In pregnancies without placenta‐related complications, reduced first‐trimester maternal haemodynamic adaptation, assessed by an increase in MAP trajectories, was associated with a higher birthweight centile, and an increase in UtA PI and UtA RI trajectories was associated with a shorter GA at birth.
Strengths and limitations
The longitudinal early first‐trimester measurements of MAP, UtA PI and UtA RI as markers of maternal haemodynamic adaptation to pregnancy are unique. Unfortunately, it was not possible to estimate both the random intercept and slope for all parameters representing haemodynamic adaptation due to the size of the study population.
Moreover, we used 3D Power Doppler ultrasound combined with VOCAL and VR as an innovative technique to measure PV, uPVV, CRL and EV. At our center there is broad experience in assessment of placental, embryonic and fetal measurements using VR. 17 We observed wide ranges of PV and uPVV values in this population. This is probably a result of (patho)physiological differences in placental development instead of inaccuracies, as these measurements of placental development were proven to be reproducible within and between observers. 17
Inclusion of participants from as early as 7 weeks gestation is unique in Doppler ultrasound. However, recruitment was performed in a tertiary hospital setting to enable this. The tertiary setting created a high‐risk and less generalisable study population, limiting external validity due to a high percentage of IVF/ICSI pregnancies (40.9%), presence of comorbidities and high median age (32 years).
In addition, for reasons of interaction with uteroplacental vascular development and inaccuracy of gestational age determination, miscarriages were excluded from analysis. As a downside this could have introduced selection bias because the data may not reflect all early first‐trimester pregnancies in the whole general population.
We corrected for a broad range of confounders. Conception mode is relevant because IVF/ICSI pregnancies are more at risk of developing pregnancy complications like FGR and pre‐eclampsia. 35 , 36 It is hypothesised that the hormonal treatment in IVF/ICSI pregnancies influences maternal vascular adaptation to pregnancy. 37 We also corrected for parity, because the placentas of nulliparous women are on average smaller at birth and nulliparous women more often develop placenta‐related complications. 38 Moreover, parity impacts UtA blood flow. 39 We also adjusted for fetal gender as it was demonstrated previously that this modifies first‐trimester placental development. However, residual confounding cannot be excluded being inherent to a prospective observational design.
Not all participants were included in the study as early as 7 weeks of gestation, resulting in missing ultrasound volumes in early pregnancy. Following critical appraisal of the quality of our 3D ultrasound data sets to ensure appropriate evaluation and generalisability, not all obtained data sets were included for assessment. The highest number of ultrasound volumes was available at 9 weeks of gestation. Subsequently, beyond 9 weeks of gestation these numbers were reduced because of increasing quality loss of the acquired ultrasound data or following a large placental size beyond this period. Moreover, obesity and/or uterine position may attenuate image quality. Therefore the included percentage of 3D data sets (70.4% for VOCAL data sets and 74.2% for VR, respectively) matched our expectations.
In general, we observed that directions of non‐significant effect estimates matched the directions of statistically significant values. Inherent to the explorative and observational design of our study, the likelihood of any Type 1 or Type 2 errors cannot be eliminated. We therefore propose that our results are considered most suitable for the purpose of hypothesis‐generating and hopefully to stimulate future investigations.
Interpretation
We hypothesised that the extent of first‐trimester maternal haemodynamic adaptation to pregnancy is associated with first‐trimester placental development, and also with fetal development and birth outcome. This is substantiated by our results, showing that in placenta‐related complicated pregnancies reduced first‐trimester maternal haemodynamic adaptation to pregnancy is associated with less optimal first‐trimester placental development and lower placental weight at birth. Available evidence of impaired maternal haemodynamic adaptation and a higher risk to develop placenta‐related complications supports these findings. 40 , 41 , 42 , 43 Moreover, previous research confirms that impaired vascular development in pre‐eclampsia or FGR in the presence of higher uterine artery Doppler indices is associated with lower serum placental growth factor and lower placental vascularisation indices. 14 , 17 , 44
Blood pressure and uterine artery blood flow are traditionally considered to be markers of placental development because increased values with advancing gestation are associated with many placenta‐related complications. 45 The direction of causality could be both ways; either less increase in uPVV as a result of reduced maternal haemodynamic adaptation, or reduced maternal haemodynamic adaptation as a response to impaired trophoblastic invasion, marked by less development of the uteroplacental vasculature. Accumulating evidence is pointing to the first direction, suggesting that changes in maternal uterine and spiral arteries, as reflected by UtA Doppler indices, are not a direct consequence from trophoblast invasion, but that pre‐pregnancy maternal haemodynamics precede placental development. 9 , 46 In future studies, other periconceptional determinants of maternal haemodynamics can be studied, e.g. cardiac function and small‐vessel endothelial function. Together they will likely contribute to improved preconception screening and treatment, thereby preventing placenta‐related complicated pregnancies. 47
Only in placenta‐related complicated pregnancies was reduced haemodynamic adaptation associated with a smaller increase in PV trajectories. This suggests that placental tissue and vasculature development are impacted differently and that the impact of first‐trimester haemodynamic (mal)adaptation seems more profound in placenta‐related complicated pregnancies. 46 Previous studies also describe reduced PV in pregnancies complicated by pre‐eclampsia or FGR. 48 , 49
Earlier work from our group revealed an inverse association between first‐trimester maternal vascular risk factors and embryonic size in pregnancies conceived after assisted reproduction. 50 In the current study, reduced first‐trimester maternal haemodynamic adaptation was not associated with embryonic or fetal development, possibly because we were challenged with a more restricted availability of embryonic/fetal measurements.
We recorded a significant association between reduced first‐trimester maternal haemodynamic adaptation and higher birthweight centile in uncomplicated pregnancies. A first explanation is the uncomplicated nature of this group and that median blood pressure levels remained within normal ranges for pregnancy (Figure 1). Second, factors impacting fetal growth during the second and third trimesters may have had a stronger influence the association between first‐trimester maternal haemodynamic adaptation and birthweight centile. Alternatively, the direction of this association could be considered a fetal compensatory phenomenon in response to a more unfavourable uteroplacental environment, which is substantiated by the developmental origins of health and disease paradigm. 10 A higher birthweight centile was not observed in placenta‐related complicated pregnancies, which advocates a reduced capacity to compensate for an unfavourable periconceptional uteroplacental environment.
The association between reduced first‐trimester maternal haemodynamic adaptation and lower placental weight in placenta‐related complicated pregnancies corresponds with previously reported findings of lower placental weight and birthweight in pregnancies complicated by pre‐eclampsia. 51 Furthermore, first‐trimester and postpartum placental size parameters are correlated with each other. 52 We attribute the absence of significance for other birth outcomes to the limited number of placenta‐related complications in this cohort and their onset beyond 32 weeks of gestation. For example, preterm birth comprised spontaneous and iatrogenic cases and the different pathophysiology of early‐onset versus late‐onset placenta‐related complications may have diluted the effect estimates.
Conclusion
This study suggests that reduced first‐trimester maternal haemodynamic adaptation to pregnancy, assessed by MAP and UtA blood flow, impairs early placental size and vascularisation, as measured by PV and uPVV, and birthweight centile. The impact was different for placenta‐related complicated pregnancies. Moreover, median values for MAP and UtA PI and UtA RI were higher in placenta‐related complicated pregnancies. Although these values were within normal ranges for the first trimester of pregnancy, 53 future investigation is necessary to study which clinical thresholds are sensitive to classify women with a vascular phenotype predisposed to placenta‐related complications. This might improve the accurate identification of those women who can benefit from preventive measures, such as daily acetylsalicylic acid administration, to diminish the risk of developing placenta‐related complications. Further, early identification of women at risk allows for implementation of a tailored scheme for antenatal surveillance already from the first trimester onwards. Also the lifestyle intervention www.smarterpregnancy.co.uk and blended lifestyle approach are promising evidence‐based interventions. 54
Assessment of first‐trimester maternal haemodynamic adaptation and placental development as performed in this study will also enhance future knowledge on the pathophysiology of placenta‐related complications. However, the added value of integrating first‐trimester parameters of maternal haemodynamic adaptation and PV and uPVV measurements into prediction models for placenta‐related complications has to be investigated in larger cohorts first.
Disclosure of interests
None. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
IR was involved in the study design, data‐acquisition, performance of measurements and data analysis, and wrote the first draft of the manuscript. AM, MK, ATMK, AHJK, SW, ES and RST were involved in the study design, data‐analysis and co‐writing of the manuscript. RST is initiator and guarantor of this work. All authors have read and approved the final version of the manuscript.
Details of ethical approval
The study was approved by the Institutional Review Board of the Erasmus Medical Centre (MEC 2015‐494) and written informed consent was obtained from all participants.
Funding
This research was funded by the Department of Obstetrics and Gynaecology of the Erasmus MC, University Medical Centre, Rotterdam, The Netherlands.
Supporting information
Figure S1. Flow chart of the Virtual Placenta Study population.
Table S1. Medians and interquartile ranges of first‐trimester maternal haemodynamic adaptation parameters and placental parameters, stratified for pregnancies with and without placenta‐related complications.
Table S2. Associations between reduced first‐trimester maternal haemodynamic adaptation and trajectories of embryonic development, stratified for pregnancies with and without placenta‐related complications.
Table S3. Associations between reduced first‐trimester maternal haemodynamic adaptation and fetal development, stratified for pregnancies with and without placenta‐related complications.▪
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank all the study participants without whom this study could not have been performed. Further, we would like to thank the Rotterdam Periconception Cohort (Predict study) team for supporting the recruitment and data collection, which contributed to the results of this study. We especially thank Nieke Kemper, Sin Mok and Anke Ummels for their contribution in the performance of the VOCAL and VR measurements.
Reijnders IF, Mulders AGMGJ, Koster MPH, Kropman ATM, Koning AHJ, Willemsen SP, Steegers EAP, Steegers‐Theunissen RPM. First‐trimester maternal haemodynamic adaptation to pregnancy and placental, embryonic and fetal development: the prospective observational Rotterdam Periconception cohort. BJOG 2022; 10.1111/1471-0528.16979.129:785–795.
Data availability statement
Data is available on request.
References
- 1. Degner K, Magness RR, Shah DM. Establishment of the human uteroplacental circulation: a historical perspective. Reprod Sci 2017;24:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridder A, Giorgione V, Khalil A, Thilaganathan B. Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci 2019;20:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meah VL, Cockcroft JR, Backx K, Shave R, Stöhr EJ. Cardiac output and related haemodynamics during pregnancy: a series of meta‐analyses. Heart 2016;102:518–26. [DOI] [PubMed] [Google Scholar]
- 4. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre‐eclampsia: pathophysiology and clinical implications. BMJ 2019;366:I2381. [DOI] [PubMed] [Google Scholar]
- 5. Steegers‐Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long‐term health of offspring: the importance of one‐carbon metabolism. Hum Reprod Update 2013;19:640–55. [DOI] [PubMed] [Google Scholar]
- 6. Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jauniaux E, Poston L, Burton GJ. Placental‐related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006;12:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet 2010;376:631–44. [DOI] [PubMed] [Google Scholar]
- 9. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–7. [DOI] [PubMed] [Google Scholar]
- 11. Wang SF, Shu L, Sheng L, Mu M, Wang S, Tao XY, et al. Birth weight and risk of coronary heart disease in adults: a meta‐analysis of prospective cohort studies. J Dev Orig Health Dis 2014;5:408–19. [DOI] [PubMed] [Google Scholar]
- 12. Sotiriadis A, Hernandez‐Andrade E, da Silva‐Costa F, Ghi T, Glanc P, Khalil A, et al. ISUOG CSC Pre‐eclampsia task force. ISUOG Practice Guidelines: role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol 2019;53:7–22. [DOI] [PubMed] [Google Scholar]
- 13. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The international Federation of Gynecology and Obstetrics (FIGO) intiative on preeclampsia: a pragmatic guide for first‐trimester screening and prevention. Int J Gynecol Obstet 2019;145:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hata T, Tanaka H, Noguchi J, Hata K. Three‐dimensional ultrasound evaluation of the placenta. Placenta 2011;32:105–15. [DOI] [PubMed] [Google Scholar]
- 15. Reus AD, El‐Harbachi H, Rousian M, Willemsen SP, Steegers‐Theunissen RP, Steegers EA, et al. Early first‐trimester trophoblast volume in pregnancies that result in live birth or miscarriage. Ultrasound Obstet Gynecol 2013;42:577–84. [DOI] [PubMed] [Google Scholar]
- 16. Eastwood KA, Patterson C, Hunter AJ, McCance DR, Young IS, Holmes VA. Evaluation of the predictive value of placental vascularization indices derived from 3‐Dimensional power Doppler whole placental volume scanning for prediction of pre‐eclampsia: a systematic review and meta‐analysis. Placenta 2017;51:89–97. [DOI] [PubMed] [Google Scholar]
- 17. Reijnders IF, Mulders AGMGJ, Koster MPH, Koning AHJ, Frudiger A, Willemsen SP, et al. New imaging markers for preconceptional and first‐trimester utero‐placental vascularization. Placenta 2018;61:96–102. [DOI] [PubMed] [Google Scholar]
- 18. Exalto N, Mulders A, Steegers E. Investigating the early human placental circulations: a challenge for anatomists and diagnostic imaging. BJOG 2021;128:1386–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rousian M, Koster MPH, Mulders AGMGJ, Koning AHJ, Steegers‐Theunissen RPM, Steegers EAP. Virtual reality imaging techniques in the study of embryonic and early placental health. Placenta 2018;64:S29–35. [DOI] [PubMed] [Google Scholar]
- 20. Reijnders IF, Mulders AG, Koster MP, Kropman AT, de Vos ES, Koning AH, et al. First‐trimester utero‐placental (vascular) development and embryonic and fetal growth: The Rotterdam Periconception cohort. Placenta 2021;108:81–90. [DOI] [PubMed] [Google Scholar]
- 21. Steegers‐Theunissen RPM, Verheijden‐Paulissen JJFM, van Uitert EM, Wildhagen MF, Exalto N, Koning AHJ, et al. Cohort profile: the Rotterdam Periconceptional Cohort (Predict Study). Int J Epidemiol 2016;45:374–81. [DOI] [PubMed] [Google Scholar]
- 22. Centraal Bureau Statistiek . Bevolking; generatie, geslacht, leeftijd en migratieachtergrond (Dutch); 2019. [https://opendata.cbs.nl/statline/#/CBS/nl/dataset/37325/table?ts=15656946583552019]. Accessed 13 August 2019. [Google Scholar]
- 23. Statistics Netherlands . The Dutch Standard Classification of Education. The Hague, The Netherlands: Statistics Netherlands; 2016. [Google Scholar]
- 24. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol 1985;151:333–7. [DOI] [PubMed] [Google Scholar]
- 25. Khalil A, Nicolaides KH. How to record uterine artery Doppler in the first trimester. Ultrasound Obstet Gynecol 2013;42:478–9. [DOI] [PubMed] [Google Scholar]
- 26. The British Medical Ultrasound Society . Guidelines for the safe use of diagnostic ultrasound equipment. 2009. [https://www.bmus.org/static/uploads/resources/BMUS‐Safety‐Guidelines‐2009‐revision‐FINAL‐Nov‐2009.pdf]. Accessed 9 August 2019. [Google Scholar]
- 27. Bhide A, Acharya G, Bilardo CM, Brezinka C, Cafici D, Hernandez‐Andrade E, et al. ISUOG Practice Guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41:233–9. [DOI] [PubMed] [Google Scholar]
- 28. Verwoerd‐Dikkeboom CM, Koning AH, Hop WC, Rousian M, van der Spek PJ, Exalto N, et al. Reliability of three‐dimensional sonographic measurements in early pregnancy using virtual reality. Ultrasound Obstet Gynecol 2008;32:910–6. [DOI] [PubMed] [Google Scholar]
- 29. Rousian M, Koning AH, van Oppenraaij RH, Hop WV, Verwoerd‐Dikkeboom CM, van der Spek PJ, et al. An innovative virtual reality technique for automated human embryonic volume measurements. Hum Reprod 2010;25:2210–6. [DOI] [PubMed] [Google Scholar]
- 30. Group BP . Overview of pregnancy complications. [https://bestpractice.bmj.com/topics/en‐us/494]. Updated September 2018. Accessed 11 February 2019.
- 31. American College of Obstetricians and Gynaecologists. ACOG practice bulletin No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol 2019;133:e1–25. [DOI] [PubMed] [Google Scholar]
- 32. Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition for placental fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. [DOI] [PubMed] [Google Scholar]
- 33. Verfaille V, de Jonge A, Mokkink L, Westerneng M, van der Horst H, Jellema P, et al. IRIS study group. Multidisciplinary consensus on screening for, diagnosis and management of fetal growth restriction in the Netherlands. BMC Pregnancy Childbirth 2017;17:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeve D, Regelmann MO, Holzman IR, Rapaport R. Small at birth, but how small? The definition of SGA revisited. Horm Res Paediatr 2016;86:357–60. [DOI] [PubMed] [Google Scholar]
- 35. Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström‐Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta‐analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- 36. Carbone IF, Cruz JJ, Sarquis R, Akolekar R, Nicolaides KH. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11–13 weeks' gestation. Hum Reprod 2011;26:1659–1664. [DOI] [PubMed] [Google Scholar]
- 37. Haavaldsen C, Tanbo T, Eskild A. Placental weight in singleton pregnancies with and without assisted reproductive technology: a population study of 536,567 pregnancies. Hum Reprod 2012;27:576–82. [DOI] [PubMed] [Google Scholar]
- 38. Rurangirwa AA, Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Hemodynamic adaptations in different trimesters among nulliparous and multiparous pregnant women; the Generation R study. Am J Hypertens 2012;25:892–9. [DOI] [PubMed] [Google Scholar]
- 39. Dane B, Batmaz G, Ozkal F, Bakar Z, Dane C. Effect of parity on first‐trimester uterine artery Doppler indices and their predictive value for pregnancy complications. Gynecol Obstet Invest 2014;77:24–8. [DOI] [PubMed] [Google Scholar]
- 40. Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol 2003;15:465–71. [DOI] [PubMed] [Google Scholar]
- 41. Browne VA, Julian CG, Toledo‐Jaldin L, Cioffi‐Ragan D, Vargas E, Moore LG. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 2015;370:20140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hafner E, Metzenbauer M, Stümpflen I, Waldhör T, Philipp K. First trimester placental and myometrial blood perfusion measured by 3D power Doppler in normal and unfavourable outcome pregnancies. Placenta 2010;31:756–63. [DOI] [PubMed] [Google Scholar]
- 43. Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension 2018;72:442–50. [DOI] [PubMed] [Google Scholar]
- 44. Eastwood KA, Hunter AJ, Patterson CC, Mc Cance DR, Young IS, Holmes VA. Placental vascularization indices and prediction of pre‐eclampsia in high‐risk women. Placenta 2018;70:53–9. [DOI] [PubMed] [Google Scholar]
- 45. Hauspurg A, Parry S, Mercer BM, Grobman W, Hatfield T, Silver RM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol 2019;221:277.e1–277.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension 2019;73:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bijl RC, Cornette JMJ, van den Bosch AE, Duvekot JJ, Molinger J, Willemsen SP, et al. Study protocol for a prospective cohort study to investigate Hemodynamic Adaptation to Pregnancy and Placenta‐related Outcome: the HAPPO study. BMJ Open 2019;9:e033083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hanchard TJ, de Vries BS, Quinton AE, Sinosich M, Hyett JA. Are first‐trimester ultrasound features prior to 11 weeks' gestation and maternal factors able to predict maternal hypertensive disorders? Ultrasound Obstet Gynecol 2020;55:629–36. [DOI] [PubMed] [Google Scholar]
- 49. Arakaki T, Hasegawa J, Nakamura M, Takita H, Hamada S, Oba T, et al. First‐trimester measurements of the three‐dimensional ultrasound placental volume and uterine artery Doppler in early‐ and late‐onset fetal growth restriction. J Matern Fetal Neonatal Med 2018;19:1–6. [DOI] [PubMed] [Google Scholar]
- 50. Wijnands KP, van Uitert EM, Roeters van Lennep JE, Koning AH, Mulders AG, Laven JS, et al. The periconception maternal cardiovascular risk profile influences human embryonic growth trajectories in IVF/ICSI pregnancies. Hum Reprod 2016;31:1173–81. [DOI] [PubMed] [Google Scholar]
- 51. Effendi M, Demers S, Giguère Y, Forest JC, Brassard N, Girard M, et al. Association between first‐trimester placental volume and birth weight. Placenta 2014;35:99–102. [DOI] [PubMed] [Google Scholar]
- 52. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 2016;96:1509–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gómez O, Figueras F, Fernández S, Bennasar M, Martínez JM, Puerto B, et al. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol 2008;32:128–32. [DOI] [PubMed] [Google Scholar]
- 54. van der Windt M, van der Kleij RM, Snoek KM, Willemsen SP, Dykgraaf RHM, Laven JSE, et al. Impact of a blended periconception lifestyle care approach on lifestyle behaviors: before‐and‐after study. J Med Internet Res 2020;22:e19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the Virtual Placenta Study population.
Table S1. Medians and interquartile ranges of first‐trimester maternal haemodynamic adaptation parameters and placental parameters, stratified for pregnancies with and without placenta‐related complications.
Table S2. Associations between reduced first‐trimester maternal haemodynamic adaptation and trajectories of embryonic development, stratified for pregnancies with and without placenta‐related complications.
Table S3. Associations between reduced first‐trimester maternal haemodynamic adaptation and fetal development, stratified for pregnancies with and without placenta‐related complications.▪
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
Data is available on request.


