Abstract
Background and objectives
Breast‐conserving surgery (BCS) is followed by reoperations in approximately 25%. Reoperations lead to an increased risk of infection and wound healing problems as well as a worse cosmetic outcome. Several technical approaches for an intraoperative margin assessment to decrease the reoperation rate are under evaluation, some of them are still experimental.
Methods
A prospective single‐arm post‐marketing study with 60 patients undergoing BCS for ductal carcinoma in situ (DCIS) and invasive breast cancer was conducted. The specimen was intraoperatively examined by the ClearSight™ system, a mobile magnetic resonance imaging system that is based on a diffusion‐weighted imaging protocol. However, the results were blinded to the surgeon.
Results
The ClearSight™ system was performed for both ductal and lobular breast cancer and DCIS, with a sensitivity of 0.80 (95% confidence interval [CI]: 0.44–0.96) and a specificity of 0.84 (95% CI 0.72–0.92), with an overall diagnostic accuracy of 80%.
Conclusion
Had the ClearSight™ been known to the surgeon intraoperatively, the reoperation rate would have been reduced by 83% for invasive carcinoma, from 10% to 2%, and 50% for DCIS, from 30% to 15% reoperations. A trial designed to examine the impact on reoperation rates is currently ongoing.
Keywords: diffusion weighted, lumpectomy, re‐excision rate, surgical margins
Abbreviations
- ADC
apparent diffusion coefficient
- BCS
breast conserving surgery
- DCIS
ductal carcinoma in situ
- DW
diffusion weighted
- IBC
invasive breast cancer
- IOUS
intraoperative ultrasound
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OCT
optical coherence tomography
- ROC
receiver operating characteristic
1. INTRODUCTION
Breast cancer is the most common invasive cancer in women worldwide, comprising 25.2% of invasive cancer in women and causing 14.2% of cancer deaths in women. 1
Breast‐conserving surgery (BCS) is one of the most common procedures performed for the treatment of breast cancer. The aim of BCS is to remove the malignant tissue with clear margins, that is, with no ink on tumor for invasive breast cancer (IBC) and with a rim of normal tissue around it in ductal carcinoma in situ (DCIS). Achieving clear margins is important to decrease the risk of local recurrence, which is 2–3 times more frequent if lumpectomy margins are not clear. 2 , 3 , 4 , 5 Reported positive margin rates after BCS for IBC and DCIS are 15%–47% 6 , 7 , 8 , 9 and 20%–81%, 10 , 11 , 12 , 13 , 14 , 15 , 16 respectively. Re‐excision and completion mastectomy rates range between 23% and 59%. 17 , 18 , 19 , 20 Reoperation for involved margins is associated with worse cosmetic outcomes, increased medical costs, and patient anxiety. Therefore, obtaining negative margins during primary BCS is essential. To date, there are a number of methods for the intraoperative assessment of surgical margins in DCIS and IBC, such as frozen section, specimen radiography, and intraoperative ultrasound (IOUS). 21 Frozen section is very time‐consuming, technically challenging since breast tissue is often fatty and hard to freeze and cut, requires evaluation by an experienced pathologist, thus, ranging sensitivity of 65%–78%. 22 The use of radiographic X‐ray mammography is limited due to limitations in detecting small noncalcific lesions and a high rate of nonspecific findings. 23 Specimen radiography found a sensitivity and specificity for detecting margin positivity of 49%–53% and 77%–84%, respectively, 24 IOUS is a well‐known tumor localization technique. However, when used for margin assessment, it is less suitable for invivible lesions and microcalcifications detection, 25 found to have a sensitivity of 59% and a specificity of 81%. 26 Both imaging methods present a valuable addition to BCS.
However, intraoperative assessment of surgical margins in DCIS remains challenging and is an unmet need. Magnetic resonance imaging (MRI) was reported to be an accurate imaging technology for breast cancer detection, with high sensitivity compared with conventional techniques, and provides highly sensitive information on DCIS. 27 , 28 , 29
The novel ClearSight™ system (ClearCut Medical Ltd.), is a CE mark‐approved intraoperative MRI‐based device designed to assess surgical specimen margins in real‐time.
The objective of this study is to evaluate the performance of the ClearSight™ system in assessing surgical margins for DCIS and IBC and assessing the ability of the ClearSight™ as an adjunct tool to reduce the re‐excision rate following primary BCS.
2. MATERIALS AND METHODS
2.1. The ClearSight™ system
The ClearSight™ system is an intraoperative mobile MRI system, designed for real‐time magnetic resonance (MR) measurement of excised breast tissue margins in the operating room.
The system is based on a diffusion‐weighted (DW) imaging protocol, which measures the random thermal Brownian diffusion of water molecules in the tissue. The rate of water diffusion is quantified by the apparent diffusion coefficient (ADC), which was shown to be a good differentiator parameter of malignant and benign breast tumors with a sensitivity of 93% and specificity of 88%. 30 , 31 The ClearSight™ system measures the T‐2 star (T2*) value, which has an inverse relationship between the cellularity and ADC, hence the ability to distinguish malignant and nonmalignant tumors in freshly excised breast tissue. The system scans a predefined 4 mm diameter and 1 mm thickness of the excised tissue surface and generates a two‐dimensional color scale (Figure 1).
Figure 1.
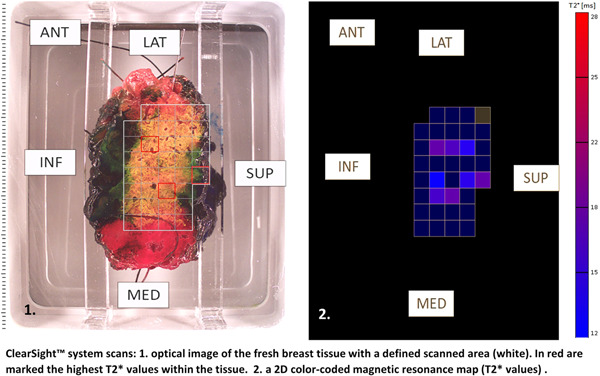
ClearSight™ system scans: (1) Optical image of the fresh breast tissue with a defined scanned area (white). In red are marked the highest T2* values within the tissue. (2) A 2D color‐coded magnetic resonance map (T2* values). 2D, two dimensional; ANT, anterior; INF, inferior; LAT, lateral; MED, medial; SUP, superior
2.2. Study design
The study was a prospective, single‐arm, blinded postmarketing study that enrolled 63 patients. It was conducted at the Agaplesion Markus Hospital, following the approval of the institutional ethics committee. Each patient signed an informed consent form before enrollment in the study. Women over 18 years diagnosed with IBC or DCIS of the breast were included in the study. All patients were eligible for BCS, had no prior systemic neoadjuvant therapy, and had no prior breast surgery or implants. The BCS included a routine lumpectomy and criteria for re‐excision were according to site and surgeon discretion using standard methods. No standardization between surgeons' surgical methods was required. All aspects of the freshly excised breast specimens were inked by the surgeon intraoperatively to uniquely define margin orientation for accurate comparison to final histopathology results. Following completion of the lumpectomy, that is, segmentectomy, multiple margins were scanned using the ClearSight™ system, generating a color‐coded map of the T2* MR values, representing the tissue's DW properties (Figure 1). The ClearSight™ was operated by either site or ClearCut personnel. As the ClearSight™ system was used following the completion of the lumpectomy/segmentectomy. All medical staff members were blinded to the ClearSight™ results, and patient management was not affected by the MR findings. Following MR scans, the specimens were sent for standard histopathology and margin assessment evaluation including routine hematoxylin and eosin staining, as per site protocols. The pathologist was unblinded to the ClearSight™ system output, to sample further tissue depicted with high restricted diffusion properties and high T2* values. Final margin status for the main lumpectomy/segmentectomy specimen margins as reported in the pathology report was compared to the margin status as determined by the T2* MR values of the ClearSight™ system, allowing for evaluation of the system's performance to detect positive margins with a subsequent potential reduction of postoperative positive margins.
2.3. Statistical analysis
The primary analysis was completed on all patients and specimen aspects with valid ClearSight™ outputs and corresponding histopathology assessments. Scans were classified as malignant or nonmalignant based on the MR signal as presented by calculated T2* values and scored according to the correlation with pathology findings ≤1 mm from the margin. The primary endpoint of the study was the ability of the ClearSight™ system to assess the presence of malignant findings within 1 mm from excised specimen margins, compared to the gold standard histopathology examination. The gold standard for margin assessment is histopathology, evaluating the microscopic distance from the tumor to each specimen margin and distinguish between tumor on‐ink and a close margin. Nevertheless, there is no consensus and there is a lack of standardization in the pathological method regarding the definition of an adequate negative margin, thus followed by various patient management.
No ink on tumor for IBC is guided by the Society of Surgical Oncology and American Society of Radiation Oncology (SSO‐ASTRO) guideline, 32 while the Association of Breast Surgery United Kingdom (ABS‐UK), 33 and the German Society for Gynecology and Obstetrics and the German Cancer Society refers to a clear margin at 1 mm from all sides of the tumor (Germany S3 guideline). 34 , 35 As for pure DCIS, both the SSO‐ASTRO and the German guideline encounter a clear margin at 2 mm, rather a 1 mm surgical margin by the ABS‐UK.
As mentioned before, the ClearSight™ system can distinguish malignant and nonmalignant tumors in freshly excised breast tissue up to 1 mm depth.
Analysis of the results included ClearSight™ system accuracy performance over time, using Receiver operating characteristic (ROC) curve analysis with the entire range of T2* values measured in the study, and sensitivity and specificity calculations.
All continuous variables were presented as mean and standard deviation, whereas categorical values were presented by frequencies and percentages, when appropriate. All statistical analyses were two‐tailed tests and significance was set at 5%. No missing data were imputed. Results were analyzed using SPSS version 23.0 (IBM, SSPS Inc).
3. RESULTS
Sixty‐three patients were enrolled in the trial between August 2017 and April 2018. Three patients did not meet inclusion criteria due to neoadjuvant therapy, formalin fixation before specimen scan, and inability to ascertain specimen margins (one each). From the 60 patients, the ClearSight™ system performed and analyzed a total of 348 scanned aspects (5.8 ± 0.5 scans per patient). The mean patient age was 61.2 years (36–80 years). Forty‐two patients (70%) had invasive ductal carcinoma, four patients (7%) had invasive ductal carcinoma with DCIS, nine patients (15%) had pure DCIS, and five (8%) had invasive lobular carcinoma. The majority of tumors were either well (33%), or moderately (64%) differentiated. Patient demographics and preoperative tumor type are listed in Table 1. BCS procedures were performed by three surgeons. Extensions were taken in 35% of cases.
Table 1.
Patient demographics and preoperative characteristics
| Parameter | Value |
|---|---|
| Age n (%) | |
| Mean (STD) | 61.2 (10.5) |
| <40 | 1 (2) |
| 40–50 | 10 (17) |
| 50–60 | 14 (23) |
| 60–70 | 20 (33) |
| >70 | 15 (25) |
| Body mass index (kg/m2), n (%) | |
| <18.5 | 8 (13) |
| 18.5–25 | 27 (45) |
| 25–30 | 22 (37) |
| >30 | 3 (5) |
| Tumor type, n (%) | |
| IDC | 42 (70) |
| ILC | 5 (8) |
| IDC with DCIS | 4 (7) |
| DCIS | 9 (15) |
| Tumor grade, n (%) | |
| I | 20 (33) |
| II | 38 (64) |
| III | 2 (3) |
Abbreviations: ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Mean calculated tumor volume (estimated by multiplying the three dimensions) was 3.1 ± 6.8 cc, and 52% (31/60) of the main lumpectomy/segmentectomy specimens contained at least one positive margin within 1 mm per histology. Of the 348 margins assessed, 15.5% (54/348) margins had a pathological finding, the majority (9.5%) being DCIS, and 6.0% with invasive ductal carcinoma at the margin. Tumor characterization based on final pathology results is presented in Table 2.
Table 2.
Specimen and tumor characteristics
| Parameter | Value |
|---|---|
| Specimen volume (cc) | |
| Mean (STD) | 48.2 (31.1) |
| Specimen weight (g) | |
| Mean (STD) | 20.1 (13.2) |
| Tumor volume (cc) | |
| Mean (STD) | 3.1 (6.8) |
| Histology margin assessment (≤1 mm) | |
| Positive margins, n (%) | 54 (16) |
| IDC | 13 (24) |
| ILC | 6 (11) |
| IDC + DCIS | 2 (4) |
| DCIS | 32 (59) |
| ADH | 1 (2) |
| Negative margins, n (%) | 294 (84) |
Note: The table presents histopathology data on the specimens.
Abbreviations: ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
The maximum ClearSight™ T2* value calculated for each specimen aspect and presented as a numerical output was 12.4 ± 5.7 ms for nonmalignant tissue and 16.7 ± 2.7 ms for malignant tissue. T2* was significantly different between the two groups (p < 0.00001). A ROC analysis, depicted in Figure 2, demonstrated the ClearSight™ performance had a sensitivity of 0.65 (95% confidence interval [CI]: 0.52–0.78) and specificity of 0.80 (95% CI: 0.75–0.85), with an overall diagnostic accuracy of 80%. When looking at different cancer types, the sensitivity for IBC and DCIS were 0.86 (95% CI: 0.71–1.00), and 0.52 (95% CI: 0.35–0.69), respectively. Out of these findings, there were seven IBC findings on‐ink, of which six (86%) were detected by the Clearsight™ system (Table 3). The system performance difference between histopathology types was found to be affected by tissue dehydration which occurs over time from excision. When analyzing system capabilities within 1 h from specimen excision (67 scans), the diagnostic performance was shown to improve and found to be the same for both histopathology subtypes, ductal and lobular IBC and DCIS, with a sensitivity of 0.80 (95% CI: 0.44–0.96) and specificity 0.84 (95% CI: 0.72–0.92). Anyway, there is no reason to wait longer than 1 h when intraoperative results are needed to continue the operation.
Figure 2.
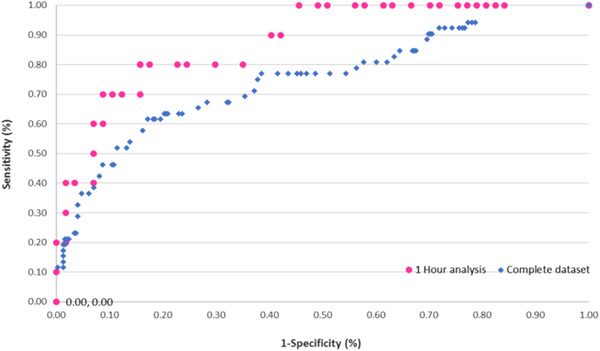
Receiver operating characteristics (ROC) curve of ClearSight™ performance (all histological subtypes). ROC curve of two different datasets: (1) complete dataset containing all samples (squares) and (2) samples scanned within 1 h from specimen excision (circles)
Table 3.
Device detection rate for tumor at the margin by histological subtypes
| Cancer histopathology | Number of samples (n) | Detected samples (n) | Detection rate (%) | |||
|---|---|---|---|---|---|---|
| Invasive cancer | ≤1 mm | On‐ink | ≤1 mm | On‐ink | ≤1 mm | On‐ink |
| IDC | 13 | 6 | 11 | 6 | 85 | 100 |
| ILC | 6 | 1 | 5 | 0 | 83 | 0 |
| IDC + DCIS | 2 | 0 | 2 | NA | 100 | NA |
| All | 21 | 7 | 18 | 6 | 86 | 86 |
| Noninvasive cancer | ||||||
| DCIS, ADH | 33 | 17 | 52 | |||
Note: The table presents a number of histology positive margins per subtypes and the corresponding margins detected by the ClearSight™ system.
Abbreviations: ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Data were further analyzed at the patient level. Results from all individual margins for a patient were compiled to yield the patient‐level classification. For a patient with a positive specimen to be considered as “successfully detected,” it was required that all pathological positive margins on the specimen were detected. In 24 out of 60 (40%) patients, the initially excised specimens were positive by histopathology and were not addressed intraoperatively by tissue re‐excision, resulting in postoperative referral for a re‐excision BCS; this comprised 6 patients with pure IBC, 13 patients with DCIS at the margin, and 5 patients with both invasive and DCIS at the margin. Of these positive specimens, the ClearSight™ successfully detected five (83%) in whom margins were involved by invasive ductal carcinoma and nine (50%) of specimens with DCIS at the margin. These detection rates characterize ClearSight™ system diagnostic performance at the per‐margin and per‐patient levels. Overall, had the ClearSight™ been used intraoperatively, the reoperation rate would have been reduced by 83% for invasive carcinoma, from 10% to 2%, and 50% for DCIS, from 30% to 15% reoperations.
Accordingly, the ClearSight™ could provide useful adjunctive intraoperative inputs and guide decision‐making, with respect to surgical margin adequacy, and contribute to improved patient outcomes.
4. DISCUSSION
Real‐time intraoperative margin assessment in BCS continues to pose a challenge, with positive margin rates requiring relumpectomy in the range of 11%–46%. 36 Novel technologies for intraoperative margin assessment are being explored, and several technologies are in various stages of development and testing.
We report herein on the results of the ClearSight™ system and have demonstrated a sensitivity and specificity of up to 80% and 84%, respectively. These results are in concordance with the results of a previously published study, 37 which showed that DW MRI is a valid technology for the assessment of tumors in freshly excised tissue. The prior study examined 6 mm sections of breast tissue after surgery and found a sensitivity and specificity of 91% and 93%, respectively. In the current study, we measured whole aspects of entire specimens and found a somewhat lower sensitivity and specificity, attributable to the fact that an entire surface was scanned, which is more heterogeneous in terms of types of tissue compared to a small (6 mm) sample. However, despite the compound tissue types at the surface of a whole specimen, the ClearSight™ system was able to differentiate benign from malignant tissue with a high level of accuracy (84%).
Other technologies which are being developed for the same purpose include radio frequency (RF) spectroscopy, bioimpedance spectroscopy, fluorescence molecular imaging, and technologies‐based optical coherence tomography (OCT).
RF spectroscopy is the technology used by the MarginProbe®, and in several clinical and postmarketing trials has been shown to reduce reoperation rate by 56% but suffered from low specificity with a 53.6% false‐positive rate. 38 , 39 The procedure involves an examination of the specimen by a handheld probe which samples a 7 mm area in each measurement, in under 1 s. Sensitivity and specificity are reported in the range of 71% and 68%, respectively. 40 , 41
Bioimpedance spectroscopy 42 measures dielectric tissue properties on the freshly excised lumpectomy specimen and can differentiate fatty and fibrotic breast tissue from tumors. In one reported trial, the device had a sensitivity of 87.3%, specificity of 75.6%, and the potential to decrease reoperation rate from 37% to 7%.
Fluorescence molecular imaging, or Cerenkov luminescence imaging, uses positron emission tomography of the specimen after injecting patients intravenously with 18F‐fluorodeoxyglucose before surgery. In a preliminary report on 22 patients, the procedure was demonstrated to be safe and potentially effective in assessing lumpectomy margins during surgery. However, this technology relies on preoperative systemic administration of exogenous radioactive dye creating potential barriers to clinical implementation. 43
OCT, a novel technique capable of high‐resolution imaging without a contrast agent, uses interferometry, creating an image based on the amount of backscattered light, with microscopic resolution. Preliminary studies have reported a high sensitivity in the range of 80%–94%, and a specificity of 87%–93% for detecting cancer in mastectomy specimens, 44 , 45 , 46 and sensitivity of 55%–65% and specificity of 68%–70% 47 in BCS, which may be related to the limited ability of the OCT or optical coherence micro‐elastography to distinguish between tumor and surrounding normal stroma. 48 Additional OCT‐based technologies yielded a sensitivity of 91.7% and specificity of 92.1% following wide local excision of 35 human breast tumors. 44
In the current study, a full evaluation of almost the entire surface of the excised specimen was performed by DW MRI scanning and compared to the histopathological findings meticulously.
The advantage of the ClearSight™ system is that it uses well‐established technology for the characterization of tissue, offers full‐surface scanning, and does not require to expertize in X‐ray interpretation or pathology evaluation. The system can be operated by the regular operation room staff with minimal training.
5. CONCLUSION
Our results suggest that the ClearSight™ system has the potential to reduce reoperation rates by up to 80% for patients undergoing BCS when used at the time of surgery on freshly excised tissue.
This study is limited by the fact that findings were not communicated back to the surgeon and were not actionable, and, therefore, did not influence intraoperative decision‐making regarding excising additional tissue.
Evaluation of the technology in a trial designed to examine the impact on reoperation rates is currently ongoing and will establish the utility of the ClearSight™ system in clinical practice. A clinical trial would also need to assess the impact of the logistics of specimen orientation and scanning on operative time and workflow in the operating room.
CONFLICT OF INTERESTS
Marc Thill received honoraria for advisory boards from ClearCut, Norgine, Neodynamics, pfm medical, RTI Surgical, and Sysmex; manuscript support from ClearCut and pfm medical; travel reimbursement from Medtronic, Norgine, pfm Medical, and RTI Surgical; and lecture honoraria from Medtronic, pfm medical, and RTI Surgical. Iris Szwarcfiter is a Clear‐Cut employee and has a financial interest in the company. Katharina Kelling received honoraria for advisory boards from pfm medical. Eyal Kolka is a Clear‐Cut's Innovator and has a financial interest in the company. Zachi Peles is a Clear‐Cut employee and has a financial interest in the company. Moshe Papa is a Clear‐Cut consultant and has a financial interest in the company. Vivane van Haasteren, Josefa Noelke, Sebastian Aulmann, and Tanir Allweis declare that there are no conflict of interests.
SYNOPSIS
The ClearSight™ system, a mobile magnetic resonance imaging system that is based on diffusion‐weighted imaging, was used in a prospective single‐arm study consisting of 60 patients who underwent breast‐conserving surgery for in situ and invasive breast cancer. The ClearSight™ system was used for intraoperative margin assessment as an adjunctive tool to the surgeon's standard of care and could have led to a reduction of the reoperation rate by 83% if the results had not been blinded to the surgeon.
ACKNOWLEDGMENT
This study was supported by ClearCut Medical Ltd.
Thill M, Szwarcfiter I, Kelling K, et al. Magnetic resonance imaging system for intraoperative margin assessment for DCIS and invasive breast cancer using the ClearSight™ system in breast conserving surgery—Results from a postmarketing study. J Surg Oncol. 2022;125:361‐368. 10.1002/jso.26721
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Cancer IAfRo . World Cancer Report. 2014.
- 2. Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast‐conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668‐1675. [DOI] [PubMed] [Google Scholar]
- 3. Leong C, Boyages J, Jayasinghe UW, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node‐negative breast carcinoma. Cancer. 2004;100:1823‐1832. [DOI] [PubMed] [Google Scholar]
- 4. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747‐752. [DOI] [PubMed] [Google Scholar]
- 5. Mullen R, Macaskill EJ, Khalil A, et al. Involved anterior margins after breast conserving surgery: is re‐excision required? Eur J Surg Oncol. 2012;38:302‐306. [DOI] [PubMed] [Google Scholar]
- 6. Lovrics PJ, Cornacchi SD, Farrokhyar F, et al. The relationship between surgical factors and margin status after breast‐conservation surgery for early stage breast cancer. Am J Surg. 2009;197:740‐746. [DOI] [PubMed] [Google Scholar]
- 7. Acosta JA, Greenlee JA, Gubler KD, Goepfert CJ, Ragland JJ. Surgical margins after needle‐localization breast biopsy. Am J Surg. 1995;170:643‐645. [DOI] [PubMed] [Google Scholar]
- 8. Gajdos C, Tartter PI, Bleiweiss IJ, et al. Mammographic appearance of nonpalpable breast cancer reflects pathologic characteristics. Ann Surg. 2002;235:246‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papa MZ, Klein E, Davidson B, et al. The effect of anesthesia type on needle localization breast biopsy: another point of view. Am J Surg. 1996;171:242‐243. [DOI] [PubMed] [Google Scholar]
- 10. Schouten van der Velden AP, Boetes C, Bult P, Wobbes T. The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am J Surg. 2006;192:172‐178. [DOI] [PubMed] [Google Scholar]
- 11. Thomas J, Evans A, Macartney J, et al. Radiological and pathological size estimations of pure ductal carcinoma in situ of the breast, specimen handling and the influence on the success of breast conservation surgery: a review of 2564 cases from the Sloane Project. Br J Cancer. 2010;102:285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fadare O, Clement NF, Ghofrani M. High and intermediate grade ductal carcinoma in‐situ of the breast: a comparison of pathologic features in core biopsies and excisions and an evaluation of core biopsy features that may predict a close or positive margin in the excision. Diagn Pathol. 2009;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahoo S, Recant WM, Jaskowiak N, Tong L, Heimann R. Defining negative margins in DCIS patients treated with breast conservation therapy: The University of Chicago experience. Breast J. 2005;11:242‐247. [DOI] [PubMed] [Google Scholar]
- 14. Melstrom LG, Melstrom KA, Wang EC, Pilewskie M, Winchester DJ. Ductal carcinoma in situ: size and resection volume predict margin status. Am J Clin Oncol. 2010;33:438‐442. [DOI] [PubMed] [Google Scholar]
- 15. Dicker AP, Williams TL, Grant DS. Targeting angiogenic processes by combination rofecoxib and ionizing radiation. Am J Clin Oncol. 2001;24:438‐442. [DOI] [PubMed] [Google Scholar]
- 16. Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45:536‐543. [DOI] [PubMed] [Google Scholar]
- 17. McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467‐475. [DOI] [PubMed] [Google Scholar]
- 18. Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44:1005‐1015. [DOI] [PubMed] [Google Scholar]
- 19. Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re‐excision among women undergoing breast‐conserving surgery for cancer. Ann Surg Oncol. 2008;15:1297‐1303. [DOI] [PubMed] [Google Scholar]
- 20. O'Donnell ME, Salem A, Badger SA, Sharif MA, Lioe T, Spence RA. Completion mastectomy after breast conserving surgery. Breast. 2008;17:199‐204. [DOI] [PubMed] [Google Scholar]
- 21. Thill M, Baumann K. New technologies in breast cancer surgery. Breast Care (Basel). 2012;7:370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RG Pleijhuis, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast‐conserving therapy for patients with early‐stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16:2717‐2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huynh PT, Jarolimek AM, Daye S. The false‐negative mammogram. Radiographics. 1998;18:1137‐1154. [DOI] [PubMed] [Google Scholar]
- 24. Lee CH, Carter D. Detecting residual tumor after excisional biopsy of impalpable breast carcinoma: efficacy of comparing preoperative mammograms with radiographs of the biopsy specimen. Am J Roentgenol. 1995;164:81‐86. [DOI] [PubMed] [Google Scholar]
- 25. Smith LF, Rubio IT, Henry‐Tillman R, Korourian S, Klimberg VS. Intraoperative ultrasound‐guided breast biopsy. Am J Surg. 2000;180:419‐423. [DOI] [PubMed] [Google Scholar]
- 26. St John ER, Al‐Khudairi R, Ashrafian H, et al. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: a meta‐analysis. Ann Surg. 2017;265(2):300‐310. [DOI] [PubMed] [Google Scholar]
- 27. Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma‐in‐situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003;10:381‐388. [DOI] [PubMed] [Google Scholar]
- 28. Hata T, Takahashi H, Watanabe K, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: a comparative study with mammography and ultrasonography. J Am Coll Surg. 2004;198:190‐197. [DOI] [PubMed] [Google Scholar]
- 29. Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485‐492. [DOI] [PubMed] [Google Scholar]
- 30. Fornasa F, Pinali L, Gasparini A, Toniolli E, Montemezzi S. Diffusion weighted magnetic resonance imaging in focal breast lesions: analysis of 78 cases with pathologic correlation. Radiol Med. 2011;116:264‐275. [DOI] [PubMed] [Google Scholar]
- 31. Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion‐weighted imaging. J Magn Reson Imaging. 2002;16:172‐178. [DOI] [PubMed] [Google Scholar]
- 32. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of surgical oncology—American Society for Radiation Oncology; consensus guideline on margins for breast‐conserving surgery with whole‐breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2015;21(3):704‐716. [DOI] [PubMed] [Google Scholar]
- 33. Association of Breast Surgery at BASO 2009 . Surgical guidelines for the management of breast cancer. Eur J Surg Oncol. 2009;35(Suppl 1):S1‐S22. [DOI] [PubMed] [Google Scholar]
- 34. Lebeau A, Denkert C, Sinn P, Schmidt M, Wöckel A. Update der S3‐leitlinie Mammakarzinom. Pathologe. 2019;40:185‐198. 10.1007/s00292-019-0578-3 [DOI] [PubMed] [Google Scholar]
- 35. Wöckel A, Festl J, Stüber T, et al. Interdisciplinary screening, diagnosis, therapy and follow‐up of breast cancer. Guideline of the DGGG and the DKG (S3‐level, AWMF registry number 032/045OL, December 2017)—part 2 with recommendations for the therapy of primary, recurrent and advanced breast cancer. Geburtsh Frauenheilk. 2018;78:1056‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thill M, Baumann K, Barinoff J. Intraoperative assessment of margins in breast conservative surgery—still in use? J Surg Oncol. 2014;110:15‐20. [DOI] [PubMed] [Google Scholar]
- 37. Papa M, Allweis T, Karni T, et al. An Intraoperative MRI System for margin assessment in breast conserving surgery initial result from a novel technique. J Surg Oncol. 2016;114(1):22‐26. [DOI] [PubMed] [Google Scholar]
- 38. Allweis TM, Kaufman Z, Lelcuk S, et al. A prospective, randomized, controlled, multicenter study of a real‐time, intraoperative probe for positive margin detection in breast‐conserving surgery. Am J Surg. 2008;196:483‐489. [DOI] [PubMed] [Google Scholar]
- 39. Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21:1589‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karni T, Pappo I, Sandbank J, et al. A device for real‐time, intraoperative margin assessment in breast‐conservation surgery. Am J Surg. 2007;194:467‐473. [DOI] [PubMed] [Google Scholar]
- 41. Thill M, Dittmer C, Baumann K, Friedrichs K, Blohmer JU. MarginProbe—final results of the German post‐market study in breast conserving surgery of ductal carcinoma in situ. Breast. 2014;23:94‐96. [DOI] [PubMed] [Google Scholar]
- 42. Dixon JM, Renshaw L, Young O, et al. Intra‐operative assessment of excised breast tumour margins using ClearEdge imaging device. Eur J Surg Oncol. 2016;42(12):1834‐1840. [DOI] [PubMed] [Google Scholar]
- 43. Grootendorst MR, Cariati M, Pinder SE, et al. Intraoperative assessment of tumor resection margins in breast‐conserving surgery using 18F‐FDG Cerenkov luminescence imaging: a first‐in‐human feasibility study. J Nucl Med. 2017;58(6):891‐898. [DOI] [PubMed] [Google Scholar]
- 44. Erickson‐Bhatt SJ, Nolan RM, Shemonski ND, et al. Real‐time imaging of the resection bed using a handheld probe to reduce incidence of microscopic positive margins in cancer surgery. Cancer Res. 2015;75:3706‐3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ha R, Friedlander LC, Hibshoosh H, et al. Optical coherence tomography: a novel imaging method for postlumpectomy breast margin assessment—a multi‐reader study. Acad Radiol. 2018;25:279‐287. [DOI] [PubMed] [Google Scholar]
- 46. Yao X, Gan Y, Chang E, Hibshoosh H, Feldman S, Hendon C. Visualization and tissue classification of human breast cancer images using ultrahigh resolution OCT. Lasers Surg Med. 2017;49:258‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zysk AM, Chen K, Gabrielson E, et al. Intraoperative assessment of final margins with a handheld optical imaging probe during breast‐conserving surgery may reduce the reoperation rate: results of a multicenter study. Ann Surg Oncol. 2015;22:3356‐3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kennedy BF, McLaughlin RA, Kennedy KM, et al. Investigation of optical coherence micro‐elastography as a method to visualize cancers in human breast tissue. Cancer Res. 2015;75:3236‐3245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


