Abstract
Context
Radioiodine refractory differentiated thyroid cancer can be effectively treated with multi-tyrosine-kinase inhibitors (MKIs). Hypocalcaemia has been reported among the side effects of these drugs, but little is known about its pathophysiology and clinical relevance.
Case report
We report the case of a 78-years-old woman with an aggressive papillary thyroid cancer infiltrating perithyroidal structures. The extent of surgery was limited to hemithyroidectomy, RAI treatment could not be performed, and she started lenvatinib treatment. After 4 months of therapy, the patient accessed the Emergency Department for a grade III hypocalcaemia (corrected serum calcium: 6.6 mg/dL, n.v. 8.1–10.4 mg/dL), due to primary hypoparathyroidism (serum PTH: 12.6 ng/L, n.v. 13–64 ng/L). The patient was treated with intravenous calcium infusions and vitamin D supplementation. After discharge, the oral dose of carbonate calcium (CaCO3) was of 6 g/day, and was titrated according to blood exams. Two weeks after discharge, while taking CaCO3 at the dose of 3 g/day, the patient experienced symptomatic grade II hypercalcemia (corrected serum calcium: 11.6 mg/dL), associated to the spontaneous reprise of PTH secretion, and leading to oral calcium withdrawal. During the subsequent follow-up, the patient remained eucalcemic without calcium supplementation.
Conclusions
Though hypocalcaemia has been described as potential side effect of MKI treatment, this is the first report of a lenvatinib-induced primary hypoparathyroidism, in a patient with a documented normal parathyroid function after surgery. The periodical assessment of calcium-phosphorus metabolism is thus warranted to prevent this potentially lethal side effect, in both post-surgical hypoparathyroid and euparathyroid patients.
Keywords: Thyroid cancer, Lenvatinib, Hypocalcemia
Introduction
Differentiated thyroid cancer (DTC) accounts for almost 90% of all thyroid cancers (TCs) and its incidence has been constantly increasing in the last 50 years [1]. DTC is firstly treated with surgery, eventually followed by radioactive iodine (RAI) therapy, and its prognosis is usually favourable [1]. Up to one-third of metastatic DTCs may become RAI refractory, with a huge reduction in life expectancy [2]. During the last decades, the treatment of advanced DTCs has benefited from the introduction of new systemic therapies acting as multi-kinase inhibitors (MKI). In particular, following the results of phase III randomized, double-blind, multicenter trials, Food and Drug Administration and European Medicines Agency have approved the use of sorafenib [3], lenvatinib [4], and cabozantinib [5].
Due to their pleiotropic mechanism of action, these drugs may cause different side effects, whose grade is defined by the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [6]. Hypocalcaemia has been reported in up to 20% of patients treated with MKIs [3–5], but little is known about its pathophysiology and clinical relevance.
Case report
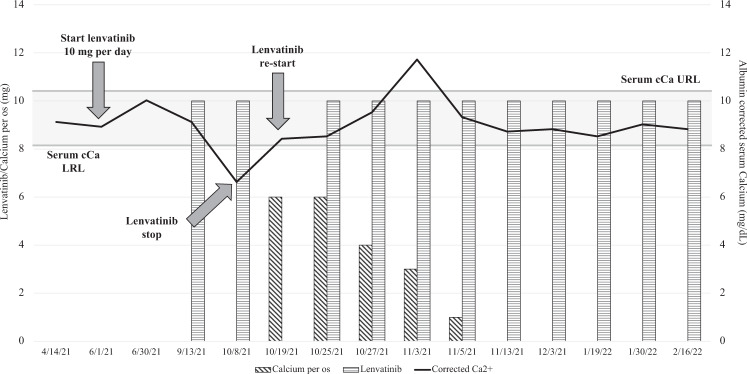
We report the clinical history of a 78-year-old woman followed-up in our tertiary care Centre for severe osteoporosis treated with denosumab 60 mg/mL, and diagnosed in December 2018 with multinodular goiter. In particular, ultrasound examination showed 2 nodules, in the left parahistmic region, measuring 40 mm, and at the basal pole of the left lobe, measuring 30 mm. No suspicious lymph-nodes were identified. The patient was euthyroid, with negative anti-thyroglobulin autoantibodies (TgAb) and calcitonin levels in the normal range. In May 2020, due to an increase in the nodules’ size and to the appearance of mild dysphagia and dyspnea, total thyroidectomy was suggested but surgery was postponed to April 2021, as a consequence of Sars-CoV2 pandemic emergency. During surgery, the left lobe nodules were found to infiltrate the adipose tissues, striated muscles, esophageal wall, the vascular and lymphatic vessels, and the procedure was thus limited to the removal of the right lobe and the isthmus, with a debulking of the left lobe. The pathological exam reported a multifocal papillary cancer, with the largest left lesion of 70 mm. The possible administration of RAI was excluded due to the high burden of the remnant neoplastic tissue and to its low avidity for RAI. No distant metastases were identified at the diagnostic whole-body scan. Therefore, on June 1st 2021, the patient started lenvatinib (LEN) and thyroxine treatment. The initial LEN dose was 10 mg per day, in order to minimize the risk of fistulization between the left neoplastic tissue and the adjacent neck structures. During the first months of therapy, the main neoplastic lesions in the neck significantly reduced and no further lesions appeared. Serum thyroglobulin (Tg) levels decreased from post-operative value of 370 μg/L to 49.6 μg/L, with negative TgAb. The patient experienced several adverse events (AEs), including grade I proteinuria and grade II anorexia, fatigue, diarrhoea, nausea, mucositis and hypertension. No significant alterations emerged at blood exams, and in particular serum parathyroid hormone (PTH), corrected calcium (cCa), phosphorus (P) were always in the normal range. On October 8th, 2021, the patient complained upper and lower limbs twitching, dyspnoea and tachycardia. Blood exams revealed severe hypocalcaemia (cCa 6.6 mg/dL, n.v. 8.1–10.4 mg/dL, grade III according to CTCAE) due to hypoparathyroidism (PTH 12.6 ng/L, n.v. 13–64 ng/L; P 4.7 mg/dL, n.v. 2.5–5.0 mg/dL). The patient was admitted to the Emergency Department and treated with carbonate calcium (CaCO3) infusions. Vitamin D and magnesium (Mg) supplementation was started as well, since hypovitaminosis D (25OH-vitamin D 8 ng/mL, n.v. ≥30 ng/mL) and hypomagnesemia (Mg 1.4 mg/dL, n.v. 1.6–2.6 mg/dL) were demonstrated. The patient was discharged after 10 days on CaCO3 6 g/day, calcitriol 0.75 μg/day, and cholecalciferol 50,000 IU/month. LEN was discontinued for the duration of hospitalization and restarted immediately after discharge. Serum cCa levels were monitored being calcitriol and CaCO3 oral supplementation titrated accordingly. On November 3rd 2021, while taking CaCO3 3 g/day and calcitriol 0.5 μg/day, the patient referred sudden worsening of anorexia and fatigue, along with confusion. Grade II hypercalcaemia was demonstrated (cCa 11.6 mg/dL), with normal PTH (20.4 ng/L) and P (3.7 mg/dL), along with mild hypomagnesemia (Mg 1.5 mg/dL). The patient was treated with 500 mL of 0.9% saline solution infusion, and calcium and calcitriol supplementations were promptly reduced and then withdrawn. Symptoms remitted immediately after, and she has been followed-up since then. At the last follow-up visit (May 2nd 2022), the patient was in good general standing, with stable disease and mild AEs. Calcium-phosphorus metabolism is regularly evaluated and no further toxicities have emerged (Fig. 1).
Fig. 1.
Serum corrected calcium trend during follow-up. cCa = corrected Calcium; URL = Upper Reference Limit; LRL = Lower Reference Limit
Discussion
We report the case of a 78-year-old woman who experienced severe hypocalcemia 4 months after LEN start for advanced DTC. To the best of our knowledge, this is the first report of a LEN-induced transient primary hypoparathyroidism, leading to hypocalcemia, occurring in a euparathyroid patient, and allows to hypothesize different mechanisms at the basis of this toxicity.
Hypocalcemia is included among the potential side effect of the MKIs approved for advanced DTC. In the DECISION trial a high incidence of hypocalcemia was found: 39 patients (18.8%) treated with sorafenib experienced low levels of serum calcium, which were severely reduced in about half of subjects [3]. A similar high prevalence of hypocalcemia (23%) was recorded during cabozantinib treatment in the COSMIC-311 trial, with 9/125 cases experiencing a grade III-IV toxicity [5]. Differently, in phase III SELECT trial, grade I-II and grade ≥ III hypocalcemia occurred in 4.2 and 2.7% of LEN-treated patients, respectively [4]. As for other toxicities, the prevalence of hypocalcemia is highly variable among registration and real-life studies (Table 1), likely due to the low number of enrolled patients, as well as to the variable presentation of this AE, which can be asymptomatic in milder cases [7]. Despite being seldomly reported, hypocalcemia during treatment with sorafenib appears to be common, with an estimated frequency of 8–41% [8–10]. In particular, Cabanillas et al. reported severe hypocalcemia in one patient with post-surgical hypoparathyroidism, out of 8 subjects treated with sorafenib [10]. This is confirmed by a recent meta-analysis, which highlights a significantly higher incidence of all grade hypocalcemia in patients treated with sorafenib compared to those on LEN treatment [11]. Unfortunately, no data from real-life studies with cabozantinib are available to date. Among LEN studies, only Aydemirli et al. reported a 10% incidence of hypocalcemia, which was severe in 1/4 patients [12]. Berends et al. described the case of a 69-year-old woman with post-surgical permanent hypoparathyroidism who experienced recurrent episodes of LEN-induced hypocalcemia [13]. Of note, with the exception of the above-mentioned cases of post-surgical hypoparathyroidism, the information about the parathyroid function in patients developing hypocalcemia is not available in both registration and real-life studies. Nevertheless, these are patients with an aggressive TC, often submitted to an extended surgical procedure and therefore likely to harbor a post-surgical hypoparathyroidism on calcium replacement chronic therapy. In our series, including 25 patients on LEN treatment, 6 developed hypocalcemia, being 5 of them initially treated with total thyroidectomy. The remaining case, here presented, underwent a partial thyroidectomy and has a particular relevance, since is the first reported with a definitely normal parathyroid function at the time of LEN start.
Table 1.
Currently available data on hypocalcemia during multi-targeted tyrosine kinase inhibitors treatment for advanced DTC
| Drug | Authors | Year | Setting | Patients (n) | Frequency of hypocalcemia (%) | Grade I-II (%) | Grade III-IV (%) |
|---|---|---|---|---|---|---|---|
| Lenvatinib | Schlumberger et al. | 2015 | Phase III trial | 261 | 18 (6.9) | 11 (61) | 7 (39) |
| Aydemirli et al. | 2020 | Real life | 39 | 4 (10.3) | 3 (75) | 1 (25) | |
| Sorafenib | Brose et al. | 2015 | Phase III trial | 207 | 39 (19) | 20 (51) | 19 (49) |
| Hoftijzer et al. | 2009 | Real life | 31 | 13 (41) | 13 (100) | 0 (0) | |
| Cabanillas et al. | 2010 | Real life | 13 | 1 (8) | 0 (0) | 1 (100) | |
| Luo et al. | 2013 | Real life | 8 | 3 (37.5) | 2 (66.7) | 1 (33.3) | |
| Cabozantinib | Brose et al. | 2021 | Phase III trial | 125 | 29 (23) | 20 (69) | 9 (31) |
Scanty data area available about the mechanisms underlying the MKI impact on calcium metabolism. Especially in hypoparathyroid cases on calcium supplementation, AEs such as nausea, vomiting, anorexia and diarrhea could lead to hypocalcemia directly or through 25OH-vitamin D and/or magnesium malabsorption [4, 5]. In euparathyroid cases, the causes of hypocalcemia are intriguing but more difficult to hypothesize. A possible mechanism is related to the occurrence of a tumor lysis syndrome (TLS), due to the sudden death of tumor cells and to the subsequent release of several metabolites into the blood stream, including phosphate ions. Hyperphosphatemia can determine calcium-phosphate salt formation, potentially leading to secondary hypocalcemia [14]. To note, the occurrence of TLS during sorafenib [15] and LEN [16] treatment for hepatocellular carcinoma has been described. Finally, as the MKIs prevent signaling through molecular pathways that are also expressed by bone osteoclasts and osteoblasts, a potential direct effect on bone metabolism and calcium levels has been hypothesized [17].
As far as the present case with a severe transient hypoparathyroidism concern, vitamin D and calcium malabsorption/increased excretion were excluded: though the patient experienced grade II gastrointestinal side effects, PTH serum levels were below the lower normal range. Moreover, in our patient serum levels of PTH appeared to be unrelated to Mg2+ serum concentration, which remained constantly low throughout the follow-up. Nevertheless, we also considered that our patient was on denosumab treatment and that hypomagnesemia could have had potentially exacerbated the risk of denosumab-induced hypocalcemia [18, 19]. Denosumab-induced hypocalcemia is reported to be more common within the first 25 days from the first dose and to induce a compensatory increase of PTH serum levels [20]. This possible mechanism was thus excluded in our patient, who received her 4th dose of denosumab five months earlier the onset of hypocalcemia and whose PTH levels were below normal range. On the other hand, TLS was ruled out, as most of its biochemical features, including hyperkaliemia, hyperuricemia, and impaired renal function [14], were not present. On the contrary, a calcium-phosphorus metabolism impairment, due to off-target inhibition of bone cells tyrosine kinase receptors [17] could not be excluded. Another possible explanation is related to a parathyroid toxicity, with hemorrhage or necrosis, possibly due to the reduced density of capillary networks, as reported in mice in some endocrine organs upon administration of anti-VEGF compounds [21, 22]. Still, further studies are required to better understand the genesis and the real prevalence of hypocalcemia and low PTH levels both in hypoparathyroid and euparathyroid patients with thyroid cancer on MKIs.
Acknowledgments
Author contributions
MT: he was involved in the collection of the data related to the present case and in the first compilation of the manuscript. CC: she was involved in the clinical evaluation of the patient. NG: she was involved in the clinical evaluation of the patient and in the collection of some clinical data. CM: she was involved in the clinical evaluation of the patient and in the collection of some clinical data. GLD: he was involved in the surgical procedures and data collection. LF: she supervised all the clinical and analytical processes and revised the paper. SDL: he followed all the processes of data analysis and revised the final version.
Funding
Research funded by the Italian Ministry of Health.
Compliance with ethical standards
Conflict of interest
L.F. consults for Eisai and Ipsen; the other authors have no conflicts to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.H. Lim, S.S. Devesa, J.A. Sosa, D. Check, C.M. Kitahara. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. (2020). 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed]
- 2.M. Schlumberger, et al., Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. (2014). 10.1016/S2213-8587(13)70215-8 [DOI] [PubMed]
- 3.M.S. Brose et al., Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. (2014). 10.1016/S0140-6736(14)60421-9 [DOI] [PMC free article] [PubMed]
- 4.M. Schlumberger, et al., Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Eng. J. Med. (2015). 10.1056/NEJMoa1406470 [DOI] [PubMed]
- 5.M.S. Brose, et al., Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2021). 10.1016/S1470-2045(21)00332-6 [DOI] [PubMed]
- 6.National Cancer Institute, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 27 May 2022.
- 7.J. Pepe, et al., Diagnosis and management of hypocalcemia. Endocrine. (2020). 10.1007/s12020-020-02324-2 [DOI] [PubMed]
- 8.H. Hoftijzer, et al., Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur. J. Endocrinol. (2009). 10.1530/EJE-09-0702 [DOI] [PubMed]
- 9.Y. Luo, et al., Sorafenib in metastatic radioactive iodine-refractory differentiated thyroid cancer: A pilot study. Mol. Clin. Oncol. (2014). 10.3892/mco.2013.199 [DOI] [PMC free article] [PubMed]
- 10.M.E. Cabanillas, et al., Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J. Clin. Endocrinol. Metab. (2010). 10.1210/jc.2009-1923 [DOI] [PubMed]
- 11.S.T. Yu, et al., Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: a systematic review and meta-analysis. Cancer Manag. Res. (2019). 10.2147/CMAR.S191499 [DOI] [PMC free article] [PubMed]
- 12.M.D. Aydemirli, et al., Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur. J. Endocrinol. (2020). 10.1530/EJE-19-0763 [DOI] [PubMed]
- 13.A. Berends, et al., Hypocalcemia induced by tyrosine kinase inhibitors: targeted treatment with ‘untargeted’ side effects. Acta Oncol. (2020). 10.1080/0284186X.2020.1726455 [DOI] [PubMed]
- 14.B. Rahmani, et al., Current understanding of tumor lysis syndrome. Hematol. Oncol. (2019). 10.1002/hon.2668 [DOI] [PubMed]
- 15.S.Z. Imam, M.F. Zahid, M.A. Maqbool. Sorafenib-induced tumor lysis syndrome in a patient with metastatic hepatocellular carcinoma. Hematol. Oncol. Stem Cell Ther. (2020). 10.1016/j.hemonc.2018.03.004 [DOI] [PubMed]
- 16.Y. Shimizu, et al., Lenvatinib-induced tumor lysis syndrome in a patient with advanced hepatocellular carcinoma: a case report. Clin. J. Gastroenterol. (2021). 10.1007/s12328-020-01306-1 [DOI] [PubMed]
- 17.J.O. Alemán, A. Farooki, M. Girotra. Effects of tyrosine kinase inhibition on bone metabolism: untargeted consequences of targeted therapies. Endocr. Relat. Cancer. (2014). 10.1530/ERC-12-0400 [DOI] [PubMed]
- 18.L.K. Laskowski, D.S. Goldfarb, M.A. Howland, K. Kavcsak, D.M. Lugassy, S.W. Smith. A RANKL Wrinkle: Denosumab-induced hypocalcemia. J. Med. Toxicol.: Off. J. Am. Coll. Med. Toxicol. (2016). 10.1007/s13181-016-0543-y [DOI] [PMC free article] [PubMed]
- 19.W.X. Qi, F. Lin, A.N. He, L.N. Tang, Z. Shen, Y. Yao, Incidence and risk of denosumab-related hypocalcemia in cancer patients: a systematic review and pooled analysis of randomized controlled studies. Curr. Med. Res. Opin. (2013). 10.1185/03007995.2013.813840 [DOI] [PubMed]
- 20.K.A. Autio, et al., Severe hypocalcemia associated with denosumab in metastatic castration-resistant prostate cancer: risk factors and precautions for treating physicians. Clin. Genitourin. Cancer. (2015). 10.1016/j.clgc.2014.11.008 [DOI] [PMC free article] [PubMed]
- 21.T. Kamba, et al., VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. (2006). 10.1152/ajpheart.00133.2005 [DOI] [PubMed]
- 22.Y. Yang, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl. Acad. Sci. U. S. A. (2013). 10.1073/pnas.1301331110 [DOI] [PMC free article] [PubMed]