Summary
The formation of Casparian strips (CS) and the deposition of suberin at the endodermis of plant roots are thought to limit the apoplastic transport of water and ions. We investigated the specific role of each of these apoplastic barriers in the control of hydro‐mineral transport by roots and the consequences on shoot growth.
A collection of Arabidopsis thaliana mutants defective in suberin deposition and/or CS development was characterized under standard conditions using a hydroponic system and the Phenopsis platform.
Mutants altered in suberin deposition had enhanced root hydraulic conductivity, indicating a restrictive role for this compound in water transport. In contrast, defective CS directly increased solute leakage and indirectly reduced root hydraulic conductivity. Defective CS also led to a reduction in rosette growth, which was partly dependent on the hydro‐mineral status of the plant. Ectopic suberin was shown to partially compensate for defective CS phenotypes.
Altogether, our work shows that the functionality of the root apoplastic diffusion barriers greatly influences the plant physiology, and that their integrity is tightly surveyed.
Keywords: apoplastic barriers, aquaporins, Arabidopsis thaliana, Casparian strips, root hydraulic conductivity, solutes diffusion, suberin, water transport
Introduction
As sessile organisms, plants strongly depend on the ability of their root system to cope with variable and possibly stressing soil conditions. Roots have evolved as plastic organs (Gruber et al., 2013), able to operate water and nutrient uptake in a wide spectrum of conditions going from deficiency to excess or toxicity. This capacity depends on multiple mechanisms, including the tuning of root system architecture, the regulation of membrane transporters and channels (Maurel et al., 2015), as well as alterations in root anatomical structures, such as the exodermis and endodermis (Líška et al., 2016). The radial transport of water and solutes from the soil to the root xylem vessels is considered as a major site of control (Steudle & Peterson, 1998). Radial transport can occur through the nonselective apoplastic pathway, or from cell‐to‐cell whereby membrane transporters and channels exert variable resistance and selectivity (Maurel et al., 2015). In contrast to the exodermis, which is lacking in certain species such as Arabidopsis thaliana (Arabidopsis), the endodermis is ubiquitous among the angiosperms and acts as the main apoplastic barrier. Two types of structures can be found in the fully developed endodermis: Casparian strips (CS) and suberin lamellae. The CS form a longitudinal belt encircling all endodermal cells, while suberin lamellae covers them, except for passage cells (Andersen et al., 2018). The CS result from a coordinated and localized impregnation of the primary cell wall by lignin (Naseer et al., 2012). This process is regulated by the MYB36 transcription factor (Kamiya et al., 2015), and its integrity surveyed by the SCHENGEN3/CIF1&2 receptor/ligand complex (Doblas et al., 2017b; Nakayama et al., 2017). Suberin is a heterogeneous biopolymer primarily composed of aliphatic monomers and some minor aromatic moieties. It is deposited around endodermal cells, eventually coating the entire endodermal cell surface (Haas & Carothers, 1975) and progressing into a suberized periderm from the pericycle (Campilho et al., 2020). Suberin is thought to create a diffusion barrier for water, gases and solutes (Enstone et al., 2002; Franke et al., 2012).
The function of endodermal CS and suberin as barriers to the radial transfer of water and solutes has been extensively questioned, notably over the last 25 yr (Peterson et al., 1993; Steudle et al., 1993; Frensch et al., 1996, and references cited therein; for recent reviews see: Geldner, 2013; Nawrath et al., 2013; Doblas et al., 2017a). A brief historical overview shows, however, that this issue is not settled yet. In 1993, mechanical disruption of maize endodermis led authors to conclude that this cell layer is not a barrier to water but to solutes, although roots were not suberized in their studies (Peterson et al., 1993; Steudle et al., 1993). In 1996, Frensch et al. concluded, also from studies in maize roots, that the suberized endodermis impedes both solute and water flow (Frensch et al., 1996). However, no clear distinction between the role of suberin and CS was made until 2000, when it was shown that suberin is a barrier to water while CS influence solute transport in maize roots (Zimmermann et al., 2000). But this conclusion was restricted to root exodermis while no role of the endodermis was found in this study. The role of suberin as a barrier to root water transport was assessed later through genetic alteration of its deposition or composition in the horst‐1 and esb1 mutants (Ranathunge & Schreiber, 2011). At this time though, the esb1 mutant was characterized for an enhanced suberin accumulation at the endodermis (Baxter et al., 2009), and it was only later that its primary defect in CS formation was established (Hosmani et al., 2013; Pfister et al., 2014; Li et al., 2017). Thorough phenotypic characterization of sgn3 mutants also pointed to the specific role of CS in altering water and solute relations (Pfister et al., 2014). However, we added to this representation that hydraulic alterations can be induced through a signaling process induced by damaged CS towards aquaporin activity (Wang et al., 2019). Therefore, a comprehensive study addressing the impact of CS and suberin on both water and solute transports at the root level would help integrate, and possibly reconcile, current knowledge. Such a study has become possible thanks to the large collection of CS and suberin‐defective mutants which is now available.
Due to the importance of CS and suberin in the control of plant water transport and mineral nutrition, variations in these structures are expected to impact shoot development. Accordingly, Baxter et al. (2009); Hosmani et al. (2013); Pfister et al. (2014); Kamiya et al. (2015); Barberon et al. (2016) and Reyt et al. (2020) showed that genotypes with CS and/or suberin disorders have both alterations in shoot ionome and shoot development. However, the causal links between modifications of the endodermal barriers and changes in shoot growth still hold many questions. In particular, are growth alterations due to disorders in water supply and/or ion provision?
The objectives of the present study were (1) to clarify the specific roles of the CS and endodermal suberin in water and/or solute transport in roots, and (2) to assess their long‐term effects on shoot growth and development. For this purpose, we used Arabidopsis wild‐type (WT) and a group of 17 Arabidopsis mutants that we classified into five distinctive groups based on standard CS and suberin characterizations.
Materials and Methods
Hydroponic experiments
Plant materials and growth conditions
Arabidopsis accession Col‐0 and 17 mutants with defects in CS and/or suberin (Supporting Information Table S1), were surface sterilized and sowed into clear polystyrene culture plates containing a 1/2 Murashige and Skoog (MS) medium (Sigma‐Aldrich, St Louis, MO, USA). Plates were kept for 2 d at 4°C, then incubated vertically for 10 d under environmentally‐controlled conditions: 60% relative humidity, 16 h d−1 of 250 µmol photons m−2 s−1, 20°C. Plants were then transferred on 35 cm × 35 cm plastic plates floating over a basins filled with 8 l of hydroponic solution (1.25 mM KNO3, 0.75 mM MgSO4, 1.5 mM, Ca(NO3)2, 0.5 mM KH2PO4, 50 μM FeEDTA, 50 μM H3BO3, 12 μM MnSO4, 0.70 μM CuSO4, 1 μM ZnSO4, 0.24 μM MoO4Na2, 100 μM Na2SiO3). Physiological and molecular determinations were done after 10–11 d of hydroponic culture (i.e. on 20–21 d‐old plants). Previously unpublished mutants are T‐DNA insertions pro‐vided by the Nottingham Arabidopsis Stock Centre (NASC): gelp51‐2, GK_016A11; anac038‐1, SALK_103716; anac038‐2, WiscDsLoxHS007‐11H (Fig. S1).
Casparian strip permeability and suberin quantification
Propidium iodide (PI) staining was performed as previously described (Alassimone et al., 2010) on 21 d‐old plants with a 1 h incubation period. Root suberin was extracted and quantified using gas chromatography following the procedures explained in Franke et al. (2005). Despite Fluorol Yellow being the standard dye for suberin staining, we faced issues when working with more mature and bigger root systems. Auramine O staining was therefore performed instead, as described in Ursache et al. (2018), on 21 d‐old plants.
Root hydraulics and root balancing pressure
Root hydraulic conductance (K r) was determined in de‐topped plants using a set of pressure chambers filled with hydroponic solution (Boursiac et al., 2005). Excised roots were subjected to 350 kPa for 10 min, followed by successive measurements at 320, 160, and 240 kPa. The value of K r was calculated as the slope of the flow to pressure relationship. The hydrostatic hydraulic conductivity (Lpr–h) was calculated by dividing K r by the root dry weight. Osmotic hydraulic conductivity (Lpr–o) was determined using the free‐exudation method. The plants were de‐topped with a razor blade and the sectioned hypocotyl immediately introduced into a 100 µl micro capillary. Dental paste (Coltène/Whaledent s.a.r.l., Lezennes, France) was used to ensure a proper seal between the hypocotyl and the capillary. The sap exuded for the first 10 min was discarded, and the sap exuded over the next 45 min was collected and analyzed. Its osmolality (as well as osmolality of the bath medium) was measured using a Vapro 5520 osmometer (Wescor, Logan, UT, USA). The value of Lpr–o was obtained by dividing the exudation rate by the root dry weight and osmotic potential gradient between the exuded sap and the bath. The contribution of the aquaporin‐related pathway to Lpr was tested by the application of 1 mM sodium azide (NaN3), a plant respiration inhibitor known to induce the gating of aquaporins (Tournaire‐Roux et al., 2003).
The passive leakage of solutes into the root was approximated by determining the root balancing pressure (P Jv0) after 1 h treatment with 100 mM sodium chloride (NaCl). The value of P Jv0 is the extrapolated intercept with the pressure axis (J v = 0) of the flow/pressure relationship obtained from pressure chamber measurements. It is related to the selectivity of the root to solutes, or reflection coefficient σ sr, as explained in Boursiac et al. (2005). Active solutes pumping by root cells, which is responsible for the free exudation of the root, may impair this measurement by mimicking solutes leakage (Knipfer & Fricke, 2010). However, we usually observed a free exudation in the range of 1/20th to 1/30th of the flow obtained under pressurization, making its influence negligible.
Total RNA isolation and aquaporin expression
RNA was isolated from 30 to 50 mg frozen roots using the RNA Isolation Kit Z3100 and DNase kit from Promega (Promega Corp., Madison, WI, USA). Total RNA was quantified by optical density measurements at 280/260 nm and stored at −80°C until use. Transcript abundance was determined by quantitative reverse transcription polymerase chain reaction (qRT‐PCR), using the sequences of primer pairs corresponding to the 13 Arabidopsis PIPs described in Sutka et al. (2011). For each gene, relative quantification was made by the Delta cycle threshold method with correction for PCR efficiency. The references genes tested were those described in Sutka et al. (2011), of which TIP41‐like, PP2A3, and SFP were selected as the most stable ones among the different mutants.
Phenopsis experiment conditions
Plant material and growth conditions
Plant phenotyping was realized using the Phenopsis platform (Granier et al., 2006). Seeds were surface sterilized and sown in pots as prescribed. The pots were filled with a loamy soil. Soil water content was automatically adjusted by replacing the water lost by evapotranspiration twice a day during 18 additional days, with a total duration of the experiment of 5 wk. Climatic conditions in the chamber were 20.5°C temperature, 65% relative humidity and 200 µmol photons m−2 s−1 with a photoperiod of 12 h.
Physiological determinations
Five weeks after sowing, the rosettes (which were close to bolting stage, n = 7) were detached from the root and were weighted prior to and after oven‐drying at 65°C for 72 h, to determine their fresh weight (FW) and dry weight (DW).
Leaves were frozen at −20°C for 2 d, pending extraction of the cellular medium with a centrifuge (8 min × 1000 g ). Next, 10 μl of the extract was transferred to an absorbent paper disc and measured using a vapor pressure osmometer (Vapro 5520; Wescor).
Whole plant transpiration was determined in seven plants with the soil covered by a plastic sheet to prevent evaporation (Granier et al., 2006). Pot weights were monitored six times a day during 2 d. Day/night transpiration per unit rosette area was determined as the slope of the pot weight loss over time (g cm−2 h−1). The rosette area was calculated based on the photographs taken at the end of the transpiration period.
Shoot elemental analysis
Briefly, 30 mg of ground dried tissue from young and old leaves of five plants per treatment and genotype (n = 5), were digested in 1 ml of 48.75% nitric acid (HNO3) and 7.5% hydrogen peroxide (H2O2) in a quartz tube at 110°C for 2 h. Cations were determined with an atomic absorption spectrophotometer (SpectrAA 220; Varian, Palo Alto, CA, USA). Results were expressed in mg g−1 DW.
Statistical analyses
All data, except for the regression analyses, were analyzed using one‐way ANOVA with the R software (R Core Team, 2020). Tukey’s post hoc adjustment was used to test mean differences between treatments at α = 0.05. Spearman's correlation analyses were performed in order to elucidate the relations between Lpr and aquaporin expression.
For the experiment at the Phenopsis platform, plants were set‐up in a random block design that was analyzed using a two‐way ANOVA together with Tukey's adjustment at α = 0.05 with R.
Results
Presentation of the mutant collection
In order to study the physiological role of CS and suberin while avoiding pitfalls related to single‐mutant studies, we gathered a collection of Arabidopsis mutants that aimed at covering various combinations of alterations. The chosen genotypes were (Table S1): (1) mutants known for their alterations in CS and the formation of ectopic suberin: myb36‐1 and myb36‐2 (Kamiya et al., 2015), esb1‐1 and esb1‐2 (Baxter et al., 2009), casp1‐1 casp3‐1 (Roppolo et al., 2011; Pfister et al., 2014). (2) Mutants with altered CS and with unaffected suberin content: sgn3‐3 esb1‐1, sgn3‐3 (Pfister et al., 2014; Wang et al., 2019), and sgn3‐4 (Tsuwamoto et al., 2008). (3) Mutants with reduced or altered suberin but no information on the functionality of CS: horst‐1 and horst‐2 (Hofer et al., 2008), and the double mutant horst‐1 ralph‐1 (present work). We added to this group a plant line expressing the CDEF1 cutinase under the control of the CASP1 promoter (pCASP1::CDEF1), which has reduced content in suberin but functional CS (Naseer et al., 2012). (4) Mutants with altered suberin composition and no differences in total suberin content: ralph‐1 and ralph‐2 (Compagnon et al., 2009). (5) A set of new, not yet described mutants, with potentially modified suberin content and unknown properties of the CS (present work). These included gelp51‐2, an insertion mutant in a GDSL‐type esterase/lipase family (GELP), and anac038‐1 and anac038‐2, insertion mutants in a NAC transcription factor gene family member. Genes encoding the latter mutants were identified based on strong in silico coexpression using suberin biosynthetic genes such as RALPH as a ‘bait’ in the ATTEDBII analysis tool (Obayashi et al., 2007) and in silico expression data showing a expression in the root endodermis (Fig. S1).
Casparian strip mutants maintain barrier defects in mature root systems
The functionality of the CS was assessed through monitoring of PI penetration into the stele (Alassimone et al., 2010). PI diffuses through the apoplast where it binds to the carboxyl groups of the cell wall homogalacturonans (Rounds et al., 2011) and thereby stains the vessels if not blocked by the CS. Assays with PI on 7 d‐old seedlings have previously revealed a defective CS in myb36‐1 and myb36‐2 (Kamiya et al., 2015), esb1‐1 and casp1‐1casp3‐1 (Baxter et al., 2009; Hosmani et al., 2013), and sgn3‐3 (Pfister et al., 2014). We performed our experiment on more mature and complex root systems of 21 d‐old plants grown for 10 d in vitro and 11 d in hydroponics. Observations were made at various zones along the root: zone of first root hairs formation, lateral root primordia (LRP) at stages I and II, first lateral root emergence, an intermediate zone, and a zone close to the base, on the primary root and lateral roots (Figs 1a, S2). While xylem or proto‐xylem vessels were stained by PI in the root hairs zone in almost all genotypes, no more staining could be observed after the stages I and II LRP in WT plants (Fig. 1b). This was observed for all genotypes but myb36‐1, esb1‐1, casp1‐1 casp3‐1, sgn3‐3 and sgn3‐3 esb1‐1 (Fig. 1c). Impermeability of the stele to PI was occasionally observed at later stages in those genotypes though. In the basal zone, no PI could penetrate in the root of any genotype (Fig. S2). Additional staining could be observed at the corners of the endodermal cells in myb36‐1 and esb1‐1 (Fig. 1c), which may relate to the deposition of ectopic cell wall, as observed in Kamiya et al. (2015). Noticeably, this ectopic cell wall material does not restore the impermeability of the stele to PI. Altogether, our analyses indicate that all the genotypes have the same high PI permeability at the root hairs zone and low PI permeability after the periderm formation. They differ in between stages I and II lateral root and intermediate zones, where the CS become fully impermeable to PI – thereafter considered as ‘functional’ – for all genotypes besides myb36, esb1, casp1 casp3, sgn3 and sgn3 esb1.
Fig. 1.
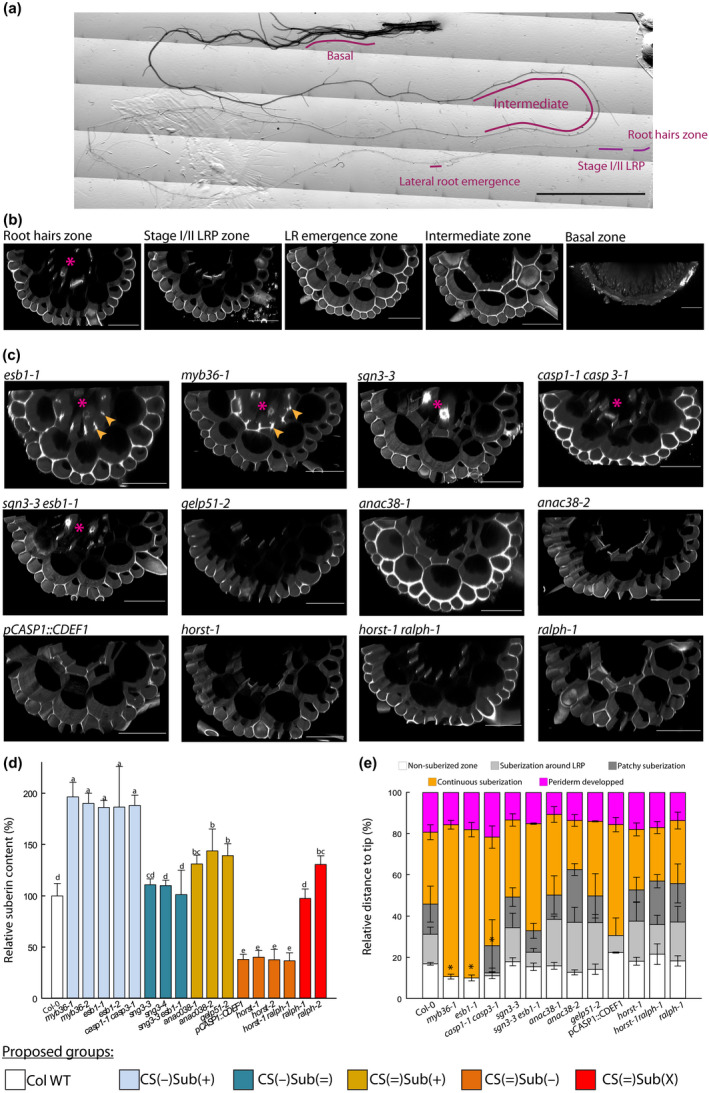
Characterization of Casparian strip permeability and suberin development. (a) Reconstituted picture of a 21 d‐old primary root, and the zones that were monitored for propidium iodide (PI) permeability. Bar, 1 cm. (b) Confocal cross‐sections of a 21 d‐old plants, PI stained, Arabidopsis root from Col‐0 at various zones: root hairs, stages I and II lateral root primordium (LRP), first lateral root (LR) emergence, intermediate, and basal. Bars, 50 µm. (c) Confocal cross‐section of PI staining in roots of 21 d‐old plant at stages I and II LRP development. Arrows highlight the staining related to ectopic deposition of cell wall polymers at the cell corners, stars indicate when the vessels are stained and hence, PI was able to penetrate through the stele. Bars, 50 µm. (d) Relative suberin content related to wild‐type Arabidopsis plants (Col‐0) of 17 Casparian strips (CS) and/or suberin mutants of 21 d‐old plants. Suberin was analyzed using gas chromatography after release by transesterification using boron trifluoride in methanol from solvent extracted root cell walls. Bars represent mean values in μg per mg dry weight ± SE (n = 3–5). *Suberin content taken from literature esb1‐2 (Baxter et al., 2009), pCASP1::CDEF1 (Barberon et al., 2016), horst‐1, horst‐2 (Hofer et al., 2008), ralph‐1, ralph‐2 (Compagnon et al., 2009). (e) Scoring of the suberin stages along the root, as a relative position from the tip ± SE, after staining with the lignin/suberin dye Auramine‐O (n = 3–5). Method detailed in Supporting Information Fig. S2. Asterisks indicate significant difference (P < 0.05) to Col‐0 plants. Colors patterns of (c) allow to visually identify the groups that are defined in the first section of the results. They are reproduced similarly over all the figures.
Suberin quantity and/or development in the mutant collection
Quantitative chemical analysis of suberin in 21 d‐old hydroponically grown plants of myb36‐1, myb36‐2, esb1‐1, esb1‐2 and casp1‐1 casp3‐1, that are mutants with ectopic suberin, confirmed an approximate 1.9‐fold increase in their root content with respect to Col‐0, while sgn3‐3 esb1‐1 and sgn3‐3 were similar to Col‐0 (Fig. 1d). The group composed of gelp51‐2, anac038‐1 and anac038‐2 was characterized by an increase in total root suberin content by 30 to 50% compared to Col‐0. Within the group with unaltered CS, horst‐1, horst‐2, horst‐1 ralph‐1, and pCASP1::CDEF1 exhibited a suberin reduction of about 60% with respect to Col‐0, while ralph‐1 and ralph‐2 showed no significant change or a slight increase in total suberin content, respectively. Auramine‐O staining was used to locate the deposition of suberin and score its development along the primary root. Although this dye stains both lignin and suberin (Ursache et al., 2018), a combination of stereo microscopy and confocal observations, as well as co‐imaging auramine‐O signal with the suberin synthesis reporter pGPAT5::NLS‐RFP was performed (Fig. S3). Since the deconvoluted Auramine O signal resembled the expression pattern of suberin genes and the staining pattern of FY during development (Beisson et al., 2007; Barberon et al., 2016), our approach allowed us to clearly distinguish between these compounds in the younger region of the roots, where periderm has not formed yet. In WT plants, a signal corresponding to suberin was first visible around the LRP, at about 20% from the tip (relative to the total root length). Further from the root tip, in between 30 and 45% of the total root length, the signal became patchy, but not necessarily around the LRP. It then evolved into a continuous signal up to 80% of the total root length where the root eventually developed a periderm (Figs 1e, S3). Similar patterns of suberin development could be observed for most of the genotypes tested (Fig. 1e). By contrast, noticeable differences were observed in myb36‐1, esb1‐1 and casp1‐1 casp3‐1, where the zones of suberization around the LRP and patchy suberized zones were absent or significantly reduced. Despite having significantly more (gelp51‐2, anac38‐1, anac038‐2) or less (horst‐1, horst‐2, horst‐1 ralph‐1) suberin, several mutants did not exhibit any major change in their suberin pattern along the primary root axis (Fig. 1e). This result indicates that the timing of suberin deposition was unaltered in these mutants and no evidence of ectopic deposition could be found.
Based on CS functionality and suberin characterization, we therefore propose a classification of our mutant collection into five groups, each comprising at least two independent members, and named as follows. CS(−)Sub(+) comprises mutants with disrupted CS, ectopic cell wall deposition, and enhanced suberin content: myb36‐1, myb36‐2, esb1‐1, esb1‐2, and casp1‐1 casp3‐1. CS(−)Sub(=) gathers genotypes with disrupted CS and similar suberin content as Col‐0: sgn3‐3, sgn3‐4, and sgn3‐3 esb1‐1. CS(=)Sub(+) comprises mutants with functional CS but with higher suberin content than Col‐0: gelp51‐2, anac038‐1 and anac38‐2. CS(=)Sub(−) comprises plants with functional CS and reduced suberin content: horst‐1, horst‐2, horst‐1 ralph‐1, pCASP1::CDEF1. Finally, CS(=)Sub(X) is formed by ralph‐1 and ralph‐2 which differ from Col‐0 in their suberin composition but not necessarily in their content. From our assays, no difference in periderm development nor periderm permeability could be identified within our mutants. Altogether, we define here a collection of genotypes that covers multiple combinations of CS and suberin defects (Table S1). Although with sometimes a limited number of alleles, such as gelp51‐2 in the CS(=)Sub(+) group, we would like to point out that the primary objective of this collection is not to determine the function of each gene. Rather, we anticipate that extracting the most salient features of each group will allow us to conclude on the role of these apoplastic barriers.
Specific effects of CS and endodermal suberin on root water transport
Root water transport capacity was characterized by measurement of root hydraulic conductivity (Lpr) on detopped plants using the pressure chamber and exudation techniques, yielding hydrostatic Lpr (Lpr–h) and osmotic Lpr (Lpr–o) conductivity, respectively (Fig. 2). Thus, Lpr–h varied among mutants by −73% to +48% compared to Col‐0 (Fig. 2a). A significant linear correlation was observed between Lpr–o and Lpr–h throughout the overall set of genotypes with the exception of sgn3‐3 esb1‐1 (Fig. 2b). Variation in Lpr was mostly consistent with the classification of mutants according to their CS and suberin characteristics, though with very few exceptions. Mutants of the CS(−)Sub(+) group showed a significant reduction in Lpr (although not statistically significant for casp1‐1 casp3‐1). CS(−)Sub(=) (sgn3‐3, sgn3‐4 and sgn3‐3 esb1‐1) and CS(=)Sub(+) (gelp51‐2, anac38‐1, anac038‐2) genotypes showed no difference in Lpr to Col‐0, although Lpr‐o, but not Lpr‐h, was lower for sgn3‐3 esb1‐1. Finally, CS(=)Sub(−) and CS(=)Sub(X) mutants (horst‐1, horst‐1 ralph‐1, pCASP1::CDEF1, ralph‐1 and ralph‐2) showed higher Lpr except for horst‐2.
Fig. 2.
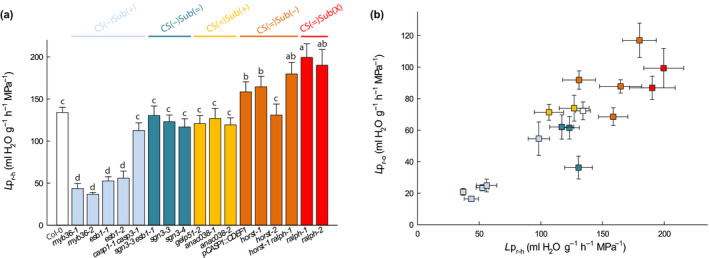
Hydrostatic root hydraulic conductivity (Lpr–h) (a), and its relation with the osmotically root hydraulic conductivity (Lpr–o) (b) in Col‐0, and in a collection of 16 Casparian strips (CS) and/or suberin mutants in Arabidopsis. The plants that were grown hydroponically for 19 to 21 d under environmental controlled conditions, and measured using pressure chambers (Lpr–h) (means ± SE, n = 15–20, n = 3) or by the exudation method (Lpr–o) (means ± SE, n = 20–25, n = 3). In (a), anac038‐2 is presented at a ‘virtual Lpr’ of 119.38 with respect to a wild‐type (WT) value of 134.08 ml g−1 h−1 MPa−1, when ‘real values’ obtained during a dedicated experiment were of 205.0 and 230.2 ml g−1 h−1 MPa−1, respectively. One‐way ANOVA and Tukey’s test were used to determine significant differences (α = 0.05). Data of Lpr–h for pCASP1::CDEF1 are the same as in Wang et al. (2019).
Based on these results, and with the exception of three genotypes out of 16 (casp1‐1 casp3‐1, sgn3‐3 esb1‐1 and horst‐2), the most important reduction in root water transport capacity occurs in plants with enhanced suberin but with defective CS. Conversely, the most important increase in root water transport capacity is found in plants with reduced or altered suberin (horst‐1, horst‐1 ralph‐1, pCASP1::CDEF1, ralph‐1, ralph‐2) and nondefective CS. Defective CS were associated to both reduced and similar Lpr in our collection. Thus, suberin quantity and composition seem to influence water transport as a barrier, while CS, per se, do not.
Reduction of Lpr–h in Casparian strip defective mutants is mediated by concomitant changes in aquaporin activity
Water transport in Arabidopsis roots is considered to be mainly contributed by aquaporins (Tournaire‐Roux et al., 2003). Possible interactions between endodermal barriers and aquaporin functionalities were analyzed by comparing Col‐0 and a subset of mutants representing the five groups identified earlier. Excised root systems were treated with NaN3, an inhibitor of aquaporin activity (Tournaire‐Roux et al., 2003; Sutka et al., 2011). By contrast to all other groups, and with the exception of casp1‐1 casp3‐1, CS(−)Sub(+) mutants only showed a slight inhibition of Lpr upon NaN3 addition, indicating a low contribution of aquaporins, as was already shown for esb1‐1 (Wang et al., 2019) (Fig. 3). The residual Lpr‐h, insensitive to NaN3, was similar to that of Col‐0. Thus, the low Lpr‐h in this group would mainly originate from downregulation of aquaporins rather than from direct physical effects of increased suberin deposition. However, mutants from the CS(=)Sub(−) and CS(=)Sub(X) groups showed an increased absolute inhibition to NaN3, together with a significantly higher residual Lpr–h, suggesting that their higher Lpr–h results from both an upregulation of aquaporins and alteration in suberin deposition or composition (Fig. 3). Finally, mutants from the CS(=)Sub(+) and CS(−)Sub(=) groups, besides sgn3‐3 esb1, behaved similarly to Col‐0.
Fig. 3.
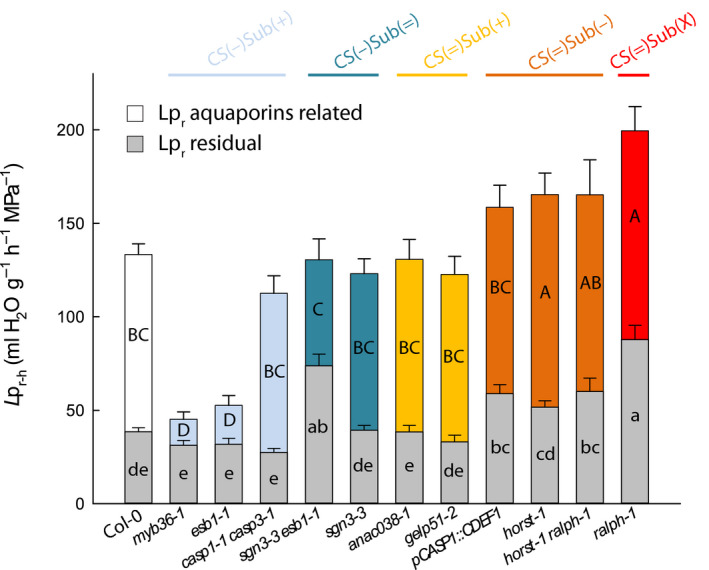
Effects of sodium azide (NaN3) on root hydraulic conductivity and effects of root barrier mutations on residual water transport. Measurements were performed in a collection of 19 to 21 d‐old Arabidopsis Col‐0 and 11 Casparian strips (CS) and/or suberin mutants (means ± SE, n = 15–20, n = 3). The aquaporin‐dependent pathway (colored bar) was derived from the substraction of xylem sap flow before (full bar) and after 40 min (residual‐Lpr, gray bars) of a NaN3 treatment. Uppercase and lowercase letters inside the bars indicate significant differences. Data were analyzed using one‐way ANOVA and Tukey’s test at α = 0.05.
Aquaporin regulation triggered by the loss of integrity of root endodermal barriers was further investigated by testing, in roots of nine of the 17 genotypes, the relationship between the messenger RNA (mRNA) abundance of 13 PIP aquaporin genes and Lpr–h. Figure 4 shows that Lpr–h was positively correlated with the expression of AtPIP1;5 (ρ = 0.7, P = 0.03) whereas it was negatively correlated with the expression of AtPIP2;1 (ρ = −0.86, P = 2.10–7). No correlation was observed with expression of other PIP genes (Fig. S4). These results indicate that modifications in root apoplastic barriers can be accompanied with changes in expression of aquaporin genes, but which cannot simply explain their hydraulic phenotype.
Fig. 4.

Correlations between root hydraulic conductivity (Lpr–h) and aquaporins AtPIP1;5 (a) or AtPIP2;1 (b) expression levels in Col‐0 and a collection of seven Arabidopsis mutants with alterations in endodermal Casparian strips (CS) and/or suberin. Spearman’s correlations are statistically significant for each gene (P < 3 × 10–2 and 2 × 10–7, respectively). Plants were grown hydroponically for 19 to 21 d (means ± SE, n = 3).
Altogether, these results indicate that the two apoplastic structures at the endodermis do not simply act as physical barriers for root water or solute transport, but also functionally interact with the aquaporin‐dependent pathway. Our results are in line with the results of Wang et al. (2019), where CS deficiency downregulates aquaporins activity and the deposition of ectopic suberin through a CIFs/SGN3 pathway (Doblas et al., 2017b). By contrast, the mechanism that possibly links a decrease in suberin content and/or composition with an upregulation in aquaporin activity or expression remains unknown.
Permeability to solutes at the endodermis is determined by the CS
Root permeability to solutes was determined for a subset of genotypes from each group, by supplying NaCl to detopped plants and measuring Lpr–h and root balancing pressure (P Jv0). The value of P Jv0 is the hydrostatic pressure required to counteract the osmotic gradient existing between the root culture medium and the xylem sap and was taken as a proxy for root selectivity to solutes. The application of 100 mM NaCl for 1 h to the root medium typically reduces Lpr–h in WT plants (Boursiac et al., 2005). Our mutant collection followed this behavior except for the CS(−)Sub(+) group (myb36‐1, myb36‐2, esb1‐1 and esb1‐2), where no major variation of the constitutively low Lpr–h could be detected (Fig. S5). We next determined P Jv0 (Fig. 5). The leakier the root to solutes, the lower the osmotic gradient across the root, and so is P Jv0. Hence, P Jv0 under NaCl treatment can be considered as an indicator of the root selectivity to Na+ and Cl− (Boursiac et al., 2005). Only mutants with defective CS showed a marked difference in P Jv0 compared to Col‐0, with a reduction in the CS(−)Sub(+) group even more marked in the CS(−)Sub(=)group. In the CS(=) groups, only two genotypes (anac38‐1 and pCASp1::CDEF1) out of eight showed a significant reduction compared to Col‐0. (Fig. 5). Our results highlight a clear link between defective CS and solute leakage into the root xylem. The role of suberin is less trivial since various configuration led to slight modifications in P Jv0, the most robust being that the increase in suberin content in CS(−)Sub(+) provided an apparent decrease in the root selectivity. Other structural factors such as suberin macromolecular structure, crosslinking or suberin associated waxes also contribute to root selectivity. An in depth analysis of the suberin associated waxes and their physical properties would shed light on this paradox.
Fig. 5.
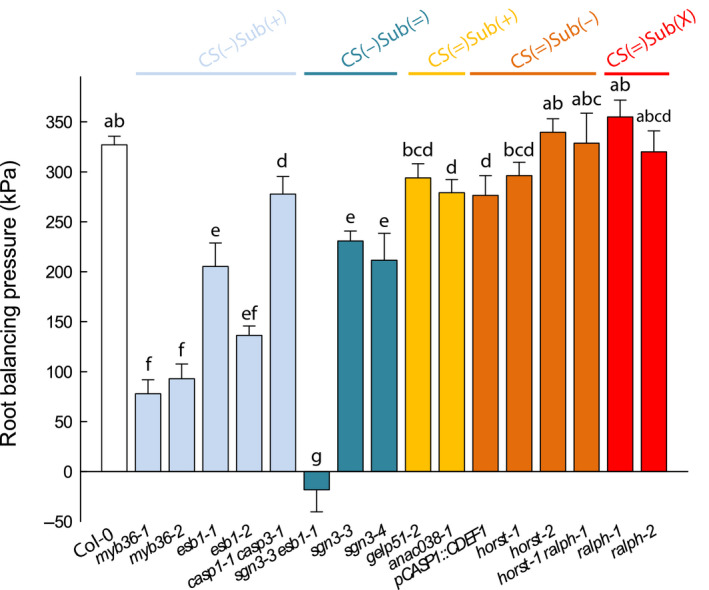
Root balancing pressure (P Jv0) in Col‐0 and in a collection of 16 Casparian strips (CS) and/or suberin mutants in Arabidopsis that were grown hydroponically for 19 to 21 d (means ± SE, n = 15–20, n = 3). The measurements were performed using pressure chambers at a constant pressure (320 kPa) and after subjecting the roots to 100 mM sodium chloride (NaCl) for 1 h. Letters above the bars indicate significant differences among means after one‐way ANOVA and post hoc Tukey's test (α = 0.05).
Rosette growth is affected by the status of CS and suberin at the endodermis, and involves hydromineral nutrition
We aimed at analyzing how the defects in root endodermal barriers affect the development of the shoots under control conditions. For this purpose, a subset of mutants was selected for each group in addition to Col‐0: myb36‐1 and esb1‐1 from CS(−)Sub(+); sgn3‐3 esb1‐1 and sgn3‐3 from CS(−)Sub(=); anac038‐1 and gelp51‐2 from CS(=)Sub(+); horst‐1 and horst‐1 ralph‐1 from CS(=)Sub(−); and ralph‐1 from CS(=)Sub(X). Plants were soil‐grown in the Phenopsis platform (Granier et al., 2006) for 5 wk until harvest. Plant rosette expansion as well as rosette biomass at harvest were determined (Figs 6, S6a). Shoot DW was lower than in Col‐0 in the groups of mutants with altered CS, CS(−)Sub(+) and CS(−)Sub(=), while mutants with altered suberin from the CS(=)Sub(+) and CS(=)Sub(−) groups reached shoot DW similar to Col‐0 (Fig. 6). Rosette area confirmed these results (Fig. S6a). It has to be noted that rosette growth after 5 wk does not predict the final rosette size, since it also depends on the cycle duration. Nevertheless, these data indicate that, under our normal soil conditions, the functionality of CS is necessary for a proper development of the aerial parts, while that of suberin layers is not.
Fig. 6.
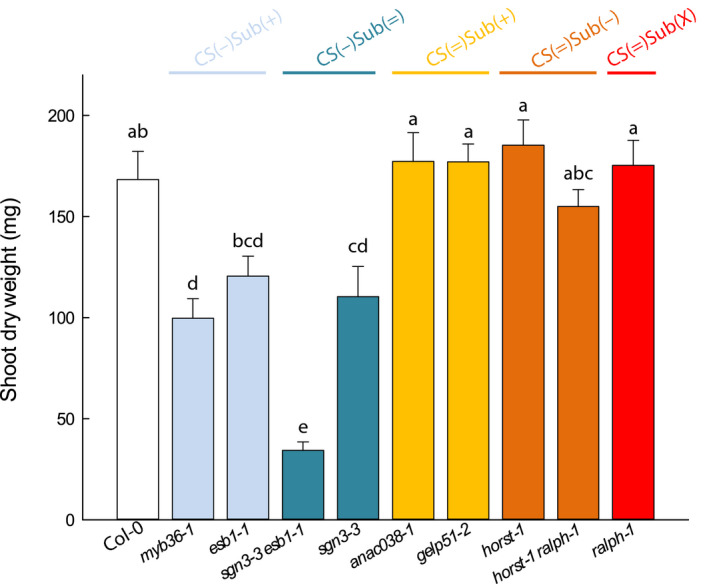
Relative shoot growth in Col‐0 and a collection of nine Casparian strips (CS) and/or suberin Arabidopsis mutants in control conditions. Plants were grown for 5 wk in a Phenopsis phenotyping platform under controlled environmental conditions (means ± SE, n = 7). Letters above the bars indicate significant differences among means after one‐way ANOVA and post hoc Tukey's test (α = 0.05).
Since both permeability to solutes and water transport capacity in roots are compromised in the CS(−) mutants with reduced growth, we aimed at identifying which one is the most influential on rosette growth and development. Previous studies showed that a change in root hydraulic conductance (K r) translates into a similar change in transpiration and growth rate in the shoot of maize or peach tree (Solari & DeJong, 2006; Ehlert et al., 2009). Similarly, the rosette FW measured at harvest in our mutant collection under control conditions varied in parallel to K r (Fig. 7a). However, transpiration rate was not different from Col‐0 under our low evaporative demand conditions (Fig. S6b). Such a reduction in leaf growth without change in transpiration had been reported in maize plants, where K r was downregulated using pharmacological aquaporin inhibition, provided that the evaporative demand was kept low (Ehlert et al., 2009). In the present work, we might face a similar scenario, where reduced growth of the CS(−) groups after 5 wk originates from the downregulation of K r although without provoking a major rebalancing of plant water relations.
Fig. 7.
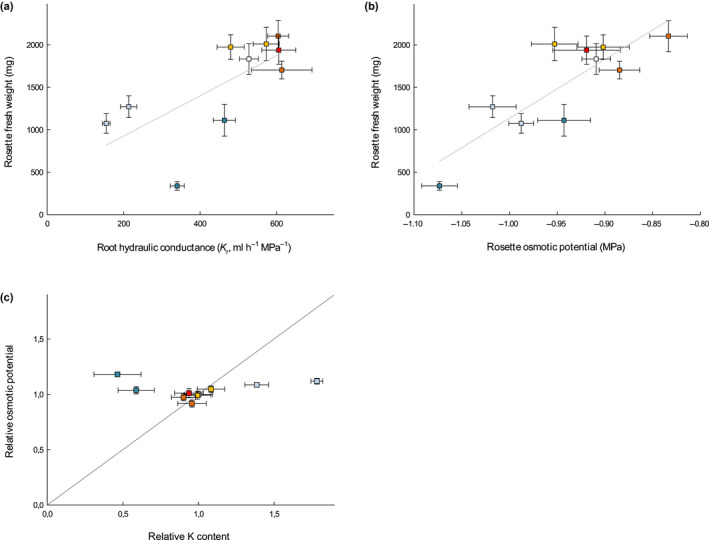
Relationship between rosette fresh weight (FW) and root hydraulic conductance (K r) (a) and rosette osmotic potential (± SEM) from elongating leaves (b) in a collection of nine Casparian strips (CS) and/or suberin Arabidopsis mutants and Col‐0 grown in soil for 5 wk in a Phenopsis phenotyping platform under controlled environmental conditions (n = 7). Pearson correlation coefficients are 0.69 (P‐value < 0.05) and 0.84 (P‐value < 0.01), for FW vs K r and FW vs shoot osmotic potential, respectively. (c) Rosette osmotic potential vs potassium (K) content for mutants grown under control conditions, relatively to Col‐0.
Additionally, there was a positive correlation between rosette osmotic potential (Πleaf) and growth across mutants in our experiments (Fig. 7b). As cell turgor is expected to vary inversely to Πleaf (the more negative Πleaf, the more positive turgor for a given total water potential), it is unlikely that variation in Πleaf was responsible for variation in growth through changes in turgor. We therefore examined whether variation in Πleaf was rather indicative of ionome disorders which could have caused variation in growth. CS(−) mutants, which showed a reduced shoot growth, also exhibited ionomic differences compared to Col‐0, with higher potassium (K) and lower calcium (Ca), as referenced in previous reports for esb1‐1 and myb36‐1 (CS(−)Sub(+) group) (Baxter et al., 2009; Kamiya et al., 2015), and a reduced K and Ca content in sgn3‐3 and sgn3‐3 esb1‐1 (CS(−)Sub(=) group) (Pfister et al., 2014), still in agreement with previous studies (Table S2). By comparison, none of the suberin mutants (CS(=)Sub(+) and CS(=)Sub(−) groups), which did not show any growth phenotype, had any alteration in their ionome profile (Table S2), similarly to the previously described ralph‐1 (group CS(=)Sub(−)) (Compagnon et al., 2009). Moreover, the relationship between K and Πleaf, which usually derives from the major role played by this mineral on the osmotic potential in cells, was not conserved across mutants (Fig. 7c). Thus, in our growth conditions, alteration of CS function provoked a reduction in rosette growth possibly associated to ionome variations, but not caused by the resulting change in Πleaf, the latter not being driven by K content.
Discussion
The present study aimed at investigating the specific role and impact on the whole plant, of each of the two main apoplastic diffusion barriers of the root: the CS and the suberin layers. For this, we used a unique collection of Arabidopsis mutants, which we categorized according to the permeability of the CS to PI and the amount and location of suberin (Figs 1, S2, S3). The characterization of multiple mutants per group ruled out the drawbacks inherent to single‐mutant analyses that could come from unforeseen genetic compensation (El‐Brolosy & Stainier, 2017). The casp1‐1 casp3‐1 double mutant typically fits into this category, being an outlier to the other members of the CS(−)Sub(+) group for many of the root parameters that were measured. Our results support the following conclusions.
Casparian strips do not directly block apoplastic water transport while suberin does. Yet, both act on aquaporin activity
Characterization of esb1‐1 revealed that CIF/SGN3 dependent signaling, which inhibits aquaporin activity, is its primary cause of Lpr downregulation (Wang et al., 2019). We generalized this observation and revealed a complex interaction between apoplastic barriers and aquaporin activity and/or expression (Figs 3, 4, S4) to regulate Lpr (Fig. 2): CS do not directly block water transport while suberin does, but alteration of both acts on aquaporin activity. Our conclusions are based on three sets of measurements.
First, functional CS were associated with higher Lpr – a paradox if we only consider CS as hydrophobic barriers – while we found a correlation between suberin alteration and Lpr in CS(=)Sub(−) and CS(=)Sub(X) mutants. Specifically, a substantial reduction in suberin (pCASP1::CDEF1, horst‐1, horst‐1ralph‐1), or a qualitative change in suberin composition (ralph‐1), potentially affecting hydrophobicity (Schreiber et al., 2005; Kreszies et al., 2019) or crosslinking and structure (Molina et al., 2009), allowed for an increased Lpr. These results extend the previous characterization of the pCASP1::CDEF1 line, for which we then observed only a trend (Wang et al., 2019). They confirm the importance of studying multiple independent mutants in a reverse genetic approach. Suberin would therefore act as a barrier to water transport. Enhanced suberin deposition, which appeared not ectopic in the CS(=)Sub(+) group, had no further effect on Lpr. This suggests that regular suberin deposition already blocks efficiently the water path in WT. In vitro measurement of the water permeability of thin layers of purified suberin would help confirming such effects.
Second, from the use of the aquaporin blocker NaN3 (Fig. 3), we derived an ‘aquaporin‐mediated Lpr’, and a ‘residual Lpr’. The former refers to the activity of aquaporins in the root, but the latter has to be interpreted with caution since it surely reflects more than apoplastic barriers, and includes transport through lipid membranes, vessels, or communication between the radial transport pathways (Steudle, 2000; Sack et al., 2004). We found significant differences in aquaporin‐mediated Lpr in our collection, that confirmed a regulation of aquaporins linked to the apoplastic barrier status (see later). Qualitatively speaking, we found no difference in residual Lpr in four out of six CS mutants, while mutants with a lower suberin content or different suberin compositions (CS(=)Sub(−), CS(=)Sub(X)) had a higher residual Lpr. We concluded that, within the root zone altered in our mutant collection, the CS is not a major barrier for water transport while suberin physically restricts this transport. In the context of disturbed CS (CS(−)Sub(+) and CS(−)Sub(=)), the comparison between esb1‐1, where ectopic suberin content can be seen as a compensatory mechanism for CS deficiency, and sgn3‐3 esb1‐1, which lacks this response, further reinforces this conclusion. The role of suberin as a hydrophobic barrier in roots has already been highlighted by previous studies in Arabidopsis (Ranathunge & Schreiber, 2011) and other species (Frensch et al., 1996; Freundl et al., 2000; Steudle, 2000; Zimmermann et al., 2000; Ranathunge et al., 2016). The originality of our work resides in the fact that, in the root of Arabidopsis, we were able to separate the role of suberin from that of the CS, and described a complementary regulation of root aquaporins. We note that the periderm defines a zone where the organization of suberin layers and lignified cell walls would challenge our interpretations. However, the initial characterization did not reveal any difference in periderm development or permeability in our collection.
Third, we found positive correlation between Lpr and AtPIP1;5 mRNA abundance. This could fit with a putative role of this isoform in root water transport, which has yet to be proven. The function of AtPIP2;5, whose expression negatively correlated to Lpr, has not been described either. In contrast, the negative correlation between Lpr and the mRNA abundance of AtPIP2;1, which is among the most abundant aquaporins in roots (Boursiac et al., 2005; Monneuse et al., 2011) and acts on osmotically‐driven root water flow (Javot et al., 2003; Péret et al., 2012), is contradictory to a major contribution of this aquaporin to water transport. Hence, our results highlight links between apoplastic barrier functionality and aquaporin expression and function, with a complex interplay between isoforms that remains to be elucidated. Future studies will have to take into account distinct cell‐specific expression patterns of isoforms (Hachez et al., 2006a, 2006b,2006a, 2006b), their regulation at the protein level, and their possible functional interactions, at the endodermis in particular (Chaumont & Tyerman, 2014).
Casparian strips are the primary barriers against passive solutes diffusion in roots, while suberin acts as a distinctive, compensatory barrier
Previous studies (Pfister et al., 2014; Barberon et al., 2016; Doblas et al., 2017b; Wang et al., 2019) have concluded that CS exerts a main barrier role in ion transport. With respect to these studies, the present work was carried out in a broader collection of mutants of different origin, and relied on quantitative measurements of balancing pressure (P Jv0, Fig. 5). Although not strictly equivalent, this parameter is indicative of the reflection coefficient (σ sr) of the root. Measurements of σ sr of Col‐0, esb1‐1 and sgn3‐3 esb1‐1 were reported by Wang et al. (2019) and agree with the alterations in P Jv0 described here. In the present study, Col‐0 plants and mutants from groups with functional CS (CS(=)Sub(+), CS(=)Sub(−) and CS(=)Sub(X)) showed very consistent P Jv0, in the range 0.57–0.73 of the total osmotic force due to NaCl, which fits with σ sr values commonly in a 0.4–0.8 range (Boursiac et al., 2005; Fritz & Ehwald, 2011; Ranathunge & Schreiber, 2011; Ranathunge et al., 2017). Mutants with altered CS (CS(−)Sub(+) and CS(−)Sub(=)), exhibited a reduction in P Jv0, down to 0–0.47 of the total osmotic force due to NaCl (with the exception of casp1‐1 casp1‐3) which confirms that the CS act as the primary barrier against solute permeation towards inner tissues. With regard to mutants with deficient CS, the higher P Jv0 of CS(−)Sub(+) members (esb1‐1 and myb36‐1) compared to sgn3‐3 esb1‐1 (CS(−)Sub(=)) suggests that deposition of ectopic suberin partially compensates for the lack of CS. This result parallels those of NaCl selectivity for esb1‐1 and pCASP1::CDEF1 esb1‐1 genotypes characterized in Wang et al. (2019). Altogether, these results indicate that CS are the main barriers to the free diffusion of solutes through the apoplast, while suberin can act secondarily as a barrier when deposited ectopically as in esb1 and myb36.
Under standard conditions, root diffusional barriers exert direct and indirect impacts on shoot development
Under control conditions, mutants of the altered CS(−) groups showed lower rosette DW and reduced surface development (Figs 6, S5). Both root hydraulic conductance and shoot solutes accumulation were correlated to rosette DW in our experiments (Fig. 7a,b). Thus, both a hydraulic defect and an alteration in solute selectivity appeared as plausible causes of the reduction in shoot growth rate.
However, the observation that plants with the lower osmotic potential are those with the lower growth rate raises an apparent paradox. Indeed, in well‐watered soil conditions and with no differences in transpiration (Fig. S6), it can be assumed that the leaf water potential is similar among the genotypes tested. Hence the lower osmotic potential in the CS(−) groups should translate into an increase in the average leaf turgor pressure. According to Lockhart’s model for plant cell expansion (Lockhart, 1965), this would increase the growth rate in the CS(−) groups, for which we observed exactly the converse (Figs 6, S5). Our results therefore suggest that other parameters involved in plant cell expansion are altered when CS are not functional, namely the yield and/or the extensibility of the cell wall.
Furthermore, we looked in more details at the elemental composition of the growing rosettes of the mutant collection in order to look for the origin of the variations in osmotic potential. Our results are in accordance with previous reports (Hosmani et al., 2013; Pfister et al., 2014; Kamiya et al., 2015), and highlight that mutants of the CS(−)Sub(+) and CS(−)Sub(=) groups had opposite phenotypes with respect to K accumulation (Table S2). This implies that the significant variations in shoot osmotic potential, while related to a reduction in shoot growth in both groups, could not be attributed to K (Fig. 7c). We therefore conclude that defective CS do not limit shoot growth through K nutrition. Quantification of other osmotic potential such as NO3 − , sugars, and organic acids would be required to find the origin of such osmotic potential variations.
Overall, the control of shoot growth by CS and suberin functionality is not simply mediated by variations in major nutrients or osmotic control of turgor in growing cells, but by indirect effects on other growth characteristics like cell wall mechanical properties. For example, Wang et al. (2019) identified that activation of the CIF/SGN3 signaling pathway in roots of CS deficient plants translates into an abscisic acid (ABA) dependent signaling in shoots, and such signaling could be at the origin of the growth inhibition highlighted in our study.
In conclusion, study of CS and suberin deficient mutants in Arabidopsis highlights that, in roots, suberin acts physically as a barrier to water transport while CS prevent the passive leakage of solutes into the stele. However, the two components appear to control aquaporin activity. In the shoots, defect in CS provokes a reduction in growth not only via an alteration in hydromineral nutrition but also via signaling, including the CIF/SGN pathway, and perhaps also via so far undiscovered pathways.
Author contributions
Design of the research: YB, CM, DES, RBF, TS, BM; data analysis: MC‐P, YB, BM, TS; performance of the research: MC‐P, ZR, MD, GR, C‐HS, RBF, YB; resources: PD, MF, GR; writing‐original draft: MC‐P, YB; writing‐review and editing: CM, BM, TS, DES, RBF, YB.
Supporting information
Fig. S1 New mutants genotyping and expression information.
Fig. S2 Propidium iodide penetration in the root of 21 d‐old Casparian strips (CS) and suberin mutants.
Fig. S3 Deconvolution of the Auramine O signal in 21 d hydroponically grown plants enables the detection and quantification of endodermal and peridermal suberin.
Fig. S4 Expression of aquaporins genes in the mutant collection.
Fig. S5 Effect of sodium chloride (NaCl) on the root hydraulic conductivity (Lpr–h) of Col‐0 and of a collection of 16 Casparian strips (CS) and suberin mutants.
Fig. S6 Kinetics of rosette development transpiration rates in Col‐0 and in a selection of Casparian strips (CS) and suberin mutants grown under environmentally controlled conditions for 5 wk.
Table S1 Table summarizing the different mutants analyzed in the present study.
Table S2 Ionomic comparisons of the shoots of Casparian strips (CS) and suberin deficient mutants.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The authors acknowledge support from the ERA‐NET Coordinating Action in Plant Sciences program project ERACAPS13.089_RootBarriers, the German Research Foundation (DFG; grant FR 1721/2‐1 to R.B.F), the AgreenSkills+ fellowship to MC‐P which has received funding from the EUs Seventh Framework Program under grant agreement no. FP7‐609398 (AgreenSkills+ contract) and FEDER‐Junta de Castilla y León, CLU‐2018‐04. Results have also been achieved within the framework of the Transnational Cooperation within the PLANT‐KBBE Initiative, with funding from the German Federal Ministry of Education to RBF. The authors want to thank Prof. Marie Barberon (University of Geneva) and Prof. Niko Geldner (University of Lausanne) for kindly providing plants expressing the pGPAT5::NLS‐RFP construct, the SAME platform from BPMP for elemental analyses, as well as Carine Alcon and the MRI imaging facility, member of the national infrastructure France‐BioImaging supported by the French National Research Agency (ANR‐10‐INBS‐04, «Investments for the future»).
References
- Alassimone J, Naseer S, Geldner N. 2010. A developmental framework for endodermal differentiation and polarity. Proceedings of the National Academy of Sciences, USA 107: 5214–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TG, Naseer S, Ursache R, Wybouw B, Smet W, De Rybel B, Vermeer JEM, Geldner N. 2018. Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells. Nature 555: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Vermeer J, De Bellis D, Wang P, Naseer S, Andersen T, Humbel B, Nawrath C, Takano J, Salt D et al. 2016. Adaptation of root function by nutrient‐induced plasticity of endodermal differentiation. Cell 164: 447–459. [DOI] [PubMed] [Google Scholar]
- Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE. 2009. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genetics 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. 2007. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19: 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu D‐T, Sorieul M, van den Dries N, Maurel C. 2005. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology 139: 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campilho A, Nieminen K, Ragni L. 2020. The development of the periderm: the final frontier between a plant and its environment. Current Opinion in Plant Biology 53: 10–14. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD. 2014. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology 164: 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnon V, Diehl P, Benveniste I, Meyer D, Schaller H, Schreiber L, Franke R, Pinot F. 2009. CYP86B1 is required for very long chain ω‐hydroxyacid and α,ω‐dicarboxylic acid synthesis in root and seed suberin polyester. Plant Physiology 150: 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas VG, Geldner N, Barberon M. 2017a. The endodermis, a tightly controlled barrier for nutrients. Current Opinion in Plant Biology 39: 136–143. [DOI] [PubMed] [Google Scholar]
- Doblas VG, Smakowska‐Luzan E, Fujita S, Alassimone J, Barberon M, Madalinski M, Belkhadir Y, Geldner N. 2017b. Root diffusion barrier control by a vasculature‐derived peptide binding to the SGN3 receptor. Science 355: 280–284. [DOI] [PubMed] [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. 2009. Aquaporin‐mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology 150: 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Brolosy MA, Stainier DYR. 2017. Genetic compensation: a phenomenon in search of mechanisms. PLoS Genetics 13: e1006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F. 2002. Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation 21: 335–351. [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L. 2005. Apoplastic polyesters in Arabidopsis surface tissues – a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658. [DOI] [PubMed] [Google Scholar]
- Franke RB, Dombrink I, Schreiber L. 2012. Suberin goes genomics: use of a short living plant to investigate a long lasting polymer. Frontiers in Plant Science 3: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch J, Hsiao TC, Steudle E. 1996. Water and solute transport along developing maize roots. Planta 198: 348–355. [Google Scholar]
- Freundl E, Steudle E, Hartung W. 2000. Apoplastic transport of abscisic acid through roots of maize: effect of the exodermis. Planta 210: 222–231. [DOI] [PubMed] [Google Scholar]
- Fritz M, Ehwald R. 2011. Mannitol permeation and radial flow of water in maize roots. New Phytologist 189: 210–217. [DOI] [PubMed] [Google Scholar]
- Geldner N. 2013. The endodermis. Annual Review of Plant Biology 64: 531–558. [DOI] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux J‐J, Rolland G, Bouchier‐Combaud S, Lebaudy A et al. 2006. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist 169: 623–635. [DOI] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von Wirén N. 2013. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiology 163: 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DL, Carothers ZB. 1975. Some ultrastructural observations on endodermal cell development in Zea mays roots. American Journal of Botany 62: 336–348. [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. 2006a. Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Molecular Biology 62: 305–323. [DOI] [PubMed] [Google Scholar]
- Hachez C, Zelazny E, Chaumont F. 2006b. Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochimica et Biophysica Acta (BBA) – Biomembranes 1758: 1142–1156. [DOI] [PubMed] [Google Scholar]
- Hofer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R. 2008. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω‐hydroxylase involved in suberin monomer biosynthesis. Journal of Experimental Botany 59: 2347–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE. 2013. Dirigent domain‐containing protein is part of the machinery required for formation of the lignin‐based Casparian strip in the root. Proceedings of the National Academy of Sciences, USA 110: 14498–14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin‐Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D et al. 2003. Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Borghi M, Wang P, Danku JMC, Kalmbach L, Hosmani PS, Naseer S, Fujiwara T, Geldner N, Salt DE. 2015. The MYB36 transcription factor orchestrates Casparian strip formation. Proceedings of the National Academy of Sciences, USA 112: 10533–10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. 2010. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytologist 187: 159–170. [DOI] [PubMed] [Google Scholar]
- Kreszies T, Shellakkutti N, Osthoff A, Yu P, Baldauf JA, Zeisler‐Diehl VV, Ranathunge K, Hochholdinger F, Schreiber L. 2019. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytologist 221: 180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Kamiya T, Kalmbach L, Yamagami M, Yamaguchi K, Shigenobu S, Sawa S, Danku JMC, Salt DE, Geldner N et al. 2017. Role of LOTR1 in nutrient transport through organization of spatial distribution of root endodermal barriers. Current Biology 27: 758–765. [DOI] [PubMed] [Google Scholar]
- Líška D, Martinka M, Kohanová J, Lux A. 2016. Asymmetrical development of root endodermis and exodermis in reaction to abiotic stresses. Annals of Botany 118: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JA. 1965. An analysis of irreversible plant cell elongation. Journal of Theoretical Biology 8: 264–275. [DOI] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu D‐T, Santoni V, Shahzad Z, Verdoucq L. 2015. Aquaporins in plants. Physiological Reviews 95: 1321–1358. [DOI] [PubMed] [Google Scholar]
- Molina I, Li‐Beisson Y, Beisson F, Ohlrogge JB, Pollard M. 2009. Identification of an Arabidopsis feruloyl‐coenzyme A transferase required for suberin synthesis. Plant Physiology 151: 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneuse J‐M, Sugano M, Becue T, Santoni V, Hem S, Rossignol M. 2011. Towards the profiling of the Arabidopsis thaliana plasma membrane transportome by targeted proteomics. Proteomics 11: 1789–1797. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa‐Ohnishi M, Matsubayashi Y. 2017. A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 355: 284–286. [DOI] [PubMed] [Google Scholar]
- Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. 2012. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proceedings of the National Academy of Sciences, USA 109: 10101–10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Schreiber L, Franke RB, Geldner N, Reina‐Pinto JJ, Kunst L. 2013. Apoplastic diffusion barriers in Arabidopsis. The Arabidopsis Book 11: e0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H. 2007. ATTED‐II: a database of co‐expressed genes and cis elements for identifying co‐regulated gene groups in Arabidopsis. Nucleic Acids Research 35: D863–D869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, Band LR, Voß U, Postaire O, Luu D‐T, Da Ines O, Casimiro I, Lucas M et al. 2012. Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology 14: 991–998. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Murrmann M, Steudle E. 1993. Location of the major barriers to water and ion movement in young roots of Zea mays L. Planta 190: 127–136. [Google Scholar]
- Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JEM, Yamazaki M, Li G, Maurel C, Takano J et al. 2014. A receptor‐like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3: e03115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ranathunge K, Kim YX, Wassmann F, Kreszies T, Zeisler V, Schreiber L. 2017. The composite water and solute transport of barley (Hordeum vulgare) roots: effect of suberized barriers. Annals of Botany 119: 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L. 2011. Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. Journal of Experimental Botany 62: 1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Bi Y‐M, Rothstein SJ. 2016. Ammonium‐induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 243: 231–249. [DOI] [PubMed] [Google Scholar]
- Reyt G, Chao Z, Flis P, Salas‐González I, Castrillo G, Chao D‐Y, Salt DE. 2020. Uclacyanin proteins are required for lignified nanodomain formation within Casparian strips. Current Biology 30: 4103–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo D, De Rybel B, Tendon VD, Pfister A, Alassimone J, Vermeer JEM, Yamazaki M, Stierhof Y‐D, Beeckman T, Geldner N. 2011. A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383. [DOI] [PubMed] [Google Scholar]
- Rounds CM, Lubeck E, Hepler PK, Winship LJ. 2011. Propidium iodide competes with Ca2+ to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiology 157: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. 2004. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134: 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Franke R, Hartmann K‐D, Ranathunge K, Steudle E. 2005. The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in the roots of rice (Oryza sativa L. cv. IR64) and corn (Zea mays L. cv. Helix). Journal of Experimental Botany 56: 1427–1436. [DOI] [PubMed] [Google Scholar]
- Solari LI, DeJong TM. 2006. The effect of root pressurization on water relations, shoot growth, and leaf gas exchange of peach (Prunus persica) trees on rootstocks with differing growth potential and hydraulic conductance. Journal of Experimental Botany 57: 1981–1989. [DOI] [PubMed] [Google Scholar]
- Steudle E. 2000. Water uptake by plant roots: an integration of views. Plant and Soil 226: 45–56. [Google Scholar]
- Steudle E, Murrmann M, Peterson CA. 1993. Transport of water and solutes across maize roots modified by puncturing the endodermis (further evidence for the composite transport model of the root). Plant Physiology 103: 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E, Peterson C. 1998. Review article. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Sutka M, Li G, Boudet J, Boursiac Y, Doumas P, Maurel C. 2011. Natural variation of root hydraulics in Arabidopsis grown in normal and salt‐stressed conditions. Plant Physiology 155: 1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire‐Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D‐T, Bligny R, Maurel C. 2003. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397. [DOI] [PubMed] [Google Scholar]
- Tsuwamoto R, Fukuoka H, Takahata Y. 2008. GASSHO1 and GASSHO2 encoding a putative leucine‐rich repeat transmembrane‐type receptor kinase are essential for the normal development of the epidermal surface in Arabidopsis embryos. The Plant Journal 54: 30–42. [DOI] [PubMed] [Google Scholar]
- Ursache R, Andersen TG, Marhavý P, Geldner N. 2018. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. The Plant Journal 93: 399–412. [DOI] [PubMed] [Google Scholar]
- Wang P, Calvo‐Polanco M, Reyt G, Barberon M, Champeyroux C, Santoni V, Maurel C, Franke RB, Ljung K, Novak O et al. 2019. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Scientific Reports 9: 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E. 2000. Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210: 302–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 New mutants genotyping and expression information.
Fig. S2 Propidium iodide penetration in the root of 21 d‐old Casparian strips (CS) and suberin mutants.
Fig. S3 Deconvolution of the Auramine O signal in 21 d hydroponically grown plants enables the detection and quantification of endodermal and peridermal suberin.
Fig. S4 Expression of aquaporins genes in the mutant collection.
Fig. S5 Effect of sodium chloride (NaCl) on the root hydraulic conductivity (Lpr–h) of Col‐0 and of a collection of 16 Casparian strips (CS) and suberin mutants.
Fig. S6 Kinetics of rosette development transpiration rates in Col‐0 and in a selection of Casparian strips (CS) and suberin mutants grown under environmentally controlled conditions for 5 wk.
Table S1 Table summarizing the different mutants analyzed in the present study.
Table S2 Ionomic comparisons of the shoots of Casparian strips (CS) and suberin deficient mutants.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


