Abstract
Since the publication of the 2018 FIGO Cancer Report, giant strides have been made in the global effort to reduce the burden of cervical cancer, with the World Health Organization (WHO) rolling out a global strategy for cervical cancer elimination, aiming for implementation by 2030. In over 130 countries, including low‐ and middle‐income countries, HPV vaccination is now included in the national program. Screening has seen major advances with wider implementation of HPV testing. These interventions will take a few years to show their impact. Meanwhile, over half a million new cases are added each year. FIGO’s revised staging of cervical cancer (2018) has been widely implemented and retrospective analyses of data based on the new staging have been published. Minimally invasive surgery has been shown to be disadvantageous in women with cervical cancer. This chapter discusses the management of cervical cancer based on the stage of disease, including attention to palliation and quality of life issues.
Keywords: cancer, cervix, FIGO Cancer Report, HPV vaccination, radiation, screening, staging, surgery
Synopsis
Elimination of cervical cancer is possible by vaccination, screening, and treatment of precancers, and management by surgery and radiation using the revised FIGO staging.
1. INTRODUCTION
Globally, cervical cancer continues to be one of the most common cancers among females, being the fourth most common after breast, colorectal, and lung cancer. GLOBOCAN 2020 estimated that, worldwide, there were approximately 604 000 new cases of cervical cancer, with 342 000 deaths annually. 1 The majority of new cases and deaths (approximately 85% and 90%, respectively) occur in low‐ and middle‐income countries (LMICs), where it is the third most common cancer among women.
2. ANATOMICAL CONSIDERATIONS
The cervix is the lowermost part of the uterus and is a cylindrical structure composed of stroma and epithelium. The ectocervix, which projects into the vagina, is lined by squamous epithelium. The endocervical canal, which extends from the internal os to the external os, is lined by columnar epithelium. Almost all cases of cervical carcinoma originate from the ecto‐ or endocervical mucosa in the transformation zone, the area of the cervix between the old and new squamocolumnar junction.
The ability to easily visualize and sample the cervix contributed to very early understanding of the natural history of cervical cancer. The fact that it needs little or no anesthesia for treatment by freezing or burning led to the development of simple outpatient techniques of screening and prevention.
3. EARLY DETECTION AND PREVENTION OF CERVICAL CANCER
Cervical cancer is a rare long‐term outcome of persistent infection of the lower genital tract by one of about 15 high‐risk HPV (hrHPV) types, which is termed the “necessary” cause of cervical cancer. Persistent HPV infection denotes the presence of the same type‐specific HPV DNA on repeated sampling after 6–12 months. More than 80% of women followed over time will acquire at least one hrHPV infection, which shows its ubiquitous nature and ease of transmission. However, only one‐tenth of all infections become persistent, and these women could develop cervical precancerous lesions. Of the estimated 604 000 new cervical cancer cases annually worldwide, HPV 16 and HPV 18 account for 71% of cases; while HPV types 31, 33, 45, 52, and 58 account for another 19% of cervical cancer cases. 2 , 3 It is well documented that nearly 90% of incident HPV infections are cleared within a period of 2 years from the acquisition of infection and persist only in about 10% of women. 4 It is debatable whether the virus is completely cleared or whether it remains latent in basal cells with the potential for reactivation in some cases.
Knowledge of HPV epidemiology and its role in causation of cancer has resulted in the development of two major strategies for prevention and early detection, namely: (1) HPV vaccination; and (2) screening for precancerous lesions. Although elimination of cervical cancer is a real possibility, the tragedy is that even today many LMICs lack effective intervention programs. The World Health Organization (WHO) has called for a global initiative for elimination of cervical cancer as a public health problem by implementing the following 90%–70%–90% triple pillar intervention strategy before the year 2030 5 :
90% of girls fully vaccinated with two doses of HPV vaccine by the age of 15 years;
70% of women screened using a high‐performance screening test at the age of 35 and 45 years; and
90% of women detected with cervical lesions to receive treatment and care.
WHO has set an incidence threshold of four cases per 100 000 women for pragmatic elimination. While high‐income countries are already well advanced in implementing the above policy, the experience in LMICs is highly variable.
3.1. Primary prevention of cervical cancer with HPV vaccination
The estimated cross‐sectional HPV prevalence worldwide among healthy women aged over 30 years is around 11.7%, with the highest in Sub‐Saharan Africa at around 24%, and country‐specific prevalence ranging between 2% and 42% globally. 6 Age‐specific cross‐sectional HPV prevalence peaks at 25% in women younger than 25 years, which suggests that the infection is predominantly transmitted through the sexual route following sexual debut. Thus, prophylactic HPV vaccination as a preventive strategy should target women before initiation of sexual activity, focusing on girls aged 10–14 years.
HPV vaccination was launched in 2006. Three prophylactic HPV vaccines are currently available for use in females and males from the age of 9 years for the prevention of premalignant lesions and cancers affecting the cervix, vulva, vagina, and anus caused by hrHPV types: a bivalent vaccine targeting HPV 16 and HPV 18; a quadrivalent vaccine targeting HPV 6 and HPV 11 in addition to HPV 16 and HPV 18; and a nonavalent vaccine targeting HPV types 31, 33, 45, 52, and 58 in addition to HPV 6, 11, 16, and 18. In addition, the last two vaccines target anogenital warts caused by HPV 6 and 11. Recently, a bivalent HPV vaccine (Cecolin; Xiamen Innovax Biotech Co., Ltd) has been licensed in China and is currently undergoing the WHO prequalification process. All the vaccines are recombinant vaccines composed of virus‐like particles and are not infectious since they do not contain viral DNA. For girls and boys aged 9–14 years, a two‐dose schedule (0.5 mL at 0 and 6–12 months, i.e. the second dose should be given 6–12 months after the first dose) is recommended. Those aged 15 years and older, and immunocompromised patients irrespective of age, must receive three doses (0.5 mL at 0, 1–2, and 6 months, as per the manufacturer's recommendation). 7 WHO has reviewed the latest data and concluded that there is no safety concern regarding HPV vaccines. 8
At the population level, there is evidence for the effectiveness of HPV vaccination in terms of reduced prevalence of hrHPV types, anogenital warts, and high‐grade cervical abnormalities (CIN2+) caused by the vaccine types among young women; with some evidence of cross‐protection against nonvaccine types also. 9 A recent systematic review and meta‐analysis involving 60 million individuals with follow up to 8 years after vaccination indicated that 5–8 years after vaccination, the following outcomes significantly declined: (1) prevalence of HPV 16 and 18 by 83% (RR 0.17; 95% CI, 0.11–0.25) in 13–19‐year‐old girls, and by 66% (RR 0.34; 95% CI, 0.23–0.49) in women aged 20–24 years; (2) prevalence of HPV 31, 33, and 45 by 54% (RR 0.46; 95% CI, 0.33–0.66) in girls aged 13–19 years; (3) anogenital warts by 67% (RR 0.33; 95% CI, 0.24–0.46) in girls aged 15–19 years, by 54% (RR 0.46; 95% CI, 0.36–0.60) in women aged 20–24 years, and by 31% (RR 0.69; 95% CI, 0.53–0.89) in women aged 25–29 years. CIN2+ decreased significantly by 51% (RR 0.49; 95% CI, 0.42–0.58) among screened girls aged 15–19 years and by 31% (RR 0.69; 95% CI, 0.57–0.84) among women aged 20–24 years. 9 Programs with multicohort vaccination and high vaccination coverage had a greater direct impact and herd effects. The impact of HPV vaccination on significantly reducing the risk of invasive cervical cancer has also been shown recently in a Swedish follow‐up evaluation of 1 672 983 girls and women who were 10–30 years of age from 2006 through 2017. 10 Cervical cancer was diagnosed in only 19 vaccinated women and in 538 unvaccinated women.
Recent studies have reported evidence for effectiveness of a single dose in preventing high‐risk HPV infections similar to three or two doses. Results from ongoing purpose‐designed, prospectively randomized clinical trials assessing the efficacy and immunogenicity of single‐dose HPV vaccination compared to currently used schedules are awaited, which will further clarify the role of one dose in preventing cervical neoplasia. 11 , 12 , 13 There is no evidence of type replacement following vaccination. 14 , 15
It is estimated that, without vaccination, the global burden of cervical cancer among young girls born between 2005–2014 birth cohorts will be 11.6 million cases by 2094. Four‐fifths of this burden will be in 25 countries in Africa (5.6 million cases) and Asia (4.5 million cases), with 51.3% of the overall expected burden of 5.9 million cervical cancer cases over a lifetime affecting birth cohorts in India, Nigeria, China, Tanzania, Indonesia, Uganda, the Democratic Republic of the Congo, Ethiopia, and Kenya. Another 2.8 million cases, corresponding to 24.2% of the total burden, would be in 17 countries, mostly in Sub‐Saharan Africa (South Africa, Malawi, Zambia, Mozambique, Angola, Zimbabwe, Madagascar, Mali, Ghana, and Burkina Faso); Asia (Pakistan, Bangladesh, and the Philippines); the Americas (Brazil, Mexico, and the USA); and Russia. The remaining 24.5% (2.8 million cases) in unvaccinated birth cohorts is expected to occur in the remaining 159 countries. It has been estimated that worldwide HPV vaccination with high coverage could prevent about 8.7 million cases by 2094. 16
3.2. Secondary prevention of cervical cancer by early detection and treatment of precancerous lesions
Screening is an important strategy in the global elimination of cervical cancer. While HPV vaccination aims to prevent cervical neoplasia by preventing HPV infection, screening aims to detect prevalent cervical precancerous lesions such as high‐grade CIN and adenocarcinoma in‐situ early, and effectively treat them to prevent invasive cancer and decrease cervical cancer mortality rates. It will therefore remain a priority for cervical cancer prevention for several decades.
Several cervical screening strategies have been used effectively in varied settings: conventional cytology (Pap smear); in recent years, liquid‐based cytology (LBC) and HPV testing; and, in LMICs, visual inspection with acetic acid (VIA). 17 While screening with Pap smear at regular intervals has resulted in substantial decline in cervical cancer risk in high‐income countries, it is resource‐intensive, needs repeated rounds to compensate for poor sensitivity, and is not feasible in low‐resource settings where poor organization, coverage, and lack of quality assurance result in suboptimal outcomes. 17 HPV‐based screening has higher sensitivity and accuracy, lower variability and better reproducibility compared with conventional or LBC. In the context of declining HPV infections in vaccinated populations, many healthcare systems are switching to primary HPV screening, whose higher negative predictive value allows extended screening intervals or even a single lifetime screening in low‐resource settings. 18 , 19 Recent European guidelines strongly recommend primary HPV‐based screening over standard cytology‐based screening. 20 Currently The Netherlands, Turkey, Finland, Italy, Sweden, and the UK are implementing HPV screening nationally or regionally. Countries such as Australia, 21 Argentina, Chile, and Mexico are implementing HPV‐based screening programs. This has increased the colposcopy referral rates, but also resulted in higher detection rates of CIN3+ lesions and cervical cancers.
VIA screening involves detection of acetowhite lesions on the cervix 1 minute after the application of 3%–5% freshly prepared acetic acid. In view of its feasibility, it has been widely implemented in opportunistic settings in many low‐income countries in Sub‐Saharan Africa. A single‐visit approach (SVA) for screening with rapid diagnosis and treatment improves coverage, eliminates follow‐up visits, and improves cost‐efficiency in low‐resource settings. 22 , 23 , 24 VIA screening is particularly suitable for SVA and WHO has issued guidelines for implementing SVA in public health settings. 25
Introducing a cervical cancer screening program in a country should be preceded by policy and managerial guidelines that clearly indicate the target age group, screening test and screening intervals, methods to reach target women, management of screen‐positive women (triaging and treating or SVA), treatment methods (cryotherapy, thermal ablation, loop electrosurgical excisions procedure [LEEP]) for CIN lesions, and criteria for type of treatment for prevalent cervical cancers detected by screening. 25 , 26 Availability of adequate infrastructure and trained human resources is critical for initiating and sustaining the various inputs of the program. A program information system supported by a database and linkage with other information systems such as cancer registration, mortality registers, and health insurance databases is important for monitoring and evaluation. The screening strategy chosen must be feasible, simple, safe, accurate, acceptable, and easily accessible to the highest‐risk women. In studies from Bangladesh and India it has been observed that following the right approach to organize several components and meticulous attention to quality is crucial for the success of a screening program and not merely the choice of a good screening test. 27 A judicious combination of HPV vaccination and screening has enormous potential to eliminate cervical cancer in the foreseeable future.
4. STAGING OF CERVICAL CANCER
Invasive cervical cancer spreads by direct extension into the parametrium, vagina, uterus, and adjacent organs, i.e. bladder and rectum. It also spreads along the lymphatic channels to the regional lymph nodes, namely, obturator, external iliac, internal iliac, and thence to the common iliac and para‐aortic nodes. Distant metastasis to lungs, liver, and skeleton by the hematogenous route is a late phenomenon.
The cervix was the first organ to be assigned a clinical staging system for cancer by FIGO in 1958. Subsequently the pathologic (TNM) staging followed, which has been used for the purpose of documenting nodal and metastatic disease status. In 2018, the FIGO Gynecologic Oncology Committee revised the staging to allow the option of clinical, radiological, or pathological findings, as available, to assign the stage. A corrigendum to this staging was published thereafter, with some modifications. The revised staging is shown in Table 1. 28 The main changes are:
The horizontal dimension of a microinvasive lesion is no longer considered.
Tumor size has been stratified further into three subgroups: IB1 ≤2 cm, IB2 >2–≤4 cm, and IB3 >4 cm.
Lymph node positivity, which correlates with poorer oncologic outcomes assigns the case to Stage IIIC—pelvic nodes IIIC1 and para‐aortic nodes IIIC2. Micrometastases are included in Stage IIIC.
TABLE 1.
FIGO staging of cancer of the cervix uteri (2018)
| Stage | Description |
|---|---|
| I | The carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy, with maximum depth of invasion ≤5 mm a |
| IA1 | Measured stromal invasion ≤3 mm in depth |
| IA2 | Measured stromal invasion >3 and ≤5 mm in depth |
| IB | Invasive carcinoma with measured deepest invasion >5 mm (greater than Stage IA); lesion limited to the cervix uteri with size measured by maximum tumor diameter b |
| IB1 | Invasive carcinoma >5 mm depth of stromal invasion and ≤2 cm in greatest dimension |
| IB2 | Invasive carcinoma >2 and ≤4 cm in greatest dimension |
| IB3 | Invasive carcinoma >4 cm in greatest dimension |
| II | The carcinoma invades beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| IIA | Involvement limited to the upper two‐thirds of the vagina without parametrial involvement |
| IIA1 | Invasive carcinoma ≤4 cm in greatest dimension |
| IIA2 | Invasive carcinoma >4 cm in greatest dimension |
| IIB | With parametrial involvement but not up to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or nonfunctioning kidney and/or involves pelvic and/or para‐aortic lymph nodes |
| IIIA | The carcinoma involves the lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or para‐aortic lymph nodes (including micrometastases) c , irrespective of tumor size and extent (with r and p notations) d |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para‐aortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV |
| IVA | Spread of the growth to adjacent pelvic organs |
| IVB | Spread to distant organs |
Imaging and pathology can be used, where available, to supplement clinical findings with respect to tumor size and extent, in all stages. Pathological findings supersede imaging and clinical findings.
The involvement of vascular/lymphatic spaces should not change the staging. The lateral extent of the lesion is no longer considered.
Isolated tumor cells do not change the stage but their presence should be recorded.
Adding notation of r (imaging) and p (pathology) to indicate the findings that are used to allocate the case to Stage IIIC. For example, if imaging indicates pelvic lymph node metastasis, the stage allocation would be Stage IIIC1r; if confirmed by pathological findings, it would be Stage IIIC1p. The type of imaging modality or pathology technique used should always be documented. When in doubt, the lower staging should be assigned.
The revised FIGO staging is closely aligned with the latest TNM staging. 29 The stage is allocated after all imaging and pathology reports are available. It is not to be altered later, for example at recurrence.
5. HISTOPATHOLOGY
It is essential that all cancers must be confirmed by microscopic examination. Cases are classified as carcinomas of the cervix if the primary growth is in the cervix. All histologic types must be included. The histopathologic types, as described in the WHO Classification of Female Genital Tumours 30 are as follows:
5.1. Squamous epithelial tumors
Squamous cell carcinoma, HPV‐associated
Squamous cell carcinoma, HPV‐independent
Squamous cell carcinoma NOS
5.2. Glandular tumors
Adenocarcinoma NOS
Adenocarcinoma, HPV‐associated
Adenocarcinoma, HPV‐independent, gastric type
Adenocarcinoma, HPV‐independent, clear cell type
Adenocarcinoma, HPV‐independent, mesonephric type
Adenocarcinoma, HPV‐independent, NOS
Endometrioid adenocarcinoma NOS
Carcinosarcoma NOS
Adenosquamous carcinoma
Mucoepidermoid carcinoma
Adenoid basal carcinoma
Carcinoma, undifferentiated, NOS
5.3. Mixed epithelial and mesenchymal tumors
Adenosarcoma
5.4. Germ cell tumors
Endodermal sinus tumor
Yolk sac tumor NOS
Choriocarcinoma NOS
6. DIAGNOSIS AND EVALUATION OF CERVICAL CANCER
6.1. Microinvasive disease
Diagnosis of Stages IA1 and IA2 is made on microscopic examination of a cone biopsy specimen, obtained by LEEP or cold knife conization, which includes the entire lesion. It can also be made on a trachelectomy or hysterectomy specimen. The depth of invasion should not be greater than 3 or 5 mm, respectively, from the base of the epithelium. The horizontal dimension is no longer considered in the 2018 revision as it has not been shown to impact survival. Note must be made of lymphovascular space involvement, which does not alter the stage, but may affect the treatment plan. The margins should be reported to be negative for disease. If the margins of the cone biopsy are positive for invasive cancer, the patient is allocated to Stage IB1. 31
6.2. Invasive disease
In the case of visible lesions, a punch biopsy may generally suffice for diagnosis, but if not satisfactory, a small loop biopsy or cone may be required. Clinical assessment is the first step in allocation of staging. FIGO 2018 staging permits the use of any of the imaging modalities according to available resources, i.e. ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), to provide additional information on tumor size, nodal status, and local or systemic spread. MRI is the best method of radiologic assessment of primary tumors greater than 10 mm. 32 However, ultrasound has also been shown to have good diagnostic accuracy in expert hands. 33 The modality used in assigning staging should be noted for future evaluation. Imaging can identify additional prognostic factors that can guide the choice of the most appropriate treatment modality.
For detection of nodal metastasis greater than 10 mm, PET‐CT is more accurate than CT and MRI, with false‐negative results in 4%–15% of cases. 34 , 35 , 36 In areas with a high prevalence of tuberculosis and inflammation, especially HIV‐endemic areas, large lymph nodes are not necessarily metastatic. The clinician may make the decision on imaging or, when possible, can use fine needle aspiration or biopsy to exclude metastases. This is especially true in advanced stages, where surgical assessment of para‐aortic lymph nodes by minimally invasive surgery or laparotomy may be used to tailor treatment according to extent of disease. 37 , 38 , 39 Surgical exclusion of para‐aortic lymph node involvement has been reported to correlate with prognosis better than radiographic exclusion alone. 40
A review of 22 articles that assessed the safety and impact of pretreatment para‐aortic lymph node surgical staging (PALNS) found that 18% (range, 8%–42%) of patients with Stage IB–IVA cervical cancer had para‐aortic lymph node metastases. 41 The mean complication rate of PALNS was 9% (range 4%–24%), with lymphocyst formation being the most common. In another study, up to 35% of clinically assessed Stage IIB and 20% of Stage III tumors were reported to have positive para‐aortic nodes. 42 In the revised staging, all these cases will be assigned to Stage IIIC as lymph node involvement confers a worse prognosis. 43 If only pelvic nodes are positive, it is Stage IIIC1; if para‐aortic nodes are also involved it is Stage IIIC2. A further notation must be added to indicate whether this allocation is based on only imaging assessment (r) or whether pathological confirmation is available (p). In due course, these data can be analyzed and reported accordingly.
FIGO no longer mandates any biochemical investigations or investigative procedures; however, in patients with frank invasive carcinoma, a chest X‐ray and assessment of hydronephrosis (with renal ultrasound, CT, or MRI) should be done. The bladder and rectum are evaluated by cystoscopy and sigmoidoscopy only if the patient is clinically symptomatic. Cystoscopy is also recommended in cases of a barrel‐shaped endocervical growth and in cases where the growth has extended to the anterior vaginal wall. Suspected bladder or rectal involvement should be confirmed by biopsy and histologic evidence. Bullous edema alone does not warrant a case to be allocated to Stage IV.
7. MANAGEMENT OF CERVICAL CANCER
Management of cervical cancer is primarily by surgery or radiation therapy, with chemotherapy a valuable adjunct.
7.1. Surgical management
Surgery is suitable for early stages, where cervical conization, simple hysterectomy, or radical hysterectomy may be selected according to the stage of disease. Table 2 shows the types of radical hysterectomy. In Stage IVA, selected cases may be suitable for pelvic exenteration.
TABLE 2.
Types of radical hysterectomy
| Simple extrafascial hysterectomy | Modified radical hysterectomy | Radical hysterectomy | |
|---|---|---|---|
| Piver and Rutledge Classification | Type I | Type II | Type III |
| Querleu and Morrow classification | Type A | Type B | Type C |
| Indication | Stage IA1 | Type IA1 with LVSI. IA2 | Stage IB1 and IB2, selected Stage IIA |
| Uterus and cervix | Removed | Removed | Removed |
| Ovaries | Optional removal | Optional removal | Optional removal |
| Vaginal margin | None | 1–2 cm | Upper one‐quarter to one‐third |
| Ureters | Not mobilized | Tunnel through broad ligament | Tunnel through broad ligament |
| Cardinal ligaments | Divided at uterine and cervical border | Divided where ureter transits broad ligaments | Divided at pelvic side wall |
| Uterosacral ligaments | Divided at cervical border | Partially removed | Divided near sacral origin |
| Urinary bladder | Mobilized to base of bladder | Mobilized to upper vagina | Mobilized to middle vagina |
| Rectum | Not mobilized | Mobilized below cervix | Mobilized below cervix |
| Surgical approach | Laparotomy or laparoscopy or robotic surgery | Laparotomy or laparoscopy or robotic surgery | Laparotomy or laparoscopy or robotic surgery |
7.1.1. Microinvasive cervical carcinoma: FIGO Stage IA
Stage IA1
The treatment is completed with cervical conization unless there is lymphovascular space invasion (LVSI) or tumor cells are present at the surgical margin. In women who have completed childbearing or in elderly women, extrafascial hysterectomy may also be recommended. 44 Any route can be chosen, i.e. abdominal, vaginal, or minimally invasive. When LVSI is evident, pelvic lymphadenectomy should be considered, along with extrafascial hysterectomy. 45 If fertility is desired, cervical conization with close follow‐up will be adequate.
Stage IA2
Since there is a small risk of lymph node metastases in these cases, pelvic lymphadenectomy is performed in addition to type B radical hysterectomy. 46 , 47 , 48 In low‐risk cases (no LVSI, sentinel node negative), simple hysterectomy or trachelectomy, combined with either pelvic lymphadenectomy or sentinel lymph node (SLN) assessment, may be adequate surgical treatment. 49 , 50 When the patient desires fertility, she may be offered a choice of the following: (1) cervical conization with pelvic lymphadenectomy (open or minimally invasive surgery [MIS]); or (2) radical trachelectomy with pelvic lymphadenectomy by abdominal, vaginal, or MIS route. 51 , 52
Post‐treatment follow‐up
After fertility sparing surgery, follow‐up with 3‐monthly Pap smears for 2 years, then 6‐monthly for the next 3 years is recommended. With normal follow‐up at 5 years, the patient can return to the routine screening schedule according to the national guidelines. 53 Other tests, including imaging are not recommended routinely and may be performed if required on a case‐by‐case basis.
7.1.2. Invasive cervical carcinoma: FIGO Stage IB1, IB2, IIA1
Surgical treatment is the preferred modality for the treatment of Stage IB1, IB2, and IIA1 lesions. It would usually consist of type C radical hysterectomy with pelvic lymphadenectomy. 54 , 55
FIGO Stage IB1
FIGO Stage IB1 is considered low risk with the following criteria: cervical stromal invasion less than 50% and no suspicious lymph nodes on imaging. The standard management is a type C radical hysterectomy but modified radical hysterectomy may be considered in these cases. Pelvic lymphadenectomy should always be included on account of the high frequency of lymph node involvement. 46 , 47 The ongoing SHAPE study (NCT01658930), an open label noninferiority trial will compare the oncologic outcomes and treatment‐related adverse events between radical hysterectomy and simple hysterectomy in low‐risk early‐stage cervical cancer. 56 Another prospective multi‐institutional study (GOG 278) is evaluating the physical function and quality of life before and after nonradical surgical therapy (extrafascial hysterectomy or cone biopsy with pelvic lymphadenectomy) in low‐risk cases. 57
A pelvic nerve‐sparing surgical procedure is recommended in patients undergoing radical hysterectomy (type C1 hysterectomy), insofar as radical curability is maintained, as intrapelvic injuries to the autonomic nerves (i.e. hypogastric nerve, splanchnic nerve, and pelvic plexus) often lead to impairment of urination, defecation, and sexual function, and consequent deterioration of the postoperative quality of life. 58 , 59
In young women desiring fertility sparing, a radical trachelectomy may be performed, indicated for Stage IA2–IB1 tumors. 60 The cervix along with the parametrium is removed followed by anastomosis of the uterus with the vaginal end. Trachelectomy can be done by open abdominal, vaginal, or by minimally invasive routes. When a vaginal approach is planned, the pelvic nodes are first removed laparoscopically and sent for frozen section to confirm node negativity; then proceed with the radical trachelectomy vaginally. Alternatively, the nodes may first be assessed by conventional pathological methods and the radical trachelectomy done as a second surgery after 1 week.
FIGO Stage IB2 and IIA1
In FIGO Stages IB2 and IIA1 cervical cancer, surgery or radiotherapy can be chosen as the primary treatment depending on other patient factors and local resources, as both have similar outcomes. The advantages of surgical treatment are: (1) that it is feasible to determine the postoperative stage precisely on the basis of histopathologic findings, thereby enabling individualization of postoperative treatment; (2) that it is possible to treat cancers that are likely to be resistant to radiotherapy; and (3) that it is possible to conserve ovarian function. Intraoperative transpositioning of the ovaries high in the paracolic gutters away from the radiation field, in case it should be required subsequently, is also feasible. The preservation of ovarian and sexual function makes surgery the preferred mode in younger women. Type C radical hysterectomy is the standard procedure for the treatment of cervical cancer, consisting of removal of the uterus, parametrium, upper vagina, and a part of the paracolpium, along with pelvic lymphadenectomy. As for the adjacent connective tissues, the anterior vesicouterine ligament (anterior and posterior leaf), lateral cardinal ligaments, and posterior sacrouterine and rectovaginal ligaments are cut from the uterus at sufficient distances from their attachments to the uterus. Pelvic lymphadenectomy is an important component of this surgical procedure. The regional lymph node excision includes the parametrial nodes, obturator nodes, external, internal, and common iliac nodes. The route of surgery used was laparotomy or MIS, either laparoscopic or robotic. However, the LACC trial (Laparoscopic Approach to Cervical cancer), a randomized trial that compared overall survival with open surgery versus laparoscopy or robotic surgery in early‐stage cervical cancer, showed a decreased overall survival in the MIS group (3 of 312 vs 19 of 319, HR 6.00; 95% CI, 1.48–20.3, P = 0.004). Disease‐free survival events were also three‐fold increased in the MIS group (7 of 312 vs 27 of 319, HR 3.74; 95% CI, 1.63–8.58, P = 0.002). Rates of intraoperative complications did not differ by treatment received (11% in both). The most common sites of recurrence were as follows: in the open arm, the vaginal vault (3/7, 43%); in the MIS arm, pelvis (7/24, 29%), pelvis along with multiple sites in abdomen (7/24, 29%). The authors concluded that hysterectomy by a minimally invasive route was associated with higher rates of recurrence than the open approach in early‐stage cervical cancer patients. 61
Subsequent to the LACC trial, several multi‐institutional observational studies have confirmed inferior survival outcomes with MIS. Melamed et al. 62 conducted a nationwide observational study in the USA and demonstrated a 4‐year mortality of 9.1% among cervical cancer patients treated with MIS and 5.3% among those who underwent open surgery (HR 1.65; 95% CI, 1.22–2.22). Uppal et al. 63 reported recurrence‐free survival outcomes for a cohort of 700 patients with open or MIS radical hysterectomy. After propensity matching, they found that the recurrence risk at 5 years was 6.1% with open surgery and 14.4% with MIS (HR 2.93; 95% CI, 1.22–7.0). A European cohort study (SUCCOR study) reviewed 1272 patients with FIGO Stage IB1 patients and found that the risk of recurrence for MIS‐treated patients was twice as high as that with open surgery (HR 2.07; 95% CI, 1.35–3.15). 64 Paik et al. 65 reviewed 738 women who underwent radical hysterectomy for FIGO Stages IB–IIA cervical cancer. They also demonstrated that MIS had inferior disease‐free survival compared with those who had open surgery (HR 2.74; 95% CI, 1.3–5.7). This evidence suggests that MIS for cervical cancer could result in an excess risk of recurrence or death compared with an open approach.
In the study by Uppal et al., 63 among tumors less than 2 cm diameter, 4.4% recurrences were noted in the open group versus 11.5% in the MIS group (HR 2.83; 95% CI, 1.1–7.18, P = 0.019) and prior conization was associated with a lower risk of recurrence (4.9% vs 16.2%, P = 0.001). However, the SUCCOR study revealed no difference in oncologic outcome between the open and MIS approach in tumors less than 2 cm. Patients who underwent MIS using a uterine manipulator had 2.76 times higher risk of relapse (HR 2.76; 95% CI, 1.75–4.33; P < 0.001) whereas MIS with protective vaginal closure had similar rates of relapse as open surgery (HR 0.63; 95% CI, 0.15–2.59; P < 0.52). 64 Currently two prospective randomized trials are evaluating the role of MIS in cervical cancer. The first is the Robot assisted Approach to Cervical Cancer (RACC), a Swedish multicenter prospective trial in which the use of a uterine manipulator is not allowed, and closure of the vagina before colpotomy is recommended but not mandatory. 66 The second is the multicenter randomized controlled trial designed in China in which the use of a uterine manipulator and the method of vaginal excision is to be reported. 67
The role of SLN mapping in cervical cancer is still experimental and needs more evidence to include it into routine practice. It may have some role in early‐stage cervical cancer, i.e. FIGO Stages IA, IB1, and IB2. 68 , 69 , 70 Dual labeling using blue dye and radiocolloid increases the accuracy of SLN detection. 71 , 72 More recently, indocyanine green dye with near infrared technique has been used in both the open and minimally invasive approaches. Pelvic lymphadenectomy needs to be considered if LVSI is present. In the only randomized trial of SLN resection alone or SLN plus pelvic lymphadenectomy (SENTICOL‐2) in early cervical cancer, among 206 patients no false‐negative case was observed in the SLN plus lymphadenectomy arm. Lymphatic morbidity was significantly lower in the SLN arm than in the SLN plus lymphadenectomy arm (31.4% vs 51.5%, P = 0.0046), as was the rate of postoperative neurological symptoms (7.8% vs 20.6%, P = 0.01, respectively). The 3‐year recurrence‐free survival was not significantly different between the two arms. 73 Currently the SENTICOL‐III study is ongoing, which will randomize 950 patients and will compare disease‐free survival and health‐related quality of life outcomes between SLN and SLN plus pelvic lymphadenectomy. 74
7.1.3. FIGO Stages IB3 and IIA2
In Stages IB3 and IIA2, the tumors are larger and the likelihood of high‐risk factors such as positive lymph nodes, positive parametria, or positive surgical margins that increase the risk of recurrence and require adjuvant radiation after surgery are high. Other risk factors that increase the risk of pelvic recurrence even when nodes are not involved include: largest tumor diameter >4 cm, LVSI, and invasion of the outer one‐third of the cervical stroma. 75 , 76 In such cases, adjuvant whole pelvic irradiation reduces the local failure rate and improves progression‐free survival compared with patients treated with surgery alone. 76 However, the dual modality treatment increases the risk of major morbidity to the patient.
The treatment modality must, therefore, be determined based on the availability of resources and tumor‐ and patient‐related factors. Concurrent platinum‐based chemoradiation (CCRT) is the preferred treatment option for Stages IB3 to IIA2 lesions. It has been demonstrated that the prognosis in terms of overall survival, progression‐free survival, and local and distant recurrences is more favorable with CCRT, rather than radical hysterectomy followed by radiotherapy as postoperative adjuvant therapy. 77 , 78
In areas where radiotherapy facilities are scarce, neoadjuvant chemotherapy (NACT) has been used with the goal of: (1) down‐staging of the tumor to improve the radical curability and safety of surgery; and (2) inhibition of micrometastasis and distant metastasis. There is no consensus as to whether it improves prognosis compared with the standard treatment. Two randomized trials, EORTC 55994 79 and the study by Gupta et al., 80 had varied outcomes. EORTC showed no difference in 5‐year overall survival between NACT and CCRT but chemotherapy‐related toxicity in the NACT arm, whereas the Gupta et al. study showed superior disease‐free survival in the CCRT arm. The extent of surgery after NACT remains the same, i.e. radical hysterectomy and pelvic lymphadenectomy. The greater difficulty is in determining the indications for adjuvant therapy, which are often kept the same as those after primary surgery. 76 , 78 However, it must be remembered that NACT may give a false sense of security by masking the pathologic findings and thus affecting evaluation of indications for adjuvant radiotherapy/CCRT. NACT surgery is best reserved for research settings or those areas where radiotherapy is unavailable. This is especially true in patients with very large tumors or adenocarcinoma, which have lower response rates. 81
7.1.4. FIGO Stage IVA or recurrence
Rarely, patients with Stage IVA disease may have only central disease without involvement up to the pelvic sidewall or distant spread. Such cases, or in the case of such a recurrence, pelvic exenteration can be considered but usually has a poor prognosis. 82 , 83 , 84
7.2. Radiation management
In LMICs, the majority of patients present with locally advanced disease, 81 where surgery plays a limited role. Over the last two decades, development of sophisticated planning and delivery techniques, and introduction of computer technology and imaging have galvanized the practice of radiotherapy, resulting in improved clinical outcome and reduced toxicity. 85 , 86
Apart from its curative role, radiotherapy can also be used as adjuvant therapy for operated patients to prevent locoregional recurrence, although the role of “dual modality” is discouraged, and as palliative therapy for alleviating distressing symptoms in patients with advanced incurable disease.
7.2.1. Radiation therapy for early‐stage disease (FIGO Stages IA, IB1, IB2, and IIA1)
Although surgery is preferred for early‐stage disease, in cases with contraindications for surgery or anesthesia, radiotherapy provides equally good results in terms of local control and survival. Treatment decision should be made on the basis of clinical, anatomic, and social factors. Patients with microinvasive disease have been treated by intracavitary radiation therapy (ICRT) alone with good results if surgery is contraindicated owing to medical problems. Selected patients with very small Stage IB1 disease (less than 1 cm) may also be treated with ICRT alone, particularly if there are relative contraindications to external beam radiation therapy (EBRT). 87 A dose of 60–65 Gy equivalent is usually prescribed to Point A. A combination of EBRT and ICRT is also an option for such patients.
Definitive radiotherapy or CCRT is preferred in patients likely to require postoperative radiotherapy to avoid compounding treatment‐related morbidity. There is a single randomized trial comparing surgery and radiotherapy 54 but none comparing surgery to CCRT, which is the current standard in patients treated by definitive radiotherapy. Landoni et al. 54 randomized patients with Stage IB or IIA cervical cancer to surgery with or without postoperative radiotherapy (PORT) versus definitive radiotherapy alone. PORT was administered to 64% of patients in the surgery arm. The two treatment arms resulted in similar overall survival (83%) and disease‐free survival (74%); severe morbidity was higher in the surgery arm (28% vs 12%), likely due to contributions from both treatment modalities. An update of the same trial with 20‐year follow‐up data has shown marginally better results with radiotherapy compared with surgery (77% vs 72%, P = 0.280). Multivariate analysis confirmed that risk factors for survival are histopathologic type (P = 0.020), tumor diameter (P = 0.008), and lymph node status (P < 0.001). 88
7.2.2. Adjuvant radiotherapy
Following radical hysterectomy, PORT with or without chemotherapy is indicated for patients with adverse pathologic factors. According to various prognostic factors, patients may be categorized into high‐, intermediate‐, or low‐risk disease. High‐risk disease includes patients with either positive surgical margins or lymph node metastases or parametrial spread, and such patients should be offered PORT with chemotherapy since the GOG 109 trial has shown overall survival advantage. 78 Intermediate‐risk patients with any two of three factors (tumor size more than 4 cm, lymphovascular invasion, deep stromal invasion) require PORT 76 , 89 and no chemotherapy should be offered to these patients. All other patients following radical hysterectomy are termed as low‐risk disease patients and do not need any adjuvant therapy.
Tumor size of more than 4 cm is a well‐known risk factor. It was incorporated in the FIGO staging system (2009) as Stage IB2 and subsequently in the FIGO 2018 staging revision as Stage IB3. Recent literature, especially with the advent of more and more fertility sparing surgery suggests tumor size more than 2 cm is a risk factor. 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 Gemer et al. 98 evaluated various clinical and pathologic risk factors that may reduce the rate of multimodality treatment of early cervical cancer. The authors observed that 89% of patients with tumors 2 cm or greater and LVSI received radiotherapy and 76% of patients with tumors 2 cm or greater and depth of invasion greater than 10 mm received radiotherapy. They suggest that in patients with early cervical cancer, evaluation of tumor size and LVSI should be undertaken before performing radical hysterectomy to tailor treatment and to reduce the rate of employing both radical hysterectomy and chemoradiation. In view of the above‐mentioned emerging literature, tumor size of more than 2 cm has been taken as the first cutoff in the FIGO 2018 revision of the staging system.
PORT consists of whole pelvic EBRT to cover the tumor bed and draining lymph node areas. A dose of 45–50 Gy is usually prescribed. Intensity modulated radiation therapy (IMRT), an advanced and refined technique of irradiation, has been explored in the postoperative setting to reduce the toxicity. 99 , 100 A recent Phase III trial 100 revealed improved patient reported outcomes at week five with IMRT, with no difference after treatment completion. Therefore, postoperative pelvic IMRT remains investigational until further data are published.
The role of vaginal brachytherapy boost following EBRT is not clear; however, it may be considered for patients with close or positive margins, large or deeply invasive tumors, parametrial or vaginal involvement, or extensive LVSI. 101 Vaginal cuff brachytherapy is usually delivered by ovoids or cylinders to the upper one‐third of the residual vagina and should include two weekly fractions of high dose rate (HDR) brachytherapy of 6 Gy each prescribed to 5 mm from the vaginal cylinder/ovoid surface.
7.2.3. Radiation therapy for FIGO Stages IB3 and IIA2
Although feasible, surgery as initial treatment is not encouraged for patients with Stage IB3 and IIA2 disease since 80% of them require PORT or CCRT. 54 It is well known that the addition of adjuvant radiotherapy to surgery increases morbidity and thus compromises quality of life. 102 , 103 Additionally, combined modality treatment will unnecessarily overburden the surgical and radiation facilities, which are already inadequate in low‐resource countries. Therefore, CCRT is the standard of care for Stage IB3 and IIA2 disease. CCRT includes external radiation and intracavitary brachytherapy. 101
7.2.4. Radiation therapy for FIGO Stages IIB–IVA
CCRT is considered the standard treatment for patients with locally advanced cervical cancer, based on the results of large randomized trials that tested addition of chemotherapy to pelvic radiation. 78 , 104 , 105 , 106 , 107 , 108 These studies demonstrated that CCRT had a significant survival advantage of 10%–15% at 5 years after treatment compared with radiotherapy alone, and also reduced local and distant recurrence. A subsequent meta‐analysis showed maximum benefit of chemoradiation of 6% in Stage IB2 (now termed IB3) to Stage IIB and only 3% benefit in Stage IIIB patients. 109
A once‐weekly infusion of cisplatin (40 mg/m2 weekly with appropriate hydration) for 5–6 cycles during external beam therapy is a commonly used concurrent chemotherapy regimen. 109 , 110 For patients who are unable to receive platinum chemotherapy, 5‐fluorouracil based regimens are an acceptable alternative. 110 , 111 , 112 Data on the toxicity associated with concurrent chemotherapy and extended field irradiation are limited. 112
Additional adjuvant chemotherapy after concurrent chemoradiotherapy is being explored in an international randomized controlled trial (OUTBACK). 113 The ongoing Phase III INTERLACE trial will assess the role of induction chemotherapy plus CCRT as first‐line treatment for LACC. 114
The combination of EBRT and ICRT maximizes the likelihood of locoregional control while minimizing the risk of treatment complications. The primary goal of EBRT is to sterilize local disease and to shrink the tumor to facilitate subsequent ICRT. Standard EBRT should deliver a dose of 45–50 Gy to the whole pelvis by the 2‐ or 4‐field box technique (Table 3) encompassing the uterus, cervix, adnexal structures, parametria, and pelvic lymph nodes. Although EBRT is commonly delivered by a Cobalt‐60 teletherapy machine in several low‐resource countries, linear accelerators are now preferred as they provide higher energy beams resulting in more homogeneous dose delivery to deep tissues with relative sparing of superficial tissues. Recently, conformal radiotherapy techniques like 3D‐CRT and IMRT are increasingly being used with encouraging results in terms of reduced toxicity owing to relative sparing of normal tissues (Figure 1). Recently, the results of MRI‐guided adapted brachytherapy in the LACC (EMBRACE‐I) study showed a 5‐year local control of 92% with 5‐year incidence of grade 3–5 morbidity as follows: 6.8% for genitourinary events, 8.5% for gastrointestinal events, 5.7% for vaginal events, and 3.2% for fistulae. 115
TABLE 3.
Field design for the pelvic radiotherapy
| Field | Border | Landmark |
|---|---|---|
| AP‐PA fields | Superior | L4–5 vertebral interspace |
| Inferior | 2 cm below the obturator foramen or 3 cm inferior to distal disease, whichever is lower | |
| Lateral | 1.5–2 cm lateral to the pelvic brim | |
| Lateral fields | Superior | Same as AP‐PA field |
| Inferior | Same as AP‐PA field | |
| Anterior | Anterior to the pubic symphysis | |
| Posterior | 0.5 cm posterior to the anterior border of the S2/3 vertebral junction. May include the entire sacrum to cover the disease extent |
FIGURE 1.
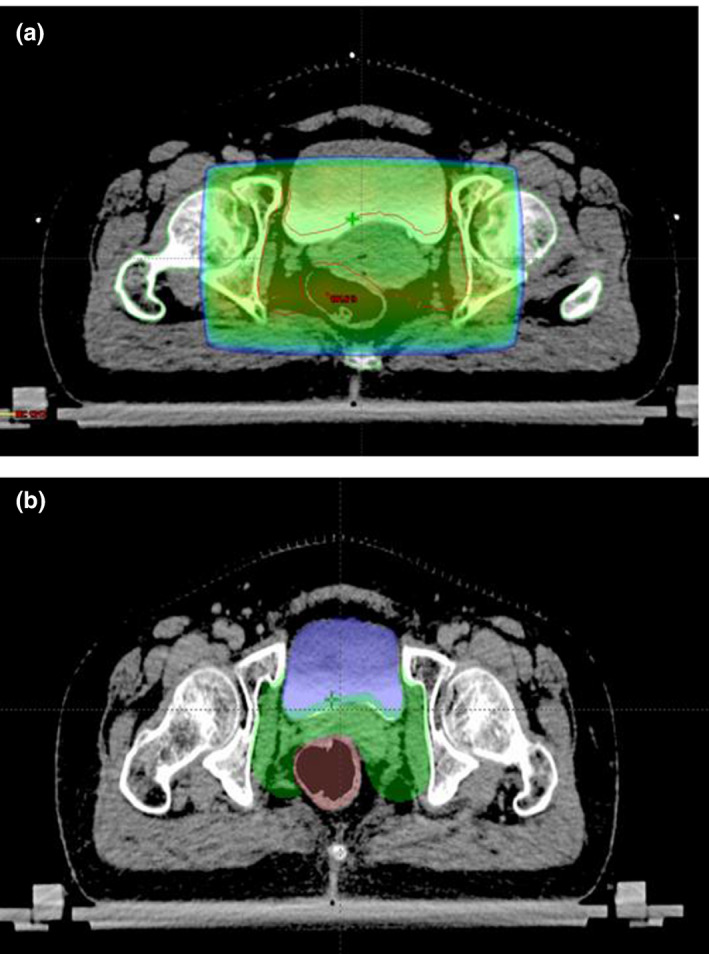
CT scan images showing radiotherapy planning using: (a) conventional four‐field box technique; and (b) intensity modulated radiation therapy (IMRT) planning. Normal tissues such as bladder and bowel are relatively spared in IMRT planning
Standard ICRT is usually performed using a tandem and two ovoids, or a tandem and ring. Any of the dose‐rate systems, namely low‐dose‐rate (LDR), HDR, or pulsed‐dose‐rate (PDR) may be practiced as all three yield comparable survival rates. 116 The dose is usually prescribed to Point A or to high‐risk clinical target volume if image‐based planning is used.
With an LDR system, a dose of 30–40 Gy is prescribed in one or two sessions. With HDR, various dose fraction schedules are used, employing a dose of 5.5–8 Gy by 3–5 weekly fractions. Owing to resource constraints and long travelling distances in low‐resource countries, delivering three instead of five fractions is often more realistic and allows for treatment of a higher number of patients. In the current COVID era, hypofractionation (increase dose per day and reduce the number of fractions) is even more necessary to reduce the number of hospital visits. The total combined dose with EBRT and ICRT should be in the range of 80–90 Gy. Although PDR is rarely used, the overall treatment time and dose in PDR remains almost the same as in LDR except that the treatment is given in multiple hourly pulses each lasting for a few minutes.
If ICRT is not feasible either due to distorted anatomy or inadequate dosimetry, then interstitial brachytherapy should be considered. Interstitial brachytherapy consists of insertion of multiple needles/catheters into the primary tumor and parametria (Figure 2) through the perineum with the help of a template. Due to the risk of trauma to normal structures like the bowel and bladder, use of ultrasound imaging (especially transrectal) is suggested during the implant procedure. 117
FIGURE 2.
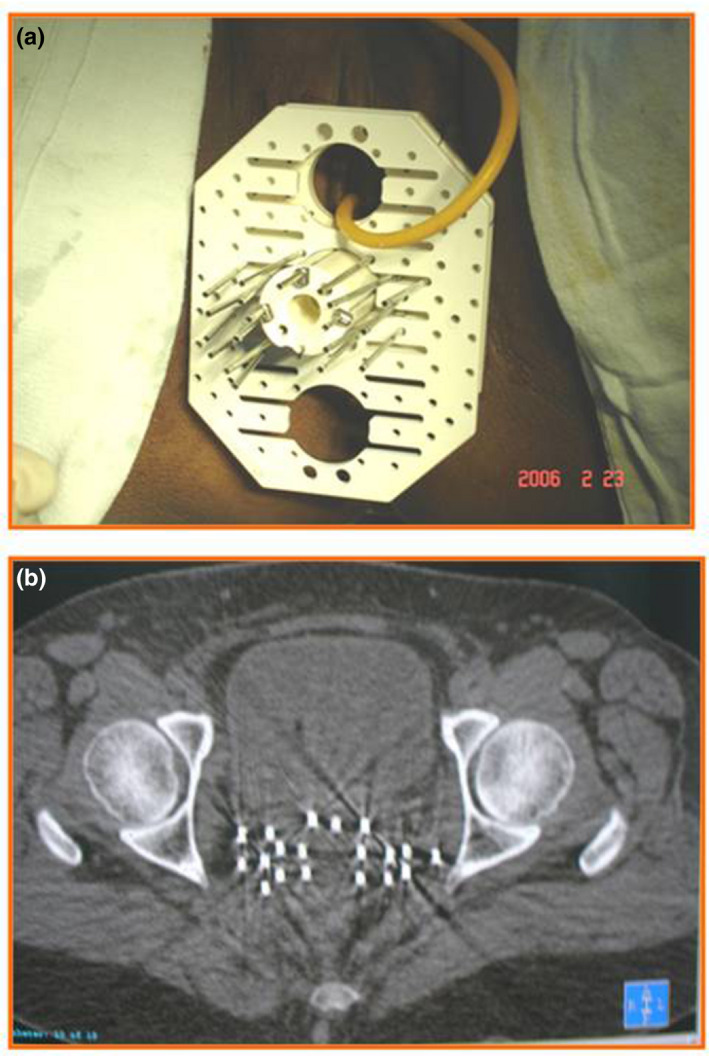
Interstitial brachytherapy implant: (a) clinical image of a patient showing the perineal template and the steel needles; (b) CT scan image showing the brachytherapy needles inserted into the pelvis
Completion of the radiotherapy protocol within the stipulated time is an important goal as it has a direct correlation on the outcome. In retrospective analyses, patients whose radiotherapy treatment times exceeded 9–10 weeks had significantly higher rates of pelvic failure when compared with women whose treatment was completed in less than 6–7 weeks. 118 , 119 The current recommendation is to complete the entire protocol of EBRT and brachytherapy within 8 weeks.
7.2.5. FIGO Stage IVB/distant metastases
Presentation with distant metastatic disease is rare, reported in about 2% of cases. A management plan should consider that the median duration of survival with distant metastatic disease is approximately 7 months.
Concurrent chemoradiation may have a better response than systemic chemotherapy with overall and disease‐free survivals of 69% and 57%, respectively, reported in patients with positive para‐aortic and supraclavicular lymph nodes. 120 Currently there is no role for prophylactic extended field radiotherapy (EFRT) in locally advanced cervical cancer. 112 When para‐aortic nodes are involved, EFRT with concurrent chemotherapy should be used. IMRT may be used in such patients to reduce the toxicity.
Despite limited response rates, cisplatin has been the standard chemotherapy used in the setting of distant metastatic disease. 121 Given low response rates to cisplatin alone after concurrent chemoradiation, recent evidence supports the use of platinum doublets over cisplatin alone, although with very modest benefits in response rates. Cisplatin may be combined with taxanes, topotecan, 5‐fluorouracil, gemcitabine, or vinorelbine. 122 Carboplatin‐paclitaxel combination has also been successful in these cases.
Patients with an ECOG (Eastern Cooperative Oncology Group) performance status of 0–2 may be considered for palliative systemic chemotherapy. Where feasible, these patients could be offered participation in clinical trials, especially when the interval to relapse is less than 12 months.
GOG 240 studied the efficacy of antiangiogenic therapy with bevacizumab—a humanized anti‐VEGF monoclonal antibody. When incorporated in the treatment of recurrent and metastatic cervical cancer, it showed increased overall survival (17.0 vs 13.3 months, HR for death 0.71; 98% CI, 0.54–0.95, P = 0.004 in a one‐sided test). 123 The treatment is expensive and patients and their families need to be counseled. Adverse effects include increased incidence of hypertension, thromboembolic events, and gastrointestinal fistula.
7.2.6. Radiation therapy after inadvertent incomplete surgery
Invasive cervical cancer may be found during pathologic evaluation of the specimen from a simple hysterectomy for an apparent benign condition. Inadvertent simple hysterectomy is considered inadequate surgery for invasive cervical carcinoma and subsequent therapy is required for all such cases. In such a situation, the extent of the disease should be assessed by a PET/CT scan if available, or a pelvic and abdominal CT or MRI scan, and chest imaging. The subsequent treatment plan is formulated based on the histologic and radiologic findings.
Although PORT for patients following inadvertent simple hysterectomy has been shown to be beneficial, 124 , 125 the outcome for such patients even after PORT remains very poor with 5‐year recurrence‐free survival of 49%, 124 and therefore CCRT is generally added. In a study from India, Sharma et al. 124 reported the results of 83 patients treated with PORT following either inadvertent simple hysterectomy (33 patients) or radical hysterectomy (50 patients). The 5‐year recurrence‐free survival was found to be significantly inferior in patients who underwent PORT after inadvertent simple hysterectomy (49% vs 72%, respectively; P = 0.04). 124 PORT, therefore, does not compensate for lack of adequate surgery.
In centers where the expertise is available, some of these patients may be found suitable for repeat laparotomy with parametrectomy and pelvic lymphadenectomy. The procedure is challenging due to previous scarring, adhesions, and distortion of anatomy, but does have the potential for curative surgery as well as to allow assessment of the need for adjuvant CCRT. 126
7.3. Post‐treatment follow‐up
In a systematic review of 17 retrospective studies that followed up women treated for cervical cancer, the median time to recurrence ranged from 7–36 months after primary treatment. 127 Therefore, closer clinical follow‐up in the first 2–3 years after treatment may be important. Routine follow‐up visits are recommended every 3–4 months for the first 2–3 years, then every 6 months until 5 years, and then annually for life. At each visit, history taking and clinical examination are carried out to detect treatment complications and psychosexual morbidity, as well as assess for recurrent disease.
Routine imaging is not indicated. Special circumstances, such as involved high pelvic lymph nodes, may justify interval imaging of the abdomen to assess for potentially curable progression of disease. In the systematic review, asymptomatic recurrent disease was detected using physical exam (29%–71%), chest X‐ray (20%–47%), CT (0%–34%), and vaginal vault cytology (0%–17%). Frequent vaginal vault cytology does not significantly improve the detection of early disease recurrence.
Women under the age of 50 years who have lost ovarian function should be considered for menopausal hormone therapy. As women age, the routine exam should also include other age‐indicated well‐woman checks to ensure quality of life, including assessment of thyroid and renal status.
7.4. Recurrent disease
Recurrences may occur locally in the pelvic or para‐aortic lymph nodes the patient may develop distant metastases, or there may be a combination thereof. The risk of both pelvic and distant failure increases in proportion to tumor volume. 128 , 129 Most recurrences are seen within 3 years and prognosis is poor as most patients die from progressive disease, with uremia the most common terminal event. 128 , 129 The treatment plan depends on the patient's performance status, site, and extent of recurrence and/or metastases, and prior treatment received. 130
If there is extensive local disease or distant metastatic disease, the patient is assigned to palliative therapy, with best supportive care. However, if the performance status is good and there is only limited metastatic disease, a trial of platinum doublet chemotherapy along with bevacizumab is justified as depicted in GOG 240 trial, 123 after counseling the patient and her family on the limited benefits in terms of response rate and progression‐free survival. 131
7.4.1. Local recurrence
The pelvis is the most common site of recurrence. Good prognostic factors are the presence of an isolated central pelvic recurrence with no involvement of the pelvic sidewall, a long disease‐free interval from previous therapy, and if the largest diameter of the recurrent tumor is less than 3 cm. 131
When the pelvic relapse follows primary surgery, it may be treated by either radical chemoradiation or pelvic exenteration. Confirmation of recurrence with a pathologic specimen obtained by biopsy is essential prior to proceeding with either therapy. Radical irradiation with or without concurrent chemotherapy may result in 5‐year disease‐free survival rates of 45%–74% with isolated pelvic failure after primary surgery. 132 , 133 , 134 The extent of recurrent disease and involvement of pelvic lymph nodes are prognostic factors for survival. 135
Concurrent chemotherapy with either cisplatin and/or 5‐fluorouracil may improve outcome. 136
Pelvic exenteration may be feasible in some patients in whom there is no evidence of intraperitoneal or extrapelvic spread, and there is a clear tumor‐free space between the recurrent disease and the pelvic sidewall. 82 , 83 , 84 Owing to its high morbidity, it is reserved for those with expected curative potential and requires careful patient selection regarding the associated physical and psychological demands. A PET/CT scan is the most sensitive noninvasive test to determine any sites of distant disease, and should be performed prior to exenteration, if possible. 137 , 138 , 139 Patient assessment and counseling regarding the implications and ability to manage stoma and ostomy sites must also be addressed prior to surgery. 140 The overall survival is 10% but careful selection of patients has been reported to yield a five‐year survival with pelvic exenteration in the order of 30%–60%, 82 , 83 , 84 and an operative mortality of less than 10%. 141
7.4.2. Para‐aortic nodal recurrence
The second most common site of recurrence is in the para‐aortic lymph nodes. Where there is isolated para‐aortic nodal recurrence, curative‐intent radiation therapy or chemoradiation can achieve long‐term survival in approximately 30% of cases. 142
7.5. Comprehensive palliative care
Symptom control is the essence of palliative care and plays a major role in maintaining dignity and quality of life. Common symptoms and signs of advanced cervical cancer include pain, ureteric obstruction causing renal failure, hemorrhage, malodorous vaginal discharge, lymphedema, and fistula. Patients require support from the corresponding clinical services as well as psychosocial care and support for their families and caregivers. Typically, a tiered approach to pain is practiced. Access to oral morphine is improving within LMICs and is an important aspect of palliative care. The availability of homecare teams in many regions and involvement of nongovernmental organizations in this effort can help minimize the need to transport the patient to hospital and save costs. In terminal cases, some patients may also require the services of a hospice facility.
7.5.1. Palliative radiotherapy
Short‐course radiotherapy is very effective in palliation of distressing symptoms. Although there is no standard dose fraction schedule, a dose of 20 Gy in five fractions over 1 week or 30 Gy in 10 fractions over 2 weeks is commonly practiced. 143 In patients with severe vaginal bleeding, a short course of EBRT may be tried and, if it fails, ICRT can be highly effective in controlling the intractable bleeding. 144 Control of bleeding is usually achieved after 12–48 h of radiotherapy.
In patients with pain arising from enlarged para‐aortic or supraclavicular nodes, skeletal metastases, 145 and symptoms associated with cerebral metastases, palliative radiotherapy should be given via larger fractions over shorter periods of time. Commonly used schedules include large single fractions, 20 Gy in five fractions, and 30 Gy in 10 fractions.
8. SPECIAL SITUATIONS
8.1. Cervical cancer during pregnancy
Adequate management of these patients requires a multidisciplinary team. The plan must be discussed with the patient, and ideally her partner too, in order to respect their wishes.
Broadly, the management of cervical cancer in pregnancy follows the same principles as in nonpregnant patients. Before 16–20 weeks of gestation, patients are treated without delay. The mode of therapy can be either surgery or chemoradiation depending on the stage of the disease. Radiation often results in spontaneous abortion of the conceptus. From the late second trimester onward, surgery and chemotherapy can be used in selected cases while preserving the pregnancy. 146 When the diagnosis is made after 20 weeks, delaying definitive treatment is a valid option for Stages IA2 and IB1 and 1B2, which has not been shown to have any negative impact on the prognosis compared with nonpregnant patients. 147 , 148 Timing of delivery requires a balance between maternal and fetal health interests. When delivered at a tertiary center with appropriate neonatal care, delivery by classical cesarean section and radical hysterectomy at the same time is undertaken no later than 34 weeks of gestation.
For more advanced disease, the impact of treatment delay on survival is not known. Neoadjuvant chemotherapy may be administered to prevent disease progression in women with locally advanced cervical cancer when a treatment delay is planned. 149 , 150
AUTHOR CONTRIBUTIONS
All authors contributed to the manuscript at all stages including design, planning, data abstraction, and manuscript writing.
CONFLICTS OF INTEREST
Outside of the submitted work, NB has received research funding through her institute from MSD, GlaxoSmithKline, and Digene/Qiagen Inc. Outside of the submitted work, DA has received honoraria from AstraZeneca, Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd, Taiho Pharmaceutical Co. Ltd, Coviden Japan Inc, Johnson & Johnson, and Takeda Pharmaceutical Co. Ltd; consulting fees from Takeda Pharmaceutical Co. Ltd, AstraZeneca, MSD, and Chugai Pharmaceutical Co. Ltd; and holds unpaid positions with the Asian Society of Gynecologic Oncology, Japan Society of Gynecologic Oncology, and the Japan Society of Obstetrics and Gynecology. RS advises on cancer prevention and early detection for Karkinos Healthcare Private Limited, a managed healthcare company in India. DNS has no conflicts of interest to declare.
ACKNOWLEDGMENTS
This chapter updates the information published in the FIGO Cancer Report 2018 (Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynecol Obstet. 2018;143 Suppl 2:22–36). Dr Jayashree Natarajan and Dr Sarita Kumari’s help in reviewing the literature is gratefully acknowledged.
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynecol Obstet. 2021;155(Suppl. 1):28–44. 10.1002/ijgo.13865
FIGO CANCER REPORT 2021
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IARC Working Group . Human Papillomaviruses: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer. Accessed May 2, 2021. https://monographs.iarc.who.int/wp‐content/uploads/2018/06/mono90.pdf [PMC free article] [PubMed] [Google Scholar]
- 4. Franco E, Villa L, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high‐risk area for cervical cancer. J Infect Dis. 1999;180:1415‐1423. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. WHO; 2020. Accessed April 17, 2021. https://www.who.int/publications/i/item/9789240014107 [Google Scholar]
- 6. Bruni L, Albero G, Serrano B, et al.: ICO/IARC. Information Centre on HPV and Cancer (HPV Information Centre) . Human Papillomavirus and Related. Diseases in the World. Summary Report 17 June 2019. Accessed May 2, 2021. https://hpvcentre.net/statistics/reports/XWX.pdf [Google Scholar]
- 7. World Health Organization . Human papillomavirus vaccines: WHO position paper, May 2017. Weekly Epidemiological Record No. 2017;19(92):241‐268. [Google Scholar]
- 8. World Health Organization . Safety of HPV vaccines. Accessed May 2, 2021. https://www.who.int/groups/global‐advisory‐committee‐on‐vaccine‐safety/topics/human‐papillomavirus‐vaccines/safety/
- 9. Drolet M, Bénard É, Pérez N, et al. Population‐level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta‐analysis. Lancet. 2019;394:497‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340‐1348. [DOI] [PubMed] [Google Scholar]
- 11. Sankaranarayanan R, Joshi S, Muwonge R, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36:4783‐4791. [DOI] [PubMed] [Google Scholar]
- 12. Whitworth HS, Gallagher KE, Howard N, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two‐dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. 2020;38:1302‐1314. [DOI] [PubMed] [Google Scholar]
- 13. Kreimer AR, Sampson JN, Porras C, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: The CVT trial. J Natl Cancer Inst. 2020;112:1038‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saccucci M, Franco EL, Ding L, et al. Non‐vaccine‐type human papillomavirus prevalence after vaccine introduction: no evidence for type replacement but evidence for cross‐protection. Sex Transm Dis. 2018;45:260‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Covert C, Ding L, Brown D, et al. Evidence for cross‐protection but not type‐replacement over the 11 years after human papillomavirus vaccine introduction. Hum Vaccin Immunother. 2019;15:1962‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonjour M, Charvat H, Franco E, et al. Global estimates of expected and preventable cervical cancers among girls born between 2005 and 2014: a birth cohort analysis. Lancet Public Health. 2021;S2468‐2667(21)00046‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sankaranarayanan R. Screening for cancer in low‐ and middle‐income countries. Ann Glob Health. 2014;80:412‐417. [DOI] [PubMed] [Google Scholar]
- 18. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385‐1394. [DOI] [PubMed] [Google Scholar]
- 19. Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet. 2014;383:524‐532. [DOI] [PubMed] [Google Scholar]
- 20. Maver PJ, Poljak M. Primary HPV‐based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020;26:579‐583. [DOI] [PubMed] [Google Scholar]
- 21. Hall MT, Simms KT, Lew JB, et al. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017–2035: example from Australia. PLoS One. 2018;13(2):e0185332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiferaw N, Salvador‐Davila G, Kassahun K, et al. The single‐visit approach as a cervical cancer prevention strategy among women with HIV in Ethiopia: successes and lessons learned. Glob Health Sci Pract. 2016;4:87‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Msyamboza KP, Phiri T, Sichali W, et al. Cervical cancer screening uptake and challenges in Malawi from 2011 to 2015: retrospective cohort study. BMC Public Health. 2016;16:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population‐level scale‐up of cervical cancer prevention services in a low‐resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 2015;10(4):e0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . WHO Guidelines for Screening and Treatment of Cervical Precancerous Lesions for Prevention. WHO; 2013. Accessed April 16, 2021. https://www.who.int/reproductivehealth/publications/cancers/screening_and_treatment_of_precancerous_lesions/en/ [PubMed] [Google Scholar]
- 26. World Health Organization . WHO Guidelines for the Use of Thermal Ablation for Cervical Pre‐Cancer Lesions. WHO; 2019. Accessed May 2, 2021. https://apps.who.int/iris/handle/10665/329299 [PubMed] [Google Scholar]
- 27. Bhatla N, Nessa A, Oswal K, et al. Program organization rather than choice of test determines success of cervical cancer screening: case studies from Bangladesh and India. Int J Gynecol Obstet. 2021;152:40‐47. [DOI] [PubMed] [Google Scholar]
- 28. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145:129‐135. Corrigendum in: Int J Gynecol Obstet. 2019;147:279–280. [DOI] [PubMed] [Google Scholar]
- 29. Olawaiye AB, Baker TP, Washington MK, et al. The new (Version 9) American Joint Committee on Cancer tumour, node, metastasis staging for cervical cancer. CA Cancer J Clin. 2021;71:287–298. [DOI] [PubMed] [Google Scholar]
- 30. International Agency for Research on Cancer (IARC) . WHO Classification of Female Genital Tumours. 5th ed. Edited by the WHO Classification of Tumours Editorial Board, International Agency for Research on Cancer (IARC); 2020. [Google Scholar]
- 31. Park KJ, Roma A, Singh N, et al. Tumour staging of endocervical adenocarcinoma: recommendations from the International Society of Gynecological Pathologists. Int J Gynecol Pathol. 2021;40:S92‐S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel‐Lippmann K, Robbins JB, Barroilhet L, et al. MR imaging of cervical cancer. Magn Reson Imaging Clin N Am. 2017;25:635‐649. [DOI] [PubMed] [Google Scholar]
- 33. Fischerova D, Cibula D, Stenhova H, et al. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int J Gynecol Cancer. 2008;18:766‐772. [DOI] [PubMed] [Google Scholar]
- 34. Yang WT, Lam WWM, Yu MY, et al. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. Am J Roentgenol. 2000;175:759‐766. [DOI] [PubMed] [Google Scholar]
- 35. Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG‐PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183‐191. [DOI] [PubMed] [Google Scholar]
- 36. Sakurai H, Suzuki Y, Nonaka T, et al. FDG‐PET in the detection of recurrence of uterine cervical carcinoma following radiation therapy‐tumour volume and FDG uptake value. Gynecol Oncol. 2006;100:601‐607. [DOI] [PubMed] [Google Scholar]
- 37. Hertel H, Köhler C, Elhawary T, et al. Laparoscopic staging compared with imaging techniques in the staging of advanced cervical cancer. Gynecol Oncol. 2002;87:46‐51. [DOI] [PubMed] [Google Scholar]
- 38. Ramirez PT, Jhingran A, Macapinlac HA, et al. Laparoscopic extraperitoneal para‐aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117:1928‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marnitz S, Köhler C, Roth C, et al. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536‐544. [DOI] [PubMed] [Google Scholar]
- 40. Gold MA, Tian C, Whitney CW, et al. Surgical versus radiographic determination of para‐aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: a Gynecologic Oncology Group Study. Cancer. 2008;112:1954‐1963. [DOI] [PubMed] [Google Scholar]
- 41. Smits RM, Zusterzeel PLM, Bekkers RLM. Pretreatment retroperitoneal para‐aortic lymph node staging in advanced cervical cancer: a review. Int J Gynecol Cancer. 2014;24:973‐983. [DOI] [PubMed] [Google Scholar]
- 42. Heller PB, Malfetano JH, Bundy BN, et al. Clinical‐pathologic study of stage IIB, III, and IVA carcinoma of the cervix: extended diagnostic evaluation for paraaortic node metastasis‐a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:425‐430. [DOI] [PubMed] [Google Scholar]
- 43. Delgado G, Bundy B. Prospective surgicopathological study of disease free interval in patients with Stage IB squamous cell carcinoma of cervix. A Gynecological Oncology Group study. Gynecol Oncol. 1990;38:352‐357. [DOI] [PubMed] [Google Scholar]
- 44. Lee SW, Kim Y‐M, Son W‐S, et al. The efficacy of conservative management after conization in patients with stage IA1 microinvasive cervical carcinoma. Acta Obstet Gynecol Scand. 2009;88:209‐215. [DOI] [PubMed] [Google Scholar]
- 45. Elliott P, Coppleson M, Russell P, et al. Early invasive (FIGO stage IA) carcinoma of the cervix: a clinico‐pathologic study of 476 cases. Int J Gynecol Cancer. 2000;10:42‐52. [DOI] [PubMed] [Google Scholar]
- 46. Webb JC, Key CR, Qualls CR, Smith HO. Population‐based study of microinvasive adenocarcinoma of the uterine cervix. Obstet Gynecol. 2001;97:701‐706. [DOI] [PubMed] [Google Scholar]
- 47. van Meurs H, Visser O, Buist MR, et al. Frequency of pelvic lymph node metastases and parametrial involvement in stage IA2 cervical cancer: a population‐based study and literature review. Int J Gynecol Cancer. 2009;19:21‐26. [DOI] [PubMed] [Google Scholar]
- 48. Costa S, Marra E, Martinelli GN, et al. Outcome of conservatively treated microinvasive squamous cell carcinoma of the uterine cervix during a 10‐year follow‐up. Int J Gynecol Cancer. 2009;19:33‐38. [DOI] [PubMed] [Google Scholar]
- 49. Bouchard‐Fortier G, Reade CJ, Covens A. Non‐radical surgery for small early‐stage cervical cancer. Is it time? Gynecol Oncol. 2014;132:624‐627. [DOI] [PubMed] [Google Scholar]
- 50. Coutant C, Cordier AG, Guillo E, et al. Clues pointing to simple hysterectomy to treat early‐stage cervical cancer. Oncol Rep. 2009;22:927‐934. [DOI] [PubMed] [Google Scholar]
- 51. Frumovitz M, Sun CC, Schmeler KM, et al. Parametrial involvement in radical hysterectomy specimens for women with early‐stage cervical cancer. Obstet Gynecol. 2009;114:93‐99. [DOI] [PubMed] [Google Scholar]
- 52. Shepherd JH, Spencer C, Herod J, Ind TE. Radical vaginal trachelectomy as a fertility‐sparing procedure in women with early‐stage cervical cancer‐cumulative pregnancy rate in a series of 123 women. BJOG. 2006;113:719‐724. [DOI] [PubMed] [Google Scholar]
- 53. Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(4):iv72‐iv83. [DOI] [PubMed] [Google Scholar]
- 54. Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib‐IIa cervical cancer. Lancet. 1997;350:535‐540. [DOI] [PubMed] [Google Scholar]
- 55. Landoni F, Maneo A, Cormio G, et al. Class II versus class III radical hysterectomy in stage IB‐IIA cervical cancer: a prospective randomized study. Gynecol Oncol. 2001;80:3‐12. [DOI] [PubMed] [Google Scholar]
- 56. Plante M. The SHAPE trial . Accessed June 10, 2021. https://clinicaltrials.gov/ct2/show/NCT01658930
- 57. Covens A. GOG Protocol. 278. Accessed June 10, 2021. https://clinicaltrials.gov/ct2/show/NCT01649089
- 58. Fujii S, Takakura K, Matsumura N, et al. Anatomic identification and functional outcomes of the nerve sparing Okabayashi radical hysterectomy. Gynecol Oncol. 2007;107:4‐13. [DOI] [PubMed] [Google Scholar]
- 59. Roh J‐W, Lee DO, Suh DH, et al. Efficacy and oncologic safety of nerve‐sparing radical hysterectomy for cervical cancer: a randomized controlled trial. J Gynecol Oncol. 2015;26:90‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abu‐Rustum NR, Sonoda Y, Black D, et al. Fertility‐sparing radical abdominal trachelectomy for cervical carcinoma: technique and review of the literature. Gynecol Oncol. 2006;103:807‐813. [DOI] [PubMed] [Google Scholar]
- 61. Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895‐1904. [DOI] [PubMed] [Google Scholar]
- 62. Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early‐stage cervical cancer. N Engl J Med. 2018;379:1905‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Uppal S, Gehrig PA, Peng K, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi‐institutional retrospective review study. J Clin Oncol. 2020;38:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 64. Chiva L, Zanagnolo V, Querleu D, et al. SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int J Gynecol Cancer. 2020;30:1269‐1277. [DOI] [PubMed] [Google Scholar]
- 65. Paik ES, Lim MC, Kim MH, et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol Oncol. 2019;154:547‐553. [DOI] [PubMed] [Google Scholar]
- 66. Falconer H, Palsdottir K, Stalberg K, et al. Robot‐assisted approach to cervical cancer (RACC): an international multi‐center, open‐label randomized controlled trial. Int J Gynecol Cancer. 2019;29:1072‐1076. [DOI] [PubMed] [Google Scholar]
- 67. Chao X, Li L, Wu M, et al. Efficacy of different surgical approaches in the clinical and survival outcomes of patients with early‐stage cervical cancer: protocol of a phase III multicenter randomised controlled trial in China. BMJ Open. 2019;9(7):e029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martínez‐Palones JM, Gil‐Moreno A, Pérez‐Benavente MA, et al. Intraoperative sentinel node identification in early stage cervical cancer using a combination of radiolabeled albumin injection and isosulfan blue dye injection. Gynecol Oncol. 2004;92:845‐850. [DOI] [PubMed] [Google Scholar]
- 69. van de Lande J, Torrenga B, Raijmakers PGHM, et al. Sentinel lymph node detection in early stage uterine cervix carcinoma: a systematic review. Gynecol Oncol. 2007;106:604‐613. [DOI] [PubMed] [Google Scholar]
- 70. Gortzak‐Uzan L, Jimenez W, Nofech‐Mozes S, et al. Sentinel lymph node biopsy vs. pelvic lymphadenectomy in early stage cervical cancer: is it time to change the gold standard? Gynecol Oncol. 2010;116:28‐32. [DOI] [PubMed] [Google Scholar]
- 71. Levenback C, Coleman RL, Burke TW, et al. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 2002;20:688‐693. [DOI] [PubMed] [Google Scholar]
- 72. Hauspy J, Beiner M, Harley I, Ehrlich L, Rasty G, Covens A. Sentinel lymph nodes in early stage cervical cancer. Gynecol Oncol. 2007;105:285‐290. [DOI] [PubMed] [Google Scholar]
- 73. Mathevet P, Lécuru F, Uzan C, et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: results of a multicentre randomised trial (SENTICOL‐2). Eur J Cancer. 2021;148:307‐315. [DOI] [PubMed] [Google Scholar]
- 74. Lecuru FR, McCormack M, Hillemanns P, et al. SENTICOL III: an international validation study of sentinel node biopsy in early cervical cancer. Int J Gynecol Cancer. 2019;29:829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow‐up of a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 2006;65:169‐176. [DOI] [PubMed] [Google Scholar]
- 76. Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177‐183. [DOI] [PubMed] [Google Scholar]
- 77. Rose PG, Ali S, Watkins E, et al. Gynecologic Oncology Group. Long‐term follow‐up of a randomized trial comparing concurrent single agent cisplatin, cisplatin‐based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2804‐2810. [DOI] [PubMed] [Google Scholar]
- 78. Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. J Clin Oncol. 2000;18:1606‐1613. [DOI] [PubMed] [Google Scholar]
- 79. Kenter G, Greggi S, Vergote I, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage Ib2‐IIb cervical cancer, EORTC 55994. J Clin Oncol. 2019;37:5503. [DOI] [PubMed] [Google Scholar]
- 80. Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36:1548‐1555. [DOI] [PubMed] [Google Scholar]
- 81. Dastidar GA, Gupta P, Basu B, et al. Is neo‐adjuvant chemotherapy a better option for management of cervical cancer patients of rural India? Indian J Cancer. 2016;53:56‐59. [DOI] [PubMed] [Google Scholar]
- 82. Morley GW, Hopkins MP, Lindenauer SM, Roberts JA. Pelvic exenteration, University of Michigan: 100 patients at 5 years. Obstet Gynecol. 1989;74:934‐943. [PubMed] [Google Scholar]
- 83. Estape R, Angioli R. Surgical management of advanced and recurrent cervical cancer. Semin Surg Oncol. 1999;16:236‐241. [DOI] [PubMed] [Google Scholar]
- 84. Benn T, Brooks RA, Zhang Q, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011;122:14‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dutta S, Nguyen NP, Vock J, et al. Image‐guided radiotherapy and brachytherapy for cervical cancer. Front Oncol. 2015;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Harkenrider MM, Alite F, Silva SR, Small W Jr. Image‐based brachytherapy for the treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2015;92:921‐934. [DOI] [PubMed] [Google Scholar]
- 87. Grigsby PW, Perez CA. Radiotherapy alone for medically inoperable carcinoma of the cervix: Stage IA and carcinoma in situ. Int J Radiat Oncol Biol Phys. 1991;21:375‐378. [DOI] [PubMed] [Google Scholar]
- 88. Landoni F, Colombo A, Milani R, et al. Randomized study between radical surgery and radiotherapy for the treatment of stage IB‐IIA cervical cancer: 20‐year update. J Gynecol Oncol. 2017;28:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow‐up of a gynecologic oncology study. Int J Radiat Oncol Biol Phys. 2006;65:169‐176. [DOI] [PubMed] [Google Scholar]
- 90. Póka R, Molnár S, Daragó P, et al. Intention‐to‐treat analysis of radical trachelectomy for early‐stage cervical cancer with special reference to oncologic failures. Int J Gynecol Cancer. 2017;27:1438‐1445. [DOI] [PubMed] [Google Scholar]
- 91. Bentivegna E, Maulard A, Pautier P, et al. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil Steril. 2016;106:1195‐1211. [DOI] [PubMed] [Google Scholar]
- 92. Hauerberg L, Høgdall C, Loft A, et al. Vaginal radical trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol Oncol. 2015;138:304‐310. [DOI] [PubMed] [Google Scholar]
- 93. Park J‐Y, Joo WD, Chang S‐J, et al. Long‐term outcomes after fertility‐sparing laparoscopic radical trachelectomy in young women with early‐stage cervical cancer: an Asan Gynecologic Cancer Group (AGCG) study. J Surg Oncol. 2014;110:252‐257. [DOI] [PubMed] [Google Scholar]
- 94. Plante M, Renaud MC, Sebastianelli A, Gregoire J. Simple vaginal trachelectomy: a valuable fertility‐preserving option in early‐stage cervical cancer. Int J Gynecol Cancer. 2017;27:1021‐1027. [DOI] [PubMed] [Google Scholar]
- 95. Ramirez PT, Pareja R, Rendón GJ, et al. Management of low‐risk early‐stage cervical cancer: should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecol Oncol. 2014;132:254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schmeler KM, Frumovitz M, Ramirez PT. Conservative management of early stage cervical cancer: is there a role for less radical surgery? Gynecol Oncol. 2011;120:321‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang Q, Li W, Kanis MJ, et al. Oncologic and obstetrical outcomes with fertility‐sparing treatment of cervical cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:46580‐46592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gemer O, Lavie O, Gdalevich M, et al. Evaluation of clinical and pathologic risk factors may reduce the rate of multimodality treatment of early cervical cancer. Am J Clin Oncol. 2016;39:37‐42. [DOI] [PubMed] [Google Scholar]
- 99. Small W, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity‐modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Klopp A, Yeung A, Deshmukh S, et al. A phase III randomized trial comparing patient‐reported toxicity and quality of life (QOL) during pelvic intensity modulated radiation therapy as compared to conventional radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96:S3. [Google Scholar]
- 101. Small W, Beriwal S, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11:58‐67. [DOI] [PubMed] [Google Scholar]
- 102. Yeo RMC, Chia YN, Namuduri RPD, et al. Tailoring adjuvant radiotherapy for stage IB‐IIA node negative cervical carcinoma after radical hysterectomy and pelvic lymph node dissection using the GOG score. Gynecol Oncol. 2011;123:225‐229. [DOI] [PubMed] [Google Scholar]
- 103. Minig L, Patrono MG, Romero N, et al. Different strategies of treatment for uterine cervical carcinoma stage IB2‐IIB. World J Clin Oncol. 2014;5:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage llB‐IVA carcinoma of the cervix with negative para‐aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339‐1348. [DOI] [PubMed] [Google Scholar]
- 105. Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para‐aortic radiation for high‐risk cervical cancer. N Engl J Med. 1999;340:1137‐1143. [DOI] [PubMed] [Google Scholar]
- 106. Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144‐1153. [DOI] [PubMed] [Google Scholar]
- 107. Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154‐1161. [DOI] [PubMed] [Google Scholar]
- 108. Sardi JE, Boixadera MA, Sardi JJ. A critical overview of concurrent chemoradiotherapy in cervical cancer. Curr Oncol Rep. 2004;6:463‐470. [DOI] [PubMed] [Google Scholar]
- 109. Vale C, Tierney JF, Stewart LA, et al. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta‐analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802‐5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kim YS, Shin SS, Nam J‐H, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high‐dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008;108:195‐200. [DOI] [PubMed] [Google Scholar]
- 111. Lanciano R, Calkins A, Bundy BN, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol. 2005;23:8289‐8295. [DOI] [PubMed] [Google Scholar]
- 112. Varia MA, Bundy BN, Deppe G, et al. Cervical carcinoma metastatic to para‐aortic nodes: extended field radiation therapy with concomitant 5‐fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015‐1023. [DOI] [PubMed] [Google Scholar]
- 113. National Cancer Institute . Clinical Trials Home Page. A Phase III Trial of Adjuvant Chemotherapy Following Chemoradiation as Primary Treatment for Locally Advanced Cervical Cancer Compared to Chemoradiation Alone: The OUTBACK Trial. Accessed June 10, 2021. https://www.clinicaltrials.gov/ct2/show/NCT01414608
- 114. National Cancer Institute . Clinical Trials Home Page. Accessed May 9, 2021. https://clinicaltrials.gov/ct2/show/NCT01566240
- 115. Pötter R, Tanderup K, Schmid MP, et al. MRI‐guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE‐I): a multicentre prospective cohort study. Lancet Oncol. 2021;22:538‐547. [DOI] [PubMed] [Google Scholar]
- 116. Sharma DN, Rath GK, Gandhi AK, et al. Low‐dose‐rate versus high‐dose‐rate versus pulsed‐dose‐rate intracavitary brachytherapy in cervical carcinoma: a mono‐institutional comparative study. Int J Radiat Oncol Biol Phys. 2015;93(Suppl):E278. [Google Scholar]
- 117. Sharma DN, Rath GK, Thulkar S, et al. Use of transrectal ultrasound for high dose rate interstitial brachytherapy for patients of carcinoma of uterine cervix. J Gynecol Oncol. 2010;21:12‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Perez CA, Grigsby PW, Castro‐Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:1275‐1288. [DOI] [PubMed] [Google Scholar]
- 119. Lanciano RM, Pajak TF, Martz K, Hanks GE. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns‐of‐care study. Int J Radiat Oncol Biol Phys. 1993;25:391‐397. [DOI] [PubMed] [Google Scholar]
- 120. Kim J‐Y, Kim J‐Y, Kim JH, et al. Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraortic and left supraclavicular lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84:741‐747. [DOI] [PubMed] [Google Scholar]
- 121. Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113‐3119. [DOI] [PubMed] [Google Scholar]
- 122. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin‐containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649‐4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open‐label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sharma DN, Rath GK, Bhatla N, et al. Postoperative radiotherapy following inadvertent simple hysterectomy versus radical hysterectomy for cervical carcinoma. Asian Pac J Cancer Prev. 2011;12:1537‐1541. [PubMed] [Google Scholar]
- 125. Saibishkumar EP, Patel FD, Ghoshal S, et al. Results of salvage radiotherapy after inadequate surgery in invasive cervical carcinoma patients: a retrospective analysis. Int J Radiat Oncol Biol Phys. 2005;63:828‐833. [DOI] [PubMed] [Google Scholar]
- 126. Kinney WK, Egorshin EV, Ballard DJ, Podratz KC. Long‐term survival and sequelae after surgical management of invasive cervical carcinoma diagnosed at the time of simple hysterectomy. Gynecol Oncol. 1992;44:24‐27. [DOI] [PubMed] [Google Scholar]
- 127. Elit L, Fyles AW, Devries MC, et al. Follow‐up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol. 2009;114:528. [DOI] [PubMed] [Google Scholar]
- 128. Eifel PJ, Jhingran A, Brown J, et al. Time course and outcome of central recurrence after radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer. 2006;16:1106‐1111. [DOI] [PubMed] [Google Scholar]
- 129. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197‐204. [DOI] [PubMed] [Google Scholar]
- 130. Van Nagell JR, Rayburn W, Donaldson ES, et al. Therapeutic implications of patterns of recurrence in cancer of the uterine cervix. Cancer. 1979;44:2354‐2361. [DOI] [PubMed] [Google Scholar]
- 131. Eralp Y, Saip P, Sakar B, et al. Prognostic factors and survival in patients with metastatic or recurrent carcinoma of the uterine cervix. Int J Gynecol Cancer. 2003;13:497‐504. [DOI] [PubMed] [Google Scholar]
- 132. Friedlander M, Grogan M, U.S. Preventative Services Task Force . Guidelines for the treatment of recurrent and metastatic cervical cancer. Oncologist. 2002;7:342‐347. [PubMed] [Google Scholar]
- 133. Grigsby PW. Radiotherapy for pelvic recurrence after radical hysterectomy for cervical cancer. Radiat Med. 2005;23:327‐330. [PubMed] [Google Scholar]
- 134. Haasbeek CJA, Uitterhoeve ALJ, van der Velden J, et al. Long‐term results of salvage radiotherapy for the treatment of recurrent cervical carcinoma after prior surgery. Radiother Oncol. 2008;89:197‐204. [DOI] [PubMed] [Google Scholar]
- 135. Piura B, Rabinovich A, Friger M. Recurrent cervical carcinoma after radical hysterectomy and pelvic lymph node dissection: a study of 32 cases. Eur J Gynaecol Oncol. 2008;29:31‐36. [PubMed] [Google Scholar]
- 136. Lee YS, Kim YS, Kim JH, et al. Feasibility and outcome of concurrent chemoradiotherapy for recurrent cervical carcinoma after initial surgery. Tumori J. 2010;96:553‐559. [DOI] [PubMed] [Google Scholar]
- 137. Husain A, Akhurst T, Larson S, et al. A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol Oncol. 2007;106:177‐180. [DOI] [PubMed] [Google Scholar]
- 138. Pallardy A, Bodet‐Milin C, Oudoux A, et al. Clinical and survival impact of FDG PET in patients with suspicion of recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2010;37:1270‐1278. [DOI] [PubMed] [Google Scholar]
- 139. Mittra E, El‐Maghraby T, Rodriguez CA, et al. Efficacy of 18F‐FDG PET/CT in the evaluation of patients with recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2009;36:1952‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ruth‐Sahd LA, Zulkosky KD. Cervical cancer: caring for patients undergoing total pelvic exenteration. Crit Care Nurse. 1999;19:46‐57. [PubMed] [Google Scholar]
- 141. Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol. 2006;7:837‐847. [DOI] [PubMed] [Google Scholar]
- 142. Niibe Y, Kenjo M, Kazumoto T, et al. Multi‐institutional study of radiation therapy for isolated para‐aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5,000. Int J Radiat Oncol Biol Phys. 2006;66(5):1366‐1369. [DOI] [PubMed] [Google Scholar]
- 143. Sharma DN, Gandhi AK, Adhikari N. Definitive radiation therapy of locally advanced cervical cancer initially treated with palliative hypofractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96(suppl):E306. [Google Scholar]
- 144. Biswal BM, Lal P, Rath GK, Mohanti BK. Hemostatic radiotherapy in carcinoma of the uterine cervix. Int J Gynecol Obstet. 1995;50:281‐285. [DOI] [PubMed] [Google Scholar]
- 145. Chow E, Zeng L, Salvo N, et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol). 2012;24:112‐124. [DOI] [PubMed] [Google Scholar]
- 146. Amant F, Brepoels L, Halaska MJ, et al. Gynaecologic cancer complicating pregnancy: an overview. Best Pract Res Clin Obstet Gynaecol. 2010;24:61‐79. [DOI] [PubMed] [Google Scholar]
- 147. Duggan B, Muderspach LI, Roman LD, et al. Cervical cancer in pregnancy: reporting on planned delay in therapy. Obstet Gynecol. 1993;82:598‐602. [PubMed] [Google Scholar]
- 148. Hunter MI, Tewari K, Monk BJ. Cervical neoplasia in pregnancy. Part 2: current treatment of invasive disease. Am J Obstet Gynecol. 2008;199:10‐18. [DOI] [PubMed] [Google Scholar]
- 149. Tewari K, Cappuccini F, Gambino A, et al. Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer. 1998;82:1529‐1534. [DOI] [PubMed] [Google Scholar]
- 150. Boyd A, Cowie V, Gourley C. The use of cisplatin to treat advanced‐stage cervical cancer during pregnancy allows fetal development and prevents cancer progression: report of a case and review of the literature. Int J Gynecol Cancer. 2009;19:273‐276. [DOI] [PubMed] [Google Scholar]


