Abstract
Aim
To compare (in the LIRA‐PRIME [NCT02730377], a randomized open‐label trial), the efficacy of liraglutide in controlling glycaemia versus an oral antidiabetic drug (OAD) in patients with uncontrolled type 2 diabetes (T2D), despite metformin use in a primary care setting (n = 219 sites, n = 9 countries).
Materials and Methods
Adults (n = 1991) with T2D (HbA1c 7.5%‐9.0%) receiving metformin were randomized 1:1 to liraglutide (≤1.8 mg/d) or one OAD, selected by the investigator, added to metformin, for up to 104 weeks. Primary endpoint: time to inadequate glycaemic control (HbA1c > 7.0%) at two scheduled consecutive visits after week 26. Outcomes were assessed for liraglutide versus a pooled OAD group, and (post hoc) liraglutide versus sodium‐glucose co‐transporter‐2 inhibitors, dipeptidyl peptidase‐4 inhibitors, and sulphonylureas individually.
Results
Among randomized patients (liraglutide, n = 996; OAD, n = 995), 47.6% were female, mean age was 57.4 years and mean HbA1c was 8.2%. Median time to inadequate glycaemic control was 44 weeks longer with liraglutide versus OAD (109 weeks [25% percentile, 38; 75% percentile, not available] vs. 65 weeks [25% percentile, 35; 75% percentile, 107], P < .0001). Changes in HbA1c and body weight at week 104 or at premature treatment discontinuation significantly favoured liraglutide over OAD. Hypoglycaemia rates were comparable between groups and few patients discontinued because of adverse events (liraglutide, 7.9% [n = 79]; OAD, 4.1% [n = 41]). Similar results were observed in the post hoc analysis for liraglutide versus individual OAD classes.
Conclusions
Glycaemic control was better maintained with liraglutide versus OAD, supporting liraglutide use when intensifying therapy in primary care patients with T2D.
Keywords: clinical trial, GLP‐1 analogue, liraglutide, primary care, type 2 diabetes
1. INTRODUCTION
Because the majority of patients with type 2 diabetes (T2D) are treated in primary care, 1 , 2 , 3 primary care clinicians have a key role in optimizing diabetes management. Improvement in, and retainment of, glycaemic control are associated with greater adherence to glucose‐lowering therapy, 4 and maintaining adequate glycaemic control reduces the long‐term risk of T2D complications. 5
The American Diabetes Association (ADA) and European Association for the Study of Diabetes guidelines recommend metformin as first‐line pharmacotherapy for patients with T2D. 6 , 7 , 8 Options for treatment intensification following failure of first‐line therapy to improve glycaemic control include a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), sodium‐glucose co‐transporter‐2 inhibitor (SGLT‐2i), dipeptidyl peptidase‐4 inhibitor (DPP‐4i), sulphonylurea (SU), thiazolidinedione, and basal insulin, or a combination of glucose‐lowering therapies. 6 , 7 , 8 The choice should be based on drug‐specific effects and patient characteristics, such as risk of atherosclerotic cardiovascular events or hypoglycaemia, need for body weight reduction, and cost. 6 , 7 , 8 , 9 However, evidence to guide intensification strategies in primary care is sparse, which may contribute to treatment inertia and suboptimal treatment. 10 Accordingly, the LIRA‐PRIME trial aimed to compare the efficacy in controlling glycaemia and safety of liraglutide versus a pooled oral antidiabetic drug (OAD) group, or the most commonly prescribed individual OAD classes in this trial (SGLT‐2i, DPP‐4i, and SU; post hoc analysis) in patients uncontrolled on metformin in a primary care setting to inform optimal antihyperglycaemic strategies.
2. MATERIALS AND METHODS
2.1. Study design
LIRA‐PRIME (NCT02730377; EudraCT: 2015‐002417‐29) was conducted in a primary care setting 11 at 219 sites across nine countries (details can be found in the supporting information: List of LIRA‐PRIME investigators). A pragmatic design was used to reflect diabetes management in primary care, including broad inclusion criteria, very limited exclusion criteria, and a follow‐up interval reflecting reality. Trial products were prescribed according to local labels, dispensed by local pharmacies or similar means and reimbursed by the sponsor. Local Institutional Review Board/Independent Ethics Committee approval was obtained before trial initiation. The trial was completed on 12 August 2019.
2.2. Patients
Patients aged 18 years or older with T2D (HbA1c 7.5%‐9.0% [58.5‐74.9 mmol/mol]) were eligible if receiving a stable dose of metformin (≥1500 mg/d or maximum tolerated dose) as monotherapy for 60 days or longer before screening and met local criteria for use of liraglutide and OAD treatment. 12 Patients who were pregnant or breastfeeding and those who had received any diabetes medication other than metformin 60 days or less before screening were excluded.
Participants were selected from volunteers responding to posters or during interactions with their personal clinicians. 12 All patients provided written informed consent and were not remunerated for participation.
2.3. Randomization and masking
An interactive web response system was used for screening and randomization. Patients were randomized using simple sequential allocation from a blocked randomization schedule without stratifying factors. After randomization, the trial was open‐label to patients and treating physicians, while medical reviewers and biostatisticians were blinded until the end of the trial.
2.4. Procedures
After a screening period of 2 weeks or less, patients were randomized (1:1) to subcutaneous liraglutide or an OAD (α‐glucosidase inhibitor, DPP‐4i, meglitinide, SGLT‐2i, SU, or thiazolidinedione), both added to metformin, for up to 104 weeks. 12 The allocated OAD was chosen by the investigator and was therefore not part of the randomization process. Doses of liraglutide were escalated from 0.6 to 1.2 mg/d up to a final dose of 1.8 mg/d. Where relevant, the OAD dose was escalated to the maximum approved/tolerated dose. For both liraglutide and OADs, a maintenance dose below the maximum approved/tolerated dose was acceptable if the patient's HbA1c was less than 7.0%. Metformin was maintained at the pretrial dose and frequency for the entire treatment period unless there was a safety concern.
Follow‐up visits were scheduled at weeks 2, 4, 16, and 26 after randomization and quarterly thereafter, to reflect the normal frequency of appointments in routine practice. 12 There was a 1‐week follow‐up period after the end of treatment (the visit schedule and assessments at each visit have been published previously). 12 Race and ethnicity were recorded based on recommendations outlined by the US Food and Drug Administration 13 to allow comparison across trials.
2.5. Outcomes
The primary endpoint was time to inadequate glycaemic control, defined as HbA1c more than 7.0% (>53.0 mmol/mol) at two scheduled consecutive visits after week 26. Accordingly, the first possible occurrence was at week 38. Patients meeting the primary endpoint before week 104 were withdrawn from the trial. A 104‐week trial duration was deemed appropriate for assessing time to inadequate glycaemic control.
Secondary endpoints included: time to premature treatment discontinuation for any reason (including inadequate glycaemic control); changes from baseline in HbA1c, fasting plasma glucose (FPG), body weight, body mass index (BMI), and blood pressure at week 104, or at premature treatment discontinuation (corresponding to the last measurement on treatment); proportion of patients who at week 104 or at premature treatment discontinuation had achieved HbA1c of 6.5% or less (≤47.5 mmol/mol) or clinically relevant composite endpoints (HbA1c ≤ 7.0% [≤53.0 mmol/mol] without weight gain, HbA1c ≤ 7.0% [≤53.0 mmol/mol] without severe or blood glucose [BG]‐confirmed symptomatic hypoglycaemia, or HbA1c ≤ 7.0% [≤53.0 mmol/mol] without severe or BG‐confirmed symptomatic hypoglycaemia or weight gain); documented symptomatic and severe hypoglycaemic episodes (both defined according to ADA criteria) 14 ; severe or BG‐confirmed symptomatic hypoglycaemic episodes (defined as severe according to ADA criteria 14 or confirmed by a plasma glucose value <3.1 mmol/L with symptoms consistent with hypoglycaemia); adverse events (AEs) leading to permanent discontinuation of the trial product; serious AEs (defined in the supporting information, Methods section); and changes from baseline in blood lipids, biochemistry, haemoglobin, and pulse.
The first and final doses of liraglutide and OADs were recorded and compared using defined daily dose (DDD, listed in Table S1). Values of DDD were taken from the World Health Organization website at the time the trial was carried out. 15
Because all the trial drugs were approved for the treatment of T2D, a selective approach to safety data collection was used. Events that occurred between the first trial‐related activity and the end of the trial that met the following definitions were collected: AEs leading to permanent discontinuation of the trial product; serious AEs; medication errors concerning trial products; and pregnancies. Investigators assessed AEs according to severity, relationship to trial product, and outcome.
2.6. Statistical methods
The sample size was calculated to detect a between‐group difference in time to inadequate glycaemic control with 90% power (5% significance level). Based on assumptions listed in the supporting information, Methods section, the necessary sample size was calculated to be 1994 patients.
Time to inadequate glycaemic control with liraglutide versus OAD was analysed using a two‐sided, non‐parametric, generalized log rank test for interval‐censored failure time data. The analysis was not based on any model assumptions or adjusted for any covariates. Possible event times were considered as a continuous variable. Similar methods were used to analyse time to premature treatment discontinuation.
A post hoc sensitivity analysis of time to inadequate glycaemic control used a proportional hazards regression model with piecewise constant baseline hazard accounting for the interval censored failure times, treatment group as a factor, and baseline HbA1c as a covariate.
Changes from baseline in HbA1c, FPG, body weight, BMI, blood pressure, amylase, lipase, and pulse were evaluated using an analysis of covariance model with treatment group and country as fixed factors, and the baseline value of the variable of interest as a covariate. For amylase and lipase, response to treatment values and the baseline values were log‐transformed in the analysis. Estimated differences between liraglutide and OAD were calculated, together with 95% confidence intervals (CIs) and two‐sided P values. Supportive analyses of the change from baseline in HbA1c were performed using a mixed model for repeated measurements.
Achievement of HbA1c of 6.5% or less (≤47.5 mmol/mol) and composite endpoints were analysed using a binary logistic regression model with treatment group as a fixed factor and baseline HbA1c as a covariate. The results were described using the odds ratios (ORs) for liraglutide versus OAD, with 95% CIs and two‐sided P values.
Hypoglycaemic episodes, AEs, serious AEs, blood lipids, biochemistry, and haemoglobin were summarized descriptively. Prespecified endpoints involving hypoglycaemic episodes were analysed using a negative binomial regression model. The results were described using the rate ratios for liraglutide versus OAD, with 95% CIs and two‐sided P values.
To investigate liraglutide versus individual OADs, a post hoc analysis was performed assessing the safety and efficacy of liraglutide versus the most commonly prescribed classes of OAD in the trial: SGLT‐2i, DPP‐4i, and SUs. This post hoc analysis was carried out to investigate liraglutide versus individual OADs, after the data showed that baseline characteristics were balanced for most variables across individual OAD subgroups. Outcomes were evaluated using similar statistical models as those for the primary and secondary analyses.
The full analysis set included all randomized patients and was used for efficacy endpoints, following the intention‐to‐treat principle and including patients according to their randomized group. The safety analysis set included all patients exposed to at least one dose of the trial product.
The main statistical analyses were based on assessments taken while the patient was on treatment, extending to 1 day after last administration of trial product, or 7 days after last administration of trial product for treatment‐emergent AEs. Statistical analyses were performed with SAS version 9.4.
3. RESULTS
3.1. Patient disposition and exposure to treatment
From March 2016 to August 2017, 1997 patients were enrolled into the study. Six patients (n = 3 in each arm) were excluded from analyses because of incomplete casebooks. Of the remaining 1991 patients, 996 and 995 were randomized to liraglutide or OAD, respectively (Figure S1). OAD classes selected by investigators were SGLT‐2is (47.9%; n = 471), DPP‐4is (39.7%; n = 391), SUs (10.8%; n = 106), thiazolidinediones (1.1%; n = 11), and α‐glucosidase inhibitors (0.5%; n = 5) (Table S2). No patients received a meglitinide as trial treatment.
Median time of exposure to trial product (min; max) was 1.7 (0.0; 2.4) patient‐years in the liraglutide group and 1.1 (0.0; 2.1) patient‐years in the OAD group (SGLT‐2i, 1.1 [0.0; 2.1]; DPP‐4i, 1.3 [0.0; 2.1]; SUs, 1.0 [0.0; 2.1]; thiazolidinediones, 1.4 [0.0; 2.0]; α‐glucosidase inhibitors 0.9 [0.8; 1.3] patient‐years). The starting dose of liraglutide was less than or equal to the DDD for most patients (978/980). The liraglutide dose was higher than the DDD (1.2 mg) at the time of premature discontinuation because of inadequate glycaemic control (rather than discontinuation for any other reason) in 91.1% of patients (331/363). In the OAD group, 55.3% of patients (545/984) initiated OAD treatment at a higher dose than the respective DDD, and the last dose was higher than DDD at the time of premature discontinuation because of inadequate glycaemic control in 68.9% of patients (325/472) (Figure S2A).
3.2. Baseline characteristics
Mean (standard deviation, SD) age was 57.4 (10.8) years, duration of diabetes was 7.2 (5.9) years, and 47.6% of patients were female. Mean (SD) baseline HbA1c was 8.2% (1.0%) (66.0 mmol/mol) and BMI was 33.5 (7.4) kg/m2. Demographics and baseline characteristics were well balanced between the liraglutide treatment group and the overall OAD treatment group (Table S3).
3.3. Time to inadequate glycaemic control and premature treatment discontinuation
Median time to inadequate glycaemic control was 44 weeks longer for liraglutide versus OAD (109 vs. 65 weeks, P < .0001) (Figure 1 and Table 1). In the sensitivity analysis, liraglutide significantly reduced the risk of inadequate glycaemic control assessed as a categorical outcome versus OAD (hazard ratio [95% CI]: 0.58 [0.51; 0.66]). Moreover, median time to premature treatment discontinuation (for any reason) was longer in the liraglutide‐treated patients versus OAD‐treated (80 vs. 52 weeks, respectively; P < .0001) (Table 1).
FIGURE 1.
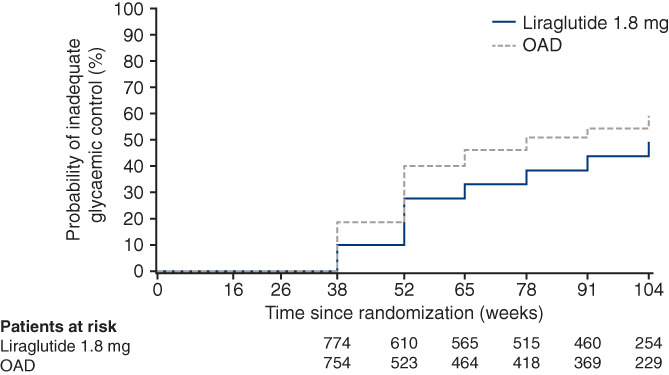
Kaplan–Meier plot of time to inadequate glycaemic control with liraglutide versus OAD*. *OADs included investigator‐selected drugs from the classes: α‐glucosidase inhibitor, dipeptidyl peptidase‐4 inhibitor, sodium‐glucose co‐transporter‐2 inhibitor, sulphonylurea, or thiazolidinedione; both liraglutide and OADs were prescribed in combination with metformin. Full analysis set. The primary endpoint of time to inadequate glycaemic control was defined as HbA1c > 7.0% (>53 mmol/mol) at two consecutive scheduled visits after the first 26 weeks of treatment and up to 104 weeks. The first possible occurrence was at the week 38 visit. Test for no treatment difference was based on using a generalized log rank test for interval censored failure time data. OAD, oral antidiabetic drug
TABLE 1.
Time to inadequate glycaemic control and time to premature treatment discontinuation with liraglutide versus OAD a
| Endpoint | Liraglutide (N = 996) | OAD a (N = 995) |
|---|---|---|
| Time to inadequate glycaemic control | ||
| Patients with event, N (%) | 416 (41.8) | 547 (55.0) |
| Median (25th; 75th percentile), weeks | 108.9 (37.7; n/a) | 64.9 (35.4; 107.4) |
| Test for no treatment difference | P < .0001 | |
| Time to premature treatment discontinuation | ||
| Patients with event, N (%) | 532 (53.4) | 624 (62.7) |
| Median (25th; 75th percentile), weeks | 80.4 (35.7; n/a) | 52.3 (35.1; n/a) |
| Test for no treatment difference | P < .0001 | |
Abbreviations: N, number of patients; OAD, oral antidiabetic drug.
OADs included investigator‐selected drugs from the classes: α‐glucosidase inhibitor, dipeptidyl peptidase‐4 inhibitor, sodium‐glucose co‐transporter‐2 inhibitor, sulphonylurea, or thiazolidinedione; both liraglutide and OADs were prescribed in combination with metformin. Full analysis set. The primary endpoint of time to inadequate glycaemic control was defined as HbA1c > 7.0% (>53.0 mmol/mol) at two consecutive scheduled visits after the first 26 weeks of treatment and up to 104 weeks. The first possible occurrence was at week 38. Possible event times were considered as a continuous variable. 25%, median (50%) and 75% percentiles for the cumulative distribution function were obtained from the Kaplan–Meier survival function. Some 75% percentiles were not estimated as the trial ended after the 104‐week treatment period and 1‐week follow‐up period.
3.4. Changes from baseline in glycaemia, body weight, and blood pressure
Changes in HbA1c, FPG, body weight, and BMI at week 104 or at premature treatment discontinuation all significantly favoured liraglutide over OAD (Table 2 and Figures S3 and S4, and Table S4). There were no significant between‐group differences in blood pressure changes.
TABLE 2.
Changes from baseline in clinical variables at week 104 or at premature treatment discontinuation
| Liraglutide – OAD a treatment difference | 95% CI | P value b | |
|---|---|---|---|
| HbA1c (%) | −0.33 | −0.43; −0.23 | <.0001 |
| HbA1c (mmol/mol) | −3.62 | −4.73; −2.52 | <.0001 |
| FPG (mmol/L) | −0.69 | −0.91; −0.46 | <.0001 |
| Body weight (kg) | −0.61 | −1.07; −0.16 | .009 |
| BMI (kg/m2) | −0.22 | −0.38; −0.06 | .007 |
| Systolic blood pressure (mmHg) | 0.24 | −0.89; 1.36 | .68 |
| Diastolic blood pressure (mmHg) | 0.21 | −0.50; 0.91 | .56 |
Abbreviations: BMI, body mass index; CI, confidence interval; FPG, fasting plasma glucose; OAD, oral antidiabetic drug.
OADs included investigator‐selected drugs from the classes: α‐glucosidase inhibitor, dipeptidyl peptidase‐4 inhibitor, sodium‐glucose co‐transporter‐2 inhibitor, sulphonylurea, or thiazolidinedione; both liraglutide and OADs were prescribed in combination with metformin. Full analysis set.
Two‐sided P value for test of no treatment difference.
3.5. Achievement of HbA1c of 6.5% or less (≤47.5 mmol/mol) and composite endpoints
Liraglutide increased the likelihood of achieving HbA1c of 6.5% or less (≤47.5 mmol/mol) compared with OAD (31.6% vs. 16.8%; OR [95% CI]: 1.88 [1.50; 2.37]; P < .0001). Significantly higher proportions of patients achieved HbA1c of 7.0% or less (≤53.0 mmol/mol) without weight gain (47.4% vs. 28.0%; OR [95% CI]: 1.69 [1.38; 2.07]), HbA1c of 7.0% or less (≤53.0 mmol/mol) without severe or BG‐confirmed symptomatic hypoglycaemia (61.2% vs. 37.5%; OR [95% CI]: 1.63 [1.34; 1.99]), and HbA1c of 7.0% or less (≤53.0 mmol/mol) without severe or BG‐confirmed symptomatic hypoglycaemia or weight gain (45.5% vs. 27.0%; OR [95% CI]: 1.68 [1.37; 2.07]) with liraglutide versus OAD (all P < .0001).
3.6. Safety
Rates of hypoglycaemia were comparable between the liraglutide and OAD groups (≤250 events per 1000 patient‐years of exposure [PYE] in both groups) (Tables S5 and S6). The rate of severe or BG‐confirmed symptomatic hypoglycaemia was 17.7 per 1000 PYE in the liraglutide group and 35.0 per 1000 PYE in the OAD group (P = .11). A severe hypoglycaemic episode was experienced by one patient receiving liraglutide and six patients receiving an OAD (P = .08).
The rate of AEs leading to permanent discontinuation was higher with liraglutide versus OAD (138.7 vs. 77.9 events per 1000 PYE), driven by gastrointestinal AEs (74.5 vs. 11.1 events per 1000 PYE) (Table S7). The rate of serious AEs was similar with liraglutide and OAD (Table 3) and all fatal events were judged as unlikely to be related to trial products.
TABLE 3.
Treatment‐emergent serious adverse events (by SOC and occurring in ≥1% of patients in any group)
| Liraglutide | OAD a | |||||||
|---|---|---|---|---|---|---|---|---|
| N | % | E | R | N | % | E | R | |
| Total | 92 | 9.4 | 145 | 107.0 | 81 | 8.2 | 140 | 111.2 |
| Cardiac disorders | 20 | 2.0 | 24 | 17.7 | 17 | 1.7 | 23 | 18.3 |
| Infections and infestations | 14 | 1.4 | 16 | 11.8 | 15 | 1.5 | 20 | 15.9 |
| Gastrointestinal disorders | 9 | 0.9 | 10 | 7.4 | 10 | 1.0 | 14 | 11.1 |
| Neoplasms (benign, malignant, and unspecified [including cysts and polyps]) | 9 | 0.9 | 10 | 7.4 | 10 | 1.0 | 10 | 7.9 |
| Nervous system disorders | 10 | 1.0 | 12 | 8.9 | 8 | 0.8 | 10 | 7.9 |
| Renal and urinary disorders | 11 | 1.1 | 13 | 9.6 | 7 | 0.7 | 9 | 7.2 |
Abbreviations: E, number of events; N, number of patients with ≥1 event; OAD, oral antidiabetic drug; R, rate (number of events divided by patient‐years of exposure multiplied by 1000); SOC, system organ class; %, percentage of patients with ≥1 event.
OADs included investigator‐selected drugs from the classes: α‐glucosidase inhibitor, dipeptidyl peptidase‐4 inhibitor, sodium‐glucose co‐transporter‐2 inhibitor, sulphonylurea, or thiazolidinedione; both liraglutide and OADs were prescribed in combination with metformin. Safety analysis set. Treatment‐emergent adverse event: defined as an event with an onset date (or increase in severity) on or after the first day of trial product administration and no later than 7 days after the last trial product administration.
There were no clinically relevant changes in fasting blood lipids (Figure S5) or biochemistry (Table S8), and liraglutide treatment resulted in mean increases in amylase and lipase (Table S9).
3.7. Liraglutide versus individual OAD classes (post hoc)
The post hoc analysis comparing liraglutide with SGLT‐2i, DPP‐4i, and SU comprised 1964 patients overall (liraglutide, 996; SGLT‐2i, 471; DPP‐4i, 391; SU, 106). Demographics and baseline characteristics were generally well balanced between treatment subgroups (Table S10), except for a slightly higher body weight at baseline in the SGLT‐2i subgroup (98.8 kg compared with 94.2, 91.2, and 93.8 kg for DPP‐4i, SU, and liraglutide, respectively). The starting dose for SU was lower than or equal to the DDD for 79.2% of patients (84/106), probably because of the inherent hypoglycaemia risk. Over half of the patients in each OAD class were on a higher dose than the DDD at the time of premature discontinuation because of inadequate glycaemic control: 65% for SGLT‐2is (147/226), 75% for DPP‐4is (134/178), and 64.4% for SUs (38/59) (Figure S2B).
Median time to inadequate glycaemic control with liraglutide (109 weeks) was 44 weeks longer than with SGLT‐2i (65 weeks), 31 weeks longer than with DPP‐4i (78 weeks), and 56 weeks longer than with SU (53 weeks) (Table S11; Figure 2). Similarly, median time to premature treatment discontinuation was longer with liraglutide (80 weeks) than with SGLT‐2i (52 weeks), DPP‐4i (63 weeks), or SU (38 weeks) (Table S11). Liraglutide was associated with significantly greater reductions in HbA1c than SGLT‐2i (−0.14% [−0.26%; −0.01%]), DPP‐4i (−0.49% [−0.62%; −0.36%]), and SU (−0.54% [−0.76%; −0.32%]) at week 104, or at premature treatment discontinuation (Table S12). Furthermore, significantly greater reductions in body weight at week 104 or at premature treatment discontinuation were observed with liraglutide than with DPP‐4i (−1.69 [−2.29; −1.10] kg) and SU (−3.24 [−4.25; −2.23] kg), but not with SGLT‐2i (0.97 [0.42; 1.53] kg) (Table S12). Rates of AEs were generally in line with those reported in the primary analysis (Tables S13 and S14), although the rate of severe or BG‐confirmed hypoglycaemic episodes varied considerably between OAD classes (11.7, 15.6, and 289.8 events per 1000 PYE in the SGLT‐2i, DPP‐4i, and SU groups, respectively) relative to the liraglutide group (23.6 events per 1000 PYE).
FIGURE 2.
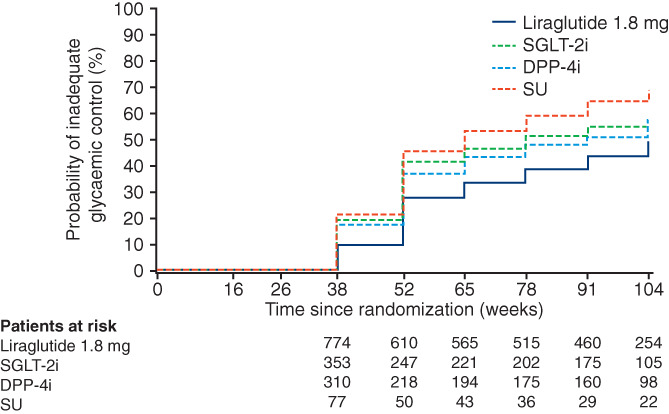
Kaplan–Meier plot of time to inadequate glycaemic control with liraglutide versus post hoc OAD subgroups*. *OADs included investigator‐selected drugs from the classes: α‐glucosidase inhibitor, DPP‐4i, SGLT‐2i, SU, or thiazolidinedione; both liraglutide and OADs were prescribed in combination with metformin. Full analysis set. The primary endpoint of time to inadequate glycaemic control was defined as HbA1c > 7.0% (>53 mmol/mol) at two consecutive scheduled visits after the first 26 weeks of treatment and up to 104 weeks. The first possible occurrence was at the week 38 visit. Test for no treatment difference was based on using a generalized log rank test for interval censored failure time data. DPP‐4i, dipeptidyl peptidase‐4 inhibitor; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulphonylurea
4. DISCUSSION
In LIRA‐PRIME, liraglutide was associated with a greater improvement in glycaemic control versus a pooled OAD group, as add‐on therapy to metformin in primary care. When patients are uncontrolled on metformin, it is important to consider which treatment will enable patients to maintain glycaemic control. Liraglutide is one of the most commonly used GLP‐1 RAs, 16 and it is therefore relevant to compare a pooled OAD group with liraglutide.
In LIRA‐PRIME, addition of either liraglutide or an OAD to metformin was associated with clinically relevant HbA1c reductions over up to 104 weeks. Trial participants were evaluated on a quarterly basis, in line with common schedules within primary care. Hence, T2D treatment intensification with liraglutide or an OAD was shown to be feasible and effective in primary care. As well as longer lasting glycaemic control, patients receiving liraglutide achieved greater HbA1c and body weight reductions versus OADs, with similar rates of hypoglycaemia. At week 104 or at premature treatment discontinuation, almost half of the patients receiving liraglutide achieved HbA1c of 7.0% or less (≤53.0 mmol/mol) without severe or BG‐confirmed symptomatic hypoglycaemia or weight gain. Importantly, no unexpected safety or tolerability issues were identified in this trial conducted in a primary care setting with a very broad patient population. This information may help guide decisions around intensifying therapy when metformin is insufficient in patients with T2D, particularly within the primary care setting. Based on results from this study, liraglutide may be favourable over a pooled OAD group for this indication.
Similar results were seen in the post hoc analysis comparing liraglutide with the most commonly prescribed OAD classes in this study (SGLT‐2i, DPP‐4i, and SUs). The exception was a larger body weight reduction with SGLT‐2i versus liraglutide. This is in contrast to results from other clinical trials, which suggest that the two drug classes do not differ significantly in terms of effect on weight. 17 , 18
Our findings are generally consistent with results from the phase 3 clinical development programme of liraglutide for T2D treatment 19 , 20 , 21 , 22 , 23 , 24 , 25 and from the LEADER cardiovascular outcomes trial. 26 , 27 Lower rates of severe and BG‐confirmed hypoglycaemia were observed with liraglutide versus placebo in LEADER. 26 , 27 To our knowledge, no trials similar to LIRA‐PRIME have been conducted for other GLP‐1RAs in primary care. Further pragmatic studies are needed to increase understanding of the relative effects and safety of different glucose‐lowering medications in this setting. Taken together with results from LIRA‐PRIME, such studies could help to address the evidence gap around intensification of T2D therapy in primary care globally, inform treatment decisions, and reduce treatment inertia.
The LIRA PRIME trial was conducted in a primary care setting at a global level. Strengths of the LIRA PRIME trial include the pragmatic design and large‐scale, multinational nature. The primary purpose of pragmatic trials is to inform decision‐makers regarding the comparative balance of benefits and risks of an intervention, in this case, within primary care. As patients were selected using broad inclusion criteria and according to indications included in the local labels, LIRA‐PRIME should provide evidence that is more reflective of a real‐world setting compared with data from randomized controlled trials conducted in specialist settings, with higher external validity. Only a small proportion of patients discontinued the study because of AEs, which should maximize the robustness of the data.
4.1. Limitations
We acknowledge the open‐label nature of the trial, investigator selection of OADs, and external funding of treatment as potential sources of bias. Because of the trial duration and the prescription of trial drugs by investigators (who could select from six different OAD classes), a double‐dummy design was deemed unfeasible. The non‐random, investigator‐led selection of OADs at baseline may have imbalanced treatment subgroup characteristics to a small extent; however, with the exception of body weight, baseline characteristics were shown to be generally well balanced. While investigators reminded patients to follow trial procedures throughout the trial, raising the importance of taking the trial drug as prescribed if necessary, treatment compliance was not enforced or considered a protocol deviation, to reflect real practice. The investigator selection of OADs also reflects clinical practice and could make the results more representative of a real‐world setting. This study focused on the comparison of efficacy and safety between liraglutide and a pooled OAD group; however, it should be acknowledged that relative cost will also be a factor in the decision to prescribe one drug over another. Moreover, although this trial was conducted in a multinational population, we cannot exclude the possibility that the efficacy of liraglutide versus OAD may vary between patients of different races or ethnicities. Finally, as the trial recruited patients aged 18 years or older who were already receiving metformin, the observed benefits and risks may not apply to patients who are younger than 18 years or treatment‐naïve.
Within global primary care settings, glycaemic control was better maintained with liraglutide than a pooled OAD group (both added to metformin).
CONFLICT OF INTEREST
JU is an advisory board member, consultant, and speakers bureau member for, and has received research support from, Novo Nordisk A/S. DCA, JKP, and KL have received research support from Novo Nordisk A/S. MZ has received research support from Novo Nordisk A/S and has presented lectures for Novo Nordisk A/S, AstraZeneca, MSD, and Eli Lilly Ltd. MK and BW are Novo Nordisk A/S employees and shareholders. CR is an employee of Novo Nordisk Service Centre India Private Ltd. ES, GY, MS, and MT have nothing to declare.
AUTHOR CONTRIBUTIONS
DCA, ES, GY, JKP, JU, KL, MS, MT, and MZ are all members of the LIRA‐PRIME Global Panel and contributed significantly to the design and conduct of the study, and acquisition of clinical data. CR performed the statistical analyses. All the authors reviewed and interpreted the data, and were involved in drafting and critically revising the manuscript. All authors approved the final version of the manuscript and take full responsibility for the content. JU is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14566.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGEMENTS
Parts of these data were presented at the ADA 2020 virtual congress, 12‐16 June 2020. The trial design and baseline characteristics were published in 2018 in Diabetes (Unger et al. Diabetes 2018:1082‐P). We thank the LIRA‐PRIME participants and all involved in the conduct of the trial. We also thank Devayani Kolhe, MSc, who as an International Project Statistician employed by Novo Nordisk was involved in data cleaning and data analysis of LIRA PRIME until 17 January 2020. Finally, we thank Sasha Walton, MSc, Alice Singleton, MSc, and Izabel James, MBBS, of Ashfield MedComms (an Ashfield Company, part of UDG Healthcare) for medical writing and editing assistance, funded by Novo Nordisk A/S. The sponsor of the study had a role in study design, medical oversight during trial conduct, data cleaning and analysis, and data interpretation. Three of the authors of this report are employees of the sponsor and, as such, were involved in the preparation, review, and approval of the manuscript.
Unger J, Allison DC, Kaltoft M, et al. Maintenance of glycaemic control with liraglutide versus oral antidiabetic drugs as add‐on therapies in patients with type 2 diabetes uncontrolled with metformin alone: A randomized clinical trial in primary care (LIRA‐PRIME). Diabetes Obes Metab. 2022;24(2):204‐211. doi: 10.1111/dom.14566
Funding information Novo Nordisk A/S
DATA AVAILABILITY STATEMENT
The patient‐level analysis datasets for the research presented in this publication are available from the corresponding author upon reasonable request. These data will be available to share from the time of publication, following approval of a proposal outlining the use of these assets.
REFERENCES
- 1. Unger J. Incretins: clinical perspectives, relevance, and applications for the primary care physician in the treatment of patients with type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 suppl):S38‐S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davidson JA. The increasing role of primary care physicians in caring for patients with type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 suppl):S3‐S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. 2017;132:169‐170. [DOI] [PubMed] [Google Scholar]
- 4. Nichols GA, Rosales AG, Kimes TM, Tunceli K, Kurtyka K, Mavros P. The change in HbA1c associated with initial adherence and subsequent change in adherence among diabetes patients newly initiating metformin therapy. J Diabetes Res. 2016;2016:9687815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heller SR, The ADVANCE Collaborative Group . A summary of the ADVANCE trial. Diabetes Care. 2009;32(suppl 2):S357‐S361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(suppl 1):S111‐S124. [DOI] [PubMed] [Google Scholar]
- 7. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khunti K, Charbonnel B, Cooper A, et al. Associations between second‐line glucose‐lowering combination therapies with metformin and HbA1c, body weight, quality of life, hypoglycaemic events and glucose‐lowering treatment intensification: the DISCOVER study. Diabetes Obes Metab. 2021;23(8):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 10. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Academy of Family Physicians Primary Care 2016. https://www.aafp.org/about/policies/all/primary-care.html#1. Accessed October 14, 2021.
- 12. Unger J, Allison DC, Carlton M, et al. Trial design and baseline data for LIRA‐PRIME: a randomized trial investigating the efficacy of liraglutide in controlling glycaemia in type 2 diabetes in a primary care setting. Diabetes Obes Metab. 2019;21(7):1543‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Food and Drug Administration Collection of Race and Ethnicity Data in Clinical Trials Guidance for Industry and Food and Drug Administration Staff, Silver Spring, MD: US Food and Drug Administration; 2016.
- 14. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization ATC/DDD Index 2021. 2020. https://www.whocc.no/atc_ddd_index/. Accessed October 14, 2021.
- 16. Divino V, Boye KS, Lebrec J, DeKoven M, Norrbacka K. GLP‐1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10(3):1067‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP‐1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev. 2019;20(6):816‐828. [DOI] [PubMed] [Google Scholar]
- 18. McKee A, Al‐Khazaali A, Albert SG. Glucagon‐like peptide‐1 receptor agonists versus sodium‐glucose cotransporter inhibitors for treatment of T2DM. J Endocr Soc. 2020;4(5):bvaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD‐1 SU). Diabet Med. 2009;26(3):268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nauck M, Frid A, Hermansen K, et al. Long‐term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2‐year results from the LEAD‐2 study. Diabetes Obes Metab. 2013;15(3):204‐212. [DOI] [PubMed] [Google Scholar]
- 21. Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once‐daily human glucagon‐like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care. 2009;32(7):1224‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374(9683):39‐47. [DOI] [PubMed] [Google Scholar]
- 25. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet. 2010;375(9724):1447‐1456. [DOI] [PubMed] [Google Scholar]
- 26. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zinman B, Nauck MA, Bosch‐Traberg H, et al. Liraglutide and glycaemic outcomes in the LEADER trial. Diabetes Ther. 2018;9(6):2383‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
The patient‐level analysis datasets for the research presented in this publication are available from the corresponding author upon reasonable request. These data will be available to share from the time of publication, following approval of a proposal outlining the use of these assets.


