Abstract
The investigation of the physical traces of memories (engrams) has made significant progress in the last decade due to optogenetics and fluorescent cell tagging applied in rodents. Engram cells were identified. The ablation of engram cells led to the loss of the associated memory, silent memories were reactivated, and artificial memories were implanted in the brain. Human engram research lags behind engram research in rodents due to methodological and ethical constraints. However, advances in multivariate analysis techniques of functional magnetic resonance imaging (fMRI) data and machine learning algorithms allowed the identification of stable engram patterns in humans. In addition, MRI scanners with an ultrahigh field strength of 7 Tesla (T) have left their prototype state and became more common around the world to assist human engram research. Although most engram research in humans is still being performed with a field strength of 3T, fMRI at 7T will push engram research. Here, we summarize the current state and findings of human engram research and discuss the advantages and disadvantages of applying 7 versus 3T fMRI to image human memory traces.
Keywords: 7T, engram, hippocampus, humans, magnetic resonance imaging, memory
1. INTRODUCTION
There have been tremendous advances in engram research in the last decade. Groundbreaking results were achieved in rodents using optogenetics. With sophisticated cell tagging techniques, researchers were able to identify cells that are part of an engram. Researchers deleted memories by deactivating engram cells and created artificial memories by recruiting cells to an engram (Josselyn & Tonegawa, 2020). Chased by such extraordinary results in rodents, where does human engram research stand and what methods are available in humans to keep up with engram research in rodents? Important developments for human engram research were made when advancing multivariate analyses techniques and machine learning techniques because these techniques allow tracking engrams on a larger scale than single cells. Another important development for human engram research occurred in the field of magnetic resonance imaging (MRI) that pushed field strength from 3 to 7T for investigations in humans. Since the mid‐2010s, 7T MRI scanners are no longer prototypes but have become commercially available and distributed around the globe (see Figure 1). Although this availability refers mostly to clinical and diagnostic use, the doors are also open for basic memory research. Memory research can profit considerably from 7T MRI, particularly when analyzed using multivariate analysis methods.
FIGURE 1.
UHF MRI scanners around the world. Data from layerfMRI blog (Huber, 2021)
The analysis of functional magnetic resonance imaging (fMRI) data can be divided into mass univariate and multivariate approaches. Multivariate approaches have become more popular in the last two decades and were first used by Haxby et al. (2001). Multivariate approaches can offer a different kind of insight into voxel activation patterns by taking the whole pattern into consideration, while mass univariate approaches provide an average activation pattern associated with an experimental condition. If two activations differ in their patterns, but not in their overall activation level within a region, then only multivariate analyses can pick up on the subtle differences between their patterns. This is of interest for engram research because every engram is instantiated by a distinct neural assembly. For instance, two episodic memories might evoke a distinct pattern of activated voxels within the hippocampus, but they may not differ regarding their local hippocampal activation level. It is possible to decode individual episodic memories based on their distinct patterns of activated voxels (Rissman et al., 2010). Tracking the reactivation (replay) of voxel patterns underlying individual memories during a resting period, which followed learning, predicted the subsequent retrieval performance (Staresina et al., 2013).
An important caveat to this analysis approach is that a distinct voxel activation pattern may not translate directly to specific representation. For example, when the decoding of a spatial position from hippocampal voxel patterns was intended, critics argued that fMRI does not provide the spatial resolution necessary to image place and grid cells. Accordingly, distinct voxel patterns might not originate from activated place or grid cells but from neurons that decode visual landmarks within the virtual environment that participants were navigating through (Nolan et al., 2018). Indeed, after the removal of the landmark‐triggered activation, the decoding of spatial position from hippocampal voxel patterns was no longer possible.
The discussion is ongoing whether large‐scale activity patterns within and between brain areas, as measured with fMRI, would offer a direct look at engrams or only an indirect look because engrams are instantiated at the cellular level. The spatial resolution of functional imaging with 7T field strength reaches the mesoscale, that is, the level of functional units of computations (De Martino et al., 2018). This resolution allows differentiating activity in individual cortical layers and hippocampal subfields. Hence, 7T fMRI can provide insights into the neural correlates of individual memories irrespective of whether the imaged signal is termed engram, as defined 100 years ago by Richard Semon, or otherwise (Schacter, 2001).
In this review, we present the first successful attempts at imaging engrams in the human at 7T field strength and summarize the merits and difficulties of 7T memory fMRI. We highlight the memory research that can profit most from 7 versus 3T fMRI and that may justify the associated financial costs. We begin by briefly describing the general potential of 7T MRI (for an extensive review see Ugurbil, 2018), and proceed to giving an overview of engram research performed at 3 and 7T field strength. We focus on three areas of engram research that might be well suited for 7T imaging given the properties and the physiological advantages of 7T imaging. The three areas are (1) high‐resolution imaging of hippocampal engrams, (2) high‐resolution imaging of layer‐specific cortical engrams, and (3) high‐resolution imaging of small subcortical structures that modulate engram formation. We present scenarios, in which the application of 7T fMRI would be most worthwhile and scenarios, where the advantages may not outweigh the costs. We finally mention theoretical debates in memory research that might be resolved using human 7T imaging.
1.1. Advantages and disadvantages of 7T fMRI
The more than two‐fold increase in magnetic field strength from 3 to 7T is the origin of most advantages but also disadvantages of 7T scanners. On the one hand, the increase in magnetic field strength causes an increase in the magnetization of any matter in the scanner bore, which leads to a stronger signal once the matter is excited by a radio frequency pulse. On the other hand, magnetic susceptibility artifacts and distortions are also enhanced. This section summarizes the advantages and disadvantages most relevant for engram research.
1.1.1. Advantages
The main advantages of 7T scanners are the increases in spatial resolution, spatial specificity, and statistical power. Each topic is discussed in the following.
Blood‐oxygen‐level‐dependent (BOLD) imaging is based on measuring magnetic field inhomogeneities (Logothetis et al., 2001; Ogawa et al., 1993). Those inhomogeneities are influenced by the ratio of oxyhemoglobin and deoxyhemoglobin within small blood vessels. The strength of magnetic field inhomogeneities rises with the strength of the main magnetic field. Thus, the BOLD signal obtained is generally stronger and the signal‐to‐noise ratio higher on 7T scanners compared to 3T scanners. It is possible to capitalize on this increase in signal strength, for example by increasing the spatial resolution, that is, decrease voxel size. Decreasing the voxel size penalizes the signal‐to‐noise ratio. The increased signal strength of 7T scanners makes it possible to decrease the voxel size below 1 mm isotropic, while maintaining a reasonably high signal‐to‐noise ratio. This makes it possible to functionally resolve hippocampal subfields (Carr et al., 2010). Additionally, instead of increasing the resolution, it is also possible to scan at lower resolutions, but faster. This is favorable when scanning patients or participants who cannot be bothered with a long scanning time.
Increasing the temporal and/or spatial resolution bears trade‐offs, especially concerning regions such as the medial prefrontal area and the medial temporal lobe (MTL), which are prone to suffer from signal loss due to field inhomogeneities. Increasing the temporal and/or spatial resolution penalizes the signal‐to‐noise ratio. If the signal loss due to inhomogeneities is strong, then a high temporal and/or spatial resolution may negate the signal gains introduced by the high field strength at 7T (Yoo et al., 2018). Increasing the temporal and/or spatial resolution should therefore be handled with care.
Additionally, some studies report that spatial smoothing can sometimes, albeit counterintuitively, benefit multivariate analyses such as multi‐voxel pattern decoding because it increases the SNR (Op de Beeck, 2010). Increasing the spatial resolution is still valuable, especially if one can increase it to the level of functional organization in the investigated brain region, because this offers the highest gains in signal/information, even if later smoothing is applied.
Spatial specificity is increased as well on 7T (Yacoub et al., 2001). This is due to the fact that smaller blood vessels contribute more to the BOLD signal than with lower field strengths, and large blood vessels contribute less (Olman et al., 2009). In addition to the increased spatial resolution, this makes 7T scanners very useful for the imaging of smaller brain structures such as individual amygdalar nuclei or hippocampal subfields.
Torrisi et al. (2018) compared a “go/no‐go” (response inhibition task) fMRI paradigm between 3 and 7T to assess the statistical power between the two systems. Their results showed large gains for 7T in the statistical power to detect small effects and effects on the group‐level. This is a result of the increased signal strength on 7T scanners. With increased signal strength the temporal signal‐to‐noise ratio (SNR of the time series) is increased as well, and thus the statistical power. The temporal SNR is an important measure and it depends on multiple factors such as physiological noise and participant movement. The increase in statistical power on the group level may be a help for research in participant groups that are inherently harder to recruit, such as amnesic patients, who are of interest for memory/engram research. The increase in statistical power makes it possible to produce meaningful results in a smaller group of participants or patients. The general gains in signal strength hold also true for the hippocampus (see Figure 2 and Theysohn et al., 2013). Here the percent signal change is often only about 0.5% above base level, and thus lower than the average signal strength in the cortex. Hence, with increased signal strength, 7T imaging has the potential to make so far unfeasible designs in the MTL feasible (Nau, 2019). If one is interested in the engram pathway, that is, in the connectivity between engram components, 7T MRI is an apt choice because its faster temporal resolution yields optimal BOLD sensitivity for functional network imaging (Yoo et al., 2018).
FIGURE 2.
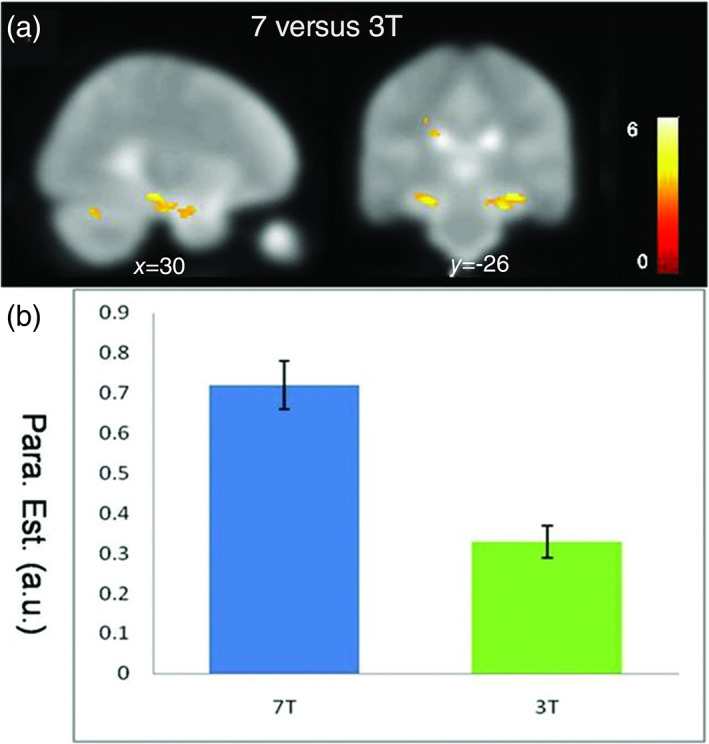
Reprinted with permission from: N. Theysohn et al., 2013. (a) Field strength comparison: A two‐sample t‐test (contrast 7 vs. 3T) shows a plus in BOLD sensitivity in bilateral hippocampus at 7T for functional activation related to associative memory encoding. Significantly higher activation in the hippocampus bilaterally. (b) Box plot comparison of 3T (right) and 7T (left) datasets. Extracted beta values of parameter estimation show significant higher intensity of the hippocampal activation at 7T (0.7 ± 0.06 at 7T vs. 0.33 ± 0.04 at 3T)
1.1.2. Disadvantages
The main disadvantages of 7T scanners are increased influences of physiological movements and increased susceptibility artifacts. In addition, tissue heating as well as other side effects can be more severe on 7T scanners. These main disadvantages are discussed in the following.
Due to the shorter wavelength of the radiofrequency fields in 7T scanners, it is more likely that local foci emerge, which can heat up the tissue (Fiedler et al., 2018). The specific absorption rate (SAR) measures how much of the radio frequency is absorbed by the tissue. It is usually averaged over the body weight. In 7T scanners, the allowed SAR is limited to prevent tissue damage. In order not to exceed those limits, the fMRI sequence may need to be restricted. For example, the number of slices may be limited, which can be a challenge in sequence design (Ladd et al., 2018).
One drawback of the higher resolution is that physiological noise becomes more dominant with decreasing voxel size. The physiological noise encompasses heart rate and breathing, which one should record during the fMRI session. A toolbox that allows including the recorded physiological data into the fMRI analysis is available (Kasper et al., 2017). Motion artifacts can also be recorded and incorporated into the data analysis, for instance as covariates in the first‐level general linear model. Foremost, participant compliance is important because it can reduce motion artifacts considerably. Low compliance may be expected when working with amnesic or demented patients. Also, from an engram research point of view, a memory paradigm that includes vocal responses such as a free recall are not feasible due to muscular activity and excessive head movements during the answer. Relatedly, studies involving shocking or scary images for emotional memory formation or recognition may induce a physical reaction in the participants, which necessitates a careful piloting, good instructions, and additional head fixation to avoid excessive movement artifacts. To control for motion artifacts, several solutions have been proposed, for example optical systems for motion tracking. One can place a camera inside the scanner bore for offline image reconstruction (Schulz et al., 2012). It was mentioned above that the BOLD signal is based on field inhomogeneities which increase at higher field strength. Thus, the BOLD signal is also stronger on higher field strengths. Ironically, the same forces that increase the signal strength also induce certain problems. Stronger effects of field inhomogeneity lead to severe image distortions and signal losses, especially at tissue boundaries (Jezzard & Clare, 1999). These so‐called susceptibility artifacts are not specific to the MTL but are particularly strong at and around the MTL, due to air cavities such as the sphenoid sinus in close proximity, which produce significant magnetic field inhomogeneities (Olman et al., 2009). This is also an issue at lower field strengths. Therefore, imaging techniques evolved that reduce the susceptibility artifacts as well as possible, although often at the cost of a good signal‐to‐noise ratio (Nau, 2019).
Unfortunately, the effect of susceptibility artifacts scales linearly with magnetic field strength and is thus even stronger on 7T scanners. Besides adjustments to the sequence itself (Brunheim et al., 2017; Marques & Norris, 2018), susceptibility artifacts can also be fought with dielectric pads, which are placed around the head of the participant in order to increase the magnetic field homogeneity, especially around the air cavities close to the MTL and medial/inferior prefrontal cortex (Yang et al., 2006). The pads should be as close as possible to the region of interest because their effect drops off at short distance (Teeuwisse et al., 2012). Software tools to design such dielectric pads for one's own use case are freely available (van Gemert et al., 2019).
Higher safety standards regarding metal in 7T (tattoos for instance) may obstruct recruiting. In addition, higher field strength makes side effects of being exposed to the magnetic field more severe. Phosphenes (perception of light flashes due to an inductive effect on the visual cortex), and vertigo (inductive effect on the vestibular system) are more pronounced. Because of stricter exclusion criteria, recruitment is generally harder with imaging at 7T scanners. Nevertheless, thanks to active shielding technology on newer 7T scanners, drop‐outs due to side‐effects are no longer common. In a study from 2008, only 5 out of 102 healthy participants ended a 7T MRI examination prematurely due to side‐effects (Theysohn et al., 2008). In a newer study using an actively shielded scanner and a large cohort of 801 subjects, less than 10% of subjects would object to another examination in the future (Hansson et al., 2020). In our own experience when using a 7T Terra Magnetom Scanner with active shielding technology, only one of 26 participants experienced discomfort (nausea); no participant terminated the MRI session prematurely.
There is no fundamental difference in the advantages and disadvantages between anatomical MRI and functional MRI at 7T. Yet, movement artifacts may have a greater impact on functional than anatomical MRI because of the prolonged acquisition times associated with fMRI. Benefits of anatomical MRI at 7T are important for the clinical use of MRI. The increased spatial resolution of anatomical MRI at 7T improves the assessment of the severity, extent, and boundaries of brain damage. In the case of dementia and amnesia, this enhanced anatomical precision may assist memory researchers. For example, with the increased specificity and resolution at 7T, it became possible to image the atrophy of the CA1 apical neuropil in the hippocampus of Alzheimer's disease patients (Kerchner et al., 2010).
This section summarized the advantages and disadvantages of 7T fMRI. While it ended on the negative points, we would like to emphasize that the increased signal‐to‐noise ratio and the resulting statistical power outweigh the pitfalls that come with higher field strength. This holds true even for regions like the hippocampal formation. While accounting for the disadvantages and problems that arise with higher field strength is possible, it does mean more work. Because new 7T MRI scanners are currently being introduced worldwide, and certain scanning sequences may not yet be available, it should be discussed whether the planned study would actually profit from 7T or is better off with an established infrastructure at 3T. The following section of this manuscript aims to present scenarios where the former was the case.
2. 7T fMRI ENGRAM RESEARCH
The following section presents existing work that successfully applied 7T fMRI to engram research (see Table 1). Here, it will become clearer when 7T fMRI is a good choice for engram research and when not. We will present work from three areas of engram research that profit most from 7T fMRI: the hippocampal engram component, cortical engram components as well as subcortical modulators of engram strength.
TABLE 1.
Overview over 7T fMRI memory studies, paradigm and scanning parameters
| Authors | N | TR (ms) | TE (ms) | Voxel size (mm) | Slices | FOV (mm) | Paradigm | ROI |
|---|---|---|---|---|---|---|---|---|
| Koster et al. (2018) | 26 | 2500 | 22 | 0.8 × 0.8 × 0.8 | 28 | 205 × 205 | Inference task (memory integration) | MTL |
| Maass et al. (2015) | 22 | 2000 | 22 | 0.8 × 0.8 × 0.8 | 28 | 205 × 205 | Visual associative memory | MTL |
| Navarro Schröder et al. (2015) | 22 | 2756 | 20 | 0.9 × 0.9 × 0.92 | 96 | 210 × 210 | Spatial navigation task | MTL (EC) |
| Shah et al. (2018) | 13 | 1000 | 24 | 2 × 2 × 2 | 64 | 192 × 192 | Resting state, functional connectivity | MTL |
| Sladky et al. (2018) | 38 | 1400 | 23 | 1.5 × 1.5 × 1 | 78 | / | Emotional face viewing | Amygdala, BNST |
| Suthana, Donix, et al. (2015) | 14 | 3000 | 19 | 1 × 1 × 2 | 21 | 200 × 200 | Visual associative memory | HC |
| Theysohn et al. (2013) | 28 | 2050 | 25 | 2.5 × 2.5 × 2 | 50 | 230 × 230 | Visual associative memory | MTL |
| Barron et al. (2020) | 22 | 1512 | 20 | 1.5 × 1.5 × 1.5 | 50 | 192 × 192 | Inference task (memory integration) | MTL |
| Hodgetts et al. (2017) | 25 | 2000 | 25 | 1.2 × 1.2 × 1.2 | 30 | 192 × 192 | Perceptual oddity task, discrimination | HC and subiculum |
| Berron et al. (2017) | 20 | 2000 | 22 | 0.8 × 0.8 × 0.8 | 28 | 205 × 205 | Scene discrimination | Hippocampus |
| Finn et al. (2019) | 15 | 2500 | 27 | 0.75 × 0.75 × 0.99 | / | 130 × 130 | Working memory, delayed response task | DLPFC |
| Lawrence et al. (2018) | 21 | 3408 | 28 | 0.8 × 0.8 × 0.8 | / | 192 × 192 | Visual working memory task | V1 |
| Margalit et al. (2020) | 7 | 2200 | 22.4 | 0.8 × 0.8 × 0.8 | 84 | 160 × 129.6 | Oddball detection task, visual stimuli | VTC |
| Murphy et al. (2020) | 30 | 3000 | 28 | 0.85 × 0.85 × 1.5 | 37 | / | Emotional face matching task | Amygdala |
| Jacobs et al. (2020) | 27 | 2000 | 19 | 1.25 × 1.25 × 1.25 | 50 | / | Emotional face matching task | LC, amygdala, HC |
Note: While all studies were memory related, not all of them specifically looked for engrams.
Abbreviations: Amy: amygdala; BNST, bed nucleus of the Stria terminalis; DLPFC, dorsolateral prefrontal cortex; EC, entorhinal cortex; FOV, field of view; HC, hippocampus; LC, locus coeruleus; MTL, medial temporal lobe; N, number of participants; ROI: region of interest, that is, investigated brain area; Sub, subiculum; V1, primary visual cortex; TE, echo time; TR, repetition time.
2.1. The engram
To make the argument that these three fields are best suited to be investigated with 7T MRI, it is important to describe how and where engrams are thought to be stored in the brain. Engrams are distributed around the whole brain and not limited to a specific anatomical location. Therefore, Tonegawa et al. (2015) introduced the term engram components. An engram component is defined as the “content of an engram stored in an individual engram cell population.” Those individual components are connected via an “Engram cell pathway” and together build the “Engram complex,” which composes the whole engram. For example, an episodic memory such as meeting a good friend consists of a sequence of multiple sensory inputs, most of which are processed and represented at least partly by cortical areas. Each of those neuronal ensembles comprises one engram component and they are connected to the hippocampus. According to the Hippocampal Memory Indexing theory, the hippocampus itself houses another, different engram component, and is both anatomically and functionally designed as a convergence zone, where neocortical input is being associated (Teyler & DiScenna, 1986). The hippocampus is necessary for the encoding of episodic memories. During the recall of a memory, the hippocampus is able to reactivate cortical engram components. Taking the given example, seeing the face of the good friend is activating a single cortical engram component in the fusiform face area. Then this memory cue activates the hippocampal engram component, which makes it possible to relive an episode by the reinstantiation of the whole engram complex (Hebscher et al., 2021; Teyler & Rudy, 2007). For engram components in the visual cortex, the strength of the cortical reinstatement at retrieval is signaled by the degree of antecedent hippocampal activation (Bosch et al., 2014).
The hippocampus is not only connected to the neocortex, but also to many subcortical areas. Subcortical nuclei and networks are functionally involved in the modulation of memories. A prominent example is the amygdaloid complex which adds both negative and positive emotional value to the engram. Another example is the subcortical dopaminergic reward network. A high reward value of a memory makes the replay of a memory trace during consolidation and retrieval success more likely (Gruber et al., 2016). The three mentioned engram components share a common feature: They are either too small to be imaged with conventional imaging methods or contain sub‐structures, which evaded precise imaging thus far. In this section, it will become clear that 7T has the potential to uncover knowledge that remained hidden with the use of conventional imaging methods.
2.2. Hippocampal engram components
2.2.1. Prior work
The MTL has already been probed with high‐resolution MRI at 3T, both anatomically and functionally. The functional heterogeneity of the hippocampal formation was proven to be considerable both regarding its subfields and regarding the anterior–posterior segments of its long axis (Zeidman & Maguire, 2016). The anterior portion of the hippocampus is anatomically distinct from the posterior portion, because the parahippocampal gyrus wraps around the most anterior portion of the hippocampus forming the uncus. Within the uncus, the hippocampal formation bends too, which leads to a more prominent CA1 region compared to more posterior CA1 regions. In addition, the firing of place cells is less concise in the anterior versus posterior CA1 cells (Kjelstrup et al., 2008). Many hypotheses about hippocampal functions are based on findings in rodents and were only later confirmed in humans, such as the differential activation during encoding versus retrieval of memories (Zeineh et al., 2000, Zeineh et al., 2003; for a review see Carr et al., 2010). As for pattern separation, Bakker et al. (2008) confirmed the important role of DG/CA3 in humans. Using 1.5. mm isotropic voxels at 3T, Bakker et al. segmented the hippocampus into its subfields and showed a clear activation bias for pattern separation in DG/CA3 while participants were encoding picture stimuli of highly similar objects. The specific activation of hippocampal neuronal populations was recently backed up by work in presurgical epilepsy patients who performed a memory paradigm (Derner et al., 2020; Suthana, Parikshak, et al., 2015). While recording from single neurons in the amygdala, parahippocampal cortex, entorhinal cortex (EC), and hippocampus, the authors could not identify any neuron showing activity for the retrieval of both item and source memory (p overlap = 1), which is in line with dual‐process models proposing that familiarity and recollection constitute two distinct processes contributing to recognition memory (e.g., Eichenbaum et al., 2007; Yonelinas, 2002).
In the light of these results, obtaining meaningful data about the function of hippocampal subfields seems important. Interestingly, both work in rodents and recent results in humans provide evidence of functional heterogeneity even within hippocampal subfields (Lee et al., 2020). In most computational models, the CA3 region—with its large number of recurrent connections—is specialized to perform pattern completion, while the dentate gyrus applies sparse coding to perform pattern separation, which reduces the correlation of similar input. In addition, the CA3 region is displaying functional heterogeneity along its transverse axis (Lee et al., 2015). While the dorsal section of the CA3 region engages in pattern completion as predicted by conventional models, the ventral section is involved in pattern separation processes together with the dentate gyrus. In the light of these results, it seems urgent for noninvasive engram research in humans to increase the spatial resolution in order to do justice to the discoveries of complexity and functional heterogeneity within the MTL. Seven Tesla fMRI serves as a promising starting point because it offers higher resolution than conventional fMRI and is thus bound to extend our knowledge about the contribution of hippocampal subfields to memory processes.
2.2.2. Prior work on 7T
The increased spatial resolution at 7T makes the segmentation of engram‐related anatomical regions easier and more reliable. The analysis of the structural and functional network architecture of the whole MTL has been achieved with 7T (Shah et al., 2018). Shah and colleagues found the bilateral hippocampus asymmetric in both volume and structural connectivity. The right hippocampus tends to have a larger volume compared to the left hippocampus. The larger right hippocampus volume was driven by the large right dentate gyrus. Resting‐state functional connectivity was surprisingly symmetrical despite the putative volumetric differences.
The different contributions of the hippocampal subfields to memory‐related processes were so far dominated by findings in rodents (Hainmueller & Bartos, 2020). Yet, research in humans recently caught up with the help of imaging at 7T. One example of anatomical and functional segmentation of the hippocampus with 7T MRI is the work by Hodgetts et al. (2017), who found evidence for the important role the subiculum plays in scene perceptual discrimination (Figure 3). Suthana, Donix, et al. (2015) restricted the field of view to the MTL. This restriction allowed them to manually segment the hippocampal subfields in order to assess the contribution of each subfield to the encoding and retrieval of associative memories in humans. The anterior CA2 and CA3 were found to be active during learning, while the posterior CA2, CA1, and posterior subiculum were active during retrieval.
FIGURE 3.
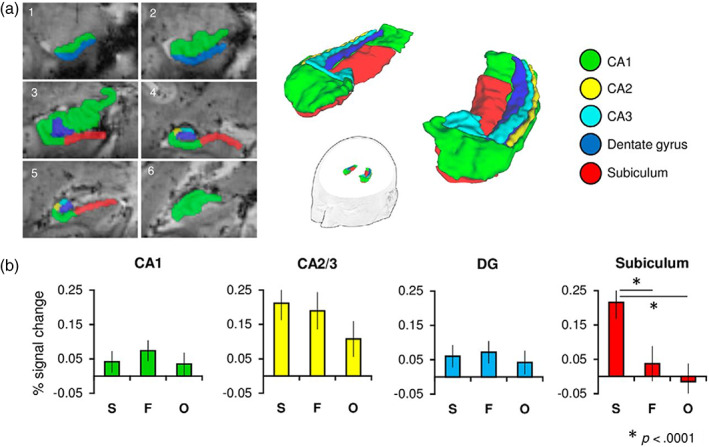
Reprinted with permission from: Hodgetts et al., 2017. (a) Hippocampal subfields (CA1, CA2, CA3, DG, and subiculum) were manually segmented on subjects' ultra‐high‐resolution T2(star)‐weighted images obtained during a visual oddity task: Selecting an oddball image from a group of three images, for example, two images of the same object from different viewpoints and a third target image of a different object). Six representative coronal slices of an individual subject's segmentation are shown (left hemisphere; 1, anterior; 6, posterior). Regions CA2 and CA3 were later concatenated as their small size precluded accurate functional localization at our coarser functional resolution of 1.2 mm isotropic. (b) Mean percentage signal change plots for correct scene (S), face (F), and object (O) judgments (relative to size baseline) for each hippocampal subfield ROI. Error bars represent SE
Today, protocols for manual and automatic segmentation of MTL subregions became available for 7T MRI (Berron et al., 2017). In fact, there are many (automated) approaches to hippocampal subfield segmentation (Giuliano et al., 2017). Those protocols allow the detection of small volumetric changes within subregions. In a study from 2000, Maguire et al. (2000) provided evidence for an association between spatial memory and hippocampus volume in a small sample of human navigation experts (taxi drivers). However, the finding of this association could not be replicated in studies with larger samples of healthy young adults (Clark et al., 2020; Weisberg et al., 2019). Still, there is evidence that hippocampal subfield volume can predict mnemonic processes such as recollection memory, even in healthy young adults (Poppenk & Moscovitch, 2011). In addition, differential volumetric changes also predict memory and cognitive impairment in healthy and pathological aging. For instance, the structure and the functional activity of the EC and the dentate gyrus can explain the performance on an associative memory task in older adults (Carr et al., 2017). In detail, the thickness of both the EC and the apical neuropil layer of the CA1 region are positively associated with memory performance. The activity in CA3/DG however, was negatively associated with memory performance, indicating a putative failure of pattern separation or completion processes. Huhn et al. (2018) reported that 7T MRI can improve the assessment of intra‐hippocampal functional connectivity and microstructure in older adults. These authors investigated the effect of the polyphenol Resveratrol on memory performance. There was no significant difference in memory performance following the administration of Resveratrol over 6 months versus a control group that received a placebo.
One recent example showcases a use case of 7T fMRI. In an attempt to illuminate the processes that encompass inference, Barron et al. (2020) investigated the cortical and hippocampal engram components at the same time, while maintaining a resolution and spatial specificity that was high enough to perform representational similarity analyses on both the hippocampal patterns as well as whole brain patterns. This study used an inference task, in which participants needed to extract commonalities of different memories. Participants first learned to associate sounds with abstract geometrical images. On the following day, they learned to associate the same geometrical images with either a monetary reward or a neutral outcome (no money). It was then tested whether participants were able to infer the associations between sounds learned on day one with the monetary outcome learned on day two, even though the sounds and rewards were never presented together. To test for indirect sound‐reward associations, the sounds were presented once more on day 3 and participants retrieved the sound‐reward associations. Interestingly, the hippocampal BOLD pattern recorded during the retrieved inference was not similar to the pattern observed during the encoding of image‐reward associations on day 2. Hence, the hippocampus did not represent the image‐reward association while performing the sound‐reward inference, but merely re‐instantiates the initial associative memory (sounds and images) during the successful inference on day 3. Using an RSA searchlight algorithm, the representation of the outcome (reward) was found in the medial prefrontal cortex. Seven Tesla imaging allowed for recording of brain volumes in a sufficient temporal and spatial resolution to perform those analyses (TR: 1512, spatial resolution: 1.5 mm isotropic, see also Barron et al., 2020 in Table 1).
The results of Barron et al. are in accordance with previous work that showed the involvement of the EC in the integration of information across episodes (Koster et al., 2018). Pattern integration also assists finding commonalities between memories or episodes. The authors hypothesized that the hippocampal system is capable of feeding itself old, retrieved memories as new input in order to find commonalities between distinct episodes. For example, the memory of a trip to the Swiss Alps with your partner might trigger the memory of a past holiday, where you visited the same hotel with your family of origin. Koster et al. suspected this recurrence of information to be processed by the EC. The superficial layers of the EC are the main input regions to the hippocampus, while the deeper layers of the EC receive output from the hippocampus. Thanks to 7T imaging, the authors could resolve the layers of the EC. They found that during inference the common element that links both memories (the hotel in the Swiss Alps) is represented by both deep and superficial layers of the EC. Additionally, the strength of functional connectivity between those two layers predicted success in the inference task.
In conclusion, the MTL lends itself for investigation with 7T anatomical and functional MRI. The MTL is still at the focus of rodent engram research. A current goal is to look for common functional specificities in rodents and humans. The MTL is a thriving field of engram research, evidenced by the recent discovery of human time cells, and provocative articles that question pattern separation in the human hippocampus (Quiroga, 2020), as it is predicted by research in animals and computational models. A high spatial resolution is necessary for the future research on the engram.
2.3. Cortical engram components
As mentioned before, whole engrams consist of engram components. During retrieval, the hippocampus reinstates the cortical representations that were present during encoding (Bosch et al., 2014; Tanaka et al., 2014). The hippocampal and cortical engram components are thus interdependent. Cortical engram components can be imaged in‐depth and in‐width using 7T MRI.
2.3.1. Probing in‐depth
The neocortex was a research focus since the very beginning of 7T neuroimaging. The layer or laminar MRI field aims to gain functional or structural knowledge of the individual cortical layers. This field profits from the advancement of ultrahigh field (UHF) MRI scanners (Norris & Polimeni, 2019).
Laminar MRI enables the differentiation between cortical layers and thus between bottom‐up and top‐down processes. Electrophysiological recordings suggest that cortical layers receive input mainly at the middle layer, while output mainly arises from the deep as well as the superficial layers (Douglas & Martin, 2004). Sharoh et al. (2019) presented evidence for this prediction using 7T neuroimaging. They were able to differentiate between top‐down and bottom‐up activity in the left occipitotemporal sulcus of participants during language processing. Furthermore, Lawrence et al. (2018) showed that holding a visual item in working memory does not activate the 4th cortical layer of the primary visual cortex. This makes sense because the 4th visual cortical layer has previously been associated with bottom‐up processing, that is, perception. Also investigating working memory processes, albeit in the dorso‐lateral prefrontal cortex, Finn et al. revealed that only superficial and deeper layers are active during a working memory task (Finn et al., 2019). The superficial cortical layers were active during the delay period of the working memory task, while deeper layers were active during the response period.
Maass et al. (2014) identified differential activity of EC and hippocampal layers for distinct kinds of memory‐related activity. While the processing of new information mainly engaged input structures of the hippocampus, namely the superficial EC and DG/CA3 layers, retrieval processes were more strongly related to output regions such as the deep layers of EC and pyramidal CA1. Two other studies applied 7T MRI to identify distinct anatomical connections and functions of the EC bridging the gap between animal and human research (Maass et al., 2015; Navarro Schröder et al., 2015). In rodents, research divides between the medial EC and the lateral EC. The medial EC is mostly connected to the parahippocampal gyrus and is believed to process visual information, the lateral EC has more connections to the perirhinal cortex, which contributes to object memory. It was difficult to assess whether this division is conserved in humans when using resolutions above 1 mm isotropic. With the help of 7T MR imaging, these authors confirmed that the human EC can be divided into an anterior‐lateral and a posterior‐medial portion, which structurally and functionally resemble the lateral and medial division of the EC in rodents, respectively.
Differentiating BOLD activity between the cortical layers of a structure is important for engram research because the layers are suspected to contribute distinctly to memory processes. While it is known and observable with 3T that encoding patterns are re‐instantiated during retrieval, fMRI at 7T can unravel the cortical layers individually. Therefore, it became possible to assign encoding‐, retrieval‐, and imagery‐related activity to distinct cortical layers. Future research could focus on how top‐down processes influence bottom‐up processes, that is, how experiences and memories can bias perception.
2.3.2. Probing in‐width
Cognitive maps were introduced by Tolman (1948). The cognitive map theory proposes that representations of concepts such as faces, or objects are mapped throughout the cortex in a cognitive or semantic space. Cortical engram components are located within these spaces; thus, cognitive maps are an important concept for engram research. Because the cognitive maps of individuals overlap and because cortical representations build the cortical engram components, memory representations may overlap as well between individuals. Indeed, Xiao et al. (2020) found with 3T MRI that the angular gyrus exhibits high representational similarity for the retrieval of shared memories across individuals. The representational similarity of memories across individuals is higher for successfully retrieved than unsuccessfully retrieved memories (Koch et al., 2020). In a further study with 3T MRI, participants watched a movie and were then asked to attempt a free recall of the whole movie, while being scanned with fMRI. Even for this uncued recall, where every participant recounted the events in their own pace and style, fMRI patterns of retrieval‐related activity for the same movie scenes were similar across participants (Chen et al., 2017). These memories seem to be transformed into a common spatial organization in high‐level cortical areas. This is suggested by the fact that for the retrieval of events, the representational similarity between participants was closer than the similarity within a participant when comparing the retrieval and the perception of an event.
Margalit and colleagues used 7T fMRI to probe object representations in the ventral temporal cortex (VTC). They presented participants with objects from five domains (e.g., faces and places) and ten categories within each domain (such as adult and child faces for the domain faces). With the high spatial resolution of 7T fMRI, they discovered differences in the representational resolution between the medial VTC and the lateral VTC. While the medial VTC was organized by both domains and categories, the lateral VTC exhibited a less specific organization dividing only by the five domains (Margalit et al., 2020).
Probing the neocortical engram component in‐width at a higher spatial resolution allows for a finer distinction of representations that lay close together within the cognitive map, supposedly because they are more closely related semantically, too. Here, the connection between hippocampal and neocortical engram components becomes apparent again. One might ask how a failure to separate two very similar but distinct memories would relate to the underlying hippocampal engram components and to the underlying neocortical engram components. Does a failure of pattern separation in the hippocampus preclude the successful distinctive activation of each of the two concepts in the neocortex? With 7T fMRI, answers to such questions do not seem too far out of reach.
2.4. Engram modulation by subcortical nuclei
The engram modulation exerted by subcortical nuclei can also be investigated with 7T fMRI. Due to the small size of many subcortical nuclei, investigations with lower spatial resolutions were not ideal. Indeed, a review detailing the general applications of 7T MRI to human brain function pointed out that subcortical areas and the cerebellum profit most from the increased resolution of 7T imaging because the functional units are small in these regions (van der Zwaag et al., 2016). Most fMRI studies of small subcortical structures lack comparability due to a high diversity in preprocessing and smoothing standards. Smaller structures are especially affected because smoothing quickly blurs the borders of small adjacent structures such as the amygdalo‐hippocampal border. Those problems are still present with 7T imaging but are worse on lower field strengths and resolutions (Murphy et al., 2020).
Colizoli et al. (2020) compared BOLD responses of the dopaminergic midbrain between 7 and 3T fMRI. The evoked responses were consistently larger at 7T.
A 7T study of the neural correlates of creative problem solving, that is, the “AHA”‐ or “EUREKA”‐moment, revealed a significant involvement of subcortical nuclei (Tik et al., 2018). Activity in the dopaminergic midbrain, caudate nucleus, and the hippocampus was related to insightful problem solving. The topographic organization of the subcortex is not part of most contemporary brain atlases and was only recently investigated (Tian et al., 2020). Tian and colleagues discovered large‐scale connectivity gradients and new areal boundaries. They delineated the subcortical area into 27 bilateral units, a result that would not have been possible without the increased spatial resolution and sensitivity of 7T imaging.
One primary region of interest for modulating engram components is the amygdala. The amygdala is involved in adding emotional valence to memories. A large body of work in rodents has shown that the amygdala and the hippocampus are distinctly involved in memory‐related processes. For example, the hippocampus is strongly involved in associative encoding, while the amygdala is involved in forming fear memories (Heldt et al., 2007). However, the two regions are anatomically intertwined and therefore prone for mislabeling in human fMRI studies at a lower resolution (Derix et al., 2014). Seven Tesla MRI may provide remedy by clearly identifying the amygdalo‐hippocampal border.
Besides the need for delimitation to the hippocampus, the amygdaloid complex itself is not united in its response to emotional stimuli, such as faces (Sladky et al., 2018). With 7T imaging, these authors showed that specifically the basolateral amygdala, the central amygdala, and the bed nucleus of the stria terminalis (BNST; part of the extended amygdala, located in the basal forebrain) do respond strongly to emotional faces. The BNST is involved in the acquisition and expression of fear memories and has recently been considered a therapeutic target for the treatment of post‐traumatic stress disorder. The treatment consists in a manipulation of the amount of stress‐related neuropeptides in the BNST (Miles & Maren, 2019). In another study, the BNST reacted strongly to novel images of fearful faces along with the hippocampus and the amygdala (Pedersen et al., 2017). Pedersen et al. noted specifically that 7T rather than lower field‐strength fMRI was necessary to resolve the BNST.
Two other prominent memory modulators are arousal and reward. Both boost memory performance and are mediated by monoaminergic signaling. In the case of arousal, the locus coeruleus (LC), a small brain stem nucleus, is the main source of cerebral norepinephrine that mediates arousal in the brain. The LC is difficult to image in humans because of its small size and its proximity to the fourth ventricle (Jacobs et al., 2018). With 7T fMRI, however, Jacobs et al. were able to image the activity of the LC while monitoring the participant's arousal (via alpha‐amylase) during an emotional memory task. The LC was active in an arousal‐related manner and amygdala activation closely followed LC activation (Jacobs et al., 2020). The LC BOLD signal was positively associated with memory performance.
We have mentioned above that the cerebellum is another structure that lends itself to the analysis with high spatial resolution because of the small size of its functional units. This applies to both the cerebellar nuclei, such as the dentate nucleus, and the cerebellar cortex that—unlike the cerebral cortex—consists of only three layers. With 7T MRI, both areas of the cerebellar cortex and of the dentate nucleus were found to be involved in working memory processes. The cerebellar cortex was specifically involved in verbal working memory (Thürling et al., 2012).
3. DISCUSSION
3.1. Conclusion
FMRI research at 7T field‐strength comes with advantages such as increased signal‐to‐noise ratio, the visualization of small structures and the detection of small physiological effects (Ladd et al., 2018). These advantages come at the price of strong magnetic field inhomogeneities, tissue heating and side‐effects like nausea and phosphenes. Currently, a 7T MRI infrastructure is not yet available at most institutions. This review points to promising applications of 7T MRI in human memory research. As we pointed out, probing cortical engram components can be rewarding when using 7T MRI. The same holds true for the hippocampal engram components, although the MRI sequence needs to be carefully adjusted because the MTL is difficult to image and prone to suffer susceptibility artifacts. If one is interested in the modulation of memory through subcortical or cerebellar nuclei, 7T MRI may provide the resolution needed to sufficiently resolve those nuclei.
Yet, the scope of 7T fMRI is larger. Thanks to the flexibility of modern 7T scanners, if adjusting the field‐of‐view and the spatial resolution between the fMRI time‐series, one can image the micro‐structure of cortical engram components in the first fMRI time‐series and their functional integration into the whole engram complex in the second fMRI time‐series, both in a single experimental session (Kuehn & Pleger, 2020). Particularly methodological work regarding brain structure and function is preferably performed using a 7T MR scanner. When trying to associate changes in structure and in the functional connectivity in pathological states, such as reported in Shah et al. (2018), any effect might be subtle, which speaks for imaging at 7T field‐strength. The decision to use a 3 or 7T MRI scanner needs not to be an exclusive choice because the fMRI task can be run with 3T MRI (if a high spatial resolution is not necessary) and the resting‐state functional connectivity and anatomical imaging can be run with 7T MRI. This could be a “best‐of‐both‐worlds” solution.
Bridging gaps between animal and human research by increasing the spatial resolution to the mesoscale is an argument in favor of 7T fMRI. However, it should not be forgotten that the temporal resolution of 7T fMRI remains noncompetitive. Although one can push the sampling rate of fMRI (TR) at 7T below 1 s, while maintaining a sub‐millimeter spatial resolution (Hendriks et al., 2020), it remains impossible to access the timescale of theta frequencies (4–7 Hz). While neural oscillations play a big role in memory processing (Herweg et al., 2020), theta frequencies and the BOLD signal do not correlate (Ekstrom et al., 2009), at least not in the hippocampus. Therefore, the electroencephalogram remains the gold standard to answer questions about the function of neural oscillations in memory. We have mentioned that the increased sensitivity of 7T MRI provides higher spatial and temporal resolutions. The higher resolutions and higher sensitivity lead to increased statistical power. The increased statistical power, in turn, allows reducing the sample size and scan time. These benefits will enrich memory research in the human.
3.2. Outlook
Seven Tesla fMRI will help resolving conceptual debates in memory research. A recent opinion article by Rodrigo Quiroga called into question whether pattern separation exists in humans in the way it is conceptualized from computational work and work in rodents (Quiroga, 2020). Quiroga argues that for similar memories the hippocampus performs pattern integration rather than pattern separation. Two memories that share overlapping content should also be represented with this overlap in hippocampal neuronal ensembles, rather than in two separated ensembles, as pattern separation models would predict. See however the response to Quiroga by Suthana et al., 2021, in which the authors argue that the focus on “concept cells”, which the intracranial single neuron recording studies had, may have overestimated the importance of generalization compared to pattern separation mechanisms. Moreover, they argue that crucial evidence for pattern separation was not considered by Quiroga. We believe that the two positions will be united: Pattern separation may not produce two distinct and completely orthogonal patterns but two patterns that are partly overlapping.
Quiroga made his point mainly based on intracranial single neuron recordings in humans. The present article mentioned two cases in which 7T fMRI provided evidence for Quiroga's pattern integration hypothesis: Barron et al. (2020) showed that the hippocampus is involved in inference and the integration of memories. They presented evidence that the overlapping part of two memories is re‐instantiated in the hippocampus at the time of retrieval. This speaks for the pattern integration hypothesis, since it is evidence that the same neuronal ensembles in the hippocampus are part of two distinct but overlapping memories. Koster et al. (2018) used an inference task as well and showed that the EC reintroduces the overlapping memory as a new input at the time of inference. Thus, high‐resolution fMRI can contribute to resolving this and other memory debates by moving the field beyond intracranial EEG and patient cohorts and toward a noninvasive data collection within the general population.
This review pointed out that for brain regions where extensive rodent, electrophysiological, and single cell data already exist, 7T imaging can be beneficial to replicate those findings because it provides higher spatial resolution than 3T fMRI. While this is a desired application of 7T fMRI, the concentration on replicating work from animal research would let other promising research ideas fall under the radar. De Martino et al. (2018) argue that the high sensitivity of 7T scanners combined with neuro‐computational methods should also be used to investigate purely “human” cognition, that is, cognitive processes that are believed to be uniquely human. Hence, UHF MRI should also be used to examine metacognition, consciousness, and language functions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
The authors thank Prof. Andrea Federspiel for comments on the manuscript. Open access funding provided by Universitat Bern.
Willems, T. , & Henke, K. (2021). Imaging human engrams using 7 Tesla magnetic resonance imaging. Hippocampus, 31(12), 1257–1270. 10.1002/hipo.23391
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Bakker, A. , Kirwan, C. B. , Miller, M. , & Stark, C. E. L. (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319(5870), 1640–1642. 10.1126/science.1152882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron, H. C. , Reeve, H. M. , Koolschijn, R. S. , Perestenko, P. V. , Shpektor, A. , Nili, H. , Rothaermel, R. , Campo‐Urriza, N. , O'Reilly, J. X. , Bannerman, D. M. , Behrens, T. E. J. , & Dupret, D. (2020). Neuronal computation underlying inferential reasoning in humans and mice. Cell, 183(1), 228–243. 10.1016/j.cell.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron, D. , Vieweg, P. , Hochkeppler, A. , Pluta, J. B. , Ding, S.‐L. , Maass, A. , Luther, A. , Xie, L. , Das, S. R. , Wolk, D. A. , Wolbers, T. , Yushkevich, P. A. , Düzel, E. , & Wisse, L. E. M. (2017). A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage: Clinical, 15, 466–482. 10.1016/j.nicl.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, S. E. , Jehee, J. F. M. , Fernandez, G. , & Doeller, C. F. (2014). Reinstatement of associative memories in early visual cortex is signaled by the hippocampus. Journal of Neuroscience, 34(22), 7493–7500. 10.1523/JNEUROSCI.0805-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunheim, S. , Johst, S. , Pfaffenrot, V. , Maderwald, S. , Quick, H. H. , & Poser, B. A. (2017). Variable slice thickness (VAST) EPI for the reduction of susceptibility artifacts in whole‐brain GE‐EPI at 7 Tesla. Magnetic Resonance Materials in Physics, Biology and Medicine, 30(6), 591–607. 10.1007/s10334-017-0641-0 [DOI] [PubMed] [Google Scholar]
- Carr, V. A. , Bernstein, J. D. , Favila, S. E. , Rutt, B. K. , Kerchner, G. A. , & Wagner, A. D. (2017). Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proceedings of the National Academy of Sciences, 114(45), 12075–12080. 10.1073/pnas.1713308114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, V. A. , Rissman, J. , & Wagner, A. D. (2010). Imaging the human medial temporal lobe with high‐resolution fMRI. Neuron, 65(3), 298–308. 10.1016/j.neuron.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Leong, Y. C. , Honey, C. J. , Yong, C. H. , Norman, K. A. , & Hasson, U. (2017). Shared memories reveal shared structure in neural activity across individuals. Nature Neuroscience, 20(1), 115–125. 10.1038/nn.4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. A. , Monk, A. M. , Hotchin, V. , Pizzamiglio, G. , Liefgreen, A. , Callaghan, M. F. , & Maguire, E. A. (2020). Does hippocampal volume explain performance differences on hippocampal‐dependant tasks? NeuroImage, 221, 117211. 10.1016/j.neuroimage.2020.117211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizoli, O. , de Gee, J. W. , van der Zwaag, W. , & Donner, T. H. (2020). Comparing fMRI responses measured at 3 versus 7 Tesla across human cortex, striatum, and brainstem. bioRxiv. 10.1101/2020.05.12.090175 [DOI]
- De Martino, F. , Yacoub, E. , Kemper, V. , Moerel, M. , Uludağ, K. , De Weerd, P. , Ugurbil, K. , Goebel, R. , & Formisano, E. (2018). The impact of ultra‐high field MRI on cognitive and computational neuroimaging. NeuroImage, 168, 366–382. 10.1016/j.neuroimage.2017.03.060 [DOI] [PubMed] [Google Scholar]
- Derix, J. , Yang, S. , Lüsebrink, F. , Fiederer, L. D. J. , Schulze‐Bonhage, A. , Aertsen, A. , Speck, O. , & Ball, T. (2014). Visualization of the amygdalo‐hippocampal border and its structural variability by 7T and 3T magnetic resonance imaging: Amygdalo‐hippocampal border at 7T. Human Brain Mapping, 35(9), 4316–4329. 10.1002/hbm.22477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derner, M. , Dehnen, G. , Chaieb, L. , Reber, T. , Borger, V. , Surges, R. , Staresina, B. , Mormann, F. , & Fell, J. (2020). Patterns of single‐neuron activity during associative recognition memory in the human medial temporal lobe. NeuroImage, 221, 117214. 10.1016/j.neuroimage.2020.117214 [DOI] [PubMed] [Google Scholar]
- Douglas, R. J. , & Martin, K. A. (2004). NEURONAL CIRCUITS OF THE NEOCORTEX. Annual Review of Neuroscience, 27(1), 419–451. 10.1146/annurev.neuro.27.070203.144152 [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. , Yonelinas, A. P. , & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30(1), 123–152. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, A. , Suthana, N. , Millett, D. , Fried, I. , & Bookheimer, S. (2009). Correlation between BOLD fMRI and theta‐band local field potentials in the human hippocampal area. Journal of Neurophysiology, 101(5), 2668–2678. 10.1152/jn.91252.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler, T. M. , Ladd, M. E. , & Bitz, A. K. (2018). SAR simulations & safety. NeuroImage, 168, 33–58. 10.1016/j.neuroimage.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Finn, E. S. , Huber, L. , Jangraw, D. C. , Molfese, P. J. , & Bandettini, P. A. (2019). Layer‐dependent activity in human prefrontal cortex during working memory. Nature Neuroscience, 22(10), 1687–1695. 10.1038/s41593-019-0487-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano, A. , Donatelli, G. , Cosottini, M. , Tosetti, M. , Retico, A. , & Fantacci, M. E. (2017). Hippocampal subfields at ultra high field MRI: An overview of segmentation and measurement methods: Segmentation and measurement methods for hippocampal subfields. Hippocampus, 27(5), 481–494. 10.1002/hipo.22717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, M. J. , Ritchey, M. , Wang, S.‐F. , Doss, M. K. , & Ranganath, C. (2016). Post‐learning hippocampal dynamics promote preferential retention of rewarding events. Neuron, 89(5), 1110–1120. 10.1016/j.neuron.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller, T. , & Bartos, M. (2020). Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nature Reviews Neuroscience, 21(3), 153–168. 10.1038/s41583-019-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, B. , Markenroth Bloch, K. , Owman, T. , Nilsson, M. , Lätt, J. , Olsrud, J. , & Björkman‐Burtscher, I. M. (2020). Subjectively reported effects experienced in an actively shielded 7T MRI: A large‐scale study. Journal of Magnetic Resonance Imaging, 52(4), 1265–1276. 10.1002/jmri.27139 [DOI] [PubMed] [Google Scholar]
- Haxby, J. V. , Gobbini, M. I. , Furey, M. L. , Ishai, A. , Schouten, J. L. , & Pietrini, P. (2001). Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science, 293(5539), 2425–2430. 10.1126/science.1063736 [DOI] [PubMed] [Google Scholar]
- Hebscher, M. , Kragel, J. E. , Kahnt, T. , & Voss, J. L. (2021). Enhanced reinstatement of naturalistic event memories due to hippocampal‐network‐targeted stimulation. Current Biology, 31(7), 1428–1437. 10.1016/j.cub.2021.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt, S. A. , Stanek, L. , Chhatwal, J. P. , & Ressler, K. J. (2007). Hippocampus‐specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular Psychiatry, 12(7), 656–670. 10.1038/sj.mp.4001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks, A. D. , D'Agata, F. , Raimondo, L. , Schakel, T. , Geerts, L. , Luijten, P. R. , Klomp, D. W. J. , & Petridou, N. (2020). Pushing functional MRI spatial and temporal resolution further: High‐density receive arrays combined with shot‐selective 2D CAIPIRINHA for 3D echo‐planar imaging at 7 T. NMR in Biomedicine, 33(5), e4281. 10.1002/nbm.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweg, N. A. , Solomon, E. A. , & Kahana, M. J. (2020). Theta oscillations in human memory. Trends in Cognitive Sciences, 24(3), 208–227. 10.1016/j.tics.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts, C. J. , Voets, N. L. , Thomas, A. G. , Clare, S. , Lawrence, A. D. , & Graham, K. S. (2017). Ultra‐high‐field fMRI reveals a role for the subiculum in scene perceptual discrimination. The Journal of Neuroscience, 37(12), 3150–3159. 10.1523/JNEUROSCI.3225-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, L . (2021). Ultra‐high field MRI scanners. Retrieved from https://www.google.com/maps/d/u/1/viewer?hl=en&ll=5.88521768432678%2C0&z=2&mid=1dXG84OZIAOxjsqh3x2tGzWL1bNU
- Huhn, S. , Beyer, F. , Zhang, R. , Lampe, L. , Grothe, J. , Kratzsch, J. , Willenberg, A. , Breitfeld, J. , Kovacs, P. , Stumvoll, M. , Trampel, R. , Bazin, P.‐L. , Villringer, A. , & Witte, A. V. (2018). Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults – A randomized controlled trial. NeuroImage, 174, 177–190. 10.1016/j.neuroimage.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Jacobs, H. I. L. , Müller‐Ehrenberg, L. , Priovoulos, N. , & Roebroeck, A. (2018). Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: A 7T MRI study. Neurobiology of Aging, 69, 167–176. 10.1016/j.neurobiolaging.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Jacobs, H. I. L. , Priovoulos, N. , Poser, B. A. , Pagen, L. H. , Ivanov, D. , Verhey, F. R. , & Uludağ, K. (2020). Dynamic behavior of the locus coeruleus during arousal‐related memory processing in a multi‐modal 7T fMRI paradigm. eLife, 9, e52059. 10.7554/eLife.52059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard, P. , & Clare, S. (1999). Sources of distortion in functional MRI data. Human Brain Mapping, 8(2–3), 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn, S. A. , & Tonegawa, S. (2020). Memory engrams: Recalling the past and imagining the future. Science, 367(6473), eaaw4325. 10.1126/science.aaw4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper, L. , Bollmann, S. , Diaconescu, A. O. , Hutton, C. , Heinzle, J. , Iglesias, S. , Hauser, T. U. , Sebold, M. , Manjaly, Z.‐M. , Pruessmann, K. P. , & Stephan, K. E. (2017). The PhysIO toolbox for modeling physiological noise in fMRI data. Journal of Neuroscience Methods, 276, 56–72. 10.1016/j.jneumeth.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Kerchner, G. A. , Hess, C. P. , Hammond‐Rosenbluth, K. E. , Xu, D. , Rabinovici, G. D. , Kelley, D. A. C. , Vigneron, D. B. , Nelson, S. J. , & Miller, B. L. (2010). Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7‐T MRI. Neurology, 75(15), 1381–1387. 10.1212/WNL.0b013e3181f736a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup, K. B. , Solstad, T. , Brun, V. H. , Hafting, T. , Leutgeb, S. , Witter, M. P. , Moser, E. I. , & Moser, M.‐B. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. 10.1126/science.1157086 [DOI] [PubMed] [Google Scholar]
- Koch, G. E. , Paulus, J. P. , & Coutanche, M. N. (2020). Neural patterns are more similar across individuals during successful memory encoding than during failed memory encoding. Cerebral Cortex, 30(7), 3872–3883. 10.1093/cercor/bhaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, R. , Chadwick, M. J. , Chen, Y. , Berron, D. , Banino, A. , Düzel, E. , Hassabis, D. , & Kumaran, D. (2018). Big‐loop recurrence within the hippocampal system supports integration of information across episodes. Neuron, 99(6), 1342–1354. 10.1016/j.neuron.2018.08.009 [DOI] [PubMed] [Google Scholar]
- Kuehn, E. , & Pleger, B. (2020). Encoding schemes in somatosensation: From micro‐ to meta‐topography. NeuroImage, 223, 117255. 10.1016/j.neuroimage.2020.117255 [DOI] [PubMed] [Google Scholar]
- Ladd, M. E. , Bachert, P. , Meyerspeer, M. , Moser, E. , Nagel, A. M. , Norris, D. G. , Schmitter, S. , Speck, O. , Straub, S. , & Zaiss, M. (2018). Pros and cons of ultra‐high‐field MRI/MRS for human application. Progress in Nuclear Magnetic Resonance Spectroscopy, 109, 1–50. 10.1016/j.pnmrs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Lawrence, S. J. , van Mourik, T. , Kok, P. , Koopmans, P. J. , Norris, D. G. , & de Lange, F. P. (2018). Laminar organization of working memory signals in human visual cortex. Current Biology, 28(21), 3435–3440. 10.1016/j.cub.2018.08.043 [DOI] [PubMed] [Google Scholar]
- Lee, H. , Goodsmith, D. , & Knierim, J. J. (2020). Parallel processing streams in the hippocampus. Current Opinion in Neurobiology, 64, 127–134. 10.1016/j.conb.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. , Wang, C. , Deshmukh, S. S. , & Knierim, J. J. (2015). Neural population evidence of functional heterogeneity along the CA3 transverse Axis: Pattern completion versus pattern separation. Neuron, 87(5), 1093–1105. 10.1016/j.neuron.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, N. K. , Pauls, J. , Augath, M. , Trinath, T. , & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157. 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Maass, A. , Berron, D. , Libby, L. A. , Ranganath, C. , & Düzel, E. (2015). Functional subregions of the human entorhinal cortex. eLife, 4, e06426. 10.7554/eLife.06426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass, A. , Schütze, H. , Speck, O. , Yonelinas, A. , Tempelmann, C. , Heinze, H.‐J. , Berron, D. , Cardenas‐Blanco, A. , Brodersen, K. H. , Enno Stephan, K. , & Düzel, E. (2014). Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nature Communications, 5(1), 5547. 10.1038/ncomms6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A. , Gadian, D. G. , Johnsrude, I. S. , Good, C. D. , Ashburner, J. , Frackowiak, R. S. J. , & Frith, C. D. (2000). Navigation‐related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, 97(8), 4398–4403. 10.1073/pnas.070039597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit, E. , Jamison, K. W. , Weiner, K. S. , Vizioli, L. , Zhang, R.‐Y. , Kay, K. N. , & GrillSpector, K. (2020). Ultra‐high‐resolution fMRI of human ventral temporal cortex reveals differential representation of categories and domains. The Journal of Neuroscience, 40(15), 3008–3024. 10.1523/JNEUROSCI.2106-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, J. P. , & Norris, D. G. (2018). How to choose the right MR sequence for your research question at 7 T and above? NeuroImage, 168, 119–140. 10.1016/j.neuroimage.2017.04.044 [DOI] [PubMed] [Google Scholar]
- Miles, O. W. , & Maren, S. (2019). Role of the Bed Nucleus of the Stria Terminalis in PTSD: Insights from preclinical models. Frontiers in Behavioral Neuroscience, 13, 68. 10.3389/fnbeh.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, J. E. , Yanes, J. A. , Kirby, L. A. , Reid, M. A. , & Robinson, J. L. (2020). Left, right, or bilateral amygdala activation? How effects of smoothing and motion correction on ultra‐high field, high‐resolution functional magnetic resonance imaging (fMRI) data alter inferences. Neuroscience Research, 150, 51–59. 10.1016/j.neures.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau, M. (2019). Functional imaging of the human medial temporal lobe. Open Science Framework. 10.17605/OSF.IO/CQN4Z [DOI]
- Navarro Schröder, T. , Haak, K. V. , Zaragoza Jimenez, N. I. , Beckmann, C. F. , & Doeller, C. F. (2015). Functional topography of the human entorhinal cortex. eLife, 4, e06738. 10.7554/eLife.06738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan, C. R. , Vromen, J. M. , Cheung, A. , & Baumann, O. (2018). Evidence against the detectability of a hippocampal place code using functional magnetic resonance imaging. eNeuro, 5(4), ENEURO.0177‐18.2018. 10.1523/ENEURO.0177-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, D. G. , & Polimeni, J. R. (2019). Laminar (f)MRI: A short history and future prospects. NeuroImage, 197, 643–649. 10.1016/j.neuroimage.2019.04.082 [DOI] [PubMed] [Google Scholar]
- Ogawa, S. , Menon, R. , Tank, D. , Kim, S. , Merkle, H. , Ellermann, J. , & Ugurbil, K. (1993). Functional brain mapping by blood oxygenation level‐dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophysical Journal, 64(3), 803–812. 10.1016/S0006-3495(93)81441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman, C. A. , Davachi, L. , & Inati, S. (2009). Distortion and signal loss in medial temporal lobe. PLoS One, 4(12), e8160. 10.1371/journal.pone.0008160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck, H. P. (2010). Against hyperacuity in brain reading: Spatial smoothing does not hurt multivariate fMRI analyses? NeuroImage, 49(3), 1943–1948. 10.1016/j.neuroimage.2009.02.047 [DOI] [PubMed] [Google Scholar]
- Pedersen, W. S. , Muftuler, L. T. , & Larson, C. L. (2017). Disentangling the effects of novelty, valence and trait anxiety in the bed nucleus of the stria terminalis, amygdala and hippocampus with high resolution 7T fMRI. NeuroImage, 156, 293–301. 10.1016/j.neuroimage.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk, J. , & Moscovitch, M. (2011). A hippocampal marker of recollection memory ability among healthy young adults: Contributions of posterior and anterior segments. Neuron, 72(6), 931–937. 10.1016/j.neuron.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Quiroga, Q. R. (2020). No pattern separation in the human hippocampus. Trends in Cognitive Sciences, 24(12), 994–1007. 10.1016/j.tics.2020.09.012 [DOI] [PubMed] [Google Scholar]
- Rissman, J. , Greely, H. T. , & Wagner, A. D. (2010). Detecting individual memories through the neural decoding of memory states and past experience. Proceedings of the National Academy of Sciences, 107(21), 9849–9854. 10.1073/pnas.1001028107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter, D. L. (2001). Forgotten ideas, neglected pioneers: Richard Semon and the story of memory. Psychology Press. https://psycnet.apa.org/record/2001-01457-000 [Google Scholar]
- Schulz, J. , Siegert, T. , Reimer, E. , Labadie, C. , Maclaren, J. , Herbst, M. , Zaitsev, M. , & Turner, R. (2012). An embedded optical tracking system for motion‐corrected magnetic resonance imaging at 7T. Magnetic Resonance Materials in Physics, Biology and Medicine, 25(6), 443–453. 10.1007/s10334-012-0320-0 [DOI] [PubMed] [Google Scholar]
- Shah, P. , Bassett, D. S. , Wisse, L. E. , Detre, J. A. , Stein, J. M. , Yushkevich, P. A. , Shinohara, R. T. , Pluta, J. B. , Valenciano, E. , Daffner, M. , Wolk, D. A. , Elliott, M. A. , Litt, B. , Davis, K. A. , & Das, S. R. (2018). Mapping the structural and functional network architecture of the medial temporal lobe using 7t MRI. Human Brain Mapping, 39(2), 851–865. 10.1002/hbm.23887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharoh, D. , van Mourik, T. , Bains, L. J. , Segaert, K. , Weber, K. , Hagoort, P. , & Norris, D. G. (2019). Laminar specific fMRI reveals directed interactions in distributed networks during language processing. Proceedings of the National Academy of Sciences, 116(42), 21185–21190. 10.1073/pnas.1907858116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky, R. , Geissberger, N. , Pfabigan, D. M. , Kraus, C. , Tik, M. , Woletz, M. , Paul, K. , Vanicek, T. , Auer, B. , Kranz, G. S. , Lamm, C. , Lanzenberger, R. , & Windischberger, C. (2018). Unsmoothed functional MRI of the human amygdala and bed nucleus of the stria terminalis during processing of emotional faces. NeuroImage, 168, 383–391. 10.1016/j.neuroimage.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Staresina, B. P. , Alink, A. , Kriegeskorte, N. , & Henson, R. N. (2013). Awake reactivation predicts memory in humans. Proceedings of the National Academy of Sciences, 110(52), 21159–21164. 10.1073/pnas.1311989110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana, N. , Ekstrom, A. D. , Yassa, M. A. , & Stark, C. (2021). Pattern separation in the human hippocampus: Response to Quiroga. Trends in Cognitive Sciences, 25(6), 423–424. 10.1016/j.tics.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana, N. A. , Donix, M. , Wozny, D. R. , Bazih, A. , Jones, M. , Heidemann, R. M. , Trampel, R. , Ekstrom, A. D. , Scharf, M. , Knowlton, B. , Turner, R. , & Bookheimer, S. Y. (2015). High‐resolution 7T fMRI of human hippocampal subfields during associative learning. Journal of Cognitive Neuroscience, 27(6), 1194–1206. 10.1162/jocna00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana, N. A. , Parikshak, N. N. , Ekstrom, A. D. , Ison, M. J. , Knowlton, B. J. , Bookheimer, S. Y. , & Fried, I. (2015). Specific responses of human hippocampal neurons are associated with better memory. Proceedings of the National Academy of Sciences, 112(33), 10503–10508. 10.1073/pnas.1423036112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K. Z. , Pevzner, A. , Hamidi, A. B. , Nakazawa, Y. , Graham, J. , & Wiltgen, B. J. (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron, 84(2), 347–354. 10.1016/j.neuron.2014.09.037 [DOI] [PubMed] [Google Scholar]
- Teeuwisse, W. M. , Brink, W. M. , & Webb, A. G. (2012). Quantitative assessment of the effects of high‐permittivity pads in 7 tesla MRI of the brain: Assessment of the effects of high‐permittivity pads. Magnetic Resonance in Medicine, 67(5), 1285–1293. 10.1002/mrm.23108 [DOI] [PubMed] [Google Scholar]
- Teyler, T. J. , & DiScenna, P. (1986). The hippocampal memory indexing theory. Behavioral Neuroscience, 100(2), 147–154. 10.1037/0735-7044.100.2.147 [DOI] [PubMed] [Google Scholar]
- Teyler, T. J. , & Rudy, J. W. (2007). The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus, 17(12), 1158–1169. 10.1002/hipo.20350 [DOI] [PubMed] [Google Scholar]
- Theysohn, J. M. , Maderwald, S. , Kraff, O. , Moenninghoff, C. , Ladd, M. E. , & Ladd, S. C. (2008). Subjective acceptance of 7 Tesla MRI for human imaging. Magnetic Resonance Materials in Physics, Biology and Medicine, 21(1–2), 63–72. 10.1007/s10334-007-0095-x [DOI] [PubMed] [Google Scholar]
- Theysohn, N. , Qin, S. , Maderwald, S. , Poser, B. A. , Theysohn, J. M. , Ladd, M. E. , Norris, D. G. , Gizewski, E. R. , Fernandez, G. , & Tendolkar, I. (2013). Memory‐related hippocampal activity can be measured robustly using fMRI at 7 tesla: Memory‐related hippocampal fMRI at 7 tesla. Journal of Neuroimaging, 23(4), 445–451. 10.1111/jon.12036 [DOI] [PubMed] [Google Scholar]
- Thürling, M. , Hautzel, H. , Küper, M. , Stefanescu, M. , Maderwald, S. , Ladd, M. , & Timmann, D. (2012). Involvement of the cerebellar cortex and nuclei in verbal and visuospatial working memory: A 7T fMRI study. NeuroImage, 62(3), 1537–1550. 10.1016/j.neuroimage.2012.05.037 [DOI] [PubMed] [Google Scholar]
- Tian, Y. , Margulies, D. S. , Breakspear, M. , & Zalesky, A. (2020). Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nature Neuroscience, 23(11), 1421–1432. 10.1038/s41593-020-00711-6 [DOI] [PubMed] [Google Scholar]
- Tik, M. , Sladky, R. , Luft, C. D. B. , Willinger, D. , Hoffmann, A. , Banissy, M. J. , Bhattacharya, J. , & Windischberger, C. (2018). Ultra‐high‐field fMRI insights on insight: Neural correlates of the Aha!‐moment. Human Brain Mapping, 39(8), 3241–3252. 10.1002/hbm.24073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman, E. C. (1948). Cognitive maps in rats and men. Psychological Review, 55(4), 189–208. 10.1037/h0061626 [DOI] [PubMed] [Google Scholar]
- Tonegawa, S. , Liu, X. , Ramirez, S. , & Redondo, R. (2015). Memory engram cells have come of age. Neuron, 87(5), 918–931. 10.1016/j.neuron.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Torrisi, S. , Chen, G. , Glen, D. , Bandettini, P. A. , Baker, C. I. , Reynolds, R. , Yen‐Ting Liu, J. , Leshin, J. , Balderston, N. , Grillon, C. , & Ernst, M. (2018). Statistical power comparisons at 3t and 7t with a GO/NOGO task. NeuroImage, 175, 100–110. 10.1016/j.neuroimage.2018.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil, K. (2018). Imaging at ultrahigh magnetic fields: History, challenges, and solutions. NeuroImage, 168, 7–32. 10.1016/j.neuroimage.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag, W. , Schafer, A. , Marques, J. P. , Turner, R. , & Trampel, R. (2016). Recent applications of UHF‐MRI in the study of human brain function and structure: A review: UHF MRI: Applications to human brain function and structure. NMR in Biomedicine, 29(9), 1274–1288. 10.1002/nbm.3275 [DOI] [PubMed] [Google Scholar]
- van Gemert, J. , Brink, W. , Webb, A. , & Remis, R. (2019). High‐permittivity pad design tool for 7T neuroimaging and 3T body imaging. Magnetic Resonance in Medicine, 81(5), 3370–3378. 10.1002/mrm.27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg, S. M. , Newcombe, N. S. , & Chatterjee, A. (2019). Everyday taxi drivers: Do better navigators have larger hippocampi? Cortex, 115, 280–293. 10.1016/j.cortex.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Zhou, Y. , Liu, J. , Ye, Z. , Yao, L. , Zhang, J. , Chen, C. , & Xue, G. (2020). Individual‐specific and shared representations during episodic memory encoding and retrieval. NeuroImage, 217, 116909. 10.1016/j.neuroimage.2020.116909 [DOI] [PubMed] [Google Scholar]
- Yacoub, E. , Shmuel, A. , Pfeuffer, J. , Van De Moortele, P.‐F. , Adriany, G. , Andersen, P. , Vaughan, J. T. , Merkle, H. , Ugurbil, K. , & Hu, X. (2001). Imaging brain function in humans at 7 Tesla. Magnetic Resonance in Medicine, 45(4), 588–594. 10.1002/mrm.1080 [DOI] [PubMed] [Google Scholar]
- Yang, Q. X. , Mao, W. , Wang, J. , Smith, M. B. , Lei, H. , Zhang, X. , Ugurbil, K. , & Chen, W. (2006). Manipulation of image intensity distribution at 7.0 T: Passive RF shimming and focusing with dielectric materials. Journal of Magnetic Resonance Imaging, 24(1), 197–202. 10.1002/jmri.20603 [DOI] [PubMed] [Google Scholar]
- Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517. 10.1006/jmla.2002.2864 [DOI] [Google Scholar]
- Yoo, P. E. , John, S. E. , Farquharson, S. , Cleary, J. O. , Wong, Y. T. , Ng, A. , Mulcahy, C. B. , Grayden, D. B. , Ordidge, R. J. , Opie, N. L. , O'Brien, T. J. , Oxley, T. J. , & Moffat, B. A. (2018). 7T‐fMRI: Faster temporal resolution yields optimal BOLD sensitivity for functional network imaging specifically at high spatial resolution. NeuroImage, 164, 214–229. 10.1016/j.neuroimage.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Zeidman, P. , & Maguire, E. A. (2016). Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nature Reviews Neuroscience, 17(3), 173–182. 10.1038/nrn.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh, M. M. , Engel, S. A. , & Bookheimer, S. Y. (2000). Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage, 11(6), 668–683. 10.1006/nimg.2000.0561 [DOI] [PubMed] [Google Scholar]
- Zeineh, M. M. , Engel, S. A. , Thompson, P. M. , & Bookheimer, S. Y. (2003). Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science, 299(5606), 577–580. 10.1126/science.1077775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


