ABSTRACT
Bone mineral density (BMD) is an established measure used to diagnose patients with osteoporosis. In clinical trials, change in BMD has been shown to provide a reliable estimate of fracture risk reduction, and achieved BMD T‐score has been shown to reflect the near‐term risk of fracture. We aimed to test the association between BMD T‐score and fracture risk in patients treated for osteoporosis in a real‐world setting. This retrospective, observational cohort study included Swedish females aged ≥55 years who had a total hip BMD measurement at one of three participating clinics. Patients were separated into two cohorts: bisphosphonate‐treated and bisphosphonate‐naïve prior to BMD measurement, stratified by age and prior nonvertebral fracture status. The primary outcome was cumulative incidence of clinical fractures within 24 months of BMD measurement, with other fracture types included as secondary outcomes. Associations between T‐score and fracture risk were estimated using proportional hazards regression and restricted cubic splines. A total of 15,395 patients were analyzed: 11,973 bisphosphonate‐naïve and 3422 bisphosphonate‐treated. In the 24 months following BMD measurement, 6.3% (95% confidence interval [CI], 5.9–6.7) of bisphosphonate‐naïve and 8.4% (95% CI, 7.5–9.4) of bisphosphonate‐treated patients experienced a clinical fracture. Strong inverse relationships between BMD T‐score and fracture incidence were observed in both cohorts. Among bisphosphonate‐naïve patients, this relationship appeared to plateau around T‐score −1.5, indicating smaller marginal reductions in fracture risk above this value; bisphosphonate‐treated patients showed a more consistent marginal change in fracture risk across the evaluated T‐scores (−3.0 to –0.5). Trends remained robust regardless of age and prior fracture status. This real‐world demonstration of a BMD–fracture risk association in both bisphosphonate‐naïve and bisphosphonate‐treated patients extends evidence from clinical trials and recent meta‐regressions supporting the suitability of total hip BMD as a meaningful outcome for the clinical management of patients with osteoporosis. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: DXA, FRACTURE PREVENTION, FRACTURE RISK ASSESSMENT, GENERAL POPULATION STUDIES, OSTEOPOROSIS
Introduction
Osteoporosis is characterized by low bone mass, decreased bone strength, and greater susceptibility to fragility fractures, which are associated with a large economic and societal burden, as well as a substantial direct burden on patients.( 1 , 2 , 3 , 4 ) There is international consensus that the overall goal of osteoporosis treatment is to minimize fracture risk.( 5 , 6 )
Bone mineral density (BMD) is recognized as a key determinant of fracture risk, with BMD accounting for 78% to 92% of whole‐bone strength.( 7 , 8 , 9 ) Change in BMD by dual‐energy X‐ray absorptiometry (DXA) in both treated and untreated patients provides a reliable estimate of fracture risk reduction, and achieved BMD (T‐score) in treated patients in clinical trials has been shown to reflect the risk of near‐term fracture.( 10 , 11 , 12 ) Indeed, earlier analyses have been extended to confirm the relationship at the individual patient level from those clinical trials. Recent meta‐regressions each analyzing 75,000 to 111,183 patients from multiple randomized control trials (RCTs) have demonstrated that total hip, femoral neck, and vertebral BMD improvements are strongly correlated with reduced fracture incidence and may be a useful surrogate for fracture risk.( 13 , 14 , 15 ) As a result, percent change in total hip BMD is being evaluated by the US Food and Drug Administration as a surrogate endpoint for reduced hip and nonvertebral fracture risk in clinical trials.( 16 ) Recent evidence from two large RCTs demonstrate that the higher the total hip BMD T‐score reached with therapy in patients with osteoporosis, the lower the observed fracture incidence; data from these studies also showed a potential plateauing improvement in fracture incidence between −2.0 and −1.5 of total hip BMD T‐scores.( 11 , 12 )
To date, evidence of the BMD–fracture risk relationship in the management of osteoporosis has largely derived from clinical trials, which may not reflect clinical practice. Furthermore, the BMD–fracture risk relationship in patients treated with bisphosphonates (BPs)—the most common therapy for osteoporosis—has received little attention. This raises an important research question, because the application of BMD as a surrogate outcome for fracture risk depends on the relationship being maintained in BP‐treated patients. The maintenance of the relationship in such patients is not inevitable, as the association could be affected by the potential effects of BP treatment on bone structure, strength, and mineralization that may occur independently of BMD change. The objective of this study was (i) to substantiate the relationship between total hip BMD T‐score and fracture risk in a real‐world setting and (ii) to compare the relationship between BMD and fracture risk between patients grouped by recent BP treatment.
Overall, this study aims to provide further evidence that can be used to assess the relevance of total hip BMD T‐score as a surrogate outcome in osteoporosis, contributing to the ongoing debate on the applicability of a treat‐to‐target approach for the management of patients with osteoporosis.
Patients and Methods
Patients and study design
This retrospective, observational cohort study included Swedish females aged ≥55 years with a total hip BMD measurement taken at one of three participating clinics during the patient selection period (Fig. 1). The date of the first BMD measurement was defined as the “index date” and any further BMD measurements were ignored for the purpose of these analyses. Women identified to have Paget's disease or any malignancies during the study period (January 1, 2001–December 31, 2015) were excluded.
Fig. 1.
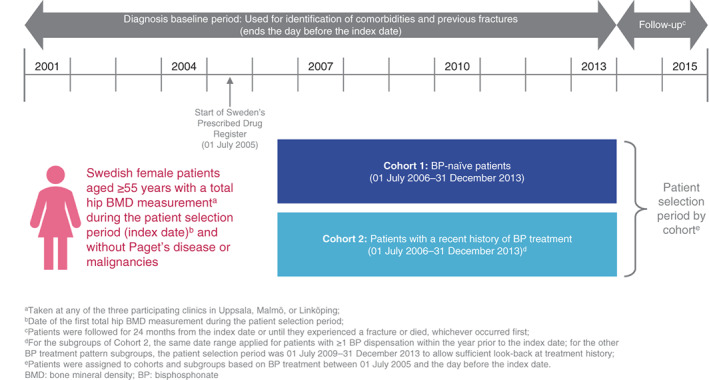
Study design.
Two cohorts were defined for the primary analysis. Cohort 1 comprised patients who had not received BP treatment between July 1, 2005 (inception of treatment records) and the day before the index date (ie, BP‐naïve for a minimum of 12 months before the earliest possible index date), hereafter referred to as “BP‐naïve” patients. Cohort 2 comprised patients who received BP treatment at any point between July 1, 2005 and the day before the index date, hereafter referred to as “BP‐treated”. A list of BP treatments considered in this study can be found in Supplemental Table S1.
For Cohort 2, the potential impact of prior BP treatment patterns was explored further in a secondary analysis; specifically, by stratifying women in Cohort 2 into those who had ≥3 or <3 years of recent (ie, since July 1, 2005 to the day before the index date) BP treatment (medication possession ratio [MPR] ≥ 80%), and into those with ≥1 or no BP dispensation within the year prior to the index date.
The patient selection period for both Cohort 1 and Cohort 2 was July 1, 2006 to December 31, 2013. For the subgroups of Cohort 2, the same date range applied for patients with one or more BP dispensation within the year prior to the index date. For the other BP treatment pattern subgroups of Cohort 2, the patient selection period was July 1, 2009 to December 31, 2013. Selection periods were set to allow for sufficient look‐back to establish eligibility for the respective cohorts and subgroups based on prior treatment. For all groups, the diagnosis baseline period in which patient characteristics, comorbidities, and previous fractures were retrieved was January 1, 2001 through the day before the patient's index date.
Patients were followed for 24 months from the index date or until they experienced a fracture or died, whichever occurred first.
Data sources
Patients were identified from total hip BMD T‐score measurements collected at three participating clinics (Uppsala, Malmö, or Linköping), which were then linked to fracture, treatment, and cause of death data from Swedish national registries governed by the Swedish National Board of Health and Welfare. Unique national person identification numbers based on birth date were used to link data across sources. Fracture codes, diagnoses dates, comorbidities, and other patient characteristics were extracted from Sweden's National Patient Register. Treatment data (drug dispensations) were obtained from Sweden's Prescribed Drug Register; extracted data included patient identification numbers, Anatomical Therapeutic Chemical Classification System (ATC) codes, prescription dates, dispensing dates, defined daily doses per prescribed package, and number of pills/injections, etc. Patients' treatment records could be linked to the other data sources from July 1, 2005 onward, as the national person identification numbers were first implemented in the Prescribed Drug Register on this date. Sweden's Cause of Death Register was used to establish patients' dates of death.
The registries used in this study are known the have a high degree of completeness, and the reporting of many extracted variables is not voluntary in Sweden.( 17 , 18 , 19 ) In cases where information necessary for the analysis (eg, regarding dates, diagnosis codes, or treatment) was absent, the patient was excluded from the study. It was considered highly unlikely that a patient's health care visit or prescription would not have been captured in the registries, although instances of such missing data would not have been possible to identify.
Fracture types assessed
The primary outcome was incidence of any clinical fracture (ie, reported clinical vertebral and nonvertebral fractures) within 0 to 24 months of the index date. Secondary outcomes included any clinical fracture within 0 to 12 months and nonvertebral, vertebral, hip, non‐hip nonvertebral, and major osteoporotic fractures, each within 0 to 12 and 0 to 24 months of the index date. Diagnosis codes for each fracture type are listed in Supplemental Table S2.
Statistical analyses
To examine fracture risk, cumulative incidence of each fracture type was assessed at 24 months (primary analysis) and 12 months (secondary analysis) following the index date, accounting for the competing risk of death for each cohort as a potential confounder. Additionally, sensitivity analyses were conducted to account for age and prior fracture status as potential confounders. Specifically, Cohorts 1 and 2 were stratified by age (≥75 years or <75 years)( 20 ) and prior nonvertebral fracture status (yes or no), and fracture incidence was also assessed within these groups. Stratification by prior nonvertebral fracture follows Ferrari and colleagues( 12 ) and reflects the weaker association between hip BMD T‐score and vertebral fractures, in part due to the underdiagnosis of vertebral fractures.
The association between T‐score and fracture risk was estimated using proportional hazards regression; Pearson's correlation coefficients were also calculated. Because the difference between T‐scores (eg, −2.5 and −1.5, and −2.0 and −1.0) may not carry the same implication in terms of fracture risk,( 12 ) restricted cubic splines were then added to the regression models to investigate and illustrate any potential nonlinear relationship between T‐score and fracture risk. Five knots were used for the splines, with the placement of the knots based on Harrell's recommendation; ie, at percentiles 5, 27.5, 50, 72.5, and 95.( 21 ) All statistical analyses were performed in MySQL (Oracle Corporation, Austin, TX, USA) and STATA 16 (StataCorp LLC, College Station, TX, USA).
Results
Patient characteristics at index date
A total of 15,395 patients were included in this study: Cohort 1 included 11,973 BP‐naïve (BP‐naïve for a minimum of 12 months prior to index date) patients and Cohort 2 included 3422 BP‐treated patients, with a mean age of 68.2 and 71.5 years, respectively (Table 1). Mean ± standard deviation (SD) total hip BMD T‐score was higher in Cohort 1 (−1.4 ± 1.2) compared to Cohort 2 (−1.7 ± 1.1). The proportion of patients with a history of prior nonvertebral fracture was greater in Cohort 1 (4138/11,973; 34.6%) than in Cohort 2 (1012/3422; 29.6%). The mean number of days since the most recent nonvertebral fracture before the index date was lower in Cohort 1 than Cohort 2 (611 versus 1315 days). The proportion of patients using glucocorticoids within the 12 months preceding the index date was lower in Cohort 1 (1374/11,973; 11.5%) than Cohort 2 (864/3422; 25.3%).
Table 1.
Patient Characteristics at Index Date
| Characteristic | Cohort 1: BP‐naïve patients | Cohort 2: BP‐treated patients |
|---|---|---|
| (n = 11,973) a | (n = 3422) | |
| Age (years), mean (SD), IQR | 68.2 (8.5), 13.0 | 71.5 (8.6), 13.0 |
| Age groups, n (%) | – | – |
| <75 years | 9009 (75.2) | 2066 (60.4) |
| ≥75 years | 2964 (24.8) | 1356 (39.6) |
| BMD T‐score, mean (SD), IQR | – | – |
| Total hip | −1.4 (1.2), 1.5 | −1.7 (1.1), 1.4 |
| Lumbar spine b | −1.4 (1.6), 1.9 | −1.7 (1.5), 1.8 |
| Total hip BMD T‐score < −2.5, n (%) | 1874 (15.7) | 772 (22.6) |
| Total hip BMD T‐score range, n (%) | – | – |
| < −4.0 | 122 (1.0) | 61 (1.8) |
| ≥ −4.0 to < −3.5 | 214 (1.8) | 87 (2.5) |
| ≥ −3.5 to < −3.0 | 510 (4.3) | 215 (6.3) |
| ≥ −3.0 to < −2.5 | 1028 (8.6) | 409 (12.0) |
| ≥ −2.5 to < −2.0 | 1660 (13.9) | 588 (17.2) |
| ≥ −2.0 to < −1.5 | 2113 (17.7) | 687 (20.1) |
| ≥ −1.5 to < −1.0 | 2080 (17.4) | 588 (17.2) |
| ≥ −1.0 to < −0.5 | 1750 (14.6) | 382 (11.2) |
| ≥ −0.5 | 2496 (20.9) | 405 (11.8) |
| History of prior fracture, n (%) | – | – |
| Any | 4407 (36.8) | 1176 (34.4) |
| Nonvertebral | 4138 (34.6) | 1012 (29.6) |
| Time since most recent fracture (days), mean (SD), IQR | – | – |
| Any | 586 (867), 619 | 1238 (1046), 1659 |
| Nonvertebral | 611 (886), 681 | 1315 (1057), 1645 |
| Osteoporosis treatment within 12 months before index date, n (%) c | 790 (6.6) | 2810 (82.1) |
| Assisted drug dispensing within 12 months before index date, n (%) d | 313 (2.6) | 98 (2.9) |
| Exposure to drugs that increase the risk of falls within 12 months before index date, n (%) | 8137 (68.0) | 2446 (71.5) |
| Glucocorticoid use per FRAX definition within 12 months before index date, n (%) e | 1374 (11.5) | 864 (25.3) |
| Initiation of BP‐treatment during follow‐up, n (%) | 4188 (35.0) | – |
| Initiation of BP‐treatment during follow‐up before occurrence of first fracture, n (%) | 4090 (34.2) | – |
| Charlson‐Quan comorbidity index score, n (%) | – | – |
| 0 | 8100 (67.7) | 2028 (59.3) |
| 1 | 3523 (29.4) | 1316 (38.5) |
| ≥2 | 350 (2.9) | 78 (2.3) |
Percentages may not sum due to rounding.
BMD = bone mineral density; BP = bisphosphonate; FRAX = Fracture Risk Assessment Tool; IQR = interquartile range; SD = standard deviation.
Patients had not received BP treatment for at least 12 months prior to index date.
n = 11,891 for Cohort 1 and n = 3393 for Cohort 2.
Includes all prior osteoporosis treatments (not exclusively BPs) within the specified timeframe.
Used as a proxy for dependency.
Exposure to ≥450 mg prednisone (or equivalent dose of other glucocorticoids).
Cumulative fracture incidence
The proportion of patients experiencing a clinical fracture within 24 months following the index date was lower in Cohort 1 than Cohort 2 for all fracture types (Table 2), though differences were not significant for vertebral and hip fractures. During the 24 months following the index date, cumulative fracture incidence was 6.3% (95% confidence interval [CI], 5.9–6.7) in Cohort 1 and 8.4% (95% CI, 7.5–9.4) in Cohort 2. Cumulative fracture incidence in Cohort 1 and Cohort 2 at 12 months (Supplemental Table S3) showed similar patterns to those at 24 months. In Cohort 1 (patients who were BP‐naïve at index date), 35.0% (4188) of patients initiated BP treatment during follow‐up.
Table 2.
Cumulative Fracture Incidence and Correlations With Hip BMD T‐Score at 24 Months
| Cohort 1: BPnaïve patients (n = 11,973) a | Cohort 2: BP‐treated patients (n = 3422) | |||||
|---|---|---|---|---|---|---|
| Fracture type | Cumulative fracture incidence | Correlation between cumulative fracture incidence and hip BMD T‐score | Hazard ratio (95% CI) for hip T‐score | Cumulative fracture incidence | Correlation between cumulative fracture incidence and hip BMD T‐score | Hazard ratio (95% CI) for hip T‐score |
| % (95% CI) | (Pearson's correlation coefficient) | % (95% CI) | (Pearson's correlation coefficient) | |||
| Any | 6.29 (5.86–6.73) | −0.92 | 0.63 (0.59–0.67) | 8.39 (7.49–9.35) | −0.94 | 0.69 (0.62–0.77) |
| Nonvertebral | 5.41 (5.02–5.83) | −0.93 | 0.65 (0.61–0.70) | 7.31 (6.47–8.21) | −0.94 | 0.69 (0.61–0.77) |
| Vertebral | 1.02 (0.85–1.21) | −0.79 | 0.50 (0.43–0.58) | 1.34 (1.00–1.77) | −0.92 | 0.68 (0.53–0.88) |
| Hip | 1.20 (1.02–1.41) | −0.72 | 0.45 (0.39–0.52) | 1.40 (1.05–1.84) | −0.61 | 0.45 (0.37–0.55) |
| Nonhip nonvertebral | 4.32 (3.96–4.69) | −0.96 | 0.73 (0.68–0.78) | 6.02 (5.26–6.85) | −0.98 | 0.79 (0.70–0.90) |
| Major osteoporotic | 4.53 (4.16–4.91) | −0.89 | 0.58 (0.54–0.62) | 6.08 (5.31–6.91) | −0.94 | 0.70 (0.62–0.79) |
BMD = bone mineral density; BP = bisphosphonate; CI = confidence interval.
Patients had not received BP treatment for at least 12 months prior to index date.
Relationship between total hip BMD T‐score and fracture risk
Strong inverse relationships between T‐score and fracture incidence were observed in both cohorts at 24 months (Table 2). For any clinical fracture, the hazard ratios (95% CI) associated with a 1‐SD greater T‐score at 24 months were comparable between Cohort 1 (BP‐naïve patients) and Cohort 2 (BP‐treated patients), at 0.63 (95% CI, 0.59–0.67) and 0.69 (95% CI, 0.62–0.77), respectively.
The relationship between BMD T‐score and fracture risk is illustrated over 24 months (Fig. 2). This figure shows that in both cohorts, higher BMD T‐scores were associated with lower incidence of any clinical fracture at 24 months. In Cohort 1 (BP‐naïve patients), this relationship appeared to plateau around a BMD T‐score of –1.5, indicating a smaller marginal reduction in fracture risk with BMD T‐score improvements above this value (Fig. 2A). In Cohort 2 (BP‐treated patients), any change in slope around BMD T‐score −1.5 was less evident, and marginal change in fracture risk appeared to be more consistent across the evaluated range of BMD T‐scores (Fig. 2B).
Fig. 2.
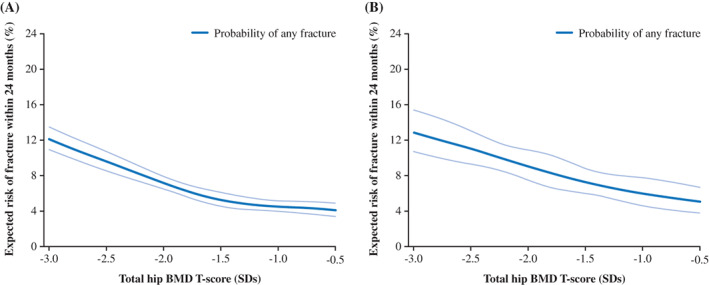
Relationship between total hip T‐score and risk of any clinical fracture at 24 months (%) in (A) Cohort 1 (BP‐naïve patients)a and (B) Cohort 2 (BP‐treated patients). aPatients had not received BP treatment for at least 12 months prior to index date. Pale blue lines show the 95% confidence interval. BMD = bone mineral density; BP = bisphosphonate; SD = standard deviation.
Similar trends were observed at 12 months (Supplemental Table S3; Supplemental Fig. S1). Trends also remained robust in both cohorts when stratified by age (Supplemental Fig. S2) and prior fracture status (Supplemental Fig. S3), as well as among patients with ≥3 years of recent BP treatment and patients with a BP dispensation within the last year (data not shown). Furthermore, the same observations were made when nonvertebral fractures were analyzed separately (data not shown). The remaining two subgroups of Cohort 2 could not be adequately assessed due to the small sample size of patients and fractures in these subgroups (data not shown).
Discussion
In this real‐world study from a large, representative Swedish cohort, higher total hip BMD Tscores were associated with lower incidence of fracture within 12 and 24 months of the BMD measurement. This relationship was observed in both patients with and without recent BP treatment. Furthermore, there was close alignment in 24‐month fracture risk across the range of evaluated total hip BMD T‐scores, irrespective of recent BP treatment status.
Despite the overall similarities between cohorts, a difference was apparent around BMD T‐score −1.5, above which a notable plateauing of fracture risk reduction was observed in the BP‐naïve cohort. In comparison, the BP‐treated cohort showed a weaker change around T‐score −1.5, and more consistent marginal decreases in fracture risk across the range of T‐scores. The difference between the cohorts was also apparent when patients were stratified by age and prior nonvertebral fracture status. The plateauing of fracture risk reduction around BMD T‐score −1.5 reflects comparable observations on nonvertebral fracture risk above T‐scores between −2.0 to −1.5 in women from the Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) and Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) clinical trials.( 11 , 12 ) The cause of these step changes in the BMD–fracture risk relationship are unclear, but they could be linked to the effects of BPs and other treatments on bone structure and strength that may not be captured by BMD.( 22 )
More broadly, the relationship between BMD and fracture risk observed in this study is consistent with findings from recent studies that have assessed the potential value of BMD as a surrogate for fracture risk.( 13 , 14 , 15 ) A meta‐regression by Black and colleagues( 15 ) found that a 2% and 6% improvement in hip BMD was predictive of a 10% and 59% reduction in hip fracture incidence, respectively (p < 0.0001). Moreover, recent reports from two RCTs involving other osteoporosis treatments have demonstrated relationships between BMD and fracture risk very similar to those observed here. In the FREEDOM trial, a relationship between total hip BMD T‐score and nonvertebral fracture in women treated with denosumab was documented.( 12 ) Similarly, in the ARCH trial assessing efficacy of romosozumab versus alendronate in postmenopausal women, higher 12‐month total hip BMD T‐score achieved with both romosozumab and alendronate was associated with lower incidence of nonvertebral fracture over the remainder of the study (p < 0.001).( 11 )
Although the weight of existing evidence strongly supports the beneficial effects of increased BMD on fracture risk, previous analyses have focused on patients enrolled in clinical trials who may not be representative of the wider population of patients with osteoporosis. Clinical trials may also not reflect the quality of BMD measurements in the real world; indeed, the rigorous quality controls that are implemented in BMD assessments within clinical trials may not be followed in clinical practice. These factors brought into question the applicability of the BMD–fracture risk relationship among patients in real‐world settings. The present study fulfilled the goal of addressing this question, demonstrating that the BMD–fracture risk relationship documented in clinical trials is also observed in a real‐world setting. Our findings are supported by recent real‐world evidence from a smaller cohort of patients with osteoporosis in Canada, in which change in total hip BMD also was associated with fracture risk over a longer mean follow‐up period of 12.1 years.( 23 )
Our results support the notion of total hip BMD T‐score as an operational surrogate for fracture risk. Our findings, in combination with existing real‐world evidence demonstrating the benefits of osteoporosis treatments on BMD and fracture risk,( 24 , 25 , 26 , 27 ) also support total hip BMD T‐score as a potentially useful treatment target in the management of patients in a clinical setting.( 6 ) Although several studies have shown that change in BMD from baseline provides useful context for treatment decisions, its use in a treat‐to‐target or goal‐oriented treatment strategy is problematic.( 10 , 12 , 15 ) This is because the relationship between change in BMD and fracture risk reduction is not linear and can be further impacted by therapy or lack of therapy, as well as type and duration of treatment, as evaluated in this study. Importantly, an equal change in BMD results in a different degree of relative and absolute fracture risk reduction for patients with different baseline BMD T‐scores.( 28 ) As a result of these issues, absolute BMD rather than change in BMD remains the most pragmatic measure when making clinical recommendations for individual patients.
Current evidence does not permit the identification of a specific T‐score to be defined for use as a therapeutic target, and this was not the goal of our study. Although it was observed in the BP‐naïve cohort, the plateau in fracture risk reduction with T‐score improvements above −1.5 in this and other studies suggests that treatment aimed at improving BMD score may have the greatest benefit on fracture risk when targeting T‐scores below this value. Further investigation is required to critically test this hypothesis in other patient populations. Regardless of the outcome of future testing, it is important to recognize that a standardized T‐score target value may not be attainable for all patients, highlighting the need for treatment strategy to be personalized to the individual. Applying personalized, goal‐orientated treatment strategies may benefit patient care by improving the assessment of treatment effectiveness and encouraging treatment modifications where appropriate, while also potentially enhancing shared decision making and improving treatment adherence.
This study evaluated a large and highly complete dataset using robust and replicable analyses, utilizing real‐world data to gain insights that are relevant for everyday clinical practice and that may lead to meaningful improvements in patient care. The study benefitted from the assessment of imminent fracture risk at 24 months—a highly clinically relevant outcome.( 29 )
Study limitations included the fact that it was not possible to validate fractures as low‐energy fractures, because data were drawn from real‐world databases. To offset this, only fractures typically associated with osteoporosis were included in our analyses. A second limitation was the likely underreporting of fractures, particularly for vertebral fractures, which often go unnoticed and unrecorded; hence, the observed incidence of fractures is likely to be an underestimate. Additionally, patients' BMD may have changed between the index and fracture dates, hindering the evaluation of the BMD–fracture risk relationship. The homogeneity of the patients included in this study was also a limitation, because Swedish women may not be fully representative of other populations. Finally, another limitation of using a real‐world dataset was that younger patients were overrepresented, possibly because BMD measurements are less common in the management of very old patients in Sweden. Although this study showed that the BMD–fracture risk relationship held across patients stratified by age or prior fracture status, future studies should aim to further explore the effects of these factors on the relationship.
Conclusion
In conclusion, irrespective of recent bisphosphonate treatment, total hip BMD T‐score was associated with fracture incidence over 12 and 24 months, with the relationship maintained across patients stratified by age or prior fracture status. This study provides the first real‐world evidence of the relationship between BMD and short‐term fracture risk, and supports the adequacy of total hip BMD T‐score as a meaningful and relevant clinical outcome in the management of patients with osteoporosis. Further studies in other real‐world populations are required to confirm our findings and test whether the plateauing of fracture risk at T‐scores above −1.5 is indicative of a therapeutic target T‐score value.
Disclosures
JB, ES, GO: Employed by and holders of stock options of Quantify Research, a contract research organization that provides consultancy services for the pharmaceutical industry, including UCB Pharma; JT, AM, CL: Employed by and stockholders of UCB Pharma; KA: Received lecture fees from Amgen Inc., Chugai Pharma, Eli Lilly, and UCB Pharma; AS: Received lecture fees from Amgen Inc., Eli Lilly and Mylan.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4448.
Supporting information
Appendix S1. Supporting information
Acknowledgments
This study was sponsored by UCB Pharma and Amgen Inc. We thank the patients, the investigators, and their teams who took part in this study. We also acknowledge Östen Ljunggren, Uppsala University Hospital, Uppsala, Sweden, for contributions to study conception and design, and contributions to the acquisition, analysis, and interpretation of data. Additionally, we acknowledge Helen Chambers, Costello Medical, Cambridge, UK, for publication coordination, and Kristian Clausen, MPH, and James Evry, MSc, from Costello Medical, Cambridge, UK, for medical writing and editorial assistance based on the authors' input and direction in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authors' roles: Substantial contributions to study conception and design: JB, JT, ES, GO, AM, KA, AS, CL; Substantial contributions to acquisition of data: JB, JT, ES, GO, AM, KA, AS, CL; Substantial contributions to analysis and interpretation of data: JB, JT, ES, GO, AM, KA, AS, CL; Drafting the article or revising it critically for important intellectual content: JB, JT, ES, GO, AM, KA, AS, CL; Final approval of the version of the article to be published: JB, JT, ES, GO, AM, KA, AS, CL. Authors who accessed and verified data: JB, ES, GO.
Data Availability Statement
Data from non‐interventional studies are outside of UCB Pharma's data sharing policy.
References
- 1. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1‐2):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Åkesson K & Mitchell P Capture the fracture: a global campaign to break the fragility fracture cycle. International Osteoporosis Foundation; 2012. Available at http://share.iofbonehealth.org/WOD/2012/report/WOD12-Report.pdf. Accessed August 4, 2021. [DOI] [PMC free article] [PubMed]
- 3. Kerr C, Bottomley C, Shingler S, et al. The importance of physical function to people with osteoporosis. Osteoporos Int. 2017;28(5):1597‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silverman SL, Minshall ME, Shen W, Harper KD, Xie S. The relationship of health‐related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum. 2001;44(11):2611‐2619. [DOI] [PubMed] [Google Scholar]
- 5. Cummings SR, Cosman F, Lewiecki EM, et al. Goal‐directed treatment for osteoporosis: a progress report from the ASBMR‐NOF Working Group on Goal‐Directed Treatment for Osteoporosis. J Bone Miner Res. 2017;32(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 6. Thomas T, Casado E, Geusens P, et al. Is a treat‐to‐target strategy in osteoporosis applicable in clinical practice? Consensus among a panel of European experts. Osteoporos Int. 2020;31(12):2303‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929‐1936. [DOI] [PubMed] [Google Scholar]
- 8. Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033‐1046. [DOI] [PubMed] [Google Scholar]
- 9. Bouxsein ML, Coan BS, Lee SC. Prediction of the strength of the elderly proximal femur by bone mineral density and quantitative ultrasound measurements of the heel and tibia. Bone. 1999;25(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 10. Berger C, Langsetmo L, Joseph L, et al. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24(2):361‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cosman F, Lewiecki EM, Ebeling PR, et al. T‐score as an indicator of fracture risk on therapy: evidence from romosozumab vs alendronate treatment in the ARCH trial. J Bone Miner Res. 2018;33(Suppl 1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T‐score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34(6):1033‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouxsein ML, Eastell R, Lui LY, et al. Change in bone density and reduction in fracture risk: a meta‐regression of published trials. J Bone Miner Res. 2019;34(4):632‐642. [DOI] [PubMed] [Google Scholar]
- 14. Black DM, Bauer DC, Vittinghoff E, et al. Treatment‐related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta‐regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672‐682. [DOI] [PubMed] [Google Scholar]
- 15. Black DM, Vittinghoff E, Eastell R, et al. Hip BMD by DXA can reliably estimate reduction in hip risk in osteoporosis trials: a meta‐regression. J Bone Miner Res. 2015;30(Suppl 1):S49. [Google Scholar]
- 16. Kehoe T. Bone quality: a perspective from the food and drug administration. Curr Osteoporos Rep. 2006;4(2):76‐79. [DOI] [PubMed] [Google Scholar]
- 17. Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer AC, Hedström M, Modig K. The Swedish Hip Fracture Register and National Patient Register were valuable for research on hip fractures: comparison of two registers. J Clin Epidemiol. 2020;125:91‐99. [DOI] [PubMed] [Google Scholar]
- 19. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register ‐ a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464‐469. [DOI] [PubMed] [Google Scholar]
- 20. Bergh C, Wennergren D, Möller M, Brisby H. Fracture incidence in adults in relation to age and gender: a study of 27,169 fractures in the Swedish Fracture Register in a well‐defined catchment area. PLoS One. 2020;15(12):e0244291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 22. Pazianas M, van der Geest S, Miller P. Bisphosphonates and bone quality. Bonekey Rep. 2014;3:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leslie WD, Martineau P, Bryanton M, Lix LM. Which is the preferred site for bone mineral density monitoring as an indicator of treatment‐related anti‐fracture effect in routine clinical practice? A registry‐based cohort study. Osteoporos Int. 2019;30(7):1445‐1453. [DOI] [PubMed] [Google Scholar]
- 24. Berry SD, Dufour AB, Travison TG, et al. Changes in bone mineral density (BMD): a longitudinal study of osteoporosis patients in the real‐world setting. Arch Osteoporos. 2018;13(1):124. [DOI] [PubMed] [Google Scholar]
- 25. Yusuf AA, Cummings SR, Watts NB, et al. Real‐world effectiveness of osteoporosis therapies for fracture reduction in post‐menopausal women. Arch Osteoporos. 2018;13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zullo AR, Lee Y, Lary C, Daiello LA, Kiel DP, Berry SD. Comparative effectiveness of denosumab, teriparatide, and zoledronic acid among frail older adults: a retrospective cohort study. Osteoporos Int. 2021;32(3):565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zullo AR, Zhang T, Lee Y, et al. Effect of bisphosphonates on fracture outcomes among frail older adults. J Am Geriatr Soc. 2019;67(4):768‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tucker G, Metcalfe A, Pearce C, et al. The importance of calculating absolute rather than relative fracture risk. Bone. 2007;41(6):937‐941. [DOI] [PubMed] [Google Scholar]
- 29. Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28(6):1765‐1769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
Data from non‐interventional studies are outside of UCB Pharma's data sharing policy.


