Abstract
Afferent lymphatics mediate the transport of antigen and leukocytes, especially of dendritic cells (DCs) and T cells, from peripheral tissues to draining lymph nodes (dLNs). As such they play important roles in the induction and regulation of adaptive immunity. Over the past 15 years, great advances in our understanding of leukocyte trafficking through afferent lymphatics have been made through time‐lapse imaging studies performed in tissue explants and in vivo, allowing to visualize this process with cellular resolution. Intravital imaging has revealed that intralymphatic leukocytes continue to actively migrate once they have entered into lymphatic capillaries, as a consequence of the low flow conditions present in this compartment. In fact, leukocytes spend considerable time migrating, patrolling and interacting with the lymphatic endothelium or with other intralymphatic leukocytes within lymphatic capillaries. Cells typically only start to detach once they arrive in downstream‐located collecting vessels, where vessel contractions contribute to enhanced lymph flow. In this review, we will introduce the biology of afferent lymphatic vessels and report on the presumed significance of DC and T cell migration via this route. We will specifically highlight how time‐lapse imaging has contributed to the current model of lymphatic trafficking and the emerging notion that ‐ besides transport – lymphatic capillaries exert additional roles in immune modulation.
Keywords: afferent lymphatics, dendritic cells, ex vivo imaging, in vivo imaging, lymphatic endothelial cells, migration, T cells, Trafficking
1. INTRODUCTION
To fulfill their function of immune defense and surveillance, most leukocytes are not stationary positioned in the body but constantly migrate within tissues or circulate between tissues and organs. 1 , 2 Considering that active interstital migration is very slow, leukocytes use blood and lymphatic vessels to rapidly move between different sites of the body. Over the past decades, the process of immune cell migration out of blood vessels has been studied in great detail, culminating in the identification of the intravascular adhesion cascade and a plethora of molecules involved in leukocyte extravasation from different blood vascular beds in steady‐state and in inflammation. 3 , 4 , 5 More recent technologic advances in intravital imaging, particularly the use of confocal and two‐photon microscopy, have opened up new ways of studying leukocyte interstitial migration and immune function in tissues like the skin, lymph nodes (LNs) or lung. 2 , 6 , 7 Compared to leukocyte extravasation from blood vessels, migration through lymphatic vessels is much less well explored. This is likely due to the fact that the lymphatic vasculature as a whole has been far less well studied in comparison to the blood vasculature. In fact, the first markers allowing to faithfully distinguish blood vessels from lymphatic vessels only emerged a bit more than 20 years ago. Ever since research on lymphatic biology has exploded and there is a growing interest in this vessel type and its involvement in health and disease. 8 , 9 , 10 Considering that one of the main functions of lymphatic vessels consists in mediating the migration and transport of leukocytes, it is not surprising that recent years have witnessed great advances in our knowledge of lymphatic trafficking. Besides deciphering the mechanisms of lymphocyte egress from LNs into efferent lymphatics, we have also gained a good mechanistic understanding of how dendritic cells (DCs) and T cells – that is, the main cell types found in afferent lymph ‐ migrate into and within afferent lymphatic vessels. In this review, we will report on how imaging has contributed to deciphering the cellular and molecular determinants of leukocyte trafficking through afferent lymphatics. We will first introduce the biology of afferent lymphatics and report on the importance of DC and T cell migration for immune function. Next, we will focus on selected recent discoveries in the field of DC and T cell trafficking via afferent lymphatics that have been achieved by time‐lapse imaging and discuss their presumed relevance for the induction and regulation of the immune response.
2. STRUCTURE OF THE AFFERENT LYMPHATIC VASCULATURE IN THE SKIN
The lymphatic system is composed of primary and secondary lymphoid organs (SLOs) and lymphatic vessels. The lymphatic vasculature comprises a hierarchically organized network of vessels that can be subdivided into three subgroups, based on their localization in tissues; afferent lymphatics, which connect peripheral tissues with draining LNs (dLNs), the lymphatic vasculature present within LNs and the efferent lymphatics, which exit from LNs. 8 , 9 , 10 Similarly to blood vessels, afferent lymphatic vessels are present in virtually all tissues of the body. They originate as blind‐ended structures, so called capillaries, which merge into a second type of vascular bed, that is, the collecting vessels. The latter subsequently fuse into even larger collectors which leave the tissue and connect with the subcapsular sinus of dLNs. Within the LN, the various LN sinuses are lined by different types of lymphatic endothelial cells (LECs) with distinct gene expression patterns and function. 11 Typically, one efferent lymphatic vessel leaves the LN and connects it to a further downstream‐located LN. As LNs are frequently arranged in chains, the efferent lymphatic exiting from one LN can at the same time be the afferent collecting vessel of another LN located further downstream in the chain. Notably, in this review we will exclusively mean lymphatic vessels originating in peripheral tissues when talking about “afferent lymphatics”. Ultimately, larger efferent collectors converge in the central region of the body to form the thoracic and lymphatic ducts. The latter fuse with the subclavian veins and release the lymphatic content – that is, a cell‐, protein‐ and lipid‐rich fluid commonly referred to as lymph ‐ into the blood circulation.
Afferent lymphatic vessels play a crucial role in the regulation of interstitial fluid homeostasis, as they return excess tissue fluid that has leaked out of blood vessels back to the circulation. Moreover, they are important for the uptake of dietary fats and vitamins from the intestine. 8 , 9 Afferent lymphatics further transport soluble antigen, inflammatory mediators as well as leukocytes from peripheral tissues to dLNs, establishing their crucial role in adaptive immunity. Much of our current knowledge of lymphatic morphology has been gained from old electron microscopy studies 12 , 13 and more recently from the analysis of tissue whole‐mounts prepared from murine skin or trachea by confocal microscopy. 14 , 15 The latter studies have revealed that LECs present in lymphatic capillaries have a characteristic oakleaf shape 14 (Figure 1). Peculiarly, capillary LECs partially overlap and are only loosely connected by button‐like cell‐cell junctions at the sites of such overlaps, thereby generating characteristic overhanging flaps. These flaps, also called primary valves, were first identified as the sites of leukocyte entry into lymphatic capillaries by electron microscopy. 14 Originally described in the murine skin and trachea, 14 the oakleaf shape of capillary LECs and button‐like cell‐cell junctions of afferent lymphatic capillaries have meanwhile been confirmed in many other murine tissues and also in human tissues like the skin. 16 , 17 Capillary LECs are further connected to the extracellular matrix (ECM) by anchoring filaments that force the vessel to expand and the flaps to open and fluid to enter when pressure increases, for example, during tissue inflammation 18 , 19 (Figure 1). Lymphatic capillaries are also surrounded by a discontinuous thin basement membrane (BM), which is composed of layers of a specialized sheet‐like ECM, that forms the supporting structure of the endothelial cells. The lymphatic BM of both capillaries and collectors is primarily composed of laminin, type IV collagen, nidogens, and perlecan. 15
FIGURE 1.
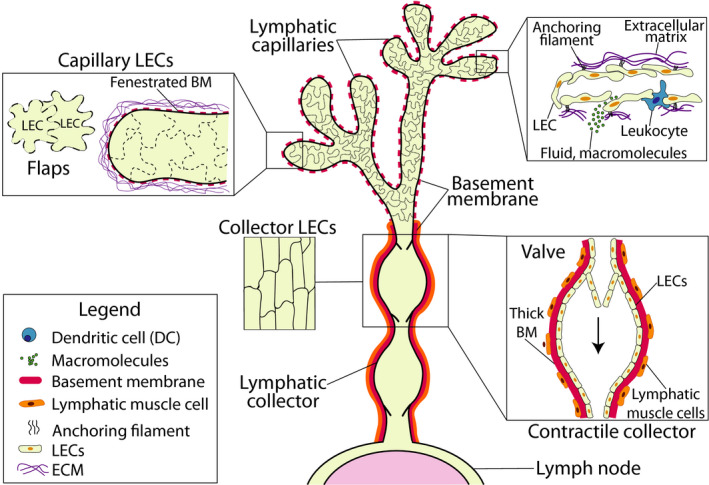
Morphology and structure of afferent lymphatics. Afferent lymphatics begin as blind‐ended capillaries present in peripheral tissues. Capillaries are composed of oakleaf‐shaped LECs that are connected by discontinuous button‐like cell‐cell junctions. These junctions create open flaps between neighboring LECs that facilitate leukocyte entry and uptake of fluids and macromolecules. LECs also possess anchoring filaments that connect to the ECM and regulate the opening of the flaps. Capillaries are further surrounded by a thin and fenestrated BM. Lymphatic capillaries subsequently merge into collecting vessels. In contrast to the capillaries, LECs in collecting vessels are elongated and tightly connected to each other by zipper‐like cell‐cell junctions. Collectors are surrounded by a thick BM as well as LMCs. Collectors further have valves, which upon vessel contraction support unidirectional fluid flow toward the dLN
Conversely, LECs in collecting vessels, that is, the more downstream‐located vessel segments, display an elongated shape and are surrounded by continuous, zipper‐like cell‐cell junctions, resembling the junctional setup of blood vessels (Figure 1). Due to the LEC‐surrounding tight junctions, collecting vessels are much less permeable compared to capillaries and hence more suited for fluid containment and transport. Collectors are also surrounded by a similar yet in comparison to capillaries thicker and less fenestrated BM and by lymphatic muscle cells (LMCs), which confer contractility. 20 Similar to veins, lymphatic collectors also contain valves that prevent fluid backflow upon vessel contraction and support lymph transport 14 , 21 (Figure 1). Because of the tree‐like, hierarchical organization of the lymphatic network, with many capillaries merging into few collecting vessels and vessel contractions occurring in lymphatic collectors, also the lymph flow is markedly increased in this segment of the lymphatic vascular bed: While lymph flow in capillaries ranges from 1 to 30 μm/s, 22 , 23 velocities of up to several mm/s can be reached in larger collectors as, for example, in the mesentery. 24 , 25
3. LYMPHATIC MARKERS
The discovery of different lymphatic markers approximately 20 years ago has propelled lymphatic research and also facilitated the development of different molecular imaging approaches. These markers nowadays allow to unambiguously distinguish lymphatics from blood vessels or to distinguish between lymphatic capillaries and collectors. All LECs express the prospero‐related homeobox 1 (Prox1), a master transcriptional regulator of lymphatic differentiation and identity and the receptor tyrosine kinase vascular endothelial growth factor (VEGF) receptor–3 (VEGFR‐3) the receptor of the lymphatic growth factor VEGF‐C. While podoplanin (also known as gp38), a mucin‐type transmembrane protein, is also expressed by all LECs, the hyaluronan receptor LYVE‐1 is only expressed by lymphatic capillaries (reviewed in 8 , 9 ). Moreover, the chemokine CCL21, which is the main attractant of leukocytes expressing the CCR7 chemokine receptor into afferent lymphatics, 26 is abundantly expressed in capillaries and displays a mosaic expression pattern in downstream collectors. 27 , 28 Over the past 15 years, various gene‐targeted mouse strains with lymphatic‐specific expression of Cre‐recombinase (eg, 29 , 30 , 31 ) or fluorescent proteins have been generated, what has greatly facilitated time‐lapse imaging of leukocyte migration into or within afferent lymphatic (Table 1). However, it is important to note that none of the described markers are exclusively expressed in lymphatics; for example, Prox‐1 is also expressed in the liver 32 and in certain muscle cells, 33 LYVE‐1 by liver sinusoidal endothelial cells 34 and certain macrophages, 35 and podoplanin by various stromal cells, 36 what may limit the applicability of reporter mice in certain tissue.
TABLE 1.
Lymphatic reporter mice and their use to study trafficking through afferent lymphatics
| Reporter mice | First report of mouse line(s) | Used to study trafficking through afferent lymphatics |
|---|---|---|
| Prox1‐GFP | 119 | 70, 88 |
| Prox1‐mOrange 2 | 120 | 88, 115 |
| Prox1‐tdTomato | 121 | No report |
| Prox1‐CreERT2 tdTomato | 31, 122 | 122 |
| Prox1‐mOrange 2 × CD11c‐YFP | 120, 123 | 115 |
| Prox1‐GFP × hCD2‐DsRed | 119, 124 | 70, 88 |
| VEGFR3‐tdTomato | 125 | No report |
| VE‐cadherin‐Cre × Rosa‐RFP | 126, 127 | 86, 88 |
| Podoplanin GFP‐Cre+ | 128 | No report |
| Lyve1 GFP‐Cre+ | 129 | No report |
| Lyve1‐CreERT2‐tdTomato | 130, 131 | No report |
4. CELL POPULATIONS MIGRATING THROUGH AFFERENT LYMPHATICS
Cannulation studies, mostly performed 20–50 years ago in larger animals like sheep and also in humans, have revealed that the prevailing cell population in afferent lymph in steady‐state conditions is T lymphocytes (80%–90%), specifically antigen‐experienced CD4+ effector memory T cells (TEMs). 37 , 38 , 39 , 40 Contrary, naive T cells, which migrate mainly between blood and SLOs, are found in low numbers in afferent lymph, since they are typically not equipped with homing molecules needed for extravasation into peripheral tissues. DCs, the major antigen‐presenting cells, constitute the second most common cell type in afferent lymph (5%–15%). Other cell types such as monocytes and granulocytes or B cells, are present in the lymph but in lower percentages (1%–10%) and rather in the context of inflammatory responses. 37 , 38 , 41 , 42 Furthermore, during inflammation, cell migration into afferent lymphatics is substantially increased. 39 Interestingly, neutrophils are the first cell type to migrate via afferent lymphatics to dLNs during an inflammatory stimulus. 43
Considering the difficulty of cannulating afferent lymphatics in smaller animals, particularly in rodents, only few cannulation experiments performed in mice or rats have been reported. 44 , 45 Lately, the use of transgenic mice expressing the photoconvertible Kaede or Kikume proteins in all body cells has provided further insights into endogenous lymphatic migration in mice. 46 , 47 Illumination of a tissue or organ with violet light induces the conversion of cells from a green‐ to a red‐fluorescent state, allowing to identify leukocytes that have emigrated from this site via afferent lymphatics in dLNs by flow cytometry. Interestingly, photoconversion experiments have revealed that under inflammatory conditions the major fraction of T cells emigrating via afferent lymphatics to dLNs are regulatory T cells (Treg). 39 , 48 , 49 However, since Treg where not yet known/analyzed for at the time when most cannulation experiments in sheep or humans were performed, this finding still warrants confirmation in larger animals and humans. Also, considering that laboratory mice comprise a much smaller pool of TEM as compared to feral mice or humans, 50 the percentage to Treg migrating in humans is likely to be smaller.
5. PRESUMED SIGNIFICANCE OF MIGRATION THROUGH AFFERENT LYMPHATICS
Although cannulation studies have indicated that DCs are less frequent in afferent lymph than T cells, the importance of their migration has been best studied. 26 DCs are professional antigen‐presenting cells (APCs) with essential roles in initiation and regulation of adaptive immunity. In peripheral tissues DCs continuously sample their environment and migrate via lymphatics to the first dLN to present peptides derived from ingested proteins on MHC molecules. In absence of an infection, the peptide pool presented will be derived from self‐proteins, and DCs will be rather immature, that is, expressing low levels of co‐stimulatory molecules. This will prevent potentially self‐reactive cognate T cells from being activated but rather induce a state of anergy or tolerance. Consequently, DC migration via afferent lymphatics is considered essential for the maintenance of tolerance. On the other hand, a recent study showed that even in germ‐free mice, where most DC migration would be expected to occur under steady‐state conditions, T cell activation in dLNs is still observed but suppressed by co‐localizing Tregs. 51 This illustrates that induction of anergy and tolerance by immature migratory DCs is likely not sufficient for preventing T cell activation across the entire T cell repertoire, highlighting the importance of additional pathways in maintaining peripheral tolerance. Conversely, in the context of infection or inflammation, DCs sense pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs), leading to their maturation and enhanced migration to dLNs and effective priming of cognate T cell responses. 26 Thus, DC migration from peripheral tissues via afferent lymphatics is considered essential for both induction of adaptive immunity and maintenance of tolerance.
In contrast to DC migration, the functional significance of T cell migration via afferent lymphatics is less clear. During an inflammatory response, for example, caused by an infection, many effector cells (Teff) are typically recruited to the affected tissue. 52 Several studies support the notion that retention of Teff at the site of infection occurs in an antigen‐specific manner: when encountering a cognate antigen in the context of a viral lung infection, CD4+ Teff have been found to loose residual expression of CCR7, making them less likely to emigrate via CCL21‐expressing lymphatics. 53 By contrast, unspecific Teff are thought to retain low levels of CCR7, allowing them to sense the chemokine gradient from afferent lymphatics and exit the tissue via this route. 54 Similar results were obtained in a delayed‐type hypersensitivity (DTH) response induced in murine skin, where CCR7‐deficiency in Teff diminished tissue egress and enhanced inflammation. 55 Modulation of CCR7 expression in Teff was also shown to impact the strength of the inflammatory response in a murine model of Morbus Crohn: adoptive transfer of CCR7‐deficient CD4+ Teff or blockade of CCR7 with antibodies exacerbated the disease. 56 Conversely, overexpression of CCR7 increased CD4+ Teff egress from inflamed skin, thereby accelerating resolution of inflammation. 56 The regulation of CCR7 expression and tissue exit of unspecific Teff via afferent lymphatic have therefore been brought forward as a mechanism for preventing overshooting tissue inflammation and immune‐mediated pathology.
The notion that T cells exiting from inflamed tissues via afferent lymphatics might serve to dampen immune‐mediated tissue inflammation is also supported by recent findings regarding Treg migration. As mentioned, in mice Treg have been identified as the major T cell subset exiting inflamed tissues. 39 , 48 , 49 In the case of the skin and colon, it was shown that Treg that have emigrated from these sites to dLNs via afferent LVs display a more suppressive phenotype and exert stronger suppressive activity than Treg that have entered the LN via high endothelial venules (HEVs). 39 , 48 , 49 Similarly, different studies in tissue allograft models showed that Treg migration via afferent lymphatics to dLNs is needed for an efficient downregulation of the adaptive immune response against the allograft. 57 , 58 , 59 Interestingly, mice lacking afferent lymphatics were shown to display multiple signs of autoimmunity, what might be a consequence of missing migration of either immature DCs in steady‐state or of Treg in inflammation. 60 However, considering that—in contrast to mice ‐ the presence and abundance of Treg have thus far not been studied/reported in human afferent lymph, the proof of the potential human relevance of these findings is still missing.
Finally, recirculation of memory T cells through peripheral tissues and their tissue exit via afferent lymphatics is thought to generally contribute to immune surveillance. In addition to tissue‐resident memory T cells (TRM) also recirculating memory T cell (TRCM) subsets have recently been identified. 61 , 62 , 63 TRCM have been identified in mice 62 and humans, where at least two different subsets were discovered. 64 As all other skin‐exiting T cells, TRCM express CCR7 and use this receptor for migration to dLNs, form where they continue to recirculate via the blood to distal tissues. In contrast, CCR7‐/‐ TRM do not migrate but remain in the tissue where they provide local and immediate protection. Several recent studies have indicated that TRM delay pathogen spread and at the same time serve to “sound the alarm” for the recruitment of TRCM. 61 However, in many experimental models of viral or bacterial infection, TRM suffice for immune protection. 61 , 63 Thus, the exact role of TRCM in immune surveillance and pathogen clearance is not fully understood at present.
6. INSIGHTS INTO LYMPHATIC MIGRATION GAINED FROM MICROSCOPY
Over the past 20 years, in vivo techniques, such as FITC painting, adoptive transfer studies and experiments in photoconvertible mice (discussed in 65 ) have greatly contributed to deciphering the molecular mechanism of lymphatic migration. One downside of these approaches is that they do not allow to dissect the particular anatomic location where a molecule contributes to migration, or how the cellular migration pattern differs at different sites in the tissue or in the vasculature. Contrary to the techniques mentioned above, microscopy allows to study immune cell migration at the single‐cell level. Particularly time‐lapse imaging approaches have therefore greatly contributed to deciphering the mechanism of cell entry into afferent lymphatics and the intralymphatic behavior of cells, revealing that lymphatic migration occurs in a stepwise manner (Figure 2). The entire process, and in particular the insights gained from imaging, shall be described in greater detail in the following parts.
FIGURE 2.
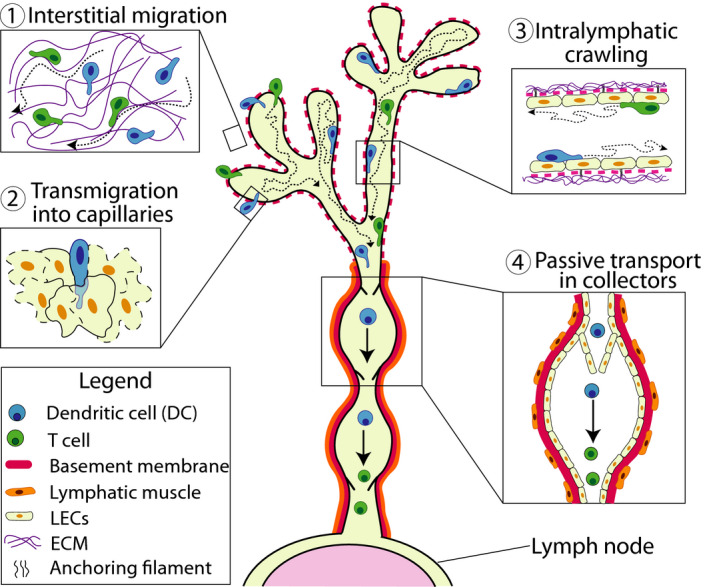
Lymphatic migration occurs in distinct steps. Leukocytes migrate in a stepwise manner from peripheral tissues via afferent lymphatics to dLNs. (1) Interstitial migration: Leukocytes squeeze and migrate through the interstitial ECM. In vicinity of the lymphatic capillaries, they start to sense the peri‐lymphatic CCL21 gradient and begin to directedly migrate toward the vessels. (2) Transmigration into capillaries: Near capillaries, leukocytes squeeze through the thin and discontinuous BM and subsequently transmigrate into the capillary lumen through the open flaps generated between neighboring LECs. (3) Intralymphatic crawling: Once inside the capillaries, leukocytes continue to actively crawl on capillary LECs and patrol the capillary lumen. (4) Passive transport in collectors: Within collecting vessel segments, lymph flow is elevated due to vessel contractions. Once leukocytes have reached this compartment, they start to detach, roll and flow, resulting in rapid transport to the dLN
6.1. Leukocyte entry into lymphatic capillaries
A breakthrough in our understanding of lymphatic migration was achieved in 2008 by the group of Michael Sixt, who introduced a new, nowadays widely used model to image DC entry into lymphatic capillaries in murine ear skin explants 66 (Figure 3A). It involves ripping murine ears along the central cartilage to expose the dermal aspects of the dorsal and ventral ear skin, in which lymphatic capillaries can be readily visualized by staining with fluorescent anti‐LYVE‐1 antibodies. When adding fluorescently labelled DCs onto the explants, DCs rapidly adhere and migrate into the tissue, allowing to visualize the lymphatic entry process by time‐lapse imaging. This first report revealed that added DCs rapidly (within 30–60 min) approached and entered into afferent lymphatic capillaries. 66 By simultaneously staining for BM components like laminin or collagen IV, the Sixt lab further reported that the capillary‐surrounding BM is discontinuous and contains preformed pores through which DCs squeeze during their approach of the lymphatic endothelium. 15 Real‐time imaging also confirmed that entry into the capillary lumen involved transmigration through the endothelial flaps formed by neighboring oakleaf‐shaped LECs, 15 in line with what had been hypothesized based on electron microscopy. 14
FIGURE 3.
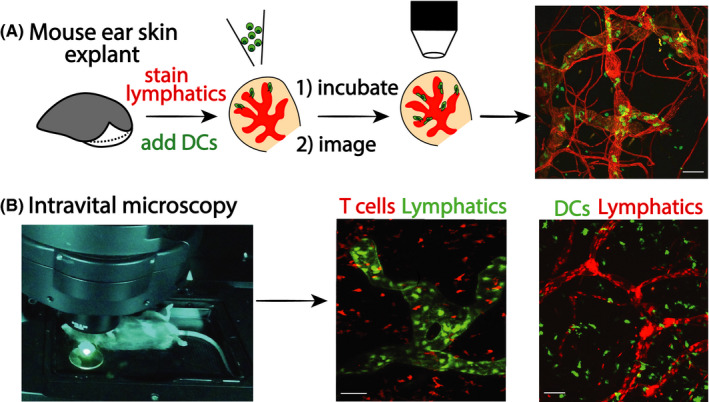
Techniques for time‐lapse imaging of leukocyte migration into and within afferent lymphatics. (A) Mouse ear skin explant model (crawl in assay). In this widely used preparation, mouse ears are ripped along the cartilage to expose the dermal tissue aspects. Lymphatics are rapidly stained by fluorescent antibodies (unless using ears of fluorescent lymphatic reporter mice). Subsequently, fluorescent leukocytes (eg, DCs) are added and left to crawl into the tissue. After washing off cells that have not entered the tissue, leukocytes are followed by time‐lapse imaging as they approach, enter or crawl within afferent lymphatics. (B) Intravital microscopy (IVM). This image technique allows us to visualize cell dynamics and cell behavior in vivo in anesthetized mice by multiphoton or confocal microscopy. Commonly used sites for imaging are the murine ear skin (depicted), footpad or cremaster muscle. IVM typically requires the use of reporter mice with fluorescent lymphatics and leukocytes, particularly for imaging endogenous leukocytes. Examples shown are from Prox1‐GFP × CD2‐Dsred mice (green lymphatics, red‐fluorescent T cells 70 ) and from Prox1‐mOrange2 × CD11c‐YFP mice (red lymphatics and yellow DCs, 115 )
6.2. Integrin requirement of lymphatic migration
Using time‐lapse imaging in ear skin explants and adoptive transfer experiments the lab of Michael Sixt further demonstrated that DCs do not require integrins for lymphatic migration in steady‐state. 66 This finding was rather surprising at the time, considering that leukocyte extravasation from blood vessels is known to be integrin dependent. The reason for this discrepancy likely is that the integrin ligands ICAM‐1 and VCAM‐1, which also mediate leukocyte extravasation from blood vessels, are much less expressed in LECs than in blood vascular endothelial cells (BECs) in steady‐state and are only upregulated under inflammatory conditions. 67 , 68 Consequently, leukocyte migration through inflamed afferent lymphatics also becomes integrin dependent, as evidence by adoptive transfer and FITC painting experiments. 68 , 69 , 70 Besides DCs, the integrin dependency of leukocyte migration from inflamed tissue to dLNs was also confirmed for T cells as well as for neutrophils. 70 , 71
6.3. CCL21 directs leukocytes toward lymphatic vessels
The dermal ear skin model also generated new insights on how interstitial DCs sense and approach lymphatic capillaries. As previously mentioned, the chemokine CCL21 is constitutively expressed by LECs 27 , 28 , 72 whereas its corresponding chemokine receptor CCR7 is induced in maturing DCs 73 and also expressed by the T cell subsets exiting from peripheral tissues. Several studies have identified the CCL21/CCR7 axis as the most important determinant of DC and T cell exit into afferent lymphatics. 26 , 62 , 74
CCL21 contains a highly positively charged C‐terminus, which confers binding to glycosaminoglycan‐containing heparan sulfates (HS‐GAGs) present on the surface of LECs and other cell types. 75 , 76 However, also other negatively charged molecules, including LEC‐expressed podoplanin 77 and BM/ECM components have been shown or suggested to bind CCL21. 78 , 79 Confocal imaging performed in ear skin revealed that most CCL21 is stored intracellularly in the trans‐Golgi network. 72 , 80 Secreted CCL21 gives rise to a peri‐lymphatic gradient that decreases toward the interstitium. As revealed by time‐lapse imaging in the ear skin model, DCs sense the peri‐lymphatic CCL21 gradient starting from approximately 90 μm distance to the vessel wall. 72 Surprisingly, a recent study revealed that LEC‐expressed HS is not necessary for the formation of the CCL21 gradient, as its loss resulted in an only modest decrease in CCL21 co‐localizing with lymphatic capillaries and did not impact DC migration toward capillaries. 78 These results suggest that other negatively charged molecules in the LEC glycocalyx or in the LEC‐surrounding BM or ECM are the major site of CCL21 anchoring. Adding to the complexity of CCL21 gradient formation, a recent study demonstrated that CCL21 in peripheral tissues exists in a full‐length and a more soluble, cleaved version lacking the positively charged C terminus. 81
The availability of extracellular CCL21 and the resulting CCL21 gradient appear to be tightly regulated: Under inflammatory conditions, for example, upon stimulation with TNFα, the release of CCL21 from LECs is increased. 80 On the other hand, DCs interacting with capillary LECs induce Ca2+ signaling in LECs and trigger the release of intracellular CCL21, thereby depositing a CCL21 track for other migratory DCs. 82 Finally, the formation of the extracellular CCL21 gradient is reportedly also regulated by the atypical chemokine receptor 4 (ACKR4), which in the skin is expressed by keratinocytes but also by some fibroblast and LECs. 81 , 83 ACKR4 scavenges CCL21 as well as CCL19, the second CCR7 ligand, which is upregulated in maturing DCs. In absence of ACKR4, DC migration from skin to dLNs was reduced. 81 , 83 Moreover, quantitative DC crawl in experiments in murine ear skin explant and intravital imaging in the footpad revealed that in absence of ACKR4 less DCs entered into dermal lymphatic capillaries, likely because of saturation of the immobilized CCL21 gradient. 81
6.4. Leukocytes actively crawl within lymphatic capillaries and flow in collectors
In addition to time‐lapse imaging in explants, also intravital microscopy (IVM) has provided valuable insights into the cellular and molecular determinants of lymphatic migration (Figure 3B, Table 1). Imaging either in the murine footpad 84 , 85 or ear skin 86 these initial studies confirmed that also in vivo DCs enter into LYVE‐1+ lymphatic capillaries. Additionally, they revealed that DCs within lymphatic capillaries were actively crawling at average velocities ranging from 2 to 12 μm/min. 84 , 85 , 86 This observation was rather surprising at the time, since in analogy to blood vessels, where leukocytes are typically transported passively with the blood flow, it was initially assumed that leukocytes would immediately detach and flow upon entry into afferent lymphatics. However, it needs to be remembered that the flow in lymphatic capillaries is several orders of magnitude lower than in blood vessels 22 , 23 likely explaining why leukocytes do not readily flow in lymphatic capillaries. Particularly the work of our group further revealed that DCs in capillaries not only crawled, but rather displayed a patrolling behavior; that is, they frequently turned and also migrated in opposite direction of drainage. 27 , 86 On the other hand, once reaching the vessel segments located deeper in the tissue, which likely were the collectors, DCs detached and were passively and rapidly transported with the lymph flow. 85 , 86 In analogy to the reports on DCs, we also found T cells to actively crawl in lymphatic capillaries ‐ at average speeds ranging from 4 μm/min in steady state to 12 μm/min in inflammation ‐ and to start rolling and flowing once they arrived in contracting lymphatic collectors. 70 Imaging in the ear skin, our group observed T cells rolling along contracting dermal collectors at a speed of approx. 300 μm/min. By contrast, in larger collecting vessels of the flank 70 or in the mesentery 25 all cells are typically observed flowing at velocities reaching 1 mm/s or higher (ie, comparable to blood vessels 24 , 25 ). These velocities reveal that leukocytes are greatly (50–10 000‐fold) accelerated once they start to roll and detach in collectors. Transition to flow is thus key for timely arrival of leukocytes in dLNs.
Using IVM, our group further characterized the process of intralymphatic crawling of DCs and T cells. We could demonstrate the involvement of the Rho‐associated protein kinase (ROCK) in intralymphatic DC crawling and in the overall migration process. 86 ROCK affected DC migration in at least two ways; by regulating nuclear contraction, ROCK was important for efficient DC migration through the interstitial space. Moreover, ROCK supported DC de‐adhesion from integrin ligands during crawling in inflamed lymphatic capillaries. Performing IVM in murine ear skin inflamed by induction of a contact hypersensitivity (CHS) response we observed that DC crawling in lymphatic capillaries, which expressed high levels of ICAM‐1, was strongly reduced in presence of the ROCK inhibitor Y27632. Conversely, intralymphatic crawling of DCs in steady‐state murine ear skin, where LECs expressed little ICAM‐1, was not compromised. 86 In a subsequent study, imaging T cells in CHS‐inflamed ear skin, we showed that the integrin ligand ICAM‐1 also supported intralymphatic crawling of T cells: during tissue inflammation the velocity of T cell crawling in inflamed capillaries was strongly increased and dependent on ICAM‐1/LFA‐1 interactions. 70 Interestingly, IVM studies performed in the inflamed ear skin or cremaster muscle recently confirmed that also neutrophils crawled within lymphatic capillaries 71 , 87 and that this process was Mac‐1‐/ICAM‐1‐dependent. 87
In sum, the ex vivo and in vivo imaging studies combinedly established that lymphatic migration starts with (1) leukocyte migration toward and (2) entry into lympahtic capillaries, followed by (3) active migration through capillaries and (4) detachment and passive transport in downstream, contracting lymphatic collectors (Figure 2).
6.5. Intralymphatic crawling is semi‐directional and guided by CCL21
Our initial IVM studies revealed that leukocytes patrol within lymphatic capillaries, that is, take frequent turns and spend significant time migrating in opposite direction of drainage. 86 This finding prompted our group to further investigate the directedness of intralymphatic migration. We addressed this by IVM and measuring directionality of either adoptively transferred DCs or endogenous DCs in dermal lymphatic capillaries. 27
Directionality of cell migration is typically measured by calculating the chemotactic index, that is, the ratio between the cellular displacement and the total path length (TPL) a cell migrated in a given time interval. However, considering that intraluminal migration is constrained by the curvature of the vessel, we had to introduce a new parameter for measuring directed cell migration in this compartment: the downstream migration index (DMI) (Figure 4A). Rather than the displacement, we measured for each tracked cell its “optimal migratory path” (OMP), which represents the shortest path along the vessel midline that it could have migrated from its starting‐point to its end‐point. In analogy to the chemotactic index, the DMI is calculated by dividing the OMP by the TPL (Figure 4A). If net migration occurred in the downstream direction of the dLN, a positive value is assigned to the DMI. Conversely, if net migration rather occurred in upstream direction of blind‐ending capillaries, a negative value is assigned. Accordingly, directed migration toward either the downstream collecting vessels or the upstream capillary‐endings would result in a DMI of 1 and −1, respectively, whereas random migration would result in a DMI of 0.
FIGURE 4.
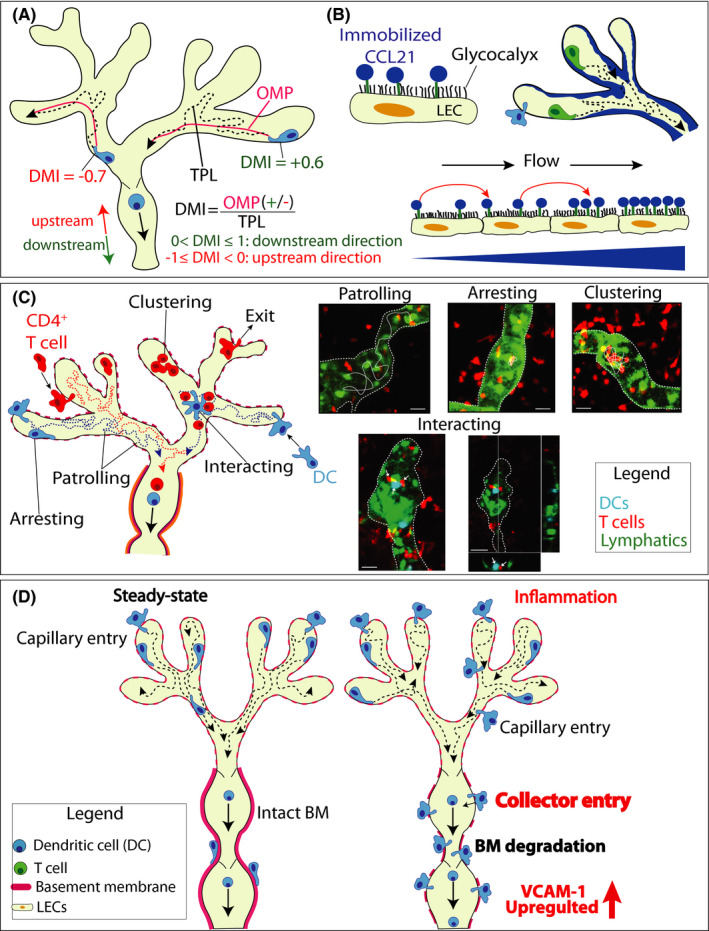
Key mechanistic insights on lymphatic migration made by time‐lapse imaging. (A‐B) An intralymphatic CCL21 gradient directs DC crawling within lymphatic capillaries in the downstream direction of the dLN. (B) The DMI serves to quantify directedness of leukocyte migration in lymphatic capillaries. Within the imaging interval, each cell covers a certain distance, referred to as the total path length (TPL). The optimal migration path (OMP) represents the shortest path that the cell could have migrated within the vessel from its starting‐point to its end‐point. The DMI is calculated by dividing the OMP by the TPL and assigning a positive value if the overall migration occurred in the downstream direction to the dLN and a negative value if it rather occurred in the upstream direction of the capillaries' blind endings. DMI of 1 or −1: completely directed movement toward the downstream collectors or the upstream capillaries. DMI of 0: random migration. The actual DMI measured for DCs was approximately 0.2, indicative of semi‐directed migration. (B) Hypothesis on how low lymph flow in capillaries helps to establish a downstream‐oriented CCL21 gradient. The positively charged C terminus of CCL21 is anchors it on the negatively charged LEC surface. Due to the constant low flow, CCL21 gets washed off from the producing LEC and rebinds to the endothelium further downstream, leading to the preferential accumulation of CCL21 in downstream capillary regions. (C) Unexpected behaviors of intralymphatic DCs and T cells revealed by IVM. In addition to actively crawling and patrolling in the capillary lumen, DCs and T cells frequently arrest for long time periods (>40 min) within lymphatic capillaries. Endogenous T cells were observed to frequently cluster in inflamed capillaries or to occasionally exit from the vessel back into the tissue. DCs were observed interacting with T cells within lymphatic capillaries. Left: schematic representation of the observations. Right: Images taken from IVM experiments as described in. 88 Scale bar: 100 μm. (D) Under inflammatory conditions DCs can directly enter into lymphatic collectors. Tissue inflammation induces degradation of the thick BM surrounding dermal collectors and preferential upregulation of VCAM‐1 in dermal collecting vessels, enabling collector entry. By directly entering into collectors, which can contract and cell rapidly detach and transition to fast flow, DCs avoid the slow migration step in capillaries. Collector‐entry therefore enables fast track for migration to dLNs
In our experiments, both adoptively transferred as well as endogenous DCs displayed a positive DMI of approx. 0.2, indicating that on average they migrated in a semi‐directed manner within lymphatic capillaries. 27 However, this low DMI also revealed that migration is rather inefficient, with only 20% of the performed movement advancing the cell in the downstream direction of the contracting lymphatic collectors, that is, where they would be expected to migrate toward in order to approach the dLNs. In contrast to another previous study, 85 we did not observe any contribution of lymphatic flow to direction sensing of DCs within capillaries. In fact, we observed that semi‐directed migration (with a DMI of approximately 0.1) was retained for DCs crawling within lymphatic capillaries in ear skin explants, that is, in complete absence of flow. Further experiments revealed that CCL21 sensing was required for semi‐directed intralymphatic migration; upon antibody‐based blockade of CCL21 or genetic deletion of CCR7 in DCs the DMI of intralymphatic migration was reduced to 0, both in vivo and in ear skin explants, pointing toward the presence of a downwards‐oriented CCL21 gradient present within lymphatic capillaries. 27
Although functionally demonstrated by our DMI data, we were thus far not able to visualize the intralymphatic CCL21 gradient, due to poor intravascular sensitivity of the staining. However, our experimental evidence suggests that such a gradient is formed by the constant low flow present in lymphatic capillaries. The latter causes dissociation of CCL21 from the surface of the producing LECs and its rebinding to further downstream‐located LECs, leading to the build‐up of CCL21 in more downstream parts of the capillary segments (Figure 4B). Specifically, we observed that a downstream‐oriented gradient could be established in vitro when CCL21‐secreting LECs were cultured for 24 hours in presence of low flow. Conversely, upon culture under high flow, mimicking the conditions in collectors, no gradient formation was observed, likely because rebinding of dissociated CCL21 was no longer occurring under high‐flow conditions. Similarly, the DMI of DCs crawling in collecting vessels segments of ear skin explants was 0, indicative of random migration and the absence of a CCL21 gradient present in collectors. 27
Overall, our study demonstrated that semi‐directed CCL21‐guided migration helps to advance DCs from capillaries to collecting vessel segments, were detachment and rapid transport can be initiated. Considering that we found DCs to migrate at an average speed of 6 μm/min in capillaries, 86 of which only 20% (DMI = 0.2) contributes to advancing them toward collectors (ie, 1.2 μm/min), DCs spend hours within this lymphatic compartment. The dwell‐time in capillaries can therefore be considered a rate‐limiting step in the migration of cells from peripheral tissues to dLNs. Interestingly, recent IVM performed in the cremaster muscle indicated that the described concept of intralymphatic crawling and direction sensing not only applies to DCs in dermal lymphatic capillaries: Also intralymphatic neutrophil crawling was found to occur in a semi‐directed manner, and direction sensing was associated with the establishment of an intralymphatic CCL21 gradient pointing in the direction of lymph flow. 87
6.6. Dendritic cells and T cells interact within murine afferent lymphatic capillaries
While we typically only observed few endogenous T cells or DCs crawling and patrolling in lymphatic capillaries in steady‐state murine skin, their numbers were strikingly increased during tissue inflammation, induced by either a CHS or DTH response. 27 , 70 , 86 Intriguingly, our IVM studies revealed that besides the continuously crawling cells, approximately 75% of DCs displayed a “stop‐and‐go” behavior, sometime arresting for long time periods (>30 min) within lymphatic vessels 88 (Figure 4C). This was observed for both adoptively transferred as well as for endogenous DCs. Similarly, performing IVM in CHS‐inflamed ear skin of Prox1‐GFP × hCD2‐DsRed mice, which feature green‐fluorescent lymphatics and red‐fluorescent T cells, we observed that also T cells remained arrested or clustered inside lymphatic capillaries for long time periods. 88 This peculiar behavior, which did not seem to contribute to migration, made us hypothesize that T cells arresting or clustering within lymphatic capillaries might be interacting with thus far not visualized intralymphatic antigen‐presenting cells. In further support of this hypothesis we observed that intralymphatic red‐fluorescent T cells were occasionally interacting with motile GFP‐expressing cells – likely DCs that had phagocytosed parts of dying Prox1‐GFP+ LECs (Figure 4C). 88 Evidence for the occurrence of intralymphatic DC‐T cell interactions also came from an adoptive transfer setup, in which a DTH response toward ovalbumin (OVA) was induced in the ear skin of Prox1‐GFP mice spiked with CD2‐DsRed‐OTII T cells. Upon adoptive transfer of fluorescent bone marrow‐derived DCs, generated from either wildtype or I‐A/I‐E‐deficient mice, pulsed with OVA‐peptide we could observe long‐lasting DC‐T cell interactions within afferent lymphatics that were formed in an antigen/MHCII‐dependent manner (Figure 4C). Interestingly, both in this adoptive transfer setup and also when observing endogenous interactions in the Prox1‐GFP × CD2RFP mice, the involved cells did not arrest for the time of the interaction, but the interacting cells (DC and T cell) continued to migrate as a duo within lymphatic vessels. 88 This type of motile interactions seems different from DC‐T cells interactions previously observed by IVM in LNs. However, it needs to be kept in mind that in contrast to the LN parenchyma, which is packed with immune cells, there are much fewer cells within afferent lymphatic vessels, leaving much more space for cell movement.
Given current knowledge of T cell trafficking through inflamed afferent lymphatics 26 , 89 we suspect that these interactions involved either CD4+ Teff or Treg. In the context of a developing or ongoing immune response, it is perceivable that some antigen‐specific CD4+ Teff will fail to encounter cognate antigen while scanning APCs in the peripheral tissue and therefore leave the tissue by entering into lymphatic capillaries. We speculate that in this case, Teff might get re‐activated upon interacting with an intralymphatic, cognate antigen‐presenting DCs, what could confer a signal to the cells to exit back into the tissue and continue searching for antigen/exert their effector functions. Indeed, in contrast to DCs which we have never observed exiting from lymphatic capillaries, we occasionally (in the low percentage range) observed T cells that transmigrated back into the tissue. 88 This exit‐behavior could contribute to enhancing the efficacy of immune recognition: by exiting the capillary, these cells would take a “short‐cut” back into the tissue, instead of recirculating through dLNs, lymphatics and blood vessels, what would represent a long journey before returning to the tissue in which their cognate antigen is located. Intriguingly, a cannulation study reported more than 20 year ago the presence of small cell clusters comprising IL‐12+ DCs and IFNγ1+ lymphocytes in cannulated human afferent lymph. 40 Although it cannot be ruled out that these clusters formed during the lymph collection process, these findings might support the occurrence of DC‐Teff cell interactions in afferent lymphatics.
On the other hand, considering that at least in mice Treg have been identified as a major fraction of T cells exiting from inflamed skin, 39 it is possible that intralymphatic DC‐T cell interactions might involve Treg. Treg are well known to suppress the antigen‐presenting functions of autoantigen‐presenting DCs. 90 DC‐Treg interactions occurring within afferent lymphatic vessels in the context of tissue inflammation might therefore help to prevent that DCs expressing high levels of co‐stimulatory molecules but presenting primary self‐antigen arrive in dLNs, where they could support activation of self‐reactive T cells. A similar DC‐suppressing function has been ascribed to Treg in dLNs. 90 However, considering the vast number of cells present in a LN, the ability to identify and suppress mature autoantigen‐presenting DCs already within afferent lymphatics could help to improve the logistic challenges of this suppressive mechanism.
6.7. Long arrest periods of DC and T cells within lymphatic capillaries
Our IVM experiments performed under both inflammatory and steady‐state conditions also revealed that intralymphatic DCs and T cells undergo long arrest periods within lymphatic capillaries 27 , 70 , 88 (Figure 4C). Considering that the cells spend several hours crawling and arresting within this compartment, this behavior might imply that leukocytes exchange signals with LECs while migrating. Indeed, several reports support the existence of such signaling events and indicate that it shapes both LECs in capillaries and the migrating leukocytes: As previously mentioned, a recent study demonstrated that transmigrating DCs may induce Ca2+ signalling in LECs, leading to the release of CCL21 from intracellular stores. 82 Similarly, interactions of DCs with collecting vessels were shown to reduce permeability and support fluid transport through larger collecting vessels. 91 Likewise, LTα1β2/LTβR‐mediated interaction of Treg with LECs were found to upregulate expression of VCAM‐1, CCL21 and other trafficking molecules in in afferent lymphatics of inflamed tissues. The latter findings suggested that Treg contribute to resolution of inflammation by supporting tissue exit of inflammatory leukocytes. 92
Similarly, several studies have identified LEC‐derived molecules that modulate the activation state of migrating DCs. In all reports thus far, the signal received from LECs lead to a suppression of the migratory DC’s maturation state and APC function: Specifically, interaction of LEC‐expressed ICAM‐1 with the integrin MAC‐1 on DCs reportedly reduced expression levels of co‐stimulatory molecule CD86 in migratory DCs, resulting in reduced T cell activation in dLNs. 93 Other studies identified prostaglandins secreted by LECs 94 and CD73 expressed on LECs 95 as negative modulators of DC maturation, dampening inflammation and immune responses in dLNs. More recently, also the LEC‐expressed adhesion molecule CLEVER‐1 (also known as Stabilin‐1 and FEEL‐1) was shown to impact both DC cell migration from the skin to the dLN as well as the maturation state of migrated DCs and their ability to induce T cell proliferation. 96 In further support of these findings, other studies have reported that migratory DCs present in dLNs in steady‐state generally have a less immune‐activating phenotype in comparison to LN‐resident DCs. 97 , 98 By contrast, under inflammatory conditions migratory DCs reportedly acquire a more stimulatory phenotype compared to LN‐resident DCs and are critical for inducing effective adaptive responses in the context of peripheral infection or vaccination. 99 , 100 , 101 , 102 This apparent discrepancy could suggest that the DC maturation‐dampening effects of LECs are more pronounced in steady‐state as compared to inflammatory conditions. Alternatively ‐ and in our opinion more likely ‐ the much stronger DC maturation‐inducing stimuli present in the peripheral tissue, for example, in context of an infection, likely override the immune‐dampening effects of LECs, explaining why migratory DCs are more mature as compared to LN‐resident DCs under the latter conditions.
With regards to T cell migration, no immunemodulatory molecules expressed by capillary LECs that impact migratory T cells have been reported so far. This is contrast to the various studies documenting the immunemodulatory and APC functions exerted by LECs in LNs 103 or in tumors. 104 For example, LN LECs and other LN stromal cells were shown to suppress T cell activation by release of nitric oxide 105 or expression of PD‐L1. 106 In addition to expressing MHCII, LN LECs were found to scavenge and cross‐present antigen drained to the LN via afferent lymphatics or to acquire antigen‐MHC complexes from migratory DCs. 103 , 107 In most cases, the responses elicited by antigen‐presenting LECs resulted in T cell anergy or conversion to a regulatory phenotype. 103 , 108 Similarly, several recent studies have highlighted the immunemodulatory properties exerted by tumor LECs, in particular via the PD‐L1/PD‐1 axis. 104 Moreover, a recent study showed that MHCII expression by tumoral LECs supported expansion and immunosuppressive activity of Treg, thereby contributing to tumor immune‐evasion. 109 Whether similar APC und immunemodulatory functions also occur in lymphatic capillaries in the context of an inflammatory response and whether this impacts tissue‐resident or emigrating T cells is not yet clear at this stage. However, it is interesting to note that, for example, PD‐L1 is strongly upregulated in LECs of afferent lymphatics during tissue inflammation 67 and so is MHCII (unpublished observations).
Overall, these findings ‐ including our observation of lengthy DC or T cell interactions with capillary LECs – contribute to the emerging notion that afferent lymphatics exert immune‐regulatory activity and generally dampen adaptive immune responses.
6.8. Lymphatic collectors support dendritic cell entry and rapid migration to lymph nodes in inflammation
IVM has revealed that leukocyte migration through capillaries is a lengthy process 27 , 86 , 88 and likely a rate‐limiting step in the overall migration from peripheral tissues to dLNs. In the case of DCs, the kinetics of migration from skin to dLNs have been studied in quite detail over the past 16 years. 46 , 110 In line with the low speed of interstitial migration, 111 , 112 the time needed for DC maturation and entry and crawling within lymphatic capillaries, these studies have revealed that even under inflammatory conditions, DC migration to dLNs remains a fairly slow process. Generally, it has been reported that dermal DCs numbers in the dLNs peak 1–2 days after induction of inflammation and epidermal Langerhans cells after 3–4 days. 46 , 110 , 113 , 114 However, even in these studies always some cells could be detected that arrived much faster in dLNs, namely within less than 8 hours. Considering the long time spent in capillaries and the inefficient mode of forward movement in this compartment, we wondered whether in the case of such early arriving, rapidly migrating DCs, entry into afferent lymphatics had occurred directly into the collecting vessel segments, where the elevated flow should favor rapid transport to the dLN. Performing in vivo migration studies and time‐lapse imaging, our group recently demonstrated that under inflammatory conditions, some DCs indeed enter directly into lymphatic collectors, allowing for their fast arrival in the dLN 115 (Figure 4D).
Specifically, we found that under in inflammatory conditions the rather tight BM surrounding dermal lymphatic collectors is degraded and adhesion molecules like VCAM‐1, CX3CL1 and CXCL12, which had all been previously shown to mediate migration to dLNs, 68 , 80 , 116 were preferentially upregulated in collector LECs but not in capillary LECs (Figure 4D). When performing time‐lapse imaging of fluorescently labeled DCs in the ear skin explant model, we could observe DC entry into LYVE‐1‐ lymphatic collectors. We could further show that entry into both capillaries and collectors was highly CCR7‐dependent, whereas loss of VCAM‐1 in collectors or genetic deletion of integrin β1 subunit in DCs selectively reduced the DC’s ability to enter into collectors but not into capillaries. Considering the difference in migration/transport speed in lymphatic capillaries and collectors, entry into collector vessels would be expected to favor fast arrival of DCs in the dLNs. In line with this assumption, when performing adoptive transfer studies with wild‐type (WT) DCs and DCs deficient in the VCAM‐1‐binding integrin subunit β1, we observed that β1‐deficiency had a strong impact on DC arrival in dLNs at early time points (eg, 8 hours after transfer). Conversely, at later time points (eg, 40 hours) at which also DCs that have entered via capillaries would be expected to have arrived in dLNs, the impact of β1‐deficiency was much less pronounced, in support of the importance of collector entry for fast arrival in dLNs. 115
These results challenge the prevailing paradigm of lymphatic trafficking assuming that leukocyte exclusively enter at the level of lymphatic capillaries. The reason why this alternative entry route was overlooked in the initial ear skin crawl in experiments 15 , 66 , 72 is likely due to the fact that the original experiments were performed in ventral murine ear skin, which in contrast to the dorsal ear skin used in our studies contains virtually only LYVE‐1+ capillaries but not collecting vessels. Also, in light of the prior knowledge of lymphatic morphology 14 it seemed/seems more intuitive that cell entry occurs through capillaries, which in contrast to collectors are surrounded by an ultrathin BM and comprise large openings, that is, primary valves, through which leukocytes can easily access the vessel lumen. The morphology of collectors, on the other hand, more resembles that of blood vessels. But considering that leukocytes also constantly extravasate from blood vessels, despite their continuous, zipper‐like endothelial cell‐cell junctions, and thick BM and surrounding pericytes, it is not so surprising that also entry into collectors is possible. Although thus far only demonstrated for DCs, we suspect that also the other cell types are capable of entering afferent lymphatics through collecting vessels in tissues. For example, we previously found that VCAM‐1 also mediated T cell migration from skin to dLNs in the context of inflammation. 70 Furthermore, neutrophils are known to be the first lymph‐borne cell type arriving in dLNs in the context of inflammation. 43 This is probably in part associated with the fact that these cells – in contrast to DCs – already arrive in the tissue in an activated and highly motile state. But preferred entry through collectors could potentially be a further explanation for their rapid migration kinetics.
The next goal will be to gain a better understanding of the occurrence of collector‐entry and its functional relevance. For example, at present, we do not know whether all or possibly only certain DC subtypes transmigrate via this route, for example, depending on their tissue location and chemotactic requirements. By entering directly into contracting collecting vessel segments, DCs will avoid the inefficient migration step in capillaries and arrive faster in dLNs. With regards to the potential functional relevance of this process, we speculate that a more rapid arrival of antigen‐bearing DCs could be an advantage in the context of infections with a rapidly dividing pathogens, as it would allow for an earlier start of the adaptive immune response. On the other hand, direct entry into collectors could not only help to avoid slow migration kinetics associated with the capillary compartment, but also bypass the immunosuppressive signals that DCs might receive from interacting with capillary LECs or potentially with intralymphatic T cells.
7. CONCLUSION AND OUTLOOK
Over the past 15 years, time‐lapse imaging studies performed in tissue explants and in vivo have identified multiple steps involved in the migration of leukocytes into and within afferent lymphatics. In addition to providing insight into the migratory process and dissecting the contribution of molecules at distinct locations in/around the vasculature, these studies have revealed unexpected behaviors of leukocytes within lymphatic capillaries. The fact that DCs and T cells spend many hours crawling, arresting and even interacting within lymphatic capillaries before migrating toward the dLN has contributed to the emerging notion that lymphatic capillaries exert immunemodulatory functions, in addition to their conventional role in leukocyte transport. In the future, it will be interesting to further investigate the immunemodulatory and potential APC functions of lymphatic capillaries using time‐lapse imaging and other techniques. One obvious challenge will be to differentiate between effects induced by LECs in lymphatic capillaries and such caused by LN LECs, that is, the second site where long‐lasting or repeated interactions of leukocytes with LECs are expected to occur. Thus far, no gene‐targeted mouse strains allowing for specific deletion of genes in LNs vs. peripheral LECs are available, but it is likely that ‐ propelled by the many single‐cell sequencing studies performed on both LN and peripheral LECs (eg, 117 , 118 ) ‐ new tools will arise from the identification of LEC subset‐specific markers. So far, time‐lapse imaging of leukocyte migration through afferent lymphatics has only been performed in steady‐state or in acute inflammation models, but not in disease models, for example, in settings of autoimmunity, graft rejection or tumor growth. Such studies are expected to deliver new insights into both aspects of migration and immunemodulation. Likewise, transferring the time‐lapse imaging protocols established for murine tissue explants to human tissues could help to compare migratory processes in both systems, thereby contributing to a better understanding of their translational potential.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
ACKNOWLEDGEMENTS
The authors thank Morgan Hunter (ETH Zurich) for providing original microscopy images (Figure 3b, Figure 4c). CH gratefully acknowledges support from the Swiss National Science Foundation (grant 310030_182528) and from ETH Zurich. Open Access funding provided by Eidgenossische Technische Hochschule Zurich
Collado‐Diaz V, Medina‐Sanchez JD, Gkountidi A‐O, Halin C. Imaging leukocyte migration through afferent lymphatics. Immunol Rev.2022;306:43–57. 10.1111/imr.13030
This article is part of a series of reviews covering Insights into Immune Function from Imaging appearing in Volume 306 of Immunological Reviews.
Collado‐Diaz and Medina‐Sanchez contributed equally to this work.
Contributor Information
Victor Collado‐Diaz, Email: cornelia.halin@pharma.ethz.ch.
Jessica D. Medina‐Sanchez, Email: cornelia.halin@pharma.ethz.ch.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research.
REFERENCES
- 1. Mackay IR, von Adrian UH. T‐Cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020‐1034. [DOI] [PubMed] [Google Scholar]
- 2. Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960‐969. [DOI] [PubMed] [Google Scholar]
- 3. Griffith JW, Luster AD. Targeting cells in motion: migrating toward improved therapies. Eur J Immunol. 2013;43(6):1430‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694‐707. [DOI] [PubMed] [Google Scholar]
- 5. Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15(11):692‐704. [DOI] [PubMed] [Google Scholar]
- 6. Lammermann T, Germain RN. The multiple faces of leukocyte interstitial migration. Semin Immunopathol. 2014;36(2):227‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lelkes E, Headley MB, Thornton EE, Looney MR, Krummel MF. The spatiotemporal cellular dynamics of lung immunity. Trends Immunol. 2014;35(8):379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21(st) century: novel functional roles in homeostasis and disease. Cell. 2020;182(2):270‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. 2020;369(6500):1–11. [DOI] [PubMed] [Google Scholar]
- 10. Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;26:31‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jalkanen S, Salmi M. Lymphatic endothelial cells of the lymph node. Nat Rev Immunol. 2020;20(9):566‐578. [DOI] [PubMed] [Google Scholar]
- 12. Leak LV. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue‐lymph interface. Microvasc Res. 1970;2:361‐391. [DOI] [PubMed] [Google Scholar]
- 13. Schmid‐Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70(4):987‐1028. [DOI] [PubMed] [Google Scholar]
- 14. Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206(13):2925‐2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang XN, McGovern N, Gunawan M, et al. A three‐dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J Invest Dermatol. 2014;134(4):965‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X, Yu Z, Liu N. Comparison of approaches for microscopic imaging of skin lymphatic vessels. Scanning. 2012;34(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 18. Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid‐Schonbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001;15(10):1711‐1717. [DOI] [PubMed] [Google Scholar]
- 19. Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat. 1966;118(3):785‐809. [DOI] [PubMed] [Google Scholar]
- 20. Scallan JP, Zawieja SD, Castorena‐Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol. 2016;594(20):5749‐5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19(3):397‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berk DA, Swartz MA, Leu AJ, Jain RK. Transport in lymphatic capillaries. II. Microscopic velocity measurement with fluorescence photobleaching. Am J Physiol. 1996;270(1 Pt 2):H330‐H337. [DOI] [PubMed] [Google Scholar]
- 23. Swartz MA, Berk DA, Jain RK. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;270(1 Pt 2):H324‐H329. [DOI] [PubMed] [Google Scholar]
- 24. Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13(7):597‐610. [DOI] [PubMed] [Google Scholar]
- 25. Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol. 2011;301(5):H1828‐H1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Permanyer M, Bosnjak B, Forster R. Dendritic cells, T cells and lymphatics: dialogues in migration and beyond. Curr Opin Immunol. 2018;53:173‐179. [DOI] [PubMed] [Google Scholar]
- 27. Russo E, Teijeira A, Vaahtomeri K, et al. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep. 2016;14(7):1723‐1734. [DOI] [PubMed] [Google Scholar]
- 28. Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid‐tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162(5):2472‐2475. [PubMed] [Google Scholar]
- 29. Martinez‐Corral I, Stanczuk L, Frye M, et al. Vegfr3‐CreER (T2) mouse, a new genetic tool for targeting the lymphatic system. Angiogenesis. 2016;19(3):433‐445. [DOI] [PubMed] [Google Scholar]
- 30. Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2009;207(1):17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bazigou E, Lyons OT, Smith A, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121(8):2984‐2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burke K, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147‐155. [DOI] [PubMed] [Google Scholar]
- 33. Kivela R, Salmela I, Nguyen YH, et al. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre‐type regulation. Nat Commun. 2016;7:13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE‐1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down‐regulation in human liver cancer and cirrhosis. Can Res. 2001;61(22):8079‐8084. [PubMed] [Google Scholar]
- 35. Xu H, Chen M, Reid DM, Forrester JV. LYVE‐1‐positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci. 2007;48(5):2162‐2171. [DOI] [PubMed] [Google Scholar]
- 36. Ward LSC, Sheriff L, Marshall JL, et al. Podoplanin regulates the migration of mesenchymal stromal cells and their interaction with platelets. J Cell Sci. 2019;132(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JB, McIntosh GH, Morris B. The traffic of cells through tissues: a study of peripheral lymph in sheep. J Anat. 1970;107(Pt 1):87‐100. [PMC free article] [PubMed] [Google Scholar]
- 38. Sokolowski J, Jakobsen E, Johannessen JV. Cells in peripheral leg lymph of normal men. Lymphology. 1978;11(4):202‐207. [PubMed] [Google Scholar]
- 39. Tomura M, Honda T, Tanizaki H, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120(3):883‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yawalkar N, Hunger RE, Pichler WJ, Braathen LR, Brand CU. Human afferent lymph from normal skin contains an increased number of mainly memory/effector CD4(+) T cells expressing activation, adhesion and co‐stimulatory molecules. Eur J Immunol. 2000;30(2):491‐497. [DOI] [PubMed] [Google Scholar]
- 41. Olszewski WL, Grzelak I, Ziolkowska A, Engeset A. Immune cell traffic from blood through the normal human skin to lymphatics. Clin Dermatol. 1995;13(5):473‐483. [DOI] [PubMed] [Google Scholar]
- 42. Bujdoso R, Hopkins J, Dutia BM, Young P, McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989;170(4):1285‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jakovija A, Chtanova T. Neutrophil interactions with the lymphatic system. Cells. 2021;10(8):2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zawieja DC, Thangaswamy S, Wang W, et al. Lymphatic cannulation for lymph sampling and molecular delivery. J Immunol. 2019;203(8):2339‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759‐765. [DOI] [PubMed] [Google Scholar]
- 46. Tomura M, Hata A, Matsuoka S, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep. 2014;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomura M, Yoshida N, Tanaka J, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein "Kaede" transgenic mice. Proc Natl Acad Sci USA. 2008;105(31):10871‐10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ikebuchi R, Teraguchi S, Vandenbon A, et al. A rare subset of skin‐tropic regulatory T cells expressing Il10/Gzmb inhibits the cutaneous immune response. Sci Rep. 2016;6:35002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakanishi Y, Ikebuchi R, Chtanova T, et al. Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol. 2017;11:437‐448. [DOI] [PubMed] [Google Scholar]
- 50. Beura LK, Hamilton SE, Bi K, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co‐localized effector and regulatory T cells. Nature. 2015;528(7581):225‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309‐320. [DOI] [PubMed] [Google Scholar]
- 53. Debes GF, Bonhagen K, Wolff T, et al. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J Virol. 2004;78(14):7528‐7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jennrich S, Lee MH, Lynn RC, Dewberry K, Debes GF. Tissue exit: a novel control point in the accumulation of antigen‐specific CD8 T cells in the influenza a virus‐infected lung. J Virol. 2012;86(7):3436‐3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomez D, Diehl MC, Crosby EJ, Weinkopff T, Debes GF. Effector T cell egress via afferent lymph modulates local tissue inflammation. J Immunol. 2015;195(8):3531‐3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McNamee EN, Masterson JC, Veny M, et al. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's‐like murine ileitis. J Leukoc Biol. 2015;97(6):1011‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brinkman CC, Iwami D, Hritzo MK, et al. Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration. Nat Commun. 2016;7:12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiong Y, Ahmad S, Iwami D, Brinkman CC, Bromberg JS. T‐bet regulates natural regulatory T cell afferent lymphatic migration and suppressive function. J Immunol. 2016;196(6):2526‐2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thomas SN, Rutkowski JM, Pasquier M, et al. Impaired humoral immunity and tolerance in K14‐VEGFR‐3‐Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189(5):2181‐2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mueller SN, Mackay LK. Tissue‐resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16(2):79‐89. [DOI] [PubMed] [Google Scholar]
- 62. Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190(3):970‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. 2019;19(8):490‐502. [DOI] [PubMed] [Google Scholar]
- 64. Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arasa J, Collado‐Diaz V, Halin C. Structure and immune function of afferent lymphatics and their mechanistic contribution to dendritic cell and T cell trafficking. Cells. 2021;10:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lammermann T, Bader BL, Monkley SJ, et al. Rapid leukocyte migration by integrin‐independent flowing and squeezing. Nature. 2008;453(7191):51‐55. [DOI] [PubMed] [Google Scholar]
- 67. Vigl B, Aebischer D, Nitschké M, et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus‐dependent manner. Blood. 2011;118(1):205‐215. [DOI] [PubMed] [Google Scholar]
- 68. Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation‐induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203(12):2763‐2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Teijeira A, Garasa S, Pelaez R, et al. Lymphatic endothelium forms Integrin‐engaging 3D structures during DC transit across inflamed lymphatic vessels. J Invest Dermatol. 2013;133(9):2276‐2285. [DOI] [PubMed] [Google Scholar]
- 70. Teijeira A, Hunter MC, Russo E, et al. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM‐1/LFA‐1 interactions. Cell Rep. 2017;18(4):857‐865. [DOI] [PubMed] [Google Scholar]
- 71. Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. Microbe‐dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun. 2015;6:7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weber M, Hauschild R, Schwarz J, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339(6117):328‐332. [DOI] [PubMed] [Google Scholar]
- 73. Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28(9):2760‐2769. [DOI] [PubMed] [Google Scholar]
- 74. Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 75. Witt DP, Lander AD. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994;4:394‐400. [DOI] [PubMed] [Google Scholar]
- 76. de Paz JL, Moseman EA, Noti C, Polito L, von Andrian UH, Seeberger PH. Profiling heparin‐chemokine interactions using synthetic tools. ACS Chem Biol. 2007;2(11):735‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15(3):603‐612. [DOI] [PubMed] [Google Scholar]
- 78. Vaahtomeri K, Moussion C, Hauschild R, Sixt M. Shape and function of interstitial chemokine CCL21 gradients are independent of heparan sulfates produced by lymphatic endothelium. Front Immunol. 2021;12:630002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276(7):5228‐5234. [DOI] [PubMed] [Google Scholar]
- 80. Johnson LA, Jackson DG. Inflammation‐induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin‐mediated dendritic cell transmigration. Int Immunol. 2010;22(10):839‐849. [DOI] [PubMed] [Google Scholar]
- 81. Bastow CR, Bunting MD, Kara EE, et al. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc Natl Acad Sci USA. 2021;118(17):e2025763118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vaahtomeri K, Brown M, Hauschild R, et al. Locally triggered release of the chemokine CCL21 promotes dendritic cell transmigration across lymphatic endothelia. Cell Rep. 2017;19(5):902‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bryce SA, Wilson RAM, Tiplady EM, et al. ACKR4 on stromal cells scavenges CCL19 to enable CCR7‐dependent trafficking of APCs from inflamed skin to lymph nodes. J Immunol. 2016;196(8):3341‐3353. [DOI] [PubMed] [Google Scholar]
- 84. Sen D, Forrest L, Kepler TB, Parker I, Cahalan MD. Selective and site‐specific mobilization of dermal dendritic cells and Langerhans cells by Th1‐ and Th2‐polarizing adjuvants. Proc Natl Acad Sci USA. 2010;107(18):8334‐8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tal O, Lim HY, Gurevich I, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208(10):2141‐2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nitschke M, Aebischer D, Abadier M, et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady‐state and inflammation. Blood. 2012;120(11):2249‐2258. [DOI] [PubMed] [Google Scholar]
- 87. Arokiasamy S, Zakian C, Dilliway J, Wang W, Nourshargh S, Voisin MB. Endogenous TNFalpha orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation. Sci Rep. 2017;7:44189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hunter MC, Teijeira A, Montecchi R, et al. Dendritic cells and T cells interact within murine afferent lymphatic capillaries. Front Immunol. 2019;10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hunter MC, Teijeira A, Halin C. T cell trafficking through lymphatic vessels. Front Immunol. 2016;7:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Plitas G, Rudensky AY. Regulatory T cells: differentiation and function. Cancer Immunol Res. 2016;4(9):721‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ivanov S, Scallan JP, Kim KW, et al. CCR7 and IRF4‐dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest. 2016;126(4):1581‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Piao W, Xiong Y, Li L, et al. Regulatory T cells condition lymphatic endothelia for enhanced transendothelial migration. Cell Rep. 2020;30(4):1052‐1062 e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Podgrabinska S, Kamalu O, Mayer L, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac‐1/ICAM‐1‐dependent mechanism. J Immunol. 2009;183(3):1767‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Christiansen AJ, Dieterich LC, Ohs I, et al. Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation. Oncotarget. 2016;7:39421‐39435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Eichin D, Pessia A, Takeda A, et al. CD73 contributes to anti‐inflammatory properties of afferent lymphatic endothelial cells in humans and mice. Eur J Immunol. 2021;51(1):231‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tadayon S, Dunkel J, Takeda A, et al. Lymphatic endothelial cell activation and dendritic cell transmigration is modified by genetic deletion of Clever‐1. Front Immunol. 2021;12:602122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13(9):888‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Idoyaga J, Fiorese C, Zbytnuik L, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123(2):844‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee HK, Zamora M, Linehan MM, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV‐1 infection. J Exp Med. 2009;206(2):359‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity. 2015;43(3):554‐565. [DOI] [PubMed] [Google Scholar]
- 101. Krishnaswamy JK, Gowthaman U, Zhang B, et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell‐dependent antibody responses. Sci Immunol. 2017;2(18):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell‐mediated immunity. Immunity. 2003;19(1):47‐57. [DOI] [PubMed] [Google Scholar]
- 103. Santambrogio L, Berendam SJ, Engelhard VH. The antigen processing and presentation machinery in lymphatic endothelial cells. Front Immunol. 2019;10:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Garnier L, Gkountidi AO, Hugues S. Tumor‐associated lymphatic vessel features and immunomodulatory functions. Front Immunol. 2019;10:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lukacs‐Kornek V, Malhotra D, Fletcher AL, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12(11):1096‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tewalt EF, Cohen JN, Rouhani SJ, et al. Lymphatic endothelial cells induce tolerance via PD‐L1 and lack of costimulation leading to high‐level PD‐1 expression on CD8 T cells. Blood. 2012;120(24):4772‐4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dubrot J, Duraes FV, Potin L, et al. Lymph node stromal cells acquire peptide‐MHCII complexes from dendritic cells and induce antigen‐specific CD4(+) T cell tolerance. J Exp Med. 2014;211(6):1153‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nadafi R, Gago de Graca C, Keuning ED, et al. Lymph node stromal cells generate antigen‐specific regulatory T cells and control autoreactive T and B cell responses. Cell Rep. 2020;30(12):4110‐4123 e4114. [DOI] [PubMed] [Google Scholar]
- 109. Gkountidi AO, Garnier L, Dubrot J, et al. MHC class II antigen presentation by lymphatic endothelial cells in tumors promotes intratumoral regulatory T cell‐suppressive functions. Cancer Immunol Res. 2021;9(7):748‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22(5):643‐654. [DOI] [PubMed] [Google Scholar]
- 111. Ng LG, Hsu A, Mandell MA, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4(11):e1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Goh CC, Li JL, Devi S, et al. Real‐time imaging of dendritic cell responses to sterile tissue injury. J Invest Dermatol. 2015;135(4):1181‐1184. [DOI] [PubMed] [Google Scholar]
- 113. Lappin MB, Weiss JM, Delattre V, et al. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. MartIn‐Fontecha A, Sebastiani S, Hopken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Arasa J, Collado‐Diaz V, Kritikos I, et al. Upregulation of VCAM‐1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J Exp Med. 2021;218(7):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kabashima K, Shiraishi N, Sugita K, et al. CXCL12‐CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171(4):1249‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takeda A, Hollmen M, Dermadi D, et al. Single‐cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity. 2019;51(3):561‐572 e565. [DOI] [PubMed] [Google Scholar]
- 118. Reynolds G, Vegh P, Fletcher J, et al. Developmental cell programs are co‐opted in inflammatory skin disease. Science. 2021;371(6527):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Choi I, Chung HK, Ramu S, et al. Visualization of lymphatic vessels by Prox1‐promoter directed GFP reporter in a bacterial artificial chromosome‐based transgenic mouse. Blood. 2011;117(1):362‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hagerling R, Pollmann C, Kremer L, Andresen V, Kiefer F. Intravital two‐photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter‐directed mOrange2 reporter mouse. Biochem Soc Trans. 2011;39(6):1674‐1681. [DOI] [PubMed] [Google Scholar]
- 121. Truman LA, Bentley KL, Smith EC, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180(4):1715‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bianchi R, Teijeira A, Proulx ST, et al. A transgenic Prox1‐Cre‐tdTomato reporter mouse for lymphatic vessel research. PLoS One. 2015;10(4):e0122976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lindquist RL, Shakhar G, Dudziak D, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5(12):1243‐1250. [DOI] [PubMed] [Google Scholar]
- 124. Veiga‐Fernandes H, Coles MC, Foster KE, et al. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446(7135):547‐551. [DOI] [PubMed] [Google Scholar]
- 125. Redder E, Kirschnick N, Hägerling R, Hansmeier N, Kiefer F. Vegfr3‐tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra‐bright red fluorescent protein in "knock‐in" Cre‐reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 127. Alva JA, Zovein AC, Monvoisin A, et al. VE‐Cadherin‐Cre‐recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235(3):759‐767. [DOI] [PubMed] [Google Scholar]
- 128. Gil HJ, Ma W, Oliver G. A novel podoplanin‐GFPCre mouse strain for gene deletion in lymphatic endothelial cells. Genesis. 2018;56(4):e23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207(1):17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Connor AL, Kelley PM, Tempero RM. Lymphatic endothelial lineage assemblage during corneal lymphangiogenesis. Lab Invest. 2016;96(3):270‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high‐throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research.


