Abstract
Imines are photoaddressable motifs useful in the development of new generations of molecular switches, but their operation with low‐energy photons and control over isomer stability remain challenging. Based on a computational design, we developed phenylimino indolinone (PIO), a green‐light‐addressable T‐type photoswitch showing negative photochromism. The isomerization behavior of this photoactuator of the iminothioindoxyl (ITI) class was studied using time‐resolved spectroscopies on time scales from femtoseconds to the steady state and by quantum‐chemical analyses. The understanding of the isomerization properties and substituent effects governing these photoswitches opens new avenues for the development of novel T‐type visible‐light‐addressable photoactuators based on C=N bonds.
Keywords: computational chemistry, isomerization, molecular dynamics, photochromism, time-resolved spectroscopy
Visible‐light‐responsive T‐type photoswitches with negative photochromism are rare and interesting candidates for a wide range of applications. Here, we describe the computer‐assisted rational design of phenylimino indolinone (PIO) and its characterization in the ground and excited state using the full toolbox of physical organic chemistry.
Photoswitches are the cornerstone of molecular machinery.[ 1 , 2 , 3 , 4 , 5 ] Upon irradiation, these photoactuators can populate a metastable state from which they can be reverted to the initial isomer using another photochemical (P‐type photoswitch) or a thermal (T‐type photoswitch) stimulus. [6] Accessing the different physical and chemical properties of the two isomers by light irradiation allows to control the photoswitch both from a spatial and a temporal point of view, thus opening countless possibilities for applications among others in optics, smart materials, molecular logic or photopharmacology.[ 1 , 7 , 8 , 9 , 10 , 11 ]
In the development of new photoactuators, the photochemical E/Z isomerization of C=N bonds is an alternative to the ones offered by the well‐established C=C and N=N motifs.[ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 ] Similar to azobenzene, [20] the presence of the nitrogen lone‐pair in the C=N systems dictates the photochemistry and thermal isomerization of imines.[ 19 , 21 ] The interconversion between the isomers is assumed to occur following two different mechanisms: an excited state rotation of the nitrogen substituent about the C=N bond in which the double bond is formally broken, or a ground state in‐plane inversion without change in the bond order. [22] Taming this complex scenario allowed the realization of the first two‐ and four‐stroke molecular motors by the group of Lehn[ 14 , 15 ] and bistable switches based on hydrazones. [16]
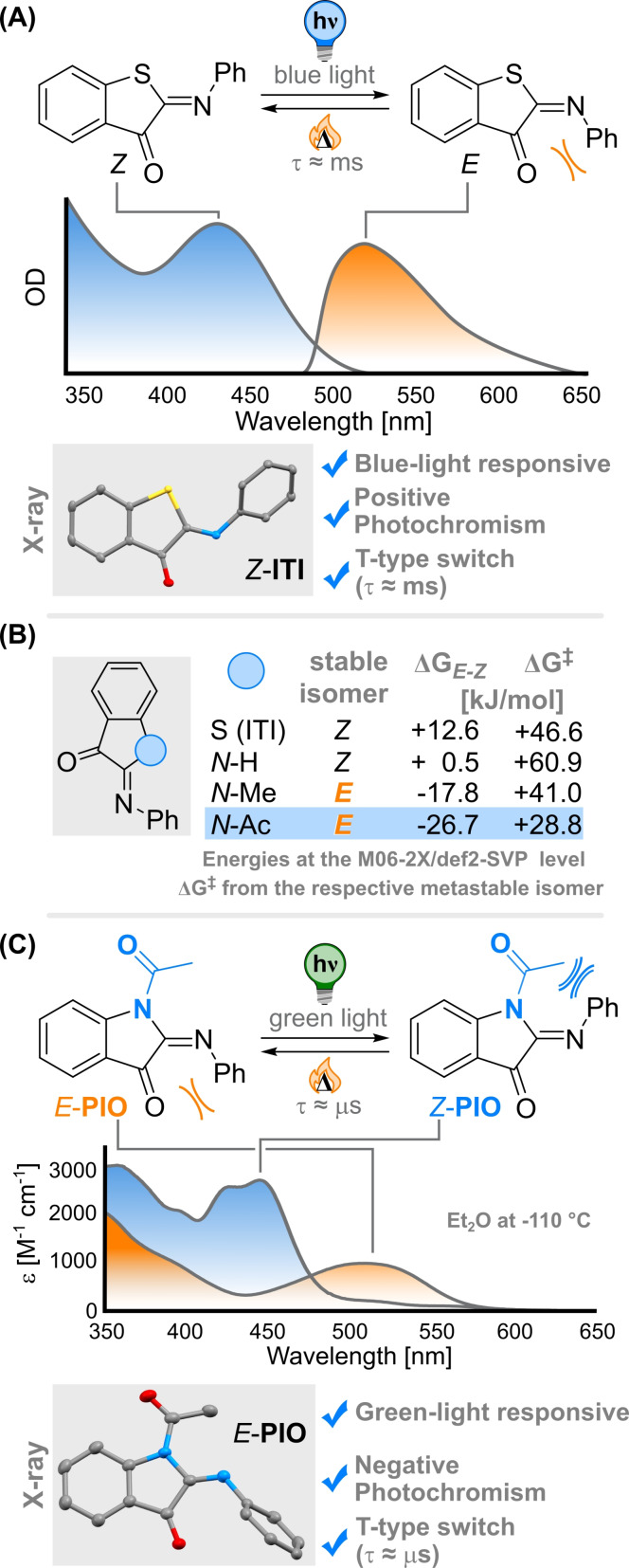
Recently, we developed the new class of iminothioindoxyl (ITI) photoswitches based on a C=N chromophore (see Figure 1 A). [23] This new photoactuator exhibits a remarkable band separation of over 100 nm between its two photoisomers, a prerogative to address each isomeric form selectively. ITI is structurally related to other indigoid photoswitches and readily absorbs light in the visible range. Irradiation with blue light excites the thermally stable Z isomer to its bright S2 state. From there, it relaxes and forms the metastable E isomer with quantum yields Φ around 5 % and showing positive photochromism. [6] The E form can be isomerized back to the stable form using orange light, or by thermal relaxation with recovery times in the ms time range making it a T‐type switch. [23] Triggering the photochemical E→Z isomerization, which in the ITI system represents the back‐switching direction, employs electromagnetic radiation of low energy and is consequently more attractive for practical applications.
Figure 1.
Iminothioindoxyl (ITI) and phenylimino indolinone switches (PIO). (A) Properties of the parent ITI photoswitch. [23] The X‐ray structure of Z‐ITI (50 % probability ellipsoids, H atoms omitted for clarity) is reported. (B) Computational screening of the principal thermal characteristics of different substituents on the original ITI core. (C) Main features of PIO, the experimental UV/Vis spectra of E‐ and Z‐PIO (the latter was extrapolated from the PSS mixture) in Et2O, together with the X‐ray structure of E‐PIO (50 % probability ellipsoids, H atoms omitted for clarity).
Hence, we were intrigued by the possibility of inverting the stability of the isomers in ITI while retaining their photophysical properties. Such a novel structure, for which the E isomer would be the stable one, would then respond to low‐energy light irradiation and, more importantly, be characterized by negative photochromism.[ 6 , 24 ]
In this respect, the light‐induced bleaching of the sample would avoid inner filter effects, a factor that limits the efficiency of photoisomerization for strongly colored photoswitches, especially at high concentrations. [24]
The reason behind the thermodynamical stability of Z‐ITI, as compared with E‐ITI (by 12.6 kJ mol−1), is the limited repulsion between the phenyl group and the sulfur atom of the benzothiophene‐3‐one moiety in Z‐ITI, whereas E‐ITI is destabilized by steric clashes between the phenyl ring and the carbonyl group (see Figure 1 B). [23] Using computational modeling, we realized that replacing the sulfur with bulkier N‐R moieties destabilizes the Z‐form and inverts the relative population of the two photoisomers, with N‐Ac affording a ΔG of 26.7 kJ mol−1 favoring the E‐form (Figure 1 B, see Supporting Information for computational analysis). Similar effects have been observed for hemiindigo P‐type photoswitches upon alkylation of the nitrogen atom. [25] In addition, the introduction of bulkier substituents reduces the predicted thermal isomerization barrier of the metastable form (see Figure 1 B) and increases its thermal lability. T‐type photoswitches characterized by a fast thermal recovery [26] are applied as photoactuators in optical lenses, [27] super‐resolution microscopy, [28] real‐time holography, [29] photomechanical systems,[ 30 , 31 ] and are designed as photopharmacological tools. [32]
Taking the challenge to reverse the stability of the isomers, i.e., from positive to negative photochromism while maintaining a large band separation in the visible, we designed phenylimino indolinone (PIO, see Figure 1 C), expected to be more sterically hindered than ITI, by the synthetically facile introduction of an N‐acetyl moiety into its core part. The synthesis of PIO consisted of a three‐step procedure starting from 1H‐indol‐3‐yl acetate with an overall 80 % yield (see SI for the detailed synthetic procedure and characterization). The synthesis afforded a purple solid absorbing at 508 nm in cyclohexane (see Table 1); characteristics that closely matched the computed TD‐DFT spectrum of the E‐isomer (see SI). The predicted difference in the absolute configuration of the stable isomers of PIO and ITI was confirmed by X‐ray analysis (see Figure 1 A and 1C). E‐PIO shows a very limited solvatochromism, in line with what has been observed in ITI, [23] and relatively low ϵ values (see Table 1).
Table 1.
Main photophysical, photochemical and thermal parameters for PIO in various solvents.
|
Solvent |
Φ EZ [a] [%] |
E λ max [nm] |
ϵ/1000 [M−1 cm−1] |
Z λ max [nm][a,b] |
τ ZE [μs][a] |
|---|---|---|---|---|---|
|
Cyclohexane |
9.0 |
508 |
0.93 |
426 |
27 |
|
Toluene |
6.7 |
510 |
0.93 |
436 |
77 |
|
DCM |
6.9 |
509 |
1.0 |
437 |
74 |
|
MeOH |
11.6 |
498 |
0.62 |
431 |
111 |
|
MeCN |
5.6 |
500 |
0.78 |
435 |
76 |
|
DMSO |
4.8 |
496 |
0.91 |
435 |
67 |
[a] Measured with ns transient absorption spectroscopy at 20 °C. [b] Data inferred from the λ max of the positive transient signal recorded with ns transient absorption spectroscopy after excitation at λ=510 nm.
Indeed, the S0→S1 transition is only partially allowed (f=0.02, predicted in vacuo at the TD‐M062X/6–311+G(2d,p) level of theory). [23] This transition has a mixed n→π* and π→π* character (see SI). On the other hand, the simulated UV/Vis spectrum of the metastable Z‐isomer predicted the expected negative photochromism, when compared with the E‐form (see Figure 1 C).
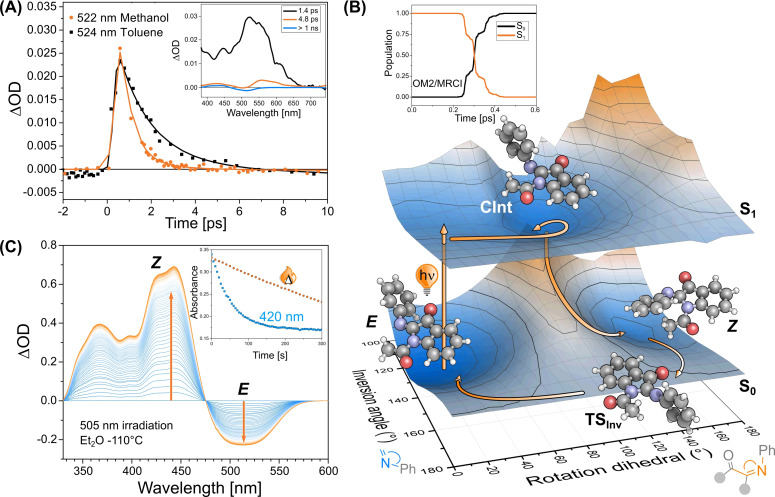
Encouraged by these results, we studied the excited‐state behavior of PIO. We recorded femtosecond transient absorption (TA) spectra of E‐PIO in different organic solvents upon excitation at 500 nm (cf. Figure 2 A for MeOH and toluene and Table S14). In toluene, directly after excitation we observed an intense and broad excited state absorption band with λ max at ca. 524 nm (see Figure 2 A, inset). The intensity of this signal is significantly reduced in about 1.4 ps (τ1). In analogy to ITI, we interpret this initial decay as the excited‐state evolution toward the S1→S0 conical intersection (CInt, see Figure 2 B). This transient evolves, forming two positive signals peaked at 425 and 550 nm. The latter band decays completely in 4.8 ps (τ2). These two positive bands are attributed to absorption from the hot ground state of Z‐ and E‐PIO, respectively. The final spectral component has a differential line shape with a negative contribution in the 470–550 nm region where ground state absorption of the initial isomer is observed, and a positive product band at shorter wavelengths (ca. 70–80 nm blue‐shifted, see Table 1). The persistence of these long‐living contributions confirms the photoisomerization and the predicted negative photochromism of PIO.
Figure 2.
Spectroscopic and computational studies on the isomerization of PIO. (A) Comparison of the short timescale kinetic traces of the excited‐state absorption band of PIO dissolved in toluene and methanol, upon excitation at 500 nm. Inset: Evolution‐associated difference spectrum (EADS) obtained from global analysis of transient absorption data recorded for PIO in toluene upon excitation at 500 nm. (B) Representation of the full isomerization cycle in PIO. Surfaces obtained as a SF‐BH&HLYP/cc‐pVDZ single point calculation on the geometry optimized at the TD‐ωB97X‐D/MIDI! (S1) or GFN2‐xTB (S0) level of theory. Inset: Population of S0 and S1 states as a function of time obtained from OM2/MRCI NAMD calculations. (C) Differential spectra of E‐PIO in Et2O (3×10−4 M) upon irradiation with a 505 nm LED. PSS was reached after 22 min. Inset: kinetics observed at 443 nm when irradiating Z‐PIO with 420 nm LED vs. thermal isomerization.
The excited‐state decay is observed to be faster in polar solvents than in non‐polar ones (see Figure 2 A and SI). The Φ EZ values are slightly higher than the ones found for ITI (see Table 1), [23] showing a tendency of the excited system to form the Z‐isomer with 5–12 % efficiency. We qualitatively reproduced this trend by mapping the ground and excited state with spin‐flip DFT (SF‐DFT) and simulating the first picoseconds after excitation using nonadiabatic molecular dynamics simulations (NAMD) at the OM2/MRCI level of theory, starting from 320 trajectories of E‐PIO in a canonical ensemble (see Figure 2 B and SI).[ 33 , 34 ] After excitation, PIO undergoes a barrierless rotation of the C=N bond [19] that drives the system toward the region of the CInt. When reaching a perpendicular arrangement of the imine bond, the molecule hops to the ground state with a predicted lifetime τS1 of 250 fs and quantum yield of 24 %, which correctly mirrors the preference of the system toward the photochemical recovery of the E‐form.
Low‐temperature NMR confirmed the predicted geometrical features of Z‐PIO (see SI). Upon irradiation at λ=505 nm, the signal attributed to the acetyl CH3 shifted upfield about 1.2 ppm, due to the shielding exerted by the phenyl ring in the Z form (see SI). The thermal recovery of Z‐PIO was followed by ns transient absorption spectroscopy. Table 1 shows that the thermal isomerization process is influenced only to a minor extent by the solvent with recovery times τ ZE ranging from 27 to 111 μs.
Irradiating E‐PIO with a 505 nm LED at −110 °C in Et2O (Figure 2 C) enabled to reach a photostationary state (PSS) with a distribution of 80:20 of the Z and E isomers as determined from low‐temperature NMR (see SI), a value higher than the one reported for ITI, as expected due to the negative photochromism of PIO. After achieving the Z‐PIO‐enriched PSS under irradiation with 505 nm light in the UV/Vis at −110 °C, we were able to switch it back to the E‐isomer by irradiating the band associated with Z‐PIO with a 420 nm LED (Figure 2 C, inset and SI). Thus, we could also prove that reversibility of the photochemical reaction can be achieved using light, by increasing the thermal stability of PIO at low temperatures for enhanced temporal control by P‐type isomerization. In addition, we followed the thermal decay of the same band with λ max at 443 nm at increasing temperatures, and consequently determined the ΔG ≠ for the thermal isomerization to be 48.3 kJ mol−1 (see SI). Using these values, a lifetime of 73 μs is extrapolated for 20 °C, which nicely agrees with the data recorded with ns TA spectroscopy at room temperature in other solvents.
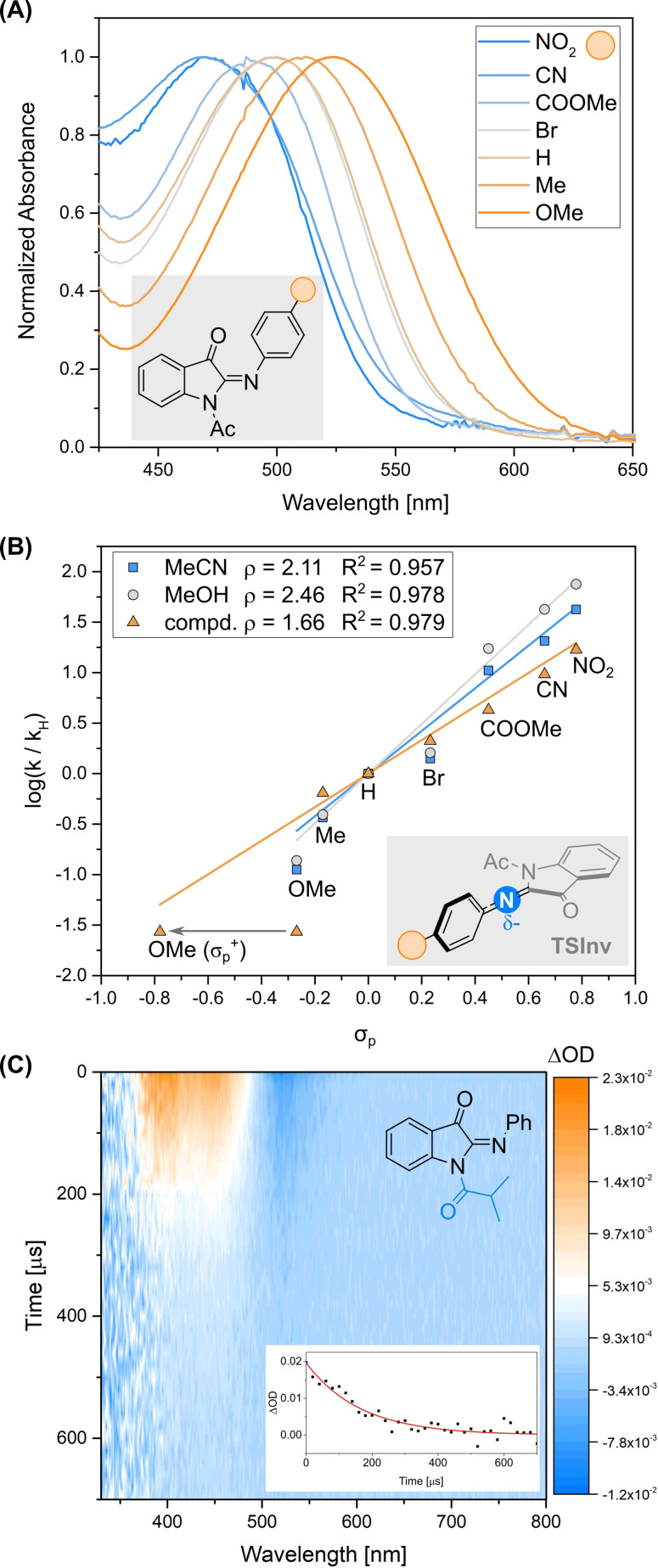
The electronic nature of the substituents on the phenyl moiety has a remarkable effect on the properties of PIO. While the UV/Vis absorption is bathochromically shifted by the presence of electron‐donating groups (see Figure 3 A), the thermal Z→E transformation can be tuned from τ=1.5 μs when NO2 is used to 800 μs for MeO (both values in MeOH, see Figure 3 B and SI). Probing the kinetics in both MeOH and MeCN for seven differently phenyl‐substituted PIOs, we obtained the two Hammett plots depicted in Figure 3 B. In both solvents, the positive ρ value (2.11 for MeCN and 2.46 for MeOH) and the relatively high linearity of the plots reflect the stabilization effect of electron‐withdrawing groups on the partial negative charge on the imine nitrogen while reaching the inversion transition state geometry (TSInv, Figure 3 B), as correctly predicted by DFT when the Z→E thermal isomerization proceeds via nitrogen inversion. For PIO, the Natural charge at the imine nitrogen obtained from NBO analysis was calculated to slightly increase going from the Z form to the transition state (see Table S16). This tendency is more marked when electron‐donating substituents are present, while it shows opposite sign in the case of electron‐withdrawing ones (see δ− and δδ− values reported in Table S16). Indeed, the substituents affect the predicted activation energies following the same experimental trend, with a computed positive ρ=1.66 in the simulated Hammett plot (see Figure 3 B).
Figure 3.
Properties of substituted PIO derivatives. (A) Normalized UV/Vis spectra of substituted PIO derivatives. (B) Hammett plots for the thermal back‐isomerisation step kinetics, obtained using the σp values for the phenyl‐substituted PIO derivatives in MeCN and MeOH (measured at 20 °C) compared to the computed values obtained at the M06‐2X/def2‐SVP level of theory in vacuo. For the latter, a better correlation was obtained employing the σp + value for OMe. (C) Heatmap of the ns transient absorption spectra of iBu‐PIO. In the inset, fit of the decay of the absorption maximum (395 nm) of the transient signal (red line) as obtained from a global analysis of the transient spectra.
To further probe the generality of this new switch towards functionalization, we focused on the amidic nitrogen and studied the synthetically easily accessible iso‐butyryl PIO derivative (iBu‐PIO, Figure 3 C). Modifications of the substituent α to the amidyl CO has only a limited effect compared to the acetyl group, both in terms of quantum yields (3–6 %) and lifetimes (in MeCN, τ=164 μs, see Figure 3 C and Table S15). The latter are similar, yet slightly longer compared to the unsubstituted PIO (Figure 3 B), an effect attributable to an increased destabilization of the TSInv, which could be correctly modelled by DFT (see Table S16), validating our predictive method for this new class of photoswitches.
In conclusion, using an extensive spectroscopic and computational characterization, we demonstrated that negative photochromism in phenyl iminoindigoid photoswitches can be achieved by increasing the steric bulk of the bicyclic core. Simultaneously, we could retain the pronounced band separation of ca. 80 nm between the two photoisomers typical of the class. In this way, we designed and synthesized PIO, a novel T‐type photoswitch that is responsive to green light.
The photochromism of PIO and its derivatives constitutes one of the few cases in which the simple molecular engineering allowed for the inversion of the stability of the isomers in the photoswitch. [25] A similar inversion was achieved for example, for azobenzenes, but requires the covalent linking of the two aromatic rings to enforce the higher stability of the usually metastable cis isomer. [35] Visible‐light responsive T‐type photoswitches with negative photochromism reported here are interesting candidates for designing new molecular machines and photoresponsive molecular systems.[ 7 , 27 , 28 , 29 , 30 , 31 , 32 ]
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
We gratefully acknowledge the generous support from the Marie Skłodowska‐Curie Actions (Individual Fellowship 838280 to S.C.), the Alexander‐von‐Humboldt Foundation (Feodor‐Lynen Fellowship to N.A.S.), the Horizon 2020 Framework Program (ERC Advanced Investigator Grant No. 694345 to B.L.F.), the Ministry of Education, Culture and Science of the Netherlands (Gravitation Program No. 024.001.035 to B.L.F.), and the Netherlands Organization for Scientific Research (NWO, VIDI grant no. 723.014.001 for W.S.). M.D.D. and S.D. acknowledge the European Union Horizon 2020 research and innovation program under grant agreement n. 871124 Laserlab‐Europe. R.T. gratefully acknowledges the Japan Society for the Promotion of Science for an Overseas Research Fellowship. We thank Prof. Browne and Dr. Klein for their support with recording the VT‐UV/Vis spectra. We would like to thank the Center for Information Technology of the University of Groningen for their support and for providing access to the Peregrine high performance computing cluster.
S. Crespi, N. A. Simeth, M. Di Donato, S. Doria, C. N. Stindt, M. F. Hilbers, F. L. Kiss, R. Toyoda, S. Wesseling, W. J. Buma, B. L. Feringa, W. Szymański, Angew. Chem. Int. Ed. 2021, 60, 25290.
Contributor Information
Dr. Stefano Crespi, Email: s.crespi@rug.nl.
Prof. Dr. Ben L. Feringa, Email: b.l.feringa@rug.nl.
Prof. Dr. Wiktor Szymański, Email: w.szymanski@umcg.nl.
References
- 1. Feringa B. L., Browne W. R., Molecular Switches, Wiley-VCH, Weinheim, Germany, 2011. [Google Scholar]
- 2. Balzani V., Credi A., Venturi M., Chem. Soc. Rev. 2009, 38, 1542–1550. [DOI] [PubMed] [Google Scholar]
- 3. Kathan M., Hecht S., Chem. Soc. Rev. 2017, 46, 5536–5550. [DOI] [PubMed] [Google Scholar]
- 4. Aprahamian I., ACS Cent. Sci. 2020, 6, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kistemaker J. C. M., Lubbe A. S., Feringa B. L., Mater. Chem. Front. 2021, 5, 2900–2906. [Google Scholar]
- 6. Bouas-Laurent H., Dürr H., Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar]
- 7. Velema W. A., Szymanski W., Feringa B. L., J. Am. Chem. Soc. 2014, 136, 2178–2191. [DOI] [PubMed] [Google Scholar]
- 8. Bruns C. J., Stoddart J. F., The Nature of the Mechanical Bond, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016. [Google Scholar]
- 9. Balzani V., Credi A., Venturi M., Molecular Devices and Machines: Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, Germany, 2008. [Google Scholar]
- 10. Welleman I. M., Hoorens M. W. H., Feringa B. L., Boersma H. H., Szymański W., Chem. Sci. 2020, 11, 11672–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Irie M., Fukaminato T., Matsuda K., Kobatake S., Chem. Rev. 2014, 114, 12174–12277. [DOI] [PubMed] [Google Scholar]
- 12. Belowich M. E., Stoddart J. F., Chem. Soc. Rev. 2012, 41, 2003–2024. [DOI] [PubMed] [Google Scholar]
- 13. Kandappa S. K., Valloli L. K., Ahuja S., Parthiban J., Sivaguru J., Chem. Soc. Rev. 2021, 50, 1617–1641. [DOI] [PubMed] [Google Scholar]
- 14. Greb L., Lehn J. M., J. Am. Chem. Soc. 2014, 136, 13114–13117. [DOI] [PubMed] [Google Scholar]
- 15. Greb L., Eichhöfer A., Lehn J.-M., Angew. Chem. Int. Ed. 2015, 54, 14345–14348; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 14553–14556. [Google Scholar]
- 16. Li Q., Qian H., Shao B., Hughes R. P., Aprahamian I., J. Am. Chem. Soc. 2018, 140, 11829–11835. [DOI] [PubMed] [Google Scholar]
- 17. Van Dijken D. J., Kovaříček P., Ihrig S. P., Hecht S., J. Am. Chem. Soc. 2015, 137, 14982–14991. [DOI] [PubMed] [Google Scholar]
- 18. Georgiev A., Yordanov D., Dimov D., Zhivkov I., Nazarova D., Weiter M., J. Photochem. Photobiol. A Chem. 2020, 393, 112443. [Google Scholar]
- 19. Lehn J.-M., Chem. Eur. J. 2006, 12, 5910–5915. [DOI] [PubMed] [Google Scholar]
- 20. Crespi S., Simeth N. A., König B., Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar]
- 21. Su Q., Li Y., Wang B., Liu M., Wang H., Wang W., Liu F., J. Phys. Chem. A 2017, 121, 2588–2596. [DOI] [PubMed] [Google Scholar]
- 22. Klán P., Wirz J., Photochemistry of Organic Compounds, John Wiley & Sons, Ltd, Chichester, UK, 2009. [Google Scholar]
- 23. Hoorens M. W. H., Medved“ M., Laurent A. D., Di Donato M., Fanetti S., Slappendel L., Hilbers M., Feringa B. L., Jan Buma W., Szymanski W., Nat. Commun. 2019, 10, 2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiken S., Edgar R. J. L., Gabbutt C. D., Heron B. M., Hobson P. A., Dyes Pigm. 2018, 149, 92–121. [Google Scholar]
- 25. Petermayer C., Thumser S., Kink F., Mayer P., Dube H., J. Am. Chem. Soc. 2017, 139, 15060–15067. [DOI] [PubMed] [Google Scholar]
- 26. Kitagawa D., Nakahama T., Nakai Y., Kobatake S., J. Mater. Chem. C 2019, 7, 2865–2870. [Google Scholar]
- 27. Inagaki Y., Kobayashi Y., Mutoh K., Abe J., J. Am. Chem. Soc. 2017, 139, 13429–13441. [DOI] [PubMed] [Google Scholar]
- 28. Deniz E., Tomasulo M., Cusido J., Yildiz I., Petriella M., Bossi M. L., Sortino S., Raymo F. M., J. Phys. Chem. C 2012, 116, 6058–6068. [Google Scholar]
- 29. Kobayashi Y., Abe J., Adv. Opt. Mater. 2016, 4, 1354–1357. [Google Scholar]
- 30. Zhu L., Tong F., Salinas C., Al-Muhanna M. K., Tham F. S., Kisailus D., Al-Kaysi R. O., Bardeen C. J., Chem. Mater. 2014, 26, 6007–6015. [Google Scholar]
- 31. Gelebart A. H., Mulder D. J., Varga M., Konya A., Vantomme G., Meijer E. W., Selinger R. L. B., Broer D. J., Nature 2017, 546, 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klaue K., Garmshausen Y., Hecht S., Angew. Chem. Int. Ed. 2018, 57, 1414–1417; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 1429–1432. [Google Scholar]
- 33. Kazaryan A., Lan Z., Schäfer L. V., Thiel W., Filatov M., J. Chem. Theory Comput. 2011, 7, 2189–2199. [DOI] [PubMed] [Google Scholar]
- 34. Thiel W., WIREs Comput. Mol. Sci. 2014, 4, 145–157. [Google Scholar]
- 35. Siewertsen R., Neumann H., Buchheim-Stehn B., Herges R., Näther C., Renth F., Temps F., J. Am. Chem. Soc. 2009, 131, 15594–15595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information