Abstract
An in vivo experiment was performed with pigs to study the inhibitory effect of fermented feed on the bacterial population of the gastrointestinal tract. Results demonstrated a significant positive correlation between pH and lactobacilli in the stomach contents of pigs in dry feed as well as in the stomach contents of pigs fed fermented feed. Furthermore, a significant positive correlation between the pH and the numbers of bacteria in the family Enterobacteriaceae in the contents of the stomach of pigs fed dry feed was found. In the stomach contents of pigs fed fermented feed, a significant negative correlation was found between the concentration of the undissociated form of lactic acid and the numbers of Enterobacteriaceae. The numbers of Enterobacteriaceae in the contents of the stomach, ileum, cecum, colon, and rectum of pigs fed fermented feed were significantly lower compared with the contents of the stomach, ileum, caecum, colon, and rectum of pigs fed dry feed. The numbers of total lactobacilli were significantly higher in the stomach contents of pigs fed fermented feed and in the ileum contents of one pig group fed fermented feed compared with the contents of pigs fed dry feed. However, the influence of lactobacilli on numbers of Enterobacteriaceae could not be demonstrated. It was concluded that fermented feed influences the bacterial ecology of the gastrointestinal tract and reduces the levels of Enterobacteriaceae in the different parts of the gastrointestinal tract.
Nowadays, there is growing attention on fermented pig feed because it might improve growth performance (8) and might influence the bacterial ecology of the gastrointestinal tract (GIT), in particular members of the family Enterobacteriaceae, including Salmonella spp. (32). At the farm level, one can divide the influence of fermented feed on Salmonella spp. in two different mechanisms, as was observed by Urlings et al. (32): the effect of fermented feed itself on Salmonella spp. and the influence of fermented feed on bacterial ecology of the GIT of the pig.
Fermented feed contains high concentrations of lactic acid, several volatile fatty acids (VFA; acetic acid, butyric acid, and propionic acid), and large numbers of lactobacilli and has a low pH. These four parameters can have, alone or combined, an effect on bacterial ecology of the GIT; lactic acid and VFA are also produced by the indigenous microflora in the GIT (3). Lactic acid and VFA are believed to play a role in reducing the numbers of Enterobacteriaceae, including Salmonella spp. (23, 35). Only the undissociated form of lactic acid and VFA can have a bactericidal or bacteriostatic effect (26). The exact concentrations of these undissociated acids and the possible effect on bacterial groups in the content of the stomach and other sites of intestinal tract in pigs fed fermented feed are not known. Fermented feed may reduce the pH in the entire GIT (5, 13, 24), thereby enhancing the effect of VFA on Enterobacteriaceae and probably on Salmonella spp.
Another hypothesis for Salmonella reduction is that lactobacilli ingested with fermented feed compete with (potential) pathogenic bacteria in the GIT by strengthening colonization resistance (21). However, the effect of feed-associated lactobacilli on GIT bacterial ecology remains unknown. Very little research has been performed in tracking feed-associated lactobacilli in the GIT of the pig and their possible influence on the bacterial ecology of the GIT. Another mechanism proposed to explain Salmonella reduction is the enhancement of digestibility of nutrients in the small intestine by feed fermentation, resulting in fewer substrates for microbial growth in the lower part of the intestinal tract (7, 19).
To study the different mechanisms discussed above, an experiment was carried out in which pigs were fed Lactobacillus plantarum-fermented feed and challenged with Salmonella spp. The metabolic activity of the microflora was investigated by the comparison of VFA concentrations in the GIT. The concentrations of undissociated VFA were calculated using the Henderson-Hasselbach equation.
MATERIALS AND METHODS
Pigs, housing, and experimental groups.
Conventionally raised piglets (10 weeks old, crossbred Landrace × Yorkshire) were obtained from a Dutch farm. The farm was Salmonella free, based on repeated testing (five times) of the herd by both serological and bacteriological techniques. The pigs were adapted to their environment for 18 days before the experiment. To avoid cross-contamination, the experimental groups were housed in separate units made of 1.5-m-high and 10-cm-thick concrete walls. The absence of Salmonella spp. in the units was checked by using swabs as described by Van den Elzen and Snijders (33).
Two groups (12 pigs per group) were fed dry pig feed, and two groups (12 pigs per group) were fed fermented pig feed. Two pigs in each group (seeder pigs) were orally challenged with one of the Salmonella isolates. The groups were as follows: group 1: dry feed, Salmonella enterica serovar Goldcoast (serovar Goldcoast); group 2: fermented feed, serovar Goldcoast; group 3: dry feed, Salmonella enterica serovar Typhimurium DT12; and group 4: fermented feed, serovar Typhimurium DT12.
Serovar Typhimurium DT12 was isolated from a pig mesenteric lymph node (kindly provided by D. L. Bagessen, Danish Veterinary Laboratory, Copenhagen, Denmark), and serovar Goldcoast was isolated from a pig slaughterhouse lairage (kindly provided by M. Swanenburg, ID-Lelystad, Lelystad, The Netherlands).
Challenge experiment.
The Salmonella strains were grown separately in 10 ml of brain heart infusion broth (Oxoid CM 225). After incubation (24 h, 37°C), the culture was spun down (10 min at 2,000 × g), the supernatant was discarded, and the pellet was resuspended in 10 ml of sterile 0.9% saline. Two pigs (seeder pigs) from each group were separated from their pen for 10 min for the inoculation. The two seeder pigs from each group received approximately 5.0 × 108 CFU of the respective strain in 25 of dry feed. Twenty hours before inoculation, all pigs were allowed to have water but no feed. Within 10 min after inoculation, the seeder pigs were replaced in their pens, and all pigs had access again to feed.
Sampling of gastrointestinal contents.
At 56 days after Salmonella inoculation, all animals in each group were euthanized with Euthesate (Apharmo, Arnhem, The Netherlands), and necropsy was performed by a pathologist. The intestinal tract was separated into three segments: the stomach, small intestine, and large intestine. The stomach was tied off at the esophageal and pyloric sphincters. The small intestine was tied off at the ileocecal junction. Contents of stomach, ileum (incision of 10 cm for the ileocecal junction), cecum, colon (incision of 10 cm after the ileocecal junction), and rectum were collected in sterile stomacher bags (Labsystem model 400).
Feed.
The pigs received a basic formulated pelleted feed. The feed contained 200 g of barley, 282 g of corn, 200 g of wheat, 105 g of soy expeller, 55 g of sugar beet, 40 g of sugar cane molasses, 8 g of animal fat, 75 g of potato protein, 12.5 g of calcium phosphate, 7.5 g of calcium carbonate, 5 g of salt, and 10 g of vitamin-mineral mix per kg. The vitamin-mineral mix contains vitamins A (9,000 IE), D3 (1,800 IE), and E (40 mg); riboflavin (5 mg); niacinamide (30 mg); d-pantothenic acid (12 mg); choline chloride (350 mg); vitamins B12 (40 μg), K (3 mg), and C (50 mg); folic acid (1 mg); and biotin (0.1 mg) (per kilogram of feed). Mineral mix contained CoSO4 · 7H2O (2.5 mg), ZnSO4 · H2O (20 mg), Na2SeO3 · 5H2O (0.2 mg), KJ (0.5 mg), FeSO4 · 7H2O (400 mg), and MnO2 (70 mg) (per kilogram of feed). Protein content was 17.2%. Moderate grinding was used. Neither antibiotic growth promoters nor copper was added. The pigs were fed ad libitum. Drinking water was given ad libitum, was not chlorinated, and did not contain any additives.
Feed fermentation.
The feed described above was used for fermentation. In a sterile 20-liter fermentor (Applicon Dependable Instruments, Schiedam, The Netherlands) 1 part feed was mixed with 2 parts sterile water (30 min at 600 rpm and 20°C). The mixing speed was lowered to 300 rpm to avoid foaming, and an L. plantarum (Purac, Gorinchem, The Netherlands; this strain was used in animal feed fermentation by Urlings et al. [31, 32], stored at −80°C in 16% sterile glycerol solution) culture (deMan-Rogosa-Sharpe broth; Merck 1.10661) (3 days, 20°C) was added in a concentration of approximately 106 CFU/ml of feed. Feed fermentation was continued for 2 days at 20°C. This fermented feed was used as a starter culture to prepare the fermented feed for the pigs. Five parts dry pelleted feed were mixed with 10 parts water and 1 part starter culture in 20-liter buckets. This product was mixed thoroughly, and the feed was fermented in closed buckets for 2 to 3 days at 20°C.
The fermentation of the feed was checked by means of L. plantarum counts on Lactobacillus-lactitol-vancomycin (LLV) plates as described by Norikatsu et al. (21). Total lactobacilli, Enterobacteriaceae counts, and the presence of Salmonella spp. were determined as described by Mossel et al. (20). VFA and lactic acid analysis was performed with high performance liquid chromatography (HPLC) as described by van Winsen et al. (36). The criteria for the fermented feed were: pH, <4.5; lactic acid, >150 mmol/liter; acetic acid, <40 mmol/liter; butyric acid, <5 mmol/liter; ethanol, <0.8 mmol/liter; total lactobacilli, >9 log CFU ml−1; L. plantarum, >9 log CFU ml−1; Enterobacteriaceae, < 1.8 log CFU ml−1; Salmonella, none in 25 ml.
Bacteriological analyses.
A total of 25 g of the respective GIT content was put into 225 ml of sterile buffered peptone water (BPW; peptone, 10 g liter−1; NaCl, 5 g liter−1; Na2HPO4 · 2H2, 4.5 g liter−1; KH2PO4, 1.5 g liter−1; pH 7.2 ± 0.1) and mixed with a stomacher (Seward stomacher 400) for 2 min. A 10-fold dilution (1 ml into 9 ml of BPW) was prepared until 10−10. The BPW dilutions were used for counts of Enterobacteriaceae, total lactobacilli, and L. plantarum Enterobacteriaceae were cultured on pour plates of violet red bile glucose (VRBG; Oxoid CM485) (20 to 24 h at 37°C) (32). For total lactobacilli counts, lactobacilli were cultured on ROGOSA plates (Oxoid CM627) as described by Snel et al. (29). All colonies on the ROGOSA plates were counted as lactobacilli. L. plantarum counts were determined on LLV plates as described by Norikatsu et al. (22), and five colonies were selected randomly from the plates and after growth in deMan-Rogosa-Sharpe broth (Merck 1.10661) and confirmed as the specific bacterial group by API-50CHL (bioMérieux, Marcy l'Etoile, France). Numbers of CFU are expressed as log CFU per gram. For Salmonella semiquantitative counts, the BPW dilutions were incubated for 20 to 24 h at 37°C. From each dilution of BPW, 0.1 ml was transferred into 10 ml of Rappaport-Vassiliadis (RV) bouillon (Oxoid CM669). After incubation of the RV (20 to 24 h at 41.5°C), selective isolation was continued by plating out on brilliant green agar (BGA; Oxoid CM329) (20 h at 37°C). Suspected Salmonella colonies were confirmed by biochemical and serological techniques (1).
VFA analysis and pH measurement of GIT contents.
Approximately 1 g of GIT content was resuspended in 4 ml of Milli-Q water (Millipore Ultra System, Etten-heur, The Netherlands). The pH of the samples was directly measured with a pH meter. After measurement, the samples were immediately frozen (−20°C) until analysis. VFA and lactate analysis was performed by HPLC as described by van der Wielen et al. (34) with some modifications for GIT contents of the pig. After thawing, the samples were mixed on a Vortex mixer and centrifuged (2,000 × g for 10 min), and 600 μl of supernatant was mixed with a 100 μl of 100 mM HC1–50 mM crotonic acid as an internal standard (crotonic acid from Acros Organics, Fairfield, N.J.).
Concentrations of undissociated lactic acid and VFA were calculated using the Henderson-Hasselbach equation (pH = pKa + 10log [A−]/[HA], where [A−] represents the concentration of dissociated acids and [HA] represent the concentration of undissociated acids), pHs, total concentration of each VFA and lactic acid, and the pKa values of acetic acid (4.75), butyric acid (4.81), propionic acid (4.87), and lactic acid (3.84) (2).
Data analyses.
Correlations between bacterial counts, pH, concentration of total and undissociated VFA, and concentration of total and undissociated lactic acid in contents of stomach, ileum, cecum, colon, and rectum were calculated and analyzed statistically with Pearson's correlation. General linear model statistics were used to compare the groups, with the gastrointestinal tract as a within factor and feed as a between factor. The data from the two seeder pigs were excluded from the Pearson's correlation and statistical procedures. Pearson's correlation and statistical procedures were calculated with the use of SPSS 7.5 software.
RESULTS
Feed.
The physical, chemical, and bacteriological results of the dry and fermented feeds are presented in Table 1. The most important differences between the dry feed and the fermented feed can be found for L. plantarum counts, lactic acid, acetic acid concentrations, and pH.
TABLE 1.
Bacteriological and physicochemical parameters of dry feed and fermented feeda
| Parameter | Normal feed (n = 35) (SD) | Fermented feed (n = 37) (SD) |
|---|---|---|
| L. plantarum (log CFU g−1) | <2.8 | 9.4 (0.26)** |
| Enterobacteriaceae (log CFU g−1) | <1.8 | <1.8 |
| Salmonella spp. | ND (in 25 g) | ND (in 25 ml) |
| pH | 5.7 (0.1) | 4.1 (0.2)** |
| Lactate (mmol/liter) | 20 (5) | 261 (20)** |
| Acetate (mmol/liter) | <0.8 | 25 (13)** |
| Butyrate (mmol/liter) | <0.8 | 2.3 (1.5)* |
| Ethanol (mmol/liter) | <0.8 | <0.8 |
| Propionate (mmol/liter) | <0.8 | <0.8 |
<0.8, <1.8, and <2.8, under detection limit. ND, not detected. Significance: *, P < 0.05; **, P < 0.01.
Numbers of total lactobacilli and L. plantarum in GIT contents.
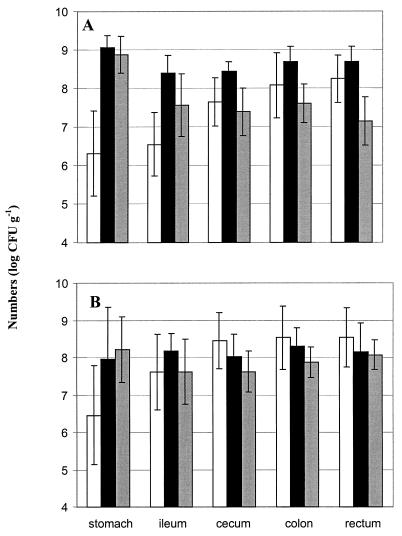
The numbers of total lactobacilli and L. plantarum in the GIT contents are shown in Fig. 1. In general, the total numbers of lactobacilli in the stomach contents (9.0 log CFU g−1) of the fermented-feed groups were significantly higher (feed effect, P < 0.01) than in the dry feed group, and the level remained the same up to the rectum. In the dry feed groups, the lactobacillus numbers were increased in ileum content and cecum content compared with the stomach content (GIT effect, P < 0.01).
FIG. 1.
Total lactobacilli and L. plantarum numbers in contents of different sites of the GIT of pigs fed dry feed or fermented feed in the presence of serovar Goldcoast (A) and in the presence of serovar Typhimurium (B). Open and solid bars, total lactobacillus numbers in GIT contents of pig fed dry feed or fermented feed, respectively; shaded bars, L. plantarum numbers in GIT contents of pigs fed fermented feed. L. plantarum numbers in GIT contents of pigs fed dry feed were under the detection level of 4 log CFU (of content)−1. Results are presented as means for 10 pigs ± standard deviation.
The L. plantarum numbers in the stomach contents of the fermented-feed groups were of the same level as the total lactobacillus counts in the stomach contents of the fermented-feed groups (Fig. 1). L. plantarum numbers were lower in the ileum contents in both fermented-feed groups. In the ileum contents, a greater decrease was observed in fermented feed group 2 compared with fermented feed group 4, and the L. plantarum numbers differed significantly compared with the total lactobacilli in the ileum content. Beyond the ileum, the L. plantarum numbers remained at the same level in contents of cecum, colon, and rectum in both fermented-feed groups.
Salmonella recovery in GIT contents.
Serovar Goldcoast was not recovered from GIT contents of pigs fed either dry or fermented feed. In the dry-feed group, serovar Typhimurium was recovered from the contents of cecum, colon, and rectum from one seeder pig. In the other seeder pig, serovar Typhimurium was recovered from stomach content. In the fermented-feed group, serovar Typhimurium was recovered from contents of cecum and colon from one seeder pig.
Numbers of Enterobacteriaceae in GIT contents.
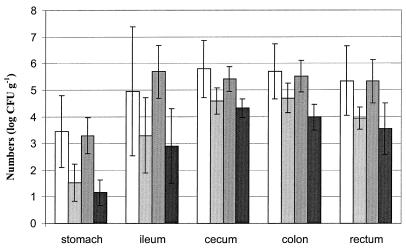
In Fig. 2, the numbers of Enterobacteriaceae in the contents of the GIT are shown. In general, in all groups the contents of the distal parts of the GIT showed higher numbers of Enterobacteriaceae (GIT effect, P < 0.01). The numbers of Enterobacteriaceae in contents of stomach, ileum, cecum, colon, and rectum were significantly lower in the fermented-feed groups (feed effect, P < 0.01) compared with the GIT contents of pigs fed dry feed.
FIG. 2.
Numbers of Enterobacteriaceae measured in gastrointestinal contents of pigs fed dry feed and fermented feed. From left to right, each group of four bars represent dry-feed group 1, light-shaded fermented-feed group 2, dry-feed group 3, and fermented-feed group 4. Results are presented as means for 10 pigs ± standard deviation.
pH in GIT contents.
The pH in stomach contents in both fermented-feed groups was significantly lower (feed effect, P < 0.01) (Table 2) compared with the pH in the stomach contents of the dry-feed groups. In the contents of ileum, cecum, and colon, no significant differences were found, except in the content of the rectum; the pH of the rectum content was significantly higher in the fermented-feed groups compared with the pH of the rectum content of the dry-feed groups (feed effect, P < 0.01).
TABLE 2.
pH and undissociated concentrations of lactate, acetate, butyrate, and propionate in gastrointestinal contents from pigs fed normal and fermented feeda
| Contents | Group | Feed | Mean pH (SD) | Mean concnb (μmol/g) (SD)
|
|||
|---|---|---|---|---|---|---|---|
| Lactate | Acetate | Butyrate | Propionate | ||||
| Stomach | 1 | Normal | 4.6 (0.9)* | 0.9 (0.2)** | 0.8 (0.5)* | < | < |
| 2 | Fermented | 3.7 (0.2) | 63.4 (13.7) | 8.7 (3.6) | < | < | |
| 3 | Normal | 4.2 (0.6)* | 2.8 (3.5)** | 0.7 (1.1) | < | < | |
| 4 | Fermented | 3.3 (0.4) | 91.8 (42.2) | 6.9 (11.9) | < | < | |
| Ileum | 1 | Normal | 7.3 (0.5) | 0 | < | < | |
| 2 | Fermented | 7.0 (0.5) | 0 | < | < | ||
| 3 | Normal | 7.2 (0.5) | 0 | < | < | ||
| 4 | Fermented | 7.3 (0.5) | 0 | < | < | ||
| Cecum | 1 | Normal | 6.5 (0.3) | 0 | 2.3 (2.2) | 0.4 (0.4) | 0.6 (0.6) |
| 2 | Fermented | 6.2 (0.4) | 0 | 3.7 (2.9) | 0.6 (0.6) | 2.4 (2.1) | |
| 3 | Normal | 6.2 (0.3) | 0 | 0.5 (0.7) | 0.5 (0.4) | 1.2 (0.9) | |
| 4 | Fermented | 6.5 (0.4) | 0 | 0.3 (0.2) | 0.3 (0.3) | 1.6 (1.6) | |
| Colon | 1 | Normal | 6.2 (0.4) | 0 | 3.6 (2.8) | 3.6 (2.8) | 1.8 (1.3) |
| 2 | Fermented | 5.9 (0.5) | 0 | 7.8 (6.2) | 1.8 (1.6) | 4.9 (3.7) | |
| 3 | Normal | 5.6 (0.2) | 0 | 8.7 (5.7) | 2.3 (1.3) | 5.7 (3.2) | |
| 4 | Fermented | 5.6 (0.2) | 0 | 9.3 (3.6) | 1.8 (0.9) | 6.5 (2.3) | |
| Rectum | 1 | Normal | 6.7 (0.2)* | 0 | 1.3 (0.5) | 0.4 (0.2) | 0.5 (0.3) |
| 2 | Fermented | 7.0 (0.3) | 0 | 0.5 (0.3) | 0.1 (0.1) | 0.3 (0.2) | |
| 3 | Normal | 6.5 (0.2)* | 0 | 1.1 (0.1) | 0.3 (0.2) | 0.8 (0.4) | |
| 4 | Fermented | 6.9 (0.3) | 0 | 0.7 (0.6) | 0.1 (0.1) | 0.4 (0.3) | |
Data are expressed as means for 10 replicates. Significance: *, P < 0.05; **, P < 0.01.
0, below calculated concentration of 10−4 μmol g−1; <, under detection limit of 0.8 μmol g−1.
Lactate and VFA concentrations in the GIT.
In both fermented-feed groups, the concentration of lactate in the stomach contents was significantly higher (mean, 127 μmol g−1) compared with the lactate concentration in stomach contents of the dry-feed groups (mean, 8 μmol g−1) (feed effect, P < 0.01). The concentration of lactate decreased along the GIT. In cecum, colon, and rectum contents, lactate was present in very low concentrations (3.7, 2.1, and under the detection limit of 0.8 μmol g−1, respectively).
In the stomach contents of the fermented-feed groups, the mean acetate concentration was approximately 10 μmol g−1 while in the dry-feed groups the acetate concentration was approximately 2 μmol g−1. In the cecum, the concentration of acetate was approximately 80 μmol g−1 in all groups (P > 0.05). Beyond the cecum, the concentrations decreased in all groups to approximately 70 μmol g−1 (P > 0.05).
Butyrate and propionate were detected in cecum (14 and 38 μmol g−1), colon (14 and 35 μmol g−1), and rectum contents (14 and 32 μmol g−1) in all groups. The concentrations were not significantly different between the feed groups (P > 0.05).
Undissociated lactate concentrations and undissociated VFA concentrations.
The concentration of the undissociated form of lactate was significantly higher in stomach contents of the fermented-feed groups than in the stomach contents of pigs fed dry feed (feed effect, P < 0.01) (Table 2). Also, the concentration of the undissociated form of acetate was found to be significantly elevated in the stomach of fermented-feed group 2 compared with the dry-feed group 1 (feed effect, P < 0.01). In the other fermented-feed group, the undissociated acetate concentration in the stomach content was not significantly different from that in the dry-feed group. In the ileum, cecum, colon, and rectum contents, undissociated lactate was below the calculated concentration of 10−4 μmol g−1. The undissociated VFA in the different contents of the GIT was not significantly different between the groups. The concentrations of VFA varied in the GIT.
Correlation analyses.
The correlations between the concentration of lactate, acetate, propionate, or butyrate and bacterial numbers and their significance were calculated. Only in the content of the stomach were significant correlations detected between pH and numbers of Enterobacteriaceae in the dry-feed groups and between undissociated lactic acid concentration and numbers of Enterobacteriaceae in the fermented-feed groups. Also between pH and lactobacillus numbers and between lactobacillus numbers and total lactic acid concentration were significant correlations determined (Table 3).
TABLE 3.
Correlations between bacterial numbers and concentrations of total lactic acid and its undissociated form in the stomach contents of pigs fed dry feed (groups 1 and 3) and fermented feed (groups 2 and 4)
| Comparison |
Ra
|
|||
|---|---|---|---|---|
| Dry (group 1) | Fermented (group 2) | Dry (group 3) | Fermented (group 4) | |
| Enterobacteriaceae versus pH | 0.684* | 0.334 | 0.656* | 0.171 |
| Enterobacteriaceae versus undissociated lactic acid | −0.231 | −0.655* | −0.489 | −0.781* |
| Lactobacilli versus pH | 0.687* | 0.827** | 0.630* | 0.765* |
| Lactobacilli versus total lactic acid | 0.709* | 0.774* | 0.861** | 0.706* |
Significance: *, P < 0.05; **, P < 0.01.
DISCUSSION
The proposed influence of lactic acid and VFA on the reduction of Enterobacteriaceae and Salmonella spp. is related to the undissociated form of the lactic acid and VFA (26). The undissociated acid is believed to be responsible for the reduction in Enterobacteriaceae because it can freely cross the bacterial membrane, while the dissociated form cannot.
Inside the bacterial cell, the acid dissociates and intracellular pH will drop. As a result, enzymatic processes will stop, and the proton motive force will collapse. In addition, the anion itself may damage the cell as well. These actions can result in cellular death (26). Several studies have shown that effects on the reduction in Enterobacteriaceae, coliforms, and E. coli numbers were related to VFA concentrations, but correlations were not determined (6, 13, 15, 16, 28). In this study, a correlation with Salmonella spp. was not determinable, since Salmonella spp. were detected only in some contents of two seeder pigs of the dry-feed group and in one seeder pig of the fermented-feed groups. A putative explanation is the measurement at 56 days after inoculation of the seeder pigs in our study. Salmonella presence in the GIT may be diminished over time, as described by Berends et al. (4). Therefore, numbers of Enterobacteriaceae were implemented as indicator bacteria. The negative correlation between undissociated lactic acid and numbers of Enterobacteriaceae in the contents of the stomach of pigs fed fermented feed supports the theory that the undissociated form of the acid inhibits the numbers of Enterobacteriaceae. We found no correlation between the numbers of Enterobacteriaceae and the undissociated concentrations of VFA in contents of ileum, cecum, colon, and rectum. In these compartments, the concentrations of the undissociated forms were very low and probably not growth inhibiting. The significantly reduced numbers of Enterobacteriaceae in the contents of cecum, colon, and rectum of pigs fed fermented feed compared with the similar contents of pigs fed dry feed suggest that other mechanisms are involved in the reduction of numbers of Enterobacteriaceae, such as nutrient availability, competition for receptor sites, and immunological responses.
pH of feces.
A significantly higher pH in feces of pigs fed fermented feed compared with pigs fed dry feed was observed, which was in agreement with Urlings et al. (32) and Fransen et al. (7). Urlings et al. hypothesized that the reduced available substrates in the large intestine resulted in less microbial growth and therefore in reduced VFA concentrations in the lower part of the GIT. In our study, however, the VFA concentrations between the different feed groups were not statistically different in contents of cecum, colon, and rectum. A higher pH in the fermented-feed groups may be the result of increased bicarbonate excretion in the colon or a change in metabolic processes in the colon due to a transition of carbohydrate to a protein fermentation (11).
Lactobacilli and L. plantarum.
In the fermented-feed groups, we observed two different results. In group 4, no significant difference was observed between the numbers of total lactobacilli and L. plantarum in the GIT. In contrast, in group 2, the L. plantarum numbers were significantly lower compared with the total lactobacillus numbers in the ileum. This indicates that other lactobacilli than L. plantarum were present. The presence of these lactobacilli can be due to changes in the available substrates or products (14, 18) or alterations in the intestine, e.g., diet-induced intestinal glycosylation (12) or epithelial proliferation by VFA (27). The influence of L. plantarum on Enterobacteriaceae in the GIT is difficult to explain from the results of this experiment. The high lactobacillus numbers in the contents of the GIT of the fermented-feed groups may have an effect on the Enterobacteriaceae level, such as strengthening of colonization resistance (reviewed by Vollaard [37]). In other studies, it was demonstrated that Lactobacillus strains inhibited the adherence of Escherichia coli in the intestinal tract (9, 25, 30).
Enterobacteriaceae.
We observed another phenomenon concerning numbers of Enterobacteriaceae, which has not been described before. The numbers of Enterobacteriaceae found in the different feed groups are running equally, i.e., the data points between the dry-feed groups and fermented-feed groups are at an almost similar distance. It seems that the reduction of Enterobacteriaceae initiates in the stomach. This reduction determines the numbers of Enterobacteriaceae in the feces. Regarding other data (where control and treatment groups are compared) concerning numbers of Enterobacteriaceae in the GIT, this trend was observed (7, 10, 15, 17, 32), although this effect had not been described. The described effect mentioned above was also found in pigs of the same age, regardless of the age of the pigs (10). Concerning the bacterial ecology of the GIT, we hypothesize that the number of Enterobacteriaceae in the contents of the stomach of pigs determines the level of Enterobacteriaceae in the feces. With the use of fermented feed, we are able to reduce the numbers of Enterobacteriaceae in the stomach content and therefore Enterobacteriaceae shedding.
The presence of Salmonella spp. in the GIT was not sufficient to state any conclusion about the effect of fermented feed on Salmonella spp. The reduced shedding of Enterobacteriaceae in the fermented-feed group might have important consequences in the infection pressure of enteropathogens belonging to this group of bacteria.
ACKNOWLEDGMENTS
We thank A. van Nes for clinical surveillance; A. Westeneng and J. van Dasselaar for taking care of the animals; M. Swanenburg, C. Bhikhie, P. Mahadew, and P. Scherpenisse for excellent technical assistance; and S. Biesterveld for critically reading the manuscript.
This study was partly funded by the European Union (FAIR contract FAIR CT 95-400), the Dutch Product Boards for Livestock, Meat and Eggs, and the Dutch Ministry of Agriculture, Nature Management and Fisheries.
REFERENCES
- 1.Anonymous. Microbiology— General guidance for the detection of Salmonella. ISO-6579. Geneva, Switzerland: International Organization for Standardisation; 1981. [Google Scholar]
- 2.Budavari S, O'Neil M J, editors. Anonymous. The Merck index: an encyclopedia of chemicals, drugs and biologicals. 11th ed. Rahway, N.J: Merck & Co., Inc; 1989. pp. 41–308. [Google Scholar]
- 3.Argenzlo R A, Southworth M. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am J Physiol. 1974;228:454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- 4.Berends B R, Urlings H A, Snijders J M, Van Knapen F. Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs. Int J Food Microbiol. 1996;30:37–53. doi: 10.1016/0168-1605(96)00990-7. [DOI] [PubMed] [Google Scholar]
- 5.Burnell T W, Cromwell G L, Stahly T S. Effects of dried whey and copper sulfate on the growth responses to organic acid in diets for weanling pigs. J Anim Sci. 1988;66:1100–1108. doi: 10.2527/jas1988.6651100x. [DOI] [PubMed] [Google Scholar]
- 6.Burnett G S, Hanna J. Effect of dietary calcium lactate and lactic acid on fecal Escherichia coli counts in pigs. Nature. 1963;197:815. [Google Scholar]
- 7.Fransen N G, Urlings B A, Bijker P G, Van Gils B G. Utilization of fermented flocculated poultry sludge as a feed constituent for pigs. Poult Sci. 1995;74:1948–1960. doi: 10.3382/ps.0741948. [DOI] [PubMed] [Google Scholar]
- 8.Geary T M, Brooks P H, Morgan T, Campbell A, Russel P J. Performance of weaner pigs fed ad libitum with liquid feed at different dry matter concentrations. J Sci Food Agric. 1996;72:17–24. [Google Scholar]
- 9.Herias M V, Hessle C, Telemo E, Midtvedt T, Hanson L A, Wold A E. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin Exp Immunol. 1999;116:283–290. doi: 10.1046/j.1365-2249.1999.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen B B. The impact of feed additives on the microbial ecology of the gut in young pigs. J Anim Feed Sci. 1998;7:45–64. [Google Scholar]
- 11.Jensen B B, Jorgensen H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl Environ Microbiol. 1994;60:1897–1904. doi: 10.1128/aem.60.6.1897-1904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly D, King T P. The influence of lactation products on the temporal expression of histo-blood group antigens in the intestines of suckling pigs: lectin histochemical and immunohistochemical analysis. Histochem J. 1991;23:55–60. doi: 10.1007/BF01886508. [DOI] [PubMed] [Google Scholar]
- 13.Kershaw G F, Luscombe J R, Cole D J A. Lactic acid and sodium acrylate: effect on growth rate and bacterial flora in the intestines of weaned pigs. Vet Rec. 1966;79:296. [Google Scholar]
- 14.Mathers J C, Goodlad J. Carbohydrate fermentation and microbial cell growth in suspensions of pig large bowel contents. Sci Aliments. 1999;19:491–497. [Google Scholar]
- 15.Mathew A G, Chattin S E, Robbins C M, Golden D A. Effects of a direct-fed yeast culture on enteric microbial populations, fermentations acids, and performance of weanling pigs. J Anim Sci. 1998;76:2138–2145. doi: 10.2527/1998.7682138x. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen L L, Jensen B B. VIIth International Symposium on Digestive Physiology in Pigs. Saint Malo, France: European Association for Animal Production; 1997. Effect of fermented liquid feed (FLF) on growth performance and microbial activity in the gastrointestinal tract of weaned piglets; pp. 639–642. [Google Scholar]
- 17.Mikkelsen L L, Jensen B B. Performance and microbial activity in the gastrointestinal tract of piglets fed fermented liquid feed at weaning. J Anim Feed Sci. 1998;7:211–215. [Google Scholar]
- 18.Moore R J, Jewell D E, Veum T L. Effect of dietary Lactobacillus on growth and performance of pigs fed a low protein low lysine diet. Univ Mo-Columb Agric Exp Stn Spec Rep. 1981;273:49–52. [Google Scholar]
- 19.Morishita Y, Ogata M. Studies on the alimentary flora of pig. V. Influence of starvation on the microbial flora. Nippon Juigaku Zasshi. 1970;32:19–24. doi: 10.1292/jvms1939.32.19. [DOI] [PubMed] [Google Scholar]
- 20.Mossel D A A, Jacobs-Reitsma W F. Microbiologisch onderzoek van levensmiddelen: strategie en methoden. 3rd ed. The Netherlands: Uitgeverij Noordervliet B. V., Zeist; 1990. [Google Scholar]
- 21.Mulder R W A W, Havenaar R, Huis in't Veld J H J. Intervention strategies: the use of probiotics and competitive exclusion microfloras against contamination with pathogens in poultry and pigs. In: Fuller R, editor. Probiotics 2: application and practical aspects. New York, N.Y: Chapman & Hall; 1997. pp. 187–207. [Google Scholar]
- 22.Norikatsu Y, Koichi W, Akito M, Yoko T, Ryuichiro T, Makoto O, Masami M. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from feces and identification using monoclonal antibodies. Int J Food Microbiol. 1999;48:51–57. doi: 10.1016/s0168-1605(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 23.Prohaszka L, Jayarao B M, Fabian A, Kovacs S. The role of intestinal volatile fatty acids in the Salmonella shedding of pigs. Zentbl Vetmed Reihe B. 1990;37:570–574. doi: 10.1111/j.1439-0450.1990.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 24.Ravindran V, Kornegay E T. Acidification of weaner pig diets: a review. J Sci Food Agric. 1993;62:313–322. [Google Scholar]
- 25.Redmond H E, Moore R W. Biologic effect of introducing Lactobacillus acidophilus into a large swine herd experiencing enteritis. Southwest Vet. 1965;18:287–288. [Google Scholar]
- 26.Russell J B, Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- 27.Sakata T, Adachi M, Hashida M, Sato N, Kojima T. Effect of n-butyric acid on epithelial cell proliferation of pig colonic mucosa in short-term culture. Dtsch Tieraerztl Wochenschr. 1995;102:163–164. [PubMed] [Google Scholar]
- 28.Shaw J N, Muth O H. The use of acidophilic milk in the treatment of dysentery of young animals. JAVMA. 1937;90:171–175. [Google Scholar]
- 29.Snel J, Van Den Brink M E, Bakker M H, Poelma F G J, Heidt P J. The influence of indigenous segmented filamentous bacteria on small intestinal transit in mice. Microb Ecol Health Dis. 1996;9:207–214. [Google Scholar]
- 30.Spencer R J, Chesson A. The effect of Lactobacillus spp. on the attachment of enterotoxigenic Escherichia coli to isolated porcine enterocytes. J Appl Bacteriol. 1994;77:215–220. doi: 10.1111/j.1365-2672.1994.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 31.Urlings H A, Bijker P G, van Logtestijn J G. Fermentation of raw poultry byproducts for animal nutrition. J Anim Sci. 1993;71:2420–2426. doi: 10.2527/1993.7192420x. [DOI] [PubMed] [Google Scholar]
- 32.Urlings H A, Mul A J, van't Klooster A T, Bijker P G, van Logtestijn J G, van Gils L G. Microbial and nutritional aspects of feeding fermented feed (poultry by-products) to pigs. Vet Q. 1993;15:146–151. doi: 10.1080/01652176.1993.9694394. [DOI] [PubMed] [Google Scholar]
- 33.Van den Elzen A M, Snijders J M. Critical points in meat production lines regarding the introduction of Listeria monocytogenes. Vet Q. 1993;15:143–145. doi: 10.1080/01652176.1993.9694393. [DOI] [PubMed] [Google Scholar]
- 34.Van der Wielen P W, Biesterveld S, Notermans S, Hofstra H, Urlings B A, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl Environ Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Schie F W. Some epidemiological and nutrional aspects of asymptomatic Salmonella infection in pigs. Ph.D. thesis. Utrecht, The Netherlands: Faculty of Veterinary Medicine, University of Utrecht; 1987. [Google Scholar]
- 36.van Winsen R L, Lipman L J A, Biesterveld S, Urlings B A, Snijders J M A, van Knapen F. Mechanism of Salmonella reduction in fermented pig feed. J Sci Food Agric. 2001;81:342–346. [Google Scholar]
- 37.Vollaard E J, Clasener H A. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]