Summary
Background
In atopic dermatitis (AD), phosphodiesterase 4 (PDE4) inhibition reduces proinflammatory mediators and cytokines. Difamilast is a new selective PDE4 inhibitor.
Objectives
To demonstrate the superiority of topical difamilast to vehicle in Japanese paediatric patients with AD.
Methods
This was a phase III randomized, double‐blind, vehicle‐controlled trial. Patients aged 2–14 years with an Investigator Global Assessment (IGA) score of 2 or 3 received difamilast 0·3% (n = 83), difamilast 1% (n = 85) or vehicle (n = 83) ointment twice daily for 4 weeks.
Results
The primary endpoint was the percentage of patients with an IGA score of 0 or 1 with improvement by at least two grades at week 4. The success rates in IGA score at week 4 were 44·6%, 47·1% and 18·1% in the difamilast 0·3%, difamilast 1% and vehicle groups, respectively. Both difamilast groups demonstrated significantly higher success rates in IGA score compared with vehicle at week 4 [difamilast 0·3% (P < 0·001); difamilast 1% (P < 0·001)]. Regarding secondary endpoints, improvements in Eczema Area and Severity Index (EASI; improvement of ≥ 50%, ≥ 75% and ≥ 90% in overall score) at week 4 were significantly higher in patients in the difamilast 0·3% and 1% groups than those in the vehicle group. EASI score in the difamilast 0·3% and 1% groups was significantly reduced compared with that of patients in the vehicle group at week 1. The significant difference between both the difamilast groups and the vehicle groups was maintained from week 1 through to week 4. Most treatment‐emergent adverse events were mild or moderate, and no serious events or deaths were reported.
Conclusions
Difamilast 0·3% and 1% ointments are superior to vehicle and well tolerated in Japanese paediatric patients with AD.

What is already known about this topic?
In atopic dermatitis (AD), increased phosphodiesterase 4 (PDE4) activity leads to a proinflammatory milieu involved in acute and chronic inflammation.
Topical inhibitors of PDE4 are available and are an alternative to topical corticosteroids or calcineurin inhibitors for AD.
Concern about the adverse effects of topical corticosteroids or calcineurin inhibitors may limit their use in the treatment of AD.
What does this study add?
This trial demonstrates the efficacy of the selective topical PDE4 inhibitor difamilast for mild‐to‐moderate AD in Japanese paediatric patients.
Difamilast is well tolerated with an adverse event profile similar to vehicle.
Linked Comment: D.M.W. Balak and E. Hajdarbegovic. Br J Dermatol 2022; 186:5–6.
Plain language summary available online
Atopic dermatitis (AD) is a chronic, fluctuating pruritic inflammatory skin disease that occurs most frequently in children, affecting one in five in industrialized countries, with onset as early as within the first 2 years of life. 1 AD is the leading nonfatal health burden caused by skin diseases. It has negative psychosocial impacts on patients and their relatives, and increases the risk of various immune‐mediated inflammatory diseases. 2 Contributing to the disease‐related burden is the high prevalence, childhood onset, impact on quality of life and substantial healthcare costs. 3
Mechanisms underlying AD are now considered to be a combination of various defects, including epidermal barrier disruption, especially that associated with filaggrin deficiency, and the activation of different T‐cell subsets, particularly T helper (Th) 2, Th17 and Th22 cells. 4 Accordingly, the main principles for the treatment of AD, as recommended by US and Japanese guidelines, are to repair epidermal barrier function, avoid trigger factors, suppress inflammation, relieve pruritus and reduce the risk of secondary cutaneous infection. 5 , 6 Topical therapies are the mainstay of AD treatment, of which the most commonly used are moisturizers and emollients to repair skin barrier function, and topical anti‐inflammatory agents to suppress inflammation. In Japan, topical corticosteroids and tacrolimus ointment (a topical calcineurin inhibitor) are used mainly to shorten and suppress the inflammation caused by AD. Although effective, both topical corticosteroids and topical calcineurin inhibitors are limited by several issues, including safety concerns and local side‐effects. 7 , 8 As a result, there is a need for new topical therapies for AD that can overcome the limitations of the existing therapies.
In AD, increased activity of phosphodiesterase 4 (PDE4) and associated reduction of intracellular cyclic adenosine monophosphate (cAMP) – a negative regulator of cytokine production – leads to enhanced generation of proinflammatory mediators and increased transcription of cytokines involved in acute and chronic inflammation. 9 , 10 Hence, the inhibition of PDE4 activity may suppress proinflammatory cytokines [e.g. tumour necrosis factor‐α, interleukin (IL)‐12 and IL‐23] by inhibiting the breakdown of cAMP. 4 , 11 Crisaborole is the first marketed PDE4 inhibitor indicated for the treatment of AD in patients aged ≥ 2 years to be approved by the US Food and Drug Administration and by the European Medicines Agency. 12
Difamilast [OPA‐15406; also referred to as MM36 (Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan)] 13 is a new selective PDE4 inhibitor that has shown significant efficacy and no safety concerns for mild‐to‐moderate AD in phase II trials of paediatric and adult patients in the USA and Japan. 14 , 15 , 16 , 17
This phase III trial was primarily designed to demonstrate the superiority of difamilast 0·3% and 1% ointments to vehicle ointment in a large number of Japanese paediatric patients aged 2–14 years with AD.
Patients and methods
Trial design
This multicentre, randomized, double‐blind, vehicle‐controlled, parallel‐group comparison trial (ClinicalTrials.gov identifier: NCT03911401) comprised a screening period (2–30 days) and a 4‐week assessment period, during which examinations were performed at baseline and then at weeks 1, 2 and 4. The trial was conducted at 30 investigational sites in Japan from May to December 2019.
This trial was performed in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Consolidated Guideline, and the applicable local laws and regulatory requirements in Japan. The protocol was approved by the institutional review board for each study site. Written informed consent was obtained from the patients’ legal guardians before participation in the trial. If possible, assent (consent not bound by the legal restrictions obtained from paediatric patients) was obtained from the patients.
Patients
Included patients were male and female Japanese outpatients aged 2–14 years who had a diagnosis of AD based on the Japanese Dermatological Association’s criteria that affected ≥ 5% to ≤ 40% of their body surface area (BSA), excluding the scalp, and had an Investigator’s Global Assessment (IGA) score of 2 (mild) or 3 (moderate) at the screening and baseline examinations. 18 , 19 Patients were excluded if they (i) had AD or a contact dermatitis flare‐up (defined as a rapid intensification of symptoms) within 28 days prior to the baseline examination; (ii) had received one of several prescribed therapies prior to the baseline examination, including ultraviolet light therapy, systemic or topical corticosteroids; and (iii) were unable to continue in the trial without changing the dosage and administration of systemic antihistamines, sodium cromoglicate, tranilast or suplatast tosilate from 7 days before the baseline examination until the week 4 examination. The full list of exclusion criteria is given in Table S1 (see Supporting Information).
Treatment
Eligible patients were randomly assigned in a 1 : 1 : 1 ratio to receive difamilast 0·3% [weight/weight (w/w)] ointment, difamilast 1% (w/w) ointment or vehicle ointment twice daily (approximately 12 h apart between morning and night administration) for 4 weeks. Both the investigator/subinvestigator and patients were blinded to the study medication. The investigator/subinvestigator entered the necessary information for the patients’ eligibility into the Interactive Web Response System (IWRS). On the day of the baseline examination, patients confirmed to be registered in the IWRS were allocated to the difamilast 0·3%, difamilast 1% or vehicle groups. In the IWRS, patients were allocated using dynamic allocation (minimization method) for balancing baseline covariates [IGA score and age (2–6 years or 7–14 years)]. The dose for each patient was calculated based on the following formula: total BSA (m2) × proportion of the treatment area (%) × 10 g m–2. The patient’s total BSA was calculated according to the Mosteller formula. 20 Patients were instructed to apply the study medication according to their own affected area. Compliance was reviewed based on the applied quantity of ointment and the number of applications. Patients were allowed to discontinue trial participation for any reason at any time. For patients who discontinued the study medication before the week 4 examination, a withdrawal examination was performed.
Outcomes
The primary endpoint was the success rate in IGA score (Table S2; see Supporting Information) 19 at week 4, defined as the percentage of patients achieving an IGA score of 0 or 1 with improvement by at least two grades.
Secondary endpoints included success rate in Eczema Area and Severity Index (EASI) 50, 21 EASI 75 and EASI 90 (improvement of ≥ 50%, ≥ 75% and ≥ 90% in overall EASI score, respectively) at week 4, and least square (LS) mean percentage change from baseline at week 4 in overall EASI score. The other secondary endpoints were LS mean changes from baseline at week 4 in overall EASI score and each subscale score (erythema, induration/papulation, excoriation and lichenification), Verbal Rating Scale (VRS) for pruritus score (only for patients aged 7–14 years), 22 Patient‐Oriented Eczema Measure (POEM) score and total affected BSA (%). 23
The safety assessments included treatment‐emergent adverse events (TEAEs), clinical laboratory tests and vital sign examinations.
Statistical analysis
The statistical analyses included all patients who received the study medication at least once.
The target sample size was set to achieve a power of 90% for the comparison of the difamilast 1% group and the vehicle group, which was firstly conducted in a closed testing procedure. In the phase II trial in paediatric patients in Japan, success rates in IGA score were 37% (n = 9/24), 40% (n = 10/25, and 8% (n = 2/24) in the difamilast 0·3%, difamilast 1% and vehicle groups, respectively. 16 However, in order to consider the trial results conservatively, it was assumed that success rates in IGA score were 36% (n = 9/25) in the difamilast 1% group and 12% (n = 3/25) in the vehicle group. Under these conditions, it was necessary to have 72 patients per group to achieve a power of 90% with a two‐sided significance level of 5%. However, in consideration of an exploratory assessment of age categories, the target sample size was set as 80 patients in each group (total 240 patients).
For the primary endpoint – the success rate in IGA score at week 4 – the efficacy of difamilast 0·3% and difamilast 1% was demonstrated vs. the vehicle group. Patients with missing IGA score data were treated as nonresponders. Overall, type I errors were controlled for using a closed testing procedure. Firstly, the difamilast 1% and vehicle groups were compared. If the comparison was significant at the two‐sided significance level of 5%, the difamilast 0·3% and the vehicle groups were then compared at the two‐sided significance level of 5%. The Cochran–Mantel–Haenszel test was conducted for comparison using baseline IGA (2 or 3) and age (2–6 years or 7–14 years) as stratification factors. The difference in success rate in IGA score and its two‐sided 95% confidence interval (CI; common risk difference adjusted by the Mantel–Haenszel method and its two‐sided 95% CI) between the vehicle group and the difamilast 0·3% or 1% group were determined.
The success rates in EASI 50, EASI 75 and EASI 90 at week 4 as secondary endpoints were analysed in the same manner as the primary endpoint. For the success rate in EASI 75, patients whose percentage change in overall EASI score from baseline decreased by ≥ 75% were treated as responders. Patients whose percentage change from baseline did not decrease by ≥ 75% or with missing EASI 75 data were treated as nonresponders. The success rates in EASI 50 and EASI 90 were defined in the same manner.
The percentage change from baseline in overall EASI score, and changes from baseline in overall EASI score and each subscale score, VRS for pruritus score, POEM score and total affected BSA (%) were analysed using a mixed‐model repeated measure.
The number and percentage of patients who experienced TEAEs were calculated by group. TEAEs were coded to preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1.
Results
Patient disposition and baseline characteristics
Patient disposition throughout the conduct of the trial is shown in Figure 1. Overall, 262 patients were screened; of these, 251 were randomized to one of three groups (difamilast 0·3%, n = 83; difamilast 1%, n = 85; vehicle, n = 83) and received at least one administration of study medication. Discontinuation was numerically higher in the vehicle group (n = 25; 30%) than either difamilast group [difamilast 0·3%, n = 7 (8%); difamilast 1%, n = 9 (11%)], which was mainly due to withdrawal by parent/guardian and lack of efficacy.
Figure 1.
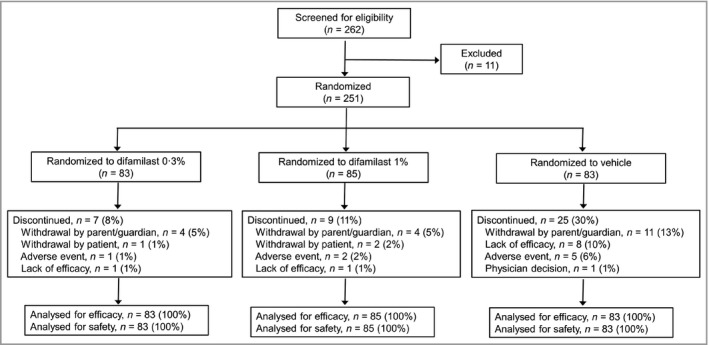
Patient disposition throughout the phases of the trial.
Mean age, mean number of years since AD onset, IGA score, severity of AD, overall EASI score and VRS for pruritus score were equivalent across treatment groups at baseline (Table 1). Overall, 39 patients (15·5%) had an IGA score of 2 and 212 (84·5%) had an IGA score of 3. Regarding the Japanese AD severity index, 6 25 patients (10·0%) had mild AD, 170 (67·7%) had moderate AD and 56 (22·3%) had severe AD (Table 1).
Table 1.
Demographic and baseline clinical characteristics
| Vehicle (n = 83) | Difamilast 0·3% (n = 83) | Difamilast 1% (n = 85) | |
|---|---|---|---|
| Mean (SD) age (years) | 7·1 (2·8) | 7·1 (3·3) | 7·2 (3·2) |
| Male | 49 (59) | 38 (46) | 48 (56) |
| Mean (SD) weight (kg) | 24·6 (9·7) | 25·7 (11·8) | 25·7 (12·5) |
| Mean (SD) height (cm) | 120·3 (17·4) | 120·8 (21·2) | 121·2 (20·7) |
| Mean (SD) BMI (kg m–2) | 16·3 (2·0) | 16·6 (2·5) | 16·5 (2·6) |
| Mean (SD) duration of AD (years) | 5·5 (2·8) | 5·0 (3·2) | 5·1 (3·0) |
| IGA score | |||
| Mild disease (2) | 12 (15) | 13 (16) | 14 (16) |
| Moderate disease (3) | 71 (86) | 70 (84) | 71 (84) |
| Severity of ADa | |||
| Mild | 8 (10) | 8 (10) | 9 (11) |
| Moderate | 55 (66) | 58 (70) | 57 (67) |
| Severe | 20 (24) | 17 (20) | 19 (22) |
| Mean (SD) EASI score | 11·3 (5·9) | 10·8 (5·5) | 11·6 (5·5) |
| Mean (SD) POEM score | 9·7 (4,7) | 11·2 (5·3) | 10·1 (5·4) |
| Mean (SD) VRS pruritus score (for patients aged 7–14 years) | 1·9 (0·6) | 1·9 (0·5) | 1·9 (0·6) |
| Affected BSA (%) | |||
| ≥ 5 to < 10 | 13 (16) | 12 (14) | 11 (13) |
| ≥ 10 to < 30 | 46 (55) | 53 (64) | 54 (64) |
| ≥ 30 | 24 (29) | 18 (22) | 20 (24) |
Data are n (%) unless otherwise indicated. AD, atopic dermatitis; BMI, body mass index; BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; POEM, Patient‐Oriented Eczema Measure; VRS, Verbal Rating Scale. aJapanese severity index of AD was based on the ‘Guidelines for the Treatment of Atopic Dermatitis 2008’ of Health and Labour Sciences Research.
Efficacy
For the primary endpoint, success rates in IGA score at week 4 were 44·6%, 47·1% and 18·1% in the difamilast 0·3%, the difamilast 1% and the vehicle groups, respectively (Table 2). Significant differences at week 4 were observed between difamilast 0·3% and vehicle (24·7%, 95% CI 11·3–38·0; P < 0·001), and between difamilast 1% and vehicle (28·7%, 95% CI 15·0–42·5; P < 0·001). Patients in the difamilast 0·3% and difamilast 1% groups also showed highly significant success rates in IGA score compared with the vehicle group at weeks 1 and 2 (Figure 2).
Table 2.
Summary of efficacy outcomes at week 4
| Vehicle | n | Difamilast 0·3% | n | P‐valuea | Difamilast 1% | n | P‐valuea | |
|---|---|---|---|---|---|---|---|---|
| Success rate in IGA score (%) | 18·1 | 83 | 44·6 | 83 | < 0·001 | 47·1 | 85 | < 0·001 |
| EASI 50 responder rateb,c | 26 (31·3) | 83 | 58 (69·9) | 83 | < 0·001 | 58 (68·2) | 85 | < 0·001 |
| EASI 75 responder rateb,c | 15 (18·1) | 83 | 36 (43·4) | 83 | < 0·001 | 49 (57·7) | 85 | < 0·001 |
| EASI 90 responder rateb,c | 6 (7·2) | 83 | 27 (32·5) | 83 | < 0·001 | 35 (41·2) | 85 | < 0·001 |
| Mean (SE) LS percentage change from baseline in overall EASI scores | –1·5 (8·6) | 59 | –43·8 (8·0) | 77 | < 0·001 | –62·6 (8·0) | 77 | < 0·001 |
| Mean (SE) LS mean change from baseline in overall EASI score | 0·35 (0·90) | 59 | –4·97 (0·84) | 77 | < 0·001 | –6·07 (0·84) | 77 | < 0·001 |
| Erythema subscale scored | –0·67 (0·28) | 59 | –2·57 (0·26) | 77 | < 0·001 | –3·10 (0·26) | 77 | < 0·001 |
| Induration/papulation subscale scored | –0·55 (0·28) | 59 | –2·17 (0·26) | 77 | < 0·001 | –2·55 (0·26) | 77 | < 0·001 |
| Excoriation subscale scored | –0·60 (0·28) | 59 | –2·27 (0·26) | 77 | < 0·001 | –2·62 (0·26) | 77 | < 0·001 |
| Lichenification subscale scored | –0·42 (0·25) | 59 | –1·97 (0·23) | 77 | < 0·001 | –2·31 (0·23) | 77 | < 0·001 |
| Mean (SE) LS change from baseline in VRS for pruritus score (for patients aged 7–14 years) | –0·33 (0·13) | 33 | –0·80 (0·12) | 38 | 0·01 | –0·68 (0·12) | 41 | 0·048 |
| Mean (SE) LS change from baseline in POEM score | –0·21 (0·64) | 59 | –3·54 (0·59) | 77 | < 0·001 | –3·98 (0·59) | 77 | < 0·001 |
| Mean (SE) LS change from baseline in percentage of affected BSA | 0·72 (1·63) | 59 | –8·37 (1·52) | 77 | < 0·001 | –10·99 (1·52) | 77 | < 0·001 |
BSA, Body Surface Area; EASI, Eczema Area and Severity Index; IGA, Investigator Global Assessment; LS, least square; POEM, Patient‐Oriented Eczema Measure; VRS, Verbal Rating Scale. a P‐values are for the comparison between each difamilast group and the vehicle group. bData are number of responders (%). cThe responder rates of EASI 50, EASI 75 and EASI 90 represent the percentages of patients who indicated at least 50%, 75% and 90% improvements in overall EASI score at week 4, respectively. dLS mean change from baseline in each EASI subscale score at week 4 (SE).
Figure 2.
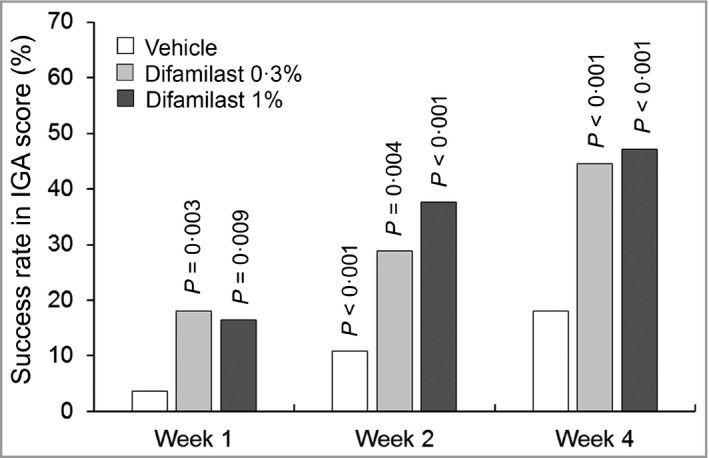
Success rate in Investigator’s Global Assessment (IGA) score. The data represent the percentage of patients achieving an IGA score of 0 or 1 with an improvement of at least two grades at each timepoint. All timepoints: vehicle, n = 83; difamilast 0·3%, n = 83; difamilast 1%, n = 85. P‐values are for the comparison between each difamilast group and the vehicle group.
The success rates in EASI 75 at week 4 were 43·4% in the difamilast 0·3% group, 57·7% in the difamilast 1% group and 18·1% in the vehicle group. Significant differences at week 4 were shown between the difamilast 0·3% and vehicle groups (23·9%, 95% CI 10·6–37·3; P < 0·001), and between the difamilast 1% and vehicle groups (38·9%, 95% CI 25·5–52·3; P < 0·001). The success rates in EASI 50 and EASI 90 at week 4 in both the difamilast groups were also significantly higher compared with the vehicle group (Table 2 and Figure 3).
Figure 3.
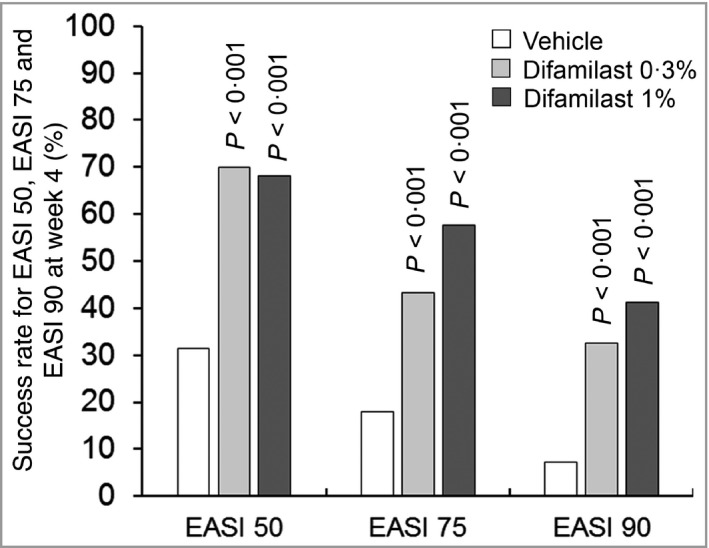
Success rate in Eczema Area and Severity Index (EASI) 50, EASI 75 and EASI 90 at week 4. The data represent the percentage of patients achieving ≥ 50% (EASI 50), ≥ 75% (EASI 75) and ≥ 90% (EASI 90) improvement in overall EASI score at week 4. Vehicle, n = 83; difamilast 0·3%, n = 83; difamilast 1%, n = 85. P‐values are for the comparison between each difamilast group and the vehicle group.
The LS mean percentage change in overall EASI score from baseline at week 1 was −48·9 for difamilast 0·3%, −51·7 for difamilast 1% and −0·1 for vehicle. Differences from vehicle in LS mean percentage changes in overall EASI score at week 1 were −48·8 for difamilast 0·3% (P < 0·001) and −51·6 for difamilast 1% (P < 0·001), and the significant reductions for both difamilast groups observed at week 1 were sustained until week 4 (Table 2 and Figure 4).
Figure 4.
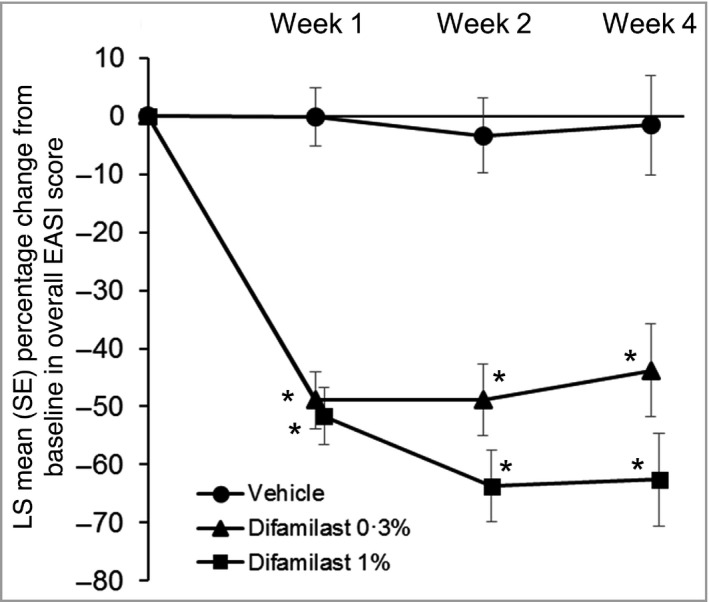
Least squares (LS) mean percentage change in overall Eczema Area and Severity Index (EASI) score from baseline at each timepoint. Week 1: vehicle, n = 81; difamilast 0·3%, n = 83; difamilast 1%, n = 85. Week 2: vehicle, n = 70; difamilast 0·3%, n = 82; difamilast 1%, n = 81. Week 4: vehicle, n = 59; difamilast 0·3%, n = 77; difamilast 1%, n = 77. P‐values are for the comparison between each difamilast group and the vehicle group. *P < 0·001.
In terms of itch, LS mean changes in VRS for pruritus score from baseline at week 1 were –0·59 in the difamilast 0·3% group, –0·54 in the difamilast 1% group and –0·14 in the vehicle group. Differences from vehicle in changes in VRS at week 1 were −0·45 for the difamilast 0·3% group (P = 0·002) and −0·40 for the difamilast 1% group (P = 0·005); the significant difference for both difamilast groups was observed at week 1 and persisted until week 4 (Table 2 and Figure 5). In addition, significant differences in all secondary endpoints [e.g. POEM score, and total affected BSA (%)] were noted between both difamilast groups and the vehicle group (Table 2).
Figure 5.
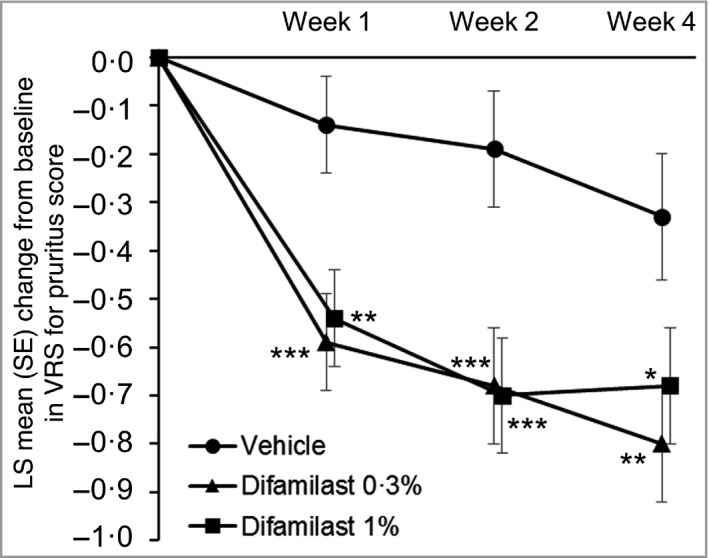
Least squares (LS) mean change in Verbal Rating Scale (VRS) for pruritus score from baseline at each timepoint. Week 1: vehicle, n = 40; difamilast 0·3%, n = 42; difamilast 1%, n = 43. Week 2: vehicle, n = 37; difamilast 0·3%, n = 42; difamilast 1%, n = 42. Week 4: vehicle, n = 33; difamilast 0·3%, n = 38; difamilast 1%, n = 41. P‐values are for the comparison between each difamilast group and the vehicle group. *P < 0·05; **P < 0·01; ***P < 0·005.
Safety
Of the 251 patients enrolled, TEAEs were experienced in 27 (33%) patients in the difamilast 0·3% group, 29 (34%) in the difamilast 1% group and 28 (34%) in the vehicle group. Most TEAEs were mild or moderate in severity, and no serious events or deaths were reported. One severe TEAE of worsening AD occurred in one (1%) patient in the difamilast 0·3% group. The most common TEAEs observed in the treatment groups were nasopharyngitis, followed by impetigo and dermatitis atopic (Table 3). Worsening AD was coded as ‘dermatitis atopic’.
Table 3.
Overall and specific treatment‐emergent adverse events (TEAEs) seen in at least two patients in any treatment group
| Vehicle (n = 83) | Difamilast 0·3% (n = 83) | Difamilast 1% (n = 85) | |
|---|---|---|---|
| Patients with any TEAE | 28 (34) | 27 (33) | 29 (34) |
| Patients with serious TEAE | 0 (0) | 0 (0) | 0 (0) |
| Patients with severe TEAE | 0 (0) | 1 (1) | 0 (0) |
| Patients who discontinued owing to TEAEs | 5 (6) | 1 (1) | 2 (2) |
| Deaths | 0 (0) | 0 (0) | 0 (0) |
| Specific events in at least two patients in any treatment group | |||
| Infections and infestations | |||
| Folliculitis | 0 (0) | 2 (2) | 2 (2) |
| Gastroenteritis | 1 (1) | 2 (2) | 2 (2) |
| Impetigo | 5 (6) | 6 (7) | 2 (2) |
| Influenza | 2 (2) | 0 (0) | 1 (1) |
| Molluscum contagiosum | 0 (0) | 0 (0) | 2 (2) |
| Nasopharyngitis | 3 (4) | 5 (6) | 7 (8) |
| Upper respiratory tract infection | 2 (2) | 0 (0) | 0 (0) |
| Injury, poisoning and procedural complications | |||
| Arthropod bite | 0 (0) | 2 (2) | 3 (4) |
| Skin abrasion | 1 (1) | 0 (0) | 3 (4) |
| Skin and subcutaneous tissue disorders | |||
| Dermatitis atopic | 4 (5) | 2 (2) | 3 (4) |
TEAEs were coded to preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1. Data are n (%).
The most frequent TEAE leading to discontinuation was worsening AD, which occurred in one (1%) patient in the difamilast 0·3% group, two (2%) in the difamilast 1% group and four (5%) in the vehicle group.
TEAEs considered to be related to study medication were reported in five (6%) patients in the difamilast 0·3% group, three (3%) in the difamilast 1% group and four (5%) in the vehicle group. The most common TEAE related to study medication was worsening AD [one (1%) patient each in the difamilast 0·3% and 1% groups and three (4%) patients in the vehicle group), followed by folliculitis [one (1%) patient each in the difamilast 0·3% and 1% groups] and impetigo [two (2%) patients in the difamilast 0·3% group].
No clinically relevant abnormalities were observed regarding clinical laboratory and vital sign assessments in any treatment group.
Discussion
In this phase III randomized, double‐blind, vehicle‐controlled trial of Japanese paediatric patients with AD, difamilast 0·3% and 1% ointments were both significantly superior to vehicle in view of the primary endpoint of the success rate in IGA score at week 4. The significant success rates with both difamilast groups compared with vehicle were evident from as early as week 1 and sustained to week 4.
EASI is an objective evaluation index to assess disease extent and severity of four clinical signs (erythema, induration/papulation, excoriation and lichenification). 21 Each difamilast group consistently demonstrated significant success rates in EASI 50, EASI 75 and EASI 90 at week 4. Furthermore – and most importantly – difamilast 0·3% and 1% showed a significant percentage change in overall EASI score compared with vehicle from as early as week 1 to week 4.
In the present trial, the success rates in IGA score at week 4 were numerically higher in patients treated with difamilast 1% vs. difamilast 0·3% and the previous phase II trial. 16 A similar phase II trial in Japanese patients aged 15–70 years and a US phase II trial in patients aged 10–70 years found that the improvement in the primary endpoint vs. vehicle was statistically significant for difamilast 1%, although the trend of improvement was also suggested in difamilast 0·3%. 15 , 17 For EASI assessments at week 4 in this trial, the trend of numerically better results was also observed in the difamilast 1% group vs. the difamilast 0·3% group. In addition to eczema severity, decreases in affected BSA were observed at week 4 in both difamilast groups.
The patient‐reported VRS for pruritus score is a measure used to assess the intensity of pruritus. 22 Significant improvement in VRS for pruritus score from as early as week 1 to week 4 was noted in the difamilast 0·3% and 1% groups, although this evaluation was conducted only for paediatric patients aged 7–14 years who could answer interview questions by the investigator or subinvestigator. The findings are meaningful, because pruritus is a characteristic and troubling symptom of AD, particularly in children. 24 The patient‐reported POEM score is used to monitor eczema severity. 23 There were significant decreases in POEM score at week 4 because of the improvement in eczema severity in paediatric patients with AD in the difamilast groups.
Results for all efficacy endpoints in both the present trial and the phase II trial in Japanese paediatric patients also largely mirrored each other, thus confirming the superiority of difamilast over vehicle observed previously.
Although the treatment period in this trial was relatively short, AD guidelines specify that patients should be evaluated for treatment effects once every 1–2 weeks, especially to maximize the drug effects and to minimize any adverse drug reaction; if necessary, the drugs and treatment methods should be adjusted. 6 Further, although long‐term adherence is critical for chronic diseases such as AD, a systematic review of studies found that a shorter time to first follow‐up visit appears to improve adherence and treatment outcomes. 25 Therefore, the rapid onset of action of difamilast, which was generally observed within 1 week, may be advantageous for clinicians, patients and parents/guardians.
Treatment with difamilast 0·3% and 1% twice daily for up to 4 weeks was safe and well tolerated in paediatric patients with AD. The incidences of TEAEs, which were mostly mild to moderate in severity, showed no appreciable difference between the treatment groups and only one severe TEAE was recorded in a patient in the difamilast 0·3% group (worsening AD). The most common TEAEs were nasopharyngitis, followed by impetigo and worsening AD. Of these, nasopharyngitis was judged not to be related to the study medication. Impetigo was seen in five patients (6%) in the vehicle group, and in six patients (7%) in the difamilast 0·3% group and in two (2%) in the difamilast 1% group. Dry skin with low barrier function in AD is reported to sometimes lead to impetigo, which is known to be common in the paediatric population. 26 , 27 Notably, application‐site pain, previously reported as skin burning and stinging with another drug, 28 was not reported in either of the difamilast groups.
Indeed, drug‐related TEAEs were infrequent and mainly consisted of worsening AD, which was understandably more common in the vehicle group (3·6%) than in the difamilast groups, where it was observed at a low frequency (1% in both groups). Treatment discontinuation due to TEAEs was also low, especially in the difamilast 0·3% (1%) and difamilast 1% (2%) groups, compared to the vehicle group (6%).
No serious TEAEs or deaths were reported, and no clinically significant changes in laboratory tests or vital signs were observed.
One of the key strengths of this trial is that the results are very similar to those of previous phase II trials in the USA and Japan. 14 , 15 , 16 , 17 The main limitations of this trial are that it only included Japanese patients and had a short treatment duration of only 4 weeks. However, phase II trials to date suggest that there are no substantial differences in difamilast efficacy and safety between Japanese and non‐Japanese patients. 14 , 15 , 16 , 17 Two phase II trials have suggested no safety issues after 8 weeks of difamilast treatment. 15 , 17 Therefore, although early effectiveness is important in the treatment of AD, further long‐term evaluation for both efficacy and safety is important. A 52‐week long‐term phase III trial of difamilast will help to address this need. 29
In conclusion, this trial demonstrated that difamilast 0·3% and 1% ointments showed superiority over vehicle and favourable safety when applied twice daily for up to 4 weeks in Japanese paediatric patients with AD. As there is currently no cure for AD, topical medications and emollients remain the mainstay for the management of paediatric patients with AD. Because safety and tolerability are essential factors, especially for paediatric patients, the selection of medication with more minimal safety concerns is important. Although the results of ongoing and additional studies are anticipated, difamilast appears to show potential as a new treatment option for paediatric patients with AD with high and rapid efficacy, and a favourable tolerability profile.
Author Contribution
Hidehisa Saeki: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (lead); Resources (equal); Supervision (lead); Validation (equal); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Naoko Baba: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (supporting); Resources (equal); Supervision (supporting); Validation (equal); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Kensuke Ito: Conceptualization (equal); Data curation (equal); Formal analysis (supporting); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (lead); Resources (equal); Supervision (lead); Validation (equal); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Daisuke Yokota: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (supporting); Resources (equal); Supervision (supporting); Validation (equal); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Hidetsugu Tsubouchi: Data curation (equal); Methodology (equal); Resources (equal); Validation (equal); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead).
Supporting information
Table S1 Full exclusion criteria.
Table S2 Investigator’s Global Assessment score.
Table S3 Study investigators and study sites.
Acknowledgments
The authors sincerely thank all study investigators (Table S3; see Supporting Information) and personnel for their involvement and contribution to the study, and all other members of the project team at Otsuka Pharmaceutical Co., Ltd who were engaged in the study. Furthermore, the authors sincerely thank Dr Jon M. Hanifin, Dr Charles N. Ellis and Dr Lawrence F. Eichenfield for their review of the manuscript. The authors also thank Yoshiko Okamoto, PhD, and Mark Snape, MBBS, of inScience Communications, Springer Healthcare, for helping write the outline and first draft of the manuscript. The medical writing assistance was funded by Otsuka Pharmaceutical Co., Ltd.
Funding sources This work was supported by Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan.
Conflicts of interest H.S. was the medical expert for the study and N.B. was the advisor. H.S. and N.B. have received fees for consultation from Otsuka Pharmaceutical Co., Ltd. K.I., D.Y. and H.T. are employees of Otsuka Pharmaceutical Co., Ltd.
Data availability The data that support the findings of this study are proprietary in nature and are not publicly available. However, the data are available from the corresponding author upon reasonable request.
Plain language summary available online
References
- 1. Roduit C, Frei R, Depner M et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr 2017; 171:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 3. Drucker AM, Wang AR, Li WQ et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017; 137:26–30. [DOI] [PubMed] [Google Scholar]
- 4. Weidinger S, Beck LA, Bieber T et al. Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 5. Eichenfield LF, Tom WL, Berger TG et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71:116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katayama I, Aihara M, Ohya Y et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int 2017; 66:230–47. [DOI] [PubMed] [Google Scholar]
- 7. Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology 2017; 233:333–43. [DOI] [PubMed] [Google Scholar]
- 8. Silverberg JI, Nelson DB, Yosipovitch G. Addressing treatment challenges in atopic dermatitis with novel topical therapies. J Dermatolog Treat 2016; 27:568–76. [DOI] [PubMed] [Google Scholar]
- 9. Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. J Am Acad Dermatol 2018; 78:S43–S52. [DOI] [PubMed] [Google Scholar]
- 10. Ahluwalia J, Udkoff J, Waldman A et al. Phosphodiesterase 4 inhibitor therapies for atopic dermatitis: progress and outlook. Drugs 2017; 77:1389–97. [DOI] [PubMed] [Google Scholar]
- 11. Malajian D, Guttman‐Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine 2015; 73:311–18. [DOI] [PubMed] [Google Scholar]
- 12. Hoy SM. Crisaborole ointment 2%: a review in mild to moderate atopic dermatitis. Am J Clin Dermatol 2017; 18:837–43. [DOI] [PubMed] [Google Scholar]
- 13. Hiyama H, Arichika N & Sakurai K Pharmacological activity of difamilast, a novel PDE4 inhibitor for the topical treatment of atopic dermatitis: comparison with other PDE4 inhibitors. Presented at the American Academy of Dermatology Annual Meeting, Washington, DC, USA, 1–5 March 2019.
- 14. Eichenfield LF, Rosenberg N, Roth S et al. Efficacy and safety of difamilast, a topical PDE4 inhibitor, in a phase 2 study of pediatric patients with atopic dermatitis. Presented at: American Academy of Dermatology Annual Meeting, Washington, DC, USA, 1–5 March 2019.
- 15. Hanifin JM, Ellis CN, Frieden IJ et al. OPA‐15406, a novel, topical, nonsteroidal, selective phosphodiesterase‐4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): a phase‐II randomized, double‐blind, placebo‐controlled study. J Am Acad Dermatol 2016; 75:297–305. [DOI] [PubMed] [Google Scholar]
- 16. Saeki H, Baba N, Oshiden K et al. Phase 2, randomized, double‐blind, placebo‐controlled, 4‐week study to evaluate the safety and efficacy of OPA‐15406 (difamilast), a new topical selective phosphodiesterase type‐4 inhibitor, in Japanese pediatric patients aged 2–14 years with atopic dermatitis. J Dermatol 2020; 47:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saeki H, Kawashima M, Sugaya S et al. Efficacy and safety of topical OPA‐15406, a new phosphodiesterase 4 inhibitor, in Japanese patients with atopic dermatitis for 8 weeks: A phase 2, randomized, double‐blind, placebo‐controlled study. J Dermatol 2019; 46:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Japanese Dermatological Association . Clinical Practice Guidelines for the Management of Atopic Dermatitis 2018. Available at: https://www.dermatol.or.jp/uploads/uploads/files/guideline/atopic_gl1221.pdf (last accessed 9 September 2021) (in Japanese).
- 19. Futamura M, Leshem YA, Thomas KS et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74:288–94. [DOI] [PubMed] [Google Scholar]
- 20. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987; 317:1098. [DOI] [PubMed] [Google Scholar]
- 21. Hanifin JM, Thurston M, Omoto M et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10:11–18. [DOI] [PubMed] [Google Scholar]
- 22. Phan NQ, Blome C, Fritz F et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92:502–7. [DOI] [PubMed] [Google Scholar]
- 23. Charman CR, Venn AJ, Ravenscroft JC et al. Translating Patient‐Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor‐based methods. Br J Dermatol 2013; 169:1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlis J, Yosipovitch G. Management of itch in atopic dermatitis. Am J Clin Dermatol 2018; 19:319–32. [DOI] [PubMed] [Google Scholar]
- 25. Bass AM, Anderson KL, Feldman SR. Interventions to increase treatment adherence in pediatric atopic dermatitis: a systematic review. J Clin Med 2015; 4:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenner J, Silverberg NB. Skin diseases associated with atopic dermatitis. Clin Dermatol 2018; 36:631–40. [DOI] [PubMed] [Google Scholar]
- 27. Furue M, Yamazaki S, Jimbow K et al. Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol 2011; 38:310–20. [DOI] [PubMed] [Google Scholar]
- 28. Paller AS, Tom WL, Lebwohl MG et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016; 75:494–503. [DOI] [PubMed] [Google Scholar]
- 29. ClinicalTrials.gov. Long‐term trial of OPA‐15406 ointment in adult and pediatric patients with atopic dermatitis. Available at: https://clinicaltrials.gov/ct2/show/NCT03961529 (last accessed 27 November 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Full exclusion criteria.
Table S2 Investigator’s Global Assessment score.
Table S3 Study investigators and study sites.


