Abstract
This review presents precisely defined amphiphilic dendrons, their self‐association properties, and their different uses. Dendrons, also named dendritic wedges, are composed of a core having two different types of functions, of which one type is used for growing or grafting branched arms, generally multiplied by 2 at each layer by using 1→2 branching motifs. A large diversity of structures has been already synthesized. In practically all cases, their synthesis is based on the synthesis of known dendrimers, such as poly(aryl ether), poly(amidoamine) (in particular PAMAM), poly(amide) (in particular poly(L‐lysine)), 1→3 branching motifs (instead of 1→2), poly(alkyl ether) (poly(glycerol) and poly(ethylene glycol)), poly(ester), and those containing main group elements (poly(carbosilane) and poly(phosphorhydrazone)). In most cases, the hydrophilic functions are on the surface of the dendrons, whereas one or two hydrophobic tails are linked to the core. Depending on the structure of the dendrons, and on the experimental conditions used, the amphiphilic dendrons can self‐associate at the air‐water interface, or form micelles (eventually tubular, but most generally spherical), or form vesicles. These associated dendrons are suitable for the encapsulation of low‐molecular or macromolecular bioactive entities to be delivered in cells. This review is organized depending on the nature of the internal structure of the amphiphilic dendrons (aryl ether, amidoamine, amide, quaternary carbon atom, alkyl ether, ester, main group element). The properties issued from their self‐associations are described all along the review.
Keywords: amphiphiles, dendrons, encapsulation, micelles, self-association
Dendrons are molecular trees, constituted of a tail (a trunk) to which several branches are attached. The different types of self‐associations of amphiphilic dendrons at the air‐water interface, as micelles or as vesicles is described. The properties of these associations of dendrons (direct or reverse micelles or vesicles) in particular as carriers of drugs or of DNA or siRNA, or as drugs by themselves is also discussed.
1. Introduction
Dendrons (dendritic wedges) are composed of a core having two different types of functions, of which one type is used for growing dendritic arms. The very first type of dendron, composed of aryl ether branches, was proposed by Fréchet et al. in 1990. [1] In the early times, dendrons were mainly synthesized with the aim of associating them to a multifunctional central core, to produce a dendrimer. [2] However, it became rapidly clear that dendrons may have their own properties, different from that of dendrimers, due to the presence of the different function linked to the core (Figure 1).
Figure 1.
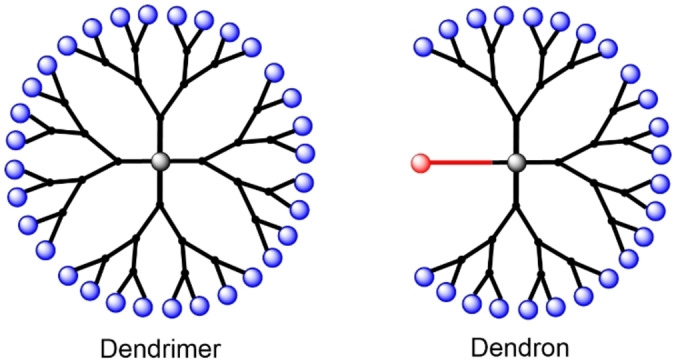
Schematized structure of a dendrimer and of a dendron.
A huge number of dendrons have been already synthesized, and there is an infinite number of possibilities concerning the type of function that can be linked to the core, the nature of the branches, and of the surface functions. In this review we will focus on well‐defined amphiphilic dendrons, suitable to self‐associate in supramolecular assemblies, generally of type micelles. Classical micelles are based on the aggregation of amphiphilic compounds having a polar/hydrophilic head and a hydrophobic tail. Amphiphilic compounds of this type are known to aggregate in water media after a certain concentration called critical micelle concentration (CMC) or critical aggregation concentration (CAC). The polar heads are located in the exterior part of the micelle in contact with water, producing an “oil‐in‐water” system. Micelles have most frequently an approximately spherical shape (Figure 2 left), even if other structures such as ellipsoids, cylinders, and bilayers are also known. [3] Comparison of the shape of micelles and of dendrimers explains why dendrimers have been considered as “unimolecular micelles”. [4] Besides normal micelles, reverse micelles are also known. They are obtained in a non‐polar solvent (“oil”), and give rise to “water‐in‐oil” systems, in which the hydrophilic head is inside the micelle, whereas the hydrophobic tail is extended away from the center (Figure 2 right). [5] Both types of micelles can be obtained with dendrons.
Figure 2.

Schematized oil‐in‐water micelle (left) and water‐in‐oil micelle (right).
Several reviews have already concerned different aspects of associations of dendrons, [6] such as their covalent association to polymers, [7] offering dendronized polymers (numerous dendrons linked to a linear polymer), [8] dendrons having a single linear polymer at the core, [9] or dendrons having polymers as surface functions (in addition to the core or not), [10] in most cases of type poly(ethylene glycol) (PEG). Most of these reviews also concern the supramolecular associations of these polymeric dendrons. However, due to the presence of polymers, none of these compounds can be considered as well‐defined, and are thus out of the scope of this review. Supramolecular assemblies of suitably functionalized dendrons may form liquid crystals having numerous original properties. [11] However, these dendrons are not amphiphile, and thus are also out of the scope of this review. Furthermore, a review has emphasized the early times of the field more than fifteen years ago. [12]
The present review will be organized depending on the nature of the branches of amphiphilic dendrons. Most of these dendrons have branches similar to that of well‐known dendrimers. The three most widely used types of amphiphilic dendrons are constituted of poly(aryl ether) branches, following the pioneering work done by Fréchet et al., [1] or poly(amido amine) branches, following the pioneering work done by Donald Tomalia et al. for the synthesis of PAMAM dendrimers, [13] or poly(amide) branches following the pioneering work done by George Newkome et al. [14] for the synthesis of “arborols” with 1→3 branching. Other types of amphiphilic dendrons are composed of branches pertaining to other families of dendrimers, such as L‐lysine, [15] glycerol, [16] ester, [17] carbosilane, [18] or phosphorhydrazone. [19] The association properties of amphiphilic dendrons and their use will be displayed all along this review. A color code is used for chemical structures in all figures: blue for surface functions, red for the function at the core, and green and pink for internal functions. Albeit being not fully exhaustive, this review aims at giving a large overview of the field.
2. Amphiphilic Aryl Ether Dendrons
Most aryl ether dendrons are issued from the method of synthesis developed by Fréchet et al., [1] based on 3,5‐dihydroxybenzyl alcohol, but several other examples have been proposed also.
2.1. Amphiphilic benzyl ether dendrons
A family of dendrons was synthesized up to generation 4 (1‐G1 to 1‐G4 ), bearing aliphatic chains as terminal functions, a single carboxylic acid at the core, and a multiplication by 3 of the number of terminal functions at each generation (Figure 3). The first generation 1‐G1 was crystalline, but all the other generations displayed liquid crystal properties. Their association occurred in the cubic phase as spherical micelle‐like shapes, comprising 12, 6, and 2 dendrons, for the second, third, and fourth generation, respectively. [20] The possible packing of these dendrons was also studied by molecular dynamic simulations at the atomistic level. [21] A fluorinated analogue of the second generation dendron was also synthesized to be studied by small‐angle X‐ray diffraction, confirming their association as spherical micelle‐like structures. [22] A very small but highly divergent dendron bearing 5 OC18H37 chains and a carboxylic salt as core (Figure 3) melt directly into a body‐centered cubic (BCC) mesophase formed by spherical micelles‐like associations. These spherical associations were found to shrink in volume upon heating and to expand upon cooling. [23]
Figure 3.
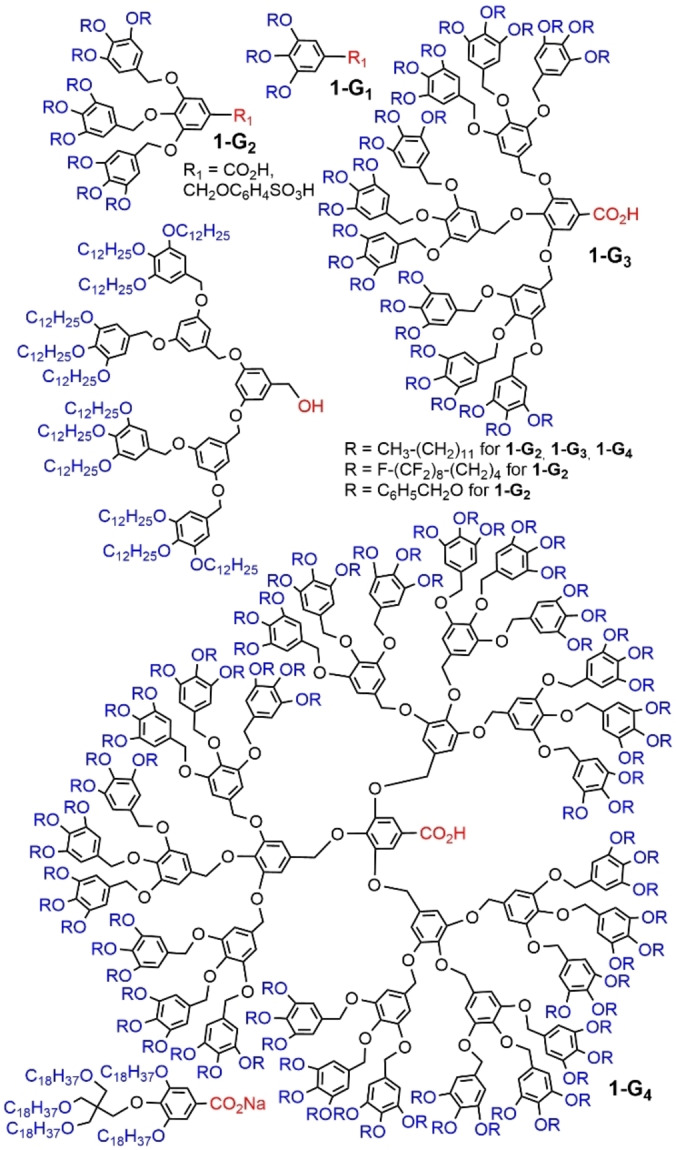
Aryl ether dendrons (1‐G1 to 1‐G4 ) bearing carboxylic acid, sulfonic acid, or alcohol at the core, and alkyl or fluorinated chains as terminal functions.
Another modification concerned the core, with an alcohol instead of the carboxylic acid, and a lower number of branches (Figure 3). The second generation was able to form quasiperiodic structures in the micellar phase, representing a new mode of organization in soft matter. [24] This association was later on directly observed by AFM (atomic force microscopy). [25] Another modification of the core concerned the attachment of a sulfonated group (1‐G2 , R1=CH2OC6H4SO3H). The second generation dendrons bearing either C12H25 alkyl chains or C6H5CH2 aryl functions were synthesized (Figure 3), and used to form micelles suitable to host aniline, which was further polymerized, to form nanotubes and nanorods, for which the micelles acted as templates. [26]
Poly(ethylene glycol) (PEG) is widely used to decorate the surface of amphiphilic dendrons, but PEGs are ill‐defined, and thus will not be considered in this review. However, short‐chain PEGs, named oligoethylene glycols (OEGs) do have a precisely defined structure and have been used as alternatives to PEGs. A second generation dendron bearing 9 OEG chains at the periphery and CO2Me at the core was synthesized. AFM demonstrated that this dendron self‐assembled into spherical micelles in water, of about 50 nm in diameter, at concentrations above the CMC. [27] The third generation of the same family of dendrons was also synthesized (Figure 4). This dendron was also able to self‐assemble into spherical micelles, of about 60 nm in diameter, at a lower concentration compared to the second generation. The CMC was 7.7×10−6 M for the third generation, 2.2×10−5 M for the second generation. These micelles of generation 3 dendrons were used for loading about 29 wt % of podophyllotoxin (POD), an anti‐cancer drug poorly soluble in water. A thermoresponsive drug release behavior was observed. [28] Another family of dendrons was obtained by grafting an OEG group at the core of dendrons, whereas the functions on the surface were carbazole derivatives (Figure 4). AFM studies revealed that these dendrons self‐assembled into spherical structures at the air‐water interface at low pressure, whereas ribbon‐like structures were obtained when the pressure increased. [29]
Figure 4.
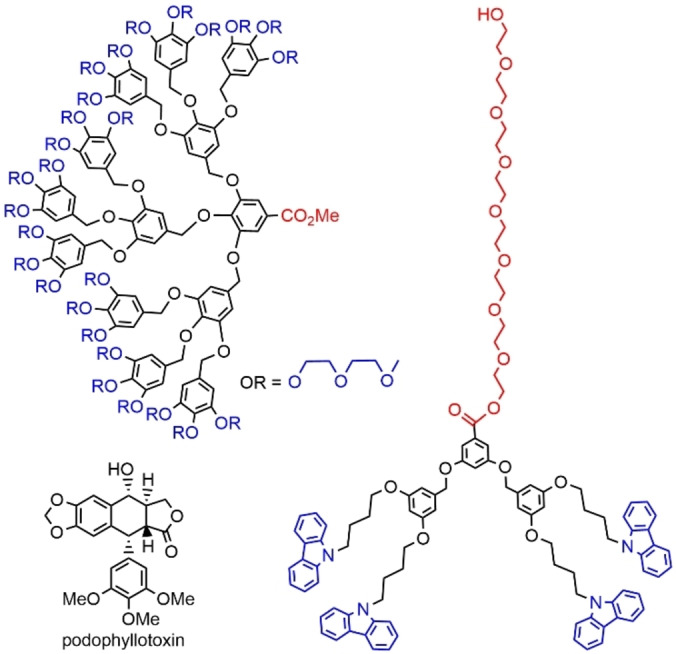
Dendrons functionalized with hydrophilic oligoethylene chains either on the surface or at the core.
A very large library of dendrons, all bearing a biphenyl group at the core, was designed and synthesized for studying the influence of the building blocks on the supramolecular assemblies of dendrons. Some of these dendrons, especially those having a carboxylic acid or an alcohol at the core could be considered as amphiphilic. Figure 5 displays some of these dendrons. Most of them self‐assembled into spherical structures, whereas others self‐assembled into long‐range 5/1 helical pyramidal columns. [30]
Figure 5.
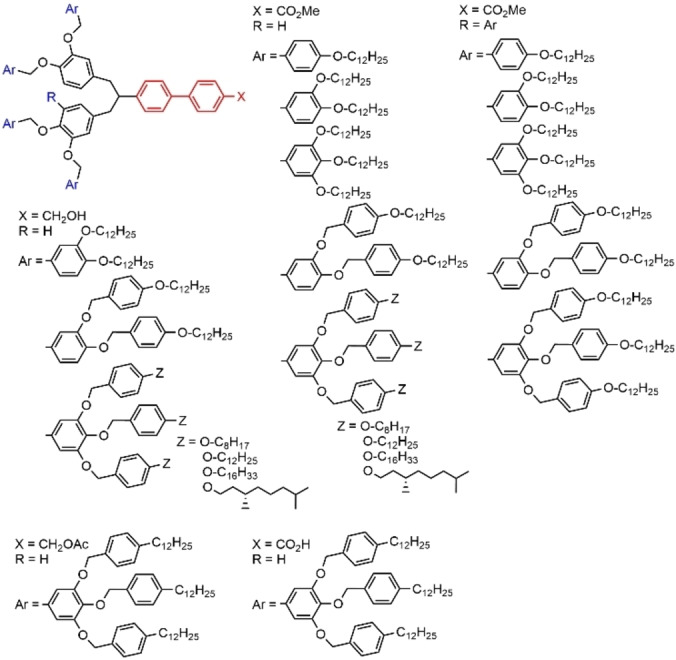
Aryl dendrons having a biphenyl spacer at the core.
Other families of poly(aryl ether) dendrons were functionalized at the core with biological entities such as single‐stranded (ss) DNA or a protein. In a first case, the dendron periphery was functionalized with dichlorobenzene, and the core with a 18‐mer ssDNA (Figure 6). Dissolving this dendron at a concentration of 20 μM in water/dichloromethane 100 : 5, and heating at 90 °C then cooling produced very stable nanofibers of associated dendrons. [31] It was proposed that in the nanofiber, the dendrons aggregated to form a hydrophobic core while the hydrophilic DNA strands assembled at the corona, with average 16.5±1.3 nm diameter with the second generation dendron, and 17.3±0.9 nm with the third generation. A carboxyfluorescein‐modified complementary strand could be hybridized with the nanofibers, producing a large amount of green nanofibers, observed under fluorescence microscopy. On the other hand, Nile Red could be encapsulated inside the fibers, producing red nanofibers. [32]
Figure 6.
Dendrons functionalized at the core with either DNA or protein.
Very recently, a series of protein‐dendron amphiphilic macromolecules was synthesized, based on one side on benzyl ether dendrons of different generations (G1‐G4) coupled to a globular protein through a monodisperse cetyl ethylene glycol having a phosphate on one side and a 1,2,3‐triazole on the other side, obtained via a click reaction (copper catalyzed azide‐alkyne cycloaddition, CuAAC). Compound 2 a shown in Figure 6 is made with the third generation dendron. [33] Very recent results have afforded other types of semi‐synthetic proteins, having different types of linkers between the dendron and the protein. In order to avoid the use of copper, a strain‐promoted azide‐alkyne cycloaddition (SPAAC) reaction was used with a shorter OEG chain, affording compound 2 b. [34] All these compounds have the ability to self‐assemble into supramolecular protein nano‐assemblies, which size depended on the generation of the dendron and the nature of the globular protein. Another method used a photosensitive 5‐hydroxy‐2‐nitro benzaldehyde group at the core of the dendron. Different generations of the dendrons, and different lengths of the OEG chain were used. The largest compound in this series is compound 2 c. The supramolecular protein nano‐assemblies exposed to light were partially disassembled. However, the full disassembly into constitutive monomers could be observed under acidic conditions. [35] The most recent example concern compounds 2 d having a disulfide functionality at the core of dendrons from generations 1 to 3, which affords redox‐sensitive protein assemblies. Treatment with DTT (dithiothreitol) induced the cleavage of the disulfide, resulting in the disassembly of the supramolecular assemblies. [36]
2.2. Internal functionalization of amphiphilic aryl ether dendrons
Several series of aryl ether dendrons having one or two types of internal functions, thanks to the incorporation of tri‐ or tetra‐functional aryl groups as constituents of the branches, have been synthesized. A trifunctional aryl group produced a dendron having a C8H17 tail and up to fourteen internal carboxyl groups (Figure 7). The carboxylate salt form of the dendron produced both normal and reverse micelles in a THF/water mixed solvent. From 90 % to 60 % of water fraction, the estimated diameters of the normal micelles increased from 100 to 400 nm. From 60 % to 45 % of water fraction, the diameters changed irregularly between 200–700 nm, producing mixed micelles (both normal and reverse). From 45 % to 0 %, very small amount of large reverse micellar particles was present; the estimated diameters were around 400 nm. [37]
Figure 7.

Internally functionalized dendron with carboxylic acid, and the normal or reverse micelles formed in water/THF mixtures.
The concept developed to introduce two different types of functions inside the structure, especially both hydrophilic and hydrophobic functions, was first disclosed in 2001 by Thayumanavan et al. The first example concerned a triethylene glycol monomethyl ether as the hydrophilic moieties, and n‐butyl group as hydrophobic moieties (compound 3 a, Figure 8). However, the self‐association properties of this family of dendrons, synthesized up to generation 4, were not studied. [38] A related dendron, having pentaethylene glycol as the hydrophilic part and a decyl chain as the hydrophobic part was then synthesized, up to the third generation (compound 3 b, Figure 8). These dendrons have the ability to form a micelle‐like assembly in polar solvents, such as water in which formation of 75–125 nm particles was observed, and an inverted micelle‐like assembly in apolar solvents, such as toluene, but only if small amounts of water were added. Furthermore, these micelles of dendrons displayed a temperature‐sensitive behavior, [39] which was studied in deeper details several years after. It was shown in particular that the size of the supramolecular assemblies significantly changed in response to temperature variations, and that the guest encapsulation stability of the dendrons decreased with decreasing temperature. [40]
Figure 8.
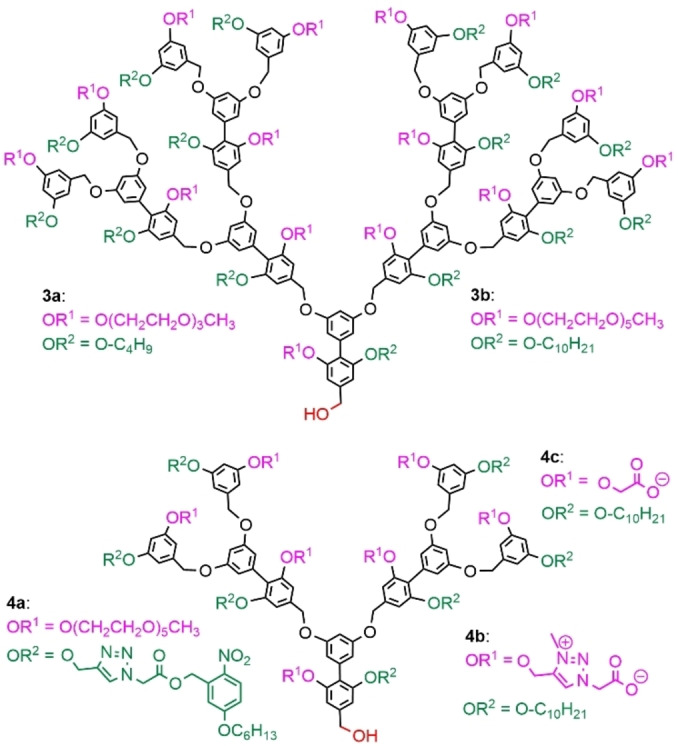
Several examples of dendrons having two types of internal functions.
The same method was applied to synthesize dendrons displaying stimuli‐sensitive properties. For this purpose, dendrons internally functionalized with both pentaethylene glycol and a photolabile 2‐nitrobenzyl ester moiety as the lipophilic unit was synthesized (compound 4 a, Figure 8). Generations 1 and 2 both displayed micellar properties in water at about 18 and 20 μm, respectively. The CMC was measured using the encapsulation of Nile Red, which was also used to monitor the disaggregation of the micelles upon irradiation at a wavelength of 365 nm, which induced the cleavage of the photolabile ester groups. The size of the aggregates was found to decrease from about 80 to 37 nm upon irradiation. [41]
This family of dendrons was expanded to charged entities as water‐solubilizing moieties instead of the OEG units. Zwitterionic moieties composed of an alkylated triazole and a carboxylic acid were one of the examples (compound 4 b, Figure 8). These dendrons also formed micelles in water of about 26 nm, with CAC of approximately 8 μM. These micelles were able to encapsulate the Nile Red dye. They were stable with time, temperature, and were not destroyed by interaction with proteins. [42] Another example concerned carboxylic acids as the sole charged moieties (compound 4 c, Figure 8). Study of the aggregation behavior of generations 1 to 3 demonstrated that the size of the aggregates clearly decreased when the generation increased, probably because of a forced curvature between the neighboring amphiphilic units within a dendron. [43]
Another family of internally difunctionalized dendrons was elaborated using another type of building unit. The aim was to synthesize dendrons having the same number of lipophilic and hydrophilic internal function than those shown in Figure 8, but with a shorter chain at each generation, to get more compact structures. Figure 9 displays the differences between each type of monomer, with arrows for comparing the linker length of each generation, and the structure of these new dendrons. [43] It was shown that they formed micelles in water and inverse micelles in toluene. However, in a mixture of these immiscible solvents, these micelles of dendrons were found to be kinetically trapped in the solvent in which they were initially assembled. This property allowed the selective extraction of peptides from water into the organic phase. The first generation was found more efficient than generations 2 and 3, but the monomer had no efficiency for the extraction. [44] Another property of these micelles concerned the sensing of metalloproteins, using a fluorescent probe encapsulated in the micelle as transducer, able to interact with the protein through electrostatic interactions. [45] All the developments of these internally functionalized dendrons were reviewed. [46]
Figure 9.
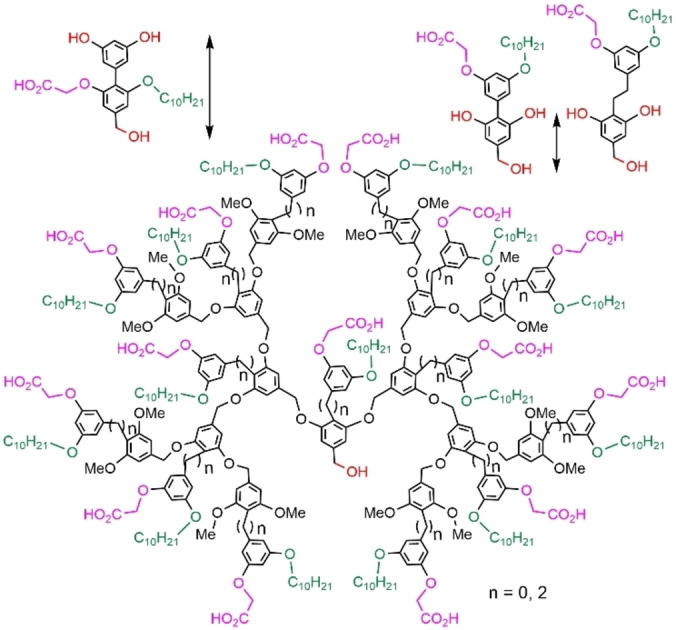
Size comparison between monomeric building blocks, and full structure of other dendrons having two types of internal functions.
Recent developments of these internally functionalized dendrons concerned the use of alkyne‐azide “click” chemistry. The alkyne was on the monomer that was used for building the dendron; it could be used at each generation, or only in a precise place. Such method was applied for labelling the dendron, for instance with 3,5‐bis(trifluoromethyl)phenyl moieties, giving birth to a large variety of 19F‐containing amphiphilic dendrons (compounds 5 a–c, Figure 10). Such labelled dendrons formed aggregates; only a very weak and broad 19F signal could be detected by NMR. However, upon exposure to an enzyme (porcine liver esterase) the ester bond present in most of these dendrons was cleaved, inducing the release of 3,5‐bis(trifluoromethyl)benzoic acid, easily detected by 19F NMR. Such cleavage induced changes in the hydrophilic‐lipophilic balance of the dendron, and a decrease in the size of the aggregates. Thus, it was possible to detect enzyme concentrations in the low nanomolar range. On the contrary, the dendrons having an amide linkage (5 a) instead of the ester (5 b,c) were not cleaved by the enzyme, and no change was observed. [47]
Figure 10.
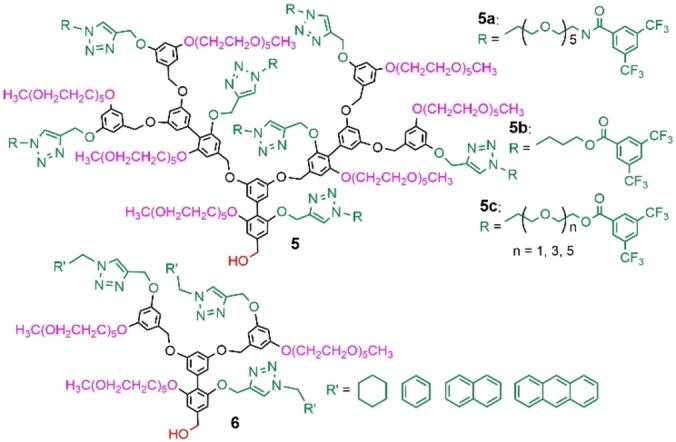
Internally functionalized dendrons using “click” chemistry.
Another series of dendrons was obtained by grafting hydrophobic functional groups, such as naphthyl or anthracyl moieties (compounds 6, Figure 10). Studying the influence of temperature on the aggregates demonstrated that the supramolecular assemblies became less sensitive to temperature changes when aromatic interactions in the aggregate were increased. In sharp contrast, the absence of aromaticity in the hydrophobic moieties (cyclohexyl) produced temperature‐sensitive aggregates. [48]
A series of dendrons functionalized with biotin in different locations was synthesized (Figure 11). Biotin has a well‐established affinity towards avidin. These dendrons formed aggregates in water that were able to encapsulate small molecules, such as Nile Red. In presence of the target protein extravidin, the dendron‐based supramolecular assembly was destabilized, and the guest molecules were released. The efficiency of the release depended on the location of biotin in the dendron. The most efficient interactions were observed when biotin was located on the surface of the dendron, being more accessible to the protein. Such process was not observed when using proteins α‐chymotrypsin, pepsin and myoglobin having lower binding affinity to biotin. [49]
Figure 11.
Different locations of biotin inside the structure of dendrons, for interaction with the protein extravidin.
3. Amphiphilic Polyamidoamine (PAMAM) Dendrons
In an early example of self‐associated PAMAM dendrons, they were functionalized at the core with 32 base‐long DNA oligonucleotides. A library of PAMAM dendrons associated by the complementary oligonucleotides at the core was established. The type and number of terminal functions of associated dendrons could be different, as illustrated in Figure 12. [50] However, the large majority of associated PAMAM dendrons formed micelles or micelle‐like aggregates.
Figure 12.
Example of dendrons associated by their DNA core.
3.1. Alkyl chain(s) at the core of PAMAM dendrons
Several generations of PAMAM dendrons (1 to 4) bearing two lipophilic chains at the core and primary amines as terminal functions were synthesized and used as transfection agents for plasmid DNA, but their self‐association properties were not studied initially. [51] However, the first generation was shown later on to form either micelles below pH 6.4, or vesicles at neutral and alkaline pHs. Indeed, this dendron contained two primary amines and two tertiary amines suitable for changing their charge in the pH region between pH 10 and pH 4. [52] The same family of dendrons was then functionalized with amide terminal groups of types IBAM (isobutylamide) or ACAM (acetamide). These dendrons have both a lipophilic tail and surface, but a hydrophilic internal structure, due to the protonation of the tertiary amines (Figure 13). This particular structure led to self‐assembled vesicles in water, which displayed a thermosensitive behavior. [53]
Figure 13.
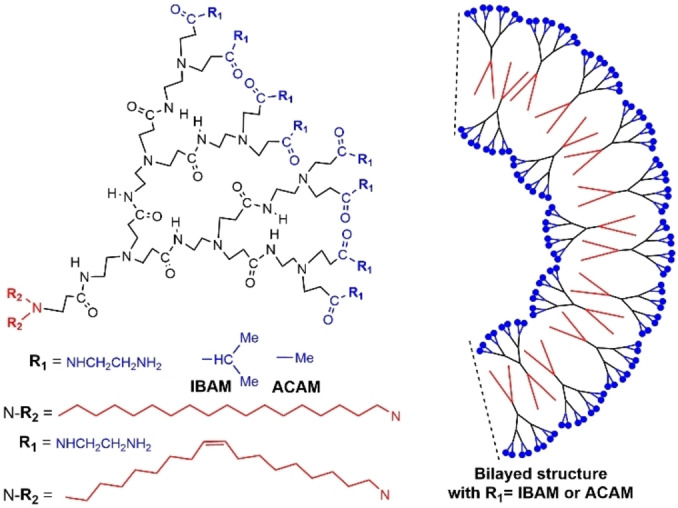
PAMAM dendrons functionalized with two alkyl chains at the core. Schematized vesicle constituted of bilayered structures.
A slight modification consisted in introducing an unsaturated bond into the alkyl chains, in view of promoting membrane fusion for the endosomal release of nucleic acids. Assemblies of these dendrons bearing ammonium terminal functions in water were shown to have broad size distributions by DLS (Dynamic Light Scattering), and TEM (Transmission Electron Microscopy). These assemblies were used for transfection in COS7 and HeLa‐S3 cells with plasmid DNA encoding the luciferase gene. The best activity was shown with the first generation dendron, whereas the second and third generations had very low activity. [54]
A recent example concerned PAMAM dendrons (generations 1 to 5) having two nitrogen atoms at the core, each of them bearing a single alkyl chain affording gemini‐type amphiphilic dendrons (Figure 14). These dendrons in water were adsorbed at the air‐water interface, and formed spherical micelles inside water, at a concentration of 5.0 mM in solution at pH 9. Surprisingly, the overall micelle size was not dependent on the generation of the dendron, but the core radius of the micelle decreased when the generation number increased. [55]
Figure 14.
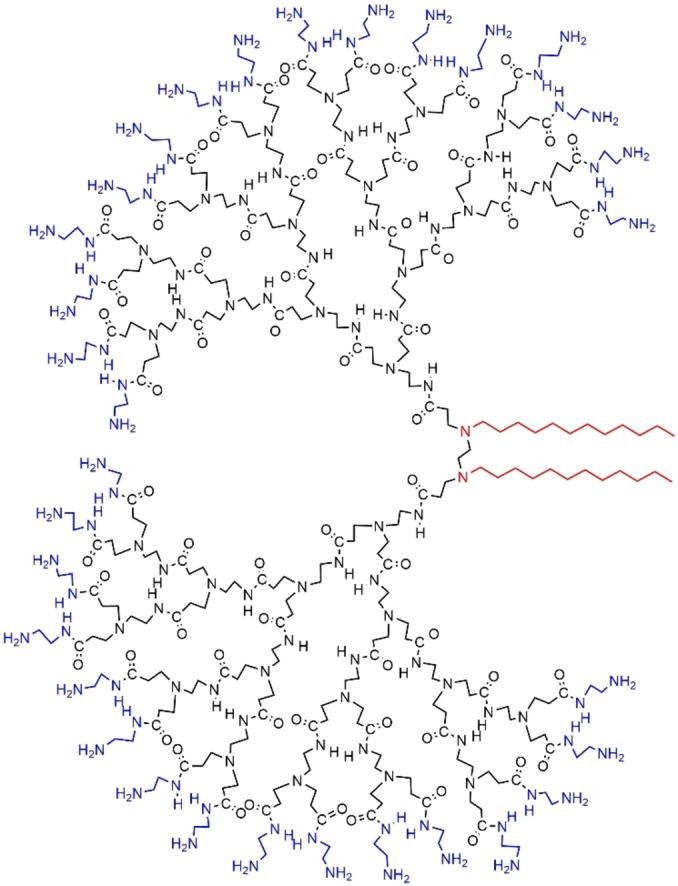
Gemini‐type amphiphilic PAMAM dendron.
A series of PAMAM dendrons having a single long alkyl chain at the core was synthesized via a click reaction between a dendron functionalized with the alkyne, and the alkyl chain functionalized with an azide. Different small generation (0, 1 or 2) dendrons bearing different lengths for the alkyl chain (C13H29, C15H31, or C17H35) and amines (2, 4, or 8) as terminal functions were synthesized (Figure 15). These amphiphilic dendrons formed micelles in water. It was shown that the most efficient dendron as vector for siRNA (small interfering RNA) molecules to silence the heat shock protein 27 (Hsp27) gene was the dendron bearing the longest alkyl chain and the largest number of amines. Tests against prostate cancer models in vitro and in vivo were carried out, and it was shown that the dendrons can successfully mediate the effective delivery of siRNA to downregulate Hsp27 and produce potent anticancer activity in vivo in mice bearing PC‐3 xenograft tumors. [56] The same dendrons were studied by molecular simulation methods, to describe their self‐assembly, depending on the generation and the tail length, and to explain the better efficiency of the largest dendron as gene carrier, in terms of binding to siRNA. [57]
Figure 15.
PAMAM dendrons with an alkyl chain of variable length as core, linked by “click” reaction.
In view of the influence of the chain length on the properties, the study was expanded to longer lengths (C19H39, C21H43, C23H47) (Figure 15). It was shown that the second generation dendron bearing the C19H39 chain was the most efficient carrier for siRNA binding and delivery for silencing of Hsp27 gene both in vitro and in vivo. [58] The same dendrons were also studied in silico, to predict the interactions between the amphiphilic dendrons and siRNA molecules. [59]
Another family of dendrons bearing a single alkyl chain was obtained by alkylation of an amine at the core, providing a quaternary ammonium (Figure 16). C12, C18, and C22 chains were linked to the core of generations 0 and 1, and the surface was functionalized with sugar groups (DL‐lactobionic acid). The CMC decreased with increasing length of alkyl chain, from 8.2×10−3 M (C12) to 4.5×10−6 M (C22) for generation 0, and from 1.0×10−2 M (C12) to 3.7×10−5 M (C22) for generation 1. [60] Another paper concerned C10, C14, and C18 chains and methylester terminal functions (Figure 16). Both CMC and surface tension of these amphiphilic dendrons were lower than those of the sugar series given just above. [61]
Figure 16.
PAMAM dendrons with an alkyl chain linked by alkylation of an amine.
Two other families of PAMAM dendrons having a single hexadecyl chain at the core were synthesized up to generation 5. These dendrons had either two or three dendritic wedges emanating from the core, either of type hexadecylamine or N‐hexadecylethylenediamine. Figure 17 displays the case of 3 dendritic wedges. Both families were shown able to adsorb at the air/water interface and to form micelles in water solution, at a concentration of 5.00 mM at pH 9. [62]
Figure 17.

PAMAM dendrons having a single hexadecyl chain at the core.
3.2. Other functional groups at the core of PAMAM dendrons
Besides alkyl chains, only a few other types of functions have been grafted to the core of PAMAM dendrons involved in supramolecular structures. An early example concerned an aniline core (compound 7 a), and the dendrons were synthesized up to the fifth generation (64 amine terminal groups). Despite the small size of the group at the core, the CMC occurred in the range 10−4–10−5 M. The aniline group was found suitable for the grafting of biotin, as a test reaction (compound 7 b, Figure 18). [63]
Figure 18.
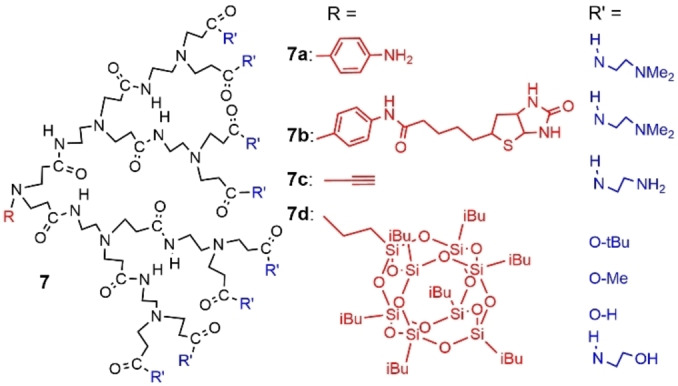
Several examples of PAMAM dendrons diversely functionalized at the core and on the surface. Only the second generation is represented, but the dendron having the alkyne at the core was synthesized up to generation 5.
Another small function at the core was an alkyne, which was linked to PAMAM dendrons up to generation 4 (Figure 18 shows the second generation of compound 7 c) in a first attempt, [64] and to generation 5 recently. [65] These dendrons were first studied in monoolein/water mixtures. It was shown that they induced a reorganization from a bicontinuous mesophase to a discontinuous reverse micellar assembly. The dendrons interacted with monoolein through their amine groups. This discontinuous reverse micellar phase of type Fd3 m was formed only in the presence of the dendron, and not in the presence of a linear analogue. [64] The fifth generation dendron in the same conditions induced the formation of a very unusual mesophase with P4332 symmetry. [65]
A last example concerned a series of PAMAM dendrons bearing as core a heptaisobutyl‐polyhedral oligomeric silsesquioxane (POSS) group, and different surface groups, such as primary amine, carboxylic acid, alcohol, or ester (compounds 7 d, Figure 18). Dendrons bearing the alcohol terminal functions formed micelles in methanol. Casting of a methanol solution of the dendron onto a glass substrate followed by heating at 100 °C for 15 min provided optically transparent films. [66]
4. Amphiphilic Polyamide Dendrons
4.1. Poly‐l‐lysine dendrons
Polyamide dendrons are mainly of type poly‐l‐lysine. Contrarily to PAMAM dendrimers, polyamide dendrons do not have protonable internal amines, and thus the amphiphilic character is essentially provided by the core and surface functional groups. A very large series of lysine dendrons (32 dendrons) was synthesized varying the number and type of terminal functions, as well as the type of function linked to the core, as shown in Figure 19A. The starting compound contained a core which was modified to possess 1, 2 or 3 alkyl groups of varying chain length, from which emanated multiple branched lysine groups, ended with either amino groups (up to 64) or with alkyl chains of varying chain lengths up to C18. Some of these dendrons were amphiphilic, with a propensity to form micelle‐like structures. Others, either in the presence or absence of cholesterol, formed vesicular structures. The cationic dendrons had the ability to condense DNA to form polyelectrolyte complexes (dendriplexes), and the efficiency depended on the structure of the dendrons. Dendrons bearing 3 alkyl chains at the core were the most efficient, and the efficiency increased with the increasing length of the alkyl chain. [67] Dendriplexes formed by the lysine dendron having 16 surface amino groups and either three C18 chains or a single C10 chain at the core with pRedN‐1 DNA (7.5 kbp), a fluorescent protein vector, were encapsulated inside biodegradable PLGA particles (poly(lactic‐co‐glycolic acid)). The encapsulation efficiency of dendriplexes in PLGA particles was found higher than that for non‐complexed DNA. [68] Analogous experiments were carried out with plasmids containing the gene for protective antigen (PA) of Bacillus anthracis. Tests carried out in vivo in mice demonstrated that the dendriplexes encapsulated in PLGA produced superior levels of anti‐PA IgG antibodies. [69]
Figure 19.
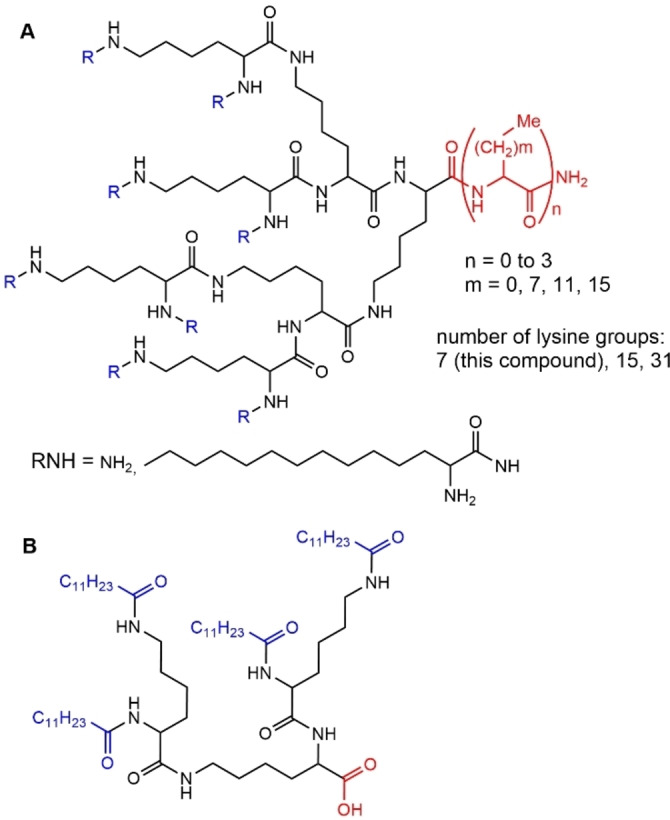
A) Poly‐l‐lysine dendron composed of 7 lysine groups. Higher generations composed of 15 or even 31 lysine groups have been also synthesized. B) Small lysine dendron able to self‐assemble in organic solvents to produce gels.
The physicochemical behavior of this family of lysine dendrons was studied in different conditions. The third‐generation polylysine dendron coupled with eight α‐amino tetradecanoic acid (C14) molecules to the surface and three pendant C14 chains coupled to the core was found to self‐assemble in water into vesicular structures. In the presence of the non‐ionic surfactant iso‐octyl phenyl poly(10)oxyethylene ether (Triton X‐100) the aggregate diameter decreased to 12–50 nm as micellar structures. [70] The aggregation number of other members of the same family was measured. The aggregation number was 33 for the dendron with three C14 chains and eight terminal NH2 groups, whereas it was 78 for the analogue with C18 chains. [71]
A small dendron functionalized with a carboxylic acid at the core and with C11 alkyl chains on the surface was shown capable to self‐assemble to form gels in toluene or cyclohexane (Figure 19B). It was proposed that the dendron could assemble effectively on its own into nanoscale structures, as a consequence of acid‐acid interactions and van der Waals interactions between the alkyl tails. [72]
Another series of polylysine dendrons bearing a single alkyl chain of variable lengths (C1‐C18) at the core was synthesized (Figure 20) and their self‐assembling properties in aqueous solutions was studied by small angle neutron scattering (SANS) and DLS. The self‐assembling properties were influenced by the pH value, demonstrating the need for positively charged amines in the head groups for the successful formation of controlled self‐assemblies. Chain lengths below C8 did not induce self‐assembly, and well‐defined micellar structures were only formed with alkyl chain lengths above C12. The dendrons showed low cytotoxicity, displaying cell viability well above 80 %, when evaluated in mouse fibroblast (NIH/3T3) and human embryonic kidney (HEK 293T) cells at 5, 10 and 20 μM concentrations. [73]
Figure 20.
Polylysine dendrons functionalized with a single alkyl chain of variable length at the core.
Very recently, another series of lysine dendrons bearing two alkyl chains at the core was synthesized. These dendrons were used for mediating effective delivery of siRNA targeting Hsp27 mRNA for treating castration‐resistant prostate cancer. The smaller dendron (4 NH2 groups, Figure 21) promoted more efficient intracellular uptake and endosome release of the siRNA complexes, as well as better siRNA releasing ability. Such behavior resulted in more potent gene silencing and anticancer effects both in vitro (PC‐3 prostate cancer cells) and in vivo (prostate cancer PC‐3 xenograft nude mouse). [74]
Figure 21.
Lysine dendrons functionalized with two alkyl chains at the core.
4.2. Other types of polyamide dendrons
A series of polyamide dendrons was synthesized by a convergent process starting from lauric acid (dodecanoic acid, a saturated fatty acid with a 12‐carbon atom chain) and N‐(3‐aminopropyl)‐1,3‐propanediamine. The secondary amine at the core could be functionalized to continue the synthesis of the dendron, but also to functionalize the focal point (the core). In first attempts, generations 1 and 2 were synthesized, and the function at the core was a methylester, which was deprotected to give a carboxylic acid (compound 8 a, Figure 22). These compounds formed self‐assembled gels in organic media, which disassociated when adding a drop of methanol. [75] This family of amide dendrons was then synthesized up to the third generation, which also formed self‐assembled gels. [76]
Figure 22.
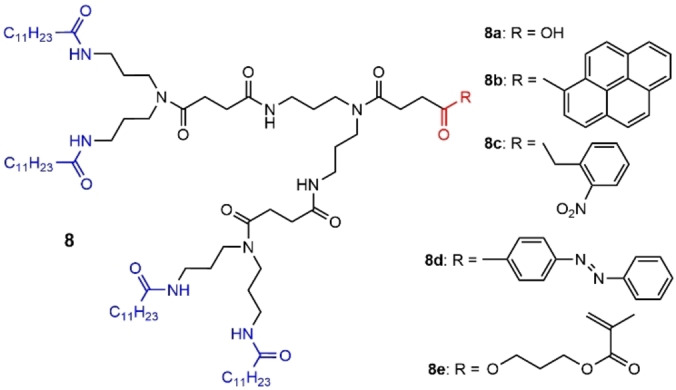
Diverse examples of polyamide dendrons with different functions at the core.
The easy possibility to modify the nature of the functional group at the core was largely exploited, first to graft a pyrene (compound 8 b, Figure 22). These dendrons self‐organized into vesicles in aqueous media. Furthermore, the focal pyrene unit could be included in the cavity of β‐ or γ‐cyclodextrin (CD) inducing the formation of self‐assembled organic nanotubes. Such assembly was reversible upon addition of poly(propylene glycol), leading again to the formation of vesicles. [77] These organic nanotubes were elaborated later on with variously functionalized cyclodextrins. Functionalization with NH2 permitted the association of the nanotubes with anionic gold nanoparticles. Functionalization with biotin afforded a biosensor. Indeed, the binding of fluorescein‐labeled streptavidin could be detected by fluorescence. [78] The core functionalization was expanded, to photosensitive compounds such as 2’‐nitrobenzyl (photo‐cleavable) and azobenzene (photo‐isomerisable) (Figure 22). Such compounds formed vesicles in water, which were able to encapsulate hydrophilic guest molecules in the inner water compartment, but also hydrophobic guest molecules from the membrane of the vesicles. The vesicles of the dendron functionalized with 2’‐nitrobenzyl (compound 8 c) underwent a large supramolecular reorganization to form a fibrous nanostructure after photolysis. Such rearrangement enabled the release of calcein molecules (a dye) entrapped in the interior of the vesicle of dendrons. The vesicles formed by the dendron functionalized with azobenzene (compound 8 d), which underwent a reversible trans‐to‐cis isomerization upon irradiation, were not noticeably deformed upon irradiation. However, the permeability of the vesicle was dependent on the photoisomerization of the focal azo moiety. The cis‐azobenzene moieties in the vesicle membrane gave rise to remarkably enhanced permeability. [79] Another example of functionalization at the core concerned the reaction of the carboxylic acid with 2‐hydroxyethyl methacrylate (HEMA). Such dendrons formed spherical vesicular assemblies in water. The methacrylate function at the core of dendron 8 e was used for radical polymerization, affording dendronized polymers. [80]
A sequel of this family of polyamide dendrons concerned compounds bearing diacetylene moieties as terminal functions, issued from 5,7‐octadecadiyn‐1‐ol, and a carboxylic acid function at the core (Figure 23). These dendrons formed organized nanostructures in organic media, lamella or columnar hexagonal structures, and vesicular structures in water. These supramolecular nanostructures could be stabilized by in situ photopolymerization of the diacetylene moieties. [81]
Figure 23.
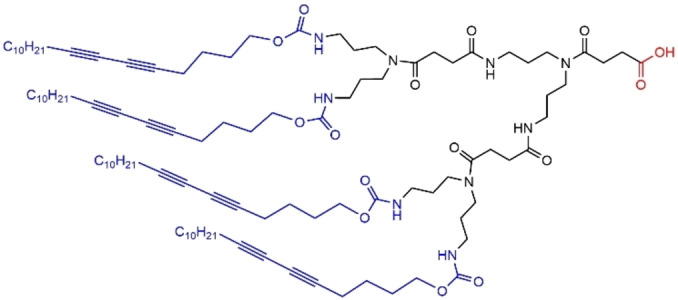
Diacetylene moieties as terminal functions of a polyamide dendron.
5. Amphiphilic Dendrons Based on Quaternary Carbon Atoms as Branching Points
Inspired by the 1→3 branching motifs pioneered and reviewed by G.R. Newkome, [82] several types of dendrons were synthesized, having different types of branches, such as amides and ethers, or only amides. The 1→3 branching offers rapidly a higher density of terminal functions compared to the 1→2 branching shown in most of the previous paragraphs.
5.1. Amide and ether branches
A different family of polyamide dendrons was functionalized on the surface with spermine, and variable lipophilic groups at the core such as a benzyl group (9 a‐G1 and 9 a‐G2 ) in the first experiments, with the aim of using these compounds for transfection, but the possibility of self‐assembly of the dendrons was not studied. [83] However, multi‐scale modeling of this family of dendrons provided an insight into the mode of self‐assembly of the dendrons, suggesting explanations for some of the unexpected observations made in the transfection experiments. [84] In a next step, one (9 b‐G1 and 9 b‐G2 ) or even two (9 c‐G1 ) cholesterol units were linked to the core, with the aim of favoring the self‐assembly of the dendrons (Figure 24). These dendrons formed self‐assemblies in which the cholesterol units were strongly packed. Among the first and second generation dendrons bearing one cholesterol units, and the first generation bearing two cholesterol units, the latter one was found the most efficient for transfection. [85] A variety of other lipophilic units were grafted to the core of these spermine dendrons, such as a dodecanoyl chain (9 d‐G1 ), a lysine derivative (9 e‐G1 ), or an aliphatic small dendron (9 f‐G1 ) for the first generation dendron, or a dodecanoyl chain (9 d‐G2 ) for the second generation, with the aim of developing a structure‐activity relationship (Figure 24). The choice of the functional group at the focal point of the dendron had a very significant impact on DNA binding. Mesoscale modeling of the dendrons self‐assembly provided a direct insight into its effect on the DNA binding process. The hydrophobic unit at the core controlled the number of dendrons in the micellar structures, and hence their diameters and surface charge density, which had a direct impact on the DNA binding affinity, and to cellular gene delivery. [86]
Figure 24.
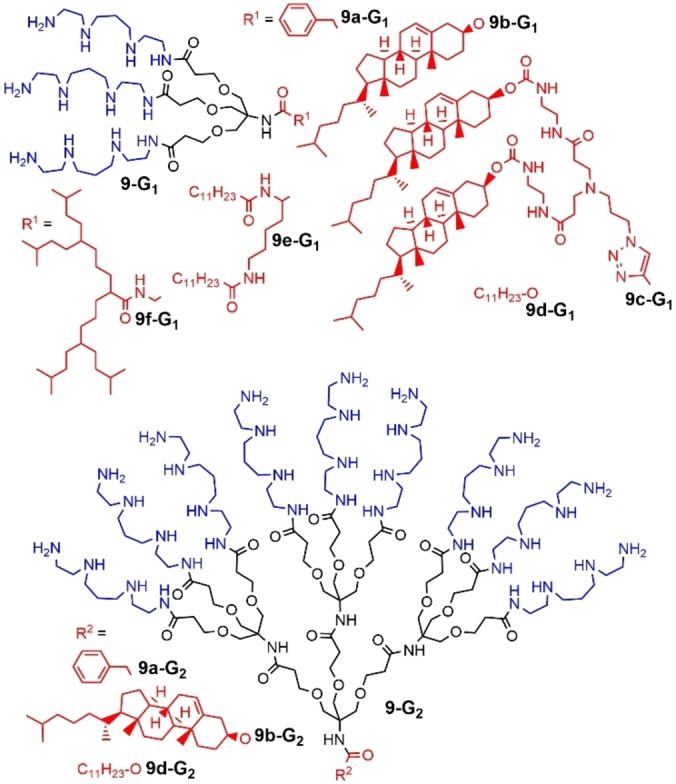
First and second generations of spermine dendrons diversely functionalized at the core.
Another family of dendrons having both amide and ether functions in their branches was functionalized at the core with a benzyl group, and on the surface with linear RGD peptides (arginylglycylaspartic acid), the most common peptide motif responsible for cell adhesion to the extracellular matrix. These dendrons were synthesized with the aim of showing the most effective integrin binding in a fluorescence polarization competition assay. The same type of dendron was also functionalized at the core with a hydrophobic C12 chain (Figure 25), which self‐assembled in aqueous phosphate buffered saline, with the CMC being calculated as ca. 300 mM. This assembly was also capable of effective integrin binding. [87]
Figure 25.

Dendrons functionalized with the RGD peptide for integrin binding.
5.2. Amide branches
A series of dendrons having a fullerene either at the level of the core (Figure 26A) or as core (Figure 26B and C) and based on 1→3 branching motifs and amide branches has been synthesized. The fullerene was linked to the core through an ester group (case A), whereas the fullerene used as core was linked through either an ester or an amide group (case B). The presence of the carboxylate terminal functions of the dendrons induced both solubility in water and the formation of small micelles at pH 7.0, as shown by DOSY NMR (Diffusion‐ordered spectroscopy) experiments. Complexation of these dendrons with cationic porphyrins was able to break apart the micelles to give monomeric species, which then afforded aggregates dendron‐porphyrin. [88] In another experiment, the porphyrin and the dendrons were covalently associated through the fullerene (Figure 26A). These particular dendrons formed micellar aggregates in water. [89]
Figure 26.
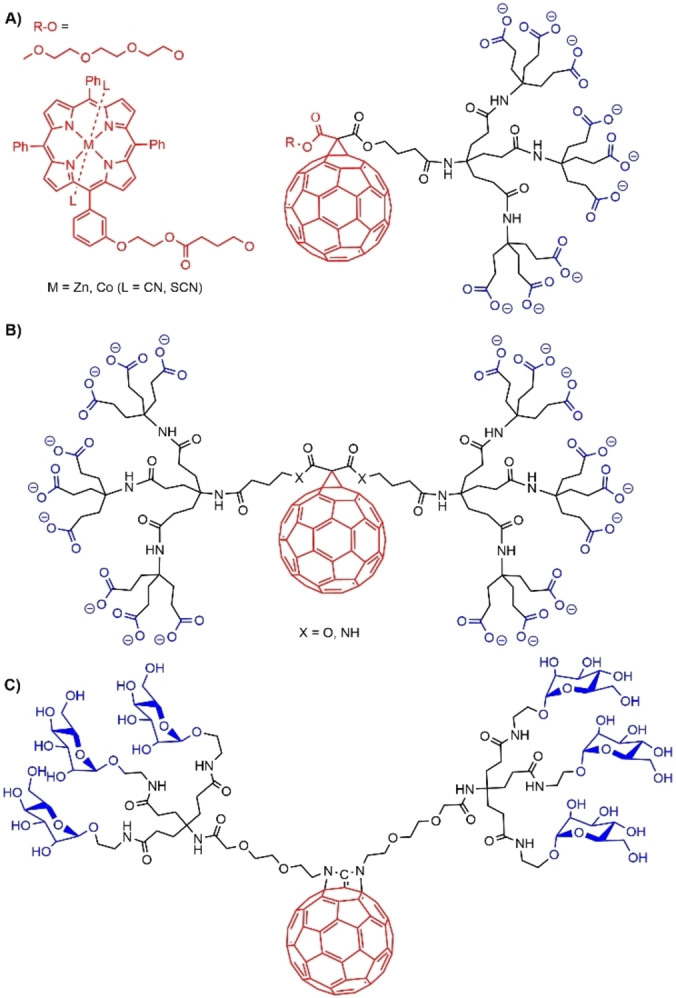
Dendrons functionalized with fullerene. A) dendrons bearing a pendant fullerene linked to the core. B) and C) Fullerene as core of dendrons.
A fullerene was functionalized with two dendritic α‐D‐mannopyranosides, connected through two adjacent imino bridges, as shown in Figure 26C. The amphiphilic nature of this dendron and its cone‐shaped structure induced the formation of small supramolecular aggregates in aqueous solutions. DOSY NMR and TEM results showed that the micellar aggregates had an extremely narrow size distribution of around 4 nm. [90]
The presence of fluorescent aromatic groups at the core of dendrons was suitable for inducing the formation of micelles in aqueous media. Both a pyrene and a perylene were used as core of small dendrons bearing carboxylic acid terminal functions (Figure 27). The dendron bearing the pyrene group formed small micelles of about 4 nm in diameter. The red perylene derivative, perylenedicarboximide, is insoluble in water. Thus, it was dissolved in diethyl ether, to be added to these micelles of dendrons. After sonication, the aqueous phase took a faint orange‐red color, showing that the dendrons were able to promote the transfer of the perylene dye in the aqueous phase. [91] The dendron based on a perylene core (Figure 27) was synthesized starting from 3,4,9,10‐perylenetetracarboxylic dianhydride. The N‐dodecyl chain was grafted to the perylene in the first step, and the dendron in the second step. This compound easily formed micelles in water at pH 7.2, with a mean diameter of 16 nm. [92]
Figure 27.
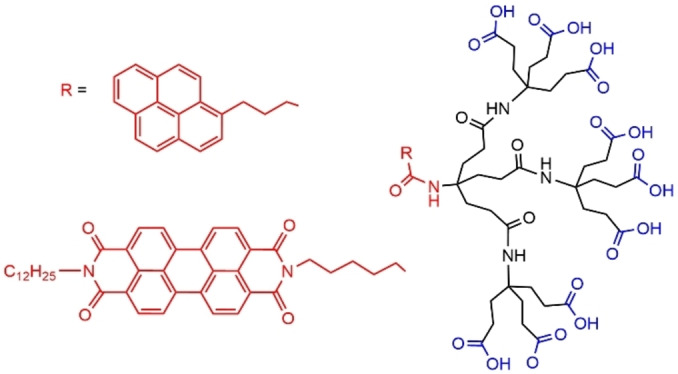
Dendrons bearing a fluorophore at the core, pyrene or perylene.
A few amide dendrons including aromatic units as branching points were also synthesized (Figure 28). The smallest one had a C16 chain at the core and 6 carboxylic acid terminal functions. These dendrons formed micelles in aqueous solution at CMC of 125 ppm. These dendrons were also able to interact with wormlike micelles of CTAT (cetyltrimethylammonium p‐toluenesulfonate). These CTAT/dendron mixtures were significantly more elastic than CTAT solutions. [93] Another example concerned cationic pyridinium branching units, surrounded by negatively charged carboxylic acids, and bearing a long alkyl chain at the core. Several dendrons were synthesized; the one shown in Figure 28 had the most pronounced tendency to form spherical micelles. [94]
Figure 28.
Amphiphilic dendrons built with aromatic branching units.
6. Amphiphilic Alkyl Ether Dendrons
A majority of amphiphilic alkyl ether dendrons are issued from the reactivity of glycerol, as mainly proposed by R. Haag et al., the others being based on ethylene glycol, as mainly proposed by M. Lee et al. In both cases, these dendrons are intrinsically non‐ionic amphiphiles.
6.1. Glycerol dendrons
A family of hydrophilic polyglycerol dendrons (generations 1 to 3) connected to hydrophobic alkyl chains through different types of spacers was synthesized, and their ability to self‐assemble in micellar aggregates was studied. The first examples concerned C11 or C16 alkyl chains, linked via a “click” reaction to the azido core of glycerol dendrons (Figure 29). The smallest dendrons (first generation) formed different structures such as ring‐like and fiber‐like micelles, whereas the second and third generations aggregated into spherical micelles of low polydispersity. It was shown that these micelles contained only 15 molecules and possessed up to 74 % solvent filled space. All these dendrons were able to solubilize hydrophobic dyes such as Nile Red or pyrene. [95] This family was expanded with the synthesis of dendrons bearing other alkyl chains (C11, C15, C21) linked through different functions such as alkyl or aryl amides, or aryl ethers (Figure 29). These dendrons were also shown able to form micelles, and they were used for the solubilization of carbon nanotubes. [96] Another linker was an alkyl ether. These dendrons associated with the Brij L4 surfactant produced a variety of honeycomb‐like patterned surfaces. Small nanoparticles (micelles of dendrons) were formed only at the inner edges of the holes. [97]
Figure 29.
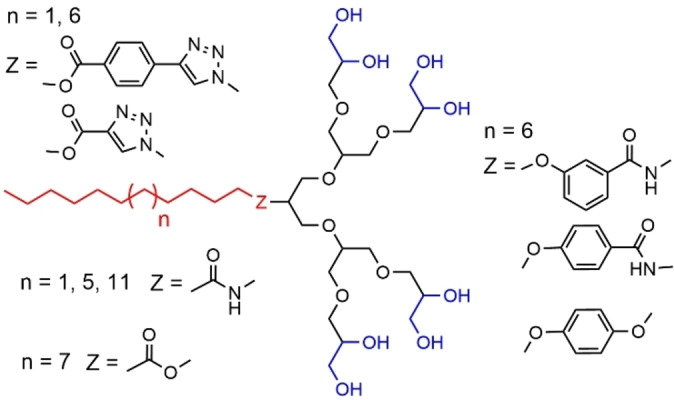
Second generation of glycerol dendrons bearing an alkyl chain of variable length at the core, linked through different types of linkers.
In a very recent paper, dendritic oligoglycerol regioisomer mixtures, differing in terms of connectivity between glycerol units in the head group, were synthesized. The alkyl chain at the core was linked via different types of functions, such as ether, amide, triazole, or aryl group. Furthermore, besides the alkyl chain, a cholesterol unit and an azobenzene group were also linked to the core (Figure 30). These dendrons were used for extracting large protein quantities from biological membranes. It was shown that these dendrons can form stable proteomicelles, and can retain the activity of membrane proteins in the absence of membranes. [98]
Figure 30.
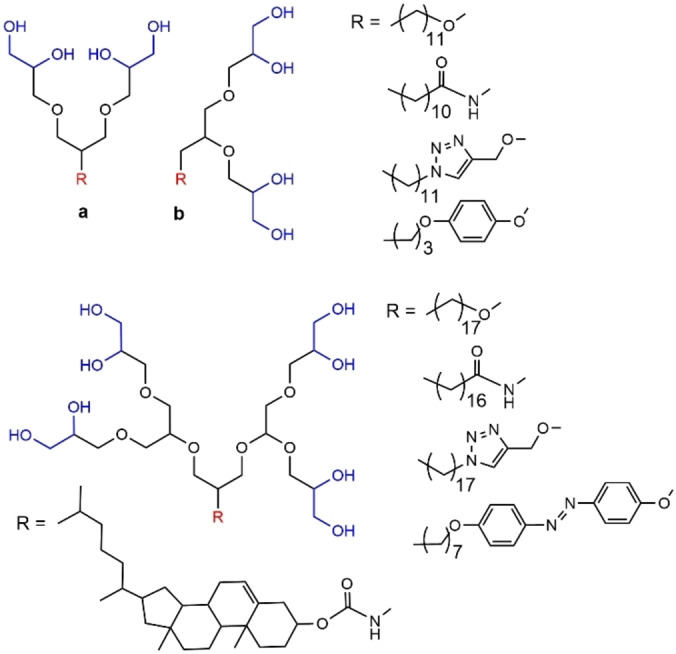
Regioisomers of first and second generations of glycerol dendrons bearing different types of functions at the core.
Besides the different types of functions for linking the tail at the core shown in the previous figures, different types of modifications of the tail were carried out. The simplest modification concerned the presence of a double bond in the middle of the aliphatic chain, issued from oleic acid, and linked through an ester bond, possibly biodegradable (compound 10 a, Figure 31). As observed previously for dendrons without the double bond, the first generation dendron formed rod shaped micellar aggregates, whereas the second and third generations formed spherical micelles. [99] Another modification of the alkyl tail concerned the grafting of two chains instead of one, either of type alkyl (10 b) or perfluoroalkyl (10 c) (Figure 31). In the first case, the behavior of these dendrons (generation 1 and 2) at the water‐air interface was studied. They were able to form well‐defined Langmuir monolayers, but also giant unilamellar vesicles (with diameters of up to 3 mm). [100]
Figure 31.
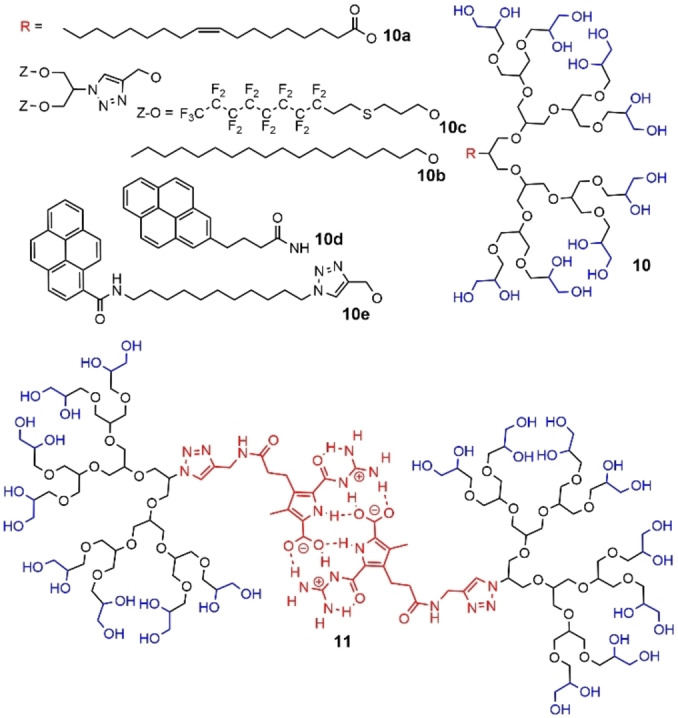
Different types of tails at the core of glycerol dendrons.
The dendrons bearing two perfluoroalkyl chains (10 c) were able to interact with polyglycerol dendrimers functionalized with a perfluorinated shell, forming highly stable supramolecular architectures in water, due to self‐assembly of the perfluorinated moieties. [101] A pyrene group linked through a short (10 d) or long (10 e) alkyl chain was also used as core (Figure 31). Surprisingly, these pyrene dendrons did not possess a single well‐defined CMC, and were found poorly efficient for solubilizing carbon nanotubes. [96] On the contrary, the presence of a self‐complementary guanidiniocarbonylpyrrole carboxylate zwitterion attached by click chemistry to the core of dendrons 11, from generation 1 to 3 induced the self‐association of two dendrons (Figure 31). The formation of these self‐assembled pseudo‐dendrimers could be controlled by the pH of the solution. At pH >8, the zwitterion was deprotonated and thus the dendrons could not self‐assemble. At neutral pH, only dimers were present. In acidic media, the monomeric cation was formed, issued from the disassociation. [102]
Besides the functionalization at the core or of the core, the functionalization of the surface of glycerol dendrons was also studied, with the aim of introducing positive charges in view of transfection experiments. The first example concerned different generations of glycine‐terminated dendrons (Figure 32). These dendrons formed micelles at a micromolar level ranging from 10 to 60 μM. The most efficient dendron vector to deliver siRNA and achieve potent gene silencing was the second generation (eight ammonium groups). The experiments were carried out to knockdown luciferase and GAPDH gene activity in HeLa cells. [103] The same dendrons were also associated with the Brij L4 surfactant, and produced a variety of honeycomb‐like patterned surfaces. [97] Another series of dendrons was decorated on the periphery with N,N‐di‐(3‐aminopropyl)‐N‐(methyl)amine (DAPMA) moieties. Several generations and two different types of linkers (biodegradable ester or non‐biodegradable triazole) between the tail and the dendron were used (Figure 32). All these dendrons formed micelles, and they were tested for carrying and delivering luciferase specific siRNA in 786‐O‐Luc tumor cells. It was shown that the ester‐linked dendrons were the most efficient, and displayed noticeable gene silencing in vitro without affecting the cell viability in the tumor cell line 786‐O. [104]
Figure 32.
Surface functionalization of glycerol dendrons with different types of ammonium groups.
6.2. Ethylene oxide dendrons
Dendrons which branches are constituted of ethylene oxide units are particularly hydrophilic, thus their tail is most generally composed of aromatic units to increase the hydrophobicity. Amphiphilic dendrons composed of an octa‐p‐phenylene rod stem, to which three ethylene oxide branching units of different sizes were attached, were synthesized. In the absence of solvent, the smallest compounds self‐assembled into a lamellar structure, whereas the largest compound (shown in Figure 33) self‐assembled into a discrete heptameric bundle that organized into a 3‐D primitive orthorhombic supercrystals. In dilute solutions (THF/water=1 : 10 v/v) these dendrons self‐assembled into capsule‐like hollow aggregates. [105]
Figure 33.
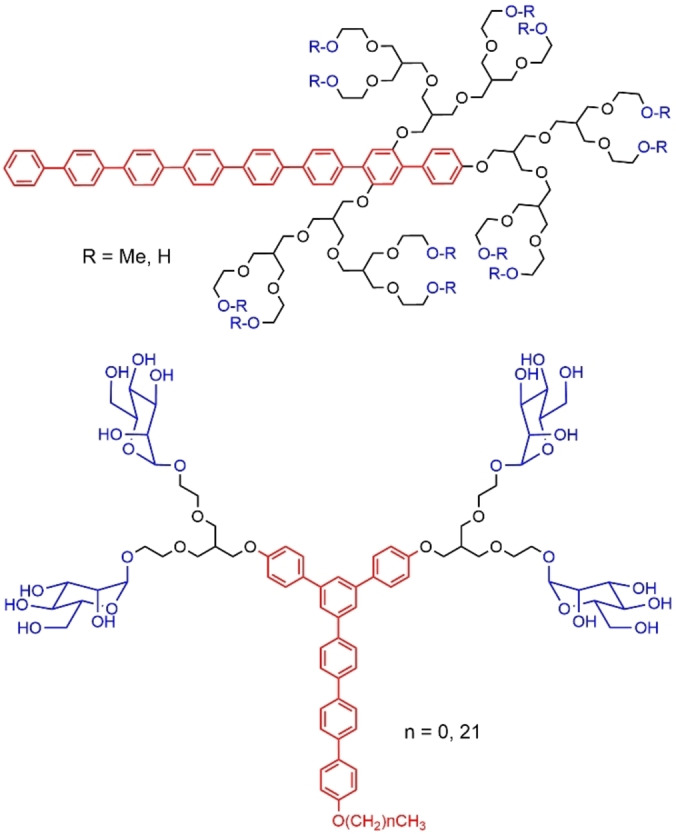
Ethylene oxide dendrons functionalized with an octa‐p‐phenylene rod stem or an aryl alkyl tail.
The ability of the same dendrons and of their analogues bearing alcohol groups instead of methoxy groups terminal functions to form two‐dimensional micellar structures on a solid surface was investigated. It was shown that the hydrophilic terminated dendron branches self‐assembled in surface monolayers, with the formation of two‐dimensional layered or circular micellar structures. [106]
Another small dendron having four mannose terminal functions and an aromatic tail functionalized or not with an alkyl chain was synthesized (Figure 33). In the solid state, the dendron having a methoxy group as tail self‐assembled into a 1D nanostructure, whereas the dendron having the alkyl chain as tail self‐assembled into 2D nanosheets. However, in aqueous solutions both dendrons self‐assembled into carbohydrate‐coated cylindrical aggregates with a uniform diameter, which reversibly transformed into spherical objects on addition of guest molecules such as Nile Red. It was shown that both dendrons could immobilize bacterial E. coli cells. [107]
Different types of ether dendrons bearing oligo ethylene oxide terminal functions were synthesized. A first family was composed of oligo(phenylene vinylene) branches with an aldehyde at the core (Figure 34). A typical micellization behavior of these dendrons in water was observed. At concentrations above the CMC, a weak maximum emission at 425 nm of these fluorescent dendrons was observed. Dilution below the CMC induced both a shift of the emission to about 480 nm, and an increase of the fluorescence intensity. [108]
Figure 34.
Ether dendrons bearing oligo ethylene oxide terminal functions and aryl groups inside their structure and as tail.
Another example concerned a T‐shape rigid internal structure to which 4 or 8 tetraethylene glycol arms were grafted (Figure 34). The dendrons having the linear aryl tail self‐assembled in water, to form nanoscale fibrils, with lengths up to several micrometers. Heating the solution above 45 °C induced a gelation of the solution. On the contrary, the dendron having a branched tail showed no apparent aggregation behavior. [109]
Different types of dendrons having chiral entities in their structure were also described. Two pentaphenylene units conjugated to an opened or closed chiral bridging group, and functionalized on the other side with oligoether dendrons, were synthesized (compound 12, Figure 35). In the bulk state, both molecules (open or closed) self‐assembled into a hexagonal columnar structure. In aqueous solution, the molecules self‐assembled into cylindrical micellar aggregates. These aggregates displayed intense signals in the circular dichroism (CD) spectra, indicative of one‐handed helical conformations, of opposite signal, depending on the closed or open structure of the core. [110]
Figure 35.

Different types of dendrons having chiral entities in their structure.
For other examples of chiral dendrons, the chirality was obtained by introducing a methyl group on the oligoether chains (compounds 13). The core contained two pyrene groups, linked through either an alkyne (13 a), or a phenyl group (13 b) (Figure 35). These molecules self‐assembled into spherical micelles with a diameter of ca. 12 nm in an aqueous solution. Despite the presence of chiral groups, no circular dichroism (CD) signals could be detected. The presence of the pyrene groups enabled the supramolecular polymerization of these micelles through charge transfer interaction by addition of an electron acceptor molecule, i. e. 2,4,5,7‐tetranitrofluorenone (TNF). Such polymerization yielded linear supramolecular polymers with controlled lengths from several tens to a few micrometers. [111]
The same chiral oligoethylene branches were grafted to an aromatic bicycle (compound 13 c, Figure 35). This compound spontaneously formed 2D porous sheets in dilute aqueous solution through the two‐dimensional self‐assembly of the aromatic macrobicycles. These 2D sheets were able to solubilize coronene in aqueous solution with preservation of their 2D structure. The maximum coronene loading per amphiphilic dendron was found equal to 0.5 equivalent. [112]
7. Ester Dendrons
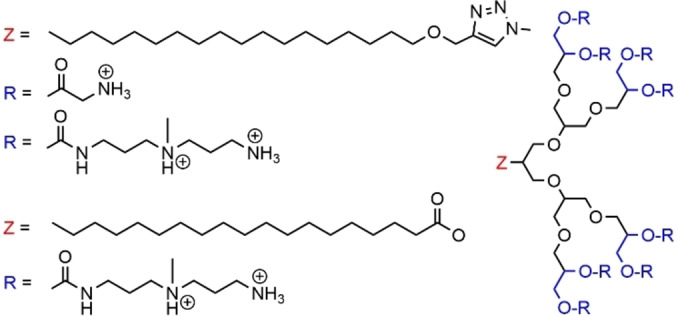
The synthesis of amphiphilic poly ester dendrons was generally carried out for having a degradable scaffold. The aim was to obtain overtime or in the presence of specific biological triggers, the breakdown of the dendron [113] and thus of its assembly in a controllable and predictable way into smaller subunits. Such behavior may enhance the biocompatibility, and lower the toxicity. This topic was essentially proposed by D.K. Smith et al. A first series of ester dendrons decorated on the surface with triamine ligands, protonated at physiological pH, for binding to DNA, and having at the core either alkyl chains (14 a) or one (14 b) (or two, 14 c) cholesterol unit were synthesized up to generation 2 (Figure 36). The first member of this family was a first generation dendron functionalized with a C22 alkyl chain at the focal point. This dendron was shown to self‐assemble at concentrations above 3.88±0.25 μM, and that it was capable of binding polyanionic heparin in a multivalent manner. [114] Later on, it was shown that the nature of the functional group at the core of the second generation dendrons had a profound effect on the dendron aggregation process. The CMC values varied from 208±56 μM for the C12 chain to 2.0±0.1 μM for the C22 chain, while one or two cholesterol units afforded the same CMC, 4.9±0.6 μM. The most effective self‐associated dendrons, i. e. those having C16 and C22 alkyl chains as well as the cholesterol at the core, enabled the strongest binding to plasmid DNA. However, levels of transgene expression were found relatively low. It was shown that these dendrons do degraded under biologically relevant conditions over a period of hours, but when bound to DNA, complete degradation became ineffective on the transfection time scale. This may explain the poor transfection performance of these dendrons. [115] The dendrons having the cholesterol unit(s) at the core were associated with a single cholesterol unit attached to a short OEG chain of 3 or 8 (CH2CH2O) units. The co‐assembly with the longest OEG chain enhanced DNA binding, whereas the smallest OEG chain inhibited the binding with DNA. [116]
Figure 36.
Ester dendrons functionalized with triamines as terminal functions.
The same family of cholesterol functionalized dendrons was modified by introducing an S−S linkage between the multivalent hydrophilic dendron and the hydrophobic cholesterol units responsible for self‐assembly (compounds 14 d and 14 e). The presence of this S−S linkage allowed these structures to undergo triggered reductive cleavage with dithiothreitol. This cleavage induced the breakdown of the self‐association of the dendrons, but also of their association with DNA, inducing the release of DNA. The two‐step double degradation (the S−S linkages and the esters) converted the large self‐assembling unit with high affinity for DNA into small units. [117]
Another family of ester dendrons was functionalized at the core with a long alkyl chain, and on the surface with either D or L lysine. Both compounds of first generation formed identical nanoscale assemblies in terms of dimensions and charge densities. However, their interaction with DNA and heparin induced different chiral binding preferences. On the contrary, the second generation dendrons (Figure 37) interacted identically with DNA and heparin, irrespectively of the chirality. [118]
Figure 37.
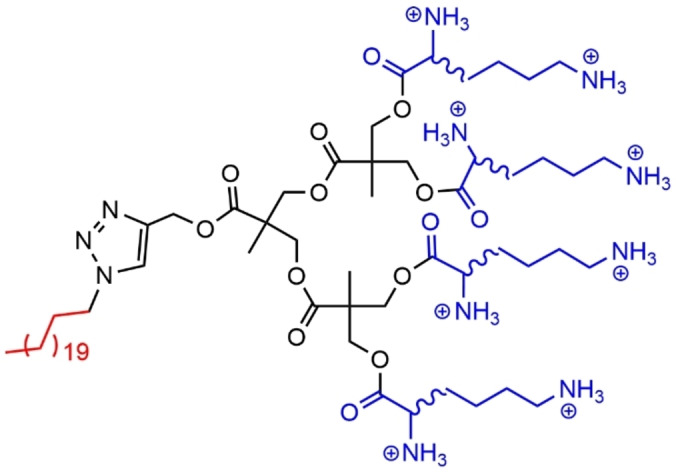
Ester dendrons functionalized with either D or L lysine on the surface.
A large series of ester dendrons from generation 0 to generation 4 was synthesized, bearing α‐galactose surface functions (up to 16 for generation 4) and two C17 alkyl chains at the core (Figure 38). The self‐assembly of these dendrons in water carried out through a solvent exchange process afforded different results depending on the generation of the dendrons. Dendrons from the zeroth through second generation resulted in vesicles, whereas micelles were obtained for the third and fourth generation dendrons. The binding of these assemblies to Griffonia simplicifolia Lectin I (GSL 1), a protein with specificity for α‐ galactose, was studied. Using GSL 1‐coated beads, it was shown that binding was enhanced for higher generation dendrons. Furthermore, these glycol‐dendrons were capable of stimulating invariant natural killer T (iNKT) immune cells. [119]
Figure 38.
Generation 4 ester dendron functionalized with α‐galactose terminal functions.
Another example of surface functionalization of ester dendrons concerned the grafting of ammonium groups to the first, second and third generations. The core was constituted of an alkyl chain of variable length (C2, C4, C6, C8, C10, C14) (Figure 39). A detailed study of the aggregation properties of these compounds was carried out. It was shown that micelles were obtained only with long alkyl chains, and this phenomenon was more pronounced when the generation of the dendron increased. Indeed, a CMC was observed only with the C14 tail in the case of the third generation. The antibacterial activities were measured for all the dendrons, by screening their inhibitory activity against E. coli and S. aureus. The antibacterial activity of these dendrons was strongly dependent on the length of the alkyl chain at the tail, whereas the dependence on generation was far less pronounced. These dendrons displayed antibacterial activities below the CMC. [120] A related work concerned dendrons functionalized on the surface by guanidinium derivatives (Figure 39). They also displayed antibacterial activities below the CMC, but they were not found better than the dendrons having the ammonium terminal functions. [121]
Figure 39.
Ester dendrons bearing positive charges as terminal functions.
8. Amphiphilic Dendrons Containing Main Group Elements
Dendritic scaffolds containing heteroatoms as branching points provide higher diversity of branching variants and better stability of a scaffold throughout the synthesis and modification as well as the exposure to biological media. To date, two major families of such kind have been reported, namely silicon‐based and phosphorus‐based dendrons.
8.1. Carbosilane dendrons
Carbosilane dendrons of generations 1–3 (15‐G1 to 15‐G3 ) containing vinyl or allyl moieties on the periphery were functionalized with cationic or anionic surface moieties and with fatty acid fragments in the focal point (Figure 40). [122] The resulting amphiphiles were self‐assembled in water media to form nanoconstructions of micellar topology. Micelles were able to retain low‐molecular cargo (procaine) and to form complexes with therapeutically relevant macromolecules (siRNA), [123] mimicking fully symmetrical dendrimers. [124] Interestingly, with the increasing of amphiphile generation, their tendency to aggregate became stronger, unlike conventional surfactants. This was due to the increasing hydrophobicity provided by the carbosilane dendritic scaffold. Also, the aggregation number (i. e., the number of amphiphilic molecules in a micelle) decreased with the increase of dendron generation. [125]
Figure 40.
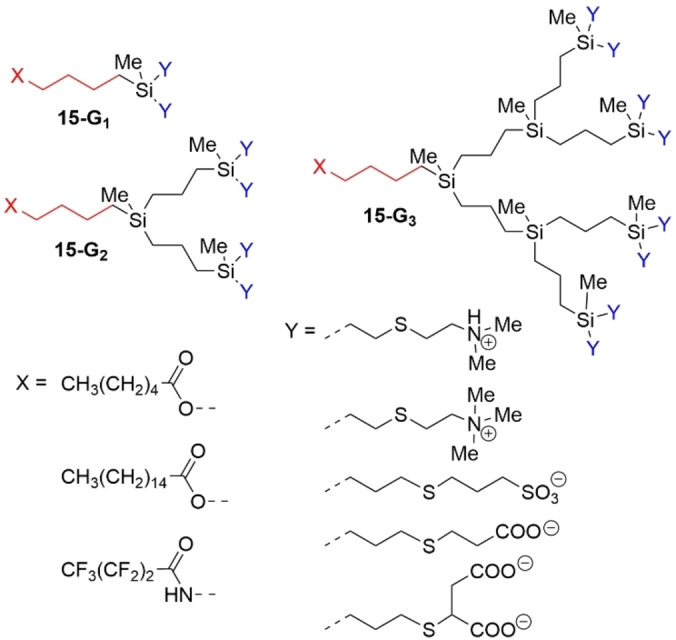
Examples of carbosilane dendrons (generations 1 to 3) bearing different types of positively or negatively charged substituents on the surface and an alkyl or perfluoroalkyl chain as tail.
The same methodology was used to prepare amphiphilic carbosilane dendrons containing perfluoroalkyl chains, linked via an amide at the focal point (Figure 40). This chemically stable fluorine‐containing dendron scaffold was available for further chemical modifications on the surface, for instance for grafting cationic derivatives. However, the CMC value of this dendron was very high, above 1 mM. [126]
By introducing carboxylate functions onto the periphery of dendrons, pH‐sensitive dendritic micelles could be assembled. Carbosilane dendrons bearing palmitoyl fragment in the focal point and propionate or succinate moieties on the periphery were shown to aggregate in the presence of salt (>10 mM) to form micelles in the generation‐dependent manner (Figure 40). Exposure of micelles to acidic media provoked their disruption, likely due to the changes of the ionization state of the periphery. [127] The micelles could be loaded with low‐molecular NSAIDs (non‐steroidal anti‐inflammatory drugs) ibuprofen, procaine, celecoxib and diclofenac, with the efficiency of loading depending on the hydrophobicity of the drug. Furthermore, these dendrons were shown to suppress the HIV‐1 activity in a generation‐dependent manner.
By branching a hydrophobic moiety in the focal point, it is possible to change the topology of dendron associates. Carbosilane dendrons functionalized with a triazine derivative possessing two long alkyl chains grafted to the dendron focal point through a piperazine‐triazine linker were shown to form vesicle‐like particles (dendrimersomes). [128] Due to the rational positioning of hydrophobic and hydrophilic fragments in the dendron structure, it was self‐assembled in a controlled manner to yield particles of low polydispersity. The triazine moiety acted as a pH‐sensitive block inducing the reorganization of dendrimersomes in slightly acidic media (pH<6.5). Dendrimersomes were loaded with low‐molecular antitumor drugs doxorubicin, methotrexate and 5‐fluorouracil; drug molecules were retained in the lipid bilayer or on the surface, depending on their own charge and hydrophobicity. Encapsulated drugs were efficiently delivered into leukemia cells inducing apoptosis (Figure 41).
Figure 41.

A carbosilane dendron bearing two alkyl chains as tail. Illustration of their self‐association properties, depending on the pH, and of the type of encapsulation of drugs (blue: doxorubicin; orange: methotrexate; green: 5‐fluorouracyl). Reprinted in part from Ref. [128], Copyright the authors.
8.2. Phosphorhydrazone dendrons
A large number of phosphorhydrazone dendrons based on AB5 derivatives of hexachlorocyclotriphosphazene has been synthesized and was recently reviewed. [129] However, very few of them can be considered as amphiphiles, and their self‐association properties have been studied only in one case. [130] A series of generations 1 and 2 dendrons bearing 10 or 20 cyclic ammonium groups on the surface, respectively, and a single hydrophobic group at the core was synthesized. The functions at the core were either a fluorescent group (maleimide or pyrene) or an azabisphosphonate. The cyclic ammoniums on the surface were either the salt of 2‐(pyrrolidin‐1‐yl)ethan‐1‐amine (five‐membered ring) or the salt of 2‐(piperidin‐1‐yl)ethan‐1‐amine (six‐membered ring) (Figure 42). The CMC of these dendrons depended mainly on the functionality at the core. The CMC for the dendrons built from the pyrene at the core was between 1 and 4 μM, whatever the generation and the type of cyclic ammoniums on the surface. The CMC values were higher in the case of the less hydrophobic azabisphosphonate functionality at the core, especially for the first generations (153.7 and 109 μM, for pyrrolidinium and piperidinium terminal groups, respectively), but also for the second generations (60 and 45.5 μM). These dendrons were tested against a panel of tumor cell lines. The most active dendron against all cell lines was the first generation dendron having the pyrene at the core and 10 piperidiniums on the surface, at concentrations slightly lower than the CMC. [130]
Figure 42.
Amphiphilic phosphorhydrazone dendrons bearing cyclic ammoniums on the surface and different types of functions at the core.
9. Conclusion
This review was focused on precisely defined amphiphilic dendrons and on their properties, and thus excluded dendrons having in their structure a polymer, either as terminal functions or at the core. The main reason is that even precisely defined polymers cannot be as well defined as dendrons, and thus the reproducibility of the experiments would have to be very carefully examined.
Despite this limitation, we have shown in this review the large variety of structures of amphiphilic dendrons already synthesized. The known methods of synthesis of all the main types of dendrimers have been applied to the synthesis of dendrons, in particular of amphiphilic dendrons. In most cases, the solubility in water is provided by the terminal functions of the dendrons, which can be positively charged (essentially ammoniums), negatively charged (essentially carboxylates), but also neutral (such as oligoethylene glycol of defined length). The function at the core is most frequently hydrophobic, essentially of type alkyl chain, but also aromatic derivatives, or fluorophores. The internal structure of the dendrons is not an innocent scaffold, [131] as it can be either hydrophilic or hydrophobic, and thus it participates in the amphiphilic balance of the full structure. The possibilities of self‐association, and in particular the CMC values are highly dependent of this hydrophilic/hydrophobic balance. The possibility to functionalize the internal structure of the dendrons, and thus to modify at will this balance is an interesting feature of the aryl ether dendrons shown in Figures 7 to 11.
Different types of self‐associations have been observed, depending on the structure of the dendrons, but also on the experimental conditions used. Self‐associations at the air‐water interface have been observed in some cases, but the large majority of amphiphilic dendrons affords micelles in water, and in some cases vesicles (generally when two alkyl chains are linked to the core). These micelles or vesicles have been found suitable for the encapsulation or carriage and delivery of low‐molecular and macromolecular bioactive entities such as DNA or siRNA. It has been shown in some cases that the transfection efficiency was impacted by the presence (or not) of the micelles. [84] However, in several cases, the biological properties were observed below the CMC. This was for instance the case for antibacterial activities, [121] or for anticancer properties. [130]
The perspectives in the field clearly concern biological uses of the dendron micelles for the dissolution and carriage of drugs, or for the delivery of biological entities. However, other uses inspired by classical micelles made of linear amphiphiles can be foreseen, such as for catalysis or in the storage of solar energy for example.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Evgeny Apartsin is Marie Skłodowska‐Curie Fellow at the Laboratoire de Chimie de Coordination (LCC) in Toulouse (France) and Assistant Professor of the Novosibirsk State University (Russia). His current research interest is on the design of dendrimer‐based assemblies for nanomedicine. He is co‐author of 24 publications, 2 book chapters and 3 patents.

Biographical Information
Anne‐Marie Caminade is Director of Research Exceptional Class at the CNRS and Deputy Director of the Laboratoire de Chimie de Coordination (LCC) in Toulouse (France). Her current research interest is on phosphorus chemistry, for the synthesis of dendrimers and dendrons, and on their properties. She is author of about 500 publications, of 55 book chapters, editor of two books, and her h index is 74.

Acknowledgements
The project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 844217 for the MSCA‐IF‐2018 “EUREKA” to E.A. This article is based upon work from COST Action CA 17140 “Cancer Nanomedicine from the Bench to the Bedside” supported by COST (European Cooperation in Science and Technology). Thanks are also due to the CNRS for financial support.
E. Apartsin, A.-M. Caminade, Chem. Eur. J. 2021, 27, 17976.
Dedicated to Professor Vincenzo Balzani on the occasion of his 85th birthday
Contributor Information
Dr. Evgeny Apartsin, Email: evgeny.apartsin@lcc-toulouse.fr.
Dr. Anne‐Marie Caminade, Email: anne-marie.caminade@lcc-toulouse.fr.
References
- 1.
- 1a. Hawker C. J., Frechet J. M. J., J. Chem. Soc. Chem. Commun. 1990, 1010–1013; [Google Scholar]
- 1b. Hawker C. J., Frechet J. M. J., J. Am. Chem. Soc. 1990, 112, 7638–7647; [Google Scholar]
- 1c. Hawker C. J., Frechet J. M. J., Macromolecules 1990, 23, 4726–4729; [Google Scholar]
- 1d. Wooley K. L., Hawker C. J., Frechet J. M. J., J. Am. Chem. Soc. 1991, 113, 4252–4261; [Google Scholar]
- 1e. Wooley K. L., Hawker C. J., Frechet J. M. J., J. Chem. Soc. Perkin Trans. 1 1991, 1059–1076; [Google Scholar]
- 1f. Gitsov I., Wooley K. L., Frechet J. M. J., Angew. Chem. Int. Ed. Engl. 1992, 31, 1200–1202; [Google Scholar]
- 1g. Hawker C. J., Frechet J. M. J., J. Am. Chem. Soc. 1992, 114, 8405–8413; [Google Scholar]
- 1h. Hawker C. J., Frechet J. M. J., J. Chem. Soc. Perkin Trans. 1 1992, 2459–2469. [Google Scholar]
- 2.
- 2a. Dendrimers. Towards Catalytic, Material and Biomedical Uses (Eds.: A. M. Caminade, C. O. Turrin, R. Laurent, A. Ouali, B. Delavaux-Nicot), John Wiley & Sons, Chichester, UK, 2011;
- 2b. Dendrimer Chemistry: Synthetic approaches towards complex architectures, (Eds.: M. Malkoch, S. Garcia-Gallego), Royal Society of Chemistry, Croydon, UK, 2020.
- 3. Chevalier Y., Zemb T., Rep. Prog. Phys. 1990, 53, 279–371. [Google Scholar]
- 4. Newkome G. R., Moorefield C. N., Baker G. R., Saunders M. J., Grossman S. H., Angew. Chem. Int. Ed. Engl. 1991, 30, 1178–1180. [Google Scholar]
- 5. Uskokovic V., Drofenik M., Adv. Colloid Interface Sci. 2007, 133, 23–34. [DOI] [PubMed] [Google Scholar]
- 6. Rosen B. M., Wilson C. J., Wilson D. A., Peterca M., Imam M. R., Percec V., Chem. Rev. 2009, 109, 6275–6540. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Dong R. J., Zhou Y. F., Zhu X. Y., Acc. Chem. Res. 2014, 47, 2006–2016; [DOI] [PubMed] [Google Scholar]
- 7b. Bolu B. S., Sanyal R., Sanyal A., Molecules 2018, 23, 1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schluter A. D., Halperin A., Kroger M., Vlassopoulos D., Wegner G., Zhang B. Z., ACS Macro Lett. 2014, 3, 991–998. [DOI] [PubMed] [Google Scholar]
- 9. Blasco E., Pinol M., Oriol L., Macromol. Rapid Commun. 2014, 35, 1090–1115. [DOI] [PubMed] [Google Scholar]
- 10. Lebedeva I. O., Zhulina E. B., Borisov O. V., Macromolecules 2019, 52, 3655–3667. [Google Scholar]
- 11. Sun H. J., Zhang S. D., Percec V., Chem. Soc. Rev. 2015, 44, 3900–3923. [DOI] [PubMed] [Google Scholar]
- 12. Al-Jamal K. T., Ramaswamy C., Florence A. T., Adv. Drug Delivery Rev. 2005, 57, 2238–2270. [DOI] [PubMed] [Google Scholar]
- 13. Tomalia D. A., Baker H., Dewald J., Hall M., Kallos G., Martin S., Roeck J., Ryder J., Smith P., Polymer J. 1985, 17, 117–132. [Google Scholar]
- 14. Newkome G. R., Yao Z. Q., Baker G. R., Gupta V. K., J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar]
- 15.
- 15a.R. G. Denkewalter, J. Kolc, W. J. Lukasavage, U. S. Patent 4 289 872, Sept. 15, 1891;
- 15b. Aharoni S. M., Crosby C. R., Walsh E. K., Macromolecules 1982, 15, 1093–1098. [Google Scholar]
- 16. Haag R., Sunder A., Stumbe J. F., J. Am. Chem. Soc. 2000, 122, 2954–2955. [Google Scholar]
- 17.
- 17a. Hawker C. J., Frechet J. M. J., J. Am. Chem. Soc. 1992, 114, 8405–8413; [Google Scholar]
- 17b. Ihre H., Hult A., Soderlind E., J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar]
- 18. Zhou L. L., Roovers J., Macromolecules 1993, 26, 963–968. [Google Scholar]
- 19.
- 19a. Launay N., Caminade A. M., Lahana R., Majoral J. P., Angew. Chem. Int. Ed. Engl. 1994, 33, 1589–1592; [Google Scholar]
- 19b. Launay N., Caminade A. M., Majoral J. P., J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar]
- 20. Balagurusamy V. S. K., Ungar G., Percec V., Johansson G., J. Am. Chem. Soc. 1997, 119, 1539–1555. [Google Scholar]
- 21. Li Y. Y., Lin S. T., Goddard W. A., J. Am. Chem. Soc. 2004, 126, 1872–1885. [DOI] [PubMed] [Google Scholar]
- 22. Dukeson D. R., Ungar G., Balagurusamy V. S. K., Percec V., Johansson G. A., Glodde M., J. Am. Chem. Soc. 2003, 125, 15974–15980. [DOI] [PubMed] [Google Scholar]
- 23. Yao X., Cseh L., Zeng X., Xue M., Liu Y., Ungar G., Nanoscale Horiz. 2017, 2, 43–49. [DOI] [PubMed] [Google Scholar]
- 24. Zeng X. B., Ungar G., Liu Y. S., Percec V., Dulcey S. E., Hobbs J. K., Nature 2004, 428, 157–160. [DOI] [PubMed] [Google Scholar]
- 25. Zhang R., Zeng X., Ungar G., J. Phys. Condens. Matter 2017, 29, 414001. [DOI] [PubMed] [Google Scholar]
- 26. Cheng C. X., Jiang J., Tang R. P., Xi F., Synth. Met. 2004, 145, 61–65. [Google Scholar]
- 27. Xu L., Shao L. D., Chen L., Hu M. Q., Bi Y. M., Chem. Lett. 2010, 39, 1177–1179. [Google Scholar]
- 28. Xu L., Shao L. D., Hu M. Q., Chen L., Bi Y. M., Can. J. Chem.-Rev. Can. Chim. 2012, 90, 600–607. [Google Scholar]
- 29. Felipe M. J., Llore N. E., Pernites R. B., Nguyen T., Ponnapati R., Advincula R. C., Langmuir 2011, 27, 9327–9336. [DOI] [PubMed] [Google Scholar]
- 30. Percec V., Won B. C., Peterca M., Heiney P. A., J. Am. Chem. Soc. 2007, 129, 11265–11278. [DOI] [PubMed] [Google Scholar]
- 31. Wang L. Y., Feng Y., Yang Z. Q., He Y. M., Fan Q. H., Liu D. S., Chem. Commun. 2012, 48, 3715–3717. [DOI] [PubMed] [Google Scholar]
- 32. Wang L. Y., Feng Y., Sun Y. W., Li Z. B., Yang Z. Q., He Y. M., Fan Q. H., Liu D. S., Soft Matter 2011, 7, 7187–7190. [Google Scholar]
- 33. Sandanaraj B. S., Bhandari P. J., Reddy M. M., Lohote A. B., Sahoo B., ChemBioChem 2020, 21, 408–416. [DOI] [PubMed] [Google Scholar]
- 34. Bhandari P. J., Reddy M. M., Rao K. J., Sandanaraj B. S., J. Org. Chem. 2021, 86, 8576–8589. [DOI] [PubMed] [Google Scholar]
- 35. Bhandari P. J., Sandanaraj B. S., ChemBioChem 2021, 22, 876–887. [DOI] [PubMed] [Google Scholar]
- 36. Bhandari P. J., Sandanaraj B. S., ChemBioChem 2021, 22, https://doi.org10.1002/cbic.202100288in press, doi.org/. [DOI] [PubMed] [Google Scholar]
- 37. Kikuzawa Y., Nagata T., Bull. Chem. Soc. Jpn. 2004, 77, 993–1000. [Google Scholar]
- 38. Bharathi P., Zhao H., Thayumanavan S., Org. Lett. 2001, 3, 1961–1964. [DOI] [PubMed] [Google Scholar]
- 39. Aathimanikandan S. V., Savariar E. N., Thayumanavan S., J. Am. Chem. Soc. 2005, 127, 14922–14929. [DOI] [PubMed] [Google Scholar]
- 40. Fuller J. M., Raghupathi K. R., Ramireddy R. R., Subrahmanyam A. V., Yesilyurt V., Thayumanavan S., J. Am. Chem. Soc. 2013, 135, 8947–8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yesilyurt V., Ramireddy R., Thayumanavan S., Angew. Chem. Int. Ed. 2011, 50, 3038–3042; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 3094–3098. [Google Scholar]
- 42. Ramireddy R. R., Subrahmanyam A. V., Thayumanavan S., Chem. Eur. J. 2013, 19, 16374–16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ambade A. V., Aathimanikandan S. V., van der Poll D., Thayumanavan S., J. Org. Chem. 2007, 72, 8167–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gomez-Escudero A., Azagarsamy M. A., Theddu N., Vachet R. W., Thayumanavan S., J. Am. Chem. Soc. 2008, 130, 11156–11163. [DOI] [PubMed] [Google Scholar]
- 45. Savariar E. N., Koppelman J., Thayumanavan S., Isr. J. Chem. 2009, 49, 41–47. [Google Scholar]
- 46. Raghupathi K. R., Guo J., Munkhbat O., Rangadurai P., Thayumanavan S., Acc. Chem. Res. 2014, 47, 2200–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang H., Raghupathi K. R., Zhuang J. M., Thayumanavan S., ACS Macro Lett. 2015, 4, 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Munkhbat O., Garzoni M., Raghupathi K. R., Pavan G. M., Thayumanavan S., Langmuir 2016, 32, 2874–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torres D. A., Garzoni M., Subrahmanyam A. V., Pavan G. M., Thayumanavan S., J. Am. Chem. Soc. 2014, 136, 5385–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeMattei C. R., Huang B. H., Tomalia D. A., Nano Lett. 2004, 4, 771–777. [Google Scholar]
- 51. Takahashi T., Kono K., Itoh T., Emi N., Takagishi T., Bioconjugate Chem. 2003, 14, 764–773. [DOI] [PubMed] [Google Scholar]
- 52. Doura T., Yamada M., Teranishi R., Yamamoto Y., Sugimoto T., Yuba E., Harada A., Kono K., Langmuir 2015, 31, 5105–5114. [DOI] [PubMed] [Google Scholar]
- 53. Kono K., Murakami E., Hiranaka Y., Yuba E., Kojima C., Harada A., Sakurai K., Angew. Chem. Int. Ed. 2011, 50, 6332–6336; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 6456–6460. [Google Scholar]
- 54. Iwashita S., Hiramatsu Y., Otani T., Amano C., Hirai M., Oie K., Yuba E., Kono K., Miyamoto M., Igarashi K., J. Biomater. Appl. 2012, 27, 445–456. [DOI] [PubMed] [Google Scholar]
- 55. Yoshimura T., Kawano N.-K., Yada S., Iwase H., Langmuir 2020, 36, 563–570. [DOI] [PubMed] [Google Scholar]
- 56. Yu T. Z., Liu X. X., Bolcato-Bellemin A. L., Wang Y., Liu C., Erbacher P., Qu F. Q., Rocchi P., Behr J. P., Peng L., Angew. Chem. Int. Ed. 2012, 51, 8478–8484; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 8606–8612. [Google Scholar]
- 57. Marquez-Miranda V., Araya-Duran I., Camarada M. B., Comer J., Valencia-Gallegos J. A., Gonzalez-Nilo F. D., Sci. Rep. 2016, 6, 29436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C., Posocco P., Liu X., Cheng Q., Laurini E., Zhou J., Liu C., Wang Y., Tang J., Dal Col V., Yu T., Giorgio S., Fermeglia M., Qu F., Liang Z., Rossi J. J., Liu M., Rocchi P., Pricl S., Peng L., Small 2016, 12, 3667–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laurini E., Marson D., Aulic S., Fermeglia M., Pricl S., Pharmaceutica 2019, 11, 324. [Google Scholar]
- 60. Torigoe K., Tasaki A., Yoshimura T., Sakai K., Esurni K., Takamatsu Y., Sharma S. C., Sakai H., Abe M., Colloids Surf. A 2008, 326, 184–190. [Google Scholar]
- 61. Yoshimura T., Saito M., Esumi K., J. Oleo Sci. 2013, 62, 213–221. [DOI] [PubMed] [Google Scholar]
- 62. Yoshimura T., Ebihara A., Iwase H., Colloids Surf. A 2017, 533, 197–203. [Google Scholar]
- 63. King A. S. H., Martin I. K., Twyman L. J., Polym. Int. 2006, 55, 798–807. [Google Scholar]
- 64. Kumar M., Patil N. G., Choudhury C. K., Roy S., Ambade A. V., Kumaraswamy G., Soft Matter 2015, 11, 5417–5424. [DOI] [PubMed] [Google Scholar]
- 65. Kumar M., Patil N., Ambade A. V., Kumaraswamy G., Langmuir 2018, 34, 6827–6834. [DOI] [PubMed] [Google Scholar]
- 66. Ogi K., Miyauchi S., Naka K., Polymer J. 2015, 47, 259–266. [Google Scholar]
- 67. Al-Jamal K. T., Ramaswamy C., Singh B., Florence A. T., J. Drug Delivery Sci. Technol. 2005, 15, 11–18. [Google Scholar]
- 68. Ribeiro S., Hussain N., Florence A. T., Int. J. Pharm. 2005, 298, 354–360. [DOI] [PubMed] [Google Scholar]
- 69. Ribeiro S., Rijpkema S. G., Durrani Z., Florence A. T., Int. J. Pharm. 2007, 331, 228–232. [DOI] [PubMed] [Google Scholar]
- 70. Al-Jamal K. T., Sakthivel T., Florence A. T., Colloids Surf. A 2005, 268, 52–59. [Google Scholar]
- 71. Ramaswamy C., Florence A. T., J. Drug Delivery Sci. Technol. 2005, 15, 307–311. [Google Scholar]
- 72. Hardy J. G., Hirst A. R., Smith D. K., Brennan C., Ashworth I., Chem. Commun. 2005, 385–387. [DOI] [PubMed] [Google Scholar]
- 73. Mirsharghi S., Knudsen K. D., Bagherifam S., Nystrom B., Boas U., New J. Chem. 2016, 40, 3597–3611. [Google Scholar]
- 74. Dong Y., Chen Y., Zhu D., Shi K., Ma C., Zhang W., Rocchi P., Jiang L., Liu X., J. Controlled Release 2020, 322, 416–425. [DOI] [PubMed] [Google Scholar]
- 75. Kim C., Kim K. T., Chang Y., Song H. H., Cho T. Y., Jeon H. J., J. Am. Chem. Soc. 2001, 123, 5586–5587. [DOI] [PubMed] [Google Scholar]
- 76. Jeon H. J., Kang M. K., Park C., Kim K. T., Chang J. Y., Kim C., Song H. H., Langmuir 2007, 23, 13109–13116. [DOI] [PubMed] [Google Scholar]
- 77. Park C., Lee I. H., Lee S., Song Y., Rhue M., Kim C., Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park C., Im M. S., Lee S., Lim J., Kim C., Angew. Chem. Int. Ed. 2008, 47, 9922–9926; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 10070–10074. [Google Scholar]
- 79. Park C., Lim J., Yun M., Kim C., Angew. Chem. Int. Ed. 2008, 47, 2959–2963; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 3001–3005. [Google Scholar]
- 80. Park C., Choi K. S., Song Y., Jeon H. J., Song H. H., Chang J. Y., Kim C., Langmuir 2006, 22, 3812–3817. [DOI] [PubMed] [Google Scholar]
- 81. Kim C., Lee S. J., Lee I. H., Kim K. T., Chem. Mater. 2003, 15, 3638–3642. [Google Scholar]
- 82. Newkome G. R., Shreiner C., Chem. Rev. 2010, 110, 6338–6442. [DOI] [PubMed] [Google Scholar]
- 83. Kostiainen M. A., Hardy J. G., Smith D. K., Angew. Chem. Int. Ed. 2005, 44, 2556–2559; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 2612–2615. [Google Scholar]
- 84. Posocco P., Pricl S., Jones S., Barnard A., Smith D. K., Chem. Sci. 2010, 1, 393–404. [Google Scholar]
- 85. Jones S. P., Gabrielson N. P., Pack D. W., Smith D. K., Chem. Commun. 2008, 4700–4702. [DOI] [PubMed] [Google Scholar]
- 86. Jones S. P., Gabrielson N. P., Wong C. H., Chow H. F., Pack D. W., Posocco P., Fermeglia M., Pricl S., Smith D. K., Mol. Pharm. 2011, 8, 416–429. [DOI] [PubMed] [Google Scholar]
- 87. Welsh D. J., Smith D. K., Org. Biomol. Chem. 2011, 9, 4795–4801. [DOI] [PubMed] [Google Scholar]
- 88. Hartnagel U., Balbinot D., Jux N., Hirsch A., Org. Biomol. Chem. 2006, 4, 1785–1795. [DOI] [PubMed] [Google Scholar]
- 89. Kovacs C., Hirsch A., Eur. J. Org. Chem. 2006, 2006, 3348–3357. [Google Scholar]
- 90. Kato H., Bottcher C., Hirsch A., Eur. J. Org. Chem. 2007, 2659–2666. [Google Scholar]
- 91. Ebel A., Donaubauer W., Hampel F., Hirsch A., Eur. J. Org. Chem. 2007, 2007, 3488–3494. [Google Scholar]
- 92. Schmidt C. D., Boettcher C., Hirsch A., Eur. J. Org. Chem. 2007, 5497–5505. [Google Scholar]
- 93. Calderon M., Velasco M. I., Strumia M. C., Lorenzo A. T., Muller A. J., Rojas M. R., Saez A. E., J. Colloid Interface Sci. 2009, 336, 462–469. [DOI] [PubMed] [Google Scholar]
- 94. Schunk T., Hirsch A., Eur. J. Org. Chem. 2012, 1130–1137. [Google Scholar]
- 95. Trappmann B., Ludwig K., Radowski M. R., Shukla A., Mohr A., Rehage H., Bottcher C., Haag R., J. Am. Chem. Soc. 2010, 132, 11119–11124. [DOI] [PubMed] [Google Scholar]
- 96. Popeney C. S., Setaro A., Mutihac R. C., Bluemmel P., Trappmann B., Vonneman J., Reich S., Haag R., ChemPhysChem 2012, 13, 203–211. [DOI] [PubMed] [Google Scholar]
- 97. De Leon A. S., Malhotra S., Molina M., Haag R., Calderon M., Rodriguez-Hernandez J., Munoz-Bonilla A., J. Colloid Interface Sci. 2015, 440, 263–271. [DOI] [PubMed] [Google Scholar]
- 98. Urner L. H., Goltsche K., Selent M., Liko I., Schweder M. P., Robinson C. V., Pagel K., Haag R., Chem. Eur. J. 2021, 27, 2537–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kumar A., Tyagi S., Singh R., Tyagi Y. K., New J. Chem. 2019, 43, 1025–1031. [Google Scholar]
- 100. Degen P., Wyszogrodzka M., Strotges C., Langmuir 2012, 28, 12438–12442. [DOI] [PubMed] [Google Scholar]
- 101. Zieringer M., Wyszogrodzka M., Biskup K., Haag R., New J. Chem. 2012, 36, 402–406. [Google Scholar]
- 102. Merschky M., Wyszogrodzka M., Haag R., Schmuck C., Chem. Eur. J. 2010, 16, 14242–14246. [DOI] [PubMed] [Google Scholar]
- 103. Malhotra S., Bauer H., Tschiche A., Staedtler A. M., Mohr A., Calderon M., Parmar V. S., Hoeke L., Sharbati S., Einspanier R., Haag R., Biomacromolecules 2012, 13, 3087–3098. [DOI] [PubMed] [Google Scholar]
- 104. Tschiche A., Staedtler A. M., Malhotra S., Bauer H., Bottcher C., Sharbati S., Calderon M., Koch M., Zollner T. M., Barnard A., Smith D. K., Einspanier R., Schmidt N., Haag R., J. Mat. Chem. B 2014, 2, 2153–2167. [DOI] [PubMed] [Google Scholar]
- 105. Yoo Y. S., Choi J. H., Song J. H., Nam-Keun H., Zin W. C., Park S., Chang T. Y., Lee M., J. Am. Chem. Soc. 2004, 126, 6294–6300. [DOI] [PubMed] [Google Scholar]
- 106. Holzmueller J., Genson K. L., Park Y., Yoo Y. S., Park M. H., Lee M., Tsukruk V., Langmuir 2005, 21, 6392–6398. [DOI] [PubMed] [Google Scholar]
- 107. Ryu J. H., Lee E., Lim Y. B., Lee M., J. Am. Chem. Soc. 2007, 129, 4808–4814. [DOI] [PubMed] [Google Scholar]
- 108. Ji T., Li S. N., Chang D. W., Dai L. M., Synth. Met. 2006, 156, 392–395. [Google Scholar]
- 109. Moon K. S., Kim H. J., Lee E., Lee M., Angew. Chem. Int. Ed. 2007, 46, 6807–6810; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 6931–6934. [Google Scholar]
- 110. Ryu J. H., Tang L., Lee E., Kim H. J., Lee M., Chem. Eur. J. 2008, 14, 871–881. [DOI] [PubMed] [Google Scholar]
- 111. Huang Z. G., Kim Y., Kim T., Lee M., Polym. Chem. 2013, 4, 268–271. [Google Scholar]
- 112. Kim Y., Shin S., Kim T., Lee D., Seok C., Lee M., Angew. Chem. Int. Ed. 2013, 52, 6426–6429; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 6554–6557. [Google Scholar]
- 113. Amir R. J., Pessah N., Shamis M., Shabat D., Angew. Chem. Int. Ed. 2003, 42, 4494–4499; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 4632–4637. [Google Scholar]
- 114. Rodrigo A. C., Barnard A., Cooper J., Smith D. K., Angew. Chem. Int. Ed. 2011, 50, 4675–4679; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 4771–4775. [Google Scholar]
- 115. Barnard A., Posocco P., Pricl S., Calderon M., Haag R., Hwang M. E., Shum V. W. T., Pack D. W., Smith D. K., J. Am. Chem. Soc. 2011, 133, 20288–20300. [DOI] [PubMed] [Google Scholar]
- 116. Barnard A., Calderon M., Tschiche A., Haag R., Smith D. K., Org. Biomol. Chem. 2012, 10, 8403–8409. [DOI] [PubMed] [Google Scholar]
- 117. Barnard A., Posocco P., Fermeglia M., Tschiche A., Calderon M., Pricl S., Smith D. K., Org. Biomol. Chem. 2014, 12, 446–455. [DOI] [PubMed] [Google Scholar]
- 118. Bromfield S. M., Smith D. K., J. Am. Chem. Soc. 2015, 137, 10056–10059. [DOI] [PubMed] [Google Scholar]
- 119. Trant J. F., Jain N., Mazzuca D. M., McIntosh J. T., Fan B., Haeryfar S. M. M., Lecommandoux S., Gillies E. R., Nanoscale 2016, 8, 17694–17704. [DOI] [PubMed] [Google Scholar]
- 120. Chen A., Karanastasis A., Casey K. R., Necelis M., Carone B. R., Caputo G. A., Palermo E. F., ACS Appl. Mater. Interfaces 2020, 12, 21270–21282. [DOI] [PubMed] [Google Scholar]
- 121. Chen A., Chen E., Palermo E. F., Polym. Chem. 2021, 12, 2374–2378. [Google Scholar]
- 122. Fuentes-Paniagua E., Pena-Gonzalez C. E., Galan M., Gomez R., de la Mata F. J., Sanchez-Nieves J., Organometallics 2013, 32, 1789–1796. [Google Scholar]
- 123. Gutierrez-Ulloa C. E., Buyanova M. Y., Apartsin E. K., Venyaminova A. G., Javier de la Mata F., Valiente M., Gomez R., Org. Biomol. Chem. 2017, 15, 7352–7364. [DOI] [PubMed] [Google Scholar]
- 124. Krasheninina O. A., Apartsin E. K., Fuentes E., Szulc A., Ionov M., Venyaminova A. G., Shcharbin D., de la Mata F. J., Bryszewska M., Gomez R., Pharmaceutica 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chiappisi L., Keiderling U., Gutierrez-Ulloa C. E., Gomez R., Valienteg M., Gradzielski M., J. Colloid Interface Sci. 2019, 534, 430–439. [DOI] [PubMed] [Google Scholar]
- 126. Mencia G., Lozano-Cruz T., Valiente M., de la Mata J., Cano J., Gomez R., Molecules 2020, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mencia G., Lozano-Cruz T., Valiente M., Jimenez J. L., de la Mata F. J., Munoz-Fernandez M. a., Cano J., Gillies E., Gomez R., Org. Biomol. Chem. 2020, 18, 9639–9652. [DOI] [PubMed] [Google Scholar]
- 128. Apartsin E., Knauer N., Arkhipova V., Pashkina E., Aktanova A., Poletaeva J., Sanchez-Nieves J., de la Mata F. J., Gomez R., Nanomaterials 2020, 10, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zibarov A., Oukhrib A., Aujard Catot J., Turrin C.-O., Caminade A.-M., Molecules 2021, 26, 4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Qiu J., Chen L., Zhan M., Laurent R., Bignon J., Mignani S., Shi X., Caminade A.-M., Majoral J.-P., Bioconjugate Chem. 2021, 32, 339–349. [DOI] [PubMed] [Google Scholar]
- 131. Caminade A. M., Fruchon S., Turrin C. O., Poupot M., Ouali A., Maraval A., Garzoni M., Maly M., Furer V., Kovalenko V., Majoral J. P., Pavan G. M., Poupot R., Nat. Commun. 2015, 6, 7722. [DOI] [PMC free article] [PubMed] [Google Scholar]