Abstract
Despite decennia of research and numerous successful interventions in the preclinical setting, renal ischemia reperfusion (IR) injury remains a major problem in clinical practice, pointing toward a translational gap. Recently, two clinical studies on renal IR injury (manifested either as acute kidney injury or as delayed graft function) identified metabolic derailment as a key driver of renal IR injury. It was reasoned that these unambiguous metabolic findings enable direct alignment of clinical with preclinical data, thereby providing the opportunity to elaborate potential translational hurdles between preclinical research and the clinical context. A systematic review of studies that reported metabolic data in the context of renal IR was performed according to the PRISMA guidelines. The search (December 2020) identified 35 heterogeneous preclinical studies. The applied methodologies were compared, and metabolic outcomes were semi‐quantified and aligned with the clinical data. This review identifies profound methodological challenges, such as the definition of IR injury, the follow‐up time, and sampling techniques, as well as shortcomings in the reported metabolic information. In light of these findings, recommendations are provided in order to improve the translatability of preclinical models of renal IR injury.
Keywords: animal models, delayed graft function, ischemia reperfusion injury, kidney failure / injury, kidney transplantation / nephrology, metabolomics, translational research / science
Short abstract
A comparison of metabolic responses in clinical and experimental renal ischemia reperfusion identifies multiple translational gaps.
Abbreviations
- AA
amino acid
- AC
acylcarnitine
- AKI
acute kidney injury
- AV
arteriovenous
- BUN
blood urea nitrogen
- CA
cardiac arrest
- CE‐MS
capillary electrophoresis–mass spectrometry
- CE‐ToFMS
capillary electrophoresis–time of flight mass spectrometry
- CPB
cardiopulmonary bypass
- CI
cold ischemia
- CIT
cold ischemia time
- dATP
deoxyadenosine triphosphate
- DGF
delayed graft function
- fDGF
functional DGF
- FA
fatty acid
- fDGF
functional DGF
- FFA
free fatty acid
- FIA‐MS/MS
flow injection analysis tandem mass spectrometry
- GC
gas chromatography
- GCxGC‐MS
two‐dimensional gas chromatography–mass spectrometry
- GFR
glomerular filtration rate
- HPLC‐Q‐ToF MS
high‐performance liquid chromatography–quadrupole–time of flight mass spectrometry
- IR
ischemia reperfusion
- ISOM
inner stripe of outer medulla
- KIM‐1
kidney injury molecule‐1
- LC
liquid chromatography
- MALDI‐MS
matrix‐assisted laser desorption/ionization mass spectrometry
- MD
microdialysis
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAG
N‐acetyl‐β‐D‐glucosaminidase
- NGAL
neutrophil gelatinase‐associated lipocalin
- NMR
nuclear magnetic resonance
- OSOM
outer stripe of outer medulla
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- Post‐I
postischemia
- Pre‐I
preischemia
- PUFA
poly unsaturated fatty acids
- qPCR
quantitative polymerase chain reaction
- RRT
renal replacement therapy
- SCr
serum creatinine
- SWATH‐MS
sequential window acquisition of theoretical spectra–mass spectrometry
- TAN
total adenine nucleotides
- TCA
tricarboxylic acid
- TGN
total guanine nucleotides
- TMAO
trimethylamine oxide
- UHPLC
ultrahigh‐performance liquid chromatography
- UPLC‐MS/MS
ultra‐performance liquid chromatography tandem mass spectrometry
- WI
warm ischemia
- WIT
warm ischemia time
1. INTRODUCTION
Ischemia reperfusion (IR) injury describes the paradoxical increase in tissue injury following reperfusion of transiently ischemic organs. IR injury contributes significantly to graft damage in the context of organ transplantation. Unfortunately, despite decades of research and numerous preclinical successes, no intervention to date successfully reduced clinical IR injury. 1 , 2
The notable contrast between preclinical successes and consistent clinical failures points toward a profound translational gap in the understanding of IR injury. Independently of each other, two recent clinical studies implied metabolic failure as the primary effector mechanism of renal IR injury. To be specific, these studies concluded that both delayed graft function (DGF) in the context of kidney transplantation as well as acute kidney injury (AKI) in the context of major cardiac surgery associate with profound, transient postreperfusion metabolic defects such as, in the case of DGF, postreperfusion normoxic glycolysis and persistent postreperfusion ATP catabolism (further details are summarized in Table 1 and Figure 1). 3 , 4
TABLE 1.
Two recent clinical studies reporting renal metabolic data after ischemia and reperfusion (IR) resulting in acute kidney injury (AKI) or delayed graft function (DGF) 3 , 4
| Article | Sample timing | IR injury definition | Results on metabolome |
|---|---|---|---|
|
Legouis et al. 2020 3 |
Blood: twice at a 30‐min interval Control group: 4–6 h post‐IR; AKI group: 2–6 days post‐IR |
AKI: KDIGO criteria | In patients experiencing AKI: switch from net renal lactate uptake to net renal lactate release, a decrease in net renal glucose release compared to that in the control group |
| Lindeman et al. 2020 4 |
Blood: renal artery: 0, 10, and 30 min post‐IR; renal vein: 0.5, 3, 5, 10, 20, and 30 min post‐IR Tissue: postischemia and 45 min post‐IR |
DGF: recipient requires dialysis in first week(s) posttransplant, excluding dialysis for hypervolemia, hyperkalemia, or hyperphosphatemia | Grafts manifesting future DGF: postreperfusion ATP/GTP catabolism (significantly impaired phosphocreatine recovery and significant persistent (hypo)xanthine production). Failing high‐energy phosphate recovery occurred despite activated glycolysis, fatty‐acid oxidation, glutaminolysis, and autophagia, and related to a defect at the level of the oxoglutarate dehydrogenase complex in the Krebs cycle |
FIGURE 1.
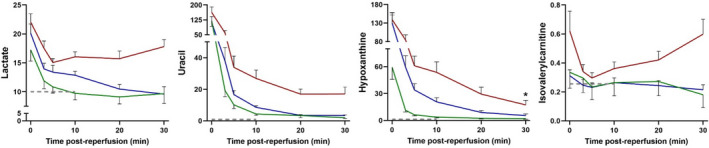
Illustration of the contrasting postreperfusion metabolic responses of kidney donor grafts with delayed graft function (DGF, IR injury, red curve) and grafts recovering without IR injury (no DGF) (green curve: living donor graft [intermediate ischemic period], blue curve: deceased donor graft [prolonged ischemic period]). The dashed line reflects the normal, non‐ischemic kidney. Figures adapted from Lindeman et al. 4 Curves represent renal vein levels of lactate (glycolysis), uracil (cellular damage), 5 hypoxanthine (ATP catabolism and metabolic incompetence), and isovalerylcarnitine (intermediate of branched‐chain amino acid oxidation [autophagia]). *Decrease at end of measurement window may reflect depletion of ATP pool
We reasoned that these unambiguous observations for clinical renal IR injury provide the opportunity to validate reported preclinical models. Therefore, we performed a systematic literature review to identify studies that report on metabolic aspects of experimental renal IR injury. Methodological aspects and reported metabolic observations in these studies were aligned with the clinical context in an attempt to map parallels and dissimilarities between preclinical models and clinical context.
2. METHODS
Systematic searches (see Supporting Information) were performed in PubMed, EMBASE, and Web of Science to identify preclinical studies reporting metabolic data following renal IR in the context of DGF and AKI. Articles were selected following recommended procedures described by PRISMA guidelines. 6 Two authors independently assessed titles and abstracts for eligibility. Full texts were consulted if it was unclear whether inclusion criteria were met.
3. RESULTS
The literature searches are summarized in two diagrams (Figure S1). Thirty‐four studies were selected based on the predefined inclusion criteria. One extra study (unidentified in both searches) was included. Thus, 35 preclinical studies were included, of which 17 explored renal IR in rats, 10 in mice, 1 in both rats and mice (included as 2 separate studies), 4 in pigs, and 2 in dogs. Almost all studies were performed in homogeneous populations of particularly young, mostly male, and healthy animals. The dog studies included an explicitly heterogeneous study population of mongrel dogs. Details of the methodology and results of the included reports are summarized in Tables 2 and 3.
TABLE 2.
Methodological details reported in the publications included by the systematic search
| Article | Species | Breed |
Age Weight |
Ischemia: transplantation, surgery, or clamping | Cold ischemia time (h) | Warm ischemia time (min) | Sample type | Sampling time points | Control(s) | Relevant measurement techniques |
|---|---|---|---|---|---|---|---|---|---|---|
| Rat | ||||||||||
| Andrianova et al. 2020 7 | Rat |
Outbred Wistar rats Male |
3–4 months 300–400 g |
Clamping Right nephrectomy, clamping of left renal vascular bundle for 40 min. |
— | 40 |
Blood (carotid artery) |
Blood: 48 h post‐IR |
Blood: intact control |
Blood metabolomics: FIA‐MS/MS Serum levels urea & creatinine: AU480 Chemistry System |
| Choi et al. 2019 8 | Rat |
Sprague‐Dawley rats Male |
3–4 months 410–450 g |
Surgery 20 min of cardiac arrest through asphyxia, then resuscitation by cardiopulmonary bypass. |
— | 20 |
Tissue |
Tissue: post‐I and 30 min post‐IR After 30 min of cardiopulmonary bypass resuscitation, rats were euthanized to recover brain, heart, kidney, and liver tissue samples. |
Tissue: intact control, no cardiac arrest, euthanized 7 min before recovering tissues. | Tissue metabolomics: LC‐MS/MS |
| Duran et al. 1990 9 | Rat |
Sprague‐Dawley rats Female |
??? 240–312 g |
Clamping Unilateral clamping of left renal artery for 1 h. |
— | 60 |
Blood (cardiac puncture) Tissue (cortex) |
Blood, tissue: 3 and 24 h post‐IR |
Blood, tissue: “control” rats Blood (for BUN): prior to IR, from tail |
Blood/tissue metabolomics: dansylation of amino acids and subsequent chromatography BUN: autoanalyzer technique |
| Gaudio et al. 1991 10 | Rat | Sprague‐Dawley rats |
??? 250 g |
Clamping Clamping of aorta proximal to both renal arteries for 45 min. |
— | 45 | Tissue | Tissue: 15 min or 2 h post‐IR, measurements of proximal tubule suspensions. | Tissue: sham‐operated rats |
ATP content: HPLC |
| Huang et al. 2018 11 | Rat | Fisher F344 rats |
??? 250–300 g |
Clamping Unilateral clamping of left renal artery for 45 min. |
— | 45 |
Blood (???) Tissue (cortex) |
Blood, tissue: 4 h and 24 h post‐IR | Blood and tissue: contralateral kidney and kidneys from healthy control rats |
Tissue metabolomics: 1H‐NMR & GCxGC‐MS |
| Lan et al. 2016 12 | Rat |
Sprague‐Dawley rats Male |
??? |
Clamping Right nephrectomy, clamping of left renal vascular pedicle for 45 min. |
— | 45 |
Blood (???) Tissue (cortex and outer stripe of outer medulla) |
Blood: daily post‐IR Tissue: 3, 7, and 14 days post‐IR |
Blood: prior to surgery Tissue: sham‐operated rats |
Tissue lactate and pyruvate levels: fluorimetry SCr:??? |
| Legouis et al. 2020 3 | Rat |
Sprague–Dawley rats Male |
8–10 weeks ??? |
Clamping Right nephrectomy, clamping of left renal artery for 25 min. |
— | 25 | Blood (left femoral artery, left femoral vein, and renal vein) | Blood: 60 and 120 min post‐IR | Blood: sham‐operated rats | Gluconeogenesis (plasma D2‐glucose enrichment after administration of D2‐glucose) measurement in blood: GC‐MS |
| Lindhardt et al. 2020 13 | Rat |
Wistar rats Male |
??? 205–290 g |
Clamping Unilateral clamping of left renal artery for 40 min. |
— | 40 |
Blood (tail vein) Tissue imaging in vivo Urine (metabolic cage) |
Blood, tissue (imaging in vivo): 24 h post‐IR Urine: 24 h post‐IR |
Blood: no control Tissue imaging in vivo: contralateral kidney Urine: no control |
Tissue metabolomics: In vivo 1H and hyperpolarized 13C MRI Urinary creatinine, BUN and SCr: COBAS 6000 device (Roche) |
| Liu et al. 2012 14 | Rat |
Sprague‐Dawley rats Male |
Adult 175–225 g |
Clamping Clamping of renal arteries for 45 min. |
— | 45 |
Blood (posterior orbital venous plexus) Tissue (cortex) |
Blood: 2, 4, 6, 12, 24, 48, 72, and 96 h post‐IR Tissue: 24 h and 96 h post‐IR |
Blood and tissue: sham‐operated rats |
Blood metabolomics: HPLC/MS |
| Nielsen et al. 2017a 15 | Rat |
Wistar rats Male |
??? 200–250 g |
Clamping Unilateral clamping of left renal artery for 40 min. |
— | 40 |
Blood (arterial) Tissue Urine (metabolic cage) |
Blood, tissue imaging in vivo, and samples: 24 h post‐IR Urine: 24 h post‐IR |
Blood: prior to surgery Tissue (imaging in vivo and samples): contralateral kidney Urine: 24 h prior to surgery |
Tissue metabolomics: In vivo 1H and hyperpolarized 13C MRI |
| Nielsen et al. 2017b 16 | Rat |
Wistar rats Male |
??? 250–290 g |
Clamping Unilateral clamping of left renal artery for 30 or 60 min. |
— | 30 or 60 |
Blood (??? before surgery and aorta post‐IR) Tissue imaging in vivo Tissue samples (cortex) |
Blood, tissue imaging in vivo, and samples: 24 h post‐IR |
Blood: prior to surgery (24 h) and sham‐operated rats Tissue: contralateral kidneys and sham‐operated rats |
Tissue metabolomics: In vivo 1H and hyperpolarized 13C MRI Tissue lactate levels: enzymatic assay BUN and SCr: COBAS 6000 device (Roche) Renal KIM−1 and NGAL expression: qPCR |
| Nielsen et al. 2020 17 | Rat |
Wistar rats Male |
??? 200–245 g |
Clamping (2 distinct procedures) Procedure 1: Unilateral clamping of left renal artery for 30 min. Procedure 2: Unilateral clamping of left renal artery for 20 or 40 min. |
— | 20, 30 or 40 |
Blood (tail vein) Tissue imaging in vivo Tissue samples (cortex and inner medulla) |
Blood: directly before tissue imaging in vivo Tissue imaging in vivo: Procedure 1 – 2 and 60 min post‐IR Procedure 2 – 1 and 7 days post‐IR Tissue samples: 60 min post‐IR and 7 days post‐IR |
Blood: no control Tissue (imaging in vivo and samples): contralateral kidney |
Tissue metabolomics: In vivo 1H and hyperpolarized 13C MRI SCr: COBAS 6000 device (Roche) |
| Peto et al. 2018 18 | Rat |
Crl:WI rats Male |
??? 342.2 ± 29.5 g |
Clamping Ligation of right renal artery and clamping of left renal vessels for 60 min. After WI, excision of right kidney and clamp removal from the left renal vessels. |
— | 60 |
Blood (left femoral artery) Tissue |
Tissue: 120 min post‐IR Blood: pre‐I, post‐I, 60 min post‐IR, and 120 min post‐IR |
Blood and tissue: prior to surgery and sham‐operated rats | Blood acid‐base parameters, glucose and electrolytes: EPOC portable blood analysis device |
| Serkova et al. 2005 19 | Rat | Lewis rats Male |
??? 200–250 g |
Transplantation After removal from living donors, kidneys were kept cold for 24 or 42 h. Implantation after removal of both kidneys from recipient. |
24 or 42 | ??? |
Blood (???) Tissue |
Blood: pre‐IR and 24 h post‐IR Tissue: pre‐IR, post‐I, and 24 h post‐IR |
Blood and tissue: recipient's kidney and blood prior to nephrectomy and transplantation | Blood/tissue metabolomics: 1H‐NMR |
| Shen et al. 2017 20 | Rat |
Sprague‐Dawley rats Female |
Adult 200–220 g |
Clamping Clamping of renal pedicles for 30 min. |
— | 30 | Tissue | Tissue: post‐IR (reperfusion time unknown) | Tissue: sham‐operated rats | Tissue metabolomics: GC/MS |
| Tani et al. 2019 21 | Rat |
Sprague‐Dawley rats Male |
6 weeks ??? |
Clamping Unilateral clamping of left renal pedicle for 30 min. |
— | 30 |
Tissue |
Tissue: 60 min after drug administration, post‐I, and 30 min post‐IR |
Tissue: “vehicle treatment group,” no surgery, 60 min after receiving 0.5 mL 0.5% methylcellulose. |
Tissue purine nucleotide concentration: HPLC Tissue metabolomics: CE‐ToFMS |
| Trifillis et al. 1984 22 | Rat |
Sprague‐Dawley rats Male |
??? 220–250 g |
Clamping Clamping of aorta above left renal artery, below the right renal artery, and superior mesenteric artery, as well as clamping right renal artery and vein for 15, 10, 60, 90, or 120 min. |
— | 5, 15, 30, 60, 90, or 120 |
Blood (aorta) Tissue |
Blood:??? Tissue: post‐I, and 0.25, 1, 6, 24, or 48 h post‐IR |
Blood:??? Tissue: control rats (no sham surgery) |
Tissue ATP/ADP/AMP/lactate measurements: specific enzymatic methods coupled with NADH or NADPH Tissue Pi measurements: modification of Fiske and SubbaRow method SCr: Beckman creatinine analyzer II BUN: Beckman urea nitrogen analyzer. |
| Varga et al. 2019 23 | Rat |
Crl:WI rats Male |
??? 301.6 ± 38.6 g |
Clamping Unilateral clamping of left renal vessels for 45 min. |
— | 45 | Blood (right femoral artery) |
Blood: pre‐I, and 30, 60, and 120 min post‐IR |
Blood: prior to surgery and sham‐operated rats | Blood acid‐base parameters, metabolites and electrolytes: EPOC portable blood analysis device |
| Mouse | ||||||||||
| Beier et al. 2020 24 | Mouse |
C57BL/6 mice Female |
??? |
Clamping Unilateral clamping of renal pedicle for 28 min. |
28 | Tissue | Tissue: 24 h post‐IR | Tissue: contralateral kidney | Tissue metabolomics: UPLC‐MS | |
| Chihanga et al. 2018 25 | Mouse | Swiss‐Webster mice |
??? 25–30g |
Clamping Clamping of renal pedicles for 30 min. |
— | 30 |
Blood (inferior vena cava for SCr, cardiac puncture for NMR) Tissue Urine (metabolic cage) |
Blood: 24 h post‐IR Tissue: 24 h post‐IR Urine: 3 days pre‐IR and 24 h post‐IR |
Blood: control mice (pre‐IR) Tissue: control mice (pre‐IR) Urine: 3 days pre‐IR |
Blood/urine metabolomics: 1H‐NMR Urinary creatinine, SCr and urinary NGAL: spectroscopy |
| Cho et al. 2017 26 | Mouse | C57BL/6 mice Male |
9 weeks ??? |
Clamping Unilateral clamping of left renal pedicle for 45 min. |
— | 45 |
Blood (???) Tissue Urine (metabolic cage) |
Blood: 24 h post‐IR Tissue: 24 h post‐IR Urine: 24 h post‐IR |
Blood, tissue, and urine: sham‐operated mice |
Blood/tissue/urine metabolomics: HPLC‐Q‐ToF MS |
| a Chouchani et al. 2014 27 |
Mouse |
C57BL/6J mice Male |
8–10 weeks ??? |
Clamping Unilateral clamping of one renal pedicle for 45 min. |
— | 45 | Tissue | Tissue: post‐I and 5 min post‐IR | Tissue:??? (normoxic controls) | Tissue metabolomics: LC‐MS |
| Fujii et al. 2019 28 | Mouse |
C57BL/6 Male |
8 weeks ??? |
Clamping Unilateral clamping of left renal artery for 1, 10, and 40 min. |
— | 1, 10, and 40 |
Blood (???) Tissue Urine (???) |
Tissue: after 1, 10, and 40 min WI, and 24 h post‐IR Blood and urine: 24 h post‐IR |
Blood and urine:??? Tissue: sham‐operated mice |
Tissue metabolomics: Matrix‐assisted laser desorption/ionization–imaging mass spectrometry (MALDI‐IMS) + data calibration by CE‐MS Serum/urine samples: “standard method” |
| Jouret et al. 2016 29 | Mouse |
C57BL/6J mice Male |
10 weeks ~20 g |
Clamping Clamping of renal pedicles for 30 min. |
— | 30 |
Blood (vena cava) Tissue Urine (metabolic cage) |
Blood, tissue, and urine: 6 h, 24 h, and 48 h post‐IR |
Blood, tissue and urine: sham‐operated mice |
Blood/tissue/urine metabolomics: 1H‐NMR Serum levels urea & creatinine: COBAS 6000 device (Roche) |
| Legouis et al. 2020 3 | Mouse |
C57Bl/6 J mice Male |
10–12 weeks 25–28 g |
Clamping Clamping of renal pedicles for 25 min. |
— | 25 |
Blood (???) Urine (???) |
Blood and urine: 24 and 48 h post‐IR Blood (for lactate clearance test): 15, 30, 60, 90, and 120 min postinjection of sodium lactate (injection at 6 h post‐IR) |
Blood and urine: sham‐operated mice |
Blood lactate and glucose: Aviva Accu‐Check glucometer and Novabio StatStrip Xpress lactate meter SCr: capillary electrophoresis BUN: quantitative colorimetric determination using Stanbio Excel analyzer |
| Poyan Mehr et al. 2018 30 | Mouse |
C57BL/6J mice Male |
8–12 weeks ??? |
Clamping Clamping of renal pedicles for 20 min. |
— | 25 |
Blood (???) Tissue Urine (???) |
Blood, urine, and tissue: 24 h post‐IR | Blood, tissue, and urine: sham‐operated mice |
Tissue/urine metabolomics: LC‐MS SCr: LC‐MS |
| Rao et al. 2016 31 | Mouse |
C57BL/6 mice Male |
10–12 weeks ??? |
Clamping Right nephrectomy, clamping of left renal pedicle for 30 min. |
— | 30 | Tissue | Tissue: 6 and 24 h post‐IR | Tissue: sham‐operated mice |
Tissue lipid concentrations: SWATH‐MS Tissue hydroxyoctadeca dienoic acid/ hydroxyeicosatetra enoic acid measurement: LC‐MS/MS |
| Wei et al. 2014 32 | Mouse |
C57BL/6 mice Male |
9 weeks ??? |
Clamping Clamping of renal pedicles for 25 min. |
— | 25 |
Blood (???) Tissue (cortex and medulla) |
Blood and tissue: 2 h, 48 h, or 1 week post‐IR | Blood and tissue: sham‐operated mice | Blood/tissue metabolomics: GC/MS and LC/MS |
| Zager et al. 2014 33 | Mouse |
CD−1 mice Male |
??? 30–45 g |
Clamping Unilateral clamping of left renal pedicle for 15, 30, or 60 min. |
— | 15, 30, or 60 |
Blood (vena cava) Tissue (cortex) |
Blood and tissue: after 15, 30, or 60 min WI, and 2 or 18 h post‐IR |
Blood: sham‐operated mice Tissue: contralateral kidney and sham‐operated mice |
Tissue/blood lactate, pyruvate, glucose and glycogen levels: enzymatic assays |
| Pig | ||||||||||
| Clendenen et al. 2019 34 | Pig |
Farm pigs Male |
??? 50–55 kg |
Clamping Clamping of renal pedicles for 30 min. |
— | 30 | Blood (renal vein) | Blood: pre‐IR, after 15 and 30 min WI, and 5 min post‐IR | Blood: pre‐IR | Blood metabolomics: UHPLC‐MS |
| Fonouni et al. 2011 35 | Pig | Landrace pigs |
??? 26–33 kg |
Transplantation Living donor left kidney explantation (30 min), implantation in recipient after 6 h CI. 120 min reperfusion. |
6 | 60 (anastomosis) |
Blood (???) Extracellular fluid (microdialysis = MD) |
Blood: during procurement, post‐I, and 120 min post‐IR MD: 10‐min intervals during kidney procurement, CI (2 samples in the first and 2 samples at the end of CI), and at 20‐min intervals during kidney implantation (WI) and postreperfusion (120 min). |
Blood and extracellular fluid: during explantation procedure |
MD: CMA 600 Microdialysis Analyzer Blood analysis: hospital laboratory |
| Hauet et al. 2000 36 | Pig |
Large White pigs Male |
??? 41–52 kg |
Transplantation Left nephrectomy, kidneys were flushed with EC solution or UW solution. After 48 h CI, heterotopic autotransplantation was performed and contralateral nephrectomy was carried out. |
48 | ??? |
Blood (right jugular vein) Urine (metabolic cage) |
Blood and urine: 2 days before kidney preservation (D−2) and at 1, 3, 5, 7, 11, and 14 days (D1–D14) post‐IR. Also examined these kidneys 30–40 min after implantation and on the sacrifice day. |
Blood and urine: control group uninephrectomized, no flushing or cold preservation | Blood/urine metabolomics: 1H‐NMR |
| Malagrino et al. 2019 37 | Pig |
Pigs Female |
Juvenile 15–20 kg |
Clamping Unilateral clamping of the right renal artery for 120 min. |
— | 120 |
Blood (inferior vena cava above the renal veins) Tissue Urine (directly from bladder) |
Tissue: 24 h post‐IR Blood and urine: pre‐IR (before occlusion), after 1 h WI, 0.5 h post‐IR, 4 or 6 h post‐IR, and 11 h post‐IR |
Tissue: contralateral kidney Blood and urine: pre‐IR samples |
Blood/urine metabolomics: 1H‐NMR |
| Dog | ||||||||||
| Maessen et al. 1989 38 | Dog | Mongrel dogs |
Adult 18–25 kg |
Transplantation Clamping of left renal vessel pedicle for 0 or 30 min. Then, kidney explantation and storage on ice. Autologous reimplantation and contralateral nephrectomy. |
24 or 48 | 0 or 30 +??? | Tissue (cortex) | Tissue: after WI, after CI, and 1 h post‐IR | Tissue: non‐ischemic control | Tissue energy metabolite levels: HPLC |
| Montañés et al. 1991 39 | Dog | Mongrel dogs |
??? 17–34 kg |
Clamping Right nephrectomy, clamping of left renal artery for 60 min. |
— | 60 |
Blood (femoral artery, left ovarian, or spermatic vein) Tissue (cortex) Urine (left ureter and metabolic cage) |
Blood, tissue, and urine: 2 days post‐IR |
Blood, tissue and urine: sham‐operated dogs |
Blood/tissue metabolomics: enzymatic assays SCr: enzymatic assay Blood/urine pH and gasses: blood gas analyzer model 168 (Corning Medical) |
For the sake of clarity, the induction of ischemia was classified as “clamping,” including several approaches to occlude blood flow, or “transplantation,” in which the kidney was removed and transplanted.
Abbreviations: AV, arteriovenous; BUN, blood urea nitrogen; CE‐MS, capillary electrophoresis–mass spectrometry; CE‐ToFMS, capillary electrophoresis–time of flight mass spectrometry; CI, cold ischemia; DGF, delayed graft function; fDGF, functional DGF; FIA‐MS/MS, flow injection analysis tandem mass spectrometry; GC, gas chromatography; GCxGC‐MS, two‐dimensional gas chromatography–mass spectrometry; HPLC‐Q‐ToF MS, high‐performance liquid chromatography–quadrupole–time of flight mass spectrometry; IR, ischemia reperfusion; KIM‐1, kidney injury molecule‐1; LC, liquid chromatography; MALDI‐MS, matrix‐assisted laser desorption/ionization mass spectrometry; MD, microdialysis; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NGAL, neutrophil gelatinase‐associated lipocalin; NMR, nuclear magnetic resonance; Post‐I, postischemia; Pre‐I, preischemia; qPCR, quantitative polymerase chain reaction; SCr, serum creatinine; SWATH‐MS, sequential window acquisition of theoretical spectra–mass spectrometry; UHPLC, ultrahigh‐performance liquid chromatography; UPLC‐MS/MS, ultra‐performance liquid chromatography tandem mass spectrometry; WI, warm ischemia; ???, data unknown.
Additionally included study.
TABLE 3.
Results reported in the publications included by the systematic search
| Article | Species | Injury definition | Renal function clinical markers | Additional damage markers | Results on metabolome |
|---|---|---|---|---|---|
| Rat | |||||
| Andrianova et al. 2020 7 | Rat |
Serum creatinine (SCr) levels: “After IR, creatinine concentration increased in all animals, varying from 50 to 200–500 µM, indicating that IR caused AKI” Note: no thresholds given. |
SCr (increase post‐IR) | — |
Only measured acylcarnitines (ACs) and amino acids (AAs). After IR, we detected 34 metabolites in blood serum, whose levels significantly changed—concentration of 31 acylcarnitines increased, while the content of 3 AAs (tyrosine, tryptophan, and proline) dropped. The most significant changes were observed for malonylcarnitine, which demonstrated a sevenfold increase compared to control, glutarylcarnitine (fivefold increase), decadienoyl carnitine (fourfold increase), hydroxybutyrylcarnitine (fourfold increase), linoleylcarnitine (fourfold increase), and methylmalonylcarnitine (fourfold increase). Other acylcarnitines showed about a twofold increase. The serum levels of the tyrosine, tryptophan, and proline concentration dropped to 60%–70% of their content. |
| Choi et al. 2019 8 | Rat | Not defined | — | — |
Principal component analysis: Significant separation following cardiac arrest (CA) and following resuscitation. Citrate, a‐ketoglutarate, malate, fumarate, and succinate significantly changed in kidney after cardiopulmonary bypass (CPB) compared with control or CA. Individual metabolite changes: ‐The only amino acids that were unaffected by CA were glutamine, threonine, alanine, and valine, remaining measured amino acids were significantly decreased. Thirty minutes of CPB resuscitation resulted in even more dysregulation; all measured amino acids with the exception of glutamate, alanine, and valine were significantly decreased. ‐Thirty minutes of CPB resuscitation resulted in a significant decrease in linoleic and linolenic acids, while stearic acid returned to control levels. The general trend of lipids in the kidney is that they increase after ischemia, but either return to or fall below control levels after resuscitation. ‐The kidney shows variations in glycolytic and TCA cycle metabolites, such as a significant elevation in 3‐phosphoglycerate and oxoglutarate after 20 minutes of CA. However, CPB resuscitation resulted in a significant increase in citrate, oxoglutarate, succinate, fumarate, malate, and oxaloacetate. ‐The urea cycle metabolites were mostly altered after 30 min of CPB resuscitation in the kidney. ‐Arginine and proline were significantly decreased after CA, but after CPB, arginine, citrulline, ornithine, and proline were all decreased. Changes in acylcarnitine species: CA resulted in elevation in only AC 16:0 when compared with control. Resuscitation resulted in a decrease from CA in AC 2:0, AC 8:0, AC 10:0, AC 14:2, AC 16:1, AC 16:0, AC 18:2, and AC 18:1. AC species that were decreased after resuscitation were AC 6:0, AC 8:0, AC 10:0, AC 14:2, AC 14:1, AC 16:2, AC 16:1, AC 18:2, and AC 18:1. The kidney was unable to normalize ACs after resuscitation, resulting in a generally diminished lipid reserve after 30 min of CPB resuscitation. Pathway analysis: In kidney tissue following resuscitation, 46 pathways remained significantly altered compared with control, with nicotinate and nicotinamide metabolism, and phenylalanine metabolism having the lowest q values. |
| Duran et al. 1990 9 | Rat | Not defined | BUN (no change post‐IR) | Kidney weight (increase post‐IR) |
Only measured AAs: The plasma concentrations of AAs were indistinguishable from control. Cellular AAs post‐IR: glutamate, glycine, phenylalanine, and serine in cortical cell water (=derived from tissue) were decreased 3 h after ischemia, but those of the remaining 12 AAs were not different from control. Concentrations of glutamate and glycine had normalized 24 h after blood reflow, leaving only 4 AAs—arginine, phenylalanine, serine and threonine—at decreased concentration. |
| Gaudio et al. 1991 10 | Rat | EM images of proximal tubule segments: “Following ischemia and 15 min of reperfusion, there were marked cellular alterations typical of ischemic injury.” | — | Histology | Forty‐five min of ischemia resulted in a significant fall in ATP levels. ATP levels had increased by only a small amount after 2h of reperfusion. |
| Huang et al. 2018 11 | Rat |
Not defined |
SCr (increase post‐IR) Lactate (tissue and plasma levels increase post‐IR) |
Histology |
Metabolomics: ‐Palmitate, stearate, linoleate, 1‐monopalmitin, 2‐monopalmitin, 2‐monostearin, and cholesterol appeared to accumulate after 4 h followed by a reduction after 24 h IR. ‐Reduced glucose levels in both 4 h IR and 4 h control (4 h‐C) kidneys that were sustained in 24 h‐IR as compared to HC (healthy control). Glucose levels were unaltered in plasma of 4 h and 24 h operated animals. Lactate levels were increased in 24 h IR as compared to HC, and were also significantly elevated in the plasma of 4 h operated animals and even to a greater extent after 24 h. ‐Blood creatinine levels were higher in both 4 h and 24 h post‐IR. ‐Compensatory changes in metabolite levels in the uninjured organ of animals subjected to kidney IR, in particular after 24 h reperfusion: a strong elevation of urea and AMP in contralateral kidneys after 24 h post‐IR, which was not observed in the injured kidney counterpart. Adenosine, glutamic acid, and glycine levels were increased in a more prominent fashion in contralateral kidneys, particularly after 24 h. Citrate appeared to be elevated in all conditions as compared to control. ATP levels were significantly decreased in 24 h IR kidney tissue as compared to 24 h C and HC. Integrated analysis: Hierarchical cluster analysis revealed the existence of five phenotypes: (i) Decreased substrates in 4 h IR and/or 4 h C compared to HC and 24 h IR/C. This includes proteins involved in fatty acid (FA) biosynthesis (Acsl6, Acsl4), metabolites involved in energy metabolism (glucose and citric acid); (ii) Decreased substrates prevalently in 24 h IR compared to the other conditions. This group: adenosine, proteins involved oxidation and reduction reactions (Por), and enzyme that play role in the TCA cycle (Pdha1/1). (iii) Metabolites increased prevalently in 24 h C animals as compared to the other conditions. This includes free fatty acids (FFAs) (2‐monostearin, 2‐monopalmitin, and linoleate), non‐essential amino acids (glutamic acid and glycine), urea, AMP, and creatinine. (iv) Proteins and metabolites prevalently increased in 24 h IR including enzymes involved in oxidative phosphorylation (Ndufa6, Ndufv1, and Ndufs1), fatty acid binding protein (Fabp4), glycolysis enzyme (Hk1), and lactate. (v) Enzymes and metabolites elevated in 4 h IR and 4 h C such as glucose transporter (Slc5a1), FA transporter (CD36), components of oxidative phosphorylation (ND−1), detoxification enzymes (Adh5, Ugt2b15), mitochondrial biogenesis (Sirt2), FFA metabolism (Cpt1a, Acadsb, Echdc3, palmitate, stearate, and 1‐monopalmitin), and ketone metabolism (Oxct1). |
| Lan et al. 2016 12 | Rat |
IR injury was monitored by SCr and renal histology. Note: no thresholds given. |
SCr (no data reported) | Histology | During reperfusion 3, 7, and 14 days after IR, lactate, and pyruvate concentrations in cortex and the outer stripe of outer medulla were increased relative to time controls. |
| Legouis et al. 2020 3 | Rat | Not defined | — | — |
Renal gluconeogenesis, selectively quantified by analysis of blood from the renal vein, was reduced in response to IR. |
| Lindhardt et al. 2020 13 | Rat | Not defined |
SCr (no control) BUN (no control) Urinary creatinine (no control) |
— | The metabolism measured in vivo by product/pyruvate ratios showed a significant decrease in the ischemic kidney compared with the contralateral kidney when considering all metabolites (lactate, alanine, and bicarbonate). |
| Liu et al. 2012 14 | Rat |
BUN and SCr were two widely used indicators of kidney Injury. There were significant differences in SCr and BUN between the control and IR groups. Note: no thresholds given. |
SCr (increase post‐IR) BUN (increase post‐IR) |
— |
‐Most important IR‐related metabolites are lysophospholipids and FFAs, including stearoyl‐glycerophosphocholine, eicosatrienoyl‐glycerophosphocholine, oleoyl‐glycerophosphocholine, palmitoyl‐glycerophosphocholine, linoleoyl‐glycerophosphocholine, linolenoyl lysolecithin, stearic acid, oleic acid, linoleic acid, arachidonic acid, and eicosapentaenoic acid. ‐Nitrotyrosine (oxidative product of tyrosine) significantly increased in the IR group. ‐Increased hydroxymethylphenidate after IR. ‐Carnitine and acetyl‐carnitine decreased during IR. ‐Saturated fatty acids and unsaturated fatty acids displayed different changes in the IR group. However, stearic acid made more contribution than polyunsaturated fatty acids (PUFAs) to discriminate between the IR and control. |
| Nielsen et al. 2017a 15 | Rat |
Functional kidney parameters showed consistent signs of renal IR injury with an elevated plasma creatinine level of 91% and a reduced creatinine clearance and BUN level of 44% and 30%, respectively, when comparing presurgery with postsurgery values. Note: no thresholds given. |
SCr (increase post‐IR) BUN (increase post‐IR) Creatine clearance (decrease post‐IR) Urine output (non‐significant increase post‐IR) |
Histology Body weight (non‐significant decrease post‐IR) Kidney weight (increase post‐IR) |
An elevated malate/fumarate ratio of 339% in the ischemic kidneys compared to that in the contralateral kidney was found. |
| Nielsen et al. 2017b 16 | Rat |
We demonstrated that increased BUN and plasma creatinine occurred in both unilateral IR groups compared with sham‐operated rats. Together, this indicates that renal IR resulted in acute renal insufficiency. Note: no thresholds given. |
SCr (increase post‐IR) BUN (increase post‐IR) |
Renal KIM−1 and NGAL mRNA expression (increase post‐IR) Body weight (decrease post‐IR) Kidney weight (increase post‐IR (60 min ischemia)) |
Significant decrease of 18–25% in the pyruvate‐to‐lactate conversion in the 60‐min postischemic kidney compared with the contralateral kidneys and kidneys from sham‐operated rats. No reduction in pyruvate‐lactate turnover was observed in the 30‐min IR Group. The alanine‐to‐pyruvate and bicarbonate‐to‐pyruvate ratio similarly showed a decrease of 44% and 59%, respectively, in the postischemic kidney, no reduction in metabolite turnover was seen in the 30‐min IR Group. The lactate‐to‐bicarbonate ratio was significantly shifted toward anaerobic glycolysis in the 60‐min postischemic kidney by 44%. No statistical difference was found between alanine metabolism and aerobic glycolysis (alanine‐to‐bicarbonate ratio), but the lactate‐to‐alanine ratio was significantly increased by 25%, and a small increase in lactate‐to‐alanine ratio of 23% was also seen in the 30‐min IR group. Pyruvate‐to‐total carbon signal and a total carbon kidney fraction were calculated for each kidney of the sham‐operated and unilateral IR rats, which yielded a significantly elevated ratio of 6% for the 60‐min postischemic kidney. Increase in lactate in the 60‐min IR group of 178%, and no significant increase in the 30‐min group. |
| Nielsen et al. 2020 17 | Rat | Not defined | SCr (increase post‐IR for longer ischemia, stable levels post‐IR for short ischemia, and [partially] recover 7 days post‐IR) | — |
Acute alterations in the ischemic re‐perfused kidneys overall metabolic phenotype were seen between the ischemic/early perfusion stage (2 min) and after 1 hour of perfusion, showing a compensatory mechanism in the contralateral kidney 60 min after reperfusion. The acute change in the lactate‐to‐bicarbonate ratio 60 min after reperfusion was not correlated with the early signature 2 min after reperfusion. The acute metabolic reprogramming seen at 60 min was driven by postrelease compensation in the contralateral kidney as well as downregulation of the lactate‐to‐bicarbonate balance in the ischemic kidney. The in vivo response that was seen 24 hours and 7 days following ischemic injury showed a similar tendency toward a general reduction in the overall metabolism in the ischemic kidney as well as a compensatory increased anaerobic metabolism shown by increased lactate production when compared to the aerobic metabolism, shown by CO2 and HCO3 production. Both 20 min and 40 min ischemia in the kidney results in a tendency toward a metabolic reprogramming from 24 hours to 7 days, with a statistically significant shift observed in the 40 min group. The metabolic phenotype at 24 hours, with reduced lactate‐to‐bicarbonate ratio, is positively correlated with the lactate‐to‐bicarbonate ratio at 7 days. A positive correlation was found in the lactate‐to‐bicarbonate ratio between 24 hours and 7 days. While no such correlation was found between the perfusion stage (2 min) and 60 min. By looking at the 20 min and 40 min group, one group with a large variance covering the 30 min acute insult, we compared the overall metabolic pattern from the initial 2 min to 7 days between the ischemic and contralateral kidney. This combination shows a significant change already at 60 min which persists throughout the 7 days. These findings are supported by a tendency toward a correlation between the lactate‐to‐bicarbonate ratio. |
| Peto et al. 2018 18 | Rat | Not defined | Lactate (blood lactate increase post‐IR and pH decrease) | — |
‐Blood lactate concentration in parallel to pH increased by the end of the observed reperfusion period in all groups. In control group, the change was not significant, but in IR, where the largest rise was found, it was. ‐No significant change in glucose concentration. |
| Serkova et al. 2005 19 | Rat |
Graft function: clinical appearance, histology, SCr, and urine output. Note: no thresholds given. |
SCr (increase post‐IR) Anuria |
Histology Clinical appearance (behavior, fur, etc.) Body weight (no change post‐IR) |
Tissue: ‐Cold storage in UW solution: significant increase in glycogen and other carbohydrates was observed at 24 and 42 hours of CI. The lactate concentration was greatly increased, up to 380% during CI. In the lipid extracts, a decrease in PUFAs was seen in CI groups versus native kidney. There were no significant differences in metabolic composition at the end of 24 and 42 hours of CIT. ‐Transplanted kidneys exposed to reperfusion for 24 hours demonstrated characteristic changes: most pronounced difference between the post‐IR and post‐I groups was the dramatic increase in allantoin. Allantoin concentrations were low in the native kidney and at the end of CI, but increased significantly after 24 h reperfusion. Stepwise logistic regression analysis revealed that from 30 metabolites quantified from kidney extracts, only two—allantoin and PUFA—were different among study groups. Blood: ‐In 6/8 animals in the native group, allantoin concentrations were below the limit of quantification for NMR. ‐ Allantoin peaks appeared in the blood in both CI groups following reperfusion. Allantoin concentrations were higher in transplanted rats with 42‐hour cold storage when compared with 24‐hour cold storage. ‐Trimethylamine oxide (TMAO) correlated well with CIT. TMAO concentration correlated well with elevated allantoin levels. ‐Uric acid concentrations were below the linear range for the assay. |
| Shen et al. 2017 20 | Rat | Renal injury confirmed by histology: disrupted kidney structure. | — | Histology |
‐All metabolites enriched in the biosynthesis of unsaturated fatty acids were augmented in IR group in contrast to the control group. ‐D‐glucose, lactic acid, and cholesterol were differentiated significantly between control and IR group. |
| Tani et al. 2019 21 | Rat | Not defined |
Lactate (tissue levels increase post‐IR) SCr (increase post‐IR) BUN (increase post‐IR) |
— |
‐Energy charge decreased upon ischemia, and increased upon reperfusion. ‐Total adenine nucleotide (TAN), ATP and ADP levels are decreased upon ischemia, and increased only slightly upon reperfusion. AMP showed hardly any difference between stationary state and ischemia, but decreased during reperfusion to a lower level. ‐Hypoxanthine, xanthine, and uric acid levels are high after ischemia, but return to baseline after reperfusion. ‐All groups in the ischemic state showed an increase in IMP level and a decrease in deoxyadenosine triphosphate (dATP) level, and a decrease in the adenine level after reperfusion. ‐TAN’ (the sum of all detectable purine metabolites excluding ATP, ADP, and AMP, thus including dATP, PRPP, adenosine, adenine, inosine, IMP, hypoxanthine, xanthine, and uric acid) increased during ischemia and dropped to approximately baseline after reperfusion again. ‐Drastic metabolic changes by the IR procedure: triphosphate compounds in purine/pyrimidine metabolism pathways, nicotinamide adenine dinucleotide (NAD+), uridine diphosphate (UDP)‐glucose, kynurenine, citrulline, and amino acids such as ornithine, isoleucine, leucine, and tryptophan. ‐Marked accumulation of hydrolysis products, such as lactate and ß‐hydroxybutyrate. |
| Trifillis et al. 1984 22 | Rat |
SCr and BUN were used as indices of renal function. Note: no thresholds given. |
SCr (increase post‐IR) BUN (increase post‐IR) |
Survival |
1 h ischemia and variable reperfusion times: ATP levels after 1 h of clamping decreased significantly to 18% of control levels. Upon release of the clamp, ATP levels began to increase after 0.25 h of reflow. ATP concentrations finally returned to control levels after 24 h of reflow. ADP levels remained relatively unchanged. AMP levels doubled after 1 h of ischemia but promptly returned to control levels after 0.25 h of reflow. Therefore, changes in AXP levels paralleled those of ATP levels, that is, AXP levels were not fully restored to control levels until 24 h of reflow. The energy charge decreased to 50% of the control value after 1 h of ischemia but returned to control levels after 6 h of reflow. Lactate levels reached 13‐fold control levels after 1 h of clamping and remained significantly elevated until 24 h of reflow when they returned to control levels. Variable ischemia times and 24 h of reperfusion: 120 min of ischemia resulted in death within the 24‐h reflow period. SCr levels increased significantly after 60 and 90 min of ischemia followed by 24 h of reflow. In the left kidney, adenine nucleotides, lactate, and inorganic orthophosphate levels were restored essentially to control levels after 30 min of ischemia followed by 24 h of reflow. Adenine nucleotide levels were partially restored but remained significantly lower than control levels after 60 and 90 min of ischemia and 24 h of reflow. Lactate levels were restored to controls after 15–90 min of ischemia followed by 24 h of reflow. Inorganic orthophosphate and the phosphorylation state were significantly different from controls only after 60 min of ischemia followed by 24 h of reflow. Adenine nucleotide, lactate, and inorganic orthophosphate content of the right kidney were not significantly different from that of the left kidney at any time period studied, that is, the time course and magnitude of metabolite restoration following 24 h of reflow was the same in both kidneys. |
| Varga et al. 2019 23 | Rat |
Not defined |
Lactate (serum levels increase post‐IR and pH decrease) SCr (increase post‐IR) |
— |
Lactate and potassium concentration significantly increased in IR (measurements after 120 min). IR decreased the pH. |
| Mouse | |||||
| Beier et al. 2020 24 | Mouse | Not defined | — | — | We found 30 metabolites elevated in IR. Among the uniquely increased metabolites in IR (compared to acute cellular rejection), the highest fold difference was observed for the lysine catabolite saccharopine. Also the downstream products 2‐aminoadipate and glutarate, but not the parent substrates lysine and α‐ketoglutarate, were increased in IR. |
| Chihanga et al. 2018 25 | Mouse |
SCr, urinary NGAL, urinary NGAL/urinary creatinine ratios, and urinary creatinine levels confirmed AKI 24 h post‐IR. Note: no thresholds given. |
SCr (increase post‐IR) Urinary creatinine (decrease post‐IR) |
Histology Urinary NGAL (increase post‐IR) Urinary NGAL/urinary creatinine (increase post‐IR) |
‐Urinary concentrations of many metabolites before IR changed dramatically after IR. Cis‐aconitate, citrate, creatine, phosphocreatine, putrescine, sarcosine, succinate, taurine, n‐nitrosodimethylamine, trimethylamine, uracil, and trimethylamine N‐oxide, galactaric acid, guanine, and hippurate all decreased following IR. Nicotinamide‐n‐oxide, trigonelline, 2‐oxoglutarate, and 2‐oxoisocaproate were absent in urine following injury. Glucose, lactate, alanine, valine, and leucine had higher urine concentrations following IR. ‐NMR spectroscopy of plasma collected 24 h post‐IR indicated no new metabolites post‐IR except for creatinine. Metabolic profiling of kidney tissue extracts indicated no new metabolites following IR. |
| Cho et al. 2017 26 | Mouse | There was a significant increase in acute tubular injury in the IR group compared to the sham group. | — | Histology |
‐The levels of adenosine and 5'‐deoxy−5'‐methylthioadenosine were higher in IR‐injured kidney. IR‐injured kidney was characterized by decreased phosphatidylethanolamine (PE) 20:3/20:4, and betaine aldehyde levels in kidney samples, as well as increased cell membrane constituents. ‐Increased serum levels of fatty acids; in particular, 4,14‐dimethyl‐hexadecanoic acid, 15‐eicosenoic acid, 3,5‐dimethyl‐tetradecanoic acid, cis−8,11,14‐eicosatrienoic acid, and 3‐oxo−2‐pentyl‐cyclopentaneoctanoic acid. The acyl‐carnitines 2‐octenoylcarnitine and 2‐hydroxylauroyl‐carnitine were decreased in the urine and serum, respectively. Levels of arachidonic acid and cis−4,7,10,13,16,19‐docosahexaenoic were lower following IR than in the sham group. |
| a Chouchani et al. 2014 27 |
Mouse |
Not defined | — | — | Increase in hypoxanthine and xanthine after ischemia. Succinate accumulation during ischemia, recovery to baseline after 5 min of reperfusion. |
| Fujii et al. 2019 28 | Mouse |
Transient ischemia for 10 min was sufficient to cause significant renal injury with increased NAG in urine and decreased creatinine clearance. No histological damage. Note: no thresholds given. |
Creatinine clearance (decrease post‐IR) |
Histology (note: no changes) Urinary NAG (increase post‐IR) |
‐In the normal kidney sections, high‐energy adenine nucleotides (ATP and ADP) were significantly rich (vs. inner medulla) in both the cortex and the outer medulla (outer stripe of outer medulla [OSOM] and inner stripe of outer medulla [ISOM]). ‐ATP and total adenylates in the cortex and OSOM decreased by transient ischemia for 10 min: ‐ATP in the cortex and the outer medulla decreased within a minute after the clipping procedure, and adenosine, inosine, and hypoxanthine increased in every region of the kidney. In particular, the content of ATP decreased by 45% within a minute of ischemia, and the decrease reached 84% during 10 min ischemia. In the inner medulla, ATP did not decrease within a minute, and significant decline first became evident at 10 min after clipping. AMP increased in every region of the kidney during the ischemia. Energy charge value decreased in the whole kidney within a minute. Accumulation of adenosine in the OSOM disappeared after the clipping procedure, which was associated with the increase in adenosine in regions of the kidney except for the OSOM. ‐Inosine and hypoxanthine increased in every region of the kidney within a minute and in parallel to AMP, and the degrading changes in adenylates progressed during 10 min ischemia. Metabolome analysis revealed increase in xanthine and uric acid in the ischemic kidney. ‐In the reperfusion sections, restoration of ATP in the renal cortex and OSOM was not complete, and ATP showed a 24% decrease when compared with sham sections. In contrast, the restoration of ATP in the ISOM and inner medulla was sufficient. Total adenylates in the cortex and OSOM decreased after 24 h reperfusion, total adenylates were maintained in the ISOM and inner medulla: total adenylates and ATP in the cortex and OSOM after 24 h reperfusion demonstrated prolonged loss. The breakdown products of ATP were increased in the whole kidney by 10 min ischemia, almost recovered to the original content after 24 h reperfusion. A prolonged loss of ATP was significant after 10 min ischemia. ‐NADH showed a fivefold increase in all regions of the kidney subjected to 10 min ischemia. The increase in NADH in the cortex and OSOM persisted during 24 h of reperfusion, whereas it significantly decreased (vs. 10 min ischemia) in the ISOM and inner medulla. |
| Jouret et al. 2016 29 | Mouse |
The renal function was monitored by SCr and serum urea levels. IR‐exposed mice showed a significant increase in both AKI parameters. Note: no thresholds given. |
SCr (increase post‐IR, recover 48 h post‐IR) Serum urea (increase post‐IR, recover 48 h post‐IR) Lactate (urinary and tissue levels increase post‐IR) |
— |
Urine: ‐Urine levels of taurine, lactate, and glucose were steadily increased after IR, urine levels of trimethylamine were significantly reduced. ‐Pathways significantly affected by renal IR: gluconeogenesis and taurine/hypotaurine metabolism at 6 and 24 h reperfusion. Protein biosynthesis, glycolysis, and galactose and arginine metabolisms appeared essential at 48 h reperfusion. Allantoin increased 24 h post‐IR, but decreased 48 h after. Tissue: ‐Similar discriminations in tissue: changes in levels of lactate, fatty acids, choline, and taurine. ‐The identification of metabolites, whose increased abundance reached significance in loading plots included fatty acids (and modified lipoproteins), lactate, and N‐acetyl groups of glycoproteins. ‐Levels of taurine and myo‐inositol were decreased in kidneys from IR mice in comparison to sham animals. ‐Analysis of metabolites at 6 h and 24 h reperfusion: taurine/hypotaurine and betaine metabolisms were significantly affected by renal IR. ‐At 48 h postreperfusion, IR‐associated cascades were protein biosynthesis, biotin, and taurine/hypotaurine metabolism. Serum: Serum analysis could not discriminate sham operated from IR‐exposed animals. |
| Legouis et al. 2020 3 | Mouse | Not defined |
SCr (increase post‐IR) BUN (increase post‐IR) |
— | Mice exposed to severe IR injury displayed increased urea and creatinine levels, together with a decrease in glucose and increase in lactate serum levels at 48 h, whereas urinary glucose and lactate were unchanged except for one outlier. Blood lactate clearance was impaired in the IR group after intraperitoneal injection of sodium lactate. Increase in blood glucose following lactate injection was reduced in the IR group. |
| Poyan Mehr et al. 2018 30 | Mouse |
SCr was measured as a measure for renal function and postischemic injury. Note: no thresholds given. |
SCr (increase post‐IR) | — | A total of 204 metabolites measured, 27 were more than twofold increased in postischemic urines compared to controls including several sugars and amino acids, a pattern consistent with tubular impairment. Among these metabolites was quinolinate, an intermediate in the de novo NAD+biosynthetic pathway from tryptophan. Many other metabolites, including amino acids and acyl‐carnitines, were differentially regulated in urine of AKI mice. |
| Rao et al. 2016 31 | Mouse |
Renal injury confirmed by SCr levels and histology. Note: no thresholds given. |
SCr (increase post‐IR) | Histology |
Only measured lipids: ‐Four lipids were changed (all increases) to a statistically significant extent at 6 h after IR. Of these, three were identified as ether‐linked phospholipids (one an abundant phosphatidylcholine (PC): PC O−38:1, and two PEs: an abundant PE O−42:3 and a minor PE O−40:4). The two abundant ether lipids were as follows: PC O−38:1 (PC O−18:0, 20:1) and PE O−42:3 (PE O−20:1, 22:2). PC O−38:1 is a plasmanylcholine, while PE O−42:3 is a plasmalogen. ‐Many more lipids were changed to a statistically significant extent at 24 h after IR. The abundant PC O−38:1 remained elevated in IR kidneys at 24 h compared with the 24‐h sham group, the low‐abundance PE O−40:4 was present at comparably low levels in kidneys of both IR and sham mice at 24 h. PE O−42:3 was present at high levels but was decreased at 24 h compared with its sham control group, this lipid was increased relative to its sham control group at 6 h post‐IR. All ether‐linked PEs and PEs detected 24 h post‐IR were reduced with AKI. ‐No statistically significant differences in major hydroxyoctadeca dienoic acids and hydroxyeicosatetra enoic acids or linoleic and arachidonic acids were detected in kidneys of sham and IR animals at 6 h post‐IR. |
| Wei et al. 2014 32 | Mouse |
Renal function: statistically significant differences in SCr or BUN levels compared to sham condition. Note: no thresholds given. |
SCr (increase post‐IR, peak 48 h post‐IR, and recover 1 week post‐IR) BUN (increase post‐IR, peak 48 h post‐IR, and recover 1 week post‐IR) Lactate (tissue and serum levels decline post‐IR, but recover) |
— |
‐The changes started in renal cortex, followed by medulla and plasma. ‐Increased allantoin levels (specifically cortex, not medulla). ‐Elevated serum β‐hydroxybutyrate levels. ‐The kidney cortex and the plasma samples showed early decreases in glucose and lactate, but recovery to near‐sham levels by 1 week reperfusion time. ‐Some metabolites, such as 3‐indoxyl sulfate, were induced at the earliest time point of renal IR. ‐There was a notable switch of energy source from glucose to lipids. ‐Decreased polyols for osmotic regulation. ‐Several pathways involved in inflammation regulation were induced. ‐Late induction of prostaglandins. |
| Zager et al. 2014 33 | Mouse |
Severity of AKI was assessed by BUN and plasma creatinine concentrations and renal cortical NGAL mRNA levels. Note: no thresholds given |
SCr (increase post‐IR) BUN (increase post‐IR) |
— | Ischemia induced persistent pyruvate depletion. During ischemia, decreasing pyruvate levels correlated with increasing lactate levels. During early reperfusion, pyruvate levels remained depressed, but lactate levels fell below control levels. During late reperfusion, pyruvate depletion corresponded to increased gluconeogenesis (pyruvate consumption). |
| Pig | |||||
| Clendenen et al. 2019 34 | Pig | Not defined |
Lactate (serum levels increase post‐IR) SCr (increase post‐IR) |
— |
‐Lactate increased in response to IR. Glutamate accumulation with a serum increase of 2.7 times from baseline occurring exclusively after reperfusion. Hypoxanthine increased 4 times baseline. IR changed arginine, proline, creatine, and polyamine metabolism. Arginine decreased. Proline increased 1.1 times baseline following IR. Creatinine with increased 1.6 times baseline. Arginine consumption and accumulation of ornithine and polyamines (putrescine, spermidine, and spermine) were observed upon IR. Spermine increased 5 times baseline levels, with spermidine following a similar trend. Putrescine increased. ‐Small molecule metabolites involved in redox homeostasis (e.g., reduced glutathione—GSH, cysteine, carnosine, kynurenine, taurine, and hypotaurine) and, in general, metabolites involved in glutathione turnover (5‐oxoproline) or sulfur metabolism (taurine, hypotaurine, methionine, and GSH) were affected. Taurine increased upon reperfusion. No substantial increases in the post‐IR circulating levels of carnitine, tryptophan, and serotonin. |
| Fonouni et al. 2011 35 | Pig | Not defined |
SCr (no “substantial” differences) BUN (no “substantial” differences) Lactate (extracellular fluid levels peak post‐IR, but recover to baseline) |
— |
Baseline (BL) value = measured parameters in donors at the beginning of the graft procurement. Baseline: Glucose 0.56 mM, lactate 0.46 mM, pyruvate 12.17 µM, glutamate 19.75 mM, and glycerol 19.58 µM Procurement: Glucose increase to 1.11 mM, lactate increase 0.54 mM, pyruvate increase 28.03 µM then decrease, glutamate increase to ~40 mM, and glycerol similar to BL CIT: Glucose decrease to 0.23 mM, lactate decrease until halfway then increase to 0.35 mM, pyruvate short increase to 20.02 µM then decrease to 4.85 µM, glutamate increase to 82.60 mM, and glycerol increase at end to 54.76 µM WIT: Glucose decrease 40 min to 0.14 mM then sharp increase to 0.48 mM, lactate increase to 0.75 mM, pyruvate increase to 10.18 µM, glutamate increase to 131 mM, and glycerol increase to 118.22 µM Reperfusion: Glucose increase 40 min 1.47 mM then decrease to 0.73 mM, lactate increase 20 min to 1.07 mM then decrease to 0.58 mM, pyruvate increase 40 min to 29.97 µM then decrease to 17.80 µM, glutamate increase 20 min to 161.60 mM then decrease 40 min to 41.03 mM and then steady, and glycerol increase 40 min to 236.70 µM then decrease to 19.60 µM |
| Hauet et al. 2000 36 | Pig |
Death: acute renal failure confirmed by histological analysis. These results were associated with prolonged oliguria or anuria. Assessment of renal function: creatinine clearance and fractional excretion of Na+demonstrated reduced renal function. Note: no thresholds given. |
Creatinine clearance (decrease post‐IR, recover slightly) |
Survival Urinary NAG excretion (increase post‐IR, recover slightly) Fractional Na+ excretion (increase post‐IR, recover to near‐baseline) |
‐The urinary lactate/Cr ratio was significantly greater in the IR groups versus control. ‐Urinary citrate/Cr level was higher in control versus ischemia. |
| Malagrino et al. 2019 37 | Pig |
The animals showed changes characteristic of AKI: increased SCr, serum NGAL, fractional excretion of sodium, potassium, and chloride, and increased glucose and protein in urine. The most important result for the diagnosis of AKI was based on histological analysis, which showed acute tubular necrosis. An increase in nitrated protein in serum and urine was also observed. Note: no thresholds given. |
SCr (increase post‐IR) |
Histology Serum NGAL (increase post‐IR) Fractional Na+, K+, and Cl− excretion (increase post‐IR) Urinary glucose (increase post‐IR) Urinary protein (increase post‐IR) |
Serum: ‐Metabolites that showed a quick increase or decrease after 60 min of ischemia, followed by a progressive return to baseline after reperfusion: L‐glutamate, L‐serine, N‐isovaleroylglycine, L‐methionine, L‐proline, 2‐aminobutyrate, and choline. These metabolites discriminate between the preischemic and ischemia periods, as well as between the ischemic and 11 h postreperfusion periods. However, as they returned to basal levels, they do not discriminate between the preischemia and reperfusion periods Urine: Focus on urinary metabolites increased immediately after the reperfusion (0.5 h postreperfusion), the result of “washing” the ischemic kidney. A sharp increase or decrease in 0.5 h postreperfusion period compared with the preischemia period: 3‐hydroxybutyrate, 3‐hydroxyisovalerate, methylguanidine, 3‐aminoisobutyrate, trigonelline, betaine, glycerol, trimethylamine, carnosine, citrate, N‐phenylacethylglycine, pyruvate, and 1‐methylnicotinamide. These metabolites were able to discriminate between the 0.5 h postreperfusion and preischemia periods. Pathway and network analysis: The metabolites identified were overrepresented in the canonical pathways of amino acids degradation, lipid metabolism, molecular transport, small molecule biochemistry, cell cycle, cellular assembly, and organization. |
| Dog | |||||
| Maessen et al. 1989 38 | Dog | Posttransplant viability | — | Survival |
Energy metabolites measured: ATP, ADP, AMP, GTP, GDP, GMP, and IMP. CI with/without WI: −24 h CI: adenine nucleotide and guanine nucleotide contents decreased 30%. IMP increased. −48 h CI: no differences with 24 h CI. −30 min WI prior to CI: adenine and guanine nucleotide content already decreased before start of CI, increase in IMP. CI of 24 h after 30 min of WI did not affect content of adenine and guanine nucleotides, but IMP levels further increased. Prolonged CI to 48 h further decreased adenine and guanine nucleotide levels, while IMP did not show an additional increase. Reperfusion: 1 h of reperfusion after 24 or 48 h of CI resulted in increase in guanine nucleotide pool. The adenine nucleotides remained unaffected in the 24 h group, but dropped in the 48 h group. IMP levels were greatly reduced in both. When 30 min WI was applied prior to CI, some different patterns were observed. In WI groups, adenine nucleotide levels dropped in 24 h groups but remained unaffected in 48 h groups after 1 h reperfusion. The effect on guanine nucleotide and IMP levels did not differ from non‐WI groups. Following reperfusion, non‐WI groups showed higher (ATP+ADP)/AMP ratios. |
| Montañés et al. 1991 39 | Dog | Not defined |
Creatinine clearance (decrease post‐IR) BUN (increase post‐IR) Lactate (systemic serum levels similar post‐IR, kidney‐specific clearance decreased post‐IR) |
— |
Systemic blood: No significant changes in pH, bicarbonate, glutamine, glutamate, alanine, lactate, and pyruvate. Kidney specific (AV sampling): No significant changes in glutamine, glutamate, alanine, and pyruvate. Decreased lactate clearance. Renal cortex: Decreased glutamine, glutamate, ADP, AMP, and TAN. No significant changes in α‐ketoglutarate, aspartate, lactate, pyruvate, alanine, and ATP. Urine: Increased fractional excretion of lactate and pyruvate. |
Results are (partially) quoted or paraphrased from the texts.
Abbreviations: AA, amino acid; AC, acylcarnitine; AV, arteriovenous; BUN, blood urea nitrogen; CA, cardiac arrest; CPB, cardiopulmonary bypass; CI, cold ischemia; CIT, cold ischemia time; dATP, deoxyadenosine triphosphate; DGF, delayed graft function; FA, fatty acid; fDGF, functional DGF; FFA, free fatty acid; IR, ischemia reperfusion; ISOM, inner stripe of outer medulla; KIM‐1, kidney injury molecule‐1; NAG, N‐acetyl‐β‐D‐glucosaminidase; NGAL, neutrophil gelatinase‐associated lipocalin; OSOM, outer stripe of outer medulla; PC, phosphatidylcholine; PE, phosphatidylethanolamine; Post‐I, postischemia; Pre‐I, preischemia; PUFA, poly unsaturated fatty acids; SCr, serum creatinine; TAN, total adenine nucleotides; TMAO, trimethylamine oxide; WI, warm ischemia; WIT, warm ischemia time.
Additionally included study.
3.1. Measures of renal IR injury
Although some variability exists for the clinical definitions of AKI and DGF (Table 4), definitions are essentially functional and outcome centered. 40 , 41 The two landmark clinical studies that report the metabolome of, respectively, AKI and DGF both used “conservative” definitions. 3 , 4 The diagnosis of AKI was based on KDIGO criteria (Table 4), 41 and DGF was defined as the need for dialysis for at least the first week after transplantation. 40 Partial or full functional recovery is an inherent aspect of both clinical definitions.
TABLE 4.
An overview of the most commonly used definitions of delayed graft function (DGF) and acute kidney injury (AKI) in clinical practice based on Mallon et al. 40 and Shin et al. 42
| Delayed graft function 40 | |
|---|---|
| Most commonly used | Requirement for dialysis in the first postoperative week |
| Dialysis based | Requirement for dialysis in the first postoperative week excluding the first 24 h |
| Requirement for two or more episodes of dialysis in the first postoperative week | |
| Requirement for dialysis in the first 10 days postoperatively | |
| Functional (creatinine based) | Failure of a fall in serum creatinine of 10% on 3 consecutive days in the first postoperative week |
| Serum creatinine at postoperative day 7 >2.5 mg/dl ( = 221 µM) | |
| Serum creatinine at postoperative day 10 >2.5 mg/dl ( = 221 µM) | |
| Fall in ratio of serum creatinine of postoperative days 1 and 2 of at least 30% | |
| Combination | Dialysis in first week or failure of serum creatinine to fall in first 24 h |
| Dialysis in the first week or serum creatinine at postoperative day 7 >2.5 mg/dl ( = 221 µM) | |
| Acute kidney injury 42 | ||||||
|---|---|---|---|---|---|---|
| RIFLE classification | AKIN classification | KDIGO classification | All | |||
| Serum creatinine | Serum creatinine | Serum creatinine | Urine output | |||
| Risk | ≥1.5 times baseline, or ≥25% decrease in GFR | Stage 1 | ≥0.3 mg/dl (; =; 56.52 µM) increase, or ≥1.5 times baseline within 48 h | Stage 1 | ≥1.5–1.9 times baseline within 7 days, or ≥0.3 mg/dl ( = 56.52 µM) increase within 48 h | <0.5 mL/kg/h for >6 h |
| Injury | ≥2 times baseline, or ≥50% decrease in GFR | Stage 2 | ≥2 times baseline | Stage 2 | ≥2.0–2.9 times baseline within 7 days | <0.5 mL/kg/h for 12 h |
| Failure | ≥3 times baseline, increase to ≥4 mg/dl, or ≥75% decrease in GFR | Stage 3 | ≥3 times baseline, or increase to ≥4.0 mg/dl ( = 353.6 µM) with acute increase of >0.5 mg/dl ( = 44.2 µM), or initiation of RRT | Stage 3 | ≥3 times baseline within 7 days, or increase to ≥4.0 mg/dl ( = 353.6 µM) with acute increase of >0.5 mg/dl ( = 44.2 µM), or initiation of RRT | <0.3 mL/kg/h for 24 h, or anuria for >12 h |
Abbreviations: GFR, glomerular filtration rate; RRT, renal replacement therapy.
The majority (23/35) of the included preclinical studies used postreperfusion serum creatinine levels as a functional measure of IR injury (Table 3). In contrast to clinical definitions of AKI, no predefined thresholds for the diagnosis of IR injury were considered. None of the preclinical studies included the need for (transient) renal replacement therapy as outcome measure. A third of the studies (11/35) reported histological grading as surrogate outcome parameter (Table 3). Other parameters used were diverse: for example, body and/or kidney weight, and expression of the damage markers kidney injury molecule‐1 (KIM‐1) and/or neutrophil gelatinase‐associated lipocalin (NGAL).
The dynamics of recovery is an inherent aspect of the clinical diagnosis of renal IR injury (Table 4), but could not be properly addressed in the majority of experimental studies due to their limited follow‐up time (Tables 2/3). In fact, most preclinical studies (24/35) relied on follow‐up times of 24 hours or less, and in 11 studies the follow‐up time was even limited to 120 min or less. Only 10/35 studies reported a follow‐up of more than 24 hours, 9 of which addressed aspects of recovery, including 1 study that exclusively relied on histology as an end point. For one study, postreperfusion follow‐up time was not reported.
IR injury was induced by unilateral clamping with the functional, contralateral kidney left in place in 14/35 studies, while 11/35 applied bilateral clamping, 5/35 performed unilateral nephrectomy prior to IR, and 1 induced transient cardiac arrest. A minority of studies (4/35) applied kidney transplantation as a model of IR injury. Autotransplantation and contralateral nephrectomy following reimplantation were performed in two studies. One study transplanted an allograft in a bilaterally nephrectomized recipient, and one study did not report whether nephrectomy was performed.
3.2. Biomaterial sampling
Diverse sampling protocols (both with regard to the type of biomaterial collected and the timing of the sampling) are applied among the studies (Table 2). The majority of studies (24/27) that include blood sampling relied on peripheral blood samples (9/24 studies did not specify sampling location). Organ‐specific measurements using renal vein blood were performed in 3/27 studies: in rats, pigs, and dogs. One of the peripheral blood sampling studies performed kidney‐specific sampling through microdialysis. Single postreperfusion sampling was reported in 13/27 studies. All other studies concerned multiple sampling (two or more consecutive postreperfusion blood samples).
Eleven studies included urine samples. Urinary sampling was generally (6/11) achieved by means of metabolic cages, and consequently cumulative samples were reported. One porcine and dog study sampled directly from the bladder or ureter, respectively. Three studies did not specify the means of urine collection.
Most studies (17/28) that included tissue‐based analysis for metabolomic profiling or as a readout of injury were based on a single sampling point, both for smaller and larger animals. Studies reporting serial time points all relied on biopsies from separate animals. The majority of the studies (18/28) used whole organs, 10/28 (including two studies that also applied in vivo magnetic resonance imaging [MRI]) performed a region‐specific analysis (i.e., cortex and medulla).
A large variety of ex vivo and in vivo analysis platforms was used among the studies. Ex vivo analysis for metabolic strategies was diverse and included mass spectrometry and/or nuclear magnetic resonance (NMR)‐based platforms, as well as more traditional biochemical assays (gas/liquid chromatography and/or enzymatic techniques). In vivo measurements were performed in 4/35 studies (all rat), and concerned 1H‐MRI combined with hyperpolarized 13C‐MRI.
An extensive metabolomic profile was included in 16/35 studies. Other studies applied a focused (targeted) approach, and reported (sub‐)aspects of the metabolome, for example, exclusively amino acids, lipids, or high energy phosphates. For the sake of clarity, extracted data were clustered along the lines of metabolic competence (energy [high energy phosphate] and/or redox status), and the primary metabolic routes (glycolysis, tricarboxylic acid [TCA] cycle, fatty acid oxidation [β‐oxidation], and amino acids) to allow for comparison of metabolic signatures.
3.3. Metabolic outcomes
Metabolic aspects (i.e., reported contrasts between cases and controls) from each experimental study are summarized in a qualitative overview (Figure 2; quantitative data were categorized to minimize interference caused by differences in measurement techniques and normal values). Data regarding metabolic recovery were only available for studies reporting successive time points (Table 2).
FIGURE 2.
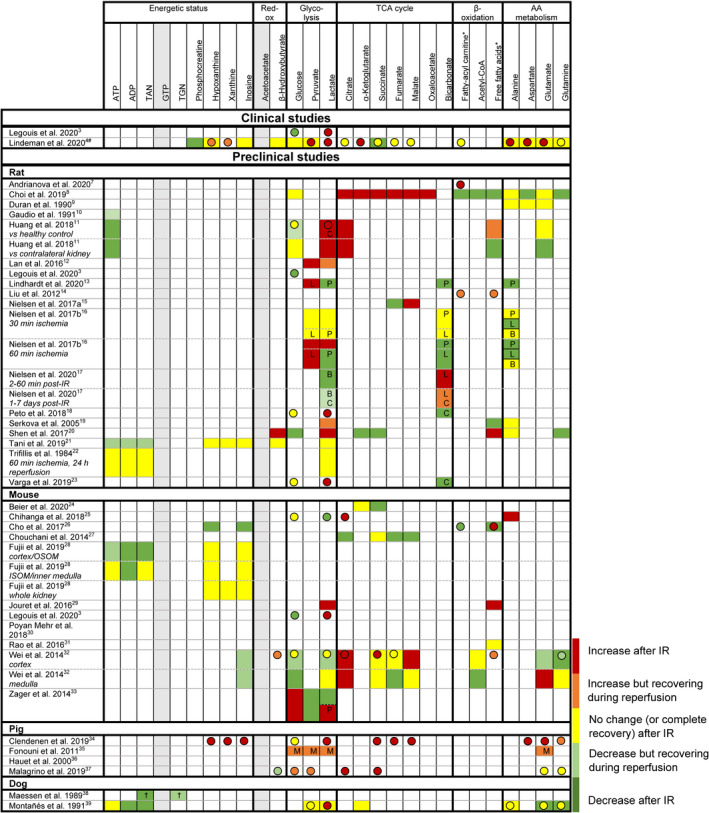
Relative differences in renal tissue (boxes) and blood (spheres, note: often systemic if no data on kidney‐specific samples) metabolite levels between control kidneys (healthy/intact control, sham‐surgery control, or contralateral kidney: see Table 2) and kidneys after ischemia reperfusion (IR). If no control data were available, data were excluded. Metabolites marked in grey were not reported for tissue or blood samples in any of the preclinical studies. Red = increase after IR. Orange = increase but recovering during reperfusion (requires multiple samples during reperfusion). Yellow = no change (or complete recovery) after IR. Light green = decrease but recovering during reperfusion (requires multiple samples during reperfusion). Green = decrease after IR. TAN: total adenine nucleotides (ATP+ADP+AMP). TGN: total guanine nucleotides (GTP+GDP+GMP). *: variety of fatty acids, results on overall trend. #: additional data on tissue levels are reported in the supplementary data, but no formal statistical evaluation was performed. OSOM: outer stripes of outer medulla. ISOM: inner stripes of outer medulla. †: ischemia time dependent. P: metabolite/pyruvate ratio. L: metabolite/lactate ratio. B: metabolite/bicarbonate ratio. C: in control (sham‐operated animal or contralateral kidney), metabolite levels showed similar dynamics following surgery. M: measured using microdialysis
Reported metabolic profiles for experimental IR injury are diffuse, and often incomplete (Figure 2). For example, the metabolic clues required to assess postreperfusion metabolic (in)competence (i.e., information on energy equivalents) were only available for a subset (10/35) of studies. Based on these studies, most (6/7) rodent studies and the dog studies indicate postreperfusion metabolic competence (i.e., recovery of high energy phosphates and/or absent release of products indicating ATP/GTP degeneration). Elevated levels of high energy phosphate breakdown products were reported in one pig study (5 min postreperfusion), but no information was available for later time points; it is therefore unclear whether this reflects postreperfusion washout or IR injury. 4 Reported aspects of the postreperfusion redox status (tissue acetoacetate/β‐hydroxybutyrate, or lactate/pyruvate ratio [plasma, serum, and dialysate]) and their dynamics following experimental IR vary substantially.
Tissue and blood glucose levels are generally reported to be decreased or recovering after experimental IR (Figure 2). Blood lactate levels are mostly increased following IR, while its tissue contents are reported variably. Among rodent studies, remarkable variations were observed in the dynamics of TCA cycle intermediates. 8 , 20 The limited data for porcine studies are relatively consistent, generally showing increased circulating levels of TCA intermediates following IR.
Aspects of β‐oxidation were only reported in a minority (n = 10) of rodent studies, and conclusions were variable (Figure 2). Similarly, reported aspects of amino acid metabolism and intermediates are limited and conclusions are variable: tissue levels are decreased or stable following IR in rats and dogs, while levels in murine tissues are highly variable.
4. DISCUSSION
Recent clinical leads for DGF and AKI point toward a universal discriminatory metabolic profile for, and possibly causal role of, metabolic aspects in renal IR injury. 3 , 4 This observation is remarkable considering the obvious mechanistic differences between DGF (warm and cold ischemia) and AKI (exclusively warm ischemia), and may imply a universal mechanism for clinical renal IR injury. It was reasoned that these observations provide a lead for validation of preclinical models of IR injury. A systematic review was performed to align the observed metabolic profiles for clinical context with those reported in preclinical studies. This review shows poor alignment of the reported preclinical metabolic data with clinical evidence, and identifies several critical methodological shortcomings in the preclinical studies.
While the phenomenon of IR injury has been known for over 50 years, a persistent translational gap in transforming the abundant preclinical therapeutic successes toward a clinical benefit remains. In fact, 10 years ago, partners in the NIH CAESAR consortium (in the context of myocardial IR injury) stated that: “for 40 years, the National Heart, Lung, and Blood Institute has invested enormous resources (at least several hundred million dollars) in preclinical studies aimed at developing infarct‐sparing therapies, and several hundred (if not thousands) therapies have been claimed to limit infarct size in preclinical models. Unfortunately, due largely to methodological problems, this enormous investment has not produced any notable clinical application." 1 Similar conclusions were expressed with respect to renal IR injury. 2 , 43
Two recent clinical studies positioned metabolic defects in the center of renal IR injury. 3 , 4 Although the conclusions of the studies do not allow discrimination between a causative mechanism or secondary defect, findings are fully discriminatory and provide insight in the early events of clinical IR injury, and imply a metabolic mechanism as driver of clinical IR injury. Alignment of the preclinical models with the clinical context identified a number of critical issues that may fundamentally interfere with translation of preclinical findings.
A first, fundamental aspect concerns the diagnosis/definition “IR injury.” Clinical studies not only identified a clear signature for IR injury but also indicated graded degrees of recovery in the absence of IR injury, that is, in the context of transplantation, an almost instantaneous functional and metabolic recovery for living donor grafts versus a more suspended recovery for deceased donor grafts (Figure 1). 4 Although less detailed information was available for AKI, different grades of AKI also associate with graded metabolic recoveries (e.g., KDIGO stage 3, demonstrating more severely impaired metabolism compared to less severe, i.e., KDIGO stage 1, injury). 3
Clinical IR injury (DGF) was accompanied by a fully discriminatory reperfusion metabolome, an aspect that allowed for discrimination between IR injury and IR damage. To be specific: IR injury is the injury triggered by the metabolic paralysis, with persistent ATP catabolism despite adequate reperfusion. IR damage, on the other hand, reflects tissue damage caused by entry of cellular and/or humoral effectors upon reestablishment of blood flow, processes that are independent from the metabolic cataplexy and may occur in response to an ischemic insult as well as IR injury.
A further aspect identified in the review were the notable variations in techniques applied to induce IR, ischemia times, timing of the postreperfusion sampling, and considerable heterogeneity concerning species, strains, and gender used. Considering the established interspecies, ‐strain, and sex variations in metabolism, the metabolic susceptibility, 44 , 45 , 46 , 47 and the impact of environmental factors on metabolic flexibility, 7 , 48 , 49 this heterogeneity may contribute to the compromised interchangeability of research findings.
Most (23/35) preclinical reports relied on serum creatinine as functional readout of kidney function/injury. Group‐wise comparisons (intervention vs. control) and single or short (≤24 hours) follow‐up times were generally applied. Consequently, appropriate ischemic controls and/or recovery profiles series required for discrimination between IR damage and injury were absent in preclinical studies. Moreover, conclusions with respect to creatinine clearance from preclinical studies that relied on cardiac arrest, bilateral clamping, or unilateral clamping/transplantation with unilateral nephrectomy are potentially interfered by a secondary prerenal kidney insufficiency resulting from a uremia‐induced somnolence with suppressed thirst reflex. Potential secondary prerenal kidney insufficiency is avoided in protocols applying unilateral clamping without contralateral nephrectomy (14/35 studies) (which obviously interferes with the use of creatinine clearance as readout) or possibly by renal replacement therapy (none of the identified studies). No study applied prespecified serum creatinine thresholds to grade the degree of injury, or corrected for over‐ or dehydration during or following surgery.
A broad palette of histological, plasma/serum, and/or urinary markers was used to further grade kidney injury. Since multiple evaluations concluded that histological examinations poorly predict outcome, 50 , 51 the question arises whether a strong reliance on histology is justified; in fact, one preclinical study reported IR injury based on serum creatinine levels in the absence of histological damage. 28
Reliance on, or the inclusion of, urinary samples in some studies is remarkable, considering that transient anuria is a key characteristic of clinical renal IR injury. Consequently, it is unlikely that the injury sustained in these studies reflects the degree of injury in clinical IR injury.
The majority of preclinical studies (23/27) that include plasma/serum measurements exclusively relied on peripheral samples for metabolic profiling (Table 2). Interpretation of this data is potentially interfered by the physiologic clearance mechanisms. 4 In fact, metabolic signals are fully absent in the peripheral (arterial) blood samples in the clinical setting, and selective postrenal venous sampling was required for distinctive signals. 4
Tissue metabolites are reported by 25/35 studies. Although metabolic information from tissue samples can be highly informative, this approach is only appropriate for non‐diffusible metabolites; a parallel evaluation of metabolic data from tissue biopsies and renal venous samples indicated washout of diffusible metabolic intermediates, with stable tissue contents. 4
Alignment of reported metabolic profiles identified contrasting findings between preclinical and clinical studies. While clinical IR injury (DGF) is characterized by energetic impairment that persists beyond the 30‐minute postreperfusion measurement window (Table 1), 4 reported data from rodent/dog studies imply reinstatement of energy equivalents following reperfusion. Hence, observations in these models align with the observations for grafts without IR injury.
Selective arteriovenous sampling identified normoxic glycolysis and impaired lactate handling as key characteristics of clinical renal IR injury (Table 1). 3 , 4 Only three experimental studies applied selective venous sampling, 3 , 34 , 39 but very limited metabolic analysis was performed.
A specific TCA cycle defect at the level of the oxoglutarate‐dehydrogenase complex with selective postreperfusion release of α‐ketoglutarate and impaired recovery of tissue succinate levels was identified in clinical IR injury (DGF) (Table 1). This finding contrasts with the metabolome of rodent IR injury, which is reportedly associated with an ischemic accumulation of succinate and its subsequent rapid re‐oxidation during reperfusion, paralleled by an oxidative burst. 27 The latter rapidly subsides upon normalization of succinate levels (within 5 min of reperfusion). 27 This brief insult contrasts with the prolonged, but reversible metabolic defects in clinical IR injury (Table 1, Figure 1). Although it cannot be excluded that a succinate‐driven mechanism contributes to the initiation of IR injury, the transient character and the apparent rapid metabolic recovery contrast with the clinical context.
In conclusion, this systematic evaluation based on reported metabolic aspects of IR injury demonstrates profound methodological variability and shortcomings in the preclinical studies. While some of these limitations are inherent to preclinical research, for example, overall interspecies differences and differences in resilience, most shortcomings can be circumvented. A key issue in preclinical studies is the inability to discriminate between IR damage and IR injury. Additional challenges in preclinical research are the strong impact of age and comorbidities on incidental AKI and DGF 52 , 53 , 54 (an aspect that is largely ignored in preclinical studies that generally use young and healthy animals), the need for kidney‐specific sampling, prolonged follow‐up, and bridging renal support to quantify the actual impact of IR. Finally, reported metabolic aspects are diffuse and often scattered. Consequently, studies to date do not allow for adequate metabolic phenotyping and/or comparison of outcomes. To fully capture the complex metabolic interactions, animal models are essential for research. Recommendations to improve the translatability of preclinical research are therefore summarized in Table 5 and Figure 3.
TABLE 5.
Recommendations for future preclinical studies investigating renal metabolism following ischemia reperfusion (IR)
| Recommendation | Rationale |
|---|---|
| Functional IR injury definition | Consensus on renal dysfunction |
| Renal replacement therapy | Exclusion of prerenal causes of renal IR injury |
| Optimal ischemia time (titration of IR injury) | Chance for both transient dysfunction, that is, DGF, and ischemic controls, that is, primary function |
| Representative study population | Higher incidence of clinical renal IR injury in older patients |
| Renal vein catheterization (excludes use of mice) | Arteriovenous kidney‐specific sampling; metabolite diffusion |
| Successive sampling | Monitoring dysfunction and functional recovery |
| Extended follow‐up time | Monitoring dysfunction and functional recovery |
| Predefined set of metabolites | Insight in metabolic competence and straightforward comparisons |
FIGURE 3.
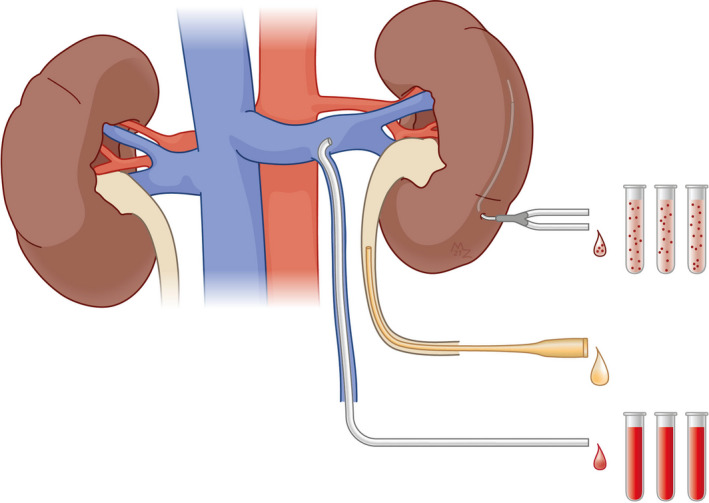
Recommendations to improve the translatability of preclinical models of renal ischemia reperfusion (IR) injury. Ischemic injury should be induced unilaterally in order to avoid interference caused by uremia. Appropriate discrimination between IR damage and IR injury critically relies on the organ‐specific assessment of metabolic competence (i.e., prolonged normoxic glycolysis, see outline in Figure 1). Kidney‐specific metabolic profiling of the injured kidney can be achieved through renal vein–specific blood sampling using the spermatic vein as access. 39 , 55 Microdialysis is a potential alternative to arteriovenous sampling, but has not yet been validated for this purpose. Ureterostomy 56 allows for selective functional monitoring of the injured kidney. The model critically relies on optimized ischemia times in order to achieve actual IR injury and avoid excess incidences of IR damage or non‐function. It is anticipated that successful implementation of these prerequisites relies on use of rats or larger laboratory animals. Illustration by Manon Zuurmond
This systemic review has some limitations. The available data are limited. Only two clinical studies are available, one concerning DGF and one on AKI. Although there is consensus that they both reflect renal IR injury, these are obviously distinct entities. Preclinical reports were extremely heterogeneous, hence no formal meta‐analysis could be performed. This study is kidney focused; given the organ‐specific difference in metabolism and metabolic rates, observations may not directly translate to other tissues.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge LUMC librarian Jan W. Schoones for his dedicated support concerning the development of the literature search queries.
Lerink LJS, de Kok MJC, Mulvey JF, et al. Preclinical models versus clinical renal ischemia reperfusion injury: A systematic review based on metabolic signatures. Am J Transplant. 2022;22:344–370. doi: 10.1111/ajt.16868
Lente J. S. Lerink and Michèle J. C. de Kok contributed equally.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lefer DJ, Bolli R. Development of an NIH Consortium for PreclinicAl AssESsment of CARdioprotective Therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther. 2011;16(3–4):332‐339. doi: 10.1177/1074248411414155 [DOI] [PubMed] [Google Scholar]
- 2. Cavaillé‐Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13(5):1134‐1148. doi: 10.1111/ajt.12210 [DOI] [PubMed] [Google Scholar]
- 3. Legouis D, Ricksten SE, Faivre A, et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab. 2020;2(8):732‐743. doi: 10.1038/s42255-020-0238-1 [DOI] [PubMed] [Google Scholar]
- 4. Lindeman JH, Wijermars LG, Kostidis S, et al. Results of an explorative clinical evaluation suggest immediate and persistent post‐reperfusion metabolic paralysis drives kidney ischemia reperfusion injury. Kidney Int. 2020;98(6):1476‐1488. doi: 10.1016/j.kint.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 5. Van Os S, De Abreu R, Hopman J, Wethly K, Liem D, Van De Bor M. Purine and pyrimidine metabolism and electrocortical brain activity during hypoxemia in near‐term lambs. Pediatr Res. 2004;55(6):1018‐1025. doi: 10.1203/01.PDR.0000125261.99069.D5 [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7): doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrianova NV, Popkov VA, Klimenko NS, et al. Microbiome‐metabolome signature of acute kidney injury. Metabolites. 2020;10(4):142. doi: 10.3390/metabo10040142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi J, Shoaib M, Yin T, et al. Tissue‐specific metabolic profiles after prolonged cardiac arrest reveal brain metabolome dysfunction predominantly after resuscitation. J Am Heart Assoc. 2019;8(17):e012809. doi: 10.1161/JAHA.119.012809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duran MA, Spencer D, Weise M, Kronfol NO, Spencer RF, Oken DE. Renal epithelial amino acid concentrations in mercury‐induced and postischemic acute renal failure. Toxicol Appl Pharmacol. 1990;105(2):183‐194. doi: 10.1016/0041-008x(90)90180-3 [DOI] [PubMed] [Google Scholar]
- 10. Gaudio KM, Thulin G, Ardito T, Kashgarian M, Siegel NJ. Redistribution of cellular energy following renal ischemia. Pediatr Nephrol. 1991;5(5):591‐596. doi: 10.1007/BF00856647 [DOI] [PubMed] [Google Scholar]
- 11. Huang H, van Dullemen LFA, Akhtar MZ, et al. Proteo‐metabolomics reveals compensation between ischemic and non‐injured contralateral kidneys after reperfusion. Sci Rep. 2018;8(1):8539. Published 2018 Jun 4. doi: 10.1038/s41598-018-26804-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lan R, Geng H, Singha PK, et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol. 2016;27(11):3356‐3367. doi: 10.1681/ASN.2015020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindhardt JL, Nielsen PM, Hansen ESS, et al. The hemodynamic and metabolic effects of spironolactone treatment in acute kidney injury assessed by hyperpolarized MRI. NMR Biomed. 2020;33(10):e4371. doi: 10.1002/nbm.4371 [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Yan S, Ji C, et al. Metabolomic changes and protective effect of (L)‐carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press Res. 2012;35(5):373‐381. doi: 10.1159/000336171 [DOI] [PubMed] [Google Scholar]
- 15. Nielsen PM, Eldirdiri A, Bertelsen LB, Jørgensen HS, Ardenkjaer‐Larsen JH, Laustsen C. Fumarase activity: an in vivo and in vitro biomarker for acute kidney injury. Sci Rep. 2017;7(1):40812. doi: 10.1038/srep40812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen PM, Laustsen C, Bertelsen LB, et al. In situ lactate dehydrogenase activity: a novel renal cortical imaging biomarker of tubular injury? Am J Physiol Renal Physiol. 2017;312(3):F465‐F473. doi: 10.1152/ajprenal.00561.2015 [DOI] [PubMed] [Google Scholar]
- 17. Nielsen PM, Qi H, Bertelsen LB, Laustsen C. Metabolic reprogramming associated with progression of renal ischemia reperfusion injury assessed with hyperpolarized [1‐13C]pyruvate. Sci Rep. 2020;10(1):8915. Published 2020 Jun 2. doi: 10.1038/s41598-020-65816-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peto K, Nemeth N, Mester A, et al. Hemorheological and metabolic consequences of renal ischemia‐reperfusion and their modulation by N, N‐dimethyl‐tryptamine on a rat model. Clin Hemorheol Microcirc. 2018;70(1):107‐117. doi: 10.3233/CH-170361 [DOI] [PubMed] [Google Scholar]
- 19. Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU. H‐NMR‐based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int. 2005;67(3):1142‐1151. doi: 10.1111/j.1523-1755.2005.00181.x [DOI] [PubMed] [Google Scholar]
- 20. Shen S, Wang JF, Wu JQ, et al. GC/MS‐based metabolomic analysis of alleviated renal ischemia‐reperfusion injury induced by remote ischemic preconditioning. Eur Rev Med Pharmacol Sci. 2017;21(4):765‐774. [PubMed] [Google Scholar]
- 21. Tani T, Okamoto K, Fujiwara M, Katayama A, Tsuruoka S. Metabolomics analysis elucidates unique influences on purine / pyrimidine metabolism by xanthine oxidoreductase inhibitors in a rat model of renal ischemia‐reperfusion injury. Mol Med. 2019;25(1):40. Published 2019 Aug 22. doi: 10.1186/s10020-019-0109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trifillis AL, Kahng MW, Cowley RA, Trump BF. Metabolic studies of postischemic acute renal failure in the rat. Exp Mol Pathol. 1984;40(2):155‐168. doi: 10.1016/0014-4800(84)90073-x [DOI] [PubMed] [Google Scholar]
- 23. Varga G, Ghanem S, Szabo B, et al. Renal ischemia‐reperfusion‐induced metabolic and micro‐rheological alterations and their modulation by remote organ ischemic preconditioning protocols in the rat. Clin Hemorheol Microcirc. 2019;71(2):225‐236. doi: 10.3233/CH-189414 [DOI] [PubMed] [Google Scholar]
- 24. Beier UH, Hartung EA, Concors S, et al. Tissue metabolic profiling shows that saccharopine accumulates during renal ischemic‐reperfusion injury, while kynurenine and itaconate accumulate in renal allograft rejection. Metabolomics. 2020;16(5):65. doi: 10.1007/s11306-020-01682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chihanga T, Ma Q, Nicholson JD, et al. NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia‐reperfusion injury. Am J Physiol Renal Physiol. 2018;314(2):F154‐F166. doi: 10.1152/ajprenal.00363.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho K, Min SI, Ahn S, et al. Integrative analysis of renal ischemia/reperfusion injury and remote ischemic preconditioning in mice. J Proteome Res. 2017;16(8):2877‐2886. doi: 10.1021/acs.jproteome.7b00167 [DOI] [PubMed] [Google Scholar]
- 27. Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431‐435. doi: 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujii K, Kubo A, Miyashita K, et al. Xanthine oxidase inhibitor ameliorates postischemic renal injury in mice by promoting resynthesis of adenine nucleotides. JCI Insight. 2019;4(22):1‐20. doi: 10.1172/jci.insight.124816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jouret F, Leenders J, Poma L, Defraigne JO, Krzesinski JM, de Tullio P. Nuclear magnetic resonance metabolomic profiling of mouse kidney, urine and serum following renal ischemia/reperfusion injury. PLoS ONE. 2016;11(9):e0163021. Published 2016 Sep 22. doi: 10.1371/journal.pone.0163021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poyan Mehr A, Tran MT, Ralto KM, et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018;24(9):1351‐1359. doi: 10.1038/s41591-018-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rao S, Walters KB, Wilson L, et al. Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. Am J Physiol Renal Physiol. 2016;310(10):F1136‐F1147. doi: 10.1152/ajprenal.00100.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei Q, Xiao X, Fogle P, Dong Z. Changes in metabolic profiles during acute kidney injury and recovery following ischemia/reperfusion. PLoS ONE. 2014;9(9):e106647. doi: 10.1371/journal.pone.0106647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zager RA, Johnson AC, Becker K. Renal cortical pyruvate depletion during AKI. J Am Soc Nephrol. 2014;25(5):998‐1012. doi: 10.1681/ASN.2013070791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clendenen N, Nunns GR, Moore EE, et al. Selective organ ischaemia/reperfusion identifies liver as the key driver of the post‐injury plasma metabolome derangements. Blood Transfus. 2019;17(5):347‐356. doi: 10.2450/2018.0188-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fonouni H, Esmaeilzadeh M, Jarahian P, et al. Early detection of metabolic changes using microdialysis during and after experimental kidney transplantation in a porcine model. Surg Innov. 2011;18(4):321‐328. doi: 10.1177/1553350610392063 [DOI] [PubMed] [Google Scholar]
- 36. Hauet T, Baumert H, Gibelin H, et al. Noninvasive monitoring of citrate, acetate, lactate, and renal medullary osmolyte excretion in urine as biomarkers of exposure to ischemic reperfusion injury. Cryobiology. 2000;41(4):280‐291. doi: 10.1006/cryo.2000.2291 [DOI] [PubMed] [Google Scholar]
- 37. Malagrino PA, Venturini G, Yogi PS, et al. Metabolomic characterization of renal ischemia and reperfusion in a swine model. Life Sci. 2016;156:57‐67. doi: 10.1016/j.lfs.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 38. Maessen JG, van der Vusse GJ, Vork M, Kootstra G. The beneficial effect of intermediate normothermic perfusion during cold storage of ischemically injured kidneys. A study of renal nucleotide homeostasis during hypothermia in the dog. Transplantation. 1989;47(3):409‐414. doi: 10.1097/00007890-198903000-00001 [DOI] [PubMed] [Google Scholar]
- 39. Montañés I, Badía A, Réngel MA, López‐Novoa JM. Renal cortical intermediary metabolism in the recovery phase of postischemic acute renal failure in the dog. Proc Soc Exp Biol Med. 1992;199(3):321‐326. doi: 10.3181/00379727-199-43363 [DOI] [PubMed] [Google Scholar]
- 40. Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. 2013;96(10):885‐889. doi: 10.1097/TP.0b013e3182a19348 [DOI] [PubMed] [Google Scholar]
- 41. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949‐1964. doi: 10.1016/S0140-6736(19)32563-2 [DOI] [PubMed] [Google Scholar]
- 42. Shin SR, Kim WH, Kim DJ, Shin IW, Sohn JT. Prediction and prevention of acute kidney injury after cardiac surgery. Biomed Res Int. 2016;2016: doi: 10.1155/2016/2985148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saat TC, van den Akker EK, IJzermans JNM, Dor FJMF, de Bruin RWF. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med. 2016;14(1): doi: 10.1186/s12967-016-0767-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montgomery MK, Fiveash CE, Braude JP, et al. Disparate metabolic response to fructose feeding between different mouse strains. Sci Rep. 2015;5(1):18474. doi: 10.1038/srep18474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleischer S, Sharkey M, Mealey K, Ostrander EA, Martinez M. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008;10(1):110‐119. doi: 10.1208/s12248-008-9011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strohl KP, Thomas AJ, St. Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82(1):317‐323. doi: 10.1152/jappl.1997.82.1.317 [DOI] [PubMed] [Google Scholar]
- 47. Reifsnyder PC, Te A, Harrison DE. Differential effects of rapamycin on glucose metabolism in nine inbred strains. J Gerontol: Series A. 2020;75(1):50‐57. doi: 10.1093/gerona/glz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short‐ and long‐term pathophysiological consequences. J Nutr. 2013;143(5):553‐562. doi: 10.3945/jn.112.172825 [DOI] [PubMed] [Google Scholar]
- 49. Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39(4):489‐517. doi: 10.1210/er.2017-00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am J Transplant. 2015;15(7):1903‐1914. doi: 10.1111/ajt.13213 [DOI] [PubMed] [Google Scholar]
- 51. Hall IE, Reese PP, Weng FL, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol. 2014;9(3):573‐582. doi: 10.2215/CJN.08270813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407‐420. doi: 10.1681/ASN.2015121308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279‐2286. doi: 10.1111/j.1600-6143.2010.03179.x [DOI] [PubMed] [Google Scholar]
- 54. da Cruz PRC, Filho ACD, Santana VBBM, Boaretto RBB, Riccetto CLZ. Donor age amplifies the detrimental effects of cold ischemia time on long‐term kidney allograft survival independently of the occurrence of delayed graft function or early acute rejection. Exp Clin Transplant. 2020;18(4):436‐443. doi: 10.6002/ect.2020.0066 [DOI] [PubMed] [Google Scholar]
- 55. Suzuki Y, Eto T. Androgens in testicular venous blood in the adult rat. Endocrinol Jpn. 1962;9:277‐283. doi: 10.1507/endocrj1954.9.277 [DOI] [PubMed] [Google Scholar]
- 56. Wang T, Yu Z, Chen C, et al. Ureteral anastomosis with a polyimide stent in rat kidney transplantation. Ren Fail. 2020;42(1):193‐199. doi: 10.1080/0886022X.2020.1726386 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


