Summary
There is currently considerable interest in the prospects for bioengineering crassulacean acid metabolism (CAM) photosynthesis – or key elements associated with it, such as increased water‐use efficiency – into C3 plants. Resolving how CAM photosynthesis evolved from the ancestral C3 pathway could provide valuable insights into the targets for such bioengineering efforts. It has been proposed that the ability to accumulate organic acids at night may be common among C3 plants, and that the transition to CAM might simply require enhancement of pre‐existing fluxes, without the need for changes in circadian or diurnal regulation. We show, in a survey encompassing 40 families of vascular plants, that nocturnal acidification is a feature entirely restricted to CAM species. Although many C3 species can synthesize malate during the light period, we argue that the switch to night‐time malic acid accumulation requires a fundamental metabolic reprogramming that couples glycolytic breakdown of storage carbohydrate to the process of net dark CO2 fixation. This central element of the CAM pathway, even when expressed at a low level, represents a biochemical capability not seen in C3 plants, and so is better regarded as a discrete evolutionary innovation than as part of a metabolic continuum between C3 and CAM.
Keywords: C3 photosynthesis, CAM photosynthesis, carboxylate, citrate, crassulacean acid metabolism, malate, malic acid, titratable acidity
Introduction
Crassulacean acid metabolism (CAM) and C4 photosynthesis represent the two modifications of the basic C3 pathway of CO2 assimilation frequently found in vascular plants, especially those associated with warmer and/or more water‐limited environments. Crassulacean acid metabolism and C4 photosynthesis are complex genetic traits, but both have arisen independently multiple times in evolution, now being found in an estimated 10% of vascular plants in total. As our understanding of the metabolic and molecular‐genetic basis of these traits has developed, interest has grown in the prospect of bioengineering these pathways – or key elements of them – into C3 crop plants to enhance their productivity and stress tolerance (e.g. Borland et al., 2014; Lim et al., 2019; Töpfer et al., 2020; Schiller & Bräutigam, 2021). This requires a detailed understanding of the core components of the respective pathways and the genetic architecture of their regulation. Progress towards these goals is being aided by recent findings from comparative evolutionary studies, whole‐genome sequencing and detailed molecular‐genetic studies of tractable model species.
Crassulacean acid metabolism plants, in particular, have long been attractive targets for studies of drought tolerance in semiarid and water‐limited habitats. Their ability to fix CO2 at night and to close their stomata during the day – associated with the accumulation and remobilization, respectively, of vacuolar malic acid – results in high water‐use efficiency and resilience to episodic drought. Many CAM plants are also phenotypically plastic, fixing considerable amounts of CO2 during the daytime when conditions are favourable, but restricting gas exchange to the night‐time under increasing water limitation. This continuous spectrum of phenotypes has led to recent suggestions that the evolution of CAM might merely require the upregulation of a low‐level acidification–deacidification cycle already present in C3 plants, and moreover that the circadian and diurnal rhythms in CAM plants are probably similar to those in C3 plants (Bräutigam et al., 2017; Schiller & Bräutigam, 2021). However, we believe this scenario overlooks the fundamental ways in which vacuolar acidification and CO2 metabolism differ in CAM plants and C3 plants, and in so doing fails to recognize the metabolic reprogramming required for the evolutionary transition from C3 to CAM. Here, we highlight the defining characteristics of the day–night cycle of carbon fixation and acid metabolism in CAM plants, describe how these contrast with C3 plants, and demonstrate that diel changes in titratable acidity are entirely lacking in a wide range of C3 species. Our results are not consistent with the existence of a low‐level CAM cycle in C3 plants and serve to highlight the discrete nature of the evolutionary innovation involved in the acquisition of CAM photosynthesis.
The CAM cycle
In essence, the CAM cycle consists of two sequential metabolic components occurring in the chlorenchymatous mesophyll cells of the leaves or stem that are temporally separated across the 24 h diel cycle, as summarized schematically in Fig. 1(a).
At night, atmospheric CO2 is fixed, following its conversion to by carbonic anhydrase, via the cytosolic enzyme phosphoenolpyruvate (PEP) carboxylase (PEPC). The three‐carbon acceptor (PEP) for this reaction is supplied by glycolytic breakdown of storage carbohydrate (either chloroplastic starch/glucan or vacuolar hexose, depending on the plant species), initially forming oxaloacetate, which is immediately reduced to malate. To remove it from its site of synthesis, this cytosolic malate is transported into the large central vacuole of the mesophyll cells in a process energized by one or both of the two tonoplast‐localized H+ pumps, the V‐ATPase and the V‐PPiase. The net result is vacuolar accumulation of malic acid, as each mol of malate accumulated can be shown by titration to be balanced by 2 mol H+ (Lüttge & Ball, 1980), a stoichiometry observed even in the submerged aquatic lycopod Isoëtes (Keeley, 1998), believed to be the earliest‐diverging CAM lineage. Sustained activity of the tonoplast H+ pumps during the night is essential to maintain cytoplasmic pH within physiological bounds in the face of a substantial acid load. For example, for a Kalanchoë leaf accumulating malic acid at an average rate of 10 mM h−1 (e.g. Lüttge et al., 1981), in mesophyll cells consisting of c. 97% vacuole and 1% cytosol by volume (Steudle et al., 1980), and assuming a typical cytoplasmic buffering capacity of 30 mmol H+ l−1 (pH unit)−1 (Kurkdjian & Guern, 1989), cytosolic pH would drop by 1 pH unit in just under a minute (54 s) in the absence of any transtonoplast H+ pumping. By the end of the night, the vacuolar sap in these assimilatory cells can reach pH 4 or even lower.
During the following daytime, the nocturnally accumulated malic acid is released from the vacuole and decarboxylated in the cytoplasm, either by NAD(P)‐malic enzyme acting in concert with pyruvate, Pi dikinase, or by PEP carboxykinase (again depending on the plant species). During the main phase of malate decarboxylation, the liberated CO2 accumulates to high internal concentrations behind closed stomata and is refixed by Rubisco in the Calvin–Benson–Bassham cycle, with the remaining three‐carbon moiety (pyruvate or PEP, respectively) being recycled back into the pool of storage carbohydrate via gluconeogenesis. Once the decarboxylation phase has finished, and environmental conditions permitting (e.g. favourable water status), the stomata may open in the latter part of the day and the plant engages in normal C3 photosynthesis, which contributes to the phenotypic plasticity in gas‐exchange patterns seen in many CAM species.
Fig. 1.
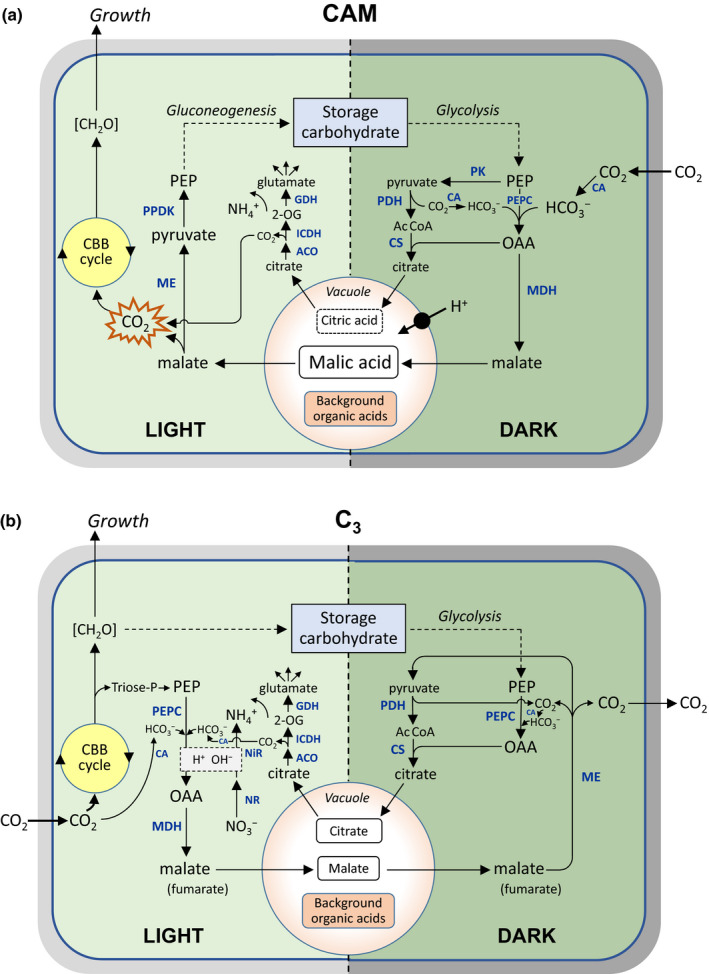
Principal day–night carbon fluxes and associated carboxylate metabolism in photosynthetic mesophyll cells, highlighting similarities and differences between crassulacean acid metabolism (CAM) plants and C3 plants. The schemes are modelled on the format used by Schiller & Bräutigam (2021) and show steady‐state operation of the cycles over the 24 h period. For simplicity, details of the energetics of the pathways, subcellular compartmentation and charges (dissociation states) of the metabolites have been omitted, but further information can be found in Winter & Smith (1996a); Holtum et al. (2005) and Shameer et al. (2018). Additional internal sources of CO2, such as the tricarboxylic acid (TCA) cycle, oxidative pentose phosphate pathway and photorespiration, are not shown explicitly. The dashed lines to and from storage carbohydrate indicate multi‐step pathways. The central vacuole is shown schematically, but in mature mesophyll cells can typically occupy ≥ 90% of the cell volume. (a) In CAM plants, net nocturnal CO2 fixation via phosphoenolpyruvate (PEP) carboxylase (PEPC) results in vacuolar accumulation of malic acid (2 H+ per malate); this is mobilized in the following daytime and decarboxylated (either via NAD(P)‐ME, as shown, or in some CAM plants via PEP carboxykinase), with the CO2 released behind closed stomata at high concentration being refixed by Rubisco in the Calvin–Benson–Bassham (CBB) cycle. In some CAM plants there is significant nocturnal synthesis and vacuolar accumulation of citric acid: this is not associated with net fixation of CO2, but on remobilization in the following daytime, the citrate supplies carbon skeletons for assimilation via glutamate dehydrogenase (GDH) and glutamine synthetase (the latter not shown explicitly). Nocturnal acid accumulation requires net influx of H+ into the vacuole, which is driven by the tonoplast H+ pump(s) (see main text). The vacuole also contains a background pool of organic acids that does not oscillate during the day–night cycle. Carbon skeletons required to support nocturnal acid accumulation are provided by glycolytic breakdown of storage carbohydrate (usually chloroplastic starch/glucan, but significant vacuolar hexose in some species), which is regenerated by gluconeogenesis in the light period. (b) In C3 plants, citrate synthesis and vacuolar accumulation also occur at night, but malate accumulation (and fumarate in some species) typically occurs during the daytime, which provides charge‐ and pH‐balancing for light‐dependent nitrate assimilation (light grey box). In some plants such as Arabidopsis thaliana, citrate and malate fluxes can be of similar magnitude, as shown here to achieve flux balance across the vacuolar membrane, but diel carboxylate accumulation and remobilization are not charge‐balanced by H+ and so do not result in vacuolar pH changes during the day–night cycle (see Table 1). AcCoA, acetyl‐CoA; ACO, aconitase; CA, carbonic anhydrase; [CH2O], sugars/carbohydrate; CS, citrate synthase; ICDH, isocitrate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; NiR, nitrite reductase; NR, nitrate reductase; OAA, oxaloacetate; 2‐OG, 2‐oxoglutarate; PDH, pyruvate dehydrogenase; PK, pyruvate kinase; PPDK, pyruvate, Pi dikinase.
The primary benefits of the CAM pathway are seen as two‐fold. First, when the initial carboxylation reaction is mediated by PEPC, CAM plants benefit (as do C4 plants) from the favourable kinetic properties of this enzyme compared with Rubisco (namely a higher effective affinity for CO2, a faster turnover number, and lack of an oxygenase activity); additionally, because PEPC in CAM plants is active at night, this means that the transpirational water loss associated with uptake of atmospheric CO2 is even further reduced. And second, the high internal CO2 concentration generated during the decarboxylation phase while stomata are closed helps to ensure that the carboxylase activity of Rubisco is maximized and its photorespiratory oxygenase minimized. These benefits come with an additional metabolic cost, mainly incurred in energizing vacuolar malic acid accumulation at night and gluconeogenesis during the day, but CAM plants generally grow in high‐light environments and so are not usually photon‐limited (Lüttge et al., 1981; Winter & Smith, 1996a; Cheung et al., 2014). Underpinning the entire CAM rhythm is thus the diel cycle of malic acid accumulation and degradation, the manifestation of which was memorably captured in the early report by Heyne (1815), who noted that: ‘The leaves of the Cotyledon calycina [Kalanchoë pinnata] … are in the morning as acid as sorrel, if not more so; as the day advances, they lose their acidity, and are tasteless about noon.’
It is worth emphasizing that net nocturnal fixation of CO2 by CAM plants can be shown – under optimal conditions with minimal recycling of respiratory CO2 – to be stoichiometrically linked to the accumulation of malic acid (e.g. Medina & Osmond, 1981). Downregulation of mitochondrial NAD‐malic enzyme activity at night in CAM plants will help to minimize futile cycling of malate synthesized via PEPC, and presumably will also mean that most respiratory substrate supplied by glycolysis feeds into the tricarboxylic acid (TCA) cycle through pyruvate kinase. Suggestions have occasionally been made for the involvement of other acids in the CAM cycle, most notably citric acid, which accumulates at night in significant amounts in some CAM species (Lüttge, 1988). However, nocturnal citric acid accumulation is not associated with net fixation of CO2, as provision of acetyl‐CoA for the citrate synthase reaction via oxidative decarboxylation of pyruvate itself releases CO2. The other major vacuolar carboxylate anion found in some CAM plants is isocitrate, but this is primarily involved in charge‐balancing inorganic cations and generally does not fluctuate significantly during the day–night cycle (Vickery, 1952; Milburn et al., 1968; Phillips, 1980; Ceusters et al., 2019). Other hypothetical schemes that might account for malate synthesis in CAM plants, such as operation of a glyoxylate cycle (Tcherkez, 2017) or a reverse citric acid cycle (Töpfer et al., 2020), have not so far received empirical support. The overwhelming body of evidence is consistent with net dark CO2 fixation in CAM plants being mediated via the canonical pathway of malic acid accumulation and degradation proposed in the late 1940s.
Do C3 plants accumulate organic acids at night?
The notion that nocturnal acid accumulation is a unique feature of CAM photosynthesis has been challenged in two recent publications by Bräutigam et al. (2017) and Schiller & Bräutigam (2021). In considering the evolutionary changes necessary for the emergence of CAM, they argue that, because ‘C3 plants store organic acids at night to fuel daytime amino acid synthesis … the framework of the CAM cycle actually is already in place in C3 species and … it is not a question of rewiring metabolism but of selecting for increased flux’ (Bräutigam et al., 2017). Indeed, the flow diagrams presented by Bräutigam et al. (2017) depicting night‐time and daytime metabolite fluxes in C3 plants and CAM plants are essentially identical, both showing nocturnal storage of malate and citrate, and differing only in the greater magnitude of the fluxes involved in CAM plants. The authors go on to propose that, because there is ‘a continuum from C3 photosynthesis to CAM … [there are] likely no general barriers to trait engineering’ (Schiller & Bräutigam, 2021). However, we believe this interpretation overlooks the fundamental differences between organic acid metabolism in C3 and CAM plants, is not consistent with a substantial body of experimental evidence, and may deflect attention away from the prime targets of metabolic engineering needed in order to express CAM‐like traits in C3 plants.
We initially address the assertion that C3 plants can reversibly accumulate organic acids in the vacuole at night, which, if true, would invalidate the use of nocturnal acidification as a diagnostic criterion for CAM. The term ‘organic acids’ is often used loosely in the literature when referring to carboxylate metabolism in plant cells, but the nature of the cation involved in charge‐balancing the carboxylate anion is important, as this has profound implications for pH regulation and ion homeostasis. In CAM, as explained earlier, the night‐time acidification resulting from net CO2 fixation is mechanistically attributable to malic acid (even if some species show significant accumulation of citric acid as well), but this differs in two important respects from the situation in C3 plants, as summarized in Fig. 1(b). First, while C3 species such as Arabidopsis may show nocturnal accumulation of citrate, there is no evidence in C3 plants for nocturnal accumulation of malate (which, by contrast, tends to accumulate during the daytime); and second, this process does not involve the free acid (citric acid), as in CAM plants, but rather the conjugate base (the citrate anion). We shall return to the question of the contrasting diel rhythms in malate content seen in CAM plants and C3 plants, but will consider first the issue of acid accumulation and its implications for cell metabolism and pH regulation.
The vacuolar sap of plant cells is typically acidic on account of its substantial content of hydroxycarboxylic acids, and there are some well‐known examples of extreme acid accumulation in C3 plants – such as oxalic acid in leaves of Begonia, Oxalis and sorrel (Rumex acetosa), and citric acid in Citrus fruits – that can result in vacuolar pH values as low as 2 (Bennet‐Clark, 1933; Hurd‐Karrer, 1939). But in most plants the vacuolar carboxylate anions are largely charge‐balanced by the strong inorganic cations K+, Na+, Ca2+ and Mg2+, with the result that the vacuolar contents are only mildly acidic, with free H+ concentrations in the micromolar range (pH 5–6). Moreover, there do not appear to have been any reports that vacuolar pH in C3 plants changes during the day–night cycle in a manner similar to CAM plants, as would be the case if C3 plants could reversibly store organic acids in the vacuole at night (Bräutigam et al., 2017).
To test for the possibility that nocturnal acidification is a widespread phenomenon in C3 plants that has been previously overlooked, we conducted a macroevolutionary common‐garden experiment with 70 species of vascular plants representing 40 families growing under field conditions in Panama (Table 1). Measurements of titratable acidity were made on tissue extracts sampled at the end of the dark period and the end of the light period, a technique capable of resolving differences in dawn–dusk acidity of the order of 1 mmol H+ kg−1 fresh mass (the smallest statistically significant dawn–dusk difference in acidity demonstrated to date appears to be 1.7 mmol H+ kg−1 fresh mass in two orchid species, Coryanthes hunteriana and Oncidium schroederianum; Silvera et al., 2005.) All species known to exhibit CAM (30 species from 14 different families) showed nocturnal acidification, to different degrees, with dawn–dusk differences in titratable acidity ranging from 452 mmol H+ kg−1 in Clusia uvitana to 7.9 mmol H+ kg−1 in Werauhia sanguinolenta. By contrast, none of the C3 plants tested (40 species from 31 different families) showed a significant difference in dawn–dusk acidity (Table 1). Several C3 species (notably Clusia spp.) had high values of tissue acidity, but did not show diel fluctuations. These results do not support the notion that nocturnal increases in organic acid content are a common feature of C3 plants, and confirm that nocturnal acidification can be regarded as a hallmark of CAM plants.
Table 1.
Titratable acidity measured at dusk and dawn in the photosynthetic tissues of 70 species of vascular plants: (a) species showing a statistically significant nocturnal increase in titratable acidity (for which dawn values are indicated in bold); species are ranked in descending order of dawn–dusk difference in acidity; (b) species showing no significant difference in dawn–dusk titratable acidity; species are arranged according to the phylogenetic position of their family (for angiosperms; APG IV, 2016).
| No. | Species | Family | Titratable acidity (mmol H+ kg−1 fresh mass) | |
|---|---|---|---|---|
| Dusk | Dawn | |||
| (a) Species with significant nocturnal H+ increase | ||||
| 1. | Clusia uvitana Pittier | Clusiaceae | 27.4 ± 11.0 | 479.7 ± 23.9 |
| 2. | Clusia minor L. | Clusiaceae | 46.2 ± 30.3 | 436.5 ± 51.2 |
| 3. | Furcraea cabuya Trel. | Asparagaceae | 22.9 ± 4.1 | 401.0 ± 28.5 |
| 4. | Alluaudia humbertii Choux | Didiereaceae | 93.8 ± 5.7 | 457.6 ± 20.7 |
| 5. | Clusia rosea Jacq. | Clusiaceae | 127.2 ± 26.0 | 454.0 ± 85.1 |
| 6. | Agave americana L. | Asparagaceae | 18.1 ± 4.6 | 331.1 ± 28.9 |
| 7. | Clusia pratensis Seem. | Clusiaceae | 99.2 ± 67.8 | 386.9 ± 126.6 |
| 8. | Kalanchoë pinnata (Lam.) Pers. | Crassulaceae | 15.6 ± 3.9 | 283.5 ± 14.6 |
| 9. | Agave angustifolia Haw. | Asparagaceae | 9.2 ± 2.3 | 273.1 ± 16.2 |
| 10. | Epiphyllum phyllanthus (L.) Haw. | Cactaceae | 8.1 ± 3.4 | 239.8 ± 11.8 |
| 11. | Kalanchoë daigremontiana Raym.‐Hamet & H.Perrier | Crassulaceae | 17.8 ± 6.4 | 234.8 ± 22.8 |
| 12. | Ananas comosus (L.) Merr. | Bromeliaceae | 10.9 ± 2.9 | 206.4 ± 33.6 |
| 13. | Tillandsia elongata Kunth | Bromeliaceae | 9.1 ± 1.2 | 203.3 ± 38.1 |
| 14. | Clusia fructiangusta Cuatrec. | Clusiaceae | 36.5 ± 11.3 | 217.3 ± 45.2 |
| 15. | Tillandsia flexuosa Sw. | Bromeliaceae | 10.6 ± 2.2 | 173.8 ± 64.9 |
| 16. | Xerosicyos danguyi Humbert | Cucurbitaceae | 7.9 ± 3.7 | 164.2 ± 6.2 |
| 17. | Sansevieria zeylanica (L.) Willd. | Asparagaceae | 13.0 ± 4.4 | 157.2 ± 30.9 |
| 18. | Coleus amboinicus Lour. | Lamiaceae | 6.3 ± 1.6 | 118.6 ± 5.7 |
| 19. | Clusia peninsulae Hammel | Clusiaceae | 248.4 ± 84.1 | 355.0 ± 64.0 |
| 20. | Vanilla pompona Schiede | Orchidaceae | 19.9 ± 2.1 | 125.9 ± 3.0 |
| 21. | Opuntia cochenillifera (L.) Mill. | Cactaceae | 8.4 ± 0.7 | 110.2 ± 18.6 |
| 22. | Crassula ovata (Mill.) Druce | Crassulaceae | 11.0 ± 2.8 | 99.5 ± 13.6 |
| 23. | Pyrrosia longifolia (Burm.f.) Morton | Polypodiaceae | 21.6 ± 2.2 | 107.8 ± 12.3 |
| 24. | Talinum fruticosum (L.) Juss. | Talinaceae | 3.6 ± 1.4 | 82.1 ± 13.8 |
| 25. | Clusia quadrangula Bartlett | Clusiaceae | 125.6 ± 19.3 | 171.5 ± 20.1 |
| 26. | Aloe vera (L.) Burm.f. | Asphodelaceae | 8.5 ± 0.8 | 48.1 ± 5.5 |
| 27. | Opuntia ficus‐indica (L.) Mill. | Cactaceae | 12.4 ± 1.2 | 40.3 ± 6.3 |
| 28. | Basella alba L. | Basellaceae | 9.2 ± 1.1 | 29.9 ± 1.5 |
| 29. | Pilea peperomioides Diels | Urticaceae | 10.5 ± 7.5 | 23.2 ± 5.4 |
| 30. | Werauhia sanguinolenta (Cogn. & Marchal) J.R.Grant | Bromeliaceae | 8.0 ± 1.0 | 15.9 ± 5.3 |
| (b) Species without significant nocturnal H+ increase | ||||
| 31. | Zamia furfuracea L.f. ex Aiton | Zamiaceae | 19.7 ± 1.3 | 20.7 ± 1.1 |
| 32. | Gnetum leyboldii Tul. | Gnetaceae | 10.4 ± 1.4 | 9.7 ± 1.0 |
| 33. | Piper arboreum Aubl. | Piperaceae | 19.0 ± 2.1 | 19.8 ± 2.5 |
| 34. | Piper marginatum Jacq. | Piperaceae | 13.8 ± 1.8 | 14.5 ± 3.9 |
| 35. | Persea americana Mill. | Lauraceae | 13.2 ± 0.7 | 13.7 ± 0.7 |
| 36. | Monstera deliciosa Liebm. | Araceae | 17.9 ± 3.2 | 13.8 ± 4.0 |
| 37. | Monstera sp. | Araceae | 20.6 ± 0.9 | 18.7 ± 3.6 |
| 38. | Hymenocallis littoralis (Jacq.) Salisb. | Amaryllidaceae | 6.1 ± 2.3 | 5.7 ± 1.4 |
| 39. | Hippeastrum puniceum (Lam.) Voss | Amaryllidaceae | 7.2 ± 1.6 | 8.4 ± 1.3 |
| 40. | Heliconia vaginalis Benth. | Heliconiaceae | 8.1 ± 0.3 | 7.9 ± 0.6 |
| 41. | Musa × paradisiaca L. | Musaceae | 5.5 ± 0.5 | 4.9 ± 0.4 |
| 42. | Andira inermis (W.Wright) DC. | Fabaceae | 12.8 ± 1.5 | 11.3 ± 1.4 |
| 43. | Dioclea guianensis Benth. | Fabaceae | 12.6 ± 2.4 | 12.9 ± 2.0 |
| 44. | Inga spectabilis (Vahl) Willd. | Fabaceae | 22.6 ± 3.7 | 22.8 ± 3.1 |
| 45. | Ormosia macrocalyx Ducke | Fabaceae | 10.0 ± 0.9 | 10.1 ± 1.3 |
| 46. | Ficus insipida Willd. | Moraceae | 0 ± 0 | 0 ± 0 |
| 47. | Cecropia peltata L. | Urticaceae | 1.0 ± 2.2 | 3.7 ± 8.3 |
| 48. | Clusia cupulata (Maguire) Maguire | Clusiaceae | 169.4 ± 72.7 | 160.4 ± 26.6 |
| 49. | Clusia valerioi Standl. | Clusiaceae | 156.9 ± 90.7 | 109.5 ± 24.2 |
| 50. | Garcinia intermedia (Pittier) Hammel | Clusiaceae | 20.5 ± 3.8 | 11.7 ± 5.8 |
| 51. | Calophyllum longifolium Willd. | Calophyllaceae | 94.3 ± 8.8 | 86.3 ± 6.2 |
| 52. | Jatropha curcas L. | Euphorbiaceae | 29.8 ± 1.4 | 26.1 ± 4.6 |
| 53. | Lagerstroemia speciosa (L.) Pers. | Lythraceae | 111.2 ± 8.9 | 97.5 ± 6.5 |
| 54. | Eucalyptus camaldulensis Dehnh. | Myrtaceae | 54.7 ± 6.1 | 50.0 ± 5.6 |
| 55. | Miconia impetiolaris D.Don | Melastomataceae | 253.8 ± 31.4 | 221.4 ± 10.9 |
| 56. | Protium stevensonii (Standl.) Daly | Burseraceae | 55.9 ± 5.2 | 53.1 ± 5.1 |
| 57. | Mangifera indica L. | Anacardiaceae | 52.3 ± 4.7 | 51.1 ± 16.2 |
| 58. | Cupania latifolia Kunth | Sapindaceae | 19.4 ± 8.1 | 16.3 ± 2.6 |
| 59. | Citrus × limon (L.) Osbeck | Rutaceae | 15.3 ± 3.5 | 16.9 ± 4.3 |
| 60. | Luehea seemannii Triana & Planch. | Malvaceae | 31.0 ± 3.9 | 30.6 ± 3.6 |
| 61. | Sterculia apetala (Jacq.) H.Karst. | Malvaceae | 15.6 ± 0.6 | 14.8 ± 1.6 |
| 62. | Carica papaya L. | Caricaceae | 15.4 ± 1.6 | 15.0 ± 3.5 |
| 63. | Coccoloba uvifera (L.) L. | Polygonaceae | 5.5 ± 2.3 | 7.1 ± 3.2 |
| 64. | Bougainvillea spectabilis Willd. | Nyctaginaceae | 10.5 ± 1.2 | 8.5 ± 2.3 |
| 65. | Isertia haenkeana DC. | Rubiaceae | 105.4 ± 5.9 | 98.6 ± 5.0 |
| 66. | Plumeria rubra L. | Apocynaceae | 22.4 ± 1.6 | 23.3 ± 2.3 |
| 67. | Nolana reichei M.O.Dillon & Arancio | Solanaceae | 2.4 ± 1.6 | 4.4 ± 3.9 |
| 68. | Thunbergia grandiflora Roxb. | Acanthaceae | 9.0 ± 2.7 | 8.4 ± 1.2 |
| 69. | Lantana camara L. | Verbenaceae | 0.9 ± 2.0 | 0.5 ± 1.2 |
| 70. | Tectona grandis L.f. | Lamiaceae | 0 ± 0 | 0 ± 0 |
Values are means ± SD (n = 5 independent samples).
The survey includes 30 known crassulacean acid metabolism (CAM)‐exhibiting species (1–30) and 40 putative C3 species (31–70). Most plants grew outdoors in the ground at the Tupper Center of the Smithsonian Tropical Research Institute (STRI) in Panama City, Republic of Panama (8°57′45″N, 79°32′36″W), or at STRI's Gamboa facilities (9°07′12″N, 79°42′07″W). Species numbers 13, 15 and 30 grew epiphytically on host trees, and species numbers 4, 18, 23, 24, 29, 31, 32, 54, 56 and 67 grew in pots/soil containers. Most species were studied during the dry season when CAM was probably expressed in species with facultative CAM. Leaf or, in the case of Epiphyllum and Opuntia, photosynthetic stem tissue was collected on sunny days at dusk and dawn (five samples at each time point per species) and boiled sequentially in 50% (v/v) ethanol and water. For determination of titratable acidity, plant extracts were titrated with 5 or 25 mM NaOH to an end‐point of pH 6.5. A one‐tailed t‐test was used to determine whether H+ values at dawn were significantly greater than those at dusk at P < 0.05.
Diel malate and citrate metabolism in C3 plants
Even if they do not show diel rhythms in acidity, leaves of C3 plants often exhibit significant day–night changes in the bulk tissue concentration of malate, which is one of the most ubiquitous carboxylate anions found in plant vacuoles. However, in photosynthetic tissues of C3 plants, carbon metabolism is widely associated with increases in malate content during the daytime (Fig. 1b), not during the night‐time as implied in the metabolic schemes of Bräutigam et al. (2017) and Schiller & Bräutigam (2021). Daytime malate production is linked to maintenance of charge balance during light‐dependent assimilation of nitrate, as the negative charge from can be transferred to malate2− during the synthesis of reduced (and approximately net‐neutral) nitrogen compounds (Osmond, 1976; Raven & Smith, 1976). In some species, such as several members of the Brassicaceae, there is also concurrent accumulation of fumarate, which in Arabidopsis thaliana is attributable to the existence of a cytosolic isoform of fumarase, FUM2 (Pracharoenwattana et al., 2010). (An ortholog of FUM2 is present in the CAM plant Agave americana, and some nocturnal fumarate accumulation is detectable in parallel with malate in Phase I of the CAM cycle, but the bulk‐tissue fumarate concentrations are two orders of magnitude lower than malate: Abraham et al., 2016.) Even in C3 plants, the quantities of carboxylates involved are large enough that almost all the malate and fumarate must be localized in the vacuole (Medeiros et al., 2017), but how the diurnal accumulation of these anions is charge‐balanced in C3 plants has been less commonly investigated. A role for inorganic cations such as K+ is possible: for example, malate is a major balancing ion for K+ accumulated in stomatal guard cells during stomatal opening. This process is in fact associated with a slight increase in vacuolar pH (Willmer & Fricker, 1996; Andrés et al., 2014), because the accumulated malate, acting as a conjugate base, increases the pH‐buffering capacity of the vacuole. Another possibility is that daytime accumulation of malate (and fumarate) in the vacuole may be counterbalanced by efflux of nocturnally accumulated citrate, as measurements of bulk tissue concentrations of these metabolites suggest these fluxes can be of similar magnitude (Zell et al., 2010; Annunziata et al., 2017). A carboxylate exchange of this type would obviate the need for movement of charge‐balancing cations and could be mediated by the tonoplast dicarboxylate transporter (tDT), which can counter‐exchange malate and citrate anions in their divalent forms (Frei et al., 2018).
The absolute concentrations of tissue carboxylates are influenced by the growth conditions of the plants (particularly light intensity and nitrogen nutrition), but the rhythms of opposing diel changes in malate and citrate content have also been observed in the leaves of other well‐studied C3 species besides A. thaliana, such as tobacco (Pucher et al., 1937; Scheible et al., 2000), tomato (Niedziela et al., 1993) and potato (Urbanczyk‐Wochniak et al., 2005). The widespread occurrence of daytime malate accumulation in C3 plants is difficult to reconcile with the proposal of Bräutigam et al. (2017), based on the labelling studies of Szecowka et al. (2013), that C3 plants possess a CAM‐like cycle involving ‘malate decarboxylation … during the day’ and subsequent refixation of the liberated CO2 by Rubisco. This would potentially represent a futile cycle of malate synthesis and degradation at a time when bulk tissue (vacuolar) concentrations of malate are increasing (even if there may be a limited role inside the chloroplast for decarboxylation of malate by NADP‐ME for the provision of pyruvate). The key observation of Szecowka et al. (2013) was that, when illuminated rosettes of A. thaliana were supplied with external 13CO2, the organic acid fraction showed only slow and very limited incorporation of the 13C label, implying that much of the daytime carboxylate metabolism was supported by remobilization of vacuolar carboxylates accumulated during the previous night. However, the plants in these experiments were grown under relatively low (‘limiting’) light conditions, had very high shoot nitrate content (224 mmol kg−1 fresh mass) and rather low concentrations of the major vacuolar carboxylates (citrate, malate and fumarate averaged 1.9, 1.8 and 1.2 mmol kg−1 fresh mass, respectively). These tissue carboxylate concentrations are an order of magnitude lower than those commonly observed in Arabidopsis (e.g. Chia et al., 2000; Dyson et al., 2016; Medeiros et al., 2017), and so may have been associated with rather slow rates of de novo synthesis and fixation of atmospheric CO2. It should also be noted that when previously illuminated C3 tissue is transferred into darkness, this gives rise to the well‐known phenomenon of light‐enhanced dark respiration (LEDR): this has been shown to be associated with a burst of malate decarboxylation via malic enzyme (Gessler et al., 2009; Gauthier et al., 2010), which again is the opposite of what occurs during the CAM cycle.
While malate (and in some cases fumarate) accumulates during the daytime in C3 plants, what is the explanation for the concomitant decline in vacuolar citrate content? Citrate is required in the daytime for conversion to 2‐oxoglutarate, in order to provide the carbon skeletons needed for light‐dependent nitrogen assimilation and amino acid synthesis. However, TCA cycle activity is strongly downregulated in the light period, so there is only limited de novo citrate synthesis via citrate synthase. Instead, labelling evidence suggests that this demand is met by remobilization in the daytime of vacuolar citrate accumulated during the previous night (Gauthier et al., 2010; Tcherkez et al., 2012; Cheung et al., 2014). Subsequent conversion of citrate to 2‐oxoglutarate in the light is also not dependent on mitochondrial metabolism, given the existence of cytosolic isoforms of aconitase and isocitrate dehydrogenase (Hanning & Heldt, 1993). The result of this downregulation of TCA cycle activity during the day is an uncoupling of malate and citrate synthesis (Tcherkez et al., 2012), leading to malate accumulation during the daytime and citrate accumulation during the night. In this regard, the nocturnal accumulation of citric acid in some CAM plants may have a similar metabolic basis, although the equivalent labelling experiments with CAM tissues are complex because of the relative pool sizes of metabolites (Tcherkez, 2017).
Malate accumulated during the light period can be remobilized from the vacuole at night and decarboxylated by mitochondrial NAD‐malic enzyme (NAD‐ME) to contribute to dark respiration (Gessler et al., 2009; Lehmann et al., 2015), but glycolytic breakdown of carbohydrate will also be important in supplying respiratory substrate for entry into the TCA cycle, either via pyruvate kinase (PK) or via PEPC/NAD‐ME. Some nocturnal PEPC activity is needed to provide OAA to support net synthesis of (1) any citrate that is removed from the TCA cycle for accumulation in the vacuole (Fig. 1b), and (2) aspartate, which together with malate is one of the major radiolabelled products following dark 14CO2 feeding in tobacco leaves (Kunitake et al., 1959). Indeed, based on the natural enrichment of 13C in aspartyl residues in protein extracted from tobacco leaves, Melzer & O’Leary (1987) estimated that more than half of cellular aspartate is synthesized by direct fixation of atmospheric CO2 (as ) by PEPC via a C4‐type pathway, rather than by refixation of CO2 originally assimilated by Rubisco. Based on this observation, Bräutigam et al. (2017) have proposed that, if at least one isoform of PEPC is active at night, the C4‐like isotope distribution in aspartate could be generated by nocturnal fixation of external CO2 by PEPC, and that this may represent a plausible precursor in C3 plants of CAM‐like dark fixation. However, it should be borne in mind that, because of dark respiration, the overall CO2 diffusion gradient at night in C3 plants is outwardly directed and the stomata are largely or partially closed at this time, so the rate of uptake of external CO2 molecules would be limited. As a more likely alternative, the distribution of ‘heavy’ 13C atoms in aspartate could be readily explained by PEPC‐mediated fixation of atmospheric CO2 during the daytime, when PEPC will be active in the light‐dependent synthesis of malate (see earlier): this could either occur by immediate transamination of the OAA formed to aspartate, or more indirectly via accumulation of malate in the vacuole during the light period, followed by its remobilization and decarboxylation by NAD‐ME in the subsequent dark period, and refixation of this ‘heavy’ CO2 via PEPC to form aspartate. The pyruvate resulting from NAD‐ME activity at night could then be used to produce acetyl‐CoA to support vacuolar citrate accumulation, as shown in Fig. 1(b), and indeed NAD‐ME loss‐of‐function mutants of A. thaliana have been shown to possess reduced citrate contents (Tronconi et al., 2008).
Thus, although at least one isoform of PEPC may be active at night in C3 plants, and this allows radiolabel from externally supplied 14CO2 in feeding experiments to appear in malate, the main function of malate at night in C3 plants is as a substrate for other reactions (citrate synthesis and respiration) and as a metabolic intermediate (in the TCA cycle). By contrast, in CAM plants in the dark there is much greater and more sustained incorporation of label into malate (Kunitake et al., 1959), reflecting its function as the metabolic end‐product of nocturnal CO2 fixation that accumulates overnight in the vacuole until being remobilized at the start of the light period in the next phase of the CAM cycle.
The discreteness of the ability to perform CAM
Schiller & Bräutigam (2021) suggest that the continuous spectrum of phenotypes observable between C3 and CAM supports their proposal that CAM photosynthesis merely requires the upregulation of fluxes already present in C3 plants. In contrast to the comparison of C3 and C4 photosynthesis, the authors suggest that CAM cannot be regarded as a binary character‐state because ‘it can be plastic with plant age and external conditions such as water availability and salinity’. However, an alternative view is that the ability to perform net dark CO2 fixation represents a discrete metabolic innovation, which has only arisen in a limited number of plant lineages, even if the phenotypic expression of CAM is strongly influenced by developmental and environmental factors. In obligate CAM plants, the assimilatory tissues show an ontogenetic progression from C3 to CAM during development (Winter et al., 2011; Winter, 2019), but even at maturity most CAM species retain the ability to acquire atmospheric CO2 directly via the C3 pathway – notably during the early morning (phase II) and late afternoon (phase IV) – when environmental conditions are favourable. The entire spectrum from C3 to CAM gas exchange is exhibited particularly clearly in facultative CAM plants as they transition from C3 to CAM in response to water deficit stress and/or high soil salinity (e.g. Winter & Holtum, 2014). It is also notable that, while performing C3 photosynthesis before the ontogenetic or environmentally induced transition to CAM, the young leaves of Kalanchoë (Amagasa, 1982) and Mesembryanthemum (Winter et al., 1978) show small daytime increases in malate content, before switching to the night‐time malate accumulation characteristic of CAM. But this phenotypic continuum does not mean that CAM is a purely quantitative trait: the ability to show net dark CO2 fixation and nocturnal acidification is not present in C3 plants (cf. Table 1), and so can be regarded as a discrete metabolic innovation only found in lineages that have evolved CAM.
An additional rationale for treating CAM as a distinct functional trait has come from ecophysiological surveys of carbon‐isotope ratios (δ13C values), which show a strongly bimodal distribution in several large families containing both C3 species and obligate CAM plants (Winter & Holtum, 2002; Winter et al., 2015). This has facilitated character‐mapping onto phylogenies as a means of investigating photosynthetic pathway evolution in families such as Bromeliaceae (Crayn et al., 2004), Orchidaceae (Silvera et al., 2009; Bone et al., 2015; Gamisch et al., 2021), Euphorbiaceae (Horn et al., 2014) and Asparagaceae (subfamily Agavoideae: Heyduk et al., 2016). Even if some substructure is discernible in the frequency histograms of δ13C values within the CAM cluster (Winter et al., 2015; Messerschmid et al., 2021), the pronounced minimum in the bimodal frequency distributions consistently seen around −20‰ (observed in at least seven families so far) is concordant with CAM being a discrete evolutionary innovation, with C3–CAM intermediate states not being favoured.
In facultative CAM species, by contrast, in which the full spectrum of phenotypes may be seen over the plant’s life cycle, a complete range of intermediate δ13C values can be found. For example, the well‐studied facultative CAM plant Mesembryanthemum crystallinum shows C3‐type δ13C values as low as −27‰ in its natural habitat soon after germination in the rainy season, and these gradually shift to values as high as −15‰ as the dry season progresses, indicative of an almost complete switch to CAM photosynthesis (Winter et al., 1978). This pattern appears to be common among annual plants of the family Aizoaceae, with the result that a bimodal distribution of δ13C values is not observed in the corresponding frequency histograms for such families (Messerschmid et al., 2021). Nevertheless, it is the genetically encoded ability to induce net dark CO2 fixation in response to water deficits that distinguishes these facultative CAM plants from C3 species. A more inclusive approach, as pointed out by Winter et al. (2015), will eventually incorporate more systematic surveys of species displaying only weakly expressed CAM, but these cannot be recognized on the basis of their δ13C values alone, and so require physiological measurements on living material under conditions when CAM activity may be expressed (e.g. Hancock et al., 2019; Torres‐Morales et al., 2020). Dawn–dusk measurements of tissue titratable acidity provide a highly sensitive method for detecting low‐level CAM activity, which in gas‐exchange studies is often discernible only as a slight reduction in the rate of net CO2 loss during the dark period in comparison with well‐watered plants.
Thus, the fact that dark CO2 fixation may contribute anywhere between 0 and 100% of total 24 h carbon gain is not incompatible with CAM being treated as a categorical trait. Certain plant lineages possess the ability to engage the CAM cycle, whereas others (the majority) do not.
Conclusions
The evidence from comparative biochemistry and genomics suggests that synthesis of malate via PEP carboxylase, and accumulation of the resulting carboxylate anion in the cell vacuole for ion homeostasis and turgor generation are ubiquitous features of central metabolism in the green plant lineage. In the heterotrophic tissues of C3 plants such as roots, PEPC activity has traditionally been associated with the ‘dark’ fixation of CO2 into malate (Lips & Beevers, 1966), but in photosynthetic tissues the accumulation of malate typically occurs during the daytime in association with light‐dependent assimilation of nitrate (e.g. Strack et al., 1986). The key metabolic innovation underpinning CAM photosynthesis is arguably the switch from daytime to night‐time synthesis of malate in the assimilatory tissue, coupled to the glycolytic breakdown of storage carbohydrate (Borland et al., 2016; Tay et al., 2021). This results in the stoichiometric fixation of CO2, with malic acid accumulating as the end‐product in the vacuole, before its remobilization and decarboxylation in the following daytime. Even when expressed at relatively low levels, this cycle would appear to involve a fundamental reprogramming of day–night carbon metabolism not easily conceptualized as part of a seamless continuum between C3 and CAM. Realization of the full water‐saving potential of the CAM pathway, as seen in obligate CAM plants, would undoubtedly involve optimization of other evolutionary modifications, but these can be viewed as contingent upon (rather than necessary precursors for) the reconfigured day–night cycle of malic acid metabolism. For example, the distinctive period of stomatal closure during phase III of the CAM cycle is a direct consequence of the wholesale remobilization and decarboxylation of the malic acid accumulated during the previous night. And the characteristic succulence of the assimilatory tissue, attributable to highly vacuolated cells with relatively thin cell walls, is important in maximizing the acid‐ and water‐storage capacity of the cells (Lüttge et al., 1982; Smith et al., 1996; Edwards, 2019; Töpfer et al., 2020; Heyduk, 2021).
Deciphering the first critical steps on the evolutionary path to net dark CO2 fixation and malic acid accumulation will certainly help to guide efforts directed at the bioengineering of the CAM trait. Schiller & Bräutigam (2021) have proposed that, considering the widespread occurrence and the plasticity of the CAM trait, there are ‘therefore likely no general barriers to trait engineering’ and that ‘[c]ircadian and diurnal rhythms in CAM plants are likely similar to those in C3 plants’, but we believe that both of these opinions are difficult to reconcile with the available evidence. Despite its dispersed taxonomic occurrence and phenotypic plasticity, CAM activity has only been detected so far in 37 out of a total of 450 families of vascular plants (Winter & Smith, 1996b; Winter et al., 2021). Acquisition of this trait by such a limited number of lineages suggests that it does not represent a facile evolutionary step requiring simply enhancement of flux pathways already present in C3 plants. Also, the switch from diurnal to nocturnal malate synthesis in CAM plants would seem, prima facie, to require significant modification of the way in which central metabolism is integrated with the circadian clock. Indeed, the ability of CAM plants to show a persistent free‐running endogenous rhythm of dark CO2 fixation provided early evidence that this core component of CAM is directly linked to the central circadian oscillator (Wilkins, 1959). This rhythm of dark CO2 fixation is now known to be attributable to the periodic activity of a specific, highly expressed isoform of PEP carboxylase, which becomes post‐translationally activated at night by a dedicated protein kinase whose expression is under endogenous circadian control (Hartwell et al., 1996; Boxall et al., 2020). Evidence of a tight interconnection between the circadian oscillator and dark CO2 fixation is also shown by the observation that suppression of PEPC expression by RNA interference results in altered expression of certain core circadian clock genes (Boxall et al., 2020). On the other hand, overexpression of the CAM isoform of PEPC in a C3 background (Arabidopsis or tobacco), while leading to increased malate content and daytime accumulation, does not itself result in a switch to night‐time acidification (Lim et al., 2019; Liu et al., 2021).
Many facets of the gene regulatory networks involved in the core CAM cycle remain to be explored. Much attention has focused so far on the regulation of PEPC, the key enzyme involved in the initial carboxylation reaction. This is encoded by a multigene family even in C3 plants, so it is plausible that one isoform of PEPC was coopted in evolution for the key function of night‐time CO2 fixation in CAM plants. But less work has been performed to date on regulation of the decarboxylation process, which will be equally critical to coordination of the CAM cycle. The α and β subunits of plant NAD‐ME, the principal decarboxylase in many CAM species, are each encoded by a single gene, and their expression is linked to the central circadian clock (Dever et al., 2015; Yang et al., 2017; Francisco et al., 2021). A fundamental reprogramming is thus needed to ensure that NAD‐ME is active during the day in the CAM cycle, rather than during the night as in C3 plants. Similarly, coordinate regulation of the key transport proteins at the vacuolar membrane with the phases of malic acid synthesis and decarboxylation will be essential to maintain cytoplasmic homeostasis and minimize futile cycling, but details of these mechanisms remain to be fully elucidated (Smith et al., 1996; Hafke et al., 2003; Ceusters et al., 2021). Further exploration of the gene regulatory networks in closely related C3 and CAM species, and especially those implicated in the C3–CAM transition in facultative species (e.g. Brilhaus et al., 2016; Heyduk et al., 2019; Wai et al., 2019), will undoubtedly help in identifying core elements of the circadian circuitry needed to bioengineer the dark CO2 fixation pathway into C3 plants.
Author contributions
KW designed the research, collected the plant material, analysed the data and wrote the initial draft. JACS revised the manuscript and designed Fig. 1. Both authors discussed, reviewed and approved the final manuscript.
Acknowledgements
This article is dedicated, on the occasion of his 85th birthday, to Professor Ulrich Lüttge, who so resolutely established the stoichiometry of malic acid accumulation as the defining characteristic of crassulacean acid metabolism. Jorge Aranda helped with plant identification and Aurelio Virgo assisted with titratable acidity measurements. This research was supported by the Smithsonian Tropical Research Institute (KW) and the Oxford Martin School, University of Oxford (JACS). We apologize to colleagues whose work was not cited here on account of the need to limit the number of references cited.
Data availability
All data required to draw the conclusions in the paper are present in the main text.
References
- Abraham PE, Yin H, Borland AM, Weighill D, Lim SD, De Paoli HC, Engle N, Jones PC, Agh R, Weston DJ et al. 2016. Transcript, protein and metabolite temporal dynamics in the CAM plant Agave . Nature Plants 2: 16178. [DOI] [PubMed] [Google Scholar]
- Amagasa T. 1982. The influence of leaf age on the diurnal changes of malate and starch in the CAM plant Kalanchoe daigremontiana Hamet et Perr. Zeitschrift für Pflanzenphysiologie 108: 93–96. [Google Scholar]
- Andrés Z, Pérez‐Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B et al. 2014. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proceedings of the National Academy of Sciences, USA 111: E1806–E1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata MG, Apelt F, Carillo P, Krause U, Feil R, Mengin V, Lauxmann MA, Köhl K, Nikoloski Z, Stitt M et al. 2017. Getting back to nature: a reality check for experiments in controlled environments. Journal of Experimental Botany 68: 4463–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APG IV . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Bennet‐Clark TA. 1933. The rôle of the organic acids in plant metabolism. Part I. New Phytologist 32: 37–71. [Google Scholar]
- Bone RE, Smith JAC, Arrigo N, Buerki S. 2015. A macro‐ecological perspective on crassulacean acid metabolism (CAM) photosynthesis evolution in Afro‐Madagascan drylands: Eulophiinae orchids as a case study. New Phytologist 208: 469–481. [DOI] [PubMed] [Google Scholar]
- Borland AM, Guo H‐B, Yang X, Cushman JC. 2016. Orchestration of carbohydrate processing for crassulacean acid metabolism. Current Opinion in Plant Biology 31: 118–124. [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Weston DJ, Schlauch KA, Tschaplinski TJ, Tuskan GA, Yang X, Cushman JC. 2014. Engineering crassulacean acid metabolism to improve water‐use efficiency. Trends in Plant Science 19: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall SF, Kadu N, Dever LVD, Knerová J, Waller JL, Gould PJD, Hartwell J. 2020. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. The Plant Cell 32: 1136–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Schlüter U, Eisenhut M, Gowik U. 2017. On the evolutionary origin of CAM photosynthesis. Plant Physiology 174: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhaus D, Bräutigam A, Mettler‐Altmann T, Winter K, Weber APM. 2016. Reversible burst of transcriptional changes during induction of crassulacean acid metabolism in Talinum triangulare . Plant Physiology 170: 102–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters N, Borland AM, Ceusters J. 2021. How to resolve the enigma of diurnal malate remobilisation from the vacuole in plants with crassulacean acid metabolism? New Phytologist 229: 3116–3124. [DOI] [PubMed] [Google Scholar]
- Ceusters N, Luca S, Feil R, Claes JE, Lunn JE, Van den Ende W, Ceusters J. 2019. Hierarchical clustering reveals unique features in the diel dynamics of metabolites in the CAM orchid Phalaenopsis . Journal of Experimental Botany 70: 3269–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CYM, Poolman MG, Fell DA, Ratcliffe RG, Sweetlove LJ. 2014. A diel flux balance model captures interactions between light and dark metabolism during day–night cycles in C3 and crassulacean acid metabolism leaves. Plant Physiology 165: 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter W‐D, Gibson SI. 2000. Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751. [DOI] [PubMed] [Google Scholar]
- Crayn DM, Winter K, Smith JAC. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences, USA 101: 3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever LV, Boxall SF, Kneřová J, Hartwell J. 2015. Transgenic perturbation of the decarboxylation phase of crassulacean acid metabolism alters physiology and metabolism but has only a small effect on growth. Plant Physiology 167: 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson BC, Miller MAE, Feil R, Rattray N, Bowsher CG, Goodacre R, Lunn JE, Johnson GN. 2016. FUM2, a cytosolic fumarase, is essential for acclimation to low temperature in Arabidopsis thaliana . Plant Physiology 172: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 223: 1742–1755. [DOI] [PubMed] [Google Scholar]
- Francisco M, Kliebenstein DJ, Rodríguez VM, Soengas P, Abilleira R, Cartea ME. 2021. Fine mapping identifies NAD‐ME1 as a candidate underlying a major locus controlling temporal variation in primary and specialized metabolism in Arabidopsis. The Plant Journal 106: 454–467. [DOI] [PubMed] [Google Scholar]
- Frei B, Eisenach C, Martinoia E, Hussein S, Chen X‐Z, Arrivault S, Neuhaus HE. 2018. Purification and functional characterization of the vacuolar malate transporter tDT from Arabidopsis . Journal of Biological Chemistry 293: 4180–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamisch A, Winter K, Fischer GA, Comes HP. 2021. Evolution of crassulacean acid metabolism (CAM) as an escape from ecological niche conservatism in Malagasy Bulbophyllum (Orchidaceae). New Phytologist 231: 1236–1248. [DOI] [PubMed] [Google Scholar]
- Gauthier PPG, Bligny R, Gout E, Mahé A, Nogués S, Hodges M, Tcherkez GGB. 2010. In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus . New Phytologist 185: 988–999. [DOI] [PubMed] [Google Scholar]
- Gessler A, Tcherkez G, Karyanto O, Keitel C, Ferrio JP, Ghashghaie J, Kreuzwieser J, Farquhar GD. 2009. On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light‐acclimated leaves in Ricinus communis . New Phytologist 181: 374–386. [DOI] [PubMed] [Google Scholar]
- Hafke JB, Hafke Y, Smith JAC, Lüttge U, Thiel G. 2003. Vacuolar malate uptake is mediated by an anion‐selective inward rectifier. The Plant Journal 35: 116–128. [DOI] [PubMed] [Google Scholar]
- Hancock LP, Holtum JAM, Edwards EJ. 2019. The evolution of CAM photosynthesis in Australian Calandrinia reveals lability in C3+CAM phenotypes and a possible constraint to the evolution of strong CAM. Integrative and Comparative Biology 59: 517–534. [DOI] [PubMed] [Google Scholar]
- Hanning I, Heldt HW. 1993. On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiology 103: 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG. 1996. Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. The Plant Journal 10: 1071–1078. [Google Scholar]
- Heyduk K. 2021. The genetic control of succulent leaf development. Current Opinion in Plant Biology 59: 101978. [DOI] [PubMed] [Google Scholar]
- Heyduk K, Hwang M, Albert V, Silvera K, Lan T, Farr K, Chang T‐H, Chan M‐T, Winter K, Leebens‐Mack J. 2019. Altered gene regulatory networks are associated with the transition from C3 to crassulacean acid metabolism in Erycina (Oncidiinae: Orchidaceae). Frontiers in Plant Science 9: 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, McKain MR, Lalani F, Leebens‐Mack J. 2016. Evolution of CAM anatomy predates the origins of crassulacean acid metabolism in the Agavoideae (Asparagaceae). Molecular Phylogenetics and Evolution 105: 102–113. [DOI] [PubMed] [Google Scholar]
- Heyne B. 1815. On the deoxidation of the leaves of Cotyledon calycina . Transactions of the Linnean Society of London 11: 213–215. [Google Scholar]
- Holtum JAM, Smith JAC, Neuhaus HE. 2005. Intracellular transport and pathways of carbon flow in plants with crassulacean acid metabolism. Functional Plant Biology 32: 429–449. [DOI] [PubMed] [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68: 3485–3504. [DOI] [PubMed] [Google Scholar]
- Hurd‐Karrer AM. 1939. Hydrogen‐ion concentration of leaf juice in relation to environment and plant species. American Journal of Botany 26: 834–846. [Google Scholar]
- Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. Botanical Review 64: 121–175. [Google Scholar]
- Kunitake G, Stitt C, Saltman P. 1959. Dark fixation of CO2 by tobacco leaves. Plant Physiology 34: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J. 1989. Intracellular pH: measurement and importance in cell activity. Annual Review of Plant Physiology and Plant Molecular Biology 40: 271–303. [Google Scholar]
- Lehmann MM, Rinne KT, Blessing C, Siegwolf RTW, Buchmann N, Werner RA. 2015. Malate as a key carbon source of leaf dark‐respired CO2 across different environmental conditions in potato plants. Journal of Experimental Botany 66: 5769–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SD, Lee S, Choi W‐G, Yim WC, Cushman JC. 2019. Laying the foundation for crassulacean acid metabolism (CAM) biodesign: expression of the C4 metabolism cycle genes of CAM in Arabidopsis . Frontiers in Plant Science 10: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips SH, Beevers H. 1966. Compartmentation of organic acids in corn roots. I. Differential labeling of 2 malate pools. Plant Physiology 41: 709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hu R, Zhang J, Guo H‐B, Cheng H, Li L, Borland AM, Qin H, Chen J‐G, Muchero W et al. 2021. Overexpression of an Agave phosphoenolpyruvate carboxylase improves plant growth and stress tolerance. Cells 10: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. 1988. Day‐night changes of citric‐acid levels in crassulacean acid metabolism: phenomenon and ecological significance. Plant, Cell & Environment 11: 445–451. [Google Scholar]
- Lüttge U, Ball E. 1980. 2H+:1 malate2−⁻ stoichiometry during crassulacean acid metabolism is unaffected by lipophilic cations. Plant, Cell & Environment 3: 195–200. [Google Scholar]
- Lüttge U, Smith JAC, Marigo G. 1982. Membrane transport, osmoregulation, and the control of CAM. In: Ting IP, Gibbs M, eds. Crassulacean acid metabolism. Rockville, MD, USA: American Society of Plant Physiologists, 69–91. [Google Scholar]
- Lüttge U, Smith JAC, Marigo G, Osmond CB. 1981. Energetics of malate accumulation in the vacuoles of Kalanchoë tubiflora cells. FEBS Letters 126: 81–84. [Google Scholar]
- Medeiros DB, Barros KA, Barros JAS, Omena‐Garcia RP, Arrivault S, Sanglard LMVP, Detmann KC, Silva WB, Daloso DM, DaMatta FM et al. 2017. Impaired malate and fumarate accumulation due to the mutation of the tonoplast dicarboxylate transporter has little effects on stomatal behavior. Plant Physiology 175: 1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E, Osmond CB. 1981. Temperature dependence of dark CO2 fixation and acid accumulation in Kalanchoë daigremontiana . Australian Journal of Plant Physiology 8: 641–649. [Google Scholar]
- Melzer E, O'Leary MH. 1987. Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiology 84: 58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmid TFE, Wehling J, Bobon N, Kahmen A, Klak C, Los JA, Nelson DB, dos Santos P, de Vos JM, Kadereit G. 2021. Carbon isotope composition of plant photosynthetic tissues reflects a crassulacean acid metabolism (CAM) continuum in the majority of CAM lineages. Perspectives in Plant Ecology, Evolution and Systematics 51: 125619. [Google Scholar]
- Milburn TR, Pearson DJ, Ndegwe NA. 1968. Crassulacean acid metabolism under natural tropical conditions. New Phytologist 67: 883–897. [Google Scholar]
- Niedziela CE Jr, Nelson PV, Peet MM, Jackson WA. 1993. Diurnal malate and citrate fluctuations as related to nitrate and potassium concentrations in tomato leaves. Journal of Plant Nutrition 16: 165–175. [Google Scholar]
- Osmond CB. 1976. Ion absorption and carbon metabolism in cells of higher plants. In: Lüttge U, Pitman MG, eds. Transport in plants II, part A Cells, Encyclopedia of Plant Physiology. Berlin, Germany: Springer, 347–372. [Google Scholar]
- Phillips R. 1980. Deacidification in a plant with crassulacean acid metabolism associated with anion–cation balance. Nature 287: 727–728. [Google Scholar]
- Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM. 2010. Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. The Plant Journal 62: 785–795. [DOI] [PubMed] [Google Scholar]
- Pucher GW, Wakeman AJ, Vickery HB. 1937. The metabolism of the organic acids of the tobacco leaf during culture. Journal of Biological Chemistry 119: 523–534. [Google Scholar]
- Raven JA, Smith FA. 1976. Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytologist 76: 415–431. [Google Scholar]
- Scheible WR, Krapp A, Stitt M. 2000. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP‐isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant, Cell & Environment 23: 1155–1167. [Google Scholar]
- Schiller K, Bräutigam A. 2021. Engineering of crassulacean acid metabolism. Annual Review of Plant Biology 72: 77–103. [DOI] [PubMed] [Google Scholar]
- Shameer S, Baghalian K, Cheung CYM, Ratcliffe RG, Sweetlove LJ. 2018. Computational analysis of the productivity potential of CAM. Nature Plants 4: 165–171. [DOI] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology 149: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Winter K. 2005. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 32: 397–407. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Ingram J, Tsiantis MS, Barkla BJ, Bartholomew DM, Bettey M, Pantoja O, Pennington AJ. 1996. Transport across the vacuolar membrane in CAM plants. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin, Germany: Springer, 53–71. [Google Scholar]
- Steudle E, Smith JAC, Lüttge U. 1980. Water‐relation parameters of individual mesophyll cells of the crassulacean acid metabolism plant Kalanchoë daigremontiana . Plant Physiology 66: 1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack D, Reinecke J, Takeuchi S. 1986. Evidence for a relationship between malate metabolism and activity of 1‐sinapoylglucose: l‐malate sinapoyltransferase in radish (Raphanus sativus) cotyledons. Planta 167: 212–217. [DOI] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, Nunes‐Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M et al. 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25: 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay IYY, Odang KB, Cheung CYM. 2021. Metabolic modeling of the C3–CAM continuum revealed the establishment of a starch/sugar–malate cycle in CAM evolution. Frontiers in Plant Science 11: 573197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G. 2017. Respiratory metabolism in CAM plants. In: Tcherkez G, Ghashghaie J, eds. Plant respiration: metabolic fluxes and carbon balance. Berlin, Germany: Springer, 227–246. [Google Scholar]
- Tcherkez G, Boex‐Fontvieille E, Mahé A, Hodges M. 2012. Respiratory carbon fluxes in leaves. Current Opinion in Plant Biology 15: 308–314. [DOI] [PubMed] [Google Scholar]
- Töpfer N, Braam T, Shameer S, Ratcliffe RG, Sweetlove LJ. 2020. Alternative crassulacean acid metabolism modes provide environment‐specific water‐saving benefits in a leaf metabolic model. The Plant Cell 32: 3689–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Morales G, Lasso E, Silvera K, Turner BL, Winter K. 2020. Occurrence of crassulacean acid metabolism in Colombian orchids determined by leaf carbon isotope ratios. Botanical Journal of the Linnean Society 193: 431–477. [Google Scholar]
- Tronconi MA, Fahnenstich H, Gerrard Wheeler MS, Andreo CS, Flügge U‐I, Drincovich MF, Maurino VG. 2008. Arabidopsis NAD‐malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism. Plant Physiology 146: 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanczyk‐Wochniak E, Baxter C, Kolbe A, Kopka J, Sweetlove LJ, Fernie AR. 2005. Profiling of diurnal patterns of metabolite and transcript abundance in potato (Solanum tuberosum) leaves. Planta 221: 891–903. [DOI] [PubMed] [Google Scholar]
- Vickery HB. 1952. The behavior of isocitric acid in excised leaves of Bryophyllum calycinum during culture in alternating light and darkness. Plant Physiology 27: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CM, Weise SE, Ozersky P, Mockler TC, Michael TP, VanBuren R. 2019. Time of day and network reprogramming during drought induced CAM photosynthesis in Sedum album . PLoS Genetics 15: e1008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB. 1959. An endogenous rhythm in the rate of carbon dioxide output of Bryophyllum: I. Some preliminary experiments. Journal of Experimental Botany 10: 377–390. [Google Scholar]
- Willmer C, Fricker M. 1996. Stomata. 2nd edn. London, UK: Chapman & Hall. [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM. 2011. Drought‐stress‐induced upregulation of CAM in seedlings of a tropical cactus, Opuntia elatior, operating predominantly in the C3 mode. Journal of Experimental Botany 62: 4037–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Garcia M, Virgo A, Smith JAC. 2021. Low‐level CAM photosynthesis in a succulent‐leaved member of the Urticaceae, Pilea peperomioides . Functional Plant Biology 48: 683–690. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2002. How closely do the δ13C values of CAM plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. 2014. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65: 3425–3441. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208: 73–78. [DOI] [PubMed] [Google Scholar]
- Winter K, Lüttge U, Winter E, Troughton JH. 1978. Seasonal shift from C3 photosynthesis to crassulacean acid metabolism in Mesembryanthemum crystallinum growing in its natural environment. Oecologia 34: 225–237. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC. 1996a. Crassulacean acid metabolism: current status and perspectives. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin, Germany: Springer, 389–426. [Google Scholar]
- Winter K, Smith JAC. 1996b. An introduction to crassulacean acid metabolism. Biochemical principles and ecological diversity. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Berlin, Germany: Springer, 1–13. [Google Scholar]
- Yang X, Hu R, Yin H, Jenkins J, Shu S, Tang H, Liu D, Weighill DA, Cheol Yim W, Ha J et al. 2017. The Kalanchoë genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nature Communications 8: 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell MB, Fahnenstich H, Maier A, Saigo M, Voznesenskaya EV, Edwards GE, Andreo C, Schleifenbaum F, Zell C, Drincovich MF et al. 2010. Analysis of Arabidopsis with highly reduced levels of malate and fumarate sheds light on the role of these organic acids as storage carbon molecules. Plant Physiology 152: 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data required to draw the conclusions in the paper are present in the main text.


