Abstract
In vitro platforms for studying the human brain have been developed, and brain organoids derived from stem cells have been studied. However, current organoid models lack three‐dimensional (3D) vascular networks, limiting organoid proliferation, differentiation, and apoptosis. In this study, we created a 3D model of vascularized spheroid cells using an injection‐molded microfluidic chip. We cocultured spheroids derived from induced neural stem cells (iNSCs) with perfusable blood vessels. Gene expression analysis and immunostaining revealed that the vascular network greatly enhanced spheroid differentiation and reduced apoptosis. This platform can be used to further study the functional and structural interactions between blood vessels and neural spheroids, and ultimately to simulate brain development and disease.
Keywords: 3D spheroid, apoptosis, differentiation, induced neural stem cell, microfluidic, spheroid‐on‐a‐chip, vascularization
Current organoid models lack 3D vascular networks, limiting proliferation and differentiation of organoid. In this study, we developed a 3D model of vascularized iNSC spheroids using an injection‐molded microfluidic chip. The vascular network in chip was perfusable and in contact with neural spheroid. Furthermore, vascularized neural spheroids showed enhanced differentiation and reduced apoptosis. We suggest that this model could be applied to the organoids‐on‐a‐chip model, and it would be a powerful tool for understanding of developmental biology and human diseases.
1. INTRODUCTION
Neural stem cells (NSCs) (somatic stem cells of the brain and spinal cord) differentiate into three major cell types (neurons, astrocytes, and oligodendrocytes) in the central nervous system (Okano, 2002). Several recent studies have reported the direct conversion of human somatic cells into stably proliferating induced neural stem cells (iNSCs). In our previous studies, we optimized iNSC generation from various human cell types, including blood cells, mesenchymal stem cells, and fibroblasts (B.‐E. Kim et al., 2018; J.‐J. Kim et al., 2017; Kwon et al., 2018; Yu et al., 2015). iNSCs exhibit great potential as a virtually unlimited source of cells for therapy and disease modeling. Patient‐specific iNSCs could serve as therapies for neurodevelopmental or neurodegenerative diseases; iNSCs can potentially replace damaged cells (Dantuma et al., 2010). Furthermore, iNSCs rapidly, provide low‐cost homogeneous populations via in vitro differentiation. Thus, models employing iNSCs are valuable alternatives to induced pluripotent stem cell (iPSC) techniques, enhancing our understanding of how the human brain works (M. S. Kim et al., 2021).
Unlike two‐dimensional (2D) cell culture, advanced 3D spheroid‐ and organoid‐based systems using NSCs or iPSCs facilitate the study of human development, and could lead to novel therapies for neurodevelopmental disorders such as microcephaly, lissencephaly, and autism (Bershteyn et al., 2017; Lancaster et al., 2013; Mariani et al., 2015). We previously used iNSC‐derived brain organoids to study the pathophysiology of Niemann–Pick disease and hypoxic injury. These models are more physiologically relevant than animal and 2D culture models (Lee et al., 2020). However, current spheroid and organoid models lack microvasculature, which compromises oxygen and nutrient delivery to the innermost regions. Spheroids and millimeter‐scale organoids consistently exhibit apoptotic cell death with long‐term culture. Efforts have been made to include vascular structures in tissue‐mimicking 3D models (Cakir et al., 2019; Mansour et al., 2018). Vascular networks play critical roles in tissue and organ development, supporting differentiation, patterning, and morphogenesis via a paracrine effect, delivering nutrients, and removing waste (Vargas‐Valderrama et al., 2020). Neurogenesis occurs in regions adjacent to blood vessels, and NSCs are closely associated with endothelial cells in both the hippocampus and subventricular zone in adults (Bond et al., 2015). Therefore, cells with a neural lineage, and a vascular network, are required when modeling the brain. An in vitro model of a vascularized neural environment is needed to fully understand human cortical development, disease mechanisms, and neurogenesis. Furthermore, functional vascularization of spheroids or organoids might reduce apoptosis and hypoxia, and thus promote neural lineage maturation.
In this study, we developed a 3D model of, vascularized “iNSC spheroids” using an injection‐molded microfluidic chip (Sphero‐IMPACT). We optimized the medium and gel used for coculture of iNSC spheroids and a perfusable, well‐formed vascular network intimately associated with the spheroids. Assays of several relevant markers revealed that the vascularized iNSC spheroids differentiated when cultured for up 14 days; the vascular network greatly enhanced differentiation and reduced apoptosis. This new organoid model will advance our understanding of the human brain.
2. MATERIALS AND METHODS
2.1. Cell culture
HUVECs (Lonza) were cultured in endothelial growth medium (EGM‐2) with 1% (wt/vol) P/S (Gibco) and were used at passages below 6. Red fluorescent protein (RFP)‐expressing HUVECs were obtained from Angio‐Proteomie. Normal human LFs (Lonza) were cultured in fibroblast growth medium (FGM‐2, Lonza) with 1% (wt/vol) P/S (Gibco) and used at Passage 6. Normal human fibroblasts (GM05659) were obtained from the Coriell Institute for Medical Research, and the iNSCs were the reprogrammed cells described in our previous study (Yu et al., 2015). These cells were maintained in NSC maintenance medium (1:1 mixture of ReNcell [Millipore] and KnockOut DMEM/F‐12 medium with the StemPro NSC SFM Supplement [Gibco], 1% (wt/vol) P/S [Gibco], bFGF [Gibco], and EGF [Gibco]). The NSC differentiation medium was Neurobasal‐A medium (Gibco) supplemented with 1% (wt/vol) P/S (Gibco), the B‐27 supplement (Gibco, 1×), the N2 supplement (Gibco, 1×), and the GlutaMAX supplement (Gibco, 1×). iNSCs were transduced with a green fluorescent protein (GFP)‐encoding retroviral vector for visualization within the chip. To prepare the retrovirus, the pMX‐GFP vector and retrovirus packaging vectors were cotransfected into 293FT cells (Invitrogen) using the FuGENE 6 transfection reagent (Promega). Viral supernatants were collected at 48 h posttransfection, filtered through a 0.45‐μm pore‐sized PVDF membrane filter, and used to directly infect iNSCs.
2.2. Spheroid preparation
On Day 0 of organoid culture, iNSCs were dissociated using Accutase (Gibco) into single‐cell suspensions and added (9000 cells/well) to an ultralow‐binding 96‐well plate (Corning) in NSC maintenance medium. The neurospheres were incubated for 3 days. On Day 4, spheroids were introduced into the Sphero‐IMPACT for vascularization.
2.3. The vascularized 3D iNSC spheroid model
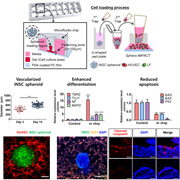
The Sphero‐IMPACT chip was used to construct the vascularized 3D iNSC spheroid model (Ko et al., 2019). We attached a silicone adhesive film (IS‐00820; IS Solutions) to the bottom of the chip and exposed the chip to plasma (Femto) for 1 min. A mixture of LFs, HUVECs, iNSC spheroids, and fibrin gel was then injected into the chip (Figure 1a). NSC differentiation medium/EGM‐2 (1:1 ratio) was then added and the cells were cultured for 14 days.
Figure 1.
The 3D iNSC spheroid model with a blood vessel network engineered using Sphero‐IMPACT. (a) Photograph of Sphero‐IMPACT, the computer‐aided design template, and the cell loading process. (b) Schematic of iNSC spheroid vascularization on a chip. Viability of iNSCs (c) and HUVECs (d) in various media, as revealed by MTT assay. (e) Left: the total vascular network area, measured by ImageJ software (NIH) during determination of the optimal gel conditions. Right: vascular network formation in various gels. Scale bar = 500 μm. (c–e) All experiments were performed in three chips (n = 24). The Kruskal–Wallis test with Dunn's post‐hoc test was used for comparisons. The data are mean ± SD. NS, not significant. *p < 0.05, **p < 0.01, and ****p < 0.0001
2.4. MTT assay
iNSCs and HUVECs were seeded at 3 × 10⁴ cells (at 24 h) or 1 × 10⁴ cells (at 72 h)/well into a 24‐well culture plate in NSC differentiation medium, EGM‐2, and a 1:1 mixture of the two. At various times, cell samples were incubated with 500 μl of MTT solution (0.5 mg/ml) for 4 h. After incubation, the MTT solution was removed and 500 μl of DMSO was added to dissolve the formazan crystals. Absorbance at 570 nm was immediately obtained using an Infinite200 PRO microplate reader (Tecan).
2.5. Immunostaining
For immunofluorescence staining, spheroids and cells were fixed in 4% (wt/vol) paraformaldehyde in phosphate‐buffered saline (PBS) for 15 min, permeabilized with 0.1% (vol/vol) Triton X‐100 in PBS for 30 min, and blocked in 3% (wt/vol) bovine serum albumin in PBS for 2 h at room temperature. Endothelial cells were marked using Ulex europaeus agglutinin 1 (Vector Laboratories) or anti‐CD31 antibody (catalog no. 303111; Biolegend). The neural spheroids were stained with Alexa Fluor 488‐conjugated anti‐olig2 antibody (MABN50A4; Sigma‐Aldrich), Alexa Fluor 594‐ conjugated anti‐SOX2 antibody (656106; Biolegend), Alexa Fluor‐488 conjugated anti‐neurofilament H antibody (801706; Biolegend), Alexa Fluor 594‐conjugated anti‐GFAP antibody (837509; Biolegend), Alexa Fluor 488‐conjugated anti‐β‐tubulin Class III antibody (560338; BD Bioscience), and Alexa Fluor 488‐conjugated anti‐MAP2 antibody (MAB3418X; Sigma‐Aldrich).
2.6. Imaging
The 3D spheroids and vessel cross sections were imaged using a confocal microscope (FV1000; Olympus). The microscope and charge‐coupled device (CCD) camera were controlled by MetaMorph time‐lapse imaging software (Molecular Devices). To assess signals, confocal microscopic images were acquired in a single session using identical exposure and gain settings.
2.7. Reverse transcriptase‐polymerase chain reaction (PCR)
To compare the gene expression level of neural spheroids in chip with or without vascular network, only neural spheroids were selected from the Sphero‐IMPACT. Total spheroid RNAs were isolated from each well using TRIzol reagent (Invitrogen) following the manufacturer's instructions, and cDNA was synthesized using Superscript reverse transcriptase (Invitrogen). Quantitative real‐time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) and the mRNA expression level of each gene was normalized to that of the housekeeping gene GAPDH. The primer sequences used are listed in Table S1.
2.8. Statistical analysis
All data are expressed as mean ± SD. The significance of differences was determined using the unpaired two‐tailed Student's t test or Kruskal–Wallis test with Dunn's post‐hoc test for multiple comparisons (as indicated in the figure legends). All analyses were performed using GraphPad Prism software (version 7.0; GraphPad Software, Inc.).
3. RESULTS
3.1. The vascularized neural spheroid‐on‐a‐chip model
To generate iNSC spheroids, equal numbers of dissociated single cells were seeded into the wells of an ultralow‐binding 96‐well plate 3 days before addition to the Sphero‐IMPACT system (Figure 1b). The chip was subjected to plasma treatment for robust cell patterning. The spheroids were embedded in a fibrin extracellular matrix with human umbilical vein endothelial cells (HUVECs) and lung fibroblasts (LFs) for vascularization, and cultured on the chip for up to 14 days. We assayed cell viability when optimizing the coculture medium (Figure 1c,d). One day after seeding, the iNSC proliferation rate was highest in NSC differentiation medium (Figures 1c and S1a), and HUVECs proliferated most readily in EGM‐2 medium (Figures 1d and S1b). These media, therefore, served as the respective controls. After 2 further days of culture, the viability of iNSCs and HUVECs in a 1:1 mixture of NSC differentiation medium and EGM‐2 did not differ from the controls. iNSCs barely proliferated in EGM‐2 medium and HUVECs did not grow in NSC differentiation medium. Therefore, we concluded that the mixed medium was optimal for coculture of neural spheroids and HUVECs. Next, we optimized the gel used for vascularization and spheroid culture. We assessed three gels: Matrigel (previously used for iNSC organoid culture) (Lee et al., 2020), fibrin (used for tumor spheroid‐on‐a‐chip culture) (Ko et al., 2019), and a 1:1 weight ratio of the two (Figure 1e). Spheroids tended to spread, and thus lose structural integrity, in Matrigel but not in the other two gels. In the fibrin gel, vessel connectivity and the vascular network area were optimal. Therefore, we concluded that the mixed medium and fibrin gel could be used for coculture of iNSC spheroids and HUVECs in the Sphero‐IMPACT system for 14 days.
3.2. Reconstruction of a perfusable vessel network in contact with neural spheroids
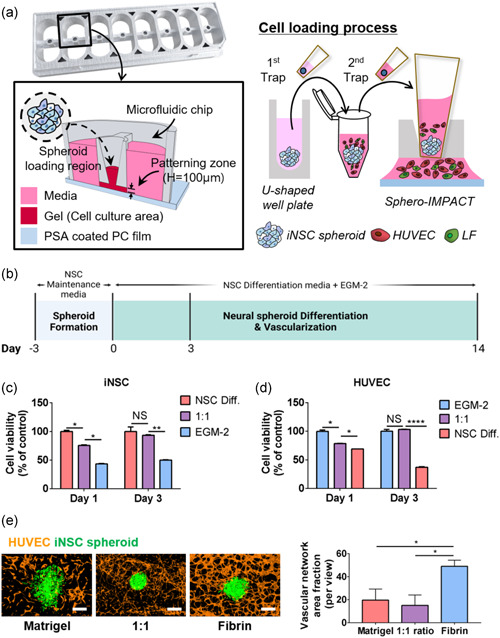
Spheroid size increased over time as differentiation and expansion proceeded (Figure 2a). Imaging on Day 14 confirmed that the vascular network was well‐formed; the microvasculature invaded into the spheroids about ∼33% (∼200 μm into the ∼600 μm diameter of spheroid) (Figures 2b and S2a, and Movie S1). The blood vessels were perfusable and lumina were evident (Figure 2c). We added PBS containing 2‐µm‐diameter fluorescent microbeads to the reservoir (Figure 2d; Movie S2). The beads moved via the blood vessels to the spheroids.
Figure 2.
Vascularization of iNSC neural spheroids in the chip. (a) Spheroid diameters on Days 3 and 14. The unpaired two‐tailed t test was used to compare mean diameters. ***p < 0.001. The data are mean ± SD. All experiments were performed in three chips (n = 24). (b) Projection of confocal image of 3D vascularized neural spheroid model. Magnified image showed the connection of vascular network and iNSC neural spheroid indicated by yellow arrows. Scale bar = 500 μm (left), 100 μm (right). (c) Cross‐sectional confocal image of the vessel network within the chip; lumina are apparent. Scale bar = 200 μm. (d) Transport of microbeads through the perfusable blood vessels was observed in real time. Scale bar = 200 μm
3.3. Differentiation and neuronal activity of the neural spheroid‐on‐a‐chip
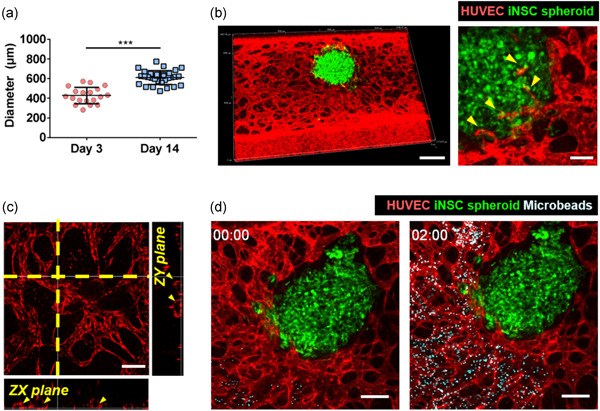
We performed quantitative PCR of specific markers of neural differentiation during culture (Figure 3a). The expression levels of genes encoding the intermediate progenitor marker TBR2, and the neuronal markers TUJ1, Neurofilament (NF), and MAP2, were higher on Day 14 than Day 3. Immunohistochemical analysis revealed differentiation toward the neural lineage over time (Figure 3b–f). SOX2, a specific marker of NSCs and progenitor cells, was expressed on Day 3 (Figure 3b). After 14 days of culture, the spheroids contained intermediate progenitor (TBR2+) cells (Figure 3c). On Day 14, immunostaining confirmed the expression of the mature neuronal markers TUJ1 (Figure 3d), Neurofilament‐H (NF‐H; Figure 3e), and MAP2 (Figure 3f), at which time the glial cell differentiation genes GFAP (an astrocyte marker) and OLIG2 (an oligodendrocyte marker) were also expressed (Figure 3g). GFAP (Figure S3a) and OLIG2 (Figure S3b) expression was confirmed by immunostaining. In contrast, iNSC spheroid could not maintain the morphology of spheroid in nonvascularized Sphero‐IMPACT (Figure S4a). Furthermore, the expression levels of differentiation markers, such as TUJ1, MAP2, GFAP, and OLIG2, were not remarkable as vascularized group on Day 14 (Figures S4b‐h). Together, the data show that vascularized iNSC neural spheroids differentiated toward the neural and glial lineages in the Sphero‐IMPACT. To demonstrate neuronal maturation, we also observed c‐fos expression of iNSC spheroid. We compared the c‐fos expression of spheroids at Day 3 and Day 14, respectively. The intensity of c‐fos expression was increased from Day 3 to 14 (Figures 3h and S3c,d). As the expression of c‐fos is induced by neuronal activity, the increment of the expression could demonstrate the neuronal activity of the organoid (Gibson et al., 2014; Hyung et al., 2019; J. Zhang et al., 2002).
Figure 3.
Differentiation and neuronal activity of iNSC neural spheroid in chip. (a) The mRNA expression of neuronal markers (TUJ1, MAP2, NF, and TBR2) was determined using quantitative PCR. The unpaired, two‐tailed t test to compare the spheroid in chip at Day 14 with the spheroid in chip at Day 3. (b–f) The expression of the neural progenitor (SOX2, b) and neuronal markers (TBR2, c; TUJ1, d; NF‐H, e; MAP2, f) was verified by immunostaining. Scale bar = 500 μm. (g) The mRNA expression of glial markers (GFAP and OLIG2) was determined using quantitative PCR. The unpaired, two‐tailed t test to compare the spheroid in chip at Day 14 with the spheroid in chip at Day 3. (h) The expression of the neuronal activity (c‐fos) was verified by immunostaining. We compared the intensity of spheroid in chip at day 14 with the spheroid in chip at Day 3. All experiments were performed in triplicate (N = 3). The results are presented as the means ± SD. **p < 0.01 and ***p < 0.001
3.4. The vascularized neural spheroid‐on‐a‐chip matured to a greater extent than spheroid in a dish
Brain vascularization is required for oxygen, nutrient, and waste exchange, as well as signal transmission. Also, the vascular network microenvironment maintains NSC homeostasis and plays essential roles in NSC self‐renewal and differentiation during embryonic development (Fantin et al., 2013; Otsuki & Brand, 2017). Therefore, we compared the vascularized neural spheroid‐on‐a‐chip to a control spheroid (cultured in a dish) to determine if the vascular network supported spheroid maturation and viability. The expression levels of genes encoding markers of neural progenitors or neurons were determined by quantitative PCR (Figure 4a,b). After 14 days of culture, the spheroid‐on‐a‐chip exhibited substantially lower expression levels of neural progenitor cell markers (SOX2 and PAX6) than the control (Figure 4a). Also, on Day 14, the expression levels of genes encoding an intermediate progenitor marker, TBR2, and the neuronal markers TUJ1, NF, and MAP2, were higher in the spheroid‐on‐a‐chip than the control (Figure 4b), as were the levels of genes encoding the glial cell differentiation markers GFAP (of astrocytes) and OLIG2 (of oligodendrocytes) (Figure 4c).
Figure 4.
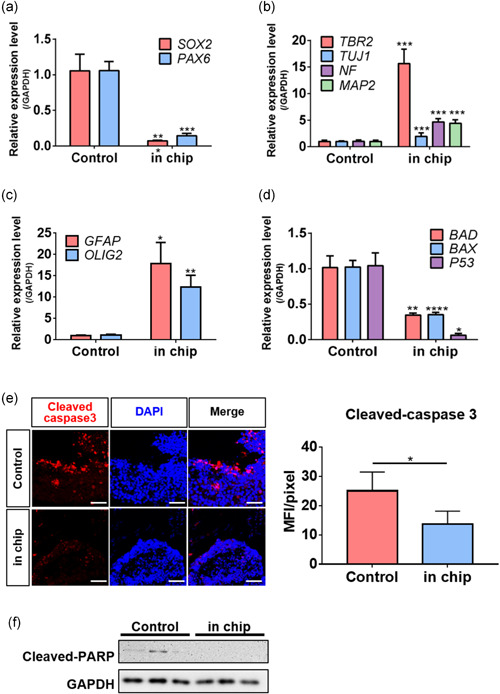
Vascularization in the chip enhanced iNSC neural spheroid differentiation and reduced apoptosis. (a–c) The levels of mRNAs encoding neural progenitor markers (SOX2 and PAX6, A), neuronal markers (TUJ1, MAP2, NF, and TBR2, b), and glial markers (GFAP and OLIG2) were determined by quantitative PCR. The unpaired two‐tailed t test was used to compare spheroids in the chip to conventional cultured spheroids on Day 14. (d) The levels of mRNAs encoding the apoptosis markers BAD, BAX, and P53 were determined by quantitative PCR. The unpaired two‐tailed t test was used to compare spheroids in the chip to conventional cultured spheroids on Day 14. (e) Immunostaining detected cleaved caspase 3 in iNSC neural spheroids, Scale bar = 100 μm. (F) The expression levels of cleaved PARP were analyzed by western blotting. All experiments were performed in three chips (n = 24). The results are means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001
3.5. Vascularization prevented apoptosis of neural spheroid
Above, we described how vasculature supported the neural differentiation of iNSC spheroids cultured on a chip. Typically, 3D spheroids and organoids stop growing when apoptosis develops in the core (where oxygen and nutrient levels are inadequate). An absence of vascularization is a major limitation of organoid culture; also, vascularization is essential for the generation of adequately sized, functional artificial tissues (S. Zhang et al., 2021). We hypothesized that vascularization of the chip would attenuate neural spheroid apoptosis. First, we evaluated the gene expression levels of BAX, BAD, and P53, which play key roles in the cell cycle and apoptosis (Chen et al., 2006) (Figure 4d). Chip culture reduced the expression levels of all three genes compared to culture in a dish. Immunostaining revealed that the number of cells positive for cleaved caspase 3, a hallmark of apoptosis, decreased with chip culture (Figure 4e). Thus, chip vascularization prevented neural spheroid apoptosis. Similarly, western blotting revealed a lower level of cleaved PARP protein with chip culture (Figure 4f). Taken together, vascularization in the chip reduced the apoptosis of neural spheroid.
4. DISCUSSION
We created a 3D model of vascularized iNSC spheroids using a microfluidic chip. Earlier brain organoid models lacked adequate nutrients and oxygen because there was no vascular network (Matsui et al., 2021), which led to apoptosis, abnormal differentiation, and proliferation. As blood vessels are required, we recently developed a microfluidic chip for construction of vascularized tumor spheroids via coculture of tumor spheroids and vessels (Ko et al., 2019). Here, iNSC spheroids were cocultured with a vascular network; this greatly enhanced differentiation and reduced apoptosis compared to dish culture.
Vascular networks facilitate neuronal outgrowth and neuronal network development by delivering adequate oxygen and nutrients (Carmeliet, 2003; Karakatsani et al., 2019; Taberner et al., 2020). In addition, endothelial cells, as a major component of vascular networks, influence NSCs via direct contact or secretion of cytokines and other factors (Goldberg & Hirschi, 2009). Brain NSCs are in direct contact with blood vessels, which maintain the NSCs via activation of Eph and Notch, and integrin signaling (Ottone et al., 2014; Shen et al., 2008). NSCs respond to factors secreted by endothelial cells; these factors promote NSC proliferation, neurogenesis, synaptogenesis, axonal growth, and neuroprotection (Goldberg & Hirschi, 2009). For example, endothelial cells secrete brain‐derived neurotrophic factor and NT‐3, which increases neurogenesis and neuronal differentiation (Delgado et al., 2014; Leventhal et al., 1999; Snapyan et al., 2009; Vilar & Mira, 2016). Endothelial cells secrete pigment epithelium‐derived factor (PEDF) which promotes neuronal proliferation or neurogenesis (Andreu‐Agulló et al., 2009; Ramírez‐Castillejo et al., 2006). Stromal cell‐derived factor 1, which is primarily secreted by blood vessels, also has been known to promote the NSC differentiation dose‐dependent manner (Caldwell et al., 2001; Cheng et al., 2017). Also, betacellulin or vascular endothelial growth factor regulates the proliferation and differentiation of NSCs (Gómez‐Gaviro et al., 2012; Sun et al., 2010). Taken together, in addition to the delivery of biological substances through microvasculature, these factors are supposed to support neuronal maturation and improve viability in our culture platform.
Several issues with this study should be noted. First, although vascularization enhanced cellular behavior, functional and structural analyses were not performed because the spheroid did not mimic a specific brain region. Second, our model is based on spheroids (micrometer scale), rather than organoids (generally millimeter scale). Thus, the chip cannot be used to engineer vascularized organoids.
5. CONCLUSIONS
The absence of vascular networks in 3D models of spheroids and organoids complicates attempts to mimic brain tissue. Here, we created a 3D model of vascularized iNSC spheroids using the Sphero‐IMPACT microfluidic system. Our vascular network wrapped around the neural spheroids and exhibited perfusion. Coculture of neural spheroids with perfusable blood vessels enhanced differentiation and reduced apoptosis. Therefore, our vascularized iNSC spheroid model represents a step toward 3D brain modeling. Neurovascular interaction is crucial for brain homeostasis, and to prevent many diseases. We suggest that this in vitro model of the neurovascular system advances our understanding of normal and pathological conditions, and could be used to develop therapeutics.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Kyung‐Sun Kang and Noo Li Jeon conceived the overall concept and design of this study. Nari Shin and Youngtaek Kim designed the figures, wrote the manuscript, and conducted data acquisition and interpretation. Jihoon Ko, Soon Won Choi, Sujin Hyung, Seung‐Eun Lee, Seunghyuk Park, and Jiyoung Song performed technical and material supports. Kyung‐Sun Kang and Noo Li Jeon supervised all work.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant nos. 2020R1A4A4078907 and 2021R1A3B1077481); and the Global PHD Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. 2018H1A2A1061663); and the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the International Cooperative R&D program (P0011266).
Shin, N. , Kim, Y. , Ko, J. , Choi, S. W. , Hyung, S. , Lee, S.‐E. , Park, S. , Song, J. , Jeon, N. L. , & Kang, K.‐S. (2022). Vascularization of iNSC spheroid in a 3D spheroid‐on‐a‐chip platform enhances neural maturation. Biotechnology and Bioengineering, 119, 566–574. 10.1002/bit.27978
Nari Shin and Youngtaek Kim contributed equally to this study.
Contributor Information
Noo Li Jeon, Email: njeon@snu.ac.kr.
Kyung‐Sun Kang, Email: kangpub@snu.ac.kr.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andreu‐Agulló, C. , Morante‐Redolat, J. M. , Delgado, A. C. , & Fariñas, I. (2009). Vascular niche factor PEDF modulates Notch‐dependent stemness in the adult subependymal zone. Nature Neuroscience, 12(12), 1514–1523. [DOI] [PubMed] [Google Scholar]
- Bershteyn, M. , Nowakowski, T. J. , Pollen, A. A. , Di Lullo, E. , Nene, A. , Wynshaw‐Boris, A. , & Kriegstein, A. R. (2017). Human iPSC‐derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell, 20(4), 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, A. M. , Ming, G.‐l , & Song, H. (2015). Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell, 17(4), 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir, B. , Xiang, Y. , Tanaka, Y. , Kural, M. H. , Parent, M. , Kang, Y.‐J. , Chapeton, K. , Patterson, B. , Yuan, Y. , He, C. S. , Raredon, M. , Dengelegi, J. , Kim, K. Y. , Sun, P. , Zhong, M. , Lee, S. , Patra, P. , Hyder, F. , Niklason, L. E. , … Park, I. H. (2019). Engineering of human brain organoids with a functional vascular‐like system. Nature Methods, 16(11), 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell, M. A. , He, X. , Wilkie, N. , Pollack, S. , Marshall, G. , Wafford, K. A. , & Svendsen, C. N. (2001). Growth factors regulate the survival and fate of cells derived from human neurospheres. Nature Biotechnology, 19(5), 475–479. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. (2003). Blood vessels and nerves: common signals, pathways and diseases. Nature Reviews Genetics, 4(9), 710–720. [DOI] [PubMed] [Google Scholar]
- Chen, L.‐H. , Hsu, C.‐Y. , & Weng, C.‐F. (2006). Involvement of P53 and Bax/Bad triggering apoptosis in thioacetamide‐induced hepatic epithelial cells. World Journal of Gastroenterology: WJG, 12(32), 5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Wang, H. , Zhang, X. , Zhao, S. , Zhou, Z. , Mu, X. , Zhao, C. , & Teng, W. (2017). The role of SDF‐1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Frontiers in Neuroscience, 11, 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma, E. , Merchant, S. , & Sugaya, K. (2010). Stem cells for the treatment of neurodegenerative diseases. Stem Cell Research & Therapy, 1(5), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, A. C. , Ferrón, S. R. , Vicente, D. , Porlan, E. , Perez‐Villalba, A. , Trujillo, C. M. , D′Ocón, P. , & Fariñas, I. (2014). Endothelial NT‐3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron, 83(3), 572–585. [DOI] [PubMed] [Google Scholar]
- Fantin, A. , Vieira, J. M. , Plein, A. , Maden, C. H. , & Ruhrberg, C. (2013). The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis. Nature Protocols, 8(2), 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, E. M. , Purger, D. , Mount, C. W. , Goldstein, A. K. , Lin, G. L. , Wood, L. S. , Inema, I. , Miller, S. E. , Bieri, G. , Zuchero, J. B. , Barres, B. A. , Woo, P. J. , Vogel, H. , & Monje, M. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science, 344(6183). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J. S. , & Hirschi, K. K. (2009). Diverse roles of the vasculature within the neural stem cell niche. Regenerative Medicine, 4(6), 879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Gaviro, M. V. , Scott, C. E. , Sesay, A. K. , Matheu, A. , Booth, S. , Galichet, C. , & Lovell‐Badge, R. (2012). Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proceedings of the National Academy of Sciences, 109(4), 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyung, S. , Lee, S. R. , Kim, Y. J. , Bang, S. , Tahk, D. , Park, J. C. , Suh, J. F. , & Jeon, N. L. (2019). Optogenetic neuronal stimulation promotes axon outgrowth and myelination of motor neurons in a three‐dimensional motor neuron–Schwann cell coculture model on a microfluidic biochip. Biotechnology and Bioengineering, 116(10), 2425–2438. [DOI] [PubMed] [Google Scholar]
- Karakatsani, A. , Shah, B. , & Ruiz de Almodovar, C. (2019). Blood vessels as regulators of neural stem cell properties. Frontiers in Molecular Neuroscience, 12, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B.‐E. , Choi, S. W. , Shin, J.‐H. , Kim, J.‐J. , Kang, I. , Lee, B.‐C. , Lee, J. Y. , Kook, M. G. , & Kang, K. S. (2018). Single‐factor SOX2 mediates direct neural reprogramming of human mesenchymal stem cells via transfection of in vitro transcribed mRNA. Cell Transplantation, 27(7), 1154–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.‐J. , Shin, J.‐H. , Yu, K.‐R. , Lee, B.‐C. , Kang, I. , Lee, J. Y. , Kim, D. H. , Seo, Y. , Kim, H. S. , Choi, S. W. , & Kang, K. S. (2017). Direct conversion of human umbilical cord blood into induced neural stem cells with SOX2 and HMGA2. International Journal of Stem Cells, 10(2), 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. S. , Kim, D.‐H. , Kang, H. K. , Kook, M. G. , Choi, S. W. , & Kang, K.‐S. (2021). Modeling of hypoxic brain injury through 3D human neural organoids. Cells, 10(2), 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, J. , Ahn, J. , Kim, S. , Lee, Y. , Lee, J. , Park, D. , & Jeon, N. L. (2019). Tumor spheroid‐on‐a‐chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab on a Chip, 19(17), 2822–2833. [DOI] [PubMed] [Google Scholar]
- Kwon, D. , Ahn, H.‐J. , & Kang, K.‐S. (2018). Generation of human neural stem cells by direct phenotypic conversion, Human neural stem cells (pp. 103–121). Springer. [DOI] [PubMed] [Google Scholar]
- Lancaster, M. A. , Renner, M. , Martin, C.‐A. , Wenzel, D. , Bicknell, L. S. , Hurles, M. E. , Homfray, T. , Penninger, J. M. , Jackson, A. P. , & Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.‐E. , Shin, N. , Kook, M. G. , Kong, D. , Kim, N. G. , Choi, S. W. , & Kang, K.‐S. (2020). Human iNSC‐derived brain organoid model of lysosomal storage disorder in Niemann–Pick disease type C. Cell Death & Disease, 11(12), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal, C. , Rafii, S. , Rafii, D. , Shahar, A. , & Goldman, S. A. (1999). Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Molecular and Cellular Neuroscience, 13(6), 450–464. [DOI] [PubMed] [Google Scholar]
- Mansour, A. A. , Gonçalves, J. T. , Bloyd, C. W. , Li, H. , Fernandes, S. , Quang, D. , Johnston, S. , Parylak, S. L. , Jin, X. , & Gage, F. H. (2018). An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology, 36(5), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, J. , Coppola, G. , Zhang, P. , Abyzov, A. , Provini, L. , Tomasini, L. , Amenduni, M. , Szekely, A. , Palejev, D. , Wilson, M. , Gerstein, M. , Grigorenko, E. L. , Chawarska, K. , Pelphrey, K. A. , Howe, J. R. , & Vaccarino, F. M. (2015). FOXG1‐dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell, 162(2), 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T. K. , Tsuru, Y. , Hasegawa, K. , & Kuwako, K. i (2021). Vascularization of human brain organoids. Stem Cells, 39, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Okano, H. (2002). Stem cell biology of the central nervous system. Journal of Neuroscience Research, 69(6), 698–707. [DOI] [PubMed] [Google Scholar]
- Otsuki, L. , & Brand, A. H. (2017). The vasculature as a neural stem cell niche. Neurobiology of Disease, 107, 4–14. [DOI] [PubMed] [Google Scholar]
- Ottone, C. , Krusche, B. , Whitby, A. , Clements, M. , Quadrato, G. , Pitulescu, M. E. , Adams, R. H. , & Parrinello, S. (2014). Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nature Cell Biology, 16(11), 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez‐Castillejo, C. , Sánchez‐Sánchez, F. , Andreu‐Agulló, C. , Ferrón, S. R. , Aroca‐Aguilar, J. D. , Sánchez, P. , Mira, H. , Escribano, J. , & Fariñas, I. (2006). Pigment epithelium–derived factor is a niche signal for neural stem cell renewal. Nature Neuroscience, 9(3), 331–339. [DOI] [PubMed] [Google Scholar]
- Shen, Q. , Wang, Y. , Kokovay, E. , Lin, G. , Chuang, S.‐M. , Goderie, S. K. , Roysam, B. , & Temple, S. (2008). Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell‐cell interactions. Cell Stem Cell, 3(3), 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapyan, M. , Lemasson, M. , Brill, M. S. , Blais, M. , Massouh, M. , Ninkovic, J. , Gravel, C. , Berthod, F. , Götz, M. , Barker, P. A. , Parent, A. , & Saghatelyan, A. (2009). Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain‐derived neurotrophic factor signaling. Journal of Neuroscience, 29(13), 4172–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Sha, B. , Zhou, W. , & Yang, Y. (2010). VEGF‐mediated angiogenesis stimulates neural stem cell proliferation and differentiation in the premature brain. Biochemical and Biophysical Research Communications, 394(1), 146–152. [DOI] [PubMed] [Google Scholar]
- Taberner, L. , Bañón, A. , & Alsina, B. (2020). Sensory neuroblast quiescence depends on vascular cytoneme contacts and sensory neuronal differentiation requires initiation of blood flow. Cell Reports, 32(2), 107903. [DOI] [PubMed] [Google Scholar]
- Vargas‐Valderrama, A. , Messina, A. , Mitjavila‐Garcia, M. T. , & Guenou, H. (2020). The endothelium, a key actor in organ development and hPSC‐derived organoid vascularization. Journal of Biomedical Science, 27, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar, M. , & Mira, H. (2016). Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Frontiers in Neuroscience, 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K.‐R. , Shin, J.‐H. , Kim, J.‐J. , Koog, M. G. , Lee, J. Y. , Choi, S. W. , Kim, H. S. , Seo, Y. , Lee, S. , Shin, T. H. , Jee, M. K. , Kim, D. W. , Jung, S. J. , Shin, S. , Han, D. W. , & Kang, K. S. (2015). Rapid and efficient direct conversion of human adult somatic cells into neural stem cells by HMGA2/let‐7b. Cell Reports, 10(3), 441–452. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, D. , McQuade, J. S. , Behbehani, M. , Tsien, J. Z. , & Xu, M. (2002). C‐fos regulates neuronal excitability and survival. Nature Genetics, 30(4), 416–420. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Wan, Z. , & Kamm, R. D. (2021). Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature. Lab on a Chip, 21(3), 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


