Summary
Background
Namodenoson, an A3 adenosine receptor (A3AR) agonist, improved liver function/pathology in non‐alcoholic steatohepatitis (NASH) preclinical models.
Aim
To evaluate the efficacy and safety of namodenoson for the treatment of non‐alcoholic fatty liver disease (NAFLD) with or without NASH
Methods
This phase 2 study included 60 patients with NAFLD (ALT ≥60 IU/L) who were randomised (1:1:1) to oral namodenoson 12.5 mg b.d. (n = 21), 25 mg b.d. (n = 19), or placebo (n = 20) for 12 weeks (total follow‐up: 16 weeks). The main efficacy endpoint involved serum ALT after 12 weeks of treatment.
Results
Serum ALT decreased over time with namodenoson in a dose‐dependent manner. The difference between change from baseline (CFB) for ALT in the namodenoson 25 mg b.d. arm vs placebo trended towards significance at 12 weeks (P = 0.066). Serum AST levels also decreased with namodenoson in a dose‐dependent manner; at 12 weeks, the CFB for 25 mg b.d. vs placebo was significant (P = 0.03). At Week 12, 31.6% in the namodenoson 25 mg b.d. arm and 20.0% in the placebo arm achieved ALT normalisation (P = 0.405). At week 16, the respective rates were 36.8% and 10.0% (P = 0.038). A3AR expression levels were stable over time across study arms. Both doses of namodenoson were well tolerated with no drug‐emergent severe adverse events, drug‐drug interactions, hepatotoxicity, or deaths. Three adverse events were considered possibly related to study treatment: myalgia (12.5 mg b.d. arm), muscular weakness (25 mg b.d. arm), and headache (25 mg b.d. arm).
Conclusion
A3AR is a valid target; namodenoson 25 mg b.d. was safe and demonstrated efficacy signals (ClinicalTrials.gov #NCT02927314).
Keywords: clinical trial, fibrosis, liver, non‐alcoholic fatty liver disease, non‐alcoholic steatohepatitis
Randomised clinical trial: A phase 2 double‐blind study of namodenoson in non‐alcoholic fatty liver disease and steatohepatitis. https://doi.org/10.1111/apt.16664
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is associated with obesity, visceral adiposity, type 2 diabetes mellitus (T2DM), and the spectrum of metabolic syndrome. 1 , 2 Non‐alcoholic steatohepatitis (NASH), the severe form of NAFLD, is characterised by lobular inflammation and hepatocellular ballooning apart from steatosis that is accompanied by fibrosis progression. 3 Long‐lasting NASH may progress to cirrhosis and hepatocellular carcinoma (HCC). 4
The prevalence of NAFLD/NASH increased dramatically in the last decades and is highly correlated to the rise of obesity and T2DM. 5 NAFLD is currently the most common aetiology for liver disease in Western countries. 6 Better understanding of the pathophysiology of NAFLD/NASH facilitated the development of therapies for these diseases. 7 Many novel therapeutics are currently under clinical development; however, the US Food and Drug Administration (FDA) has approved none so far. 7 , 8 , 9 , 10 , 11
A3 adenosine receptor (A3AR) is one of four receptors that mediate extracellular adenosine signalling. It is overexpressed in liver cells derived from inflammatory and tumour tissues, but not in normal liver cells. 12 , 13 , 14 Namodenoson is a synthetic highly selective A3AR agonist with robust anti‐inflammatory and anti‐cancer effects as demonstrated in experimental animal models of autoimmune hepatitis and in orthotopic and xenograft liver cancer models. 13 , 14 In two clinical studies in advanced HCC, namodenoson showed anti‐tumour activity with an excellent safety profile. 15 , 16
The effect of namodenoson on liver inflammation and fibrosis was demonstrated in NAFLD/NASH preclinical models. 17 Namodenoson significantly decreased the NAFLD activity score in the STAM model. It also improved alanine aminotransferase (ALT), adiponectin, and leptin values, as well as ameliorated liver inflammation/fibrosis in the carbon tetrachloride model. 17 Mechanistic studies suggest that namodenoson exerts this effect by de‐regulation of the phosphoinositide 3‐kinase (PI3K)/NF‑κB/Wnt/β‑catenin signalling pathway. 17 Namodenoson also acts as a protective agent in models of liver ischaemia and partial hepatectomy. 12 , 14
The anti‐NAFLD/NASH effect of namodenoson observed in the preclinical models alongside its hepatoprotective and anti‐cancer effects prompted its clinical development as a treatment for NAFLD/NASH since these patients are at high risk of progression to cirrhosis and HCC. This phase 2 randomised, placebo‐controlled, dose‐finding study investigated the efficacy and safety of namodenoson in NAFLD (with/without NASH).
2. METHODS
2.1. Study design and treatment
This multicentre randomised, double‐blind, placebo‐controlled study was conducted in three centres in Israel (Hadassah Hebrew University Medical Centre, Rabin Medical Centre, and the Holy Family Hospital). The study randomised patients with NAFLD and ALT ≥60 IU/L (1:1:1, using a central randomisation schedule generated by an independent biostatistician) to oral namodenoson 12.5 mg BD, 25 mg BD, or placebo BD for 12 weeks, and the total follow‐up was 16 weeks. The randomisation was stratified by the presence/absence of T2DM. Patients were evaluated regularly for safety, and indicators of efficacy were measured at baseline and Week 12.
All relevant national regulatory authorities and local Ethics Committees/Institutional Review Boards approved the study. The study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all patients. All authors had access to the study data, and reviewed and approved the final manuscript. ClinicalTrials.gov #NCT02927314.
2.2. Study participants
The study included patients aged ≥18 years with a diagnosis of NAFLD defined as hepatic steatosis ≥10% as determined by magnetic resonance imagining‐determined proton‐density fat‐fraction (MRI‐PDFF), and serum ALT ≥60 IU/L. Key inclusion criteria included ≥2 metabolic comorbidities out of (a) obesity defined as body mass index (BMI) ≥25 and ≤40 kg/m2 or waist circumference >102 to <200 cm for men and >88 to <200 cm for women; (b) T2DM; (c) controlled hypertension; (d) hypertriglyceridemia defined as >150 mg/dL; and (e) reduced high‐density lipoproteins cholesterol defined as <40 mg/dL in men or <50 mg/dL in women. Additionally, patients had to be with an acceptable hepatic metabolic/synthetic function (serum albumin ≥3.5 g/dL, international normalised ratio ≤1.4, and serum total bilirubin ≤2.0 mg/dL); absence of cirrhosis (FibroScan score of ≤F4 defined as liver stiffness measurement [LSM] of <12 kPa); absolute neutrophil count ≥1.0 × 109/L; platelet count ≥100 × 109/L; and serum creatinine ≤2.0 mg/dL. Key exclusion criteria included the presence of ascites; hepatic encephalopathy or other clinical evidence of cirrhosis; other active acute/chronic liver disease; familial dyslipidaemia; weight loss of >5% in the previous 6 months; bariatric surgery in the previous 5 years; type I diabetes; average daily alcohol intake >20 g/d for women and 30 g/d for men; haemoglobin A1c > 9.0%; treatment with vitamin E at ≥800‐1000 IU daily; and treatment with certain anti‐diabetic drugs (DPP‐4 inhibitor, GLP‐1 receptor agonists, pioglitazone, or SGLT2 inhibitors) unless dose and regimen have been stable for ≥3 months prior to screening.
2.3. Assessments
The main efficacy endpoint involved serum ALT at Week 12. Other endpoints included mean CFB in serum aspartate aminotransferase (AST) levels, the proportion of patients achieving ALT normalisation (ie, ALT≤upper limit of normal [ULN], which was defined as 33 and 41 IU/L for female and male patients, respectively), and CFB in adiponectin levels. Supplemental exploratory efficacy variables included liver fat volume and liver fat fraction as determined by MRI‐PDFF; liver stiffness, as measured by FibroScan; Fibrosis‐4 (FIB‐4) scores; controlled attenuation parameter (CAP) scores; FibroScan‐aspartate transaminase (FAST) scores, and body weight. Since liver biopsies were not performed, NASH presence was defined as having a FAST score >0.67. 18 Safety was also a main endpoint and was monitored through the assessment of adverse events (AEs) using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Changes from baseline in vital signs, laboratory parameters, and electrocardiograms were also assessed.
2.4. Biomarkers
The peripheral blood expression of A3AR was measured at baseline and Week 12. A3AR mRNA expression in white blood cells was determined from blood collected to PAXgene RNA tubes (Qiagen), using the QuantiGene Plex 2.0® assay (Thermo Fisher). β‐actin was used as a reference control, and the oligonucleotide probe sets were designed by Thermo Fisher. Luminescence from each specific probe set was captured by Glomax Multi (Promega). A3AR was expressed in units, where one unit was defined as the mean of A3AR expression in healthy subjects, as previously determined. 16
2.5. Statistical analysis
Power calculation determined that assuming a standard deviation of 30% and a difference of 30% in percent change from baseline in serum ALT levels between either dose of namodenoson and placebo, 17 patients per group provide 80% power for a 2‐sided test at a significance level of 0.05. Assuming a standardised between‐treatment difference of 0.93, 20 patients/group provide 80% power for a 2‐sided test at level 0.05.
Efficacy analyses were performed on the intent‐to‐treat (ITT) population (all treated patients with ≥1 post‐baseline efficacy assessment). Safety analysis was performed using the safety population (all patients who received at ≥1 dose of study medication). Descriptive statistics were used to summarise patient characteristics and safety. Analyses of CFB for each of the variables were performed using ANCOVA by visit. Ad hoc analysis utilised t‐tests for these analyses with the assumption of unequal variances. Ad hoc analysis of CFB in FAST score (within each arm) was performed on the ITT population using the sign test. All statistical tests were 2‐sided and P ≤ 0.05 was considered statistically significant. Statistical analyses were conducted using SAS® 9.4 (SAS Institute Inc).
3. RESULTS
3.1. Patient disposition and characteristics
A total of 60 patients were enrolled between November 2017 and October 2019, and were randomly assigned to namodenoson 12.5 mg BD (n = 21), namodenoson 25 mg BD (n = 19), or placebo (n = 20). Two patients were withdrawn from the namodenoson 12.5 mg BD arm (one due to non‐compliance and the other due to pregnancy of the patient's partner) and two from the 25 mg BD arm (one due to non‐compliance and the other was lost to follow up) (Figure 1). All 60 patients were included in the ITT and safety populations.
FIGURE 1.
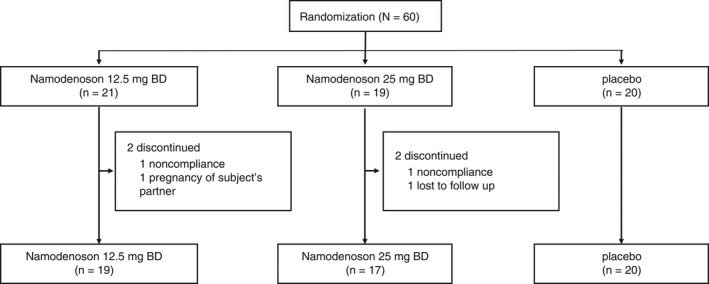
Patient disposition
Baseline demographics and disease characteristics were generally similar between the groups (Table 1). Overall, the mean (SD) age was 45 (12) years, the majority of patients were males (77%), and a quarter (25%) had T2DM. All patients except for one (in the placebo group) were White/Caucasian. At Screening, three patients (18.8%) in the 12.5 mg BD arm, four patients (28.6%) in the 25 mg BD arm, and none in the placebo arm had NASH, as defined in the current study (FAST score >0.67).
TABLE 1.
Baseline patient characteristics
|
Namodenoson 12.5 mg BD n = 21 |
Namodenoson 25 mg BD n = 19 |
Placebo n = 20 |
|
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 40.8 (12.7) | 47.9 (10.7) | 45.0 (12.4) |
| Median (range) | 39 (20‐72) | 48 (27‐68) | 47 (27‐66) |
| Male, n (%) | 16 (76.2%) | 14 (73.7%) | 16 (80.0%) |
| Race, n (%) | |||
| White/Caucasian | 21 (100.0%) | 19 (100.0%) | 19 (95.0%) |
| Black or African | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 1 (5.0%) |
| Diabetic (type 2) status, n (%) | |||
| Yes | 5 (23.8%) | 4 (21.1%) | 6 (30.0%) |
| No | 16 (76.2%) | 15 (78.9%) | 14 (70.0%) |
| Mean ALT (SD), IU/L | 71.9 (25.0) | 70.1 (23.4) | 64.4 (13.5) |
| Mean AST (SD), IU/L | 52.0 (34.6) | 41.3 (14.1) | 36.7 (11.7) |
| Mean adiponectin (SD), ng/mL a | 3652 (1564) | 3812 (2013) | 3149 (1990) |
| Mean liver fat volume (SD), mL b | 573 (281) | 554 (383) | 582 (285) |
| Mean liver MRI‐PDFF (SD), % c | 24.6 (8.2) | 23.5 (10.4) | 24.5 (6.9) |
| Mean liver stiffness (SD), kPa d | 8.4 (2.0) | 8.5 (2.5) | 7.6 (1.8) |
| Patients with CAP ≥331, n/n (%) e | 8/16 (50.0%) | 6/14 (42.9%) | 5/15 (33.3%) |
| Mean FIB‐4 score (SD) f | 1.19 (1.13) | 1.31 (0.55) | 1.02 (0.503) |
| Mean weight (SD), kg | 91.8 (18.3) | 96.7 (15.8) | 97.0 (13.4) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BD, twice a day; MRI‐PDFF, magnetic resonance imagining‐determined proton‐density fat‐fraction; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; SD, standard deviation.
Data were missing for 5, 4, and 3 patients in the 12.5 mg, 25 mg, and placebo arms, respectively.
Data at screening. Data were missing for 6, 9, and 3 patients in the 12.5 mg, 25 mg, and placebo arms, respectively.
Data at screening. Data were missing for 2, 2, and 1 patients in the 12.5 mg, 25 mg, and placebo arms, respectively.
Data at screening. Data were missing for 2 patients in the 12.5 mg and in the 25 mg arm.
Data at screening.
Data at screening. Data were missing for 1 patient in the placebo arm.
Mean treatment compliance rates were similar between the namodenoson arms (87.7% and 87.5% in the 12.5 mg BD and the 25 mg BD arms, respectively) and slightly higher in the placebo arm (93.6%). The mean duration of treatment was similar between the namodenoson 12.5 mg BD and the placebo arms (86.0 and 88.4 days, respectively). One patient in the 25 mg BD was lost to follow up; after excluding him, the mean duration of treatment for the 25 mg BD was also similar at 81.1 days.
3.2. Anti‐inflammatory effects of namodenoson
Analysis of the main endpoint revealed a decrease in serum ALT over time with namodenoson. The effect was more pronounced in the 25 mg BD arm (Figure 2A). The difference between the CFB for namodenoson 25 mg BD and placebo trended towards significance at 12 weeks (P = 0.066; ad hoc analysis). Although at Week 12, the difference in the proportion of patients achieving ALT normalisation between the namodenoson 25 mg BD arm (31.6%) and the placebo arm (20.0%) was not statistically significant (P = 0.405); at Week 16, the difference demonstrated statistical significance (36.8% vs 10.0%; P = 0.038; Figure 2B). In the namodenoson 12.5 mg BD arm, 19.0% of patients achieved ALT normalisation at 12 weeks and 23.8% at 16 weeks; however, the difference vs placebo (at both timepoints) was not statistically significant. Serum AST levels also decreased over time with namodenoson treatment in a dose‐dependent manner. The difference between the namodenoson 25 mg BD arm and placebo in CFB was statistically significant at Week 12 (P = 0.03; ad hoc analysis) (Figure 2C). In an analysis of the biomarker adiponectin levels (where data were missing for 20% of the patient population), increased adiponectin levels were noted between baseline and Week 12, further suggesting an anti‐inflammatory effect of namodenoson. At Week 12, CFB was statistically significantly higher in the namodenoson 12.5 mg arm compared to placebo (mean, 539 ng/mL vs −78 ng/mL, P = 0.032). CFB was also numerically higher in the 25 mg BD arm compared to placebo (mean, 220 ng/mL vs −78 ng/mL); however, the difference was not statistically significant (P = 0.216) (Figure 2D).
FIGURE 2.
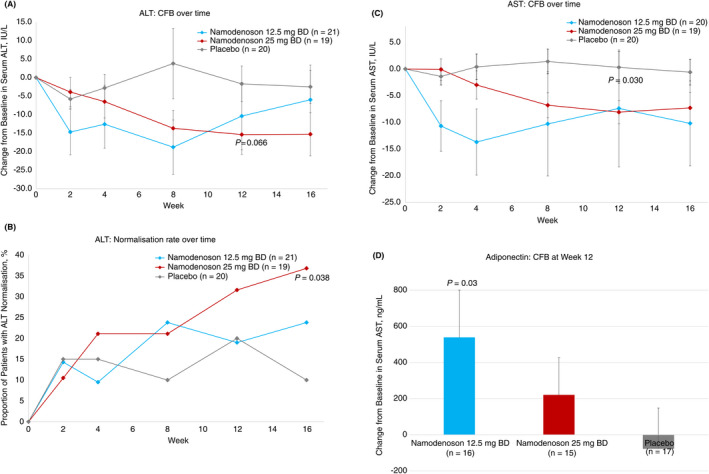
Changes from baseline (CFB) in inflammation‐related parameters. A, CFB in ALT levels over time. B, The proportion of patients achieving normalisation of ALT levels over time. C, CFB in AST levels over time. D, CFB in adiponectin levels at Week 12. Error bars represent SE P‐values for ALT and AST CFB were derived from ad hoc analyses
3.3. Effects of namodenoson on liver fat volume, liver fibrosis, and body weight
Measures of liver content and fibrosis were determined relative to screening (as patients underwent MRI and FibroScan then). Mean fat liver volume decreased over time with the greatest decrease observed with namodenoson 25 mg BD, for which the comparison to placebo trended towards significance (mean CFB at Week 12 −158.0 mL; P = 0.065 vs placebo; Figure 3A). MRI‐PDFF analysis revealed a numeric decreased in the fat fraction after 12 weeks in all study arms (mean [SE] MRI‐PDFF for namodenoson 12.5 mg BD at screening and Week 12: 24.6 [1.9] and 23.2 [2.2], respectively; for namodenoson 25 mg BD: 23.5 [2.5] and 19.5 [2.3], respectively; and for placebo: 24.5 [1.6] and 20.8 [1.9], respectively). Percent CFB (PCFB) was not statistically significantly different between the 12.5 mg BD arm and the 25 mg BD namodenoson arm vs placebo (P = 0.342 and P = 0.642, respectively).
FIGURE 3.
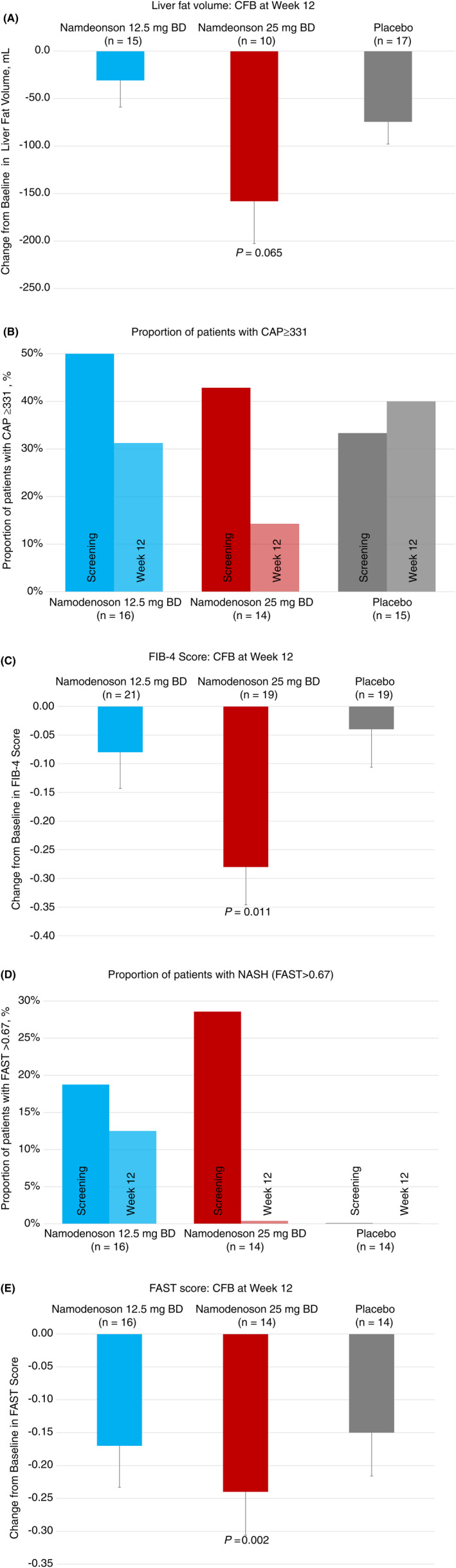
Changes from baseline in liver content and fibrosis‐related parameters. A, CFB in liver fat volume at Week 12. B, The proportion of patients with CAP ≥ 331 at screening and Week 12. C, CFB in FIB‐4 score at Week 12. D, The proportion of patients with NASH (FAST > 0.67) at screening and Week 12. E, CFB in FAST scores at Week 12. Error bars represent SE. Screening data were used as baseline
The findings were also supported by a decrease in the proportion of patients with high steatosis scores (CAP ≥331) in both the namodenoson 12.5 mg BD and the namodenoson 25 mg BD arms at Week 12 compared to screening (from 50% to 31% and from 43% to 14%, respectively), in contrast to the placebo arm where the proportion of such patients increased in this timeframe (from 33% to 40%), although the differences between the treatment arms and the placebo arm were not statistically significant (Figure 3B). Liver stiffness, as measured by FibroScan was overall similar at screening and Week 12 in all study arms (mean [SE] liver stiffness for namodenoson 12.5 mg BD at screening and Week 12: 8.4 [0.5] and 7.6 [0.6] kPa, respectively; for namodenoson 25 mg BD: 8.5 [0.6] and 8.4 [0.95] kPa, respectively; and for placebo: 7.6 [0.4] and 7.1 [0.5] kPa, respectively). PCFB was not statistically significantly different between the 12.5 mg BD arm and the 25 mg BD namodenoson arm vs placebo (P = 0.782 and P = 0.710, respectively).
A significant decrease in Fib4‐scores was observed between screening and Week 12 in the namodenoson 25 mg BD arm (Figure 3C; Week 12 mean CFB: −0.08, −0.28, and −0.04 for the 12.5 mg BD, 25 mg BD, and placebo, respectively; P = 0.011 for 25 mg BD vs placebo) suggesting a potential dose‐dependent inhibitory effect of namodenoson on fibrosis progression. Reduced liver fibrosis with namodenoson is also suggested by the decreased proportion of patients with NASH (as defined by FAST scores >0.67) from screening to Week 12 in both the namodenoson 12.5 mg BD and the 25 mg BD arms (Figure 3D). Specifically, all four patients with NASH at Screening in the 25 mg BD arm had their NASH resolved by Week 12 and no new patient in this study arm developed NASH; of the three patients with NASH at Screening in the 12.5 mg BD arm, one had the NASH resolved by Week 12 and no new patient in this study arm developed NASH. In the placebo arm, there were no patients with NASH at screening or Week 12. The difference between the namodenoson and placebo arms with respect to the CFB in the proportion of patients with NASH was not statistically significant. Within group analysis evaluating FAST scores at Screening and Week 12 demonstrated a statistically significant reduction in FAST scores in the 25 mg BD group (P = 0.002, sign test) and a reduction that trended towards significance in the 12.5 mg BD group (P = 0.077, sign test). The reduction observed in the placebo arm was non‐significant (Figure 3E).
A decrease in body weight over the study period was observed in both namodenoson arms and was more pronounced in the 25 mg BD arm (Figure 4). At Week 12, patients in the namodenoson 12.5 mg BD arm lost a mean (SE) of 1.6 (0.7) kg and those in the namodenoson 25 mg BD arm lost a mean (SE) of 2.1 (0.7) kg, whereas those in the placebo arm lost a mean (SE) of 0.5 (0.7) kg. The differences in CFB between the namodenoson arms and placebo were not statistically significant (P = 0.232 and P = 0.122 for the 12.5 mg BD and 25 mg BD arms vs placebo, respectively).
FIGURE 4.
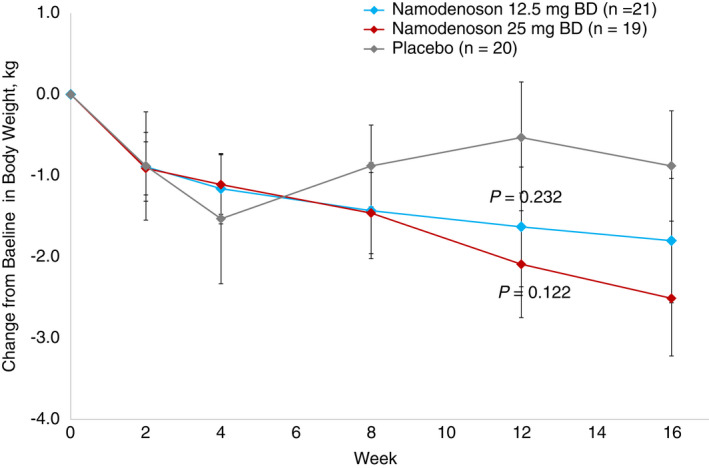
CFB in body weight with namodenoson treatment over time. Error bars represent SE P‐values were derived from ad hoc analyses
3.4. A3AR expression over time
A3AR expression levels were stable over time in all study arms. The mean (SD) CFB at Week 12 was 0.076 (0.344), 0.093 (0.335), and 0.027 (0.528) units for the namodenoson 12.5 mg BD, 25 mg BD, and the placebo arms, respectively.
3.5. Safety
Namodenoson was well‐tolerated at both doses. No patient withdrew due to toxicity. No drug‐drug interactions were reported. No severe treatment‐related adverse events (TEAEs) were reported and three patients (all in the 12.5 mg BD group) had moderate severity TEAEs (Table 2). In the namodenoson arms, three TEAEs (7.5%) were considered as possibly related to treatment, the remaining TEAEs were considered not‐related to study treatment (Table 2). The three TEAEs considered possibly related to study treatment included myalgia in the 12.5 mg BD arm, and one case each of muscular weakness and headache in the 25 mg BD arm (Table 2). No hepatotoxicity was observed and no deaths occurred during the study.
TABLE 2.
Adverse events
|
Namodenoson 12.5 mg BD n = 21 |
Namodenoson 25 mg BD n = 19 |
Placebo n = 20 |
|
|---|---|---|---|
| Patients with at least 1 TEAE, n (%) | 11 (52.4%) | 7 (36.8%) | 4 (20.0%) |
| Highest AE severity grade, n (%) a | |||
| Mild | 9 (42.9%) | 7 (36.8%) | 4 (20.0%) |
| Moderate | 3 (14.3%) | 0 | 0 |
| Severe | 0 | 0 | 0 |
| Strongest relationship of AE, n (%) | |||
| Not related | 10 (47.6%) | 5 (26.3%) | 4 (20.0%) |
| Possibly | 1 (4.8%) | 2 (10.5%) | 1 (5.0%) |
| Probably or definitely | 0 | 0 | 0 |
| Gastrointestinal disorders, n (%) | 1 (4.8%) | 1 (5.3%) | 2 (10.0%) |
| Abdominal distension | 0 | 1 (5.3%) | 2 (10.0%) |
| Abdominal pain | 1 (4.8%) | 0 | 0 |
| Diarrhoea | 0 | 0 | 1 (5.0%) |
| Dyspepsia | 0 | 0 | 1 (5.0%) b |
| Toothache | 0 | 1 (5.3%) | 0 |
| Vomiting | 1 (4.8%) | 0 | 0 |
| General disorders and administrative site conditions, n (%) | 1 (4.8%) | 1 (5.3%) | 2 (10.0%) |
| Chest pain | 0 | 1 (5.3%) | 0 |
| Fatigue | 0 | 1 (5.3%) | 0 |
| Infections and infestations, n (%) | 3 (14.3%) | 1 (5.3%) | 0 |
| Otitis media | 2 (9.5%) | 0 | 0 |
| Tonsillitis | 1 (4.8%) | 0 | 0 |
| Upper respiratory tract infection | 1 (4.8%) | 0 | 0 |
| Viral infection | 0 | 1 (5.3%) | 0 |
| Injury, poisoning and procedural complications, n (%) | 3 (14.3%) | 0 | 0 |
| Face injury | 1 (4.8%) | 0 | 0 |
| Paternal exposure during pregnancy | 1 (4.8%) | 0 | 0 |
| Skin laceration | 1 (4.8%) | 0 | 0 |
| Musculoskeletal and connective tissue disorders, n (%) | 3 (14.3%) | 1 (5.3%) | 2 (10.0%) |
| Arthralgia | 1 (4.8%) | 0 | 0 |
| Back pain | 1 (4.8%) | 0 | 1 (5.0%) |
| Gouty arthritis | 0 | 0 | 1 (5.0%) |
| Muscular weakness | 0 | 1 (5.3%) b | 0 |
| Myalgia | 1 (4.8%) b | 0 | 0 |
| Nervous system disorders, n (%) | 1 (4.8%) | 2 (10.5%) | 0 |
| Headache | 0 | 1 (5.3%) b | 0 |
| Syncope | 1 (4.8%) | 0 | 0 |
| Transient ischaemic attack | 0 | 1 (5.3%) | 0 |
Abbreviation: TEAE, treatment‐emergent adverse events.
Patients could be counted twice if their AEs were in different system organ classes.
TEAEs considered possibly related to treatment.
4. DISCUSSION
Our multicentre phase 2 study suggests that namodenoson may confer clinical benefit in NAFLD (with or without NASH) including improvements in various hepatic parameters such as ALT, AST, and adiponectin alongside a favourable safety profile.
The effect of namodenoson on increased ALT/AST levels (which are known to be associated with hepatocellular injury) and adiponectin (an anti‐inflammatory/anti‐fibrotic cytokine) suggests an anti‐inflammatory effect of namodenoson. Notably, more than a third (36.8%) of patients in the namodenoson 25 mg BD group achieved serum ALT normalisation at Week 16, which was significantly more than in the placebo group (10.0%, P = 0.038). Furthermore, approximately a quarter (23.8%) of patients in the namodenoson 12.5 mg BD arm achieved serum ALT normalisation at Week 16 suggesting a continued anti‐inflammatory effect after treatment discontinuation.
Namodenoson also had a clinical benefit on other hepatic parameters, including the proportion of patients with high steatosis scores (CAP ≥331), a decrease in Fib4‐scores and in the proportion of patients with NASH (as defined by FAST scores >0.67 18 ), and a decrease in FAST scores from Screening to Week 12 within the namodenoson arms. Notably, as the duration of the study was 12 weeks, the favourable effect of namodenoson on some of these parameters likely stems from the effect of namodenoson on ALT and AST. Still, these observed benefits suggest an inhibitory effect of namodenoson on the progression of liver fibrosis. Interestingly, patients across all arms in our study exhibited a loss in body weight. A reduction in body weight has secondary benefits on cardiovascular morbidity, which, in turn, could also improve liver health. Body weight loss is clinically important, since NAFLD/NASH patients are characterised by a high prevalence of obesity, T2DM and metabolic syndrome, and since the cause of death in these patients is often related to cardiovascular disease (reviewed in Ref. [19]). The cardioprotective and neuroprotective characteristics of namodenoson, established in preclinical models, 20 , 21 may contribute to its clinical benefits in NAFLD/NASH. The potential synergistic effect of namodenoson with medications to treat cardiovascular disease or metabolic syndrome was not investigated in the current study and is worth exploring.
The effects of namodenoson were, for the most part, dose‐dependent, with the 25 mg BD demonstrating more benefit than the 12.5 mg BD dose. From a safety perspective, both doses were equally safe, with no severe TEAEs, hepatotoxicity, or deaths reported during the study. Therefore, future studies in NAFLD/NASH patients will be conducted with the 25 mg BD dose.
Our results regarding safety and efficacy are consistent with the known differential effect of namodenoson on pathologic vs normal cells in preclinical studies, where A3AR activation by namodenoson induced apoptosis of inflammatory and cancer cells. The mechanism of action entails deregulation of both the NF‐κB and the Wnt/β‐catenin signalling pathways leading to inhibition of inflammatory cytokine production, whereas normal cells were not affected. 13 , 14 Our safety results are also consistent with findings from clinical studies investigating namodenoson in advanced HCC. 15 As in prior studies, no clinically meaningful drug‐drug interactions were reported, which is key, as NAFLD/NASH patients typically receive additional therapies for the abovementioned prevalent comorbidities such as T2DM.
At present, no therapeutic has been FDA‐approved for NAFLD/NASH. The therapies that are currently under clinical development vary with respect to mechanism of action, and no other drug candidate targets A3AR. 8 , 9 , 10 , 11 Namodenoson is also different than some of the other investigated drugs as it is an orally administered drug (unlike the GLP‐1 inhibitor liraglutide that is administered via subcutaneous injection 22 ).
Notably, since A3AR belongs to a family of Gi protein‐associated receptors that were shown to undergo desensitisation/re‐sensitisation in response to agonist treatment, 23 we examined A3AR expression levels before and after treatment. Consistent with our previous clinical study in HCC, 16 we found that A3AR expression remained stable after 12 weeks of treatment suggesting that A3AR is a valid target that is not desensitised by chronic exposure to namodenoson.
The study is limited by the relatively small number of patients in each study arm and the homogenous patient population (all patients except one were White). The study is also limited by the difference in the proportion of patients with FAST score >0.67 at randomisation and the paucity of data in some measurements (eg, adiponectin levels at Week 12). Another limitation is the short duration of active treatment (12 weeks), which impeded our ability to observe changes in liver stiffness (eg, changes observed in FAST likely stemmed from changes in AST levels). Also, in the current study, patients did not undergo post‐treatment liver biopsy to determine the impact of the study drug on fibrosis or NASH. A longer‐term study with a larger sample size, a more diverse patient population, and that includes post‐treatment liver biopsy is required to fully assess the potential clinical utility of namodenoson in NAFLD/NASH. Such a study is currently underway (ClinicalTrials.gov # NCT 04697810).
In conclusion, our results suggest that namodenoson 25 mg BD has signs of efficacy as a treatment for NAFLD/NASH alongside a good safety profile. Further clinical evaluation of namodenoson 25 mg BD for this indication is underway.
AUTHORSHIP
Guarantor of the article: PF.
Author contributions: RS and PF contributed to the study concept and design. RS, MB, AF, YM, MM, DH, WH, AI and NL were investigators, supervised study‐related activities at their study sites, and contributed to data acquisition, analysis and interpretation of data. ZH, MF, II, ABS, MHS and PF contributed to analysis and interpretation of data. All authors contributed to the drafting, critical revision for intellectual content, and final approval of the manuscript.
ACKNOWLEDGEMENTS
Declaration of personal interests: Rifaat Safadi and Michael H. Silverman are consultants and shareholders at Can‐Fite BioPharma Ltd; Avital Bareket‐Samish is a consultant and a medical writer for Can‐Fite BioPharma Ltd. Zivit Harpaz, Motti Farbstein, Inbal Itzhak, and Pnina Fishman are employed by and shareholders at Can‐Fite BioPharma Ltd; the remaining authors declare no conflict of interest.
Declaration of funding interests: The study was funded by Can‐Fite BioPharma Ltd.
Safadi R, Braun M, Francis A, et al. Randomised clinical trial: A phase 2 double‐blind study of namodenoson in non‐alcoholic fatty liver disease and steatohepatitis. Aliment Pharmacol Ther. 2021;54:1405–1415. doi: 10.1111/apt.16664
The Handling Editor for this article was Dr Rohit Loomba, and it was accepted for publication after full peer‐review.
Funding information
The work was supported by Can‐Fite BioPharma, Ltd.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ratziu V, Bellentani S, Cortez‐Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372‐384. [DOI] [PubMed] [Google Scholar]
- 2. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 3. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 4. Angulo P, Kleiner DE, Dam‐Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kabbany MN, Selvakumar PKC, Watt K, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112:581‐587. [DOI] [PubMed] [Google Scholar]
- 6. Liu A, Galoosian A, Kaswala D, et al. Nonalcoholic fatty liver disease: epidemiology, liver transplantation trends and outcomes, and risk of recurrent disease in the graft. J Clin Transl Hepatol. 2018;6:420‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vuppalanchi R, Noureddin M, Alkhouri N, et al. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2021;18:373‐392. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi Y, Sugimoto K, Inui H, et al. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:3777‐3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillaume M, Ratziu V. Pharmacological agents for nonalcoholic steatohepatitis. Hepatol Int. 2013;7:833‐841. [DOI] [PubMed] [Google Scholar]
- 10. Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol. 2015;62:S65‐75. [DOI] [PubMed] [Google Scholar]
- 11. Cave MC, Clair HB, Hardesty JE, et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 2016;1859:1083‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohana G, Cohen S, Rath‐Wolfson L, et al. A3 adenosine receptor agonist, CF102, protects against hepatic ischemia/reperfusion injury following partial hepatectomy. Mol Med Rep. 2016;14:4335‐4341. [DOI] [PubMed] [Google Scholar]
- 13. Cohen S, Stemmer SM, Zozulya G, et al. CF102 an A3 adenosine receptor agonist mediates anti‐tumor and anti‐inflammatory effects in the liver. J Cell Physiol. 2011;226:2438‐2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bar‐Yehuda S, Stemmer SM, Madi L, et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de‐regulation of the Wnt and NF‐kappaB signal transduction pathways. Int J Oncol. 2008;33:287‐295. [PubMed] [Google Scholar]
- 15. Stemmer SM, Benjaminov O, Medalia G, et al. CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open‐label, dose‐escalation study. Oncologist. 2013;18:25‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stemmer SM, Manojlovic NS, Marinca MV, et al. Namodenoson in advanced hepatocellular carcinoma and Child‐Pugh B cirrhosis: randomized placebo‐controlled clinical trial. Cancers. 2021;13:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fishman P, Cohen S, Itzhak I, et al. The A3 adenosine receptor agonist, namodenoson, ameliorates nonalcoholic steatohepatitis in mice. Int J Mol Med. 2019;44:2256‐2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newsome PN, Sasso M, Deeks JJ, et al. FibroScan‐AST (FAST) score for the non‐invasive identification of patients with non‐alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glass LM, Hunt CM, Fuchs M, et al. Comorbidities and nonalcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36:64‐71. [PMC free article] [PubMed] [Google Scholar]
- 20. Cross HR, Murphy E, Black RG, et al. Overexpression of A(3) adenosine receptors decreases heart rate, preserves energetics, and protects ischemic hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1562‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G‐J, Harvey BK, Shen H, et al. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848‐1855. [DOI] [PubMed] [Google Scholar]
- 22. Liraglutide [Package Insert]. Novo Nordisk Inc; 2020. [Google Scholar]
- 23. Bünemann M, Lee KB, Pals‐Rylaarsdam R, et al. Desensitization of G‐protein‐coupled receptors in the cardiovascular system. Annu Rev Physiol. 1999;61:169‐192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


