Summary
Many plant species simultaneously interact with multiple symbionts, which can, but do not always, generate synergistic benefits for their host. We ask if plant life history (i.e. annual vs perennial) can play an important role in the outcomes of the tripartite symbiosis of legumes, arbuscular mycorrhizal fungi (AMF), and rhizobia.
We performed a meta‐analysis of 88 studies examining outcomes of legume–AMF–rhizobia interactions on plant and microbial growth.
Perennial legumes associating with AMF and rhizobia grew larger than expected based on their response to either symbiont alone (i.e. their response to co‐inoculation was synergistic). By contrast, annual legume growth with co‐inoculation did not differ from additive expectations. AMF and rhizobia differentially increased phosphorus (P) and nitrogen (N) tissue concentration. Rhizobium nodulation increased with mycorrhizal fungi inoculation, but mycorrhizal fungi colonization did not increase with rhizobium inoculation. Microbial responses to co‐infection were significantly correlated with synergisms in plant growth.
Our work supports a balanced plant stoichiometry mechanism for synergistic benefits. We find that synergisms are in part driven by reinvestment in complementary symbionts, and that time‐lags in realizing benefits of reinvestment may limit synergisms in annuals. Optimization of microbiome composition to maximize synergisms may be critical to productivity, particularly for perennial legumes.
Keywords: arbuscular mycorrhizal fungi (AMF), inoculation, mutualism, plant life history, rhizobia, symbiosis, synergism, tripartite
Introduction
Microbial symbionts can play a critical role in plant fitness, and the benefits plants derive from associations with these symbionts can have consequences for plant population dynamics, community composition, and ecosystem function (Friesen et al., 2011). While the effects of individual symbionts on plant performance are widely documented, in nature plants often interact with multiple symbionts simultaneously. The outcomes of multiple symbioses may vary greatly, with plant hosts showing synergistic growth responses, additive responses, or even reduced growth (Larimer et al., 2010; Afkhami et al., 2020). Understanding the conditions under which plants receive synergistic benefits from interacting with multiple symbionts would be a significant step toward management of the plant microbiome to optimize agronomic and environmental applications.
Plants derive synergistic benefits from associating with multiple symbionts when their growth exceeds additive expectations based on their growth with individual symbionts (Fig. 1). This has been demonstrated in many studies of legume–arbuscular mycorrhizal fungi (AMF)–rhizobia interactions (i.e. Jin et al., 2010; Abd‐alla et al., 2014; Larimer et al., 2014; Afkhami & Stinchcombe, 2016), in which AMF and rhizobia enhance plant growth by increasing availability of complementary, limiting nutrients (phosphorus (P) and nitrogen (N), respectively). Yet a meta‐analysis of studies examining the interactive effects of plant microbial symbionts found that in general, AMF and rhizobia do not act synergistically (Larimer et al., 2010). Variation in outcomes of this symbiosis likely depends on several factors, including abiotic environmental conditions. For example, if soil P or N are not limiting, plants may invest fewer resources in AMF or rhizobia (Shantz et al., 2016), lessening the likelihood of microbial synergism. Life history strategies of host plants may also influence microbial synergism, as plants are known to vary in their dependence on and investment in interactions with microbial symbionts (Bever et al., 1996; Koziol & Bever, 2015; Bauer et al., 2018). Notably, the previous meta‐analysis showing no overall synergism in legume–AMF–rhizobia interactions was dominated by studies of annual plants (Larimer et al., 2010), while many examples of synergism come from studies of perennial plants (Larimer et al., 2014; Ren et al., 2016; Primieri et al., 2021), suggesting that plant life‐history in particular may influence the outcome of plant–microbe interactions.
Fig. 1.

Graphical representation of synergism. Bars represent an average response variable (e.g. plant biomass) of uninoculated plants, plants inoculated with arbuscular mycorrhizal fungi (AMF) or rhizobia, and plants inoculated with both symbionts. Plants inoculated with both symbionts show synergistic growth responses if they perform better than expected based on the independent effects of AMF and rhizobia. Blue bars, rhizobia effects; yellow bars, AMF effects; orange bars, synergism effects.
Symbionts may be more likely to act synergistically when interacting with perennial plants because of differences between perennials and annuals in terms of life cycle. Long‐lived, late‐successional plant species have been shown to be more responsive to mycorrhizal fungi than early‐successional species (Siqueira & Saggin‐Júnior, 2001; Pasqualini et al., 2007; Koziol & Bever, 2015; Bauer et al., 2018) and more sensitive to mycorrhizal fungal species and/or strain identity (Koziol & Bever, 2016; Cheeke et al., 2019). Less is known about whether similar patterns exist in plant–rhizobium interactions. Differences between long‐ and short‐lived plants may arise due to the costs of associating with microbial symbionts and time lags in receiving benefits derived from them. For example, mycorrhizal fungi associations have been shown to be costly to plants in early stages of growth but provide long‐term benefits to plants in later growth stages (Johnson et al., 1997), as young plants have to invest carbon (photosynthate, lipids, etc.) in symbionts to initiate interactions, but may not immediately receive benefits (nutrients) from the symbionts. If reinvestment of gain from one symbiont into the complementary symbiont is necessary for realization of synergism, then there may be an additional lag in realization of this benefit. Annual legumes can have very short life‐spans and investment in roots can be reduced with initiation of flowering (Portes & Araújo, 2012), when plants begin preferentially allocating carbon to flower and fruit production, potentially reducing the opportunity to realize synergism compared to perennial legumes.
In addition to using legume–AMF–rhizobia interactions to examine context dependency in synergistic benefits, we can also explore potential mechanisms underlying synergism. Symbionts are predicted to act synergistically when they provide distinct functional benefits to plants (Stanton, 2003; Afkhami et al., 2014, 2020). In the case of the nutritional symbioses in legume–AMF–rhizobia interactions, synergism may reflect stoichiometric requirements of the legume, as has been argued for mycorrhizal fungal benefits (Johnson, 2010). Although AMF and rhizobia can provide more to their hosts than P and N, respectively (Qin et al., 2011; Delavaux et al., 2017), if we accept the widely held expectation that improved P nutrition is a primary benefit of AMF (Smith & Read, 2008) and improved N nutrition the primary benefit of rhizobia, associating with AMF or rhizobia alone will increase plant growth through increased access to P or N, respectively. However, growth of a legume associating with only one symbiont may become limited by the nutrient not provided by that symbiont (i.e. growth of a legume only associating with rhizobia may be limited by P, as N fixation is a P‐demanding process (Udvardi & Poole, 2013)). Associating with AMF and rhizobia simultaneously provides the plant with both of these potentially limiting, complementary nutrients (Afkami et al., 2020), which can be used for growth and further investment in each mutualism. This stoichiometric complementarity may then lead to growth greater than expected from the sum of the benefits of the two symbionts alone, because neither N nor P is limiting. Examining the N and P tissue concentration of legumes could provide insight into whether resource complementarity underlies synergistic outcomes: when grown in nutrient‐limited soil, plants associating with only rhizobia that become P limited would show increased tissue N concentration compared to uninoculated plants, and plants only associating with AMF that become N limited would show increased tissue P concentration. If synergistic growth is a result of simultaneous relaxation of N and P limitation, we may expect plants deriving synergistic growth benefits to have lower N and P tissue concentrations than plants associating with rhizobia or AMF alone, because instead of accumulating these nutrients, they can invest them in further growth. Examining plant investment in each mutualist (rhizobia nodule number/AMF colonization) will also help determine whether synergistic outcomes are linked to a greater ability of legumes to invest in their mutualists.
To explore context dependence in synergistic outcomes in legume–AMF–rhizobium interactions and the potential mechanisms underlying synergism, we conducted a meta‐analysis. Specifically, we asked (1) whether annual and perennial legumes differ in the benefits they derive from AMF and/or rhizobia; (2) whether N and P tissue concentrations support a stoichiometric complementarity mechanism underlying synergism; (3) whether co‐inoculation increases investment in AMF or rhizobium, and whether this differs between annual or perennial plant species; and (4) whether plant investment in symbionts is correlated with realized synergism. We hypothesized that: (1) perennial legumes derive more benefits than annuals from rhizobia and/or AMF, and are more likely to derive synergistic benefits from dual‐inoculation; (2) plants associating with rhizobia or AMF will display higher N and P tissue concentrations, respectively, than plants associating with both symbionts; (3) co‐inoculation will increase symbiont investment more in perennial plants than in annuals; and (4) investment in symbionts is positively correlated with synergistic growth benefits.
Materials and Methods
Literature search and data compilation
We searched articles using ISI Web of Science (All Databases; Thomson Reuters 2020) in September 2020, using the topic search terms ‘mycorr* AND rhiz* AND nitrogen fix*’. This initial search yielded 776 studies. The use of the ‘*’ character ensured that words such as mycorrhizae, mycorrhizas, mycorrhizal, rhizobia and rhizobium were included. Additionally, we surveyed references used in meta‐analysis (Larimer et al., 2010; Morris et al., 2007), and in Portuguese language (Carvalho & Moreira, 2010), which yielded 80 and 49 additional studies, respectively. We screened studies following the Preferred Reporting Items for Meta‐Analyses (PRISMA) guidelines (Moher, 2009; Supporting Information Fig. S1). Our criteria for inclusion of a paper in the meta‐analysis was that the study (1) included a legume plant, a rhizobium and an AMF; (2) included an experiment that followed a full‐factorial design, with a noninoculated control, a rhizobium and AMF individual inoculum, and a combined treatment (AMF + rhizobia); and (3) reported mean biomass and/or N and P tissue concentration and sample size of plants grown under each experimental treatment level. Thus, 88 studies fit our criteria for inclusion in the meta‐analysis (Notes S1).
When studies included multiple species of either legumes or AMF/rhizobia, we extracted multiple data points, generating more than one effect size for the same study, similar to the approach taken in other meta‐analyses (i.e. Lekberg & Koide, 2005; Larimer et al., 2010; He & Dijkstra, 2014; Hoeksema et al., 2018). For studies that provided data on more than one time point, we only used the final time point. For each data point in each study, we recorded the plant species examined, experiment type (glasshouse or field), species/strain of symbionts manipulated, duration of experiment, substrate type, and substrate sterilization method. Plant species were categorized by life history (annual or perennial; Table 1). In total, the data included 24 perennial species and 12 annual species (Table 1), associating with many AMF and rhizobia species (Fig. S2). Therefore, 17% and 83% of data points came from field and glasshouse studies, respectively, and 27% and 73% of studies used unsterilized and sterilized substrates, respectively, with roughly the same percentage of annual and perennial species represented in each category. We recorded plant response as total mean dry biomass (root and shoot, or only shoot when root biomass was unavailable), height, N and P content, nodulation (i.e. number of nodules and nodule dry weight), mycorrhizal total colonization (i.e. percent AMF colonization) and included sample size, mean effect, and standard deviation (SD)/error (we converted reported standard error to SD for analysis). For data presented in graphical format, we used ImageJ (http://rsbweb.nih.gov/ij/) to accurately measure means and errors. When studies failed to report the SD or other metrics that could be used to compute it, we followed the methods of Hoeksema et al. (2018) to estimate . Briefly, we calculated the coefficient of variation () for every study that did report error, and used these studies to compute a median coefficient of variation that was then assigned to studies that did not report error. This method is similar to the prognostic method used by Ma et al. (2008), but scales deviation values by the mean, which was needed as data from different studies spanned several orders of magnitude.
Table 1.
Species of plants included in the meta‐analysis, categorized by plant life history.
| Perennial | Annual |
|---|---|
| Acacia auriculiformis A. Cunn. ex Benth. | Cicer arietinum L. |
| Acacia crassicarpa A. Cunn. ex Benth. | Glycine max (L.) Merr |
| Acacia cyclops A. Cunn. ex G. Don | Lathyrus sativus L. |
| Acacia mangium Willd. | Lens culinaris Medik. |
| Acacia saligna (Labill.) | Medicago truncatula Gaertn. |
| Acacia tortillis (Forsk.) | Phaseolus lunatus L. |
| Amorpha canescens Pursh | Phaseolus vulgaris L. |
| Anadenanthera macrocarpa (Benth.) Brenan | Pisum sativum L. |
| Anadenanthera peregrina (L.) Speg. | Sesbania cannabina (Retz.) |
| Anthyllis cytisoides L. | Vicia faba L. |
| Cajanus cajan (L.) Millsp. | Vigna radiata (L.) R. Wilczek |
| Calliandra calothyrsus Meisn. | Vigna unguiculata (L.) Walp. |
| Dalbergia sissoo Roxb. ex DC. | |
| Glycyrrhiza uralensis Fisch. ex DC. | |
| Medicago sativa L. | |
| Mimosa caesalpiniifolia Benth. | |
| Mimosa scabrella Benth. | |
| Onobrychis viciifolia Scop. | |
| Piptadenia gonoacantha (Mart.) J.F. Macbr. | |
| Piptadenia paniculata Benth. | |
| Prosopis juliflora (Sw.) | |
| Pterocarpus santalinus L.f. | |
| Robinia pseudoacacia L. | |
| Trifolium repens L. |
Calculation of effect sizes
We used the natural log of the response ratio (LRR) as a metric of the effect size in the meta‐analysis (Hedges et al., 1999). For a given study, we used the average response of plants inoculated with the focal symbiont (AMF or rhizobia) and the average response of uninoculated plants to calculate the effects of AMF (LRRamf) and rhizobia (LRRrhiz) inoculation as follows:
| (Eqn 1) |
| (Eqn 2) |
where a represents the mean of the noninoculated treatment, b and c are the means of inoculated treatments by rhizobia and AMF, respectively, and d is the mean of the combined treatment (rhizobia + AMF). We calculated the synergism effect () of co‐inoculation of AMF and rhizobia as the statistical interaction term
| (Eqn 3) |
Each of these three metrics are independent of each other and are directly analogous to the estimation of the two main effects and their interaction in a 2 × 2 full factorial ANOVA (Crawford et al., 2019). We calculated LRRs in this manner for all measured response variables (plant biomass, height, N and P content; see Methods S1 for details). We also calculated LRRs for symbiont response to co‐inoculation for mycorrhizal colonization () and nodulation (nodule number and biomass) () as
| (Eqn 4) |
| (Eqn 5) |
Estimates of pooled variances of averaged effects were calculated as
| (Eqn 6) |
| (Eqn 7) |
| (Eqn 8) |
where for each average effect x = a, b, c, d, we used nx to designate the sample size, the mean across studies and Var x the pooled variance of across studies.
Statistical analyses
To assess the overall effect of microbes on plant/microbial responses (the LRRs described earlier) we used multi‐level random effects models. We tested for consistency of patterns across studies, plant species, and symbiont identity by including the following five random effects in our models: study ID, experiment ID nested within study ID, plant species nested within plant life history, AMF species, and rhizobia strain nested within rhizobia species. These random effects account for nonindependence of inclusion of multiple data points from individual studies as well as multiple studies of the same plant species, fungal species or rhizobia isolate. To assess how plant life history (annual/perennial), substrate sterilization method (sterilized/unsterilized) and experiment type (glasshouse/field) affected plant/microbial responses, we used meta‐regression models with these factors as fixed effects, and included the same random effects listed earlier. Although we also recorded data on experiment duration and substrate type when available, not enough of the studies provided this information for us to be able to test these factors in our models. Models were calculated using the rma.mv function in the metafor package (Viechtbauer, 2010) in R v.4.0.2 (R Core Team, 2020), using a restricted maximum likelihood estimator. In a small number of cases, models would not converge when all random effects were included, at which point we removed rhizobia strain nested within rhizobia species (but kept rhizobia species) as a random effect to get models to converge.
To test whether there were differences between different levels of fixed effects, we used tests of moderators, results presented as Q M (Wald‐type chi‐square tests to determine whether a fixed effect is statistically significant at P < 0.05) (Viechtbauer, 2010). We visualized the effect sizes by setting the other explanatory factors to their average values. We depict results using mean response ratios and standard errors. To further illustrate the mean synergism response ratio (Eqn 3) as in Fig. 2, we back‐calculated estimates of average individual experimental means (see Methods S1 for derivation; Table S1). To determine whether symbiont responses (mycorrhizal colonization, nodulation) are correlated with synergism in plant growth and whether this varies with plant life history, we calculated meta‐regression models similar to those described earlier but with LRR symbiont response, plant life history, and their interaction as fixed effects.
Fig. 2.

Effects of inoculation on plant biomass (a), shown as the log response ratio (LRR ± SE) calculated according to Eqn 1 for LRRamf, Eqn 2 for LRRrhiz and, Eqn 3 for LRRsyn. A positive LRR for plants inoculated with individual symbionts indicates a positive effect of inoculation on plant biomass compared to controls. A positive LRR synergism indicates that co‐inoculation had a greater effect on plant biomass than expected based on additive effects of individual symbionts. Panels (b, c) represent biomass synergism for perennial plants (b) and lack of biomass synergism (statistically not different from additive effects) for annuals (c) in terms of biomass results in a regular full‐factorial graph. Blue bars, the rhizobia effect; yellow bars, the arbuscular mycorrhizal fungi effect; orange bars, the synergism effect; green bars, the lower than additive effect of co‐inoculation. ‘n’ represents the number of data points/studies in each group. Asterisks represent significant differences between annual and perennial plants in a given treatment as calculated by meta‐regression analysis (***, P < 0.001). Dotted lines in (b, c) represent the additive expectation from co‐inoculation.
We assessed potential publication bias by examining funnel plots (effect size variance plotted against residual values) for each model (Fig. S3), although these plots should be interpreted with caution when using mixed‐effects models as nonindependent data points may cluster (Lau et al., 2006). We also conducted modified Egger's regression (Egger et al., 1997) by adding the sample variance to our models as a moderator; a significant variance effect would indicate asymmetry in funnel plots and suggest publication bias. We examined sensitivity of all models by fitting them with and without influential outliers. We considered effect sizes with standardized residual values > 3 and hat values (standardized measures of deviation from expectation) greater than two times the mean hat value to be influential outliers (Habeck & Schultz, 2015).
Results
Plant responses to symbionts
Inoculation with AMF and/or rhizobia significantly increased legume biomass compared to uninoculated controls, but across all plants, these effects were strictly additive when plants were grown with both symbionts (Figs S4, S5). However, the magnitude of these responses depended on plant life history. Perennial species derived greater benefits from inoculation with either symbiont than annual species (AMF Q M = 30.35, P < 0.0001; rhizobia Q M = 29.72, P < 0.0001; Fig. 2a). Similarly, perennial and annual plants grown with both symbionts differed in benefits (synergism Q M = 15.39, P < 0.0001; Fig. 2a), with perennials showing synergistic growth benefits (Fig. 2b), while growth benefits in annuals did not differ from additive expectations from individual effects (Fig. 2c). Substrate sterilization and experiment type had no significant effects on plant biomass.
Across all plants, inoculation with AMF significantly increased plant P and N tissue content and inoculation with rhizobia significantly increased N tissue content (Fig. S6). The magnitude of AMF inoculation effects varied with plant life history, but rhizobia inoculation effects did not (Fig. 3a,b). Specifically, AMF inoculation increased P and N tissue concentration more in perennial plants than in annuals (P Q M = 6.19, P = 0.0128; N Q M = 26.94, P < 0.0001). Similarly, the effect of co‐inoculation on P concentration differed between perennials and annuals (Q M = 8.35, P = 0.0038), with lower than expected P concentration in perennials (negative synergism), but not annuals (Fig. 3a). The effects of co‐inoculation on N concentration did not differ from additive expectations (Fig. 3b). Results for N and P accumulation were generally qualitatively similar (Fig. S7a,c). The N : P ratio decreased with AMF inoculation and increased with rhizobia inoculation, and the magnitude of these effects were significantly greater in perennial plants (AMF Q M = 14.06, P = 0.0002; rhizobia Q M = 10.58, P = 0.0011; Fig. 3c). The N : P ratios of co‐inoculated plants did not differ from additive expectations (Fig. 3c). Substrate sterilization and experiment type did not have any effects on plant nutrient concentrations, except for N concentration of dual‐inoculated plants, where plants grown in sterile soil had lower than expected N concentrations, while plants grown in nonsterilized soil did not (Q M = 4.17, P = 0.0407).
Fig. 3.
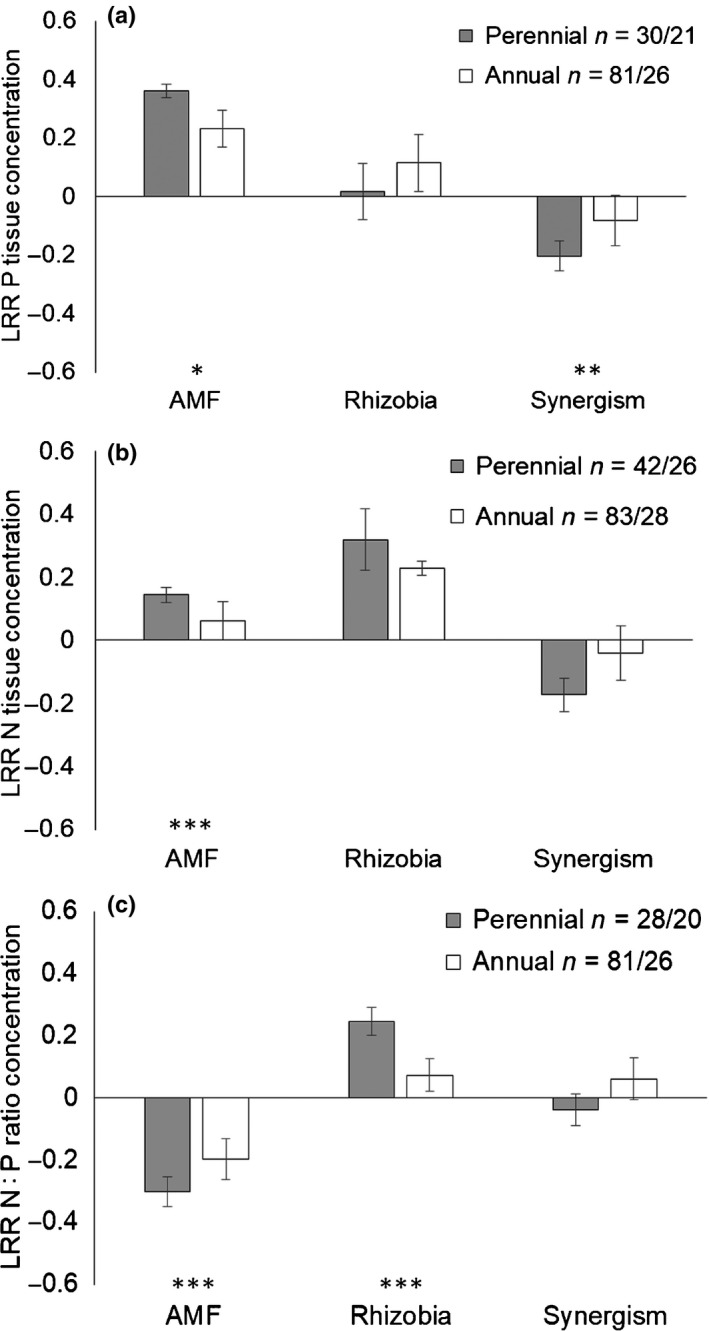
Effect of inoculation with arbuscular mycorrhizal fungi (AMF) and rhizobia, and synergism on (a) phosphorus (P) tissue concentration, (b) nitrogen (N) tissue concentration and (c) N : P ratio of perennial and annual legume species, shown as the log response ratio (LRR ± SE) calculated according to Eqn 1 for AMF, Eqn 2 for rhizobia and, Eqn 3 for synergism. ‘n’ represents the number of data points/studies in each group. Asterisks represent significant differences between annual and perennial plants in a given treatment as calculated by meta‐regression analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Microbial responses
Co‐inoculation of rhizobia and AMF significantly increased nodule number and nodule biomass compared to plants inoculated with rhizobia alone (Fig. 4), and this effect did not differ between perennial and annual species. By contrast, co‐inoculation did not have an effect on AMF colonization compared to plants inoculated with AMF alone (Fig. 4). Substrate sterilization and experiment type did not have significant effects on any microbial response variables.
Fig. 4.

Effect of arbuscular mycorrhizal fungi (AMF) and rhizobia co‐inoculation on nodule number, nodule biomass, and AMF colonization shown as the log response ratio (LRR ± SE) calculated according to Eqn 4 for nodule number and biomass and Eqn 5 for AMF colonization. A positive LRR indicates a higher response in a given variable when plants are co‐inoculated than plants inoculated with rhizobia or AMF alone. ‘n’ represents the number of data points/studies in each group. Asterisks represent significant differences between annual and perennial plants for a given variable as calculated by meta‐regression analysis (***, P < 0.001).
Both LRRnodbiomass (nodule biomass) and LRRcol (mycorrhizal colonization) were positively correlated with LRRsyn of plant biomass (Fig. 5b,c) across all plants. LRRnodnum (nodule number) was also positively correlated with LRRsyn of plant biomass, but only in annual plants (Fig. 5a).
Fig. 5.
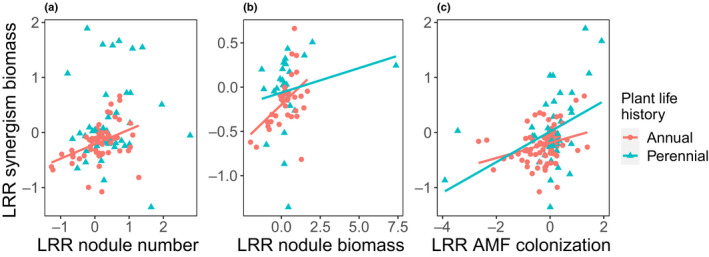
Relationships between the effect size (log response ratio (LRR)) for synergism plant biomass vs nodule number (a), nodule biomass (b), and arbuscular mycorrhizal fungal (AMF) colonization (c), with annual plants represented in red and perennial plants in teal. Figures show raw data fitted with regression lines, which indicate statistically significant relationships determined by meta‐regression models examining correlations between LRR synergism biomass and microbial responses.
Sensitivity analyses and additional variables
We detected publication bias in our P concentration (P = 0.0054), nodule number (P = 0.0039), nodule biomass (P < 0.0001), and height data (P = 0.0004). We detected one influential outlier in our nodule biomass data, but models calculated with and without this data point generated similar results. A small subset of studies included plant height, and the results of meta‐analysis of this variable are presented in Fig. S6. Substrate sterilization and experiment type had some significant effects on plant height (Fig. S6).
Discussion
As most plants interact with multiple symbionts simultaneously, understanding the potential for, and expected patterns of, synergistic benefits of co‐inoculation represents a critical issue in microbiome–plant interactions and in the optimization of plant microbiomes for production. Our meta‐analysis reveals that plant life history can play an important role in the outcomes of legume symbioses with both rhizobia and AMF. While all plants associating with individual symbionts generally grew larger than uninoculated plants, the magnitude of this effect was greater in perennial species. Perennials also derived synergistic growth benefits from co‐inoculation, while annual species did not. Rhizobia increased in abundance in the presence of AMF regardless of the life history of host plants, while AMF colonization did not consistently increase with the presence of rhizobia. Yet for both microbial symbionts, the magnitude of the benefit to the complementary microbial partner predicted the strength of the synergistic growth benefit in all plants. Together, these results advance our understanding of the mechanisms and context dependence of synergisms.
Synergism may occur in legume–rhizobia–AMF interactions because rhizobia and AMF help plants acquire complementary resources (N and P, respectively). These resources are potentially co‐limiting, as translation of acquired P into plant fitness may require additional N and vice versa. Moreover, rhizobia nodules have high P requirements (Sulieman & Tran, 2013), and AMF impose high demands on N, as well as plant derived lipids and carbohydrates (Johnson, 2010). Therefore, associating with one symbiont may enhance a plant's ability to invest in, and benefit from, the other symbiont. Our results partially support this hypothesis. When plants were inoculated with both symbionts, they produced more nodules and greater nodule biomass compared to when plants were inoculated with rhizobia alone, but AMF colonization did not consistently increase with co‐inoculation compared to inoculation with AMF alone (Fig. 4). In both cases, however, the responses of AMF colonization, nodule number, and nodule biomass to co‐inoculation were significantly positively correlated with synergistic plant growth – that is, as the benefits of co‐inoculation were realized by complementary symbionts, synergistic growth benefits increased (Fig. 5). These results suggest that while symbionts generally benefit from co‐inoculation regardless of plant life‐history, increased microbial abundance may translate to synergistic growth benefits in long‐lived legumes. Better estimates of microbial fitness, such as fungal spore production (Pringle & Taylor, 2002), would provide more insight into how closely plant synergistic growth and microbial fitness are correlated.
Reinvestment in complementary symbionts could result in a time‐lag in the realization of synergism, which may contribute to the differences in synergism that we observed between annuals and perennial legumes. While individual studies show synergistic benefits in annuals (e.g. Abd‐Alla et al., 2014; Afkhami & Stinchcombe, 2016), our meta‐analysis results identify that annual legumes are commonly not able to translate co‐inoculation into even the expected levels of growth promotion from the individual effect. The differences we found between perennials and annuals are consistent with a time‐lag in benefit accumulation for host plants (Johnson et al., 1997). To establish associations with symbionts, plants must invest resources in symbionts that can be costly during early stages of growth. These associations can then become more beneficial to plants over time, as symbionts alleviate resource limitation by N or P to the plants, allowing plants to not only continue investing in the symbioses, but to translate increased access to N or P to enhanced biomass production. Perennial plants continue to invest in growth throughout the first growing season (and longer for long‐lived perennials), allowing them to reinvest complementary resources in the two mutualisms. However, as annuals shift resources from growth to reproduction within the first growing season (Portes & Araújo, 2012), they may not have as long to realize synergistic growth benefits, making this realization vulnerable to the time lag in growth benefits generated by reinvestment in complementary symbionts. As a small number of individual studies of annual legumes do show evidence of synergism (e.g. Afkhami & Stinchcombe, 2016), more research on the context‐dependency of synergism is needed to determine under what conditions annuals (particularly agriculturally important species) can experience synergism, which could potentially be used to enhance productivity in agricultural and natural systems.
While we find clear differences between perennial and annual plants with regard to synergistic growth outcomes in our study, these results need to be interpreted with caution given other differences between our two plant groups. Namely, the annual plants in our meta‐analysis are almost all domesticated crop species, while the perennial plants are mostly nondomesticated species. Decreases in genetic diversity as a result of domestication, selection on certain traits, and the environmental conditions of agricultural systems may lead to changes in how domesticated plants interact with rhizosphere microbes comparted to nondomesticated plants (Pérez‐Jaramillo et al., 2016). For example, comparisons of several domesticated species to their wild ancestors have shown that domesticated species can be less able to support AMF (Xing et al., 2012), or more limited in their interactions with rhizobia (Mutch & Young, 2004). By contrast, a meta‐analysis showed that newer domesticated crop genotypes were more responsive to AMF compared to ancestral genotypes (Lehmann et al., 2012), suggesting that the effects of domestication on plant–microbe interactions may vary. Further work examining legume–AMF–rhizobia interactions in nondomesticated annual plants is needed to tease apart the effects of life‐history from possible effects of domestication on synergistic outcomes. However, a study of nondomesticated grassland species found that annuals were relatively unresponsive to mycorrhizal fungi compared to perennials (Reinhart et al., 2012), indicating that life history itself can influence the outcomes of plant–microbe interactions, and suggesting that our findings in this meta‐analysis may not only be the result of differences between domesticated and wild species.
In addition to teasing apart potential effects of plant life history and domestication, further work is needed to specifically explore the role of soil nutrients in synergistic outcomes in the legume–AMF–rhizobium system. The studies in our analyses included legumes grown in a variety of substrates, which likely varied in nutrient levels. Soil N and P levels may greatly influence the benefits derived by legumes from rhizobia and AMF (Johnson et al., 1997; Lau et al., 2012), and thus the likelihood of synergistic outcomes. This could influence the results of our meta‐analyses, particularly if annual/domesticated legumes were grown under conditions approximating typical agricultural soil fertility levels, which are often more N and P rich than under natural conditions. While we only included control data points from studies that manipulated soil N and P, we were unable to control for natural variation in soil fertility across studies. In addition, most data points in our study came from glasshouse experiments (83%), where conditions likely differ greatly from agricultural or natural communities, and where factors such as light could be a limiting factor for plant growth, which could affect the benefits derived by legumes from rhizobia and AMF (Ballhorn et al., 2016). However, we note that our analyses show that experiment type (glasshouse vs field) had limited influence on measures of synergism. More studies examining synergism in natural plant communities and/or agricultural settings, especially in the field, are needed to confirm the patterns in synergism observed in our study and to make our findings more generalizable to natural communities.
In addition to exploring the effect of plant life history on synergistic growth outcomes, our analyses also find support for N and P co‐limitation and reinvestment in symbionts being a mechanism by which synergistic benefits occur. When plants were inoculated with either rhizobia or AMF, they had higher N and P tissue concentrations, respectively, than uninoculated plants, but co‐inoculation resulted in N and P tissue concentrations that were lower than the additive expectation for annuals and perennial legumes (Fig. 3a,b). The absence of positive synergism with co‐inoculation in N and P concentration is expected because legumes are able to translate increased access to N and P into faster growth thereby diluting tissue resource concentrations, and because resources have been invested in complementary symbionts (i.e. N into increased AMF colonization and P into rhizobia nodulation). Our results then, are consistent with balanced N : P tissue stoichiometry mediating synergistic growth in legumes. We note, however, that this interpretation must be qualified, as the subset of studies of perennials that included measurements of N and P concentrations did not show strong synergism in growth promotion (Fig. S7b,d). Further work is required to demonstrate that perennials do not have positive synergistic patterns in resource concentrations or total content, while producing synergistic benefits in total biomass.
Microbial symbionts can be hugely influential on plant fitness, but the outcomes of plant–microbial interactions are notoriously context dependent. Our meta‐analysis demonstrates that plant life history can be an important factor determining when plants derive synergistic growth benefits by interacting with multiple mutualists. Moreover, we find support for a general framework that synergistic benefits emerge from microbial symbionts that provide complementary resources because of constraints on growth imposed by tissue stoichiometry. Further investigation of other context‐dependencies for synergistic growth benefits of co‐inoculation, such as dependence on the relative availability of soil N and P, would lead to a better understanding of how and when plants benefit from synergistic interactions. Our results identify synergistic benefits of multiple, complementary symbionts can be critically important for legume productivity, particularly for perennial plant species, and therefore realization of these synergisms should be a priority for management and breeding of perennial legumes.
Author contributions
JDB, SLS and SP planned and designed the research. SP and SMM conducted literature search and data collection. SMM and SP performed the analysis. JDB and TK interpreted mathematical derivation. JDB, SMM and SP wrote the manuscript and all co‐authors edited the manuscript.
Supporting information
Fig. S1 PRISMA 2009 Flow diagram detailing the article selection process for eligible articles to include in the meta‐analysis.
Fig. S2 Histograms showing the number of data points associated with each plant species, arbuscular mycorrhizal fungi species, and rhizobia species in the meta‐analysis data set.
Fig. S3 Funnel plots showing residuals plotted against standard error as a visual diagnostic of publication bias for each response variable.
Fig. S4 Log response ratio (LRR ± SE) of overall individual effects and overall synergistic effects of symbionts on plant biomass.
Fig. S5 Forest plot showing log response ratio synergism for each data point from every study included in the meta‐analysis.
Fig. S6 Log response ratio (LRR ± SE) of height, nitrogen (N) content and phosphorus (P) content.
Fig. S7 Effect of inoculation with arbuscular mycorrhizal fungi or rhizobia, and synergism on (a) phosphorus and (c) nitrogen accumulation. Inoculation effect on plant biomass from the subset of studies.
Methods S1 Mathematical derivation of log response ratio results.
Notes S1 Articles reviewed.
Table S1 Worksheet to estimate the results of a tripartite experiment (legumes, rhizobia and arbuscular mycorrhizal fungi) based on the meta‐analysis outcome.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This work was supported in part by funding from the National Science Foundation (DEB‐1556664, DEB‐1738041 and OIA 1656006), and USDA (2019‐67012‐29534). SP thanks the Instituto Federal de Santa Catarina (IFSC) for supporting a study leave at the University of Kansas. SLS would like to thank the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) for a Research Assistantship (Process 307.995‐2019‐4) and Universidade Regional de Blumenau (FURB) for supporting a sabbatical leave at the University of Kansas. Three anonymous reviewers provided insightful, detailed comments that greatly improved the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abd‐Alla MH, El‐Enany AE, Nafady NA, Khalaf DM, Morsy FM. 2014. Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiological Research 169: 49–58. [DOI] [PubMed] [Google Scholar]
- Afkhami ME, Almeida BK, Hernandez DJ, Kiesewetter KN, Revillini DP. 2020. Tripartite mutualisms as models for understanding plant–microbial interactions. Current Opinion in Plant Biology 56: 28–36. [DOI] [PubMed] [Google Scholar]
- Afkhami ME, Rudgers JA, Stachowicz JJ. 2014. Multiple mutualist effects: conflict and synergy in multispecies mutualisms. Ecology 95: 833–844. [DOI] [PubMed] [Google Scholar]
- Afkhami ME, Stinchcombe JR. 2016. Multiple mutualist effects on genomewide expression in the tripartite association between Medicago truncatula, nitrogen‐fixing bacteria and mycorrhizal fungi. Molecular Ecology 25: 4946–4962. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Schädler M, Elias JD, Millar J, Kautz S. 2016. Friend or foe – light availability determines the relationship between mycorrhizal fungi, rhizobia, and lima bean (Phaseolus lunatus L.). PLoS ONE 11: e0154116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JT, Koziol L, Bever JD. 2018. Ecology of floristic quality assessment: testing for correlations between coefficients of conservatism, species traits and mycorrhizal responsiveness. AoB Plants 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever JD, Morton JB, Antonovics J, Schultz PA. 1996. Host‐dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. Journal of Ecology 84: 71–82. [Google Scholar]
- de Carvalho TS, Moreira FM de S. 2010. Simbioses tripartites; leguminosas, fungos micorrízicos e bactérias fixadoras de nitrogênio nodulíferas. In: Siqueira JO, de Souza FA, Cardoso EJBN, Tsai SM, eds. Micorrizas: 30 anos de pesquisas no Brasil. Lavras, Brazil: Ufla, 716. [Google Scholar]
- Cheeke TE, Zheng C, Koziol L, Gurholt CR, Bever JD. 2019. Sensitivity to AMF species is greater in late‐successional than early‐successional native or nonnative grassland plants. Ecology 100: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KM, Bauer JT, Comita LS, Eppinga MB, Johnson DJ, Mangan SA, Queenborough SA, Strand AE, Suding KN, Umbanhowar J et al. 2019. When and where plant‐soil feedback may promote plant coexistence: a meta‐analysis. Ecology Letters 22: 1274–1284. [DOI] [PubMed] [Google Scholar]
- Delaveaux CS, Smith‐Ramesh LM, Kuebbing SE. 2017. Beyond nutrients: a meta‐analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 98: 2111–2119. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. 1997. Bias in meta‐analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, Martinez‐Romero E. 2011. Microbially mediated plant functional traits. Annual Review of Ecology, Evolution, and Systematics 42: 23–46. [Google Scholar]
- Habeck CW, Schultz AK. 2015. Community‐level impacts of white‐tailed deer on understory plants in North American forests: a meta‐analysis. AoB Plants 7: plv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Dijkstra FA. 2014. Drought effect on plant nitrogen and phosphorus: a meta‐analysis. New Phytologist 204: 924–931. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. 1999. The meta‐analysis of response ratios in experimental ecology. Ecology 80: 1150–1156. [Google Scholar]
- Hoeksema JD, Bever JD, Chakraborty S, Chaudhary VB, Gardes M, Gehring CA, Hart MM, Housworth EA, Kaonongbua W, Klironomos JN et al. 2018. Evolutionary history of plant hosts and fungal symbionts predicts the strength of mycorrhizal mutualism. Communications Biology 116: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Sun X, Wang X, Shen Y, Hou F, Chang S, Wang C. 2010. Synergistic interactions of arbuscular mycorrhizal fungi and rhizobia promoted the growth of Lathyrus sativus under sulphate salt stress. Symbiosis 50: 157–164. [Google Scholar]
- Johnson NC. 2010. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist 185: 631–647. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH, Smith FA. 1997. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytologist 135: 575–585. [Google Scholar]
- Koziol L, Bever JD. 2015. Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology 96: 1768–1774. [DOI] [PubMed] [Google Scholar]
- Koziol L, Bever JD. 2016. AMF, phylogeny, and succession: specificity of response to mycorrhizal fungi increases for late‐successional plants. Ecosphere 7: 1–11. [Google Scholar]
- Larimer AL, Bever JD, Clay K. 2010. The interactive effects of plant microbial symbionts: a review and meta‐analysis. Symbiosis 51: 139–148. [Google Scholar]
- Larimer AL, Clay K, Bever JD. 2014. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 95: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Lau JA, Bowling EJ, Gentry LE, Glasser PA, Monarch EA, Olesen WM, Waxmonsky J, Young RT. 2012. Direct and interactive effects of light and nutrients on the legume–rhizobia mutualism. Acta Oecologica 39: 80–86. [Google Scholar]
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. 2006. The case of the misleading funnel plot. British Medical Journal 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A, Barto EK, Powell JR, Rillig MC. 2012. Mycorrhizal responsiveness trends in annual crop plants and their wild relatives – a meta‐analysis on studies from 1981 to 2010. Plant and Soil 355: 231–250. [Google Scholar]
- Lekberg Y, Koide RT. 2005. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta analysis of studies published between 1988 and 2003. New Phytologist 168: 189–204. [DOI] [PubMed] [Google Scholar]
- Ma J, Weiru L, Hunter A, Zhang W. 2008. Performing meta‐analysis with incomplete statistical information in clinical trials. BMC Medical Research Methodology 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Maron JL, Mitchell CE, Parker IM, Power AG et al. 2007. Direct and interactive effects of enemies and mutualists on plant performance: a meta‐analysis. Ecology 88: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Mutch LA, Young JPW. 2004. Diversity and specificity of Rhizobium leguminosarum biovar viciae on wild and cultivated legumes. Molecular Ecology 13: 2435–2444. [DOI] [PubMed] [Google Scholar]
- Pasqualini D, Uhlmann A, Stürmer SL. 2007. Arbuscular mycorrhizal fungal communities influence growth and phosphorus concentration of woody plants species from the Atlantic rain forest in south Brazil. Forest Ecology and Management 245: 148–155. [Google Scholar]
- Pérez‐Jaramillo JE, Mendes R, Raaijmakers JM. 2016. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Molecular Biology 90: 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portes TA, Araújo BRB. 2012. Comparison of the allocation of phytomass in soybean and bean and its potential role in biological nitrogen fixation. Acta Scientiarum. Agronomy 34: 285–292. [Google Scholar]
- Primieri S, Santos JCP, Antunes PM. 2021. Nodule‐associated bacteria alter the mutualism between arbuscular mycorrhizal fungi and N2 fixing bacteria. Soil Biology and Biochemistry 154: doi: 10.1016/j.soilbio.2021.108149. [DOI] [Google Scholar]
- Pringle A, Taylor JW. 2002. The fitness of filamentous fungi. Trends in Microbiology 10: 474–481. [DOI] [PubMed] [Google Scholar]
- Qin L, Jiang H, Tian J, Zhao J, Liao H. 2011. Rhizobia enhance acquisition of phosphorus from different sources by soybean plants. Plant and Soil 349: 25–36. [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/. [Google Scholar]
- Reinhart KO, Wilson GWT, Rinella MJ. 2012. Predicting plant responses to mycorrhizae: integrating evolutionary history and plant traits. Ecology Letters 15: 689–695. [DOI] [PubMed] [Google Scholar]
- Ren C‐G, Bai Y‐J, Kong C‐C, Bian B, Xie Z‐H. 2016. Synergistic interactions between salt‐tolerant Rhizobia and Arbuscular mycorrhizal fungi on salinity tolerance of sesbania cannabina plants. Journal of Plant Growth Regulation 35: 1098–1107. [Google Scholar]
- Shantz AA, Lemoine NP, Burkepile DE. 2016. Nutrient loading alters the performance of key nutrient exchange mutualisms. Ecology Letters 19: 20–28. [DOI] [PubMed] [Google Scholar]
- Siqueira JO, Saggin‐Júnior OJ. 2001. Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 11: 245–255. [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn. London, UK: Academic Press. [Google Scholar]
- Stanton ML. 2003. Interacting guilds: moving beyond the pairwise perspective on mutualisms. American Naturalist 162: S10–S23. [DOI] [PubMed] [Google Scholar]
- Sulieman S, Tran LP. 2013. Asparagine: an amide of particular distinction in the regulation of symbiotic nitrogen fixation of legumes. Critical Reviews in Biotechnology 33: 309–327. [DOI] [PubMed] [Google Scholar]
- Udvardi M, Poole PS. 2013. Transport and metabolism in legume‐rhizobia symbioses. Annual Review of Plant Biology 64: 781–805. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 36: 1–48. [Google Scholar]
- Xing X, Koch AM, Jones AMP, Ragone D, Murch S, Hart MM. 2012. Mutualism breakdown in breadfruit domestication. Proceedings of the Royal Society B: Biological Sciences 279: 1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 PRISMA 2009 Flow diagram detailing the article selection process for eligible articles to include in the meta‐analysis.
Fig. S2 Histograms showing the number of data points associated with each plant species, arbuscular mycorrhizal fungi species, and rhizobia species in the meta‐analysis data set.
Fig. S3 Funnel plots showing residuals plotted against standard error as a visual diagnostic of publication bias for each response variable.
Fig. S4 Log response ratio (LRR ± SE) of overall individual effects and overall synergistic effects of symbionts on plant biomass.
Fig. S5 Forest plot showing log response ratio synergism for each data point from every study included in the meta‐analysis.
Fig. S6 Log response ratio (LRR ± SE) of height, nitrogen (N) content and phosphorus (P) content.
Fig. S7 Effect of inoculation with arbuscular mycorrhizal fungi or rhizobia, and synergism on (a) phosphorus and (c) nitrogen accumulation. Inoculation effect on plant biomass from the subset of studies.
Methods S1 Mathematical derivation of log response ratio results.
Notes S1 Articles reviewed.
Table S1 Worksheet to estimate the results of a tripartite experiment (legumes, rhizobia and arbuscular mycorrhizal fungi) based on the meta‐analysis outcome.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


