Abstract
Background.
Persistent ascites after orthotropic liver transplantation has numerous causes and can be challenging to manage. This study aimed to determine the outcomes associated with conservative and endovascular intervention of posttransplant ascites after deceased donor liver transplantation.
Methods.
Adult (≥18 y) liver transplant recipients (between 2006 and 2019) who underwent hepatic venous pressure studies to investigate posttransplant ascites were included in this retrospective study. Comparisons were made between those who were managed with conservative therapy versus endovascular intervention and were also based on hepatic venous wedge pressure gradient (normal [≤10 mm Hg] versus elevated [>10 mm Hg]).
Results.
A total of 30 patients underwent hepatic venography to investigate ascites during the study period. The median time from transplant to venography was 70 d. At least 1 endovascular intervention was performed in 18 of 30 patients (62%), and 12 of 30 patients (38%) were managed conservatively. Endovascular interventions included angioplasty (n = 4), hepatic vein stenting (n = 9), or a transjugular intrahepatic portosystemic shunt (n = 7). The mean (range) hepatic venous wedge pressure gradient for the conservative and endovascular intervention groups was 12 mm Hg (3–23) and14 mm Hg (2–35), respectively. At a 6-mo follow-up, ascites resolved in 6 of 12 patients (50%) and 11 of 18 patients (61%) in the medical management and endovascular groups, respectively. The graft survival rates at 6 and 12 mo were (7/12 [58%] versus 17/18 [94%], P = 0.02) and (7/12 [58%] versus 14/18 [78%], P = 0.25), respectively.
Conclusions.
Despite medical or endovascular intervention, resolution of ascites is achieved in <60% of patients with persistent ascites. Biopsy findings and venographic pressure studies should be carefully integrated into the management of posttransplant ascites.
In the early postoperative period after liver transplantation, it is common for ascitic fluid to accumulate. As graft function improves and the pressure within the portal circulation normalizes, the ascites usually resolves. However, persistent posttransplant ascites can be troublesome and is associated with renal impairment, intrabdominal infections, prolonged hospitalization, and subsequent graft loss.1 Persistent ascites after liver transplantation has been reported to occur in up to 6% of patients, and effective management has been associated with improved outcomes.2-5
The reasons for persistent ascites post–liver transplantation are multifactorial and include vascular complications, graft failure, renal dysfunction, acute rejection, bacterial peritonitis, or fungal peritonitis.3,5 Hepatic venous outflow obstruction (HVOO), also known as piggyback syndrome, is thought to have an incidence of 1% after whole liver transplantation.2,4,6 Persistent ascites is a cardinal feature of HVOO and is the most common indication for hepatic venography after liver transplantation.7,8 Portal vein stenosis, splenic artery steal syndrome, and left-sided portal hypertension are additional vascular causes of persistent ascites.2,4,9 Preoperative factors that result in persistent post–liver transplantation ascites include preexisting ascites, encephalopathy, hepatitis C cirrhosis, prolonged cold ischemic time, and a history of spontaneous bacterial peritonitis.3,10 Early detection of the cause, followed by timely intervention, will give the best opportunity for optimal outcomes.
Treatment options are dependent on the cause and include medical therapy such as fluid restriction, diuretics to increase fluid excretion, albumin replacement, or immunosuppression to treat graft rejection. In patients with vascular complications, endovascular intervention such as balloon angioplasty, hepatic vein (HV) stenting, or a transjugular intrahepatic portosystemic shunt (TIPS) aims to reduce the high vascular resistance and improve blood flow. Hepatic venography provides important diagnostic information regarding venous pressures and graft outflow anatomy. An elevated hepatic venous wedge pressure (HVWP) indicates portal hypertension, and an elevated hepatic venous wedge pressure gradient (HVWPG) suggests site of increased resistance is within the parenchyma of the liver. The aim of this study was to describe the hepatic venography findings, subsequent management, and the outcomes associated with conservative and radiological intervention in a series of deceased donor liver transplant recipients investigated for persistent ascites.
MATERIALS AND METHODS
Adult (≥18 y) recipients of liver transplants performed at our institution between January 2008 and December 2019 were considered for inclusion in this retrospective study. Patients were eligible if they underwent investigation with hepatic venography and posttransplant ascites was the main indication. All included patients were required to have had an ultrasound and computed tomography scan that did not demonstrate a cause of their ascites. Patient demographics and clinical variables including transplant indication, UK model for end-stage liver disease and model for end-stage liver disease scores, graft characteristics, presence of pretransplant ascites, renal and liver function tests, hepatic venography, and liver biopsy results were collected from electronic medical records. Patients were grouped according to the subsequent management into either a conservative management group or an endovascular intervention group. The patients were then subgrouped on the basis of either a normal (<10 mm Hg) or elevated (≥10 mm Hg) HVWPG. The primary outcome was resolution of ascites at 6 mo. Additional outcomes assessed were graft and patient survival at 90 d, 6 mo, and 12 mo, and changes in serum creatinine, alanine aminotransferase (ALT), bilirubin, and alkaline phosphatase (ALP).
During hepatic venography, 3 pressure measurements are generally taken at different locations: inferior vena cava (IVC), free HV, and HVWP that reflects the pressure in the portal circulation. The difference between the HVWP and the free HV is the HVWPG. A pressure gradient >5 mm Hg across the anastomosis (HV-IVC) signifies HVOO.11 An elevated HVWP indicates portal hypertension, and an elevated HVWPG suggests the site of increased resistance is within the parenchyma of the liver.
Statistical analysis was performed using SPSS (Version 25.0, IBM Corp, Armonk, NY). Continuous variables from each group were compared using independent samples t tests if they were normally distributed or a Mann-Whitney U test if they did not follow the normal distribution. Categorical variables were compared using the Fisher exact test. Two-sided tests of significance were used, and a P value of ≤0.05 was considered statistically significant.
RESULTS
During the study period, 30 patients were identified as being subject to hepatic venography, with the primary indication being posttransplant ascites. These patients did not have a clear cause of their ascites on either computed tomography or ultrasound. During this period, 1718 adult transplants were performed; therefore, the incidence of this presentation was 1.7% (30/1718). The demographics and transplant indications for the included patients are demonstrated in Table 1. All patients received a whole liver graft. The median age (interquartile range) was 54 (43–64) y, and 11 of 30 (37%) patients were female. Graft steatosis of ≥10% was present in 16 of 30 (53%). All 30 patients had piggyback caval anastomoses, with 22 of 30 patients (73%) undergoing a modified piggyback (side-side) and 8 of 30 patients (27%) undergoing classical piggyback (end-side). Acute rejection had occurred in 4 of 12 patients (33%) and 7 of 18 patients (39%) before ascites onset in the conservative and endovascular group, respectively (P = 0.54). The median (interquartile range) time between transplantation and hepatic venography was 70 (41–137) d for the entire series, 54 (27–121) d for the conservative group, and 82 (47–152) d for the endovascular group. A periprocedural graft biopsy was performed in 29 of 30 patients (97%), with 22 of 29 biopsies (76%) being performed at the time of the venography. In the remaining 7 cases, 4 were performed before and 3 were performed after the venography.
TABLE 1.
Sample demographics and transplant characteristics
| Total cohort (N = 30) | Conservative management (N = 12) | Endovascular intervention (N = 18) | P | |
|---|---|---|---|---|
| Age (y), median (range) | 54 (43–63) | 57 (23–68) | 49 (33–69) | 0.18 |
| Female (%) | 11/30 (37%) | 5/12 (42%) | 6/18 (33%) | 0.71 |
| BMI, median (range) | 28 (19–34) | 29 (24–33) | 27 (19–48) | 0.8 |
| UKELD, median (range) | 53 (43–64) | 58 (49–63) | 52 (42–64) | 0.09 |
| MELD, median (range) | 15 (6–29) | 19 (8–29) | 12 (6–26) | 0.02 |
| Preoperative ascites | 17/30 (57%) | 6/12 (50%) | 10/18 (56%) | 0.82 |
| Indication for transplant | 0.56 | |||
| ArLD | 4/30 (13%) | 1/12 (8%) | 3/18 (17%) | |
| Primary sclerosing cholangitis | 4/30 (13%) | 2/12 (17%) | 2/18 (11%) | |
| Primary biliary cirrhosis | 5/30 (17%) | 2/12 (17%) | 3/18 (17%) | |
| Nonalcoholic fatty liver disease | 3/30 (10%) | 2/12 (17%) | 1/18 (6%) | |
| Hepatitis C virus | 6/30 (20%) | 3/12 (25%) | 3/18 (17%) | |
| Polycystic liver disease | 2/30 (7%) | – | 2/18 (11%) | |
| Other | 6/30 (20%) | 2/12 (17%) | 4/18 (22%) | |
| DCD (%) | 6/30 (20%) | 3/12 (25%) | 3/18 (17%) | |
| CIT (min), median (range) | 362 (286–693) | 438 (274–624) | 490 (252–734) | |
| Technique | ||||
| Classical piggyback | 8/30 (27%) | 3/12 (25%) | 5/18 (28%) | |
| Modified piggyback | 22/30 (73%) | 9/12 (75%) | 13/18 (72%) | |
| Rejection before ascites onseta | 11/30 (37%) | 4/12 (33%) | 7/18 (39%) | 0.53 |
| IVC pressure (mm Hg) | 10 (4–22) | 11 (4–18) | 11 (4–22) | 0.42 |
| HVP (mm Hg) | 12 (4–27) | 11 (4–19) | 12 (4–27) | 0.26 |
| HVWP (mm Hg) | 24 (7–44) | 24 (7–36) | 28 (13–44) | 0.16 |
| HVWPG, median (range) | 12 (0–35) | 12 (3–23) | 14 (2–35) | 0.74 |
| Normal HVWPG (<10 mm Hg) | 13 (43%) | 5 (42%) | 8 (44%) | |
| Elevated HVWPG (≥10 mm Hg) | 17 (57%) | 7 (58%) | 10 (56%) | |
| HV-IVC gradient (mm Hg) | 2 (0–14) | 1 (0–4) | 2 (0–14) | 0.04 |
| Biochemistry,b median (range) | ||||
| Creatinine (µmol/L) | 112 (52–531) | 109 (65–531) | 129 (52–308) | 0.58 |
| ALT (IU/L) | 19 (5–481) | 29 (5–481) | 12 (5–59) | 0.01 |
| Bilirubin (µmol/L) | 14 (3–524) | 30 (3–524) | 13 (4–43 | 0.18 |
| ALP (IU/L) | 184 (56–848) | 207 (64–848) | 179 (56–389) | 0.58 |
aBiopsy-proven acute rejection.
bBiochemistry at the time of initial venography to investigate ascites.
Numbers in bold represent statistically significant findings at the alpha level of 0.05.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ArLD, alcohol-related liver disease; BMI, body mass index; CIT, cold ischemic time; DCD, deceased circulatory death; HV, hepatic vein; HVP, hepatic vein pressure; HVWP, hepatic venous wedge pressure; HVWPG, hepatic vein wedge pressure gradient; IVC, inferior vena cava; MELD, model for end-stage liver disease; UKELD, United Kingdom model for end-stage liver disease.
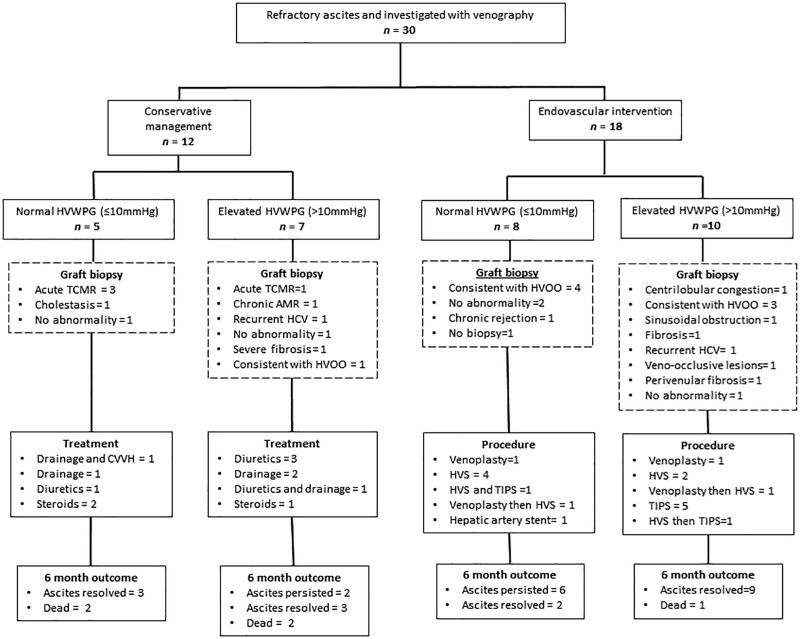
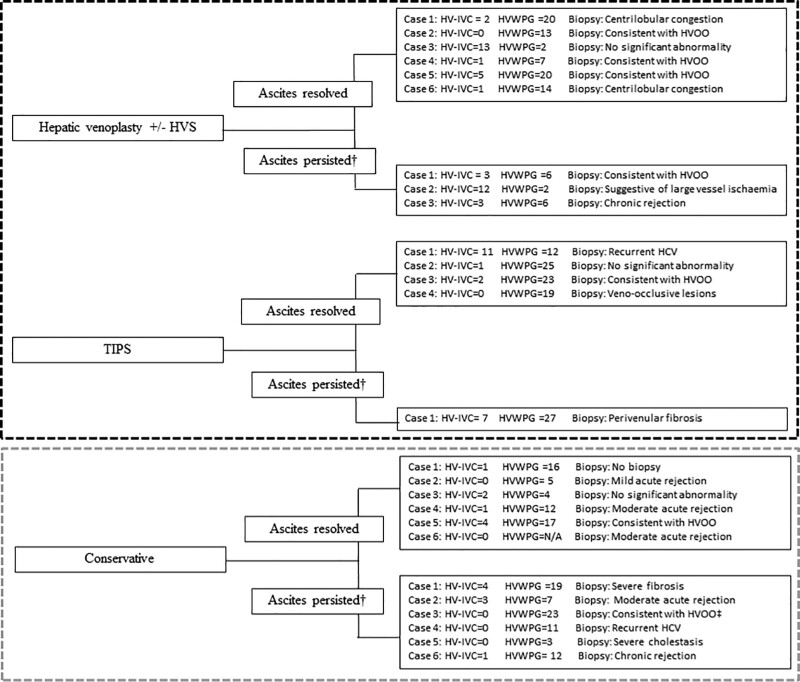
The conservative and endovascular groups comprised 12 of 30 (40%) and 18 of 30 (60%), respectively (Figure 1). The demographic and transplant characteristics of each group did not differ significantly, with the exception of the pretransplant model for end-stage liver disease score (Table 1). Patient characteristics are also demonstrated in Figure 1. A significant difference existed (P = 0.04) between the HV-IVC gradient of the medical therapy and endovascular intervention groups (1 [0–4] versus 2 [0–14] mm Hg). In the group of subjects who were alive at a 6-mo follow-up (n = 25), 17 of 25 patients (68%) had resolution of their ascites at this time point. The proportion of patients with resolution of their ascites did not differ between groups (6/12 [50%] versus 11/18 [61%], P = 0.31; Figure 1). The HV-IVC gradient, HVWPG, and biopsy results for each patient in the series are demonstrated in Figure 2 and Figure S1 (SDC, http://links.lww.com/TXD/A434).
Figure 1.
Study flow and results. Patient had TIPS attempted but was unsuccessful. AMR, antibody mediated rejection; CVVH, continuous venovenous hemofiltration; HCV, hepatitis C virus; HVOO, hepatic venous outflow obstruction; HVS, hepatic vein stent; HVWPG, hepatic vein wedge pressure gradient; TCMR, T cell–mediated rejection; TIPS, transjugular intrahepatic portosystemic shunt.
Figure 2.
Details of each case according to intervention and resolution of ascites at 6 mo. †Ascites present at 6-mo follow-up or failed to survive 6 mo after venography. TIPS attempted but not technically possible flowchart showing the cohort stratified by intervention and outcome at 6 mo. Patients who underwent multiple different endovascular procedures were excluded from this figure. HV, hepatic vein; HCV, hepatitis C virus; HVOO, hepatic venous outflow obstruction; HVP, hepatic vein pressure; HVS, hepatic vein stent; HVWPG, hepatic vein wedge pressure gradient; IVC, inferior vena cava; TIPS, transjugular intrahepatic portosystemic shunt.
Conservative Management Group
In the group of patients who received medical therapy, 5 of 12 patients (42%) had a normal and 7 of 12 patients (58%) had an elevated HVWPG. A periprocedural biopsy was performed in all of these groups, and either acute or chronic rejection was the most common finding (4/11; 36%). Other biopsy findings in the medical therapy group included recurrent hepatitis C virus (HCV), cholestasis, fibrosis, and sinusoidal congestion suggestive of HVOO (Figure 1). The median IVC, free HV, and HVWP are demonstrated in Table 1. Diuretic treatment was initiated in 5 patients, and 1 of these also had drainage via paracentesis. One patient had percutaneous drainage of the ascites and hemofiltration for fluid removal rather than diuretics because this patient was anuric. Two patients had paracentesis only. High-dose steroid therapy was commenced for acute rejection in 3 patients (Figure 1). At a 6-mo follow-up, 8 of 12 patients (66%) of the conservative management group were alive, and the ascites had persisted in 3 of 8 patients (33%). One patient in this group was retransplanted within 6 mo of initial venography. The HV-IVC gradient, HVWPG, and biopsy result of each case in this group are demonstrated in Figure 2. There was no patient in this group who had an HV-IVC gradient ≥5 mm Hg. The biopsy findings of the 4 patients who did not survive to a 6-mo follow-up were acute rejection (1), chronic rejection (1), cholestasis (1), and sinusoidal congestion (1) suggestive of HVOO. The cause of death for these 4 patients was sepsis (1) and multiorgan failure secondary to persistent graft dysfunction (3).
Endovascular Intervention Group
In the group of patients who underwent endovascular intervention (18/30; 60%), 21 procedures were performed that comprised venoplasty (n = 4), HV stenting (n = 9), TIPS (n = 7), and hepatic artery stent (n = 1; Figure 1). One patient with an elevated HVWPG on venography also required stenting of a stenosing lesion in his hepatic artery. A normal HVWPG was present in 8 of 18 patients (44%), and 4 out of these 8 patients (50%) had biopsy findings consistent with HVOO. An elevated HVWPG was present in 10 of 18 patients (56%), and 3 of 10 patients (30%) had biopsy findings consistent with HVOO. The HV-IVC gradient, HVWPG, and biopsy result of each case in this group are demonstrated in Figure 2 and stratified according to procedure and the resolution of ascites at 6 mo. Three patients had resolution of ascites after HV stenting or venoplasty, despite not having an elevated HV-IVC gradient (Figure 2). The most common endovascular procedure performed in the subgroup with an elevated HVWPG was a TIPS, ultimately being required in 6 of 10 patients (60%). At a 6-mo follow-up, 17 of 18 patients (94%) were alive at 6 mo after venography and the ascites had resolved in 11 of 17 patients (65%).
Biochemical Parameters, Graft Survival, and Patient Survival
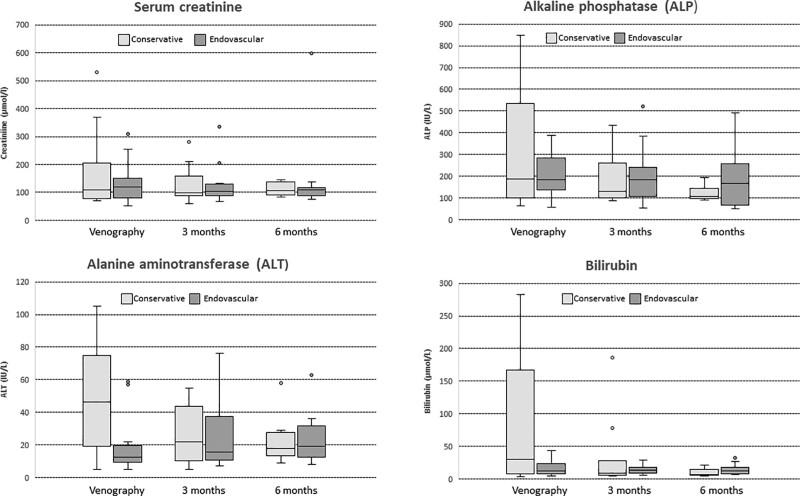
Patients in the conservative group had higher levels of ALT, bilirubin, and ALP at the time of venography (Table 1), which improved during the subsequent 6-mo follow-up. The change in median creatinine, ALT, bilirubin, and ALP during the 6 mo after venography was the greatest for the medical group, with the sharpest decline being in the first 3 mo (Figure 3). There was a significant difference between the graft survival (7/12 [58%] versus 17/18 [94%], P = 0.02) and patient survival (8/12 [67%] versus 17/18 [94%], P = 0.05) at 6 mo but not at 12 mo (Table 2).
Figure 3.
Biochemical trends (mean) of both the conservative management and endovascular intervention groups. Graphs of biochemical trends during the 6 mo after venography for posttransplant ascites. ALP, alkaline phosphatase; ALT, alanine aminotransferase.
TABLE 2.
Short-term graft and patient survival
| Medical management | Endovascular intervention | P | |
|---|---|---|---|
| 90-d graft survival | 9/12 (75%) | 17/18 (94%) | 0.13 |
| 90-d patient survival | 10/12 (83%) | 17/18 (94%) | 0.32 |
| 6-mo graft survival | 7/12 (58%) | 17/18 (94%) | 0.02 |
| 6-mo patient survival | 8/12 (67%) | 17/18 (87%) | 0.05 |
| 12-mo graft survival | 7/12 (58%) | 14/18 (78%) | 0.25 |
| 12-mo patient survival | 7/12 (58%) | 15/18 (83%) | 0.13 |
Graft and patient survival of patients who received conservative or endovascular management for ascites. Statistical comparison was performed with the Fisher exact test.
Numbers in bold represent statistically significant findings at the alpha level of 0.05.
DISCUSSION
This study highlights the numerous different causes of posttransplant ascites and the approach to investigations and management at our institution. Comprised of patients with posttransplant ascites who underwent venography, this series likely represents a select group that did not have a cause of ascites identified on less invasive investigations. Hepatic venography with pressure measurements is an important investigation in the setting of posttransplant ascites because it provides the most useful information evaluating the vascular anastomosis and parenchymal resistance to portal flow. HVOO after piggyback-type caval anastomoses has previously been reported to present with persistent ascites and is best diagnosed at venography based on the gradient across the anastomosis and the rate of emptying of the hepatic venous contrast to vena cava.12 Several studies reported that patients with HVOO had persistent ascites as a presenting feature; however, the rates of resolution of the ascites after endovascular intervention are not well reported.12 The incidence of HVOO obstruction posttransplant ranges from 0.5% to 9.5%, with a higher rate after living donor liver transplants.12,13 It is important to detect and treat HVOO because it causes graft dysfunction and irreversible injury that may lead to graft loss or patient death.13 In this series, the resolution of ascites, patient survival, and graft survival were better in the endovascular intervention group, indicating that the outcomes are best when a correctable cause could be identified. Pressure studies are useful in identifying the presence of HVOO, and the periprocedural biopsy findings, namely sinusoidal congestion, were only present in a minority of cases.
A TIPS procedure aims to reduce the portal venous pressure by reducing the resistance to vascular flow within the sinusoids of the liver and can be used in both a native and transplanted liver.4 Persistent ascites has been reported to be the most common indication for TIPS posttransplant.14 In a systematic review and pooled analysis, Chen et al reported TIPS to either completely or partially resolve persistent posttransplant ascites in 57% (96/168). However, the results of venography and pressure measurements were not reported by these authors. The majority of patients (6/7; 86%) in this series who underwent a TIPS procedure had an elevated HVWPG indicating elevated portal venous pressures. In this series, the resolution of ascites at 6 mo was the greatest in the group with a raised HVWPG who received endovascular intervention. A known risk with performing a TIPS procedure is the onset (or worsening) of hepatic encephalopathy, and in the posttransplant setting, this has been reported to occur in 33% of cases.14 Additionally, it has the potential to worsen graft ischemia because of a more limited ability of the hepatic artery to compensate in the posttransplant setting.4 A total of 6 out of 7 patients in this series had resolution of ascites after TIPS procedure. Therefore, TIPS should be performed after giving due consideration to all the investigations only in significantly symptomatic patients with isolated elevation in the HVPG and no other causes of ascites.
The significantly higher level of ALT in the group managed with medical therapy likely reflects the greater proportion of patients with an intrinsic graft problem (acute or chronic rejection, fibrosis, and cholestasis). The group managed with endovascular therapy had a mean bilirubin and ALT within normal range with minimal change during the 6-mo follow-up. The findings suggest that causes other than HVOO should be strongly considered in a patient with posttransplant ascites and significantly elevated transaminase levels.
In this series, 5 patients died within 6 mo of the initial venography, and 4 of these were in the conservative management group. The higher rate of graft loss and mortality at 6 mo in the patients who did not receive endovascular intervention likely reflects the poor prognosis associated with the type of disease evident on biopsy (recurrent HCV, chronic antibody-mediated rejection, and severe fibrosis). Additionally, this group likely already had more established graft failure and renal dysfunction as evidenced by greater elevation in their liver function tests and creatinine, respectively. The longer-term survival did not differ significantly between groups, and this is likely a type II error because of the small sample size. The other limitation of this study is its retrospective nature and small sample resulting in potential selection bias. However, the significance of integrating the venographic pressure studies along with biochemical and biopsy results in the management of posttransplant ascites is highlighted in the study. Overall, the 5-y survival of this series is comparable with the 70% reported by other authors for persistent ascites after deceased donor liver transplantation.5 The findings show that patients with posttransplant ascites because of intrinsic graft-related issues have a poorer 6-mo survival in comparison with those with outflow obstruction. However, endovascular intervention frequently fails to resolve posttransplant ascites, as indicated by nearly 40% in this series having persistent ascites despite undergoing endovascular intervention.
ACKNOWLEDGMENTS
A.H. would like to acknowledge the Royal Australasian College of Surgeons and the research funding provided through the Catherine Marie Enright Kelly Memorial Research Scholarship.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
M.A.-Z., M.A.l, and A.H. collected data and prepared the first draft of article. H.M., S.K., P.M., M.Ab., H.H., K.R., D.F.M., and J.R.I. reviewed and contributed to the article. B.V.M.D. conceptualized the research project, prepared the first draft, and edited the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Cirera I, Navasa M, Rimola A, et al. Ascites after liver transplantation. Liver Transpl. 2000;6:157–162. [DOI] [PubMed] [Google Scholar]
- 2.Khorsandi SE, Athale A, Vilca-Melendez H, et al. Presentation, diagnosis, and management of early hepatic venous outflow complications in whole cadaveric liver transplant. Liver Transpl. 2015;21:914–921. [DOI] [PubMed] [Google Scholar]
- 3.Gotthardt DN, Weiss KH, Rathenberg V, et al. Persistent ascites after liver transplantation: etiology, treatment and impact on survival. Ann Transplant. 2013;18:378–383. [DOI] [PubMed] [Google Scholar]
- 4.Pereira K, Salsamendi J, Fan J. An approach to diagnosis and endovascular treatment of refractory ascites in liver transplant: a pictorial essay and clinical practice algorithm. Exp Clin Transplant. 2015;13:387–393. [PubMed] [Google Scholar]
- 5.Nishida S, Gaynor JJ, Nakamura N, et al. Refractory ascites after liver transplantation: an analysis of 1058 liver transplant patients at a single center. Am J Transplant. 2006;6:140–149. [DOI] [PubMed] [Google Scholar]
- 6.Kubo T, Shibata T, Itoh K, et al. Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantation. Radiology. 2006;239:285–290. [DOI] [PubMed] [Google Scholar]
- 7.Pitchaimuthu M, Roll GR, Zia Z, et al. Long-term follow-up after endovascular treatment of hepatic venous outflow obstruction following liver transplantation. Transpl Int. 2016;29:1106–1116. [DOI] [PubMed] [Google Scholar]
- 8.El Atrache M, Abouljoud M, Sharma S, et al. Transjugular intrahepatic portosystemic shunt following liver transplantation: can outcomes be predicted? Clin Transplant. 2012;26:657–661. [DOI] [PubMed] [Google Scholar]
- 9.Schneider N, Scanga A, Stokes L, et al. Portal vein stenosis: a rare yet clinically important cause of delayed-onset ascites after adult deceased donor liver transplantation: two case reports. Transplant Proc. 2011;43:3829–3834. [DOI] [PubMed] [Google Scholar]
- 10.Stewart CA, Wertheim J, Olthoff K, et al. Ascites after liver transplantation—a mystery. Liver Transpl. 2004;10:654–660. [DOI] [PubMed] [Google Scholar]
- 11.Sze DY, Semba CP, Razavi MK, et al. Endovascular treatment of hepatic venous outflow obstruction after piggyback technique liver transplantation. Transplantation. 1999;68:446–449. [DOI] [PubMed] [Google Scholar]
- 12.Arudchelvam J, Bartlett A, McCall J, et al. Hepatic venous outflow obstruction in piggyback liver transplantation: single centre experience. ANZ J Surg. 2017;87:182–185. [DOI] [PubMed] [Google Scholar]
- 13.Chu HH, Yi NJ, Kim HC, et al. Longterm outcomes of stent placement for hepatic venous outflow obstruction in adult liver transplantation recipients. Liver Transpl. 2016;22:1554–1561. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Wang W, Tam MD, et al. Transjugular intrahepatic portosystemic shunt in liver transplant recipients: indications, feasibility, and outcomes. Hepatol Int. 2015;9:391–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.