Abstract
Background
Infections following abdominal surgery remain a significant problem. Although preoperative antibiotic prophylaxis is a primary strategy used to reduce postoperative infections, they are typically prescribed based upon standardized protocols, without attention to previous infection or antibiotic history. Patients with a previous infection after surgery may be at higher risk for infectious complications after subsequent operations owing to antibiotic resistance. We hypothesized that a previous postoperative infection is a significant risk factor for the development of infection following a second unrelated surgery.
Study Design
We performed a retrospective study of patients who had undergone two unrelated abdominal operations at a tertiary care center from 2012-2018. Clinical variables and microbiological culture results were abstracted. Univariate and multivariable regression models were constructed.
Results
Of 758 patients, 15.0% (n=114) developed an infection after the first operation. After the second operation, 22.8% (n=26) of those with a previous infection developed another infection, whereas the incidence of an infection following the second operation was only 9.5% (n=61) in patients that did not develop an infection following the first operation. Multivariable analysis demonstrated that previous infection (OR 2.49, 95% CI1.46-4.25) was associated with future infection risk. Microbiological analysis found that infections following the second surgery were significantly more common to be antibiotic resistant compared to infections following the first surgery (82.3% vs 64.1%; p=.036). Strikingly, 49% of infections after the second surgery were resistant to the antibiotic prophylaxis given at the time of incision.
Conclusions
Previous postoperative infection is an independent risk factor for a subsequent postoperative infection and is associated with resistance to standard prophylaxis. Individualization of antibiotic prophylaxis in patients with a previous postoperative infection is warranted.
Keywords: Postoperative infection, antibiotic resistance, bacterial resistance
PRECIS
Patients with a previous postoperative infection may be at higher risk for infectious complication after subsequent operations owing to antibiotic resistance or other patient factors. We found that a previous postoperative infection is an independent risk factor for a subsequent postoperative infection and is associated with resistance to standard prophylaxis.
INTRODUCTION
Infections after abdominal surgery are frequent and include surgical site infection, urinary tract infection or pneumonia. Although the incidence of each type of infection varies based upon the type of abdominal procedure, all are associated with increased morbidity, hospital length of stay, readmissions, and health care costs(1–4). Despite advances in preoperative optimization, surgical technique, and enhanced recovery programs, postoperative infections continue to cause significant patient morbidity and mortality.
Prophylactic perioperative antibiotics are a cornerstone of efforts to decrease the risk of postoperative infections(5). While the use of prophylactic antibiotics has routinely been shown to decrease the incidence of postoperative infections in appropriate settings, they are often prescribed on standardized protocols, without attention to previous infection or antibiotic history(6). It has been well known that previous antibiotics may promote colonization with antibiotic resistant organisms(7). Furthermore, exposure to antibiotics coupled with surgical stress and critical illness has been demonstrated to deplete the commensal microbiota and increase the risk for pathogen colonization(8). Recently, Guidry et al, found that previous antibiotic exposure is an independent risk factor for the development of postoperative infection following elective surgery(9). Whether a history of infection after previous surgery, or antibiotic resistance from previous treatment, portends a greater risk of infection after a second operation is poorly studied.
We hypothesized that a history of a postoperative infection is a significant risk factor for the development of another postoperative infection following an unrelated second surgery, and may be associated with the presence of resistant bacteria to protocolized prophylactic antibiotics. The aims of this study were: (1) to determine if a previous postoperative infection is an independent risk factor for an infection following a second unrelated operation and (2) to compare the bacterial culture and resistance profiles between a first and second postoperative infection.
METHODS
Study Design and Data Collection
This was a single center, retrospective cohort study of patients undergoing abdominal surgery at an urban tertiary care academic center. Adult patients over 18 years of age who had undergone two elective unrelated abdominal surgeries between January 1, 2012 to July 21, 2018 were included for this study. An unrelated second operation was defined as one that occurred greater than 30 days following the initial operation and was not indicated to correct a postoperative infection following the first operation. All colorectal, gastric, hepatobiliary, or small bowel procedures with a predetermined Current Procedural Terminology (CPT) code were included (Supplemental Digital Content 1). To ensure adequate time for resolution of the initial postoperative infection, patients for whom the second operation was less than 30 days from the index operation were excluded. To include only postoperative infections, patients were excluded if a postoperative infection was the indication for either operation or infection was present upon admission for the procedure.
Postoperative infection was initially screened for using International Classification of Diseases (ICD) 9 and 10 codes for postoperative infections within 30 days of the procedure date (Supplemental Digital Content 2). Infection was then confirmed by individual chart review and defined using the American College of Surgeons National Surgical Quality Improvement Project Definitions (NSQIP)(10). Additionally, demographic information and known risk factors for a postoperative infection, such as a diagnosis of diabetes, use of corticosteroids, use of minimally invasive surgery (MIS) and smoking status were manually abstracted from the medical record. Prescription of preoperative antibiotic prophylaxis was also abstracted by manual chart review.
Microbial Culture Analysis
For patients that developed a postoperative infection after either operation, data from microbial cultures, including the source of the culture, organisms isolated, and antibiotic susceptibility and resistance were abstracted. Microbial antibiotic resistance was defined as resistance to one or more class of antibiotics on the microbial laboratory results. Microbial identification and resistance was performed at the University of Chicago Clinical Microbiological laboratory.
Statistical Analysis
For demographic information, comorbidities, and surgical characteristics, appropriate cutoffs for continuous variables were selected. Categorical variables were analyzed using the chi-square test or Fisher’s exact test when five or fewer events were expected. Ordinal variables were analyzed using the Wilcoxon rank sum test. Infection rates after the second operation were compared between those who did and did not have an infection after their first operation, and a multivariable logistic regression for the outcome of infection after the second operation was built.
Logistic regression was performed for the primary outcome presence of postoperative infection after the second operation. A priori confounders of the relationship between presence of infection after the first operation and presence of infection after the second operation were considered based on prior evidence of their effect on postoperative infection. These a priori variables included in the multivariable model were a diagnosis of diabetes, smoking status, and whether a laparoscopic approach was used. Variables that differed significantly between those who did develop infections after the first operation and those that did not develop an infection were also included in the model. Additional confounders were first tested with a univariate regression and added to the model when a significant relationship with the primary outcome was found, or when adding the variable to the multivariable model changed the beta coefficient of presence of infection after the second operation by greater than 10%.
Culture results were compared between infections developed after the first operations and after the second operations. Two sample tests of proportions were used to compare sources of positive culture and rates of antibiotic resistance and resistance to preoperative antibiotic prophylaxis. All analyses were performed using STATA statistical software (StataCorp) 15 using an alpha value of 0.05 for significance.
RESULTS
Study Population
840 adult patients met the inclusion criteria for the study and had undergone two abdominal operations during the study period. From this cohort, 82 adults had an infection that was the indication for surgical intervention and thus were excluded from the study. This resulted in a final group of 758 adult patients for analysis.
Of the 758 patients, 15.0% (n=114) had a confirmed postoperative infection following their first operation. 8 patients had an ICD9/10 codes consistent with an infection but on manual chart review an infection could not be confirmed and were placed into the non-infection cohort. Data on patient demographics, comorbidities, and surgical indication and operative approach is presented in Table 1. Patients who developed an infection after this index operation were more likely to be diabetic and undergo an open procedure. Of the 114 patients who had a postoperative infection after the first operation, 22.8% (n=26) had a postoperative infection after their second operation. Of the 644 patients who did not have an infection after the initial operation, only 9.5% (n=61) had a postoperative infection after their second operation. There were no significant differences in the demographics and comorbidities between patients who had an infection versus those that did not following their second operation (Table 2). Further, there were no significant differences in the location of infection between infection 1 and infection 2: intra-abdominal abscess 35.9% vs. 37.9%, p=0.77; bacteremia 35.1% vs. 42.5%, p=0.28; skin 21.9% vs. 27.6%, p=0.35; urinary 12.2% vs. 17.2%, p=0.32; pneumonia 4.2% vs. 4.6, p=0.83. The mean time between the first procedure and the second procedure was 366.4 days (SD 421.6), and there was significant differences in time to the second procedure between those who developed a second infection and those that did not (mean 328.5 days, SD 350.8 vs. 371.1, SD 429; p=0.38).
Table 1.
Baseline Characteristics of the Cohort after Operation 1
| Characteristic | Total cohort (n = 758) | Postoperative infection | p Value | |
|---|---|---|---|---|
| No (n = 644) | Yes (n = 114) | |||
| Age, y, mean (SD) | 49.4 (16.5) | 49.2 (16.3) | 50.6 (16.6) | 0.84 |
| Sex, m, n (%) | 359 (47.4) | 311 (57.9) | 48 (42.1) | 0.22 |
| Race, n (%) | ||||
| White | 509 (67.2) | 435 (67.6) | 74 (64.1) | 0.87 |
| African American | 183 (24.1) | 152 (23.6) | 31 (27.2) | |
| Other | 51 (6.7) | 44 (6.8) | 7 (6.1) | |
| Unknown | 15 (2.0) | 13 (2.0) | 2 (1.8) | |
| Diabetes, n (%) | 114 (15.0) | 90 (13.8) | 24 (22.4) | 0.02* |
| Smoking, n (%) | ||||
| Never | 335 (44.2) | 285 (44.3) | 50 (43.9) | 0.91 |
| Former | 169 (22.3) | 141 (21.9) | 28 (24.6) | |
| Current | 94 (12.4) | 80 (12.4) | 14 (12.3) | |
| Unknown | 160 (21.1) | 138 (21.4) | 22 (19.3) | |
| Steroid use, n (%) | 338 (44.6) | 288 (44.7) | 50 (43.9) | 0.86 |
| BMI, kg/m2, mean (SD) | 26.7 (8.1) | 26.8 (8.2) | 26.5 (7.2) | 0.71 |
| ASA, n (%) | 118 (15.6) | 101 (15.7) | 17 (14.9) | |
| 1 | 16 (2.1) | 13 (2.0) | 3 (2.6) | 0.36 |
| 2 | 231 (30.5) | 199 (30.9) | 32 (28.1) | |
| 3 | 357 (47.1) | 305 (47.4) | 52 (45.6) | |
| 4 | 26.7 (7.6) | 26.8 (8.16) | 26.5 (4.11) | |
| 5 | 34 (4.5) | 25 (3.9) | 9 (7.9) | |
| Unknown | 2 (0.3) | 1 (0.2) | 1 (0.9) | |
| Operation, n (%) | ||||
| HPB | 69 (9.1) | 61 (9.5) | 8 (7.0) | <0.01* |
| CRS | 334 (44.1) | 279 (43.3) | 55 (48.3) | |
| Gastric | 47 (6.2) | 44 (6.8) | 3 (2.6) | |
| SB | 152 (20.1) | 116 (18.0) | 36 (31.6) | |
| Exploratory | 156 (20.6) | 144 (22.4) | 12 (10.5) | |
| MIS, n (%) | 405 (53.4) | 374 (58.1) | 31 (27.2) | <0.01* |
| LOS, d, mean (SD) | 8.5 (10.2) | 7.6 (9.9) | 13.6 (9.8) | <0.01* |
p Value < 0.05
ASA, American Society of Anesthesiologists Class, CRS, colorectal surgery; HPB, hepatobiliary surgery; LOS, length of stay; MIS, minimally invasive surgery; SB, small bowel surgery
Table 2.
Baseline Characteristics of the Cohort after Operation 2
| Characteristic | Surgery 1: Infection | Surgery 1: No infection | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 114) | and Surgery 2: Infection | p Value | Total (n = 644) | and Surgery 2: Infection | p Value | |||
| No (n = 88) | Yes (n = 26) | No (n = 583) | Yes (n = 61) | |||||
| Age, y, mean (SD) | 51.2 (15.4) | 49.4 (13.2) | 50.9 (14.5) | 0.49 | 49.8 (14.2) | 50.8 (14.8) | 49.7 (15.9) | 0.55 |
| Sex, m, n (%) | 48 (42.1) | 39 (44.3) | 9 (34.6) | 0.49 | 311 (48.3) | 281 (48.2) | 30 (49.2) | 0.88 |
| Race, n (%) | ||||||||
| White | 74 (64.9) | 60 (68.2) | 14 (53.9) | 0.38 | 435 (67.6) | 390 (66.9) | 45 (73.8) | 0.09 |
| AA | 31 (27.2) | 21 (23.9) | 10 (38.5) | 152 (23.6) | 144 (24.7) | 8 (13.11) | ||
| Other | 7 (6.1) | 5 (5.7) | 2 (7.7) | 44 (6.8) | 37 (6.4) | 7 (11.5) | ||
| Unknown | 2 (1.8) | 2 (2.3) | 0 (0.0) | 13 (2.0) | 12 (2.1) | 1 (1.6) | ||
| Diabetes, n (%) | 90 (79.0) | 17 (19.3) | 7 (26.9) | 0.40 | 83 (12.9) | 76 (13.0) | 7 (11.5) | 0.84 |
| Smoking, n (%) | ||||||||
| Never | 50 (43.9) | 36 (40.9) | 14 (53.9) | 0.18 | 285 (44.3) | 262 (44.9) | 23 (43.9) | 0.61 |
| Former | 28 (24.6) | 25 (28.4) | 3 (11.9) | 141 (21.9) | 128 (22.0) | 13 (21.3) | ||
| Current | 14 (12.3) | 9 (10.2) | 5 (19.2) | 94 (14.6) | 80 (12.2) | 14 (14.8) | ||
| Unknown | 22 (19.3) | 18 (20.5) | 4 (15.4) | 138 (21.4) | 122 (20.9) | 16 (26.2) | ||
| Steroid use, n (%) | 50 (43.9) | 39 (44.3) | 11 (42.3) | 0.85 | 288 (44.7) | 263 (45.1) | 25 (41.0) | 0.54 |
| BMI, kg/m2, mean (SD) | 26.7 (5.7) | 26.2 (7.9) | 26.9 (6.5) | 0.41 | 27.1 (7.4) | 26.8 (8.1) | 27.6 (5.4) | 0.75 |
| ASA, n (%) | ||||||||
| 1 | 1 (0.9) | 1 (1.1) | 0 (0.0) | 0.19 | 12 (1.9) | 10 (1.7) | 2 (3.3) | 0.23 |
| 2 | 35 (30.7) | 30 (34.1) | 5 (19.2) | 229 (35.6) | 212 (36.4) | 17 (27.9) | ||
| 3 | 65 (57.0) | 48 (54.6) | 17 (65.4) | 362 (56.2) | 323 (55.4) | 39 (63.9) | ||
| 4 | 10 (8.8) | 7 (8.0) | 3 (11.5) | 30 (4.7) | 27 (4.6) | 3 (4.9) | ||
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | ||
| Unknown | 3 (2.6) | 2 (2.3) | 1 (3.9) | 10 (1.6) | 10 (1.7) | 0 (0.0) | ||
| Operation, n (%) | ||||||||
| HPB | 13 (11.4) | 11 (12.5) | 2 (7.7) | 0.11 | 61 (9.5) | 54 (9.3) | 7 (11.5) | 0.36 |
| CRS | 68 (59.7) | 54 (61.4) | 14 (53.9) | 319 (49.5) | 292 (50.1) | 27 (44.3) | ||
| Gastric | 3 (2.6) | 3 (3.4) | 0 (0.0) | 47 (7.3) | 42 (7.2) | 5 (8.2) | ||
| SB | 17 (14.9) | 14 (15.9) | 3 (11.5) | 72 (11.2) | 61 (10.5) | 11 (18.0) | ||
| Exploratory | 13 (11.4) | 6 (6.8) | 7 (26.9) | 145 (22.5) | 134 (23.0) | 11 (18.0) | ||
| MIS, n (%) | 31 (27.2) | 25 (28.4) | 6 (23.1) | <0.01* | 374 (58.1) | 359 (61.5) | 15 (24.5) | <0.01* |
| LOS, d, mean (SD) | 13.4 (9.8) | 13.2 (8.9) | 14.1 (12) | 0.67 | 8.5 (10.2) | 8.3 (10.0) | 10 (10.8) | 0.21 |
p Value < 0.05
ASA, American Society of Anesthesiologists Class; CRS, colorectal surgery; HPB, hepatobiliary surgery; LOS, length of stay; MIS, minimally invasive surgery; SB, small bowel surgery
Incidence of Postoperative Infection
We next compared the incidence of infection across the various cohorts (Figure 1). The 30-day postoperative infection rate was 15.0% for the first operation and 11.5% for the second operation. The incidence of infection following the second operation was significantly higher in those patients who developed an infection after the initial operation compared to those that did not develop an infection after the first operation (22.8% vs 9.5%; p<0.001). The infection rate after the second operation also significantly associated with infection after the first operation: those with a previous infection had a significantly higher rate of infection after the second operation (22.8% and 15.0%; p=0.035), whereas those without a previous infection had a significantly lower rate of infection after the second operation (9.9% and 15.0%; p<0.001)
Figure 1.
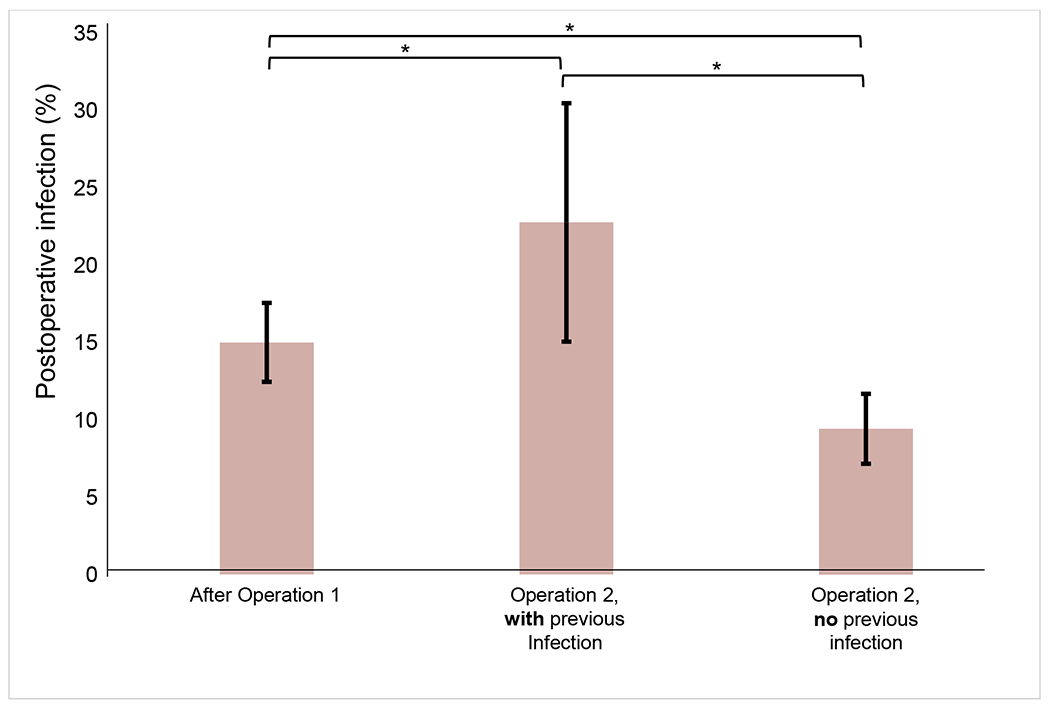
30-day postoperative infection rate after first abdominal operation and after second operation, stratified by history of postoperative infection after previous operation. Error bars indicate the 95% CI for rate of infection. Brackets above correspond to the infection rates compared. *p Value < 0.05.
Logistic Regression
To determine if having a previous postoperative infection was an independent risk factor for a postoperative infection following a second operation, a multivariable logistic regression was built (Table 3). Diabetes, operation category, and MIS approach, which were found to be significantly associated with the presence of a postoperative infection after the first operation, were included in the multivariable model. Results demonstrated that infection following the first operation was independently associated with development of an infection following the second operation (OR 2.49; 95% CI 1.46-4.25). Additionally, a MIS approach was protective against a postoperative infection (OR 0.2; 95% CI 0.10-0.41). When the other a priori cofounders that were tested in the univariate analysis were added stepwise to this model, no other variables had a significant relationship with occurrence of infection after the second operation.
Table 3.
Multivariable Logistic Regression for Odds of Postoperative Infection after a Second Abdominal Surgery.
| Variable | Operation 2: risk of infection, total n (%) | Odds ratio (95% CI) |
|---|---|---|
| Infection after operation 1 | ||
| No | 61 (9.4) | Ref |
| Yes | 26 (22.8) | 2.49 (1.46-4.25)* |
| Diabetes | ||
| No | 73 (12.6) | Ref |
| Yes | 14 (15.1) | 1.00 (0.52-1.93) |
| Smoking | ||
| Never | 37 (12.4) | Ref |
| Former | 16 (10.5) | 0.81 (0.42-1.54) |
| Current | 14 (17.5) | 1.26 (0.63-2.49) |
| Unknown | 20 (14.3) | 1.18 (0.65-2.15) |
| MIS | ||
| No | 78 (15.7) | Ref |
| Yes | 9 (3.4) | 0.20 (0.10-0.41)* |
| Operation | ||
| HPB | 9 (12.2) | 0.96 (0.40-2.28) |
| CRS | 41 (10.6) | 0.81 (0.44-1.49) |
| Gastric | 5 (10.0) | 0.86 (0.30-2.47) |
| SB | 14 (15.7) | 1.25 (0.58-2.70) |
| Exploratory | 18 (11.4) | Ref |
p Value < 0.05
CRS, colorectal surgery; HPB, hepatobiliary surgery; MIS, minimally invasive surgery; SB, small bowel surgery
Microbiology of Postoperative Infections
Microbiological cultures were available in 71.1% (n=143) of the patients who had an infection (Table 4). The source and organisms isolated of microbial cultures from infections after the first and second operations were similar. Tissue or fluid was the most common source of positive cultures. The most common organisms isolated were Enterococcus species, Staphylococcus species, and Escherichia coli, and the majority of positive cultures isolated more than one organism. Infection with Enterobacter species were observed more frequently in infections developed after the second operation (p=0.035).
Table 4.
Microbiological Results
| Variable | Operation 1: infection | Operation 2: infection | p Value |
|---|---|---|---|
| Source | |||
| DSSI | 37 (46.8) | 29 (46.8) | 0.29 |
| SSSI | 16 (20.3) | 12 (19.4) | |
| Urine | 13 (16.5) | 11 (17.7) | |
| Respiratory | 6 (7.8) | 4 (6.5) | |
| Blood | 4 (5.1) | 5 (8.1) | |
| Other | 3 (3.8) | 1 (1.6) | |
| Culture | |||
| Enterococcus spp. | 22 (27.5) | 27 (42.9) | 0.16 |
| Escherichia coli | 17 (21.3) | 9 (14.3) | 0.28 |
| Staphylococcus spp. | 14 (17.6) | 19 (30.1) | 0.29 |
| Bacteroides spp. | 13 (16.3) | 7 (11.1) | 0.38 |
| Candida spp. | 12 (15.0) | 10 (15.9) | 0.89 |
| Klebsiella sp | 6 (7.5) | 10 (15.9) | 0.12 |
| Streptococcus spp. | 4 (5) | 3 (4.8) | 0.67 |
| Enterobacter spp. | 2 (2.5) | 7 (11.1) | 0.04 |
Data presented as n (%)
DSSI, deep surgical site infection; SSSI, superficial surgical site infection
Antibiotic resistance was significantly more common after the second operation compared to infections following the first operation (64.1% and 82.3%, p=0.036) (Table 5). There was no significant difference in the time between the first and second operation between patients who developed a resistance infection verses those that did not (mean 311.1 days, SD 324.7 vs. 333.3, SD 359.8; p=0.82. Resistance to a class of antibiotics given as preoperative prophylaxis was observed more frequently after second operations (32.1% and 49.0%, p=0.078). Of the 25 patients that had a second infection and showed resistance to preoperative prophylaxis given to them during their second procedure, 84% (21/25) had an antibiotic resistant infection following their first operation.
Table 5.
Antibiotic Resistance Observed in Microbial Cultures of Postoperative Infection after First and Second Abdominal Operation
| Resistance | Operation 1 infection | Operation 2 infection | p Value |
|---|---|---|---|
| None | 19 (35.9) | 9 (17.7) | 0.03* |
| Single-drug | 11 (20.8) | 10 (19.6) | 0.87 |
| Multi-drug | 34 (64.1) | 42 (82.3) | 0.03* |
| To drugs given for preoperative antibiotic prophylaxis | 17 (32.1) | 25 (49.0) | 0.07 |
Data presented as n (%)
p < 0.05
DISCUSSION
Infections following gastrointestinal surgery cause significant resource utilization, increased healthcare costs, and prolonged length of stay(1–3). Identifying patients at an increased risk of infection may be pivotal in reducing patient morbidity following abdominal surgery. In this study, we demonstrated that a previous postoperative infection is an independent risk factor for the development of another postoperative infection after a second, unrelated gastrointestinal operation. Given that nearly 60% of surgical patients will undergo more than one abdominal surgery over their lifetime, this finding has important clinical implications(11).
Why patients are at risk for a second infection if they have a history of a postoperative infection is unclear. Preoperative antibiotics given prior to incision, are one of the main modalities to prevent postoperative infections and has proven to be very efficacious across disciplines(12–14). For example, a recent Cochrane review of 260 trials showed that preoperative prophylactic antibiotics significantly decreased the incidence of surgical site infections in patients undergoing colorectal surgery (risk ratio 0.34; 95% confidence interval 0.28 - 0.41)(15). Societies such as the Surgical Infection Society (SIS), American College of Surgeons (ACS), and the Centers of Disease Control (CDC) all have given recommendation on the class of preoperative antibiotic based on the location of the incision, wound classification, and local resistance patterns(16, 17). In our study, we found that 50% of the infections following the second operation were resistant to the prophylactic antibiotics that were prescribed preoperatively; almost all of these patients demonstrated antibiotic resistant organisms following their first operation. While this observation is associative, it suggests that the resistant bacteria that colonized during the first infection, may play a causative role in the development of the second infection. If so, further investigation is needed to determine if the risk of a second infection may be reduced by giving preoperative antibiotics tailored to the resistance pattern of the first operation.
The most common clinical situation in which antibiotics are altered due to preoperative resistance is when vancomycin or clindamycin is prescribed to patients that are high-risk for- or colonized with methicillin-resistant Staphylococcus aureus (MRSA). This strategy has been shown to be efficacious in reducing postoperative infections and is recommended in most societal guidelines(18, 19). Yet, there is little data as to if other drug-resistant pathogens that recently colonized a patient undergoing surgery should be directly targeted with preoperative antibiotics. Cohen et al. found that a culture proven infection within 90 days of a surgical procedure was associated with the development of a postoperative infection(20). While the authors did not evaluate if the resistance included antibiotics given as perioperative prophylaxis, it does suggest that, as in the case with preoperative MRSA colonization, administrating antibiotics that covered all resistant pathogens from previous infections could decrease the risk of postoperative infection.
Prescription of antibiotics to surgical patients, either as prophylaxis or treatment, must be balanced with the risk of the development of antibiotic resistance. It is well known that antibiotic resistant infections have significantly increased over the last decade(21, 22). Sixty-four percent of patients in our cohort had an antibiotic resistance infection following their first operation, which is in line with previous reports(20). Strikingly, the incidence of antibiotic resistance in our cohort following the second infection was nearly 85%. This alarmingly high rate of antibiotic resistance is likely due to the cumulative exposure of antimicrobials over the course of two surgeries and two postoperative infections as even a single dose or short courses of antibiotics can promote resistance(23, 24). In patients whom have had multiple prior procedures, the risk of an antibiotic resistance infection is extraordinarily high.
Our study has multiple limitations. Culture results were not available in every patient. At our institution, acquisition of microbial cultures is up to the discretion of the surgeon, and thus could have led to selection bias in which the more severe infections had cultures. Further, the retrospective nature of our study relied upon chart review. While we performed a manual chart review to confirm infections and gather microbiological data, irregularities in either coding for the included procedures or infections could have unintentionally omitted patients.
Taken together, our study demonstrates that a previous infection is an independent risk factor for another postoperative infection following an unrelated abdominal surgery. These patients may benefit from an individualized approach where previous culture data drives the choice of prophylactic antibiotics.
CONCLUSIONS
In this manuscript we have demonstrated that a previous postoperative infection is an independent risk factor for a subsequent postoperative infection and is associated with resistance to standard prophylaxis. Individualization of antibiotic prophylaxis in patients with a previous postoperative infection may be warranted.
Supplementary Material
Supplemental Digital Content 1. Current Procedure Terminology Codes Used to Query the Electronic Record for Patients Who Underwent 2 Abdominal Operations During the Study Period
Supplemental Digital Content 2. ICD Codes Used to Screen Patients for Infection Within 30 Postoperative Days.
Support:
This work was supported by NIH grant [K08 CA248957]. Dr Feldt is supported by National Cancer Institute grant [R25CA240134].
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the 15th Annual Academic Surgical Congress, Orlando, FL, February 2020.
REFERENCES
- 1.Marchetti A, Rossiter R. Economic burden of healthcare-associated infection in US acute care hospitals: societal perspective. J Med Econ 2013;16:1399–404. [DOI] [PubMed] [Google Scholar]
- 2.Lee MJ, Daniels SL, Wild JRL, et al. Readmissions after general surgery: a prospective multicenter audit. J Surg Res 2017;209:53–59. [DOI] [PubMed] [Google Scholar]
- 3.Mujagic E, Marti WR, Coslovsky M, et al. Associations of Hospital Length of Stay with Surgical Site Infections. World J Surg 2018;42:3888–3896. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien WJ, Gupta K, Itani KMF. Association of Postoperative Infection With Risk of Long-term Infection and Mortality. JAMA Surg 2020;155:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Dumville JC, Norman G, et al. Intraoperative interventions for preventing surgical site infection: an overview of Cochrane Reviews. Cochrane database Syst Rev 2018;2:CD012653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedrick TL, Heckman JA, Smith RL, et al. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg 2007;205:432–8. [DOI] [PubMed] [Google Scholar]
- 7.Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Covington A, Pamer EG. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 2017;279:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidry CA, Shah PM, Dietch ZC, et al. Recent Anti-Microbial Exposure Is Associated with More Complications after Elective Surgery. Surg Infect (Larchmt) 2018;19:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anon. American College of Surgeons. 2021. Available at: https://www.facs.org/quality-programs/acs-nsqip/participant-use. Accessed August 9, 2021. [Google Scholar]
- 11.Strik C, Stommel MWJ, Schipper LJ, et al. Risk factors for future repeat abdominal surgery. Langenbeck’s Arch Surg 2016;401:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659–695. [DOI] [PubMed] [Google Scholar]
- 13.Roos D, Dijksman LM, Tijssen JG, et al. Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery. Br J Surg 2013;100:1579–88. [DOI] [PubMed] [Google Scholar]
- 14.Schuster KM, Holena DN, Salim A, et al. American Association for the Surgery of Trauma emergency general surgery guideline summaries 2018: acute appendicitis, acute cholecystitis, acute diverticulitis, acute pancreatitis, and small bowel obstruction. Trauma Surg acute care open 2019;4:e000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane database Syst Rev 2014:CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017;224:59–74. [DOI] [PubMed] [Google Scholar]
- 17.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 18.Branch-Elliman W, Ripollone JE, O’Brien WJ, et al. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study. PLoS Med 2017;14:e1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reineke S, Carrel TP, Eigenmann V, et al. Adding vancomycin to perioperative prophylaxis decreases deep sternal wound infections in high-risk cardiac surgery patients. Eur J Cardiothorac Surg 2018;53:428–434. [DOI] [PubMed] [Google Scholar]
- 20.Cohen ME, Salmasian H, Li J, et al. Surgical Antibiotic Prophylaxis and Risk for Postoperative Antibiotic-Resistant Infections. J Am Coll Surg 2017;225:631–638.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laxminarayan R, Van Boeckel T, Frost I, et al. The Lancet Infectious Diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect Dis 2020;20:e51–e60. [DOI] [PubMed] [Google Scholar]
- 22.Nji E, Kazibwe J, Hambridge T, et al. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci Rep 2021;11:3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil D, Hultin M, Rashid MU, Lund B. Oral microflora and selection of resistance after a single dose of amoxicillin. Clin Microbiol Infect 2016;22:949.e1–949.e4. [DOI] [PubMed] [Google Scholar]
- 24.Archer GL, Armstrong BC. Alteration of staphylococcal flora in cardiac surgery patients receiving antibiotic prophylaxis. J Infect Dis 1983;147:642–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Current Procedure Terminology Codes Used to Query the Electronic Record for Patients Who Underwent 2 Abdominal Operations During the Study Period
Supplemental Digital Content 2. ICD Codes Used to Screen Patients for Infection Within 30 Postoperative Days.


