Abstract
Nanoparticle-assisted laser desorption/ionization mass spectrometry (LDI-MS) is a powerful tool for the analysis of a wide range of molecules. Many of the drawbacks in the matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) can be avoided with the application of nanomaterials as matrices as well as substrates for the LDI-MS to achieve a low background noise in low m/z region and high reproducibility. Surface-assisted LDI (SALDI)-MS, especially the nanoparticle-based LDI-MS, has emerged as a promising technique for the analysis of trace amounts of substances in various biological samples due to their high surface area for analyte enrichment, efficient desorption/ionization, and homogeneous crystallization of sample. Therefore, it is highly useful in clinical, forensic, medical, food and drug analyses, disease diagnosis, and various other fields. In this review, we briefly discuss the application of various nanomaterials, which include metal-based, carbon-based, silicon-based nanomaterials and nanocomposites, as matrices and substrates for LDI-MS based drug and metabolite analyses and possible detection strategies. Also, we discuss the idea of using “mass tag” for signal amplification for drug and metabolite detection using nanoparticle assisted LDI-MS.
Keywords: Drugs, Laser desorption and ionization, Mass spectrometry, Matrix Metabolites, Nanoparticles
1. Introduction
Laser desorption/ionization (LDI) is an ionization technique that desorbs and ionizes substances through heating them by pulse laser irradiation. LDI is one of the most prevalent ionization techniques; only second to electrospray-based ionization (ESI), due to its high speed of analysis, simple spectra and its capacity to analyze wide category of substances. Early LDI-mass spectrometry (LDI-MS) techniques had limitations in the type of substances due to low laser absorption and ionization efficiencies, easy fragmentation and thermal degradation of analytes. Organic molecule (nicotinic acid) as matrix to assist the LDI process of the analyte was introduced in the 1980's by Hillenkamp and Karas [1]. The matrix enhances the desorption and ionization of the analytes by absorbing laser energy and transferring it to analytes while preventing their direct fragmentation. This technique, named matrix-assisted laser desorption/ionization (MALDI), has greatly expanded the category of analytes, especially large molecules, that can be analyzed by LDI-MS [2–4]. In a typical process, both the matrix and the analyte molecule are desorbed and ionized by protonation or deprotonation. Although MALDI improves the signal of large biomolecules by preventing fragmentation to an extent and increases ionization efficiency, the matrices create intense fragmentation signals in the <700 m/z region of the mass spectrum [5]. Thus, matrix fragmentation results in poor analysis of smaller molecules even though matrices can be modified to reduce matrix effects [6]. In addition, the uneven crystallization of the matrix and the analyte molecules may cause heterogeneous sample distribution on the MALDI plate and affect the shot-to-shot and sample-to-sample reproducibilities. Another variation of the LDI-MS, the surface assisted laser desorption/ionization (SALDI), has emerged as the technique of choice for the analysis of small molecules, such as drugs and metabolites [7–11]. SALDI is an ionization technique that utilizes inorganic substances, nanomaterials or composites to assist in the ionization of target analyte [12]. SALDI has significantly lower matrix interferences in the low m/z region with more homogeneous analyte distribution and higher salt tolerance in comparison with MALDI [13–15].
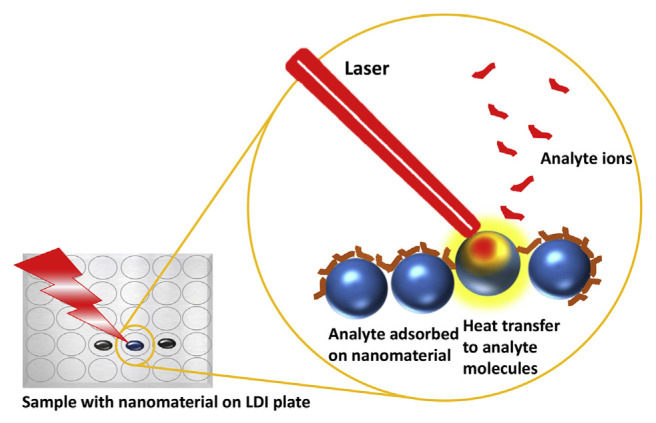
Many nanostructured inorganic materials such as silica, metal and metal oxide nanoparticles, semiconductor nano-particles and some carbon-based nanomaterials like graphene oxide are efficient substrates in SALDI-MS analysis for drug and metabolite analysis due to their excellent photo-absorption coefficient and heat transfer efficiency [7,16–22]. In a SALDI process, the applied laser beam heats up the substrate instead of the analyte. The substrate then transfers heat to the analyte, leading to the desorption and ionization of the latter. Thus, the desorption and ionization of the analyte highly depend on the photothermal conversion and heat transfer efficiencies of the substrate [23]. The mechanism of SALDI process is illustrated in Fig. 1. Recently, Picca et al. have reported a concise review on the mechanism of nanophase induced LDI in nanoparticle assisted LDI-MS [24]. Since there is very low fragmentation of nanostructure-based substrates in SALDI process, the interference due to the fragments is highly reduced. As a result, the low background noises of SALDI-MS at low m/z region allow for the analysis of a large category of small molecules. Common pollutants, illegal or medicinal drugs and their metabolites tend to have small molecular sizes, which can be analyzed by SALDI-MS. Other important aspects in mass spectrometry based detection of metabolite are the metabolomics profiling and targeted approach [25–28]. For selective ionization of the analytes, SALDI matrices are engineered through structural and surface modification, which has the advantages of maximizing the specificity and sensitivity of MS methods. The nano-particle surfaces modified with specific molecules, such as antibodies, proteins, and aptamers, highly reduce the background noise and interference from the undesired molecules from the biological samples. Therefore, combination of separation or enrichment of analytes and efficient SALDI is critical in nanoparticle-based LDI-MS for drugs and metabolites analysis.
Fig. 1.
Schematic representation of a SALDI process mechanism.
In this review, we mainly discuss the detection of drugs and metabolites using nanomaterial-assisted LDI-MS. A summary of the various types of naomaterial substrates and analytes discussed in this review is presented in Table 1. Based on the category of nanomaterials employed as substrate, different names have been coined to the LDI processes for convenience, by various authors. For example, nano-assisted LDI (NALDI) [16], desorption/ionization on silicon (DIOS) [17], silicon nanopost arrays-assisted LDI (NAPA-LDI) [29], colloidal graphite-assisted LDI (GALDI) [30], carbon dot assisted LDI (CALDI) [31], etc. However, the role of these substrates in the LDI application is almost the same. We briefly discuss the type of nanomaterial substrates and their important properties which improve the LDI process and application of “mass tag” for LDI-MS signal amplification, in drug and metabolite analysis.
Table 1.
Various types of nanomaterial substrates used for the detection of drugs or metabolites by LDI-MS.
| Category | Probe/substrate | Nanomaterial properties or LDI process | Analytes | LODa | References |
|---|---|---|---|---|---|
| Metal-based | Au nanoshell | plasmonic, generation of hot carriers conductive, nanostructured, and transparent | amino acids and carbohydrate | 3–30 pmol | [8] |
| ITO | lipids, glycerol, and creatinine | 6 mg L−1 | [9] | ||
| TiO2 nanowire | photoabsorption | benzylpenicillin | 0.4 ng mL−1 | [18] | |
| TiO2 NPs | photoabsorption | 18 candidate metabolites from bacteria | n/a | [46] | |
| HSA-Fe3O4 NPs | affinity towards analytes molecules | phenytoin, ibuprofen, camptothecin, warfarin | 5 μM | [19] | |
| ZnO, TiO2, Fe2O3, CeO2 | small size and large surface area, strong UV absorption | amitriptyline hydrochloride, imipramine hydrochloride, nortriptyline hydrochloride, promazine hydrochloride | n/a | [44] | |
| Au NPs, Ag NPs, Pd NPs, Pt NPs | photoabsorption, binding affinity, internal energy transfer | benzylpyridinium chloride | n/a | [39] | |
| Au NPs | photoabsorption, high salt tolerance | progesterone, testosterone, cortisol, ribose, glucose, maltose, 5-HIAA, tryptophan, GM1, bradykinin, angiotensin I/II, substance P | 46.5–5115.7 nM | [40] | |
| Au NPs | photoabsorption | palmitic acid, oleic acid, stearic acid, verapamil | n/a | [41] | |
| Au NPs/KBr | photoabsorption | acetaminophen, noscapine, loratadine, coptisine, berberine, palmatine | n/a | [42] | |
| ARRO SupraNano™ | photoabsorption | cocaine, methadone, aspirin, paracetamol, caffeine | n/a | [43] | |
| TiO2-dopamine monolith | dopamine enhanced UV absorbance, selective binding of Lewis bases | fatty acids, cholesterols, ceramides, diacylglycerols, phosphatidylethanolamine, amino acids, alkaloids, peptides, lipids | n/a | [45] | |
| Carbon-based | graphene, GO | photoabsorption, electron transport | scutellarin, wogonin, ferulic acid | n/a | [67] |
| aptamer modified GO | enrichment, photoabsorption | cocaine, adenosine | n/a | [21] | |
| graphite rods | photoabsorption | 9-phenylacridine | 0.1 pmol | [30] | |
| C-Dots | in situ energy transfer via functional groups | mefenamic acid | 0.5 ng | [31] | |
| GO/MWCNT hybrid films | photoabsorption; functional groups of GO | cellobiose, leuenkephaline, glutamine, glucose, leucine, lysine, D-mannitol, phenylalanine | n/a | [33] | |
| CNT, C60, PGC, G, HOPG, ND | phase transition | nenzylpyridinium halide derivatives | n/a | [34] | |
| Graphene, GO, rGO | size dependent, photoabsorption | flavonoids, coumarin derivatives | 10 ppm | [35] | |
| Graphene, GO | photoabsorption, electron transport | tetracycline | 2 nM | [51] | |
| Graphite dots | strong UV absorption, hydroxyl groups provide high stability and favorable ΔG for analyte deprotonation | glucose, Ala-Gln, oligosaccharides; puerarin, daidzein, dihydrodaidzein | n/a | [52] | |
| N-doped graphene | proton transfer, photoabsorbance process | fatty acid, peptide, epiandrosterone, testosterone, methyltestosterone, nilotinib | n/a | [53] | |
| Silicon/Silica/Semiconductor | Nanostructured silicon | desorption ionization on porous silicon mass spectrometry imaging (DIOS-MSI) | methadone, heroine, EDDP | n/a | [17] |
| Mesoporous germanium | photoabsorption | cocaine | 1.7–3.5 ng mL−1 | [20] | |
| Silicon nanopillar arrays | photoabsorption | peptides, methadone | 32 ng mL−1 | [22] | |
| Nanoporous GaN–Ag composite | high UV absorption | R6G, adenine | <50 pmoles | [23] | |
| Silicon nanopost arrays | nanophotonic interaction | cAMP, acetylcholine, glucose, cholesterol, amino acids, small organic acids, phospholipids, fatty acids | n/a | [29] | |
| Porous silicon | high UV absorption | methadone | 14.74–19.50 ng mL−1 | [36] | |
| Nanostructured Silicon | nanostructure-initiator mass spectrometry (NIMS) | rapamycin, tigylglycine, N-acetyl-glutamic acid, uridine monophosphate, isobutyrylcarnitine, stearic acid, D-glucose | n/a | [37] | |
| Ordered silicon nanocavity arrays | desorption ionization on porous silicon-mass spectrometry (DIOS-MS) | benzylpyridinium salts, DPPC, angiotensin III | n/a | [56] | |
| Ordered silicon nanocavity arrays | desorption ionization on porous silicon-mass spectrometry (DIOS-MS) | methamphetamine, cocaine, MDMA | n/a | [57] | |
| Nanoporous silicon microparticles | photoabsorption | methamphetamine, cocaine, MDMA, methadone, EDDP | ~20 ng mL−1 | [58] | |
| hydrophobic silica powder | photoabsorption | nicotine | n/a | [60] | |
| DHB + porous silicon | matrix-enhanced nanostructure initiator mass spectrometry (ME-NIMS) | pentamidine | 0.005 μM | [61] | |
| Nanostructured Silicon | morphology-driven controlled nanostructure-initiator mass spectrometry (NIMS) | arginine, palmitylcarnitine, streptomycin, bradykinin, angiotensin, neurotensin | n/a | [62] | |
| Nanoporous silicon films | perfluoro coating assisted desalting | taurine, aspartic acid, malic acid, glutamic acid, histidine | ~1 μM | [63] | |
| Composite-based | PAN/Nafion®/CNT | photoabsorption | verapamil, methotrimeprazine, propranolol, chloroprothixene | 220 fM | [13] |
| Au/PAA-GO film | photoabsorption, thermal conductivity | cellobiose, mannitol, glucose, leucine, phenylalanine, glutamine, leuenkephalin | n/a | [50] | |
| SiO2@Ag particles | plasmonic resonance | mannitol, glucose | 100 ng μL−1 | [65] | |
| Ag-DIOS | silver adduct assisted | bromoisatin, lipids, cholesterol | n/a | [66] | |
| plasmonic gold chip | plasmon resonance, hot carrier production | amino acids, carbohydrates, metabolites | n/a | [64] | |
| Mass tag | thiolalkane-Au NPs | thermal desorption of ligand | enrofloxacin, ciprofloxacin | 50 mg kg−1 | [7] |
| Fib–Au NPs–MCEM | NP fragmentation | thrombin, anticoagulants | n/a | [47] |
n/a: not available.
2. Nanomaterials as a matrix for SALDI-MS
The major role of nanomaterial in nanomaterial-based LDI-MS is the enrichment of the analyte molecules and their efficient desorption and ionization. Therefore, nanomaterials with large surface area, porous structure, easy functionalization, high photoabsorption properties and heat transfer efficiency are highly favored. Metal nanoparticles, especially gold nanoparticles (Au NPs) were found to transfer heat efficiently to the analyte molecules under pulse laser irradiation, enabling more effective ionization [32]. In addition, Au NPs' surface favors easy functionalization especially with sulfur containing molecules. Nanostructured metal oxides tend to have porous structure which enhances the analyte enrichment. Carbon materials such as carbon nanotube (CNT), graphene oxide (GO), graphite, etc. have hydrophobic as well as hydrophilic properties, and their surface can be easily modified. They are excellent choices as substrates for LDI-MS [33–35]. Similarly, silicon based nanomaterials, especially porous structured materials, are highly useful in enriching analyte molecules from the sample, which are found to be applied for detection of many drugs and metabolites [36,37]. For convenience, the discussion in this review has been divided into sections based on the category of nanomaterials employed for the analysis of drugs and metabolites. Most of the nanomaterial based LDI-MS follow SALDI-principle, in which either analyte ions or mass tag ions are produced; however, in some cases the nanomaterial substrate itself undergoes explosion and produces cluster ions, which are considered as the signal response for analyte determination. A detailed review of such LDI process is available in our earlier review article [32].
2.1. Metal-based nanomaterial
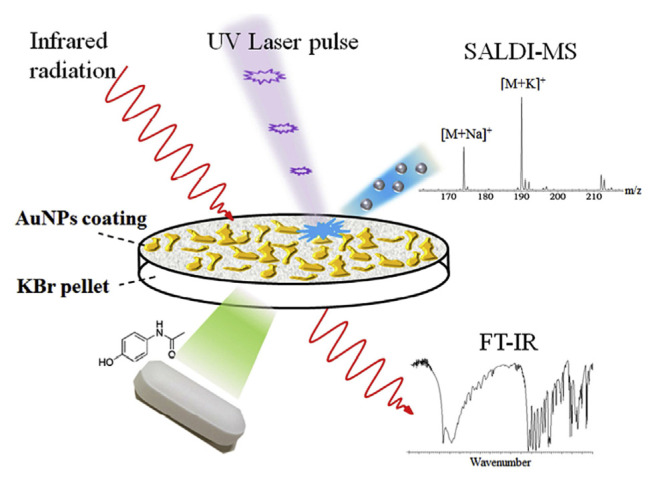
Metal-based nanomaterials are one of the earliest nano-materials to be developed and utilized as matrices in LDI-MS. Tanaka et al. demonstrated the use of ultrafine cobalt powder with a diameter of about 300 Å for the analysis of proteins and polymers with m/z values up to 100,000 by LDI-TOF-MS [38]. The discrete electronic levels in small-size metal nano-particles create quantum effects, and thus they show increased photo-absorption efficiency than that of bulk metals. Many metal nanoparticles have excellent UV absorption profile, and high melting point, which can promote thermal desorption process. Additionally, the ability of metal to form alloys, whether by chemical or physical processes, allows easy control of nanoparticle properties for optimization of analyte desorption. The mechanism of ionization of analytes by metallic nanoparticles are well addressed by Ng et al. who conducted the experiments on different small-size noble metal nanoparticles (Ag, Au, Pd and Pt NPs) [39]. The study concluded that many factors might affect the desorption/ionization efficiency of analytes, including thermal conductivity, internal energy transfer, thermal absorption, melting point and analyte-nanoparticle binding affinity. They suggest that substrates with a low melting point could enhance the ionization through phase transition. Phase transition increases entropy and favors ionization. The high affinity between analyte and the substrate could reduce the ionization efficiency. Therefore, to achieve efficient ionization of the analytes, high photo-absorption and low melting point of the substrate material, and moderate affinity between the analytes and substrate are highly favorable. Metal nanoparticles, Au NPs in particular have a long history in SALDI-MS detection of drugs and metabolites. Wu et al. reported the use of Au NPs in the detection of biomolecules including urea, creatinine, uric acid, and glucose in complicated urine samples [40]. In this report, Au NPs have been proved to be an excellent substrate, providing great shot-to-shot reproducibility [relative standard deviation (RSD) values from 0.03 to 3.48%], and is capable of detecting a multitude of compounds including neutral steroids (testosterone, progesterone and cortisol), carbohydrates (ribose, glucose and maltose), indoleamines (5-hydroxyindole acetic acid and tryptophan), etc. Tang et al. demonstrated that Au NPs when coupled with LDI-MS could also be applied for mass spectrometric detection of endogenous (palmitic acid, oleic acid, and stearic acid) and exogenous (verampil) chemicals in latent fingerprint (LFP) and mass spectrometric imaging of LFP based on the distribution of exogenous compounds [41]. The Au NPs are deposited on the ridges and grooves of the fingerprint with different degree of aggregation, resulting in a clear pattern of distribution. Mass spectrometric molecular imaging could reveal the chemical information of endogenous and exogenous compounds in the fingerprint, and if coupled with optical imaging it allows for personal identification. Chau et al. demonstrated that Au NPs could be directly applied as a coating on over-the-counter drug and traditional concentrated Chinese medicine granules in KBr pellets for LDI-MS detection of their active ingredients, without interfering the usage of the same pellets for FT-IR analysis (Fig. 2) [42]. They showed that the thin layer of Au NPs do not interfere with FT-IR analysis (difference in transmittance less than 2%), and also allow for LDI-MS detection of several minor active ingredients (e.g. noscapine and loratadine) and phytochemicals of Coptidis rhizoma (i.e. berberine, palmatine and coptisine) that are undetected by FT-IR.
Fig. 2.
Schematic representation of Au NPs coated KBr pellet for SALDI-MS and FT-IR analysis of traditional Chinese medicine. (Reproduced with permission from Ref. [42]).
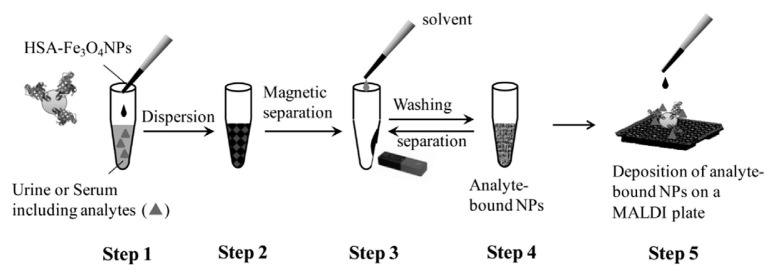
Metal oxide nanoparticles also assist LDI process and some of them have porous structure and high surface area, which are favorable for the enrichment of analyte molecules. Human serum albumin-modified Fe3O4 magnetic nanoparticles (HSA–Fe3O4 NPs) are found to be an efficient affinity SALDI probe to capture traces of drugs with low molecular weight, such as phenytoin, ibuprofen, camptothecin, and warfarin present in urine and serum by LDI-MS, whereas traditional MALDI can only detect warfarin [19]. Fig. 3 shows the schematic representation of affinity-SALDI-MS procedure for the enrichment and detection of drugs using HSA–Fe3O4 NPs as SALDI substrate, in serum or urine samples. The magnetic property of the nanoparticles enables the easy separation of the analytes from the background molecules. In this approach, first the analyte molecules are enriched using the affinity probe-functionalized Fe3O4 magnetic nanoparticles, then they are subjected to SALDI process. In such a case, the analysis follows a SALDI principle, in which analyte ions are produced. The authors further demonstrated that denatured-HSA (using NaBH4 for denaturation) exhibits higher sensitivity for the detection of drugs than HSA, probably due to the reduced S–S bonding in denatured-HSA leading to more open structure and enhanced interaction with drugs. Interestingly, LDI-MS can be successfully applied for the detection of drugs in latent fingerprint using the fingerprint powder itself as LDI substrate. Sundar et al. applied LDI-MS for the detection of two drugs of abuse (cocaine and methadone) and three therapeutic drugs (aspirin, paracetamol and caffeine) in latent fingerprint and imaging using commercial ARRO SupraNano™ MS black magnetic powder [43]. Very recently, Amin et al. used various metal oxide nanoparticles, such as ZnO, TiO2, Fe2O3, and CeO2 nanoparticles for the identification of small drug molecules, such as amitriptyline hydrochloride, imipramine hydrochloride, nortriptyline hydrochloride, promazine hydrochloride in latent fingerprint by SALDI-MS [44]. They found that all the nanoparticles reduce the background noise and increase the signal intensity, among which Fe2O3 exhibits the maximum sensitivity.
Fig. 3.
Schematic representation of enrichment and detection strategy for the analysis of phenytoin, ibuprofen, camptothecin, and warfarin in urine and serum samples using human serum albumin-modified Fe2O3 nanoparticles. (Reproduced with permission from Ref. [19]).
TiO2 is widely used in various analytical applications. Kim et al. utilized TiO2 nanowires for the detection of benzylpenicillin in milk [18]. This report revealed that the optimal TiO2 crystalline structure to be served as LDI matrix is anatase. The nanowire has proven to be excellent for the detection of two model amino acids (asparagine and alanine). Penicillin spike in milk is detectable after simple centrifugation treatment with a detection limit of 0.4 ng mL−1, which is one order lower than the cut-off value of 4 ng mL−1, according to the EU directive. Dopamine modified TiO2 (TiO2-DA) monoliths have been reported to exhibit better UV absorption in comparison with unmodified TiO2 particles [45]. The TiO2-DA monolith-assisted LDI-MS imaging of mouse brain tissue exhibits lower background signals, higher selectivity and sensitivity for some Lewis base compounds, such as fatty acids, cholesterols, ceramides, diacylglycerols, and phosphatidyl-ethanolamine, compared to traditional MALDI-MS imaging with 2,5-dihydroxybenzoic acid (DHB) as matrix. The Lewis acid site in TiO2 (Ti4+ possess very strong affinity to Lewis bases. More than 100 molecules, including amino acids, alkaloids, free fatty acids, peptides, and lipids, localized in mouse brain sections could be detected by TiO2-DA monolith-assisted LDI-MS imaging. Moreover, this approach demonstrates the analysis of aging-related neurochemical changes in the brain by comparing the presence and localization of those molecules in young and old mouse brains. Recently, Zhang et al. showed the potential of TiO2-assisted LDI-MS to distinguish drug-resistant bacteria based on the profiling of candidate metabolite biomarkers from intact bacterial cells [46].
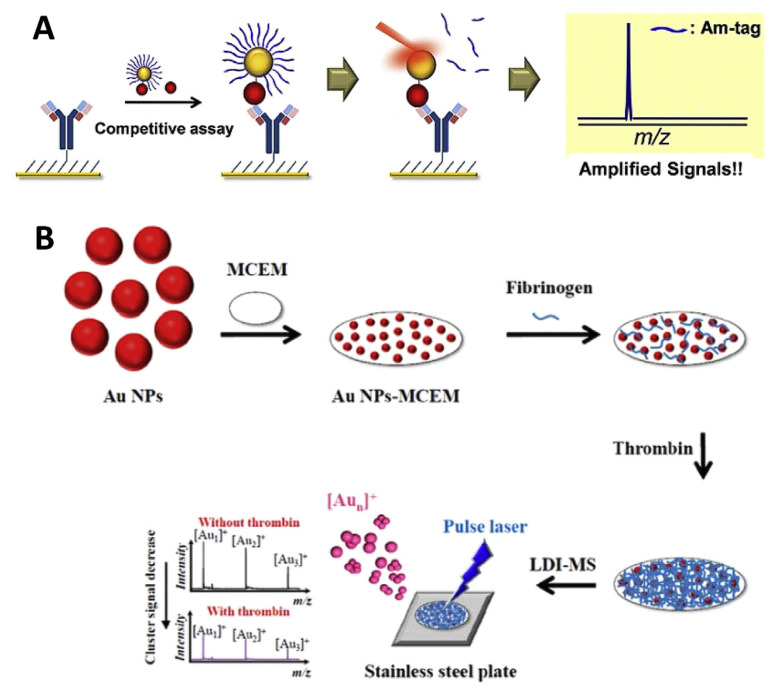
“Mass tags” or “amplification tags” are used in the SALDI-MS analysis for signal amplification and to reduce interference. Amplification tags, generally, are easily ionizable molecules immobilized on the probe nanoparticles. Depending on the number of tags on each nanoparticle and the type of assay, the signal can be highly amplified for improving the sensitivity. In this case, instead of monitoring the analyte peaks, the mass tag peaks are monitored. However, the assay preparation is a little more complicated, involving the functionalization of the nanoparticles with mass tags and selective enrichment of the analyte, than that of normal SALDI-MS analysis. Thioalkanes have unique affinity with gold through the strong Au–S bond (180 kJ mol−1) and their ionization efficiency enable them as mass reporter tags in LDI-MS [7]. Ha et al. utilized Au NPs with mass tags of thiolalkane ligands for the detection of enrofloxacin, a commonly used antibiotic, and its metabolite ciprofloxacin (Fig. 4A) [7]. The enrofloxacin or ciprofloxacin molecules are chemically anchored on thiolated-Au NPs with the carboxylate terminal by EDC/NHS reaction, whereas the tri(ethylene glycol)-terminated thioalkanes are used as the mass reporter tags. The analytes (enrofloxacin and ciprofloxacin) and functional Au NPs are competitively captured by antibody coated coverslips and subjected to LDI-TOF MS. Therefore, the tag signal deceases upon increasing the concentration. This competitive assay could selectively detect enrofloxacin and ciprofloxacin with a limit of detection of 0.5 mg kg−1.
Fig. 4.
Schematic representation of SALDI-MS coupled with (A) thioalkane as amplification tag for detection of enrofloxacin and ciprofloxacin, and (B) gold cluster ions as amplification tag for monitoring of thrombin activity and anticoagulant screening. (Reproduced with permission from Refs. [7] and [47], respectively).
Other than organic molecules, metal-based nanoparticles also can be applied as mass tags or amplification tags. In this case, the metal nanoparticles serve as substrate as well as amplification tag for LDI-MS. Metallic nanoparticles are known to produce metal cluster ions [Mn]+ under pulse laser irradiation. Several studies have shown that Au NPs can produce intense gold cluster ions ([Aun]+) signals in LDI-MS [47]. Nano- and femto-second pulsed laser can produce [Aun]+ by thermal evaporation and columbic explosion, respectively [32]. Fig. 4B shows the principle of the LDI-MS using metallic cluster ions as mass tags. In 2014, our research group demonstrated the use of Au NPs as metallic barcode for anticoagulant drugs screening [47]. We utilized Au NPs-modified mixed cellulose ester membrane (Au NPs–MCEM) conjugated with fibrinogen (Fib) as a probe (Fib/Au NPs–MCEM) for the detection of the activity of thrombin. The Fib/Au NPs–MCEM coupled with LDI–MS sensing system provides very clean mass spectra, allowing the monitoring of Au cluster ions ([Aun]+; n = 1–3) under pulse LDI process. The deposition of fibrin on Au NPs–MCEM triggered by thrombin is found to suppress the signal of [Aun]+. As a result, the intensity of the [Aun]+ is correlated with the thrombin concentration. The probe could specifically detect thrombin with a limit of detection of 2.5 pM in human plasma samples. Moreover, the sensing system is employed for the detection of direct thrombin inhibitors (DTI), such as thrombin-binding-aptamers, hirudin and argatroban. Due to the fact that monometallic nanoparticles can only produce certain cluster ions of certain m/z after fragmentation, like ligand amplification mass tags, metallic barcodes also have the potential for multiplex detection. Our research group demonstrated the use of different metal nanoparticles (Au, Ag and Pt NPs) for the detection of different proteins (thrombin, VEGF, PDGF) [48]. By using appropriate recognition ligands, multiple metal cluster ion bar codes have huge potential for the analysis of multiple metabolites and drugs simultaneously.
In summary, metal-based nanomaterials including noble metal nanoparticles and oxides of transitional metals are widely used in both global and targeted detection of a variety of metabolites and drugs. Further, they are successfully applied for the detection of drugs in latent fingerprints. Interestingly, metal-based nanomaterials can provide high resolution and visual contrast (due to plasmon resonance) that can be applied for dual modal fingerprint imaging. Many transitional metal oxides are favored for their high melting point and magnetic properties, providing better thermal desorption and easier sample enrichment. Thermal desorption processes are highly important for metal and metal oxide particles; however, recent reports suggest that surface chemical properties and phase transition processes are also important. In addition, metallic nanoparticles and their surface ligands can also act as mass tags for signal amplification. However, for selective enrichment and ionization of analytes in targeted approach, the functionalization of the nano-particle followed by analyte enrichment usually is complicated, time consuming or expensive.
2.2. Carbon-based nanomaterials
Carbon materials as matrices for LDI-MS were developed quite early in the history of SALDI-MS. Sunner et al. demonstrated the use of graphite as SALDI substrate for the analysis of peptides and proteins by SALDI-TOF-MS [49]. They obtained slightly different spectra in wet and dry conditions for peptides and proteins and were comparable to that of conventional MALDI spectra, even though a few background peaks were observed in the low molecular weight region. Carbon nanomaterials, due to their low cost, excellent UV absorption, tunable surface functional groups and electronic properties, have been sought after as potential LDI matrices. One of the greatest advantage of carbon nanomaterial in LDI application is their ease of conjugation and range of interactions with many analytes. Simple oxidation treatment can introduce various functional groups onto the surface of carbon nanomaterials. Functional groups such as carboxylate, epoxy and hydroxyls offer sites for chemical modification/physical adsorption of ligands/analytes. In addition, carbon nanomaterials with sp2 hybridization allow for physical interaction with analytes through π-π interaction, enabling enrichment. That is, nonoxidized sites can also contribute by interacting with hydrophobic analytes. A hybrid film of poly(allylamine hydrochloride)-functionalized graphene oxide (GO) and Au NP is found to be an efficient substrate for the LDI-MS detection of small molecules [50]. GO can efficiently dissipate the heat and avoid the fragmentation of the analytes. Multi-layer GO films have good photo-absorption and heat transfer efficiency [33]. Tang et al. conducted a study on the SALDI mechanism of carbon-based nanomaterials [carbon nanotubes (CNT), buckminsterfullerene (C60), nanoporous graphitic carbon (PGC), non-porous graphite particles (G), highly oriented pyrolytic graphite (HOPG), or nanodiamonds (ND)] [34]. Their work demonstrated the desorption efficiency is in the order of CNT ≈ C60 > PGC > G > HOPG > ND, which in general exhibits an opposite trend to the extent of internal energy transfer (CNT < C60 ≈ PGC < G ≈ HOPG < ND). This result indicates that increasing the extent of internal energy transfer in the SALDI process may not enhance the ion desorption efficiency. Furthermore, the authors demonstrated that thermal desorption (determined mainly by photoabsorption efficiencies) accounts for only a small part in the desorption efficiency of carbon substrates, the main mechanism for the improved ionization efficiency may be due to the phase transition/destruction of the substrates. Phase transition in essence is the melting and vaporization of the substrates which eject the adsorbed analyte into the vacuum. Another study further proposed that the thermal-driven desorption plays a significant role in the ion-desorption process [39]. The phase transition of nanoparticles during laser ablation could reduce the activation energy (ΔG) of the ion-desorption of the analyte by increasing the entropy (ΔS) through vaporization and phase explosion of the substrates [39].
Gulbakan et al. have reported the detection of cocaine by cocaine aptamer covalently conjugated GO as the matrix [21]. By using the matrix effect of GO combined with the enrichment ability of cocaine aptamer, they have successfully detected cocaine in plasma samples. Graphene, GO and reduced graphene oxide (rGO) can be used as matrices for the detection of various flavonoids, such as kaempferol, morin hydrate, myricetin, quercitrin, quercetin-3-b-D-glucoside and rutin in the negative ion mode [35]. At low concentrations of flavanoids, GO exhibits higher sensitivity than graphene and reduced GO, due to the abundant carboxyl functional groups, indicating its efficiency for ionizing the analytes. Liu et al. reported the use of graphene and GO for the detection of tetracycline antibiotics (tetracycline, oxytetracycline, demeclocycline, and chlortetracycline) in milk [51]. In addition, due to the large surface area and interaction with the analytes, the enrichment of tetracycline on the matrices are high, enabling a limit of detection as low as 2 nM.
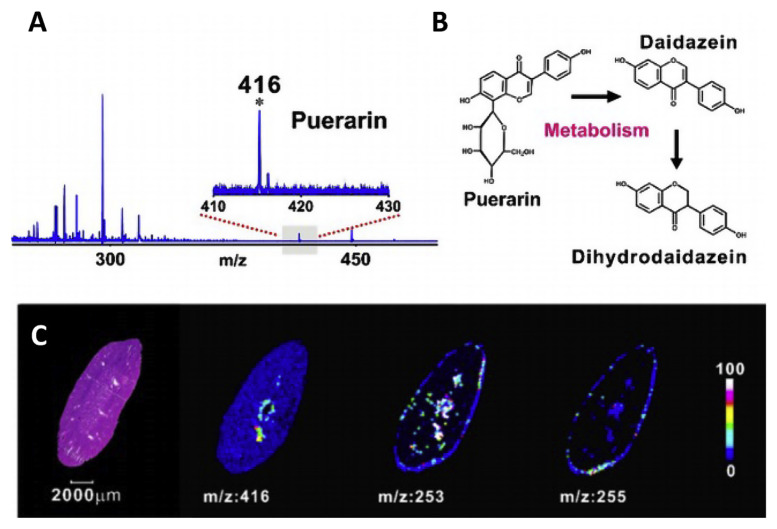
Carbon dots (C-dots) are a new class of interesting carbon materials for LDI-MS analyses. Gedda et al. employed C-dots synthesized from citric acid as matrix for the detection of low molecular weight nonsteroidal anti-inflammatory drug, mefenamic acid (MFA), which is difficult to detect with other spectrometric methods without complex sample preparation [31]. C-dots are found to be effective in detecting MFA in serum in both positive and negative ion mode with a limit of detection of ca. 0.5 ng. Shi et al. conducted a detailed research on using hydroxyl-group dominated graphite dots (GDs) as substrates for the detection, in situ imaging and real time monitoring of many small molecules [52]. GDs have been found to be excellent matrices for analysis of extracts of Morinda officinalis, which is commonly used in traditional Chinese medicine, and identification of several oligosaccharides [52]. Furthermore, GDs are also capable of detecting puerarin in mouse serum and that by applying GDs on kidneys, metabolites of puerarin (daidzein and dihydrodaidzein) can be tracked in situ through mass spectrometry imaging (MSI) (Fig. 5). The hydroxyl rich GDs have favorable energy dissociation pathways for non-carbon groups, which indicates lower background noise and higher structural stability.
Fig. 5.
MSI of puerarin and its metabolites in rat kidney. (A) MALDI mass spectrum (negative-ion mode) of mouse serum after intraperitoneal administration of puerarin. Inset: Zoomed-in view of the spectrum in m/z 410–430, characteristic peak of puerarin: [M – H] at m/z 416. (B) Metabolic pathways showing puerarin and its two metabolites, (C) MALDI-MS images of the drug and its metabolites (puerarin, m/z: 416; daidzein, m/z: 253; and dihydrodaidzein, m/z: 255) in a kidney tissue slice with an optical micrograph of an H&E-stained consecutive slice as a reference. The color bar encodes the signal intensities of the three small molecules in MSI. Reproduced with permission from Ref. [52].
Similarly, nitrogen-doped graphene (NG) has been found to be an excellent matrix for the detection of anabolic androgenic steroids (AAS) and anticancer drugs in negative ion mode, which is currently not often used in MS analysis, producing [M]− and [M–H]− ions [53]. This study reveals NG as a superior matrix for detection of a wide range of low-molecular weight analytes including amino acids, fatty acids, peptides, anabolic androgenic steroids as well as anticancer drugs, with an extraordinary LDI efficiency over traditional α-cyano-4-hydroxycinnamic acid (CHCA) and other carbon-based materials in the negative ion detection mode. The [M–H]− ions are proposed to arise mainly from proton transfer from the analyte to the pyridinic nitrogen while the [M]− are the result of electron transfer from the graphitic nitrogen species to the analyte on the NG. The results show that NG is an excellent matrix in negative ion mode, providing both less matrix interferences and lower matrix fragmentation. The good salt tolerance and high sensitivity of NG based SALDI-MS allow for the detection of anticancer drug nilotinib in the spiked human serum, down to 1 μM, which meets the demand of assessing drug level in the patient serum. The above reports clearly reveal that carbon materials are excellent matrices/substrates for drug and metabolites analysis due to their functional and hydrophobic properties and high desorption/ionization efficiency emanating from phase transition during laser ablation.
In summary, carbon-based nanomaterials are also excellent SALDI matrices in both global and targeted profiling. Compared to metal nanoparticles, the core structure, phase transition processes and surface functional groups of carbon nanomaterials are shown to play a more significant role in the ionization mechanism. Moreover, carbon nanomaterials are shown to have more potential in both positive and negative ion mode detection. The functional properties of carbon nanomaterials and π-π interaction with analytes are advantages in enrichment of some specific analytes. However, a small disadvantage of using carbon nanomaterials as substrate for LDI-MS is that, they produce carbon cluster peaks in the low molecular weight region at high laser fluence. Although there had been some success in reducing carbon cluster signal by modifying functional groups, precise control of the surface functional groups of carbon materials remains challenging.
2.3. Silicon-based nanostructures
Silicon oxides are known to produce porous structures, which provide greater area of contact. Since silica can be easily functionalized with a variety of molecules, it has been used for the extraction of analytes prior to mass spectrometry analysis for better sensitivity and selectivity. For example, Rejeeth et al. reported the application of hyaluronic acid (HA) functionalized Fe3O4–SiO2 core shell nanocomposite for the extraction and profiling of various serum biomarkers by mass spectrometry [54]. The silica shell enables easy functionalization with HA, which is otherwise difficult to functionalize HA directly on Fe3O4. Even though silica materials are developed later for LDI-MS [55], they revolutionized SALDI-MS detection and are currently the most used matrices for small molecule analysis. They have a variety of LDI derivatives such as DIOS which operates mainly on direct analyte desorption process (the “dry process”) and indirect desorption process from the fast vaporization of residue solvents in the pores (the “wet process”) [56]. Dry process dominates when the porosity is low, while wet process dominates when the porosity is high. DIOS chip of thin porous silicon (pSi) film functionalized with pentafluorophenylpropyl chlorodimethyl silane (F5PhPr) has been employed for the detection of nicotine, methamphetamine, codeine, methadone, and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (metabolite of methadone) in fingerprint [17,57]. This DIOS-MSI has been further utilized for the analysis of exogenous and endogenous drug compounds from fingerprints.
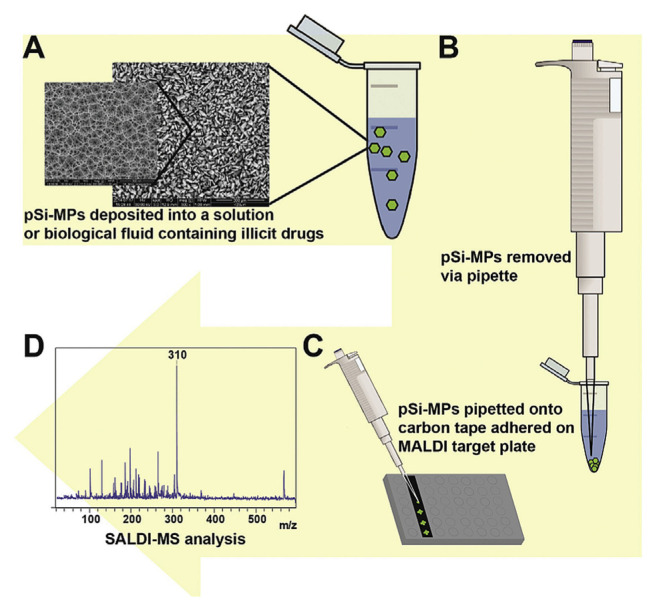
Hydrophobically functionalized porous silicon microparticles (pSi-MPs)-based DIOS has been reported as an efficient matrix for the detection of illicit drugs, such as methamphetamine, cocaine and 3,4-methylenedioxy-methamphetamine (Fig. 6) [58]. The study reveals that particle size, pore diameter, pore depth and functionalization of pSi-MP all have effect on the detection of the illicit drugs. The optimized pSi-MPs allow for the extraction and detection of methadone from spiked saliva and urine samples. More recently, the same group further demonstrated pSi-MPs as efficient substrates for the detection of methadone and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), in clinical urine and plasma samples [36]. Similarly, hydrophobic silica with sub-micron size doped with carbon black has been found to be an effective fingerprint-developing agent as well as a SALDI substrate for the detection of nicotine and cotinine in latent fingermark of smokers [59,60].
Fig. 6.
Schematic for (A) pSi-MP extraction of illicit drugs compounds from biological fluids, (B) pSi-MPs removed from the sample solution, (C) deposition onto standard MALDI target plate using double sided carbon tape and (D) analysis using SALDI-MS. (Reproduced with permission from Ref. [58]).
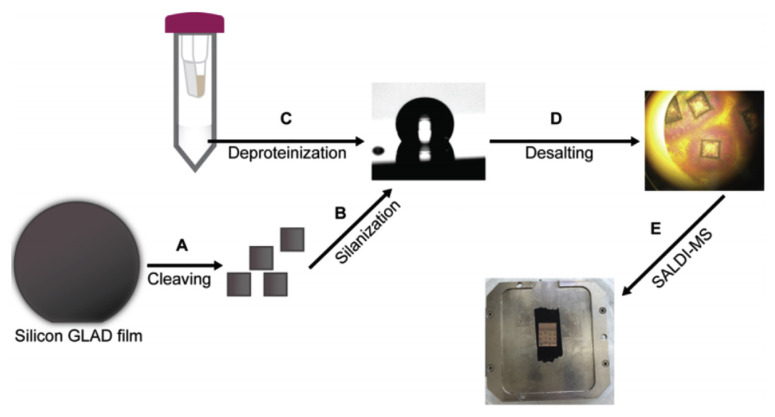
Another strategy for silicon based substrate in LDI-MS is the nanostructure initiator mass spectrometry (NIMS), which uses perfluorinated compounds as liquid initiators [16,61,62]. In addition to better adsorption of analytes due to hydrophobic interaction, these initiators do not absorb UV light, usually do not ionize or crystallize with the analyte allowing for reducing matrix interferences. This technique combines the benefits of traditional MALDI and NIMS, using a porous substrate to improve desorption efficiency at lower flux and providing a proton rich environment with acid matrix. The study by Guinan et al. on the DIOS, NIMS and NALDI process for the detection of amphetamines, benzodiazepines, opiates and tropane alkaloids suggests these matrices have comparable performance, however, cheaper manufacturing cost for DIOS and NIMS may be a better alternative for commercialization [16]. Perfluoro-coated (1H,1H,2H,2H-perfluorooctyl) trichlorosilane or (1H,1H,2H,2H-perfluorooctyl) dimethyl-chlorosilane nanostructured surfaces can also be employed to selectively segregate hydrophilic analytes from biofluidic electrolytes [63]. The work demonstrated that by controlling the contact angle of fluorinated silane substrate between 120 and 150°, background electrolytes can be made to segregate from hydrophilic analytes during a drying step on the surface of a highly nanoporous thin film (Fig. 7). By this way, the high concentration salts are separated, while the analytes can enter the pores. Coupling with LDI-MS, this strategy enables more sensitive detection of metabolites such as amino acids even in complicated samples such as cerebrospinal fluid and serum. With this on-chip desalting, histidine spiked in cerebrospinal fluid could be detected within a range from 1 to 50 μM. Moreover, this SALDI-MS shows both good reproducibility for the quantification of five highly polar metabolites (taurine, aspartic acid, malic acid, glutamic acid, and histidine) in serum and the results obtained on the desalted serum sample spots are comparable with those of NMR and liquid chromatography–mass spectrometry. In addition to silicon, some semiconductors such as mesoporous germanium (meso-pGe), synthesized by bipolar electrochemical etching method, has been demonstrated as an efficient matrix for the detection of cocaine by LDI-MS [20]. The report suggests that the substrate has high stability and reproducibility and it is capable of detecting cocaine down to 1.7 ng mL−1 in DI water and 5.3 ng mL−1 in spiked saliva.
Fig. 7.
Workflow for biofluid analysis by silicon GLAD film. (A) Cleaving silicon GLAD film into workable wafers. (B) Modifying Si GLAD film with pFMe2SiCl solution to obtain a perfluoro coated surface with optimal contact angle. (C) Preparation of deproteinated human serum sample. (D) On-chip desalting and (E) mounting the SALDI chips on a custom MALDI plate for MS analysis. (Reproduced with permission from Ref. [63]).
In summary, silicon-based materials are widely used as matrices for analysis of small compounds including drugs and metabolites. The porosity of silicon nanomaterials is easy to control and a variety of materials (such as fluorocarbons) can be functionalized or coated onto the silica materials for improved detection of certain analytes making them extremely useful for small molecule detection. DIOS and its many variants are now a staple for SALDI detection of small molecule. However, preparation of various types of silicon substrates requires a more complicated procedure than that of metal- or carbon-based LDI substrates.
2.4. Nanocomposites
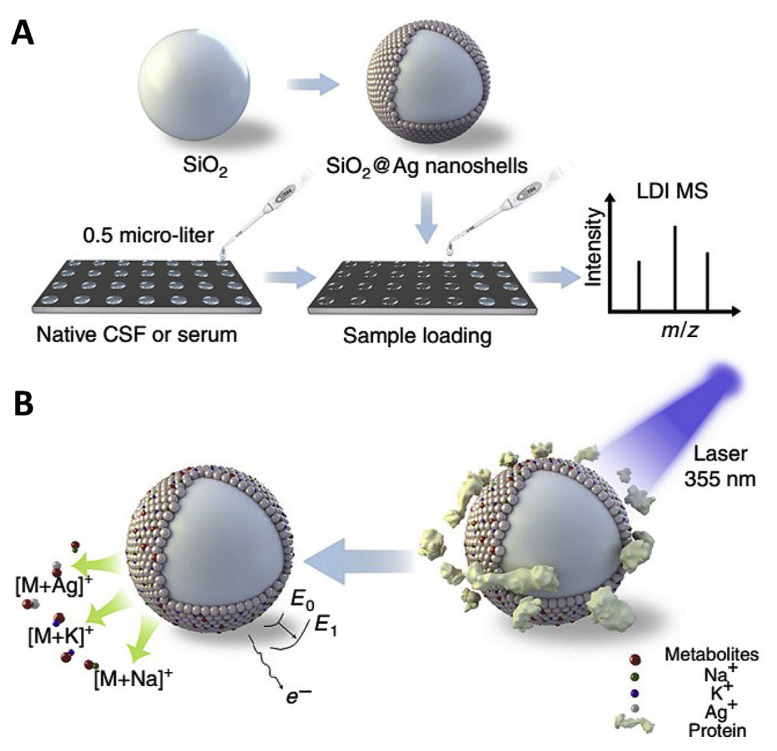
Recently, composite nanoparticles have been found to be very useful as substrates for LDI-MS analysis of drugs and metabolites. Nanocomposites having tunable band gap and surface properties for the absorption of laser light and interaction with analytes endow them as potential LDI substrates. Nie et al. demonstrated the use of nanoporous GaN–Ag composite material with moderate band-gap and high density of pores (~109 cm−2) as LDI-substrate for the detection of cholesterol and nucleotides [23]. The moderate band gap enables strong photo absorption and GaN–Ag composite has good heat transfer efficiency. In addition, the Ag nanostructures in the porous GaN-Ag composite material act as cationization agents and also facilitate the thermal desorption process, resulting in high efficiency of LDI process. Wei et al. synthesized SiO2 core with plasmonic gold nanoshells (Au nanoshells) for the detection of serum metabolites [8]. The Au nanoshell exhibits higher ionization efficiency and less fragmentation in the low m/z region, than those of gold nanorod, nanosphere and traditional organic matrices. Au nanoshells are shown to be highly effective in the detection of six representative amino acids (valine, lysine, methionine, arginine, tryptophan and phenylalanine) with a detection limit of 3–30 pmol, and also various amino acids and carbohydrates (glucose, mannitol) in serum. A more recent article by Sun et al. reveals the potential of plasmonic gold nanoshells coated microarray chips for the metabolite fingerprinting of biofluids and exosomes [64]. The authors suggest that plasmonic properties of the gold nanoshell with silica core has significant influence in the LDI processes such as hot carrier production, local heating, and photodesorption. Such efficient LDI substrate can handle samples with sample volume as low as 400 pL. The plasmonic gold chip has the potential to diagnose early stage non-small cell lung cancer, by differentiating them from healthy controls. Huang et al. reported the use of SiO2 with plasmonic silver nanoshells (SiO2@Ag) nanocomposites for the detection of selected metabolites such as glucose and mannitol (dynamic range 100–600 ng μL−1) in biofluid (cerebrospinal fluid and serum) volume of just 0.5 μL (Fig. 8) [65]. Furthermore, their research demonstrated that SiO2@Ag nanocomposites can be used to determine glucose concentration in cerebrospinal fluid for identifying patients with postoperative brain infection and mannitol distribution in blood and cerebrospinal fluid systems to validate the function and permeability of blood–brain/cerebrospinal fluid-barriers.
Fig. 8.
Schematic representation of (A) work flow and (B) LDI-MS process using SiO2@Ag nanoshells as substrate for the detection of drugs and metabolites (Reproduced with permission from Ref. [65]).
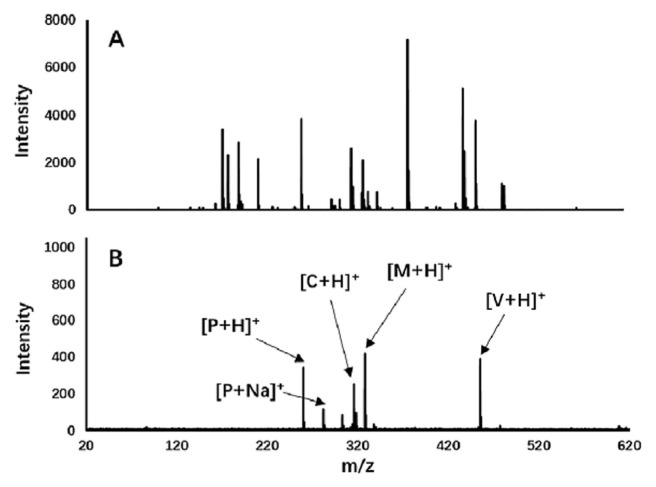
Gustafsson et al. reported the use of silver coated desorption/ionization on silicon (Ag-DIOS) for detection and imaging of metabolites with low ionization efficiency such as fatty acids and esters in tissue and fingerprints [66]. Other than the enhanced desorption of the analyte, Ag layer on the DIOS substrate can produce [Agn]+ clusters, which can act as calibration standards for improved mass accuracy and can form adducts with compounds such as fatty acids and cholesterol which are hard to detect with DIOS otherwise. Carbon nano-tube and polymer composites also have been found to be excellent substrate for LDI-MS analysis of drugs providing clean mass spectra, enabling simultaneous detection. Bian et al. demonstrated electrospun polyacrylonitrile/Nafion®/ carbon nanotube (PAN/Nafion®/CNT) fiber as SALDI substrate for the detection of four drugs, namely verapamil, chlorprothixene, methotrimeprazine and propranolol [13]. The carbon nanotube composite material extends the duration of signals of analyte ions. A comparison of the complicated MALDI spectrum using α-cyano-4-hydroxycinnamic acid as matrix and clean SALDI spectrum using PAN/Nafion®/CNT, of four drugs is given in Fig. 9, which clearly indicates the advantages of SALDI-MS using nanocomposite for the detection of low molecular weight molecules over MALDI-MS [13].
Fig. 9.
(A) MALDI-MS spectrum using α-cyano-4-hydroxycinnamic acid as matrix and (B) SALDI-MS spectrum using polyacrylonitrile/Nafion®/carbon nanotube composite as substrate for the detection of verapamil (V), chlorprothixene (C), methotrimeprazine (M) and propranolol (P). (Reproduced with permission from Ref. [13]).
Nanocomposites combine several advantages of different materials and are well suited for detection of many drugs and metabolites. However, designing composite materials require in depth knowledge of ionization mechanism, which is rather lacking in current SALDI research. As of present Ag/Au and silicon/silica composite materials appear to be an attractive option, providing easy access to cationization (via Ag+ addition), controlled plasmonic resonance and/or porous structure. Recent design of SALDI nanocomposite have advanced from nanoparticle dispersions into nanostructured target plate or chip fabrication, which effectively resolves matrix deposition issue such as uneven distribution of substrate and analytes on the plate. The better shot-to-shot reproducibility provided by this design may be a reason why silicon–metal composites are so commonly used, as their nanostructures can be easily controlled by electrochemical etching and sputtering.
3. Conclusions
A thorough study of literature suggests that nanomaterial-assisted LDI-MS is a promising technique for the analysis of drugs and metabolites. Nanomaterials offer several advantages including high surface area, homogeneous co-crystallization, low matrix interference, analyte enrichment, and tunable physical properties. Many of these characteristics are absent in traditional organic matrices and often encounter low shot-to-shot and sample-to-sample reproducibility. The nanomaterials serve as substrates and not only reduce the fragmentation of analytes and background noises, but also increase selectivity through enrichment of the analyte on its surface. However, moderate affinity between the nano-substrates and analytes should be maintained for efficient LDI process. Metal based nanoparticles such as Au NPs, Fe3O4 NPs, and TiO2 NPs coupled with LDI-MS is especially useful in analyzing the low molecular weight region. Carbon and silica nanomaterials are also successful SALDI matrices with advantages over metal-based substrates due to their functional properties which enable efficient enrichment of analytes and easy ionization. In particular, silicon nanostructure (such as DIOS, NIMS) have been explored more than other nano-materials for LDI-MS for drugs and metabolites. Development of new nanomaterials and nanocomposites for LDI-MS and elucidation of their mechanisms are urgent in the future for simultaneous detection of biomacromolecules (protein and DNA) and small molecules in clinical diagnosis. Understanding the nanomaterial-assisted LDI process mechanism, especially that of nanocomposites, will facilitate more systematic and logical design of new effective nanomaterial matrices compared to traditional method of continuous trial and error. On the other hand, employing mass tags as amplification system is a promising leap in applying SALDI-MS for trace analysis of biomolecules in various fields including food, drug, clinical and forensic analyses. However, this technique is relatively new and only a few reports are available, and needs to be explored for its potential use in drugs and metabolite analyses. A selective detection assay coupled with SALDI-MS and mass tag will provide a better strategy to increase the selectivity, sensitivity and reduce the interference.
Acknowledgement
This study was supported by the Ministry of Science and Technology of Taiwan under the contracts 104-2628-M-019-001-MY3, 104-2622-M-019-001-CC2, and 103-2627-M-007-002-MY3 and by the Center of Excellence for the Oceans, National Taiwan Ocean University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Funding Statement
This study was supported by the Ministry of Science and Technology of Taiwan under the contracts 104-2628-M-019-001-MY3, 104-2622-M-019-001-CC2, and 103-2627-M-007-002-MY3 and by the Center of Excellence for the Oceans, National Taiwan Ocean University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
References
- 1. Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988;60:2299–301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- 2. Cobo F. Application of MALDI-TOF mass spectrometry in clinical virology: a review. Open Virol J. 2013;7:84–90. doi: 10.2174/1874357920130927003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duncan MW, Nedelkov D, Walsh R, Hattan SJ. Applications of MALDI mass spectrometry in clinical chemistry. Clin Chem. 2016;62:134–43. doi: 10.1373/clinchem.2015.239491. [DOI] [PubMed] [Google Scholar]
- 4. Francese S, Bradshaw R, Denison N. An update on MALDI mass spectrometry based technology for the analysis of fingermarks – stepping into operational deployment. Analyst. 2017;142:2518–46. doi: 10.1039/c7an00569e. [DOI] [PubMed] [Google Scholar]
- 5. Mandal A, Singha M, Addy PS, Basak A. Laser desorption ionization mass spectrometry: recent progress in matrix-free and label-assisted techniques. Mass Spectrom Rev. 2017:1–19. doi: 10.1002/mas.21545. [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Liu JA, Chen Y, Xiong S, Wang G, Chen J, et al. A novel strategy for MALDI-TOF MS analysis of small molecules. J Am Soc Mass Spectrom. 2010;21:154–60. doi: 10.1016/j.jasms.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 7. Ha MS, Seo H, Bae DH, Yeo WS. Detection of enrofloxacin and its metabolite ciprofloxacin using gold nanoparticles and laser desorption/ionization time-of-tlight mass spectrometry. Anal Sci. 2014;30:451–5. doi: 10.2116/analsci.30.451. [DOI] [PubMed] [Google Scholar]
- 8. Wei X, Liu Z, Jin X, Huang L, Gurav DD, Sun X, et al. Plasmonic nanoshells enhanced laser desorption/ionization mass spectrometry for detection of serum metabolites. Anal Chim Acta. 2017;950:147–55. doi: 10.1016/j.aca.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 9. López de Laorden C, Beloqui A, Yate L, Calvo J, Puigivila M, Llop J, et al. Nanostructured indium tin oxide slides for small-molecule profiling and imaging mass spectrometry of metabolites by surface-assisted laser desorption ionization MS. Anal Chem. 2015;87:431–40. doi: 10.1021/ac5025864. [DOI] [PubMed] [Google Scholar]
- 10. Lin Z, Cai Z. Negative ion laser desorption/ionization time-of-flight mass spectrometric analysis of small molecules by using nanostructured substrate as matrices. Mass Spectrom Rev. 2018:1–16. doi: 10.1002/mas.21558. [DOI] [PubMed] [Google Scholar]
- 11. Abdelhamid HN. Nanoparticle assisted laser desorption/ ionization mass spectrometry for small molecule analytes. Microchim Acta. 2018;185:200. doi: 10.1007/s00604-018-2687-8. [DOI] [PubMed] [Google Scholar]
- 12. Chiang CK, Chen WT, Chang HT. Nanoparticle-based mass spectrometry for the analysis of biomolecules. Chem Soc Rev. 2011;40:1269–81. doi: 10.1039/c0cs00050g. [DOI] [PubMed] [Google Scholar]
- 13. Bian J, Olesik SV. Surface-assisted laser desorption/ ionization time-of-flight mass spectrometry of small drug molecules and high molecular weight synthetic/biological polymers using electrospun composite nanofibers. Analyst. 2017;142:1125–32. doi: 10.1039/c6an02444k. [DOI] [PubMed] [Google Scholar]
- 14. Wu CY, Lee KC, Kuo YL, Chen YC. Revisiting the quantitative features of surface-assisted laser desorption/ionization mass spectrometric analysis. Philos Trans R Soc A Math Phys Eng Sci. 2016;374:20150379. doi: 10.1098/rsta.2015.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arakawa R, Kawasaki H. Functionalized nanoparticles and nanostructured surfaces for surface-assisted laser desorption/ionization mass spectrometry. Anal Sci. 2010;26:1229–40. doi: 10.2116/analsci.26.1229. [DOI] [PubMed] [Google Scholar]
- 16. Guinan T, Ronci M, Vasani R, Kobus H, Voelcker NH. Comparison of the performance of different silicon-based SALDI substrates for illicit drug detection. Talanta. 2015;132:494–502. doi: 10.1016/j.talanta.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 17. Guinan T, Della Vedova C, Kobus H, Voelcker NH. Mass spectrometry imaging of fingerprint sweat on nanostructured silicon. Chem Commun. 2015;51:6088–91. doi: 10.1039/c4cc08762c. [DOI] [PubMed] [Google Scholar]
- 18. Kim JI, Park JM, Noh JY, Hwang SJ, Kang MJ, Pyun JC. Analysis of benzylpenicillin in milk using MALDI-TOF mass spectrometry with top-down synthesized TiO2 nanowires as the solid matrix. Chemosphere. 2016;143:64–70. doi: 10.1016/j.chemosphere.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 19. Iwaki Y, Kawasaki H, Ryuichi A. Human serum albumin- modified Fe3O4 magnetic nanoparticles for affinity-SALDI-MS of small-molecule drugs in biological liquids. Anal Sci. 2012;28:893–900. doi: 10.2116/analsci.28.893. [DOI] [PubMed] [Google Scholar]
- 20. Abdelmaksoud HH, Guinan TM, Voelcker NH. Fabrication of nanostructured mesoporous germanium for application in laser desorption ionization mass spectrometry. ACS Appl Mater Interfaces. 2017;9:5092–9. doi: 10.1021/acsami.6b14362. [DOI] [PubMed] [Google Scholar]
- 21. Gulbakan B, Yasun E, Shukoor MI, Zhu Z, You M, Tan X, et al. A dual platform for selective analyte enrichment and ionization in mass spectrometry using aptamer-conjugated graphene oxide. J Am Chem Soc. 2010;132:17408–10. doi: 10.1021/ja109042w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alhmoud HZ, Guinan TM, Elnathan R, Kobus H, Voelcker NH. Surface-assisted laser desorption/ionization mass spectrometry using ordered silicon nanopillar arrays. Analyst. 2014;139:5999–6009. doi: 10.1039/c4an01391c. [DOI] [PubMed] [Google Scholar]
- 23. Nie B, Duan BK, Bohn PW. Nanoporous GaN–Ag composite materials prepared by metal-assisted electroless etching for direct laser desorption-ionization mass spectrometry. ACS Appl Mater Interfaces. 2013;5:6208–15. doi: 10.1021/am401132s. [DOI] [PubMed] [Google Scholar]
- 24. Picca RA, Calvano CD, Cioffi N, Palmisano F. Mechanisms of nanophase-induced desorption in LDI-MS. A short review. Nanomaterials. 2017;7:75. doi: 10.3390/nano7040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal Chem. 2016;88:524–45. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
- 26. Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. 2011;286:25435–42. doi: 10.1074/jbc.R111.238691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao JF, Zhou B, Ressom HW. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Anal Chem TRAC. 2012;32:1–14. doi: 10.1016/j.trac.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda F. Technical challenges in mass spectrometry-based metabolomics. Mass Spectrom. 2016;5:S0052. doi: 10.5702/massspectrometry.S0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korte AR, Stopka SA, Morris N, Razunguzwa T, Vertes A. Large-scale metabolite analysis of standards and human serum by laser desorption ionization mass spectrometry from silicon nanopost arrays. Anal Chem. 2016;88:8989–96. doi: 10.1021/acs.analchem.6b01186. [DOI] [PubMed] [Google Scholar]
- 30. Liu P, Hu Y, Chen J, Yang Q. Direct detection of the anti-cancer drug 9-phenylacridine in tissues by graphite rod laser desorption vacuum-ultraviolet post-ionization mass spectrometry. Rapid Commun Mass Spectrom. 2015;29:1328–34. doi: 10.1002/rcm.7226. [DOI] [PubMed] [Google Scholar]
- 31. Gedda G, Pandey S, Bhaisare ML, Wu HF. Carbon dots as nanoantennas for anti-inflammatory drug analysis using surface-assisted laser desorption/ionization time-of-flight mass spectrometry in serum. RSC Adv. 2014;4:38027–33. [Google Scholar]
- 32. Unnikrishnan B, Chang CY, Chu HW, Anand A, Huang CC. Functional gold nanoparticles coupled with laser desorption ionization mass spectrometry for bioanalysis. Anal Methods. 2016;8:8123–33. [Google Scholar]
- 33. Kim YK, Min DH. Mechanistic study of laser desorption/ ionization of small molecules on graphene oxide multilayer films. Langmuir. 2014;30:12675–83. doi: 10.1021/la5027653. [DOI] [PubMed] [Google Scholar]
- 34. Tang HW, Ng KM, Lu W, Che CM. Ion desorption efficiency and internal energy transfer in carbon-based surface-assisted laser desorption/ionization mass spectrometry: desorption mechanism(s) and the design of SALDI substrates. Anal Chem. 2009;81:4720–9. doi: 10.1021/ac8026367. [DOI] [PubMed] [Google Scholar]
- 35. Liu CW, Chien MW, Su CY, Chen HY, Li LJ, Lai CC. Analysis of flavonoids by graphene-based surface-assisted laser desorption/ionization time-of-flight mass spectrometry. Analyst. 2012;137:5809–16. doi: 10.1039/c2an36155h. [DOI] [PubMed] [Google Scholar]
- 36. Guinan TM, Neldner D, Stockham P, Kobus H, Della Vedova CB, Voelcker NH. Porous silicon mass spectrometry as an alternative confirmatory assay for compliance testing of methadone. Drug Test Anal. 2017;9:769–77. doi: 10.1002/dta.2033. [DOI] [PubMed] [Google Scholar]
- 37. O'Brien PJ, Lee M, Spilker ME, Zhang CC, Yan Z, Nichols TC, et al. Monitoring metabolic responses to chemotherapy in single cells and tumors using nanostructure-initiator mass spectrometry (NIMS) imaging. Cancer Metab. 2013;1:4. doi: 10.1186/2049-3002-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y, Yoshida T, et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1988;2:151–3. [Google Scholar]
- 39. Ng KM, Chau SL, Tang HW, Wei XG, Lau KC, Ye F, et al. Ion-desorption efficiency and internal-energy transfer in surface-assisted laser desorption/ionization: more implication(s) for the thermal-driven and phase-transition-driven desorption process. J Phys Chem C. 2015;119:23708–20. [Google Scholar]
- 40. Wu HP, Yu CJ, Lin CY, Lin YH, Tseng WL. Gold nanoparticles as assisted matrices for the detection of biomolecules in a high-salt solution through laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 2009;20:875–82. doi: 10.1016/j.jasms.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 41. Tang HW, Lu W, Che CM, Ng KM. Gold nanoparticles and imaging mass spectrometry: double imaging of latent fingerprints. Anal Chem. 2010;82:1589–93. doi: 10.1021/ac9026077. [DOI] [PubMed] [Google Scholar]
- 42. Chau SL, Tang HW, Ng KM. Gold nanoparticles bridging infra-red spectroscopy and laser desorption/ionization mass spectrometry for direct analysis of over-the-counter drug and botanical medicines. Anal Chim Acta. 2016;919:62–9. doi: 10.1016/j.aca.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 43. Sundar L, Rowell F. Detection of drugs in lifted cyanoacrylate-developed latent fingermarks using two laser desorption/ionisation mass spectrometric methods. Analyst. 2014;139:633–42. doi: 10.1039/c3an00969f. [DOI] [PubMed] [Google Scholar]
- 44. Amin MO, Madkour M, Al-Hetlani E. Metal oxide nanoparticles for latent fingerprint visualization and analysis of small drug molecules using surface-assisted laser desorption/ionization mass spectrometry. Anal Bioanal Chem. 2018 doi: 10.1007/s00216-018-1119-2. [DOI] [PubMed] [Google Scholar]
- 45. Wu Q, Chu JL, Rubakhin SS, Gillette MU, Sweedler JV. Dopamine-modified TiO2 monolith-assisted LDI MS imaging for simultaneous localization of small metabolites and lipids in mouse brain tissue with enhanced detection selectivity and sensitivity. Chem Sci. 2017;8:3926–38. doi: 10.1039/c7sc00937b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang R, Qin Q, Liu B, Qiao L. TiO2-assisted laser desorption/ ionization mass spectrometry for rapid profiling of candidate metabolite biomarkers from antimicrobial-resistant bacteria. Anal Chem. 2018;90:3863–70. doi: 10.1021/acs.analchem.7b04565. [DOI] [PubMed] [Google Scholar]
- 47. Li YJ, Chiu WJ, Unnikrishnan B, Huang CC. Monitoring thrombin generation and screening anticoagulants through pulse laser-induced fragmentation of biofunctional nanogold on cellulose membranes. ACS Appl Mater Interfaces. 2014;6:15253–61. doi: 10.1021/am503615c. [DOI] [PubMed] [Google Scholar]
- 48. Chang CY, Chu HW, Unnikrishnan B, Peng LH, Cang J, Hsu PH, et al. Pulse laser-induced generation of cluster codes from metal nanoparticles for immunoassay applications. APL Mater. 2017;5:053403. [Google Scholar]
- 49. Sunner J, Dratz E, Chen YC. Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions. Anal Chem. 1995;67:4335–42. doi: 10.1021/ac00119a021. [DOI] [PubMed] [Google Scholar]
- 50. Kim YK, Min DH. Preparation of the hybrid film of poly(allylamine hydrochloride)-functionalized graphene oxide and gold nanoparticle and its application for laser-induced desorption/ionization of small molecules. Langmuir. 2012;28:4453–8. doi: 10.1021/la204185p. [DOI] [PubMed] [Google Scholar]
- 51. Liu J, Liu Y, Gao M, Zhang X. High throughput detection of tetracycline residues in milk using graphene or graphene oxide as MALDI-TOF MS matrix. J Am Soc Mass Spectrom. 2012;23:1424–7. doi: 10.1007/s13361-012-0400-4. [DOI] [PubMed] [Google Scholar]
- 52. Shi R, Dai X, Li W, Lu F, Liu Y, Qu H, et al. Hydroxyl-group-dominated graphite dots reshape laser desorption/ionization mass spectrometry for small biomolecular analysis and imaging. ACS Nano. 2017;11:9500–13. doi: 10.1021/acsnano.7b05328. [DOI] [PubMed] [Google Scholar]
- 53. Min Q, Zhang X, Chen X, Li S, Zhu JJ. N-doped graphene: an alternative carbon-based matrix for highly efficient detection of small molecules by negative ion MALDI-TOF MS. Anal Chem. 2014;86:9122–30. doi: 10.1021/ac501943n. [DOI] [PubMed] [Google Scholar]
- 54. Rejeeth C, Pang X, Zhang R, Xu W, Sun X, Liu B, et al. Extraction, detection, and profiling of serum biomarkers using designed Fe3O4@SiO2@HA core–shell particles. Nano Res. 2018;11:68–79. [Google Scholar]
- 55. Wei J, Buriak JM, Siuzdak G. Desorption–ionization mass spectrometry on porous silicon. Nature. 1999;399:243–6. doi: 10.1038/20400. [DOI] [PubMed] [Google Scholar]
- 56. Xiao Y, Retterer ST, Thomas DK, Tao JY, He L. Impacts of surface morphology on ion desorption and ionization in desorption ionization on porous silicon (DIOS) mass spectrometry. J Phys Chem C. 2009;113:3076–83. [Google Scholar]
- 57. Guinan T, Ronci M, Kobus H, Voelcker NH. Rapid detection of illicit drugs in neat saliva using desorption/ionization on porous silicon. Talanta. 2012;99:791–8. doi: 10.1016/j.talanta.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 58. Guinan TM, Kirkbride P, Della Vedova CB, Kershaw SG, Kobus H, Voelcker NH. Direct detection of illicit drugs from biological fluids by desorption/ionization mass spectrometry with nanoporous silicon microparticles. Analyst. 2015;140:7926–33. doi: 10.1039/c5an01754h. [DOI] [PubMed] [Google Scholar]
- 59. Benton M, Rowell F, Sundar L, Jan M. Direct detection of nicotine and cotinine in dusted latent fingermarks of smokers by using hydrophobic silica particles and MS. Surf Interface Anal. 2010;42:378–85. [Google Scholar]
- 60. Benton M, Chua MJ, Gu F, Rowell F, Ma J. Environmental nicotine contamination in latent fingermarks from smoker contacts and passive smoking. Forensic Sci Int. 2010;200:28–34. doi: 10.1016/j.forsciint.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 61. Moening TN, Brown VL, He L. Matrix-enhanced nanostructure initiator mass spectrometry (ME-NIMS) for small molecule detection and imaging. Anal Methods. 2016;8:8234–40. [Google Scholar]
- 62. Gao J, Louie KB, Steinke P, Bowen BP, Raad MD, Zuckermann RN, et al. Morphology-driven control of metabolite selectivity using nanostructure-initiator mass spectrometry. Anal Chem. 2017;89:6521–6. doi: 10.1021/acs.analchem.7b00599. [DOI] [PubMed] [Google Scholar]
- 63. Zhou Y, Peng C, Harris KD, Mandal R, Harrison DJ. Salt segregation and sample cleanup on perfluoro-coated nanostructured surfaces for laser desorption ionization mass spectrometry of biofluid samples. Anal Chem. 2017;89:3362–9. doi: 10.1021/acs.analchem.6b03934. [DOI] [PubMed] [Google Scholar]
- 64. Sun X, Huang L, Zhang R, Xu W, Huang J, Gurav DD, et al. Metabolic fingerprinting on a plasmonic gold chip for mass spectrometry based in vitro diagnostics. ACS Cent Sci. 2018;4:223–9. doi: 10.1021/acscentsci.7b00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang L, Wan J, Wei X, Liu Y, Huang J, Sun X, et al. Plasmonic silver nanoshells for drug and metabolite detection. Nat Commun. 2017;8:220. doi: 10.1038/s41467-017-00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gustafsson OJR, Guinan TM, Rudd D, Kobus H, Benkendorff K, Voelcker NH. Metabolite mapping by consecutive nanostructure and silver-assisted mass spectrometry imaging on tissue sections. Rapid Commun Mass Spectrom. 2017;31:991–1000. doi: 10.1002/rcm.7869. [DOI] [PubMed] [Google Scholar]
- 67. Liu Y, Liu J, Yin P, Gao M, Deng C, Zhang X. High throughput identification of components from traditional Chinese medicine herbs by utilizing graphene or graphene oxide as MALDI-TOF-MS matrix. J Mass Spectrom. 2011;46:804–15. doi: 10.1002/jms.1952. [DOI] [PubMed] [Google Scholar]