Abstract
The rapid development of nanotechnology has transformed many domains of food science, especially those that involve the processing, packaging, storage, transportation, functionality, and other safety aspects of food. A wide range of nanostructured materials (NSMs), from inorganic metal, metal oxides, and their nanocomposites to nano-organic materials with bioactive agents, has been applied to the food industry. Despite the huge benefits nanotechnology has to offer, there are emerging concerns regarding the use of nanotechnology, as the accumulation of NSMs in human bodies and in the environment can cause several health and safety hazards. Therefore, safety and health concerns as well as regulatory policies must be considered while manufacturing, processing, intelligently and actively packaging, and consuming nano-processed food products. This review aims to provide a basic understanding regarding the applications of nanotechnology in the food packaging and processing industries and to identify the future prospects and potential risks associated with the use of NSMs.
Keywords: Nanostructured materials (NSMs), Food safety, Food preservation, Functional food, Packaging, Human health
1. Introduction
Nanotechnology integrates several disciplines, including physics, chemistry, biotechnology, and engineering, and refers to the use of nanomaterials whose nanoscale structures range from 1 to 100 nm [1]. In these nano-sizes, materials adopt unique properties that were not present when the materials were in their original form. Understanding these unique properties in order to develop new and improved products through green processes is the goal of nanoscientists across the globe [2].
Nanomaterials or nanostructure materials exhibit different dimensions for their structural elements, clusters, molecules, or crystallites, such as zero dimensions (nanoparticles, nanoclusters, and quantum dots), one dimension (nanorods and nanotubes), two dimensions (nano-thin films), and three dimensions (nanomaterials) in the 1–100 nm range [3,4]. Combining nanostructure materials with other polymer, biomolecule, and other nanostructure material or existing in the aggregate form can result relatively in a larger particle size material (>100 nm) leading to the formation of nanocomposite [5]. These nanomaterials having high surface volume ratio exhibit inimitable physio-chemical characteristics, such as solubility, toxicity, strength, magnetism, diffusivity, optics, color, and thermodynamics [6,7].
The applications of these nanomaterials are growing in various sectors, including agriculture, medicine, clothing, cosmetics, food, and public health due to their unique ability to increase solubility and bioavailability and to protect bioactive components while they are being processed and stored [4,8,9]. Due to the excellent physiochemical nature and the antimicrobial potential of nanomaterials, they are widely used against various pathogenic microbes and in healthcare, crop protection, water treatment, food safety, and food preservation [8,10]. In addition, nanostructured materials (NSMs) are being applied in the food industry as a nanosensor, new packaging material, and encapsulated food component. In general, this review concerns the use of nanotechnology, specifically nanoparticles and constructed nanostructures, in the food sector.
Nanotechnology has a wide range of food-related applications. In these applications, a specific type of nanomaterial is incorporated into a specific food product in order for that food product to develop certain desired properties [2]. The field of nanotechnology has also been an integral part of research and development for the large-scale manufacturing of agricultural products and processed foods and drinks, as well as for food packaging across the world [3]. Several reports confirm that these nanomaterials can successfully improve food safety by enhancing the efficacy of food packaging, shelf-life, and nutritional value as additives without changing the taste and physical characteristics of food products [13,14]. Although they have much potential to frame innovative products and production processes in the food industry, nanotechniques are facing a major challenge in using cost-effective processing operations to create edible and non-toxic nano-delivery systems and to develop effective formulations that are safe for human consumption. Therefore, due to the increased use of these NSMs, there have been growing concerns regarding the development of biocompatible, safe, and non-toxic nanostructures from food-grade ingredients using simple, green, and cost-effective strategies, including the layer-by-layer technique [15].
United states-based safety evaluation agencies such as FDA and EPA, including the Directorate of European Health and Consumer Protection, and many other regulatory authorities, have released several guidelines on potential human health risks associated with the use of nanostructure materials in food [16]. These potential risks are determined by nanoscientists using nanotoxicology, which is a branch of toxicology and an interdisciplinary field that concerns varied toxicity aspects of nanomaterials [15]. Nanotoxicity mediated via generation of reactive oxygen species(ROS) is considered a crucial mechanism leading to successive human oxidative stress [8]. Also, there is a huge interest in using nanomaterials in various fields, including their safety concerns. Consequently, there is a compelling demand to address these issues so as to expand our knowledge on the use of nanomaterials/nanostructures in terms of their biocompatibility, safety, and toxicity in the food sector.
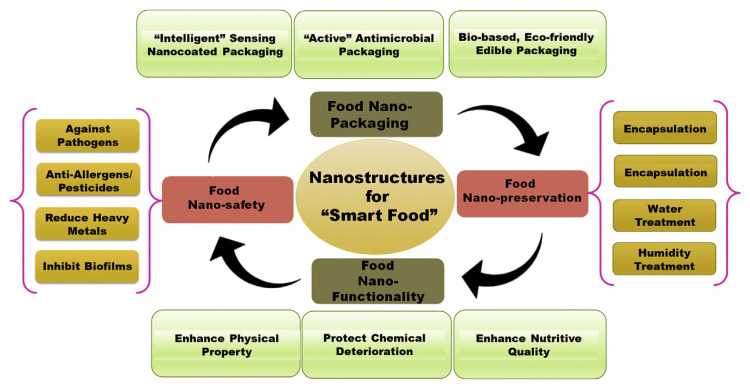
Although nanotechnology has applied green-synthesized NSMs in several ways in the food sector, on a few occasions, the use of NSMs has been controversial, as NSMs are scientifically uncertain and can impart long-term detrimental effects on human health and the environment [17]. In this regard, the complexity and limitations in the field of nanotechnology in terms of toxicity and accumulation (in appropriate doses) can be overcome only by elucidating the physiochemical and biological properties of NSMs through extensive research [18]. In light of the effects of nanomaterials on the food industry, this review has been formulated as a “snapshot” to address the current status of the techniques and implementations of nanotechnology in food preservation, safety, and security criteria and to identify the underlining mechanistic actions regarding safety or packaging issues. In addition, the review focuses on the current and future prospects of nanotechnology and discusses the strengths and weaknesses (i.e. the risks related to toxicological effects) of existing regulatory authorities. Moreover, the review discusses the current efforts to address these weaknesses and other issues related to the development, understanding, and promotion of nanotechnology. Fig. 1 illustrates an overview of the important usages of nanoparticles/nanomaterials and nanostructures in the food sector.
Fig. 1.
Systematic representation of application of nanoparticles in various areas of food industry.
2. Natural and synthetic nanostructures in the food system
The food system naturally contains a variety of nano-sized elements that include self-assembled higher order structures, such as carbohydrates and fats [19]. These ingredients are different from synthetically manufactured nanomaterials/ nanostructures thus can be used to make nanoemulsion, nano-encapsulate, and food-grade polymer [19]. During food processing, manufacturing, or thermal treatments, nano-structures are used to produce, which are not associated with modern nanotechnology (e.g. coagulation, emulsifying, or homogenizing). In brief, food proteins, including polysaccharides and lipids, are globular particles whose size can vary between 10 and several hundred nanometers. Coagulation and emulsification are based on the formation of reticular, zero, one, two and three-dimensional nanostructures. When corn starch is boiled to make custard-like food, small, three-dimensional crystalline structures that are only tens of nanometers in thickness are melted [20]. Fresh milk and milk-based products naturally contain nanostructures, such as milk proteins and casein. When milk is homogenized, fat globules that are about 100 nm in size are produced [20].
Nanotechniques play an essential role in the functional and nutraceutical food sectors. Encapsulation of effective molecules or components in NSMs can enhance the bio-availability and solubility of coloring agents and nutritional ingredients such as minerals and vitamins, thus facilitating controlled release and protecting biologically active substances and micro-nutrients during their processing. Currently, the most usable NSMs are nanocapsules (e.g. micelles and liposomes) and nanoemulsions, including a few carbon-based, green-synthesized, ecofriendly nanomaterials.
3. Nanotechnology in food packaging and security
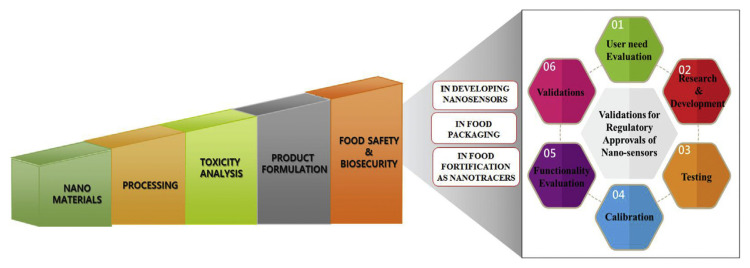
The packaging of food is one of the most critical steps in terms of food safety. Natural substances, atmospheric gases and water vapors are absolutely impermeable to no packaging materials [21,22]. However, completing blocking the migration and permeability of gases is not desirable in the case of packaging fresh fruits and vegetables that undergo cellular respiration [21]. In contrast, the packaging of carbonated beverages should eliminate the flow of oxygen and carbon dioxide (CO2) to prevent oxidation and de-carbonation [21]. The flow of CO2, oxygen, and water vapors varies according to the food matrices and the packaging materials that are used. Hence, these complexities in food packaging can be addressed and overcome by employing various nanocomposite materials, including polymers [24]. Nanoparticle having a diameter of less than 100 nm is thousand-times thinner than about 100,000 nm thick book page or hundred-times thinner than about 10,000 nm thick human hair. With an appropriate regulatory processing approval, different structures of nano-particles can be of significant use in various fields of food and pharmaceutical industries, especially for the research programs associated with food science-based research (Fig. 2).
Fig. 2.
Steps for processing and utilizing of nanomaterials in food sectors.
Recently, the use of nano-biocomposites in food packaging has enhanced the ability of food packaging to act as a barrier against gases [25]. The applications of various nanoparticles in food packaging are briefly shown in Table 1. Recent trends in food packaging are encouraging the use of biodegradable polymers reinforced with nanofillers, which are eco-friendly [26]. However, there is a major concern regarding the ingestion of these nano-compounds during consumption of the food. Hence, it is essential to investigate how these nanoparticles migrate within the human body as well as these nanoparticles’ toxic and immunogenic effects [27]. Another concern is related to the biodegradability of these nanofilled, biodegradable polymers [28]. These concerns are seriously considered by researchers across the world who are searching for human and environmentally friendly nanomaterials [29].
Table 1.
Nanoparticles for application in food packaging.
| Types of nanoparticles | Matrix | Application | Reference |
|---|---|---|---|
| Silver | Asparagus, Orange juice, Poultry meat, Fresh-cut melon, Beef meat exudates | Retards the growth of aerobic psychrotrophics, yeasts and molds; antimicrobial effect against Escherichia coli and Staphylococcus aureus | [165–169] |
| Zinc oxide | Orange juice, Liquid egg albumen | Effectively reduces Lactobacillus plantarum, Salmonella, yeast and mold counts without changes in quality parameters | [169–171] |
| Titanium oxide | Chinese jujube, Strawberry | Reduces browning, slow-down ripening, senescence and decay | [172] |
| Silver oxide | Apple slice | Retards microbial spoilage | [173] |
3.1. Nanocoatings as intelligent packaging for surfaces
In food packaging, NSMs, with biopolymers, can either enhance the property characteristics of neat polymer or improve the functional properties of active and intelligent packaging. Packaging can be categorized as “Improved”, “Active,” and “Intelligent” packaging, which represent which kind of packaging material could be used for what applications [30]. However, official restrictions have been imposed by the European Union (EU) using “active” and “intelligent” packaging materials in foods except using titanium nitride in plastic bottles [31].
For in-packaging detection of oxygen, Mill [32] developed nano-sized titanium dioxide (TiO2) or tin dioxide particle-based favorable photo-indicator intelligent ink using a redox-activate methylene blue dye, and this detector was gradually able to change the color in response to minor changes in oxygen quantity. In recent years, the packaging industry has focused on the various types of nanostructures and specified nanomaterials, including nano-clay particulates due to their easy accessibility, low-cost, easy process ability, and great performance. In addition, graphene nano-sheets and carbon nano-tubes are also encouragingly developed as a carbon-based nanomaterial [33]. Polymeric substrate-based flexible or static films and bottles (various materials, such as glass and plastic etc.) in combination with vacuum-coated thin inorganic layers have great demand in material packaging due to their ability to block out oxygen and aromas. While aluminum foil and aluminum-metallization used to be the material and method of choice, respectively, new materials are being used more frequently.
Thick and uniform layers (10–100 nm coating thickness) are broadly referred to as “nanocoatings” [34]. In recent years, few researchers have developed an intelligent type of nanocoating film that can indicate whether any contamination has occurred during storage. Gas content and non-invasive detection methods have also shown great ability to continually and easily monitor the gas content, excess moisture, and oxygen content of a package-headspace thus providing effective means to evaluate the quality and safety of food even after the production process [35,36]. Presence of oxygen inside the packaging can cause shelf-life threatening of food due to the ability of oxygen to create healthy environment for microbial growth [37]. Yu et al. [38] developed polyvinyl alcohol/chitosan polymer-based biodegradable and cost-effective films using a nanocomposite of silica in-situ which caused significant reduction in the permeability of oxygen and moisture by 25.6% and 10.2%, respectively, suggesting that the films extended the preservation time of cherries by three-fold compared to normal packaging. Swaroop and Shukla [39] developed a food packaging material using a combination of nanostructures of magnesium oxide (MgO) and polylactic acid biopolymer, and found that the material effectively protected against bacterial bio-films. Furthermore, Foltynowicz et al. [40] synthesized zero-valent iron particles to act as oxygen scavengers in food packaging.
3.2. The mechanisms of nanoparticles/nanocapsules as antimicrobial agents for active packaging
The outstanding use of nanoparticles with antimicrobial properties has, to some extent, protected our food products from foodborne illness outbreaks due to the consumption of spoiled packaged food. When compared with conventional food-packagings, an active food-packaging not only has the ability of being a passive barrier, but also helps to release antioxidant and antimicrobial compounds through the direct interaction with the food as well as helps to remove some negative factors, such as oxygen or water vapor. These interactions usually improve food stability [41].
Active packaging contains specific molecules which have the ability to absorb or release the components into or from the surrounding environment of the packaged food. Today, active polymer nanomaterials in food packaging have mainly been developed for antimicrobial packaging applications [42]. Overall, in order to improve the functionality and effectiveness of food packages, various bioactive substances can be incorporated into the packaging material by capsulation, capping the material with nanomaterials, or employing other nano-techniques [43]. To effectively apply antimicrobial active packaging to food products, food providers should select effective antimicrobial substances-based unique methodology to ensure the quality of the packaged products, such as their visual appearance and sensory levels [44].
The encapsulation of nutraceuticals and functional antimicrobial ingredients is essential for the preservation and bioavailability of bioactive ingredients, as it is extremely important in food processing, food storage, and passage through the gastrointestinal tract [5]. The macromolecule-based nanoparticles in food not only improve the bioavailability of bioactive polyphenolics, such as resveratrol, epigallocatechin-3-gallate, and curcumin, but also enhance the solubility of these polyphenols and thus prevent their degradation in the gastrointestinal environment [9]. There are various encapsulation techniques that have been used to produce nano or micro-particulate systems, such as nano-emulsion [45], coacervation [46,47], the extrusion method [48], fluidized bed coating [49], spray cooling [50], and spray drying [51].
Nano-encapsulation allows for the direct contact of nano-materials during the consumption of food. In a commonly known example, silicon dioxide (SiO2) is widely applied as a fragrance carrier in various food products [52]. Several lipid-based, nano-encapsulation systems have been developed to increase the activity of antioxidant components by improving their bio-availability and solubility [53] as well as their targeted, site-specific delivery with efficient absorption [54]. Studies have found that nano-sized edible coating is an effective alternative for preservation and shelf-life extension of food, as well as prevention of food from microbial spoilage [55]. Efficacy of coatings made with gelatin incorporating nano-crystals of cellulose [56], chitosan or nano-silica [57], chitosan with nano-silica [58], and alginate or lysozyme nano-laminate has been found very effective for the preservation of the quality of fresh foods during prolonged storage [59]. Additionally, the nano-packaging-based method that incorporates blended polyethylene with nano-powders such as Ag, kaolin, anatase TiO2, and rutile TiO2 has been considered to be a novel and facile technique to preserve fruits, such as strawberries (Fragaria ananassa Duch.) [60]. Despite the tremendous research in nano-encapsulation using various materials [61], the precise delivery of foods and their safety has not been studied in detail. Therefore, future research should examine the long-term toxicity of nano-encapsulated foods [62].
In addition to this, Johnston [63] employed nanostructured calcium silicate (NCS) for the absorption of Ag+ ions from a solution, which formed an NCS–Ag complex that was successfully used as an antimicrobial agent for food packaging purposes. Similarly, TiO2 is commonly utilized in surface-coating as a photocatalytic disinfecting material [64] and to inactivate several pathogenic bacteria in food [65]. The antibacterial potential of chitosan-capsulated nanoparticles could be mediated through the interaction between the positively-charged chitosan and the negatively-charged cell membranes. These interactions lead to enhanced membrane permeability and cause the rupture and eventual leakage of the intracellular material [66].
Sarwar et al. [67] developed polyvinyl alcohol (PVA), nanocellulose and Ag nanocomposite films and applied them to antimicrobial food packaging. These films profound antimicrobial effects against both Methicillin Resistant Staphylococcus aureus (MRSA) and DH5-alpha strain of Escherichia coli. Valerini et al. [68] also developed nanostructured, and aluminum-doped zinc oxide-based antimicrobial coatings functionalized with polylactic acid using sputtering power. The coatings exhibited strong antibacterial potential against E. coli and were proposed as promising materials for environment-friendly active packaging. Moreover, Lu et al. [69] prepared an antimicrobial nano-emulsion that was 100 nm by encapsulating citral essential oil using ultrasonic power, and as a result, the designed nano-emulsion was significantly effective in delivering an antimicrobial agent (citral essential oil) in the food system.
Metal and metal oxide nanoparticles (NPs)-based active packagings could be effective tools of nano-composites in terms of their antimicrobial potential. Among all metal nanoparticles, silver (Ag+) nanoparticles are widely used in commercial sectors. Silver nanoparticles are toxic for various food pathogens [70] because silver nanoparticles increase cell membrane permeability via cell surface adherence and by degrading lipopolysaccharide [71]; in this way, the silver nanoparticles can penetrate the bacterial cell, damage its DNA [72], and release antimicrobial Ag+ ions [73], which bind to sulfur, oxygen, or nitrogen containing electron donor groups in molecules and thus inhibiting synthesis of adenosine triphosphate (ATP) and DNA replication, eventually leading the cell to die [74]. Millimolar concentrations of Ag+ ions can easily cause cytoplasm shrinkage; separation of the cell wall membrane; the devastation of the peptidoglycan in the cell wall; denaturation of ribosomes; and DNA compression, which inhibits DNA synthesis, ruptures the cell membrane, and causes cell death [74]. Basically, the three major mechanisms of the bacterial toxicity of metal-containing NPs that are widely accepted include the uptake of metal ions, which causes the intracellular ATP depletion; ROS production, which causes oxidative cellular damage, and disruption of bacterial membrane [4].
Similarly, reports have confirmed incorporation, coating, immobilization and surface modification of antimicrobial components onto packaging material [75]. Also, the higher antimicrobial effect can be achieved via direct incorporation of antimicrobial components into packaging films. Similarly, films coated with various effective antimicrobial components can result in higher antimicrobial potential. While incorporating these antimicrobial agents (volatile and non-volatile) into film packaging, these molecules can be dispersed onto the food surfaces via adopting migration, evaporation or diffusion abilities [76]. Recently, Arfat et al. [77] designed a bio-nanocomposite film based on fish skin gelatin and silver-copper bimetallic NPs. The film showed significant antibacterial potential against Listeria monocytogenes and Salmonella enterica. Several silver-based, antimicrobial master batches, such as Bactiblock®, Aglon®, Surfacine®, d2p®, IonPure®, Irgaguard®, and Biomaster® have been used to retard the growth of Campylobacter, Salmonella, and E. coli.
4. Nanotechnology in food functionality
4.1. Nanoparticles for protection from chemical deterioration
The main reason behind deterioration in the quality of food lies in an array of chemical reactions that occur between various components present in food and the external environment. Researchers have identified several nanomaterials that can control these unwanted reactions in various food matrices. However, some of these metal and metal oxide nanomaterials cause nano-toxicity, primarily because they can form ROS and cause oxidative stress, which leads to an imbalanced redox state in the cell [78]. Therefore, relatively less reactive nanomaterials are employed as an antioxidant carrier [18,79,80]. Polymeric nanoparticles are suitable for the encapsulation of bioactive compounds, such as vitamins and flavonoids, and for the release of these compounds in an acidic environment, such as in the stomach [81]. The application of antioxidants and edible coatings control the browning, the conversion of phenolics into dark-colored pigments in the presence of oxygen of fresh-cut fruits [82]. Although few nanomaterials have been used directly as anti-browning agents, nano-ZnO has been used as coated active packaging to improve the shelf-life of fresh-cut Fuji apples [83].
Nanotechnology can enhance food functionality either by eliminating chemical toxicants or by enhancing nano-sized nutritional supplements. For instance, some of the nutra-ceuticals incorporated in the carriers consist of lycopene, β-carotenes, and phytosterols in order to avoid cholesterol accumulation in body [84]. It is well-known that food itself has few nanostructures that are responsible for self-mediated nano-effects. For example, green tea has many health benefits because of its nano-selenium content, which facilitates the effective uptake of selenium.
Nano-encapsulation involves a nanoscale process of packing material by using nanocapsules, and it ensures the functionality of the final product, which includes the controlled release of the core. Therefore, encapsulated forms of ingredients exhibit numerous advantages, including consecutive delivery of multiple active ingredients, shelf-life extension, increased stability, and pH-triggered controlled release [85]. Further, Liang et al. [86] encapsulated epigallocatechin gallate (EGCG in zein/chitosan) nanoparticles for controlled, effective, functional applications in the food system. It was observed that the release of EGCG from zein/chitosan (CS) NPs and the DPPH scavenging ability of the zein/CS NPs was relatively higher than that of the nanoparticles that did not have the zein/CS coating in fatty simulant of 95% ethanol. These findings confirmed that the antioxidant activity and controlled-release of EGCG from zein/CS NPs in fatty simulant of 95% ethanol protect fatty foods from chemical deteriorates via enhancing antioxidant efficiencies and thus may help fatty foods to protect from oxidation for longer period.
4.2. Nanoparticles for enhancing the physical properties of food and packaging materials
Nanomaterials developed have shown enormous ability to increase the physical properties of both food and packaging materials [87]. In the 1990s, polymer nanocomposites with layered silicates that had several important characteristics, such as high flame resistance [88] and protection from UV rays, were developed [89]. Moreover, a number of NPs have been developed to increase the physical attributes of food, such as color and appearance. TiO2 was approved as a coloring food additive by the USFDA with the agreement that the limit of TiO2 should not exceed 1% (w/w) as an additive ingredient [90]. Besides TiO2, mixture of color additives may contain SiO2 and/or Al2O3, but the use of carbon black is strictly prohibited by the USFDA as a food coloring additive [91]. On the other hand, SiO2 is used as an anti-caking agent to maintain the flow properties in powdered products and as a carrier of aroma in food and non-food products. Nano-sized SiO2 is widely employed in food products and has been registered within the EU as a food additive, as per Directive E551 [52].
β-Carotene is a nutraceutical component that acts as a coloring agent and as provitamin A, but its incorporation into foods is limited because of its low chemical stability and hydrophobicity. To improve the physicochemical stability of β-carotene, Mehrad et al. [92] synthesized nanomaterial by encapsulating it into solid lipid nanoparticles (SLNPs) that contained palmitic acid and corn oil and stabilized the material using a whey protein isolate (WPI). In this nanostructure, the encapsulated β-carotene was protected by a solid shell made by palmitic acid crystals covering the surface of the oil droplets. Corn oil was able to decrease the exclusion of β-carotene from the solid lipid matrix to the surface of the SLNPs, while WPI increased the stability of the colloidal system, thus improving the oxidative stability of the β-carotene.
5. Nanotechnology in food safety
Globally, food safety is a growing public health concern. The primary goal of food safety is to ensure that food will not cause any harm to the consumer during preparation and consumption [93]. Food must be protected from any type of physical, chemical, and biological contamination during processing, handling, and distribution [94]. Recent developments in nanotechnology have revolutionized the food industry with its various applications in food processing, safety, and security, as well as its strides in enhancing nutraceutical value, extending shelf-life, and reducing packaging waste [94]. Today, food safety is a major concern due to rapid changes in food recipes and food habits. Foodborne pathogens, toxins, and other contaminants can cause serious threats to human health. The conventional methods for the detection of pathogens and their toxins are labor intensive and time consuming. Advances in nanotechnology have expedited the addressing of food safety issues in microbial contaminants and have improved toxin detection, shelf-life, and packaging strategies [84]. In addition, nanomaterials, including metal nanoparticles, carbon nanotubes, quantum dots, and other active nanomaterials can be used to develop biosensors for the quantification of microbes and other tests for food safety applications [84,94].
5.1. Nanoparticles for the detection of foodborne pathogens
Nano-biosensors are bioanalytical devices that are developed using various NSMs and biological receptors in an integrated system design [96]. Various types of biosensors have been developed to detect foodborne pathogens and food spoiling materials [97,98]. Surface enhanced Raman scattering (SERS) is employed as a nano-biosensing tool to rapidly and accurately detect microbial pathogens [99,100]. Silver nano-colloids are commonly used in SERS [101] to detect bacteria, as silver nano-colloids enhance Raman signals. Besides silver nano-colloids, graphene oxide [102], magnetic beads [103], carbon nanotubes [104], plasmonic gold [44], and silver nanoparticles [24] are commonly used to detect food-associated bacterial pathogens. In addition, synthetic DNA molecular beacons tagged with color-coded probes are used as nano-barcodes to identify food pathogens [72].
The direct detection of E. coli in food samples has also become possible by the measurement and detection of light that is scattered by the cells. This type of sensor operates on the basis of binding with a known protein and is characterized as a bacterium onto a silicon chip, which can further bind with any other E. coli bacteria present in the food sample [105]. Chen and Durst [106] devised an array-based immunosorbent assay to detect E. coli O157:H7, Salmonella spp., and L. monocytogenes using protein G-liposomal nano-vesicles in pure and mixed cultures. The researchers showed that protein G-liposomal nanovesicles can be successfully used in immunoassays for simultaneous detection of foodborne pathogens and demonstrated the effectiveness of protein G-liposomal nano-vesicles as universal immunoassay reagents. Further, DeCory et al. [107] developed an immunomagnetic bead-immunoliposome fluorescence assay to rapidly detect E. coli O157:H7 in aqueous samples. The findings demonstrated the feasibility of using immunomagnetic beads in combination with sulforhodamine B encapsulated in immunoliposomes to rapidly detect E. coli O157:H7 in aqueous samples. Additionally, liposome-based methods have also been investigated by other researchers for pathogen detection [108,109]. Nano-sensors, such as nano-cantilevers, employ silicon-based materials to recognize proteins and detect pathogens that vibrate at different frequencies depending on their biomass [110].
In recent years, various new nanoparticle-based detection platforms have been developed. Tominaga [111] developed lateral-flow immune test strips with palladium nanoparticles against Klebsiella, which allowed for the specific binding and visualized detection of specific bacteria. Further, Thakur et al. [112] detected single E. coli bacterial cell using a reduced graphene, nanoparticle-based, field-effect transistor device. Recently, our research group developed an electrochemical sensing platform based on graphene oxide–gold nanoparticles to detect Cronobacter sakazakii, which is a bacterium that is hazardous to infants, in infant formula powder with a detection limit of 2.0 × 101 cfu/mL [113]. Also, Song et al. [114] developed a fluorescence sensing platform using immunomagnetic nano-particles coupled with liposome nanoparticles to detect Cronobacter sp. at the genus level with a detection limit of 5.9 × 103 cfu/ mL. Additionally, our research team developed an aptamer and gold nanoparticle-based optical detection platform to detect Salmonella in contaminated pork samples [115]. The various types of nanoparticles for the detection of various foodborne pathogens and their detection limits are shown in Table 2.
Table 2.
Nanoparticles employed for the detection of foodborne pathogens.
| Nanoparticles | Pathogens | Detection limit | Reference |
|---|---|---|---|
| Gold nanoparticle | Salmonella enterica serotype Typhi | 98.9 CFU/mL | [174] |
| Gold/silicon nanorod | Salmonella enterica serotype Typhi; Respiratory syncytial virus | Not reported | [175] |
| Gold nanorod | Escherichia coli O157:H7 | 1–10 CFU/mL | [159] |
| Quantum dot | Salmonella enterica serotype Typhi, E. coli O157:H7, Listeria monocytogenes | 103–106 cells/mL | [160–163] |
| Magnetic bead/quantum dot | E. coli 0157:H7 | 103 CFU/mL | [176] |
| RuBpy doped silica | E. coli O157:H7 | 1 cell/mL | [164,176] |
| Single walled carbon nanotube | E. coli | Not reported | [177] |
| Magnetic nanoparticle | E. coli O157:H7, S. aureus, S. epidermidis | 104 CFU/mL, 8 CFU/mL, 10 CFU/mL | [177] |
| Immunomagnetic liposome nanoparticle | Cronobacter sakazakii | 103 CFU/mL | [108] |
| Aptamer conjugated gold nanoparticles | Salmonella typhimurium | 104 CFU/mL | [115,120] |
| Liposome nanoparticles | Salmonella typhimurium | 102 CFU/mL | [173] |
5.2. Nanoparticles for protection from allergens
Nanotechnology has been employed as a basic tool to control and manage food allergens [116,117]. Despite these efforts, certain nanomaterials cause allergic pulmonary inflammation [118,119] in humans. For instance, SiO2 nanoparticles have been reported to cause allergen-specific, Th2-type immune responses in vivo in female BALB/c mice [118]. The immunotherapy of allergies using aluminum hydroxide (alum) as an adjuvant displayed several side effects, including swelling, indurations, erythema, cutaneous nodules, and granulomas at the injection site [119]. To overcome this, researchers have investigated the use of polymeric nanoparticles [120] and substances that target toll-like receptors (TLR) as alternative adjuvants [121]. For instance, the protamine-based nano-particles with TLR-9 ligand cytosine phosphate guanine (CpG)-oligodeoxynucleotides (ODNs) can be used as an adjuvant. Protamines are arginine-rich peptides of approximately 4 kDa found in the sperm of salmon and are isolated from the mature testicles of salmons. Protamines are biodegradable and efficiently utilized for the reversal of heparin activity during surgical operations [122] or as an insulin-additive [123]. Additionally, protamine-based nanoparticles with CpG-ODN counteract the Th2-dominated immune response induced by an allergen, thus have shown remarkable potential in allergen immunotherapy as a novel carrier system [124].
Similarly, Gamazo et al. [125] reported immunotherapy that employed nanoparticles as an allergen delivery system, which facilitated the administration, reduced the dose, and diminished the allergen exposure to the immunoglobulin E that was bound to mast cells and/or basophils. Moreover, in recent years, bioinspired NSMs are being explored that are known to have little to no toxic effects and can thereby be potentially exploited in the food industry [126]. Localized surface plasmon resonance (LSPR)-based label-free biosensing methods have gained huge attention due to their high sensitivity, simplicity, and relatively low cost efficacy. However, the challenges have been observed using these methods for in situ analysis of real samples due to the instability of colloidal nanoparticles at certain levels of pH and salt concentration. Recently, Lee et al. [127] developed a simple and cost-effective optical fiber coated with aptamer-modified gold nanorods for easy detection of ochratoxin A in grape juice samples (food mycotoxin causing allergy) and subjected the fiber to LSPR analysis, which demonstrated a significant detection limit of 12.0 pM. Zhang et al. [128] reported a magnetic, nanoparticle-assisted, aptamer-based fluorescence assay to detect allergens in food matrices. As a result, under optimal conditions, the linear range was recorded as 0.4–5 g/mL (R2 = 0.996), and a low limit of detection was calculated as 77 ng/mL with efficient selectivity. Moreover, Brotons-Canto et al. [129] evaluated the positive effects of poly-(anhydride) nanoparticles (150 nm) as oral vehicles for immunotherapy against experimental peanut allergies. Several other studies demonstrated the promising applications of nanotechnologies in vaccinology and in allergen immunotherapy [130]. In the near future, nanoparticle-based formulations can be applied to allergen immunotherapy to solve its unmet needs [131].
5.3. Nanoparticles for preventing heavy metal reduction
The release of heavy metals from nanomaterials poses a high risk of toxic outbreaks [80]. The release of these metals in food products has adverse effects in the case of long-term accumulation. Metal and metal oxide-based nanomaterials, such as ZnO [132], Ag [133], and CuO [134], increase the intracellular ROS level [132] and ultimately cause lipid peroxidation and DNA damage. Cationic surfactant coated silica-modified magnetite nanoparticles act as adsorbents for the microextraction and have ability to determine trace amount of Cu, Ni, Co, Cd, Pb, and Mn from environmental samples. The silica-coated NPs synthesized using cetylpyridinium bromide, can solubilize metal ions after being complexed with 8-hydroxyquinoline [135]. While many nanomaterials have tremendous potential for the remediation of contaminants, magnetite nanoparticles have proven to be the most attractive and inexpensive substrates for the recovery of heavy metals [136] from various sources. Zhang et al. [137] fabricated Fe@Fe2O3 core/shells, nanowires, and nano-necklaces to remove chromium from aqueous solutions.
Recently, Lin et al. [138] demonstrated the ability of aminated magnetic iron oxide nanoparticles, especially Cu2+, Ni2+, Pb2+, and Zn2+, to act as adsorbents to remove aqueous heavy metal ions. The results of the study displayed that the increase in the degree of amination had a positive effect with a simultaneous increase in the adsorption capacity and the initial rate of adsorption of heavy metal ions. MgO nano-particles synthesized via sol–gel and calcination processes showed great potential against bacterial infections and were able to remove heavy metal ions from contaminated water samples [139]. These findings anticipated that nano-sized MgO particles may have potential for the treatment of bacterial and heavy metal contaminated wastewater due to their high removal efficiency, low cost, facile preparation, and environmentally friendly characteristics.
In recent years, Lingamdinne et al. [140] demonstrated how synthesized, reusable, recoverable iron oxide nanoparticles could reduce heavy metals without losing their stability. In addition, over the years, various carbon nanoparticles with high fluorescence properties such as carbon dots (C-dots) with a particle size of smaller than 10 nm and carbon nanoparticles (CNPs) of about 10 nm size or larger have shown a broad range of biological propertieswithlowtoxicityandgoodbiocompatibility. Recently, through a thermal process in the presence of H3PO4, Simpson et al. [141] fabricated, characterized, and approved carbon nanoparticles (66 nm) with glycerol for the detection of heavy metal ions, with a detection limit as low as 0.30 ppm.
5.4. Nanoparticles for the inhibition of biofilm formation
Biofilms are tightly-packed bunch of bacterial cells that adhere to many substrates and produce a polymeric extracellular matrix that is extremely difficult to penetrate [142]. The biofilm formation begins with the attachment of free floating micro-organisms to the surface by employing van der Waals forces that create problems such as biocorrosion, biofouling, and accumulation in the food processing industries [143]. Glycerol monolaurate (GML), which is recognized as safe by the USFDA, acts as an antimicrobial agent against many Gram-positive bacteria, including Bacillus anthracis [144]. GML has been shown to inhibit the biofilm formation of three different strains of S. aureus and of MRSA [145]. Besides this, the use of antimicrobials throughout the nano-fibers of filter membranes is employed to prevent biofilm formation [137]. Moreover, Shahrokh and Emtiazi [146] demonstrated that nanosilver particles at low concentrations (0.2 ppm) enhanced bacterial metabolism and thus suggested the use of an optimum concentration of nanosilver particles for various nanomaterials to prevent biofilm formation.
Further, nickel oxide nanoparticles (NiO NPs) have been proposed as prospective antibacterial and anti-tumor agents. Through a green approach and using Eucalyptus globulus leaf extract, Saleem et al. [147] synthesized NiO-NPs that were 10–20 nm in size and assessed their anti-biofilm activity. In addition,Gambino etal.[148] demonstratedthebio-applicability of zinc oxide nanoparticles for hindering fungal biofilm (Alternaria alternata, Penicillium chrysogenum, P. pinophilum, and Aspergillus niger) that had developed in an ancient Egyptian tomb. Chlorhexidine-conjugated gold nanoparticles exhibited biofilm inhibitory effects against Klebsiella pneumonia [149].
Bacillus subtilis has shown enormous potential in various industrial processes for the production of value-added products. Fermentation process of B. subtilis is directly related to its ability to form biofilm resulting in operational issues and major process. Ranmadugala et al. [150] evaluated the effects of two types of super paramagnetic iron oxide (IONs), such as naked IONs and IONs coated with 3-aminopropyltriethoxy silane (IONs@APTES) against B. subtilis in terms of their ability to inhibit its biofilm formation, bacterial growth, and cell viabilities. The results showed that a significant reduction in the total biomass of the bacterial biofilm was observed without affecting the cell viabilities. These results suggested that these nano-particles could be applied in several industries to combat the growth of bacterial biofilm. Moreover, Thuptimdang et al. [151] suggested that controlling the growth conditions to alter the biofilm physical structure was a possible approach to reduce the impact of silver nanoparticles (AgNPs) on biofilms in engineered and natural systems. These recent findings are increasing the demand for researchers to explore the use of nanoparticles in several other industries, including medicine.
6. Are nanotechnology and big data effective enough for next industrial revolution for securing “Smart Food”?
The engineering applications of nanotechnology will herald a new digital future characterized by improvements in food safety, shelf-life performance, and food reliability. These advancements are frequently referred to as big data information and open new fields of research and applications that will increasingly affect all parts of society. There is a huge complicated response to outbreaks associated with foodborne diseases due to the globalization of food supply chains. Generation of big data in the food industry may help to facilitate encouraging advancement in food safety and security at the global scale [156]. Big data is represented as high volume, high velocity, high veracity, and/or high variety information assets requiring new processing formats so as to enhance the process of decision making, process optimization, and insight discovery [157]. Although the big data analysis and large datasets in food safety and quality have focused on improving root cause and retrospective analyses, there is a huge growth in the advancement and utilization of predictive analytics in food security sector [157]. For example, whole genome sequencing data for L. monocytogenes isolates obtained from ice cream in Kansas became publicly available soon after a listeriosis outbreak linked to ice cream was reported in early 2015. Other omics datasets, such as metagenomics data, have also been used to identify and characterize food spoilage issues. It is likely that these types of data sources will become increasingly available to personnel in the food industry.
Recently, the World Health Organization (WHO) has enfold the big data information approach to deal with the food-safety process of decision making, which has brought about a new food-safety platform known as “FOSCOLLAB,” which integrates different sources from various disciplines [158]. On this platform, organized and nano-structured information from numerous areas, for example, food, horticulture, animals, public health, and economic index, are incorporated and accessible to the users by means of a few committed dashboards [158]. The information sources associated in FOSCOLLAB are assessments of the Joint FAO/WHO Expert Committee on Food Additives and the Joint FAO/WHO Meeting on Pesticide Residues databases on chemical risk assessment, the WHO database on Collaborating Centers, and the Global Environment Monitoring System (GEMS) food databases on chemical occurrence and food consumption. There is no restriction of utilizing big data information in the fields of food safety and food quality to facilitate improved methodologies.
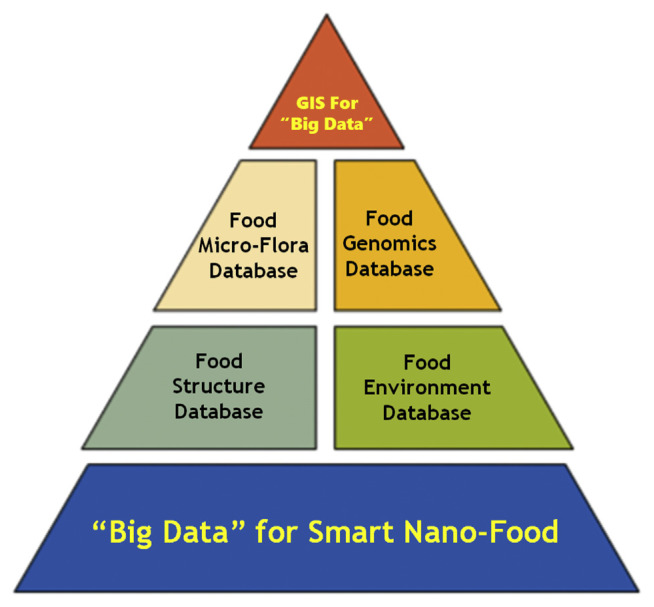
Finally, input food industries often work worldwide, are R&D intensive, and deal with new technologies, including genetics, robotics, informatics, and nanotechnology. Besides the challenge of feeding the world, there is the issue of sustainability, which is mainly an issue of farming and transport. Together with the relationship between food and health (e.g. obesity concerns), food safety directly affects food processors, retailers, and consumers. The ultimate objective is to estimate risks to farms and to build up a preventive way to deal with food safety issues. The use of geographical information system technology (GIST) to create food security has demonstrated incredible practices, for example, helping producers settle on more informed choices about field practices and establish the programs of targeted pathogen surveillances. Overall, the utilization of GIST to yield big data information in food safety investigations has created remarkable measures of new data with respect to the ecology of various life forms in the food environment and data on different contamination episodes. In the era of big data information, GIST is one of the data tools that enables scientists to store, analyze, process, capture, and visualize extensive datasets. Additionally, the use of GIST to complex food safety/security issues is being demonstrated for further integration of various large data sets. Essential components associated with big data requirements in nano-food safety are presented in Fig. 3.
Fig. 3.
Essential components for producing “big data” for safety and security of smart nano-food.
7. Future perspectives and potential risks of nanotechnology
There have been tremendous developments in the application of nanotechnology in food science and research. Nanotechnology helps in the detection of pesticides [159], pathogens [160], and toxins [161], and tracking-tracing-monitoring helps in the maintenance of food quality. A massive amount of interest is being on using carbon nano-tubes for incorporating them into packaging materials for the detection of toxic proteins, microorganisms, and food spoilage [162]. Furthermore, carbon nanotube can transform our future food packaging materials to active and intelligent packaging systems [163]. Along with the incessant research on the application of nanomaterials, several potential risks and toxicity issues associated with the application of nanomaterials have been revealed, and these concerns must be addressed [104,161]. The effect of these nanoscale particles on human beings, animals, and the environment are unpredictable due to changes over time in their properties. Some nanoparticles can even cross biological barriers, such as the blood–brain barrier, and enter various cells and organs [164].
The evidences for the health risks associated with the inhalation of ultrafine and nanoparticles have been increasing every day [60]. In addition, bioaccumulation of nanomaterials, such as nano-silver, derived from either nanopackaging or plants and animals has been confirmed in food and human being [62]. The use of nanomaterials as activation catalysts, pesticides, and microbicides always pose an unknown associated risk, and hence risk assessment procedures must be strictly followed while processing food [57,58]. Even with the advent of nanotechnology, the challenges to the development of a healthy and sustainable food industry remain. With the introduction and development of nanotechnology in the food system, the public should be educated regarding the associated health, safety, and environmental effects of nanotechnology.
Acknowledgements
We acknowledge financial support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016R1A2B4013374 and 2014R1A5A1009799).
Funding Statement
We acknowledge financial support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016R1A2B4013374 and 2014R1A5A1009799).
References
- 1.Chandra P.Nanobiosensors for personalized and onsite biomedical diagnosis. The Institution of Engineering and Technology. 2016. [DOI]
- 2. Sandoval B. Perspectives on FDA's regulation of nanotechnology: emerging challenges and potential solutions. Compr Rev Food Sci Food Saf. 2009;8:375–93. [Google Scholar]
- 3. Moriarty P. Nanostructured materials. Rep Prog Phys. 2001;64:297. [Google Scholar]
- 4. Pathkoti K, Manubolu M, Hwang HM. Nanostructures: current uses and future applications in food science. J Food Drug Anal. 2017;25(2):245–53. doi: 10.1016/j.jfda.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhushan B. Springer handbook of nanotechnology. Berlin; Heidelberg: Springer Science & Business Media; 2010. [Google Scholar]
- 6. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–41. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- 7. Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA. Application of nanotechnology in food Science: perception and overview. Front Microbiol. 2017;8:e1501. doi: 10.3389/fmicb.2017.02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu PP. Introduction to the special issue: nanomaterials – toxicology and medical applications. J Food Drug Anal. 2014;22:1–2. doi: 10.1016/j.jfda.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu B, Liu X, Zhang C, Zeng X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J Food Drug Anal. 2017;25:3–15. doi: 10.1016/j.jfda.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baranwal A, Srivastava A, Kumar P, Bajpai VK, Maurya PK, Chandra P. Prospects of nanostructure materials and their composites as antimicrobial agents. Front Microbiol. 2018;9:e422. doi: 10.3389/fmicb.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das MP, Livingstone JR, Veluswamy P, Das J. Exploration of Wedelia chinensis leaf-assisted silver nanoparticles for antioxidant, antibacterial and in vitro cytotoxic applications. J Food Drug Anal. 2017;26:917–25. doi: 10.1016/j.jfda.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He X, Hwang HM. Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal. 2016;24:671–81. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adabi M, Naghibzadeh M, Adabi M, Ali Zarrinfard M, Esnaashari SS, Seifalian AM, et al. Biocompatibility and nanostructured materials: applications in nanomedicine. Artif Cells Nanomed Biotechnol. 2017;45:833–42. doi: 10.1080/21691401.2016.1178134. [DOI] [PubMed] [Google Scholar]
- 16. He X, Aker WG, Pelaez M, Lin Y, Dionysiou DD, Hwang HM. Assessment of nitrogenefluorine-codoped TiO2 under visible light for degradation of BPA: implication for field remediation. J Photochem Photobiol Chem. 2016;314:81–92. [Google Scholar]
- 17. Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, et al. Acute toxicity of nano- and microscale zinc powder in healthy adult mice. Toxicol Lett. 2006;16:115–23. doi: 10.1016/j.toxlet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18. He X, Aker WG, Hwang HM. An in vivo study on the photoenhanced toxicities of S-doped TiO2 nanoparticles to zebra fish embryos (Danio rerio) in terms of malformation, mortality, rheotaxis dysfunction, and DNA damage. Nanotoxicology. 2014;8(S1):185–95. doi: 10.3109/17435390.2013.874050. [DOI] [PubMed] [Google Scholar]
- 19. Peters RJ, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci Technol. 2016;54:155–64. [Google Scholar]
- 20.Nanotechnology information statement. IFST – Institute of Food Science & Technology; 2006. [Google Scholar]
- 21.Robertson GL. Food packaging: principles and practice. 2nd ed. New York: CRC Press Taylor & Francis Group; 2006. [Google Scholar]
- 22.Finnigan B. Barrier polymers. In: Yam KL, editor. The wiley encyclopedia of packaging technology. New York: John Wiley and Sons Inc; 2009. pp. 103–9. [Google Scholar]
- 24. Abbaspour A, Norouz-Sarvestani F, Noori A, Soltani N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of Staphylococcus aureus. Biosens Bioelectron. 2015;68:149–55. doi: 10.1016/j.bios.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 25. Ghanbarzadeh B, Oleyaei SA, Almasi H. Nano-structured materials utilized in biopolymer based plastics for food packaging applications. Crit Rev Food Sci Nutr. 2014;7:37–41. doi: 10.1080/10408398.2012.731023. [DOI] [PubMed] [Google Scholar]
- 26. Abdollahi M, Rezaei M, Farzi G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J Food Eng. 2012;111(2):343–50. [Google Scholar]
- 27.Azeredo HMC, Mattoso LHC, McHugh TH. Nanocomposites in food packaging—a review. In: Reddy B, editor. Advances in diverse industrial applications of nanocomposites. InTech; 2011. p. 550. [DOI] [Google Scholar]
- 28. Klaine SJ, Koelmans AA, Horne N, Carley S, Handy RD, Kapustka L. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ Toxicol Chem. 2012;31(1):3–14. doi: 10.1002/etc.733. [DOI] [PubMed] [Google Scholar]
- 29. Klaine SJ. Considerations for research on the environmental fate and effects of nanoparticles. Environ Toxicol. 2009;28(9):1787–8. doi: 10.1897/09-203.1. [DOI] [PubMed] [Google Scholar]
- 30. Hannon JC, Kerry J, Cruz-Romero M, Morris M, Cummins E. Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends Food Sci Technol. 2015;43:43–62. [Google Scholar]
- 31. Echegoyen Y, Nerin C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem Toxicol. 2013;62:16–22. doi: 10.1016/j.fct.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 32. Mills A. Oxygen indicators and intelligent inks for packaging food. Chem Soc Rev. 2005;34:1003–11. doi: 10.1039/b503997p. [DOI] [PubMed] [Google Scholar]
- 33. Arora A, Padua GW. Review: nanocomposites in food packaging. J Food Sci. 2010;75:43–9. doi: 10.1111/j.1750-3841.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- 34. Stormer A, Bott J, Kemmer D, Franz R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci Technol. 2017;63:39–50. [Google Scholar]
- 35.Arndt GW. Leak testing. In: Yam KL, editor. The wiley encyclopedia of packaging technology. 3rd ed. New York: John Wiley and Sons, Inc; 2008. [Google Scholar]
- 36. Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci 2011. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ariyarathna IR, Rajakaruna RMPI, Nedra Karunaratne D. The rise of inorganic nanomaterial implementation in food applications. Food Control. 2017;77:251–9. [Google Scholar]
- 38. Yu Z, Lib B, Chud J, Zhang P. Silica in situ enhanced PVA/ chitosan biodegradable films for food packages. Carbohydr Polym. 2017;184:214–20. doi: 10.1016/j.carbpol.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 39. Swaroop C, Shukla M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int J Biol Macromol. 2018;113:729–36. doi: 10.1016/j.ijbiomac.2018.02.156. [DOI] [PubMed] [Google Scholar]
- 40. Foltynowicz Z, Bardenshtein A, Sängerlaub S, Antvorskov H, Kozak W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag Shelf Life. 2017;11:74–83. [Google Scholar]
- 41. Dasgupta N, Ranjan S, Mundekkad D, Ramalingam C, Shanker R, Kumar A. Nanotechnology in agrofood: from field to plate. Food Res Int. 2015;69:381–400. [Google Scholar]
- 42. Silvestre C, Duraccio D, Cimmino S. Food packaging based on polymer nanomaterials. Prog Polym Sci. 2011;36:1766–82. [Google Scholar]
- 43. Ghaani M, Cozzolino CA, Castelli G, Farris S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci Technol. 2016;51:1–11. [Google Scholar]
- 44. Fang Z, Zhao Y, Warner RD, Johnson SK. Active and intelligent packaging in meat industry. Trends Food Sci Technol. 2017;61:60–71. [Google Scholar]
- 45. Silva HD, Cerqueira MA, Vicente AA. Nanoemulsions for food applications: development and characterization. Food Bioprocess Technol. 2012;5(3):854–67. [Google Scholar]
- 46. Tamjidi F, Nasirpour A, Shahedi M. Physicochemical and sensory properties of yogurt enriched with microencapsulated fish oil. Food Sci Technol Int. 2012;18(4):381–90. doi: 10.1177/1082013211428212. [DOI] [PubMed] [Google Scholar]
- 47. De Conto LC, Grosso CRF, Gonçalves LAG. Chemometry as applied to the production of omega-3 microcapsules by complex coacervation with soy protein isolate and gum Arabic. LWT – Food Sci Technol. 2013;53(1):218–24. [Google Scholar]
- 48. Li XY, Chen XG, Sun ZW, Park HJ, Cha DS. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr Polym. 2011;83(4):1479–85. [Google Scholar]
- 49.Zuidam NJ, Shimoni E. Encapsulation technologies for active food ingredients and food processing. Springer Science Business Media LLC; 2010. Overview of microencapsulates for use in food products or processes and methods to make them. [Google Scholar]
- 50. Gibbs BF, Kermash S, Alli I, Mulligan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 2010;199:213–24. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- 51. Murugesan R, Orsat V. Spray drying for the production of nutraceutical ingredients – a review. Food Bioprocess Technol. 2012;5(1):3–14. [Google Scholar]
- 52. Dekkers S, Krystek P, Peters RJ, Lankveld DX, Bokkers BG, van Hoeven-Arentzen PH, et al. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5:393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- 53. Mozafari MR, Flanagan J, Matia-Merino L, Singh H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in food. J Sci Food Agric. 2006;86(13):2038–45. [Google Scholar]
- 54. Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2013;6:628–47. [Google Scholar]
- 55. Flores-Lopez ML, Cerqueira MA, de Rodriguez DJ, Vicente AA. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng Rev. 2015;5:1–14. [Google Scholar]
- 56.Fakhouri FM, Casari ACA, Mariano M, Yamashita F, Mei LI, Soldi V, et al. Effect of a gelatin-based edible coating containing cellulose nanocrystals (CNC) on the quality and nutrient retention of fresh strawberries during storage, IOP conference series: materials science and engineering. Conference 1, 2nd International Conference on Structural Nano Composites (NANOSTRUC 2014; 2014 20–21 May 2014; Madrid, Spain. [Google Scholar]
- 57. Shi S, Wang W, Liu L, Wu S, Wei Y, Li W. Effect of chitosan/ nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J Food Eng. 2013;118:125–31. [Google Scholar]
- 58. Yu YW, Zhang SY, Ren YZ, Li H, Zhang XN, Di JH. Jujube preservation using chitosan film with nano-silicon dioxide. J Food Eng. 2012;113:408–14. [Google Scholar]
- 59. Medeiros BS, Souza MP, Pinheiro AC, Bourbon AI, Cerqueira MA, Vicente AA, et al. Physical characterization of an alginate/lysozyme nano-laminate coating and its evaluation on ‘Coalho’ cheese shelf life. Food Bioprocess Technol. 2014;7:1088–98. [Google Scholar]
- 60. Yang FM, Li HM, Li F, Xin ZH, Zhao LY, Zheng YH, et al. Effect of nano-packaging on preservation quality of fresh strawberry (Fragaria ananassa Duch. cv Fengxiang) during storage at 4 °C. J Food Sci. 2010;75(3):236–40. doi: 10.1111/j.1750-3841.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 61. Elgadir MA, Uddin MS, Ferdosh S, Adam A, Khan AJ, Saraker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23(4):619–29. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jovanovic B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr Environ Assess Manag. 2015;11:10–20. doi: 10.1002/ieam.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnston CT. Probing the nanoscale architecture of clay minerals. Clay Miner. 2010;45(3):245–79. [Google Scholar]
- 64. Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev. 2000;1:1–21. [Google Scholar]
- 65. Chawengkijwanich C, Hayata Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int J Food Microbiol. 2008;123:288–92. doi: 10.1016/j.ijfoodmicro.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 66. Qi LF, Xu ZR, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339:2693–700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 67. Sarwar MS, Niazi MBK, Jahan Z, Ahmad T, Hussain A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr Polym. 2018;184:453–64. doi: 10.1016/j.carbpol.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 68. Valerinia D, Tammaroa L, Di Benedettoa F, Vigliottab G, Capodiecia L, Terzia R, et al. Aluminum-doped zinc oxide coatings on polylactic acid films for antimicrobial food packaging. Thin Solid Films. 2018;645:187–92. [Google Scholar]
- 69. Lu WC, Huang DW, Wang CCR, Yeh CH, Tsai JC, Huang YT, et al. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26:82–9. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol. 1997;25:279–83. doi: 10.1046/j.1472-765x.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 71. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 72. Li Y, Tseng YD, Kwon SY, Despaux L, Bunch JS, Mceuen PL. Controlled assembly of dendrimer-like DNA. Nat Mater. 2004;3:38–42. doi: 10.1038/nmat1045. [DOI] [PubMed] [Google Scholar]
- 73. Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 74. Karimi M, Sadeghi R, Kokini J. Human exposure to nanoparticles through trophic transfer and the biosafety concerns that nanoparticle-contaminated foods pose to consumers. Trends Food Sci Technol. 2018;75:129–45. [Google Scholar]
- 75. Suppakul P, Miltz J, Sonneveld K, Bigger SW. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J Food Sci. 2003;68:408–20. [Google Scholar]
- 76. Ahmed I, Lin H, Zou L, Brody AL, Li Z, Qazi IM, et al. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control. 2017;82:163–78. [Google Scholar]
- 77. Arfat YA, Ahmed J, Hiremath N, Auras R, Joseph A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocolloids. 2017;62:191–202. [Google Scholar]
- 78. Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. 2013;15:e942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pathakoti K, Morrow S, Han C, Pelaez M, He X, Dionysiou DD, et al. Photo-inactivation of Escherichia coli by sulfur and nitrogen-fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ Sci Technol. 2013;47:9988–96. doi: 10.1021/es401010g. [DOI] [PubMed] [Google Scholar]
- 80. He X, Aker WG, Huang MJ, Watts JD, Hwang HM. Metal oxide nanomaterials in nanomedicine: applications in photodynamic therapy and potential toxicity. Curr Top Med Chem. 2015;15:1887–900. doi: 10.2174/1568026615666150506145251. [DOI] [PubMed] [Google Scholar]
- 81. Pool H, Quintanar D, de Dios Figueroa J, Mano CM, Bechara JEH, Godinez LA, et al. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater. 2012;2012:e145380. [Google Scholar]
- 82. Rojas-Grau MA, Soliva-Fortuny R, Martin-Belloso O. Edible coatings to incorporate active ingredients to fresh cut fruits: a review. Trends Food Sci Technol. 2009;20:438–47. [Google Scholar]
- 83. Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45(5):1977–83. doi: 10.1021/es102624t. [DOI] [PubMed] [Google Scholar]
- 84. Inbaraj BS, Chen BH. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24:15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Momin JK, Jayakumar C, Prajapati JB. Potential of nanotechnology in functional foods. Emir J Food Agric. 2013;25:10–9. [Google Scholar]
- 86. Liang J, Yan H, Wang X, Zhou Y, Gao X, Puligundla P, et al. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017;231:19–24. doi: 10.1016/j.foodchem.2017.02.106. [DOI] [PubMed] [Google Scholar]
- 87. Youssef AM. Polymer nanocomposites as a new trend for packaging applications. Polym Plast Technol Eng. 2013;52:635–60. [Google Scholar]
- 88. Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta JM, Dubois P. New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng R Rep. 2009;63:100–25. [Google Scholar]
- 89. Lizundia E, Ruiz-Rubio L, Vilas JL, Leon LM. Poly (l-lactide)/ ZnO nanocomposites as efficient UV-shielding coatings for packaging applications. J Appl Polym Sci. 2016;133:e42426. [Google Scholar]
- 90.USFDA; Office USGP, editor. Code of federal regulations title 21dfood and drugs. Washington, DC: U.S. FDA; 2002. [Accessed 18, 07, 16]. Listing of color additives exempt from certification. [Google Scholar]
- 91.USFDA. Color additive status list. 2015. [Accessed 18, 07, 16]. http://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.html .
- 92. Mehrad B, Ravanfar R, Licker J, Regenstein JM, Abbaspourrad A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res Int. 2018;105:962–9. doi: 10.1016/j.foodres.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 93. Pal M. Nanotechnology: a new approach in food packaging. J Food – Microbiol Safety Hyg. 2017;2:121. [Google Scholar]
- 94. Wesley SJ, Raja P, Sunder Raj AA, Tiroutchelvamae D. Review on nanotechnology applications in food packaging and safety. Int J Eng Res. 2014;3:645–51. [Google Scholar]
- 96. Chandra P, Noh HB, Won MS, Shim YB. Detection of daunomycin using phosphatidylserine and aptamer co-immobilized on Au nanoparticles deposited conducting polymer. Biosens Bioelectron. 2011;26(11):4442–9. doi: 10.1016/j.bios.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 97. Thakur MS, Ragavan KV. Biosensors in food processing. J Food Sci Technol. 2013;50(4):625–41. doi: 10.1007/s13197-012-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li YS, Church JS. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J Food Drug Anal. 2014;22(1):28–48. doi: 10.1016/j.jfda.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jarvis RM, Goodacre R. Discrimination of bacteria using surface enhanced Raman spectroscopy. Anal Chem. 2004;76:40–7. doi: 10.1021/ac034689c. [DOI] [PubMed] [Google Scholar]
- 100. Kahraman M, Yazici MM, Sahin F, Culha M. Convective assembly of bacteria for surface-enhanced Raman scattering. Langmuir. 2008;24:894–901. doi: 10.1021/la702240q. [DOI] [PubMed] [Google Scholar]
- 101. Baranwal A, Mahato K, Srivastava A, Maurya PK, Chandra P. Phytofabricated metallic nanoparticles and their clinical applications. RSC Adv. 2016;6(107):105996–6010. [Google Scholar]
- 102. Zuo P, Li X, Dominguez DC, Ye BC. A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip. 2013;13:3921–8. doi: 10.1039/c3lc50654a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Holzinger M, Le Goff A, Cosnier S. Nanomaterials for bio sensing applications: a review. Front Chem. 2014;2:e63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang M, Peng Z, Ning Y, Chen Y, Zhou Q, Deng L. Highly specific and cost-efficient detection of Salmonella paratyphi combining aptamers with single-walled carbon nanotubes. Sensors. 2013;13:6865–81. doi: 10.3390/s130506865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bhattacharya S, Jang J, Yang L, Akin D, Bashir R. Biomems and nanotechnology based approaches for rapid detection of biological entities. J Rapid Meth Autom Microbiol. 2007;15:1–32. [Google Scholar]
- 106. Chen CS, Durst RA. Simultaneous detection of Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes with an array-based immunosorbent assay using universal protein G-liposomal nanovesicles. Talanta. 2006;69:232–8. doi: 10.1016/j.talanta.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 107. DeCory TR, Durst RA, Zimmerman SJ, Garringer LA, Paluca G, DeCory HH, et al. Development of an immunomagnetic bead immunoliposome fluorescence assay for rapid detection of Escherichia coli O157:H7 in aqueous samples and comparison of the assay with a standard microbiological method. Appl Environ Microbiol. 2005;71:1856–64. doi: 10.1128/AEM.71.4.1856-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shukla S, Lee G, Song X, Park S, Kim M. Immunoliposome-based immunomagnetic concentration and separation assay for rapid detection of Cronobacter sakazakii. Biosens Bioelectron. 2016;77:986–94. doi: 10.1016/j.bios.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 109. Shukla S, Leem H, Kim M. Development of a liposome-based immunochromatographic strip assay for the detection of Salmonella. Anal Bioanal Chem. 2011;401:2581–90. doi: 10.1007/s00216-011-5327-2. [DOI] [PubMed] [Google Scholar]
- 110. Jain KK. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn. 2003;3(2):153–61. doi: 10.1586/14737159.3.2.153. [DOI] [PubMed] [Google Scholar]
- 111. Tominaga T. Rapid detection of Klebsiella pneumoniae, Klebsiella oxytoca, Raoultella ornithinolytica and other related bacteria in food by lateral-flow test strip immunoassays. J Microbiol Meth. 2018;147:43–9. doi: 10.1016/j.mimet.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 112.Thakur B, Zhou G, Chang J, Pu H, Jin B, Sui X, et al. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens Bioelectron. 2018. [DOI] [PubMed]
- 113. Shukla S, Haldorai Y, Bajpai VK, Rengaraj A, Hwang SK, Song X, et al. Electrochemical coupled immunosensing platform based on graphene oxide/gold nanocomposite for sensitive detection of Cronobacter sakazakii in powdered infant formula. Biosens Bioelectron. 2018;30:139–49. doi: 10.1016/j.bios.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 114. Song X, Shukla S, Kim M. Detection of Cronobacter species in powdered infant formula using immunoliposome-based immunomagnetic concentration and separation assay. Food Microbiol. 2018;72:23–30. doi: 10.1016/j.fm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 115. Oh SY, Heo NS, Shukla S, Cho HY, Ezhil Vilian AT, Kim J, et al. Development of gold nanoparticle aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci Rep. 2017;7:e10130. doi: 10.1038/s41598-017-10188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kumar S, Dilbaghi N, Barnela M, Bhanjana G, Kumar R. Biosensors as novel platforms for detection of food pathogens and allergens. BioNano Sci. 2012;2:196–217. [Google Scholar]
- 117. Pilolli R, Monaci L, Visconti A. Advances in biosensor development based on integrating nanotechnology and applied to food-allergen management. Trends Anal Chem. 2013;47:12–26. [Google Scholar]
- 118. Yoshida T, Yoshioka Y, Fujimura M, Yamashita K, Higashisaka K, Morishita Y, et al. Promotion of allergic immune responses by intranasal-administrated nanosilica particles in mice. Nanoscale Res Lett. 2011;6:1–6. doi: 10.1186/1556-276X-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vogelbruch M, Nuss B, Korner M, Kapp A, Kiehl P, Bohm W. Aluminium induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy. 2000;55:883–7. [PubMed] [Google Scholar]
- 120. De Souza Reboucas J, Esparza I, Ferrer M, Sanz ML, Irache JM, Gamazo C. Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J Biomed Biotechnol. 2012:e474605. doi: 10.1155/2012/474605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hedayat M, Takeda K, Rezaei N. Prophylactic and therapeutic implications of toll-like receptor ligands. Med Res Rev. 2012;32:294–325. doi: 10.1002/med.20214. [DOI] [PubMed] [Google Scholar]
- 122. Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37–48. doi: 10.1016/j.tmrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 123. Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Therapeut. 2011;13(S1):S5–14. doi: 10.1089/dia.2011.0068. [DOI] [PubMed] [Google Scholar]
- 124. Pali-Scholl I, Szollosi H, Starkl P, Scheicher B, Stremnitzer C, Hofmeister A. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur J Pharm Biopharm. 2013;85:656–64. doi: 10.1016/j.ejpb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 125. Gamazo C, Gastaminza G, Ferrer M, Sanz ML, Irache JM. Nanoparticle based-immunotherapy against allergy. Immunotherapy. 2014;6(7):885–97. doi: 10.2217/imt.14.63. [DOI] [PubMed] [Google Scholar]
- 126. Prasad A, Mahato K, Chandra P, Srivastava A, Joshi SN, Maurya PK. Bioinspired composite materials: applications in diagnostics and therapeutics. J Mol Eng Mater. 2016;4:e1640004. [Google Scholar]
- 127. Lee B, Park JH, Byun JY, Kim JH, Kim MG. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens Bioelectron. 2018;102:504–9. doi: 10.1016/j.bios.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 128. Zhang Y, Wu Q, Sun M, Zhang J, Mo S, Wang J, et al. Magnetic-assisted aptamer-based fluorescent assay for allergen detection in food matrix. Sensor Actuator B Chem. 2018;263:43–9. [Google Scholar]
- 129. Brotons-Cantoa A, Gamazo C, Martín-Arbella N, Abdulkarim M, Matías J, Gumbleton M, et al. Evaluation of nanoparticles as oral vehicles for immunotherapy against experimental peanut allergy. Int J Biol Macromol. 2018;110:328–35. doi: 10.1016/j.ijbiomac.2017.09.109. [DOI] [PubMed] [Google Scholar]
- 130. Di Felice G, Barletta B, Bonura A, Butteroni C, Corinti S, Colombo P. Nanoparticles adjuvants in allergology: new challenges and pitfalls. Curr Pharmaceut Des. 2015;21:4229–39. doi: 10.2174/1381612821666150901102822. [DOI] [PubMed] [Google Scholar]
- 131. Di Felice G, Colombo P. Nanoparticle–allergen complexes for allergen immunotherapy. Int J Nanomed. 2017;12:4493–504. doi: 10.2147/IJN.S134630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fukui H, Horie M, Endoh S, Kato H, Fujita K, Nishio K. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem Biol Interact. 2012;198:29–37. doi: 10.1016/j.cbi.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 133. McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Karlsson HL, Cronholm P, Hedberg Y, Tornberg M, Battice LD, Svedhem S, et al. Cell membrane damage and protein interaction induced by copper containing nanoparticles-importance of the metal release process. Toxicology. 2013;313:59–69. doi: 10.1016/j.tox.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 135. Karatapanis AE, Fiamegos Y, Stalikas CD. Silica-modified magnetic nanoparticles functionalized with cetylpyridinium bromide for the preconcentration of metals after complexation with 8-hydroxy-quinoline. Talanta. 2011;84:834–9. doi: 10.1016/j.talanta.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 136. Amin MT, Alazba AA, Manzoor U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng. 2014:1–24. [Google Scholar]
- 137. Zhang L, Luo J, Menkhaus TJ, Varadaraju H, Sun Y, Fong H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J Membr Sci. 2011;369:499–505. [Google Scholar]
- 138. Lin S, Xu M, Zhang W, Hu X, Lin K. Quantitative effects of amination degree on the magnetic iron oxide nanoparticles (MIONPs) using as adsorbents to remove aqueous heavy metal ions. J Hazard Mater. 2017;335:47–55. doi: 10.1016/j.jhazmat.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 139. Cai Y, Li C, Wu D, Wang W, Tan F, Wang X, et al. Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. Chem Eng J. 2017;312:158–66. [Google Scholar]
- 140. Lingamdinne LP, Chang YY, Yang JK, Singh J, Choi EH, Shiratani M, et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem Eng J. 2017;307:74–84. [Google Scholar]
- 141. Simpson A, Pandey RR, Chusuei CC, Ghosh K, Patel R, Wanekaya AK. Fabrication characterization and potential applications of carbon nanoparticles in the detection of heavy metal ions in aqueous media. Carbon. 2018;127:122–30. [Google Scholar]
- 142. Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23. doi: 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 143. Shakeri S, Kermanshahi R, Moghaddam M, Emtiazi G. Assessment of biofilm cell removal and killing and biocide efficacy using the microtiter plate test. Biofouling. 2007;23:79–86. doi: 10.1080/08927010701190011. [DOI] [PubMed] [Google Scholar]
- 144. Vetter SM, Schlievert PM. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis. Antimicrob Agents Chemother. 2005;49:1302–5. doi: 10.1128/AAC.49.4.1302-1305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schlievert PM, Peterson ML. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One. 2012;7:e40350. doi: 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shahrokh S, Emtiazi G. Toxicity and unusual biological behavior of nanosilver on Gram positive and negative bacteria assayed by microtiter-plate. Eur J Biol Sci. 2009;1:28–31. [Google Scholar]
- 147. Saleem S, Ahmed B, Khan MS, Al-Shaeri S, Musarrat J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb Pathog. 2017;111:375–87. doi: 10.1016/j.micpath.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 148. Gambino M, Ali Ahmed MA, Villa F, Cappitelli F. Zinc oxide nanoparticles hinder fungal biofilm development in an ancient Egyptian tomb. Int J Biodeterior Biodegrad. 2017;122:92–9. [Google Scholar]
- 149. Ahmed A, Khan AK, Anwar A, Ali SA, Shah MR. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumonia. Microb Pathog. 2016;98:50–6. doi: 10.1016/j.micpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 150. Ranmadugala D, Ebrahiminezhad A, Manley-Harris M, Ghasemid Y, Berenjian A. The effect of iron oxide nanoparticles on Bacillus subtilis biofilm, growth and viability. Process Biochem. 2017;62:231–40. [Google Scholar]
- 151. Thuptimdang P, Limpiyakorn T, Khan E. Dependence of toxicity of silver nanoparticles on Pseudomonas putida biofilm structure. Chemosphere. 2017;188:199–207. doi: 10.1016/j.chemosphere.2017.08.147. [DOI] [PubMed] [Google Scholar]
- 156. King T, Cole M, Farber JM, Eisenbrand G, Zabaras D, Fox EM, et al. Food safety for food security: relationship between global megatrends and developments in food safety. Trends Food Sci Technol. 2017;68:160–75. [Google Scholar]
- 157.Wiedmann M.Can big data revolutionize food safety? Food quality and safety. 2015. Retrieved from: http://www.foodqualityandsafety.com/article/can-big-datarevolutionize-food-safety/
- 158.WHO – World Health Organization. FOSCOLLAB. Global platform for food safety data and information. 2015. http://www.who.int/foodsafety/foscollab_dashboards/en/
- 159. Fu J, Park B, Siragusa G, Jones L, Tripp R, Zhao Y. An Au/Si hetero-nanorod-based biosensor for Salmonella detection. Nanotechnology. 2008;15:e155502. doi: 10.1088/0957-4484/19/15/155502. [DOI] [PubMed] [Google Scholar]
- 160. Hahn MA, Keng PC, Krauss TD. Flow cytometric analysis to detect pathogens in bacterial cell mixtures using semiconductor quantum dots. Anal Chem. 2008;3:864–72. doi: 10.1021/ac7018365. [DOI] [PubMed] [Google Scholar]
- 161. Yang L, Li Y. Quantum dots as fluorescent labels for quantitative detection of S. typhimurium in chicken carcass wash water. J Food Protect. 2005;6:1241–5. doi: 10.4315/0362-028x-68.6.1241. [DOI] [PubMed] [Google Scholar]
- 162. Tully E, Hearty S, Leonard P, O'Kennedy R. The development of rapid fluorescence-based immunoassays, using quantum dot-labelled antibodies for the detection of L. monocytogenes cell surface proteins. Int J Biol Macromol. 2006;1–3:127–34. doi: 10.1016/j.ijbiomac.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 163. Wang C, Irudayaraj J. Gold nanorod probes for the detection of multiple pathogens. Small. 2008;12:2204–8. doi: 10.1002/smll.200800309. [DOI] [PubMed] [Google Scholar]
- 164. Su SL, Li Y. Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157:H7. Anal Chem. 2004;76(16):4806–10. doi: 10.1021/ac049442+. [DOI] [PubMed] [Google Scholar]
- 165. An J, Zhang M, Wang S, Tang J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT – Food Sci Technol. 2008;41(6):1100–7. [Google Scholar]
- 166. Fernandez A, Soriano E, Lopez-Carballo G, Picouet P, Lloret E, Gavara R. Preservation of aseptic conditions in absorbent pads by using silver nanotechnology. Food Res Int. 2009;42(8):1105–12. [Google Scholar]
- 167. Fernandez A, Picouet P, Lloret E. Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. Int J Food Microbiol. 2010a;142(1–2):222–8. doi: 10.1016/j.ijfoodmicro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 168. Fernandez A, Picouet P, Lloret E. Reduction of the spoilage-related microflora in absorbent pads by silver nanotechnology during modified atmosphere packaging of beef meat. J Food Protect. 2010b;73(12):2263–9. doi: 10.4315/0362-028x-73.12.2263. [DOI] [PubMed] [Google Scholar]
- 169. Emamifar A, Kadivar M, Shahedi M, Soleimanian-Zad S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innovat Food Sci Emerg Technol. 2010;11(4):742–8. [Google Scholar]
- 170. Emamifar A, Kadivar M, Shahedi M, Soleimanian-Zad S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control. 2011;22(3–4):408–13. [Google Scholar]
- 171. Jin T, Gurtler JB. Inactivation of Salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. J Appl Microbiol. 2011;110(3):704–12. doi: 10.1111/j.1365-2672.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 172. Li H, Li F, Wang L, Sheng J, Xin Z, Zhao L, et al. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd) Food Chem. 2009;114(2):547–52. [Google Scholar]
- 173. Zhou L, Lv S, He G, He Q, Shi BI. Effect of PE/AG2O nano-packaging on the quality of apple slices. J Food Qual. 2011;34(3):171–6. [Google Scholar]
- 174. Vikesland PJ, Wigginton KR. Nanomaterial enabled biosensors for pathogen monitoring – a review. Environ Sci Technol. 2010;10:3656–69. doi: 10.1021/es903704z. [DOI] [PubMed] [Google Scholar]
- 175. Dungchai W, Siangproh W, Chaicumpa W, Tongtawe P, Chailapakul O. S. typhi determination using voltammetric amplification of nanoparticles: a highly sensitive strategy for metalloimmunoassay based on a copper-enhanced gold label. Talanta. 2008;2:727–32. [Google Scholar]
- 176. Yang L, Li Y. Quantum dot bioconjugates for simultaneous detection of Escherichia coli O157:H7 and Salmonella Typhimurium. Analyst. 2006;131:394–401. doi: 10.1039/b510888h. [DOI] [PubMed] [Google Scholar]
- 177. Zhao X, Hilliard L, Mechery S, Wang Y, Bagwe R, Jin S. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci USA. 2004;42:15027–32. doi: 10.1073/pnas.0404806101. [DOI] [PMC free article] [PubMed] [Google Scholar]