Abstract
Phyllanthus emblica L. fruit contains abundant bioactive components and exhibits a variety of biological activities. In this study, the hepatoprotective effect of water extract of P. emblica (WEPE) on nonalcoholic steatohepatitis (NASH) was evaluated. C57BL/6 mice were fed methionine and choline-deficiency diet (MCD diet) for 4 or 8 weeks to induce NASH. Results showed that administration of WEPE could significantly reduce serum AST and ALT as compared to MCD diet-alone group. Administration of WEPE could significantly decrease lipid peroxidation and CYP2E1 mRNA expression, and elevate the antioxidant activities in mice livers. In addition, administration of WEPE after 8 weeks could significantly decrease the mRNA expressions of TNF-α and IL-1β in mice livers, but have less improving effect of hepatic steatosis and mononuclear cell infiltration. Taken together, MCD diet might cause serious hepatic steatosis and mild inflammation in mice livers, but administration of WEPE could ameliorate the rapid progression of NASH.
Keywords: Oxidative stress, Lipid peroxidation, Nonalcoholic steatohepatitis, Methionine and choline-deficiency diet, Phyllanthus emblica
1. Introduction
Nonalcoholic steatohepatitis (NASH) related with histopathologic abnormalities including steatosis with inflammation and fibrosis is recognized as a common liver injury [1]. The two-hit hypothesis is a vital theoretical framework for understanding the pathophysiology of nonalcoholic fatty liver disease (NAFLD) and NASH. The ‘first hit’, an excessive accumulation of fat in the liver, increases susceptibility of the liver injury mediated by ‘second hits’, such as oxidative stress, lipid peroxidation, and pro-inflammatory cytokines, which in turn lead to steatohepatitis and/or fibrosis [2]. Free radical damage is vital for in the pathogenesis of NASH [3–6], therefore, the improvement of the oxidant status may be a benefit on NASH pathology [5–8]. The MCD diet is extremely effective as the main model for developing and studying NASH [9]. The MCD diet-induced NASH model has been used to induce oxidative stress and TNF-α that symptoms are resemble to the human NASH [10].
Phyllanthus emblica L. exhibited anti-oxidative, hypolipidemic, hypoglycemic, anti-microbial, anti-tumor, anti-inflammatory, and hepatoprotective activities [2]. Previous studies showed that the fruit of P. emblica contain tannins, lignans, flavonoids, alkaloids, vitamin C, mucic acid, gallic acid, and ellagic acid [2,11,12]. Numerous reports have revealed that flavonoids, vitamin C, gallic acid, and ellagic acid exert hepatoprotective effects, which might be related to their anti-inflammatory and antioxidant properties [2,13–15]. Huang et al. [16] showed that water extract of P. emblica fruit (WEPE) could significantly decrease the adipose tissue weights of peritoneal fat and epididymal fat, enhance the antioxidant enzyme activities, and improve steatosis in the liver of high fat diet (HFD) fed rats. However, to the best of our knowledge, there is no prior report on the protective effects of P. emblica fruit on MCD diet-induced systemic liver injury. Thus, the aim of this study was to investigate the anti-NASH effect of WEPE.
2. Materials and methods
2.1. Chemicals and reagents
NaN3, NADPH, GSH reductase, 1-chloro-2,4-dintrobenzene, GSSG, KCl, H2O2, Triton™ X-100, 1-bromo-3-chloropropane, and TRI Reagent® were obtained from Sigma–Aldrich (St. Louis, MO, USA). Chloroform was obtained from Thermo Fisher Scientific (New Jersey, USA). Methanol, isopropanol, and acetic acid were obtained from Echo Chemical (Miaoli, Taiwan). NaOH was obtained from Merck (Darmstadt, Germany). Triglycerides assay kit was obtained from Fortress Diagnostic (Northern Ireland, UK). Al(NO3)3·9H2O was obtained from Ferak Berlin (Berlin, Germany). Bovine serum albumin (BSA), Bio-Rad protein dye, and iScript™Reverse Transcription Supermix for RT-qPCR were obtained from Bio-Rad (Hercules, CA, USA). KH2PO4, K2HPO4, NaH2PO4·H2O, and Na2HPO4 were obtained from Avantor (PA, USA). 24% Formaldehyde, diethyl ether, and Na2CO3 were obtained from Union Chemical (Hsinchu, Taiwan). EDTA and MgCl2·6H2O were obtained from Showa (Tokyo, Japan). Phosphoric acid and NaCl were obtained from Wako (Tokyo, Japan). UltraPure™ DEPC-Treated Water was obtained from Invitrogen (Carlsbad, CA, USA). 50X TAE Buffer, 10X Marker Ready-Run Buffer, 2X PCR Dye Master Mix, and HealthView nucleic acid stain were obtained from Faith Biotechnology (Taipei, Taiwan). Glutathione peroxidase Assay Kit, Glutathione reductase Assay Kit, Glutathione S-transferase Assay Kit, and Superoxide Dismutase Assay Kit were obtained from Cayman (Michigan, USA).
2.2. Preparation of WEPE
P. emblica fruit was generously supplied by the Miaoli District Agricultural Research and Extension Station, Council of Agricultural, Executive Yuan (Miaoli, Taiwan). The dry powder of P. emblica fruit was extracted with reverse osmosis (RO) water on a rotary shaker (120 rpm) for 24 h at room temperature. The extract was filtered through Whatman qualitative filter paper No. 1 and then was vacuum evaporated to dryness on a rotary evaporator (waterbath temperature below 40 °C). All dried extracts were stored at −20 °C before use. The extract source is the same with our previous study [2].
2.3. Animal treatment
Male C57BL/6JNarl mice with specific pathogen-free conditions were purchased from BioLASCO (A Charles River Licensee Corp., Yi-Lan, Taiwan). The experimental animals were given 1 week to acclimatize to the environment and diet. The experimental design was depicted as in Fig. 1. The experimental animals were given 2 weeks to acclimatize to the environment and diet. 5-week-old male C57BL/6JNarl mice (20 ± 2 g) were used for the experiments. The mice were given with food and water ad libitum, and divided into six groups (n = 8 mice/group). To study the effect of WEPE on NASH, mice were fed methionine and choline-deficiency diet (MCD diet) to induce NASH. At the same time, 100 mg/kg BW of gallic acid (GA) or 125 (low dose of WEPE, L-WEPE), 250 (medium dose of WEPE, M-WEPE) and 500 (high dose of WEPE, H-WEPE) mg/kg BW of WEPE were given daily by oral gavage to the mice for 4 or 8 weeks to evaluate their improving effects on NASH. Previous studies have indicated that 125 mg/kg BW of WEPE improves high fat diet-induced nonalcoholic fatty liver disease(NAFLD)in SD rats[16]. Therefore, the dosage of 100, 125, and 250 mg/kg BW was chosen for WEPE treatment of mice in this study. The mice were fed a standard chow diet (Lab diet 5001) as the normal control group and MCD diet for 4 or 8 weeks as the MCD diet-induced group. At the end of the experiments, mice were sacrificed using isoflurane anesthesia, and then liver were collected, weighted and rinsed extensively in cold phosphate-buffered saline (PBS). All animal experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of National Chung Hsing University, Taichung, Taiwan, and the study conformed to the guidelines of the protocol IACUC Approval No: 102-87 approved by the IACUC ethics committee.
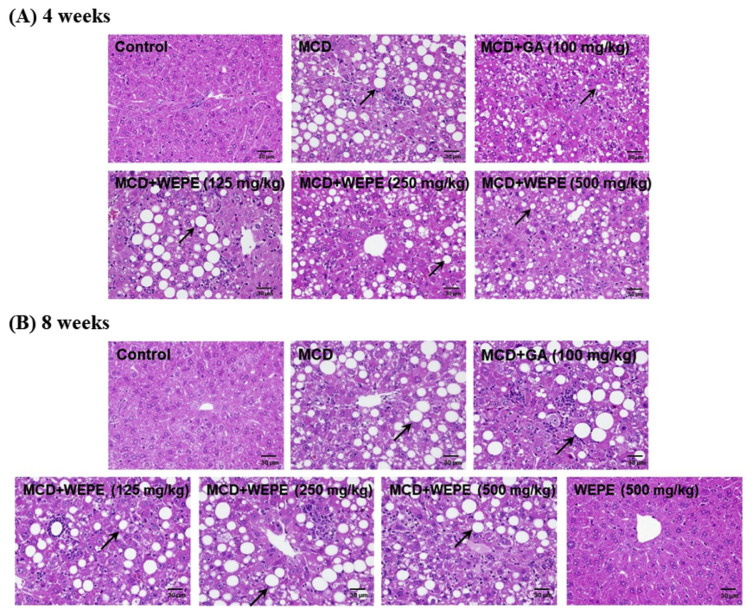
Fig. 1.
Effects of WEPE and GA on hepatosteatosis of C57BL/6 mice fed by MCD diet after (A) 4 or (B) 8 weeks. Liver specimens were stained with hematoxylin and eosin (H&E), 400X. Black arrows showed the fat accumulation in the liver.
2.4. Measurement of biochemical variables
Blood samples were collected and serum was separated by centrifugation at 4 °C and stored at −20 °C for biochemical analysis. Serum aspartate transaminase (AST), alanine aminotransferase (ALT), cholesterol (CHOL), and triglycerides (TG) were analyzed using ADVIA 1800 (Siemens, USA).
2.5. Histological examination
The left liver was immersed in 10% neutral buffered formalin for a week, embedded in paraffin, and sectioned (5 μm thickness) before staining with hematoxylin-eosin (HE). The histological analyses were conducted by a light microscope attached to an image-analysis system (Image-Pro Plus; Media Cybernetics). The technique was analyzed as described previously [16].
2.6. Analysis of total protein concentration
In order to express the antioxidant enzyme activities as U per milligram of protein or nanomoles per minute per milligram of protein, total protein concentration of liver tissue was determined colorimetrically using a commercial protein reagent kit (Bio-rad, Hercules, CA, USA). The total protein concentration was analyzed as described previously [16].
2.7. Measurement of antioxidant enzymes
SOD activity was assayed using a SOD assay kit-WST (Dojindo Molecular Technologies Inc., Gaithersburg, MD, USA) as specified by the manufacturer. The GST activity was measured using a commercial kit (Cayman Chemical Co., Ann Arbor, MI, USA). The activities of catalase, GSH peroxidase (GPx), and glutathione reductase (GRd) antioxidant enzymes were assayed according to previously reported methods [13,17].
2.8. Measurement of liver TBARS
The thiobarbituric acid reactive substances (TBARS) of livers were measured using a commercial kit for TBARS (Cayman, Michigan, USA). The absorbance change at 535 nm was recorded and the amount of TBARS was expressed as malondialdehyde (MDA) equivalents as micromoles MDA per milligram of protein. The liver TBARS was measured according to previously reported methods [18].
2.9. RNA extraction and RT-PCR
Liver tissues were washed three times with PBS, and Trizol (Invitrogen) was used to extract the intracellular RNA. Next, iScript™ Reverse Transcription Supermix for RT-qPCR kit (Bio-Rad, Hercules, CA, USA) was used to translate RNA into cDNA. The primers used to amplify CYP2E1, TNF-α, IL-1β, and 18S rRNA were as follows: CYP2E1, forward: GTGTCTGGAGCT-CATGAGTTTGTTC, reverse: GGCAGTTGATGTCCAGTGACT-TAAG; TNF-α, forward: GGCAGGTCTACTTTGGAGTCATTGC, reverse: CTGAGTAGTTGTTGAAAGCTCTGAG; IL-1β, forward: GCAGCAGCACATCAACAAGAGCTTC, reverse: GAACGTCACA-CACCAGCAGGTTATC; 18S rRNA, forward: GTAACCCGTT-GAACCCCATT, reverse: CCATCCAATCGGTAGTAGCG. The PCR products were electrophoresed on a 1.5% agarose gel and the cDNA of 18S rRNA was used as an internal control. The RT-PCT method was analyzed as described previously [16].
2.10. Statistical analysis
The data were presented as mean ± SD. An ANOVA was used to evaluate differences between multiple groups with Dun-can’s test. P value < 0.05 was considered statistically significant.
3. Results
3.1. Effects of WEPE and GA on dietary intake, body weight, and tissue weights in MCD diet-induced NASH mice
P. emblica fruit contains abundant natural bioactive components including tannins, lignans, flavonoids, alkaloids, vitamin C, gallic acid, mucic acid, and ellagic acid [2,11,12]. The content of gallic acid in WEPL was 1.8 mg/g of extract, and thus it is a major component of P. emblica fruit [2]. As a major component, gallic acid (GA) has a number of phytomedicines with diverse biological and pharmacological activities, including antioxidant, antimutagenic, anticarcinogenic, hepatoprotective, anti-obesity, and anti-diabetic activity [13,16]. In this study, GA is not only the major component of WEPE, but also the well-known hepatoprotective compound (the positive control of this study). Thus, this study was to investigate whether WEPE and its major component, GA, could improve NASH using a MCD diet-induced NASH model. All of the experimental groups fed with MCD diet, including treatments with H2O, GA, L-WEPE, M-WEPE, and H-WEPE had no significant differences on body weight and food intake for both 4 and 8 weeks. Although the experimental groups significantly decreased body weight and food intake compared with the normal control group (p < 0.05). However, treatments with GA, L-WEPE, M-WEPE, and H-WEPE didn’t improve the body weight and food intake when compared with the MCD diet-alone group (data not shown).
3.2. Effects of WEPE and GA on hepatic damage levels in MCD diet-induced NASH mice
Serum aminotransferase activities have long been considered effective indicators of hepatic injury. The protective effects of GA and WEPE on serum AST and ALT activities in MCD diet-induced NASH mice are presented in Table 1. The serum AST and ALT activities of the MCD diet-induced NASH mice after 4 weeks were dramatically elevated to 386.83 and 620.67 U/L, while these values were 92.17 and 41.33 U/L, respectively, in the normal control group. However, the groups treated with GA, L-WEPE, M-WEPE, and H-WEPE significantly decreased serum AST and ALT activities (p < 0.05), with the values of 283.20 and 454.23 U/L, 235.33 and 322.83 U/L, 292.83 and 415.67 U/L, and 239.00 and 293.83 U/L, respectively. In addition, there were no significant differences among the groups treated with GA, L-WEPE, M-WEPE, and H-WEPE. Furthermore, the results of serum AST and ALT activities on WEPE and GA in the MCD diet-induced NASH mice after 8 weeks were similar with the protective effects on the MCD diet-induced NASH mice after 4 weeks. Thus, whatever for 4 or 8 weeks, MCD diet clearly increased serum AST and ALT compared with the normal control group (p < 0.05). However, serum AST and ALT of MCD + GA, MCD + L-WEPE, MCD + M-WEPE, and MCD + H-WEPE groups in mice fed by MCD diet after 4 or 8 weeks were significantly decreased compared with the MCD diet-alone group (p < 0.05). Consistent with serum AST and ALT, the histological examination of the mice fed by MCD diet after 4 or 8 weeks showed severe hepatic steatosis and mononuclear cell infiltration, while the normal control group had histologically normal livers. However, WEPE and GA treatments could slightly reduce the hepatic damage (Fig. 1).
Table 1.
Effects of WEPE and GA on serum biochemical values of C57BL/6 mice fed by MCD diet after 4 or 8 weeks.
| Control | MCD | MCD + GA | MCD + L-WEPE | MCD + M-WEPE | MCD + H-WEPE | Control + H-WEPE | ||
|---|---|---|---|---|---|---|---|---|
| 4 week | AST (U/L) | 92.2 ± 45.6c | 386.8 ± 127.9a | 283.2 ± 106.0b | 235.3 ± 53.8b | 292.8 ± 59.4b | 239.0 ± 49.4b | |
| ALT (U/L) | 41.3 ± 15.2c | 620.7 ± 199.8a | 454.2 ± 170.3b | 322.8 ± 90.4b | 415.7 ± 66.0b | 293.8 ± 74.1b | ||
| CHOL (mg/dL) | 91.5 ± 4.3a | 29.7 ± 5.4c | 30.0 ± 5.1c | 33.5 ± 6.8bc | 31.5 ± 2.7bc | 37.7 ± 6.5b | ||
| TG (mg/dL) | 78.7 ± 8.1a | 45.8 ± 6.1b | 47.2 ± 5.2b | 41.0 ± 8.1b | 40.8 ± 9.1b | 42.7 ± 5.0b | ||
| 8 week | AST (U/L) | 96.8 ± 28.9de | 521.7 ± 335.6a | 443.3 ± 18.9ab | 285.2 ± 73.7bc | 300.7 ± 90.2bc | 231.8 ± 87.6cd | 56.8 ± 14.8e |
| ALT (U/L) | 39.0 ± 7.8c | 760.7 ± 368.8a | 542.8 ± 121.1b | 386.2 ± 129.8b | 495.0 ± 223.6b | 347.7 ± 140.9b | 36.7 ± 8.9c | |
| CHOL (mg/dL) | 74.3 ± 12.0a | 14.3 ± 5.7bc | 12.3 ± 3.7c | 15.2 ± 5.2bc | 15.8 ± 3.4bc | 22.0 ± 4.6b | 76.5 ± 2.7a | |
| TG (mg/dL) | 57.8 ± 6.9a | 42.0 ± 5.6c | 43.0 ± 6.1c | 50.8 ± 16.9ab | 46.5 ± 8.0ab | 47.3 ± 16.0ab | 40.7 ± 6.7c |
Each value was expressed as the mean ± SD (n = 8/group).
The letters indicated statistically significant differences between the groups (p < 0.05).
3.3. Effects of WEPE and GA on serum cholesterol and triglycerides levels in MCD diet-induced NASH mice
The effects of WEPE and GA on serum cholesterol and triglycerides in MCD diet-fed mice are shown in Table 1. The MCD diet fed to mice after 4 or 8 weeks markedly decreased serum cholesterol (a 67.6% or 80.7% decrease, respectively, compared with the normal control group, p < 0.05) and triglycerides (a 41.7% or 27.4% decrease, respectively, compared with the normal control group, p < 0.05). The results obtained herein are similar to those obtained by Hellerstein & Neese [19], who found that methionine and choline deprivation affected hepatic lipid metabolism through inhibiting triglyceride secretion. Moreover, H-WEPE group fed by MCD diet after 4 weeks significantly increased serum cholesterol compared with the MCD diet-alone group (p < 0.05). In addition, MCD + L-WEPE, MCD + M-WEPE, and MCD + H-WEPE groups after 8 weeks significantly increased serum triglycerides compared with the MCD diet-alone group (p < 0.05).
3.4. Effects of WEPE and GA on antioxidant enzymes in MCD diet-induced NASH mice
The activities of SOD, catalase, GPx, GRd, and GST in the livers of mice fed by MCD diet after 4 or 8 weeks are shown in Table 2. After 4 weeks, MCD diet caused significant reduction of SOD (0.58 U/mg of protein), catalase (320.36 nmol/min/mg protein), GPx (5.42 nmol/min/mg protein), and GST (6.77 nmol/min/mg protein) activities when compared with those in the normal control group, showing values of 1.74 U/mg of protein, 590.90 nmol/min/mg protein, 13.50 nmol/min/mg protein, and 12.67 nmol/min/mg protein, respectively (p < 0.05). Enhancement of catalase activity was observed in the GA group (364.32 nmol/min/mg protein) compared with the mice fed by MCD diet-alone after 4 weeks (320.36 nmol/min/mg protein) (p < 0.05). Enhancement of GPx activity was observed in the group treated with H-WEPE compared with the mice fed by MCD diet-alone after 4 weeks (p < 0.05). Except for L-WEPE group, the groups treated with GA (9.21 nmol/min/mg protein), M-WEPE (9.13 nmol/min/mg protein), and H-WEPE (9.60 nmol/min/mg protein) significantly increased GST activity when compared with the mice fed by MCD diet-alone after 4 weeks (p < 0.05).
Table 2.
Effects of WEPE and GA on antioxidant enzymes in the livers of C57BL/6 mice fed by MCD diet after 4 or 8 weeks.
| U/mg protein | nmol/min/mg protein | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SOD | Catalase | GPx | GRd | GST | ||
| 4 week | Control | 1.74 ± 0.72a | 590.90 ± 59.33a | 13.50 ± 1.87a | 3.83 ± 2.14a | 12.67 ± 1.91a |
| MCD | 0.58 ± 0.16b | 320.36 ± 31.15cd | 5.42 ± 1.69c | 3.35 ± 0.77a | 6.77 ± 1.72c | |
| MCD + GA | 0.70 ± 0.13b | 364.32 ± 27.26b | 6.75 ± 1.56c | 5.61 ± 2.97a | 9.21 ± 1.57b | |
| MCD + L-WEPE | 0.53 ± 0.23b | 340.16 ± 21.74bc | 6.09 ± 1.91c | 4.34 ± 3.03a | 7.52 ± 2.02bc | |
| MCD + M-WEPE | 0.60 ± 0.08b | 296.05 ± 10.36d | 6.84 ± 0.85c | 6.76 ± 6.22a | 9.13 ± 2.00b | |
| MCD + H-WEPE | 0.77 ± 0.10b | 289.91 ± 32.66d | 9.05 ± 0.78b | 6.10 ± 4.41a | 9.60 ± 1.39b | |
| 8 week | Control | 0.34 ± 0.06a | 316.34 ± 50.89a | 11.33 ± 2.40a | 11.27 ± 6.95a | 11.04 ± 0.94ab |
| MCD | 0.10 ± 0.08c | 77.62 ± 25.64d | 11.25 ± 0.75a | 7.68 ± 2.62a | 8.60 ± 0.71c | |
| MCD + GA | 0.25 ± 0.12ab | 111.77 ± 26.42cd | 12.16 ± 1.63a | 11.55 ± 6.34a | 10.38 ± 1.30bc | |
| MCD + L-WEPE | 0.23 ± 0.09ab | 98.85 ± 37.70cd | 11.62 ± 1.83a | 7.94 ± 4.26a | 10.20 ± 1.67bc | |
| MCD + M-WEPE | 0.21 ± 0.04b | 123.04 ± 27.95cd | 11.30 ± 2.13a | 11.08 ± 4.83a | 8.78 ± 0.64c | |
| MCD + H-WEPE | 0.31 ± 0.11ab | 132.67 ± 44.72c | 13.34 ± 1.59a | 7.83 ± 4.65a | 9.19 ± 2.33bc | |
| Control + H-WEPE | 0.32 ± 0.12ab | 245.39 ± 52.45b | 12.40 ± 2.59a | 11.54 ± 7.02a | 12.56 ± 1.93a | |
Each value was expressed as the mean ± SD (n = 8/group).
The letters indicated statistically significant differences between the groups (p < 0.05).
After 8 weeks, MCD diet caused the significant reduction of SOD (0.10 U/mg of protein), catalase (77.62 nmol/min/mg protein), and GST (8.60 nmol/min/mg protein) activities when compared with those in the normal control group, showing values of 0.34 U/mg of protein, 316.34 nmol/min/mg protein, and 11.04 nmol/min/mg protein, respectively (p < 0.05). However, the groups treated with GA (0.25 U/mg of protein), L-WEPE (0.23 U/mg of protein), M-WEPE (0.21 U/mg of protein), and H-WEPE (0.31 U/mg of protein) significantly increased SOD activity when compared with the mice fed by MCD diet-alone after 8 weeks (p < 0.05). Enhancement of catalase activity was observed in the group treated with H-WEPE compared with the mice fed by MCD diet-alone after 8 weeks (p < 0.05). Consequently, these results suggested that oxidative stress induced by MCD diet was slightly blocked by the supplementation of GA or WEPE.
3.5. Effects of WEPE and GA on lipid peroxidation in MCD diet-induced NASH mice
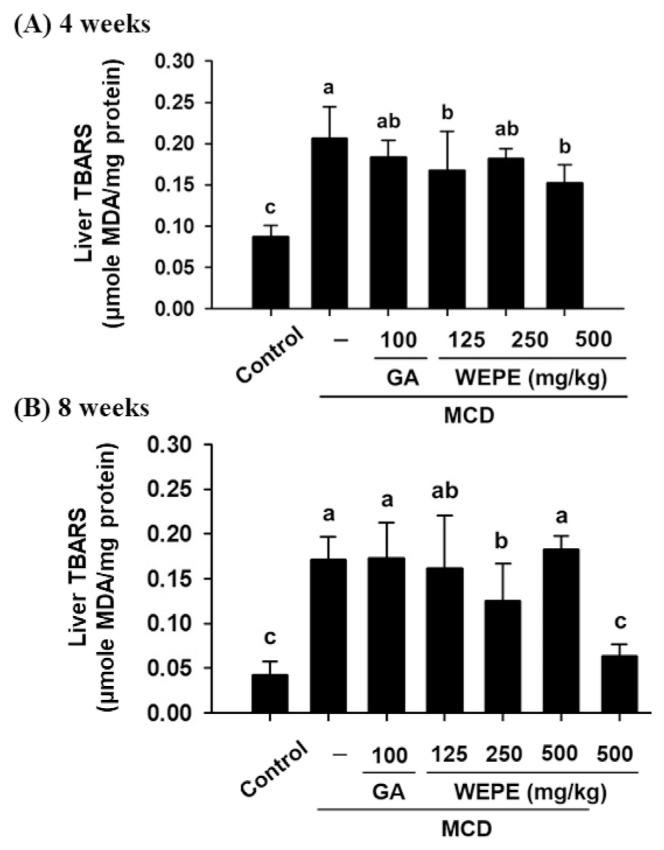
Fig. 2 shows the formation of TBARS in the livers of mice fed by MCD diet after 4 or 8 weeks. The content of TBARS was significantly increased by MCD diet after 4 or 8 weeks (0.21 or 0.17 μM, respectively) as compared with that of the normal control group (0.09 or 0.04 μM, respectively). However, mice treated with L-WEPE (0.17 μM) and H-WEPE (0.15 μM) significantly reduced TBARS content in the liver of mice fed by MCD diet after 4 weeks (p < 0.05). Moreover, the TBARS content was decreased in M-WEPE group (0.13 μM) compared with the mice fed by MCD diet-alone after 8 weeks (0.17 μM) (p < 0.05).
Fig. 2.
Effects of WEPE and GA on the liver TBARS (MDA) of C57BL/6 mice fed by MCD diet after (A) 4 or (B) 8 weeks. Values were presented as the means ± SD (n = 8/group). The letters indicated statistically significant differences between the groups (p < 0.05).
3.6. Effects of WEPE and GA on hepatic CYP2E1 expression in MCD-induced NASH mice
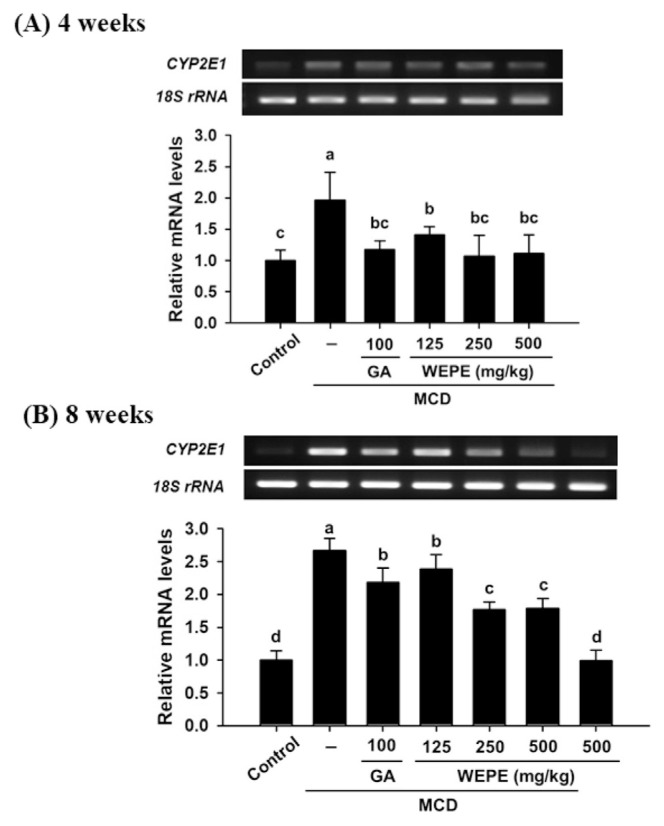
Fig. 3 shows the mRNA expressions of CYP2E1 in the liver tissues of mice fed by MCD diet after 4 or 8 weeks. Mice fed by MCD diet after 4 or 8 weeks significantly increased hepatic CYP2E1 level relative to that observed in the normal control group (p < 0.05). However, the groups treated with GA, L-WEPE, M-WEPE, and H-WEPE all significantly decreased hepatic CYP2E1 levels relative to that in the mice fed by MCD diet after 4 or 8 weeks (p < 0.05).
Fig. 3.
Effects of WEPE and GA on CYP2E1 gene expressions in the livers of C57BL/6 mice fed by MCD diet after (A) 4 or (B) 8 weeks. 18S rRNA is an internal control. Values were presented as the means ± SD (n = 8/group). The letters indicated statistically significant differences between the groups (p < 0.05).
3.7. Effects of WEPE and GA on the inflammatory levels in MCD diet-induced NASH mice
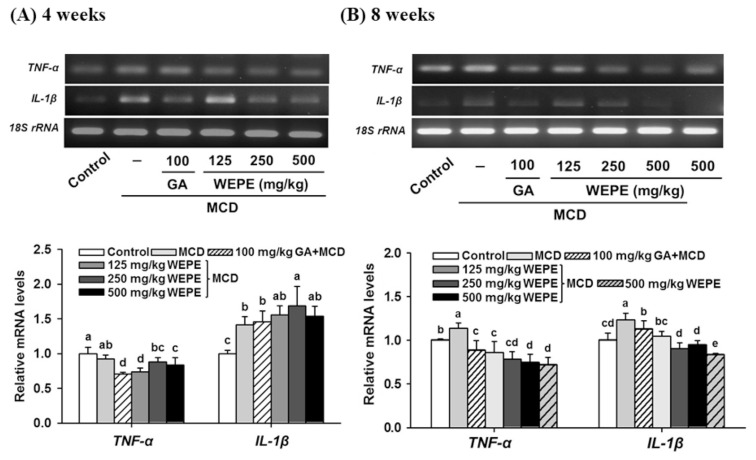
Fig. 4 shows the mRNA expressions of pro-inflammatory genes (IL-1β and TNF-α) were measured. IL-1β gene expression was significantly increased in the liver tissue of mice fed by MCD diet after 4 or 8 weeks (p < 0.05). In addition, the mice fed by MCD diet after 8 weeks resulted in high expression of hepatic TNF-α compared with the normal control group (p < 0.05). However, treatments with GA, L-WEPE, M-WEPE, and H-WEPE after the MCD diet for 4 or 8 weeks could reduce TNF-α mRNA expression relative to that in mice fed the MCD diet only. IL-1β mRNA expression in the liver tissues of mice fed by MCD diet for 8 weeks showed the similar result with TNF-α mRNA expression. These results displayed WEPE and GA may have anti-inflammation potential through reducing the expressions of TNF-α and IL-1β in the liver.
Fig. 4.
Effects of WEPE and GA on TNF-α and IL-1β gene expressions in the liver of C57BL/6 mice fed by MCD diet after (A) 4 or (B) 8 weeks. 18S rRNA is an internal control. Values were presented as the means ± SD (n = 8/group). The letters indicated statistically significant differences between the groups (p < 0.05).
4. Discussion
NASH is increasingly recognized as a chronic liver injury associated with abnormal liver histopathologic findings including steatosis with inflammation and fibrosis [1]. NASH often accompanies with obesity, type 2 diabetes, and metabolic syndrome [20–22]. Transformation from NAFLD to NASH is the two-hit hypothesis, wherein the first hit is the accumulation of hepatic lipids, and the second hit promotes lipid peroxidation, toxin, hepatocyte injury and fibrosis [3,23]. The MCD diet-induced NASH could develop hepatic steatosis and steatohepatitis, which histological features is similar to the clinical and pathological features of human NASH [10,24].
The abundant phenolic compounds of P. emblica possess potent antioxidant and anti-inflammatory properties [12,25]. Recent studies showed that phenolic compounds have hepatoprotective activity, and their ability might be associated with the antioxidant and anti-inflammatory properties [26–28]. The present study demonstrated that WEPE possesses hepatoprotective activity through decreasing AST, ALT, lipid peroxidation, as well as increasing the antioxidant enzymes.
After the administration of MCD diet for 4 or 8 weeks, MCD diet significantly increased AST and ALT levels in the serum, and TBARS, CYP2E1, TNF-α and IL-1β in the liver, as well as decreased serum cholesterol and triglycerides in the serum, and SOD, catalase, GPx and GST in the liver. Markedly increased serum AST and ALT, and liver pro-inflammatory cytokines, and lipid peroxidation parameters were used as evidence of hepatic damage in this study. These results obtained herein were similar to those obtained by Leclercq et al. [10], who found that MCD diet-induced NASH is closely associated with the generation of oxidative stress, proinflammatory cytokines and pro-apoptotic factors. Lee et al. [29] also showed that MCD diet impairs mitochondrial β-oxidation, elevates the generation of ROS, mitochondrial DNA damage and apoptotic cell death, activates hepatic stellate cells, and deposits extracellular matrix components.
In the present study, general indicators of MCD diet-induced NASH such as histopathological lesions and the activities of liver-specific enzyme (e.g. AST and ALT) were evident. In addition, serum AST and ALT, and liver TNF-α and IL-1β in the groups of MCD + L-WEPE, MCD + M-WEPE, and MCD + H-WEPE were significantly lower than that in the MCD diet-alone group. Moreover, GST activity was increased by GA, M-WEPE and H-WEPE after the MCD diet for 4 weeks, as well as SOD activity was increased by GA, L-WEPE, M-WEPE and H-WEPE after the MCD diet for 8 weeks. Accordingly, the concentration of TBARS was significantly decreased following WEPE administration (p < 0.05). CYP2E1, one of the main enzymes responsible for initiating oxidative stress, is involved in dietary hepatitis induced by an MCD diet [10]. Previous studies showed that the expression of CYP2E1 is increased in the human and animal models of NASH [10,30]. The expression of CYP2E1 in MCD diet mice was markedly increased when compared with the normal control mice; however, WEPE could lowered the expression of CYP2E1 in MCD diet-induced mice. Furthermore, WEPE ameliorated liver enzymes, increased antioxidant enzymes, and decreased lipid peroxidation and pro-inflammatory genes, as well as thus significantly reduced MCD diet-induced liver injury. Therefore, WEPE could improve the second-hit risk factors of the two-hit hypothesis and further decreased the formation of NASH.
5. Conclusion
WEPE decreases the severity of NASH via mechanisms likely to involve blockade of pro-inflammatory genes, and resultant up-regulation of antioxidant enzymes and down-regulation of lipid peroxidantion and liver-specific enzymes. Thus, WEPE markedly improved NASH in the MCD diet-induced NASH model. It is therefore suggested that WEPE may have a potent effect on ameliorating biochemical and histopathological markers of the liver damage.
Acknowledgements
This research work was supported in part by the Council of Agriculture, Taiwan, under grant 101AS-3.1.3-FD-Z1(1), and by the Ministry of Education, Taiwan, R.O.C. Under the ATU plan.
Funding Statement
This research work was supported in part by the Council of Agriculture, Taiwan, under grant 101AS-3.1.3-FD-Z1(1), and by the Ministry of Education, Taiwan, R.O.C. Under the ATU plan.
References
- 1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2. Lu CC, Yang SH, Hsia SM, Wu CH, Yen GC. Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis and liver fibrosis in vitro. J Funct Foods. 2016;20:20–30. [Google Scholar]
- 3. Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289–93. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- 4. Imeryuz N, Tahan V, Sonsuz A, Eren F, Uraz S, Yuksel M, et al. Iron preloading aggravates nutritional steatohepatitis in rats by increasing apoptotic cell death. J Hepatol. 2007;47:851–9. doi: 10.1016/j.jhep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 5. Tahan V, Eren F, Avsar E, Yavuz D, Yuksel M, Emekli E, et al. Rosiglitazone attenuates liver inflammation in a rat model of nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:3465–72. doi: 10.1007/s10620-007-9756-x. [DOI] [PubMed] [Google Scholar]
- 6. Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed] [Google Scholar]
- 7. Gulbahar O, Karasu ZA, Ersoz G. Treatment of nonalcoholic steatohepatitis with N-acetylcysteine. Gastroenterology. 2000;118:1444A. [Google Scholar]
- 8. Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–8. [PubMed] [Google Scholar]
- 9. London RM, George J. Pathogenesis of NASH: animal models. Clin Liver Dis. 2007;11:55–74. doi: 10.1016/j.cld.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–75. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang YJ, Tanaka T, Yang CR, Kouno I. New phenolic constituents from the fruit juice of Phyllanthus emblica. Chem Pharm Bull. 2001;49:537–40. doi: 10.1248/cpb.49.537. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Cui C, Zhao M, Wang J, Luo W, Yang B, et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 2008;109:909–15. doi: 10.1016/j.foodchem.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 13. Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–35. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 14. Peres W, Tuñón MJ, Collado PS, Herrmann S, Marroni N, González-Gallego J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol. 2000;33:742–50. doi: 10.1016/s0168-8278(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 15. Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 16. Huang CZ, Tung YT, Hsia SM, Wu CH, Yen GC. The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced nonalcoholic fatty liver disease (NAFLD) in SD rats. Food Funct. 2017;8:842–50. doi: 10.1039/c6fo01585a. [DOI] [PubMed] [Google Scholar]
- 17. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 18. Tu PS, Tung YT, Lee WT, Yen GC. Protective effect of camellia oil (Camellia oleifera Abel.) against ethanol-induced acute oxidative injury of the gastric mucosa in mice. J Agric Food Chem. 2017;65:4932–41. doi: 10.1021/acs.jafc.7b01135. [DOI] [PubMed] [Google Scholar]
- 19. Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276:E1146–70. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 20. Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–8. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 21. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 22. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–72. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 23. Angulo P, Lindor KD. Insulin resistance and mitochondrial abnormalities in NASH: a cool look into a burning issue. Gastroenterology. 2001;120:1281–5. doi: 10.1053/gast.2001.23591. [DOI] [PubMed] [Google Scholar]
- 24. Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–93. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 25. Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559–70. doi: 10.1211/jpp.58.12.0001. [DOI] [PubMed] [Google Scholar]
- 26. Tung YT, Wu JH, Huang CC, Peng HC, Chen YL, Yang SC, et al. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem Toxicol. 2009;47:1385–92. doi: 10.1016/j.fct.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 27. Hung MY, Fu TY, Shih PH, Lee CP, Yen GC. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food Chem Toxicol. 2006;44:1424–31. doi: 10.1016/j.fct.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28. Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 29. Lee HS, Son WC, Ryu JE, Koo BA, Kim YS. Standardized Salvia miltiorrhiza extract suppresses hepatic stellate cell activation and attenuates steatohepatitis induced by a methionine-choline deficient diet in mice. Molecules. 2014;19:8189–211. doi: 10.3390/molecules19068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–33. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]