Abstract
Because of its tumor origin from nerve sheath cells (the Schwann cells), the pathogenesis of an extraordinary rare intramedullary schwannoma, which should not have any Schwann's cells in nature, is controversial and still in debate. We report a case of a 63-year-old man diagnosed with a cervical cord intramedullary schwannoma with an interesting intraoperative finding that could support one of the theories on its genesis.
Keywords: subpial schwannoma, intramedullary schwannoma, pathogenesis, cervical cord tumor
Key Message
An intraoperative finding in our study and some previous case reports support the theory of tumoral growth of the Schwann cells at a critical site in which the dorsal root transit into the pia mater, which sometimes could be called “subpial schwannoma.”
Introduction
Spinal schwannoma accounts for approximately 10% of all spinal tumors. 1 While extradural or intradural extramedullary lesions are more common, intramedullary schwannoma is extremely rare representing approximately 1% of the overall incidence of spinal schwannoma. 2 3 4 5 Subpial schwannoma, which its intraoperative finding confirmed that the tumor is underneath the pia mater, was scarcely reported. Herein, we report a case of subpial schwannoma of the cervical spinal cord which might support a specific theory on the origin of an intramedullary schwannoma. To report this study, the case report (CARE) guidelines were followed. 6
Case Presentation
A 63-year-old man presented with severe spastic tone, progressive weakness, and numbness in both hands and legs in the past 5 years. Physical examination revealed paraesthesia and motor power weakness of both upper and lower limbs with knee jerk reflex of 3 + . There were no skin lesions (e.g., café-au-lait spots, neurofibromas, and freckles), no history of optic glioma, no other sites of the brain or spinal cord tumors, no hearing problems, or any other manifestations that could lead to the suspicious of neurofibromatosis. Furthermore, the patient has no family history of neurofibromatosis or any other congenital diseases and as a result, we did not further workup for neurofibromatosis or any other congenital diseases in this patient.
The magnetic resonance imaging (MRI) demonstrated homogeneously well-circumscribed mass from the C4 to C6 levels on the sagittal series which showed low signal intensity on T1-weighted images and iso- to high signal intensity on T2-weighted images. The mass was enhanced after the contrast injection. The lesion was appeared to be intramedullary, more pronounced on the right side of the spinal cord ( Fig. 1 ). Initially, intramedullary spinal cord tumors, such as astrocytoma or ependymoma, were included in the differential diagnosis.
Fig. 1.
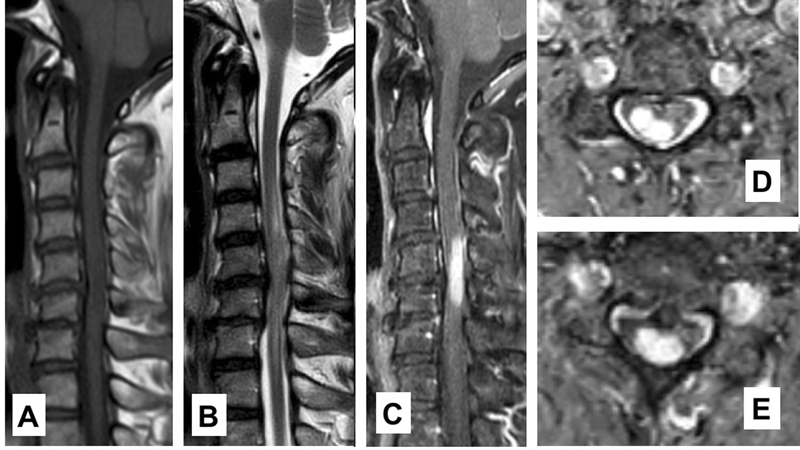
( A ) T1-weighted sagittal image shows iso- and slightly low-intensity lesion at C5–6 level. ( B ) T2-weighted sagittal image shows a mixed iso-to high-intensity signal mass with mild peritumoral edema. ( C ) Contrast-enhanced sagittal image shows a homogenously well-enhanced mass. ( D, E ) Axial images shows a well-defined mass on the right side of the spinal canal.
Deciding to proceed with the treatment, an operation was performed. A standard posterior approach from midline was done. After the laminectomy of affected levels was achieved, the dura mater was opened meticulously. A well-defined border of yellowish mass with a firm and rubbery consistency was located at the right posterolateral aspect of C4–6 cord level, beneath the pia mater. The tumor was involving those dorsal rootlets of right C5 and C6 spinal nerves and compressing the spinal cord from the posterior. The tumor was located underneath pia mater and origin of nerve root was arose from right posterolateral part of the tumor. We can partially identify the tumor plane from the spinal cord at the caudal site. However, the ventral plane of the tumor had severely being adhered to the spinal cord and its true cleavage plane could not be identified clearly ( Fig. 2 ). The tumor was excised. Both intraoperative somatosensory-evoked potentials (SSEPs) and motor-evoked potentials (MEPs) monitoring were used. No abnormal signal was being detected throughout the case.
Fig. 2.
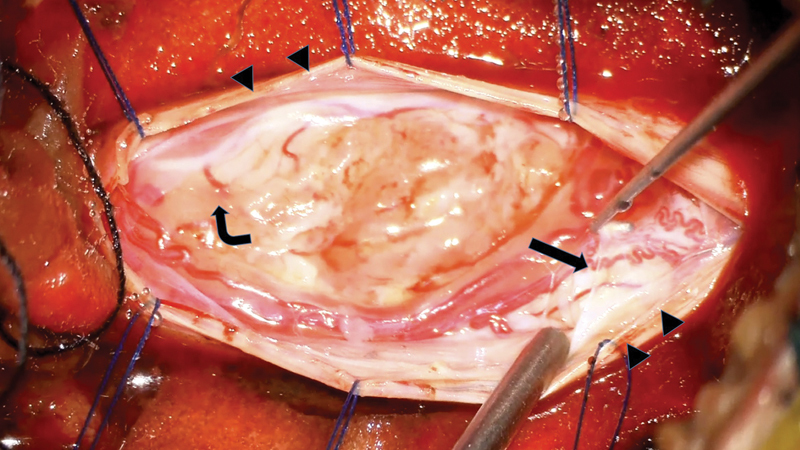
The tumor was located underneath the origin of nerve root origin ( bend arrow ). Arachnoid was opened and sutured with the dura ( arrow heads ). Pia mater was elevated and separated ( straight arrow ).
Pathological examination showed alternating hyper- and hypocellular areas of spindle cells (the Antoni A and Antoni B, respectively). The hypocellular area shows a loose myxoid stroma. The neoplastic spindle cells contain enlarged hyperchromatic, mild-to-moderate pleomorphic nuclei, small nucleoli, as they are palisaded in rows ( Fig. 3 ). Additional immunohistochemistry study showed strongly and diffusely positive S-100 stained ( Fig. 4 ). The final pathological diagnosis was confirmed as a schwannoma (the World Health Organization [WHO] grade I).
Fig. 3.
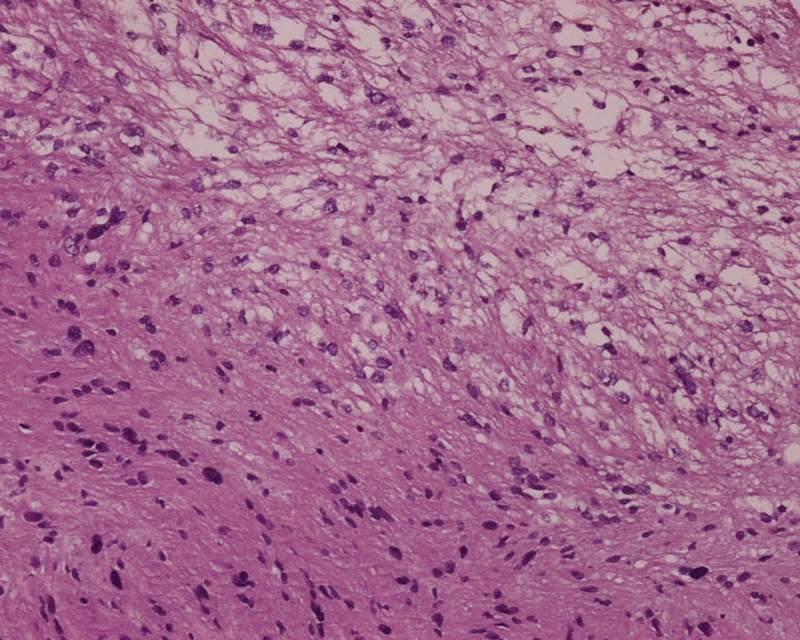
Pathological study shows alternating area of spindle cells hypercellularity (Antoni's A) and hypocellularity (Antoni's B).
Fig. 4.
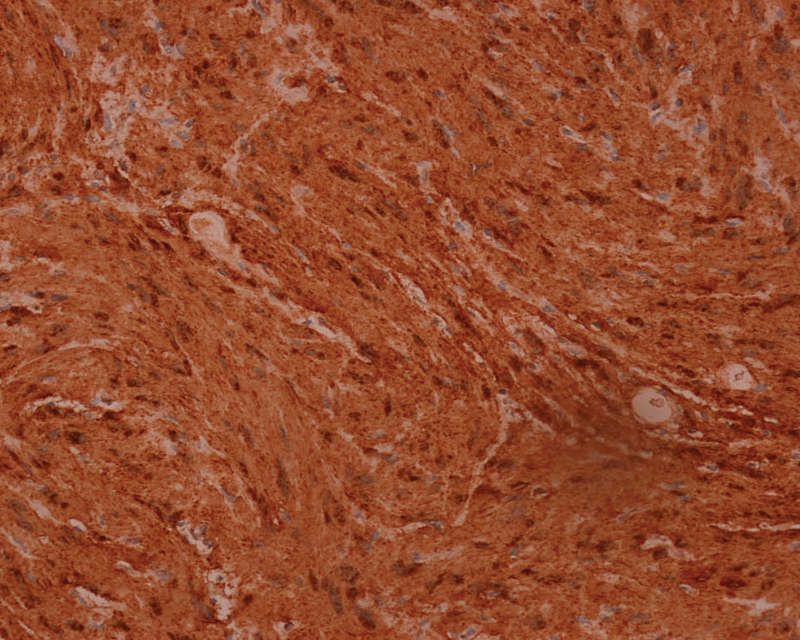
Immunohistochemistry study showed strongly, and diffusely positive S-100 stained which confirmed the diagnosis of schwannoma.
The patient was then discharged from the hospital uneventfully. No complication was observed. At the last follow-up at 6-month postoperatively, the patient's function was improved, including better handgrip power and ambulation compared with before surgery. Unfortunately, because the patient has been loss to follow-up after that, follow-up images of the tumor after surgery could not be done.
Discussion
Intramedullary schwannoma was first reported in 1932. 7 Since then, according to a recent extensive review, 166 cases have been reported. 8 But for the term “subpial schwannoma” has been reported in four studies so far. 9 10 11 12 Because of the dispute over its origin, the pathogenesis of intramedullary schwannoma has been under debate for a long time. Several explanations have been purposed as follows: (1) the Schwann cells migration into the central nervous system during embryogenesis 13 14 ; (2) the atypical Schwann cells residing around the intramedullary myelin fibers 15 ; (3) extension of the Schwann cells along the intramedullary perivascular nervous plexus 14 16 ; (4) transformation of pial cells from the neuroectoderm into the Schwann cells 17 ; and (5) the Schwann cells becoming a tumor on a dorsal root located at the critical area where the dorsal root loses its cover and enters the pia mater. 14 18 19 The fifth hypothesis, in which the term “subpial schwannoma” was coined, has been reported by several authors in the last decades. A study by Ozawa et al demonstrated a rare case of a subpial schwannoma of the cervical cord which initially mimicked an intramedullary tumor on MRI. The surgical finding was a well-demarcated yellowish lesion on the dorsal side of the spinal cord located in the subpial space and invading the spinal cord. 9 This finding suggested that the tumor might have originate from the subpial space that the authors stated that this could support the fifth hypothesis. Another recent case report by Suematsu et al, 12 demonstrated a case of concurrent dorsal subpial schwannoma and ventral meningioma arising at the same upper cervical level of C1–2. From our study, an intraoperative finding of a well-demarcated yellowish mass, involving the dorsal rootlets of C5 and C6 levels at the subpial layer could also support this hypothesis that the Schwann cells become a tumor on a dorsal root at the point of its transition into the pia mater.
Although our finding supports the fifth hypothesis, it does not completely rule out the other possibilities. While the fifth theory is satisfactorily better at explaining the strict relation between the dorsal root and the findings of a spinal schwannoma, it does not always have to be true. Kim et al 11 reported a case of thoracic subpial intramedullary schwannoma involving a ventral nerve root which is even more exceedingly rare than usual cases of the dorsal root involvement. Thus, because of its rarity in origin, future investigation of intramedullary schwannoma and its finding would still be needed for a better understanding of the disease.
Conclusion
Intramedullary schwannoma is rare, and its origin is still in question. The intraoperative finding in our study supports the pathogenesis theory of intramedullary schwannoma, a tumoral growth of the Schwann cells at a critical site in which the dorsal nerve transit into the pia mater.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authors' Contributions
P.D. contributed to the concepts, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, manuscript preparation, manuscript editing, manuscript review, and guarantor. S.S. contributed to the concepts, design, definition of intellectual content, literature search, clinical studies, data acquisition, data analysis, manuscript preparation, manuscript editing, manuscript review, and guarantor. P.N. contributed to the concepts, design, definition of intellectual content, literature search, manuscript editing, manuscript review, and guarantor. W.T. contributed to the concepts, design, definition of intellectual content, literature search, manuscript editing, manuscript review, and guarantor.
References
- 1.Ottenhausen M, Ntoulias G, Bodhinayake I. Intradural spinal tumors in adults-update on management and outcome. Neurosurg Rev. 2019;42(02):371–388. doi: 10.1007/s10143-018-0957-x. [DOI] [PubMed] [Google Scholar]
- 2.Ross D A, Edwards M S, Wilson C B. Intramedullary neurilemomas of the spinal cord: report of two cases and review of the literature. Neurosurgery. 1986;19(03):458–464. doi: 10.1227/00006123-198609000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Lee D Y, Chung C K, Kim H J. Thoracic intramedullary schwannoma. J Spinal Disord. 1999;12(02):174–176. [PubMed] [Google Scholar]
- 4.Riffaud L, Morandi X, Massengo S, Carsin-Nicol B, Heresbach N, Guegan Y. MRI of intramedullary spinal schwannomas: case report and review of the literature. Neuroradiology. 2000;42(04):275–279. doi: 10.1007/s002340050885. [DOI] [PubMed] [Google Scholar]
- 5.Kodama Y, Terae S, Hida K, Chu B C, Kaneko K, Miyasaka K. Intramedullary schwannoma of the spinal cord: report of two cases. Neuroradiology. 2001;43(07):567–571. doi: 10.1007/s002340100540. [DOI] [PubMed] [Google Scholar]
- 6.CARE Group . Gagnier J J, Kienle G, Altman D G, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(01):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Del Rio-Hortega P, Penfield W. New York, NY: Hoeber Inc.; 1932. Cytology & Cellular Pathology of the Nervous System. [Google Scholar]
- 8.Swiatek V M, Stein K P, Cukaz H B. Spinal intramedullary schwannomas-report of a case and extensive review of the literature. Neurosurg Rev. 2021;44(04):1833–1852. doi: 10.1007/s10143-020-01357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozawa N, Tashiro T, Okamura T, Koyama K, Ohata K, Inoue Y. Subpial schwannoma of the cervical spinal cord mimicking an intramedullary tumor. Radiat Med. 2006;24(10):690–694. doi: 10.1007/s11604-006-0090-6. [DOI] [PubMed] [Google Scholar]
- 10.Houkin K, Abe H, Iwasaki Y, Tsuru M, Miyasaka K. Spinal sub-pial schwannoma. Case report [in Japanese] Neurol Med Chir (Tokyo) 1983;23(05):370–374. doi: 10.2176/nmc.23.370. [DOI] [PubMed] [Google Scholar]
- 11.Kim S D, Nakagawa H, Mizuno J, Inoue T.Thoracic subpial intramedullary schwannoma involving a ventral nerve root: a case report and review of the literature Surg Neurol 20056304389–393., discussion 393 [DOI] [PubMed] [Google Scholar]
- 12.Suematsu Y, Tsuji O, Nagoshi N. Concurrent dorsal subpial schwannoma and ventral meningioma arising at the same upper cervical level: a case report. Spinal Cord Ser Cases. 2020;6(01):64. doi: 10.1038/s41394-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason T H, Keigher H A. Intramedullary spinal neurilemmoma: case report. J Neurosurg. 1968;29(04):414–416. doi: 10.3171/jns.1968.29.4.0414. [DOI] [PubMed] [Google Scholar]
- 14.Rout D, Pillai S M, Radhakrishnan V V. Cervical intramedullary schwannoma. Case report. J Neurosurg. 1983;58(06):962–964. doi: 10.3171/jns.1983.58.6.0962. [DOI] [PubMed] [Google Scholar]
- 15.Scott M, Bentz R. Intramedullary neurilemmoma (neurinoma) of the thoracic cord. A case report. J Neuropathol Exp Neurol. 1962;21:194–200. doi: 10.1097/00005072-196204000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann H D, Neuss M, Winkler D. Intramedullary spinal cord tumors resected with CO2 laser microsurgical technique: recent experience in fifteen patients. Neurosurgery. 1988;22(03):518–522. doi: 10.1227/00006123-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Roka L. Clinical aspects and pathologic anatomy of tumors of the oblongata and cord in the region of the foramen magna [Article in Undetermined language] Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1951;186(04):413–436. doi: 10.1007/BF00716069. [DOI] [PubMed] [Google Scholar]
- 18.Prakash B, Roy S, Tandon P N. Schwannoma of the brain stem: case report. J Neurosurg. 1980;53(01):121–123. doi: 10.3171/jns.1980.53.1.0121. [DOI] [PubMed] [Google Scholar]
- 19.Shalit M N, Sandbank U. Cervical intramedullary schwannoma. Surg Neurol. 1981;16(01):61–64. doi: 10.1016/s0090-3019(81)80069-9. [DOI] [PubMed] [Google Scholar]


