Abstract
Membrane permeabilization due to pulsed electric field (PEF) treatment of gram-positive Lactobacillus cells was investigated by using propidium iodide uptake and single-cell analysis with flow cytometry. Electric field strength, energy input, treatment time, and growth phase affected membrane permeabilization of Lactobacillus plantarum during PEF treatment. A correlation between PEF inactivation and membrane permeabilization of L. plantarum cells was demonstrated, whereas no relationship was observed between membrane permeabilization and heat inactivation. The same results were obtained with a Lactobacillus fermentum strain, but the latter organism was more PEF resistant and exhibited less membrane permeabilization, indicating that various bacteria have different responses to PEF treatment. While membrane permeabilization was the main factor involved in the mechanism of inactivation, the growth phase and the acidity of the environment also influenced inactivation. By using flow cytometry it was possible to sort cells in the L. plantarum population based on different cell sizes and shapes, and the results were confirmed by image analysis. An apparent effect of morphology on membrane permeabilization was observed, and larger cells were more easily permeabilized than smaller cells. In conclusion, our results indicate that the ability of PEF treatment to cause membrane permeabilization is an important factor in determining inactivation. This finding should have an effect on the final choice of the processing parameters used so that all microorganisms can be inactivated and, consequently, on the use of PEF treatment as an alternative method for preserving food products.
A high-voltage pulsed electric field (PEF) can inactivate microorganisms under reduced-temperature conditions. Consequently, food products have a fresher appearance and lose less flavor and other functional food components, factors that are currently in high demand by consumers (1, 16, 27). PEF treatment is the application of pulses with very high field strength for a short time (microseconds) to foods placed between two electrodes. Due to technical and technological developments during the last few years, it is now possible to perform PEF treatment in a continuous-treatment chamber. This has increased the efficiency of the treatment process and offers more possibilities for scaling up the technology (4, 26, 40), which has enhanced interest by the food industry.
Recently, microbial inactivation kinetics were systematically studied under a range of conditions in continuous-PEF systems (8, 29, 39). Furthermore, inactivation kinetics were determined under close-to-isothermal conditions to study the effect of field strength and energy input independent of heat (12). It was concluded that electric field strength and the amount of energy input, (i.e., the number of pulses) were important in determining the inactivation level. Other important process factors were pulse length and inlet temperature (39). Product factors (pH and conductivity) and the physiological state of the microorganisms also play a role in determining inactivation kinetics (38, 39).
The underlying mechanism of inactivation of microorganisms by PEF treatment has not been fully elucidated. Knowledge of the mechanism of inactivation is essential in order to develop better equipment and define conditions for inactivating microorganisms in food products with this technology. The most commonly accepted theory is that local instabilities in the membranes of the microorganisms are formed by electromechanical compression and electric field-induced tension, which causes pores to form in the membrane (electroporation) (1, 13, 34, 37). One major consequence of electroporation is a phenomenon called electropermeabilization, which is a dramatic increase in permeability (or conductivity) and, in some cases, mechanical rupture of the membrane. It has been determined that mechanical instability of membranes occurs only when the applied electric field induces a certain critical membrane potential. Electropermeabilization has been demonstrated to be reversible or irreversible depending on the degree of membrane organizational changes (30, 34, 36). Strong electric fields result in an irreversible effect and ultimately in cell death (11, 31). However, it is still not clear whether cell death occurs because of localized rapid rupture of a portion of the cell membrane or because of chemical stress associated with molecular transport. Only limited data about the correlation between cell viability and electropermeabilization of prokaryotes are available (33). Factors that influence membrane permeabilization of Saccharomyces cerevisiae have been investigated, and it has been shown that the growth phase, ion composition, concentration of the extracellular medium, and PEF conditions affect electropermeabilization (2, 9, 22–24). Membrane permeabilization can be studied by flow cytometric measurement (FCM) of the uptake of the fluorescent probe propidium iodide (PI), which is a nucleotide-binding probe excluded by intact cells. PI is a strongly hydrophilic, small molecule (Mr, 660) which has been shown to be a good indicator of membrane integrity and has been used to label dead cells (18, 35). Flow cytometry allows rapid simultaneous measurement of scattered light and fluorescence emission from individual cells as they pass by a laser illumination point (6).
In this study we determined the effects of different process conditions on membrane permeabilization of Lactobacillus plantarum during PEF treatment as measured by PI uptake using FCM. We correlated membrane permeabilization with induced inactivation to gain insight in the mechanisms of inactivation of vegetative bacteria. Moreover, we studied inactivation and membrane permeabilization of a second, more PEF-resistant Lactobacillus species to evaluate the validity of our findings. Membrane permeabilization was studied as a function of electric field strength, energy input, treatment time, treatment medium conductivity and pH, growth phase, and morphology of the microorganisms. Heat treatment was used as a control treatment to determine whether the permeabilization induced during PEF treatment was due to the electric field applied or to the increased temperature induced during PEF treatment.
MATERIALS AND METHODS
Bacteria, growth conditions, and media.
L. plantarum LA 10-11 was obtained from the Unilever culture collection; this strain was isolated from spoiled onion ketchup and was identified by the American Type Culture Collection (it has no American Type Culture Collection number). Lactobacillus fermentum PW7 was also obtained from the Unilever collection; it was isolated from a tomato paste production line and was identified by the Culture Collection Laboratory for Microbiology, University of Ghent, Ghent, Belgium. Inocula were prepared 3 days before each experiment by starting with a culture that was stored in vials with 10% (vol/vol) glycerol at −20°C. The strains were inoculated into 10 ml of De Man-Rogosa-Sharpe (MRS) broth (Difco Laboratories, Detroit, Mich.) and incubated for 18 h at 30°C. The cultures were reinoculated (0.4%) into 50 ml of MRS broth and incubated for 48 h at 30°C to obtain cells which were in the stationary phase for a prolonged time. Cells were incubated until the optical density at 660 nm was 0.1, at which point they were in the exponential growth phase. Both cell suspensions were harvested by centrifugation at 13,000 × g for 10 min at 5°C and were resuspended in 5 ml of 50 mM HEPES (pH 7.0). Each inoculum was kept on ice until inoculation (maximum time, 15 min). In addition, all media and solutions used in this study were filter sterilized (pore size, 0.1 μm) prior to use to minimize particle interference during FCM.
PEF treatment.
Cells were treated in a CoolPure CPS-4 apparatus (PurePulse Technologies Inc., San Diego, Calif.), which is a laboratory-scale continuous-PEF apparatus with a maximum capacity of 10 liters per h. The system has a colinear continuous-flowthrough treatment chamber with a gap of 3.4 mm, an inside diameter of 3 mm, and a volume of 0.024 ml. The average residence time in the treatment chamber was 0.024 s at a flow rate of 3.6 liters/h. The total residence time (at a certain temperature) after PEF treatment and before placement on ice was estimated to be approximately 30 s. The high-voltage power supply of the PEF apparatus can deliver 4 kJ/s and a maximum output voltage of 12.6 kV. The electric field strength, pulse length, and pulse wave shape were determined with an oscilloscope (model TDS 220; Tektronix). The applied voltage was measured with a voltage probe that was located as close as possible to the treatment chamber. Care was taken so that the measured voltage across the treatment chamber was almost equal to the applied voltage. The system was able to generate a pulse with a square wave shape. The pulse duration was set at 2.3 μs. The PEF treatment medium was a phosphate buffer prepared from 0.5 M Na2HPO4 · 2H2O and 0.5 M NaH2PO4 · 2H2O. The conductivity of the solution was adjusted to 0.4 or 1.5 S/m at 25°C by using demineralized water, and the pH was adjusted at 4.3, 4.5, or 6.8 by using either 4 N H2SO4 or 4 N KOH. Experiments were also performed with a commercial ultra-high-temperature-treated tomato juice (Zontomaatje; Riedel, Ede, The Netherlands) with a pH of 4.3 and a conductivity of 1.5 S/m. The final pH and conductivity of the treatment medium were measured before and after each experiment. The phosphate buffer was sterilized together with the PEF system and cooled to room temperature. During the start-up procedure, the desired system settings (i.e., flow rate, charge voltage, inlet medium temperature, and pulse frequency) were set. When the system was running steadily, the treatment medium was inoculated with the microorganisms. The inlet temperature of the treatment medium was adjusted to 30°C by using a heat exchanger prior to the PEF treatment. During each experiment the inlet and outlet medium temperatures were measured continuously and automatically entered into a computer. The measured total energy input was calculated from the difference between the inlet and outlet medium temperatures and was multiplied by the heat capacitance of water (i.e., 4.18 J/ml/K) or the tomato juice (4.14 J/ml/K). PEF treatments with increasing energy input up to 120 J/ml were performed, which corresponded to a maximum temperature increase of approximately 28°C and a final temperature of 58°C. The pulse wave shape of each PEF treatment was also entered into the computer. To determine the inoculum level in the buffer, samples were taken before and after PEF treatment from the vessel containing the inoculum. After pulsing the samples were immediately placed in ice water, and they were analyzed within 1 h. A control experiment was performed to test the effect of storage at room temperature for 5 h on membrane permeabilization and inactivation after PEF treatment. The concentrations of viable cells, expressed as the number of CFU per milliliter, were determined before and after the PEF treatment by using pour plates containing MRS agar (Merck, Darmstadt, Germany). The plates were incubated aerobically at 30°C for 5 days All experiments were done at least in duplicate, and each experiment was performed on a different day with a new inoculum.
Heat treatment.
Heat treatments, performed as control experiments, were done by using the method described by Kooiman and Geers (21). The relationship between membrane permeabilization and the inactivation kinetics of L. plantarum LA 10-11 was investigated for heat treatments consisting of 3 min at temperatures ranging from 45 to 95°C in a phosphate buffer prepared from 0.5 M Na2HPO4 · 2H2O and 0.5 M NaH2PO4 · 2H2O. A wide temperature range was chosen in order to find temperatures that could cause levels of membrane permeabilization similar to those caused by PEF treatment. The conductivity of the buffer solution was adjusted to 1.5 S/m at 25°C by using demineralized water, and the pH was adjusted to 4.3 with 4 N H2SO4. After each heat treatment, the tubes were immediately placed in ice water. The concentrations of viable cells were determined before and after heat treatment by using pour plates containing MRS agar; the plates were incubated aerobically for 5 days at 30°C. Membrane permeabilization was analyzed as described below. All experiments were done in duplicate, and each experiment was performed on a different day with a new inoculum.
Fluorescence labelling with PI.
To test whether a treatment caused membrane damage, cells were incubated after the treatment with the DNA-binding probe PI, which cannot pass through intact membranes. Upon cell entry after membrane damage, binding to DNA increases the fluorescence of PI by a factor of 40. Stock solutions of PI (Molecular Probes, Leiden, The Netherlands) were prepared in distilled water at a final concentration of 10 μg/ml and were stored in the dark at 4°C. PI was added to a final concentration of 0.5 μg/ml. The concentrations of organisms in samples were adjusted for flow cytometric analysis to approximately 106 CFU/ml in order to ensure that the events detected really represented single cells. The cells were incubated with the probe for exactly 5 min at room temperature before FCM.
Flow cytometric analysis of L. plantarum and L. fermentum.
All FCM were performed with a Coulter EPICS ELITE flow sorter at an excitation wavelength of 488 nm by using a 15-mW argon laser with a 100-μm flow cell at a sheath pressure of 12 lb/in2, as described in detail by Nebe-von Caron et al. (25). Filter-sterilized (pore size, 0.1 μm) 0.9% (wt/vol) saline was used as the sheath fluid. Upon excitation at 488 nm, PI gives an emission signal in the red region at 617 nm. In addition to the log fluorescence parameter, log forward scatter was monitored, which is an indicator of cell size (5, 17). Samples were processed so that 10,000 events were collected for each sample, and the event rate was less than 1,000 events s−1 to avoid detection of coincident events. Untreated stained cells were used as negative controls and cells treated for 3 min at 80°C were used as positive controls for voltage adjustment and assignment of PI-negative and -positive histogram regions. A histogram analysis of PI fluorescence caused by membrane damage (expressed as a percentage of the total population) was performed by using the Coulter Epics analysis software, version 4.02.
Image analysis.
Populations of cells sorted by the FCM technique were analyzed microscopically by using a Zeiss Axioplan microscope. Digital image recording and analysis of 100 cells of each population were performed with the Qwin software (version 2.3; Leica) running under Windows 98 on a Leica Q5001W computer. Observations were made in the transmitted-light mode by using a Zeiss Plan Neofluar lens (magnification, ×100) with a numerical aperture of 1.3. An image size of 760 by 573 pixels was used; this resulted in a 98- by 74-μm field of view (the pixel size was 0.13 μm).
Statistical analysis.
Data were analyzed by using the statistical analysis package of Microsoft Excel 5.0.
RESULTS
Membrane permeabilization of L. plantarum as a function of electric field strength during PEF treatment.
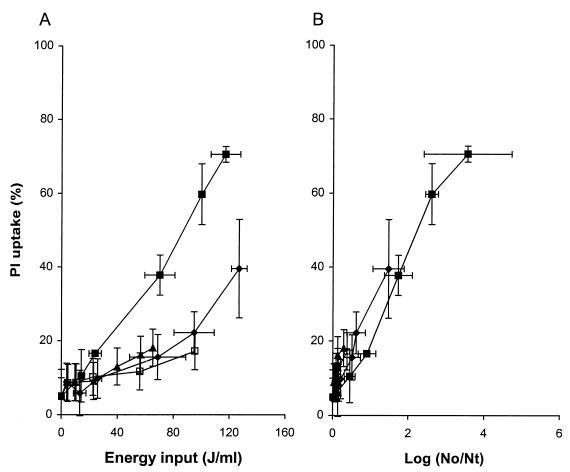
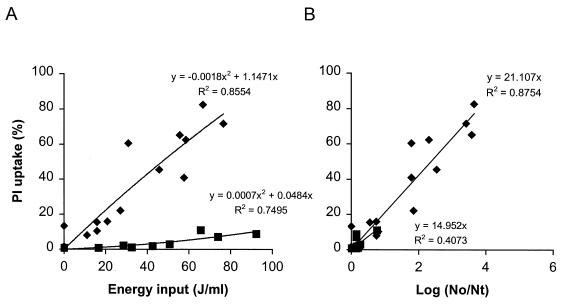
Membrane permeabilization of L. plantarum was determined by measuring the amount of fluorescent PI uptake by flow cytometry. FCM allows determination of PI uptake in cultures on a single-cell basis. The number of cells in the total population of L. plantarum cells that showed PI uptake after PEF treatment is indicated in Fig. 1. An increase in electric field strength resulted in more PI uptake (Fig. 1A). Furthermore, an increase in the energy input as a result of applying more electric pulses resulted in a larger number of permeabilized cells. Membrane permeabilization was plotted against log reduction in order to investigate whether the observed reduction in viability of L. plantarum after PEF treatment was directly caused by membrane permeabilization (Fig. 1B). At lower field strengths (1.0 and 1.2 V/μm) there was very little inactivation and less membrane permeabilization. However, when electric field strengths of 1.5 and 2.5 V/μm were used, there appeared to be a linear relationship between the number of inactivated cells and the number of permeabilized cells at least up to a 3.6-log reduction. Only after a severe PEF treatment (2.5 V/μm and an energy input of 120 J/ml) was a small decrease (5%) in the number of cells measured by flow cytometry, which indicated that a small portion of the cells in the population were completely ruptured. To determine if the observed membrane damage was reversible, the PEF-treated cells were incubated for 5 h in ice water or for 5 h at room temperature, and PI uptake was measured again. Neither treatment influenced the amount of PI uptake (data not shown). This information is further evidence that the PEF treatment used induced irreversible membrane damage. Simultaneously, plate counts were determined, and the incubation temperature did not affect the colony counts under PEF process conditions which resulted in outlet temperatures in the range from 30 to 50°C. However, PEF conditions that resulted in temperatures greater than 50°C resulted in slightly higher levels of inactivation (maximum, 0.8 log) if the samples were not immediately placed in ice water but were kept at room temperature (data not shown). This increased inactivation was due to the thermal effect, and therefore we decided to place all samples in ice water immediately to avoid such additional inactivation.
FIG. 1.
Effect of electric field strength on membrane permeabilization of stationary-growth-phase L. plantarum LA 10-11 cells as a function of energy input (A) and inactivation (B) after PEF treatment. Symbols: □, 1.0 V/μm; ▴, 1.2 V/μm; ⧫, 1.5 V/μm; ■, 2.5 V/μm. The pulse length was 2.3 μs, the flow rate was 3.6 liters/h, the start temperature was 30°C, the pH of the phosphate buffer was 4.5, and the conductivity was 1.5 S/m. The inoculum contained 3.3 × 107 CFU/ml. The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; No, number of cells before treatment.
Membrane permeabilization as a function of treatment medium conductivity and pH.
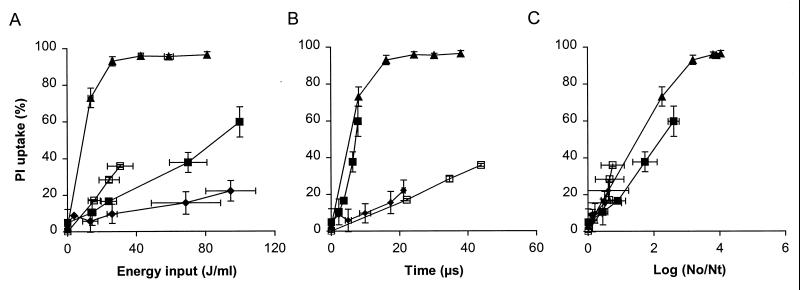
Membrane permeabilization of L. plantarum was studied with treatment medium conductivities of 0.4 and 1.5 S/m at electric field strengths of 1.5 and 2.5 V/μm (Fig. 2). L. plantarum cells suspended in a phosphate buffer having a conductivity of 0.4 S/m showed significantly more membrane permeabilization after PEF treatment at 2.5 V/μm (Fig. 2A). The energy input required to obtain 70% membrane-permeabilized cells was 14 J/ml for cells that were treated with PEF in a phosphate buffer having a conductivity of 0.4 S/m, whereas the corresponding value was 100 J/ml for cells in a phosphate buffer having a conductivity of 1.5 S/m (Fig. 2A). However, the treatment times required to obtain this level of membrane permeabilization were about the same for the two buffer solutions (Fig. 2B). A similar trend was observed with an electric field strength of 1.5 V/μm. The treatment medium conductivity had an effect on the relationship between the number of permeabilized L. plantarum cells and the number of inactivated L. plantarum cells after PEF treatment at an electric field strength of 2.5 V/μm, whereas at 1.5 V/μm this was not observed (Fig. 2C). When an electric field strength of 2.5 V/μm was used, less membrane permeabilization was necessary to obtain a certain inactivation level in the phosphate buffer having a conductivity of 1.5 S/m than was necessary to obtain the same level in the phosphate buffer having a conductivity of 0.4 S/m.
FIG. 2.
Membrane permeabilization of stationary-growth-phase L. plantarum LA 10-11 cells as function of energy input (A), treatment time (B), and inactivation (C) after PEF treatment in pH 4.5 phosphate buffer. Symbols: □, 0.4 S/m and 1.5 V/μm; ⧫, 1.5 S/m and 1.5 V/μm; ▴, 0.4 S/m and 2.5 V/μm; ■, 1.5 S/m and 2.5 V/μm. The inoculum for the 1.5-S/m buffer contained 3.3 × 107 CFU/ml, and the inoculum for the 0.4-S/m buffer contained 4.4 × 108 CFU/ml. The pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C. The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; No, number of cells before treatment.
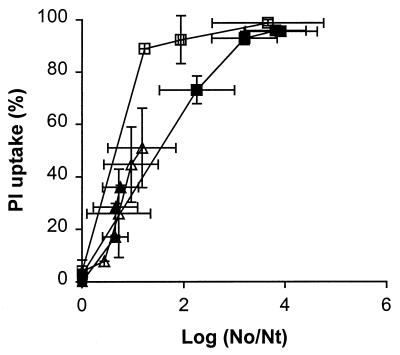
The effect of the pH of the treatment medium on membrane permeabilization and its relationship to inactivation of L. plantarum were studied at pH 4.5 and 6.8 by using the phosphate buffer having a conductivity of 0.4 S/m and electric field strengths of 1.5 and 2.5 V/μm (Fig. 3). After PEF treatment at 2.5 V/μm, cells in pH 6.8 phosphate buffer exhibited less inactivation and a similar amount of PI uptake compared to cells which were treated in pH 4.5 phosphate buffer. At the lower field strength, 1.5 V/μm, the pH did not influence the relationship between inactivation and membrane permeabilization.
FIG. 3.
Effect of pH of the treatment medium on the relationship between membrane permeabilization and inactivation of stationary-growth-phase L. plantarum LA 10-11 cells after PEF treatment. Symbols: ▴, pH 4.5 and 1.5 V/μm; ▵, pH 6.8 and 1.5 V/μm; ■, pH 4.5 and 2.5 V/μm; □, pH 6.8 and 2.5 V/μm. The conductivity of the buffer was 0.4 S/m, the pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C. The inoculum for the pH 6.8 buffer contained 9.5 × 108 CFU/ml, and the inoculum for the pH 4.5 buffer contained 4.4 × 108 CFU/ml. The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; No, number of cells before treatment.
Membrane permeabilization of exponential- and stationary-growth-phase cells of L. plantarum.
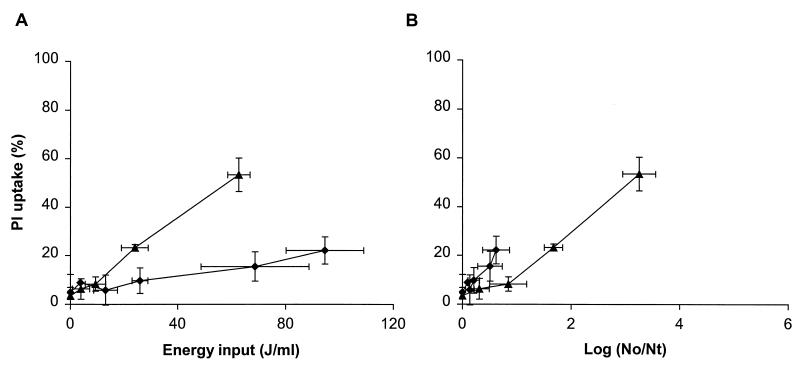
We compared exponentially growing L. plantarum cells and cells that were in a prolonged stationary growth phase in terms of membrane permeabilization and inactivation after PEF treatment in pH 4.5 phosphate buffer having a conductivity of 1.5 S/m. (Fig. 4). PEF treatment with an electric field strength of 1.5 V/μm and an energy input of 95 J/ml resulted in 23% permeabilized cells when the cells were in the stationary growth phase, whereas an energy input of only 24 J/ml was required to obtain a similar level of membrane permeabilization with cells in the exponential growth phase (Fig. 4A). The cells in the stationary-phase L. plantarum population 23% of which showed PI uptake, were inactivated with only a 0.5-log reduction, whereas the cells in the exponentially growing population with the same amount of PI uptake were inactivated with a 1.7-log reduction (Fig. 4B). A similar trend was observed with an electric field strength of 2.5 V/μm (data not shown). These results indicate that despite similar membrane permeabilization levels and lower energy input, more cells were inactivated in an exponentially growing L. plantarum culture.
FIG. 4.
Effect of PEF treatment on membrane permeabilization (A) and its relationship to inactivation (B) of exponential-phase (▴) and stationary-phase (⧫) L. plantarum LA 10-11 cells. The electric field strength used was 1.5 V/μm, the pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C with pH 4.5 phosphate buffer having a conductivity of 1.5 S/m. The inoculum of exponentially growing cells contained 6.2 × 105 CFU/ml. The inoculum of prolonged stationary-growth-phase cells contained 3.3 × 107 CFU/ml. The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; No, number of cells before treatment.
Effect of cell morphology on membrane permeabilization after PEF treatment.
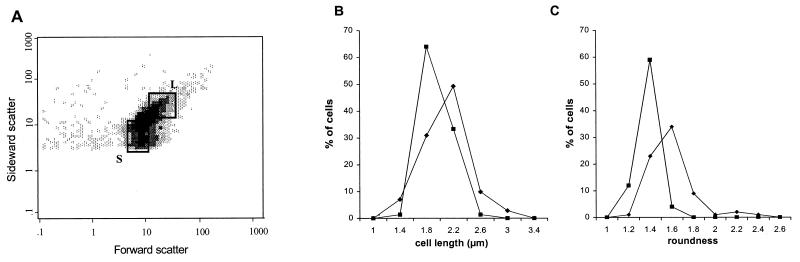
Cell size and shape in the L. plantarum population were estimated by measuring the forward and sideward scatter by the FCM method. By specifying regions of small and large cells with the software of the FCM equipment, it was possible to determine the amount of membrane damage, as reflected by PI uptake, for the cells in the specified regions (Fig. 5A). Regions L and S were the areas of cells that were high and low, respectively in terms of forward and sideward scatter. Following sorting of the cells with the FCM technique, we confirmed using image analysis that the two populations could be distinguished from each other on the basis of size distribution and cell shape; the latter was reflected by the roundness parameter, which gave a minimum value of unity for a circle (Fig. 5B and C). Interestingly, the distribution of cell size between the large-cell and small-cell populations differed (Fig. 5B). The cells of the large-cell population had a greater variety of cell lengths (Fig. 5B), and there were more longitudinal cells (Fig. 5C). In contrast, the population of cells designated small contained mainly (64%) short cells (1.8 ± 0.2 μm) (Fig. 5B) and more round cells (Fig. 5C).
FIG. 5.
(A) Flow cytometric dot plot of the forward scatter and sideward scatter (in arbitrary units) for stationary-growth-phase L. plantarum LA 10-11 cells. The arbitrary populations of small cells (square S) and large cells (square L) are indicated. (B and C) Size (B) and roundness (shape) (C) distributions of the population S (■) and L (⧫) stationary-growth-phase L. plantarum LA 10-11 cells.
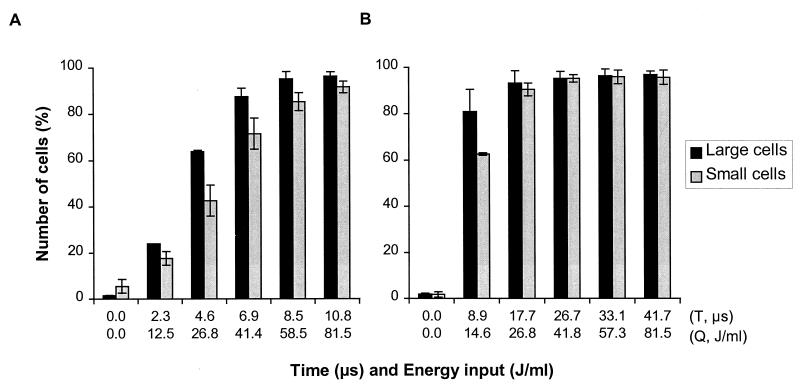
The effect of cell morphology on the number of membrane-permeabilized cells is illustrated in Fig. 6. For verification of PI uptake independent of cell size and shape, the ratio of log fluorescence to log forward scatter was calculated (data not shown) by using the software FCS Express, version 1.0 (De Novo Software, Cambridge, Mass.). A clear distinction was observed between the number of membrane-permeabilized cells in the large-cell population and the number of membrane-permeabilized cells in the small-cell population after a 2.5-V/μm PEF treatment in pH 4.5 phosphate buffer having a conductivity of 1.5 S/m (Fig. 6A). The small cells appeared to be less vulnerable to membrane permeabilization by the PEF treatment. However, after the time of treatment or the energy input of the PEF was increased, the difference between the numbers of permeabilized cells in the small- and large-cell populations gradually disappeared in the 1.5-S/m buffer and rapidly disappeared in the 0.4-S/m buffer (Fig. 6).
FIG. 6.
Effect of cell morphology on membrane permeabilization of stationary-growth-phase L. plantarum LA 10-11 cells in phosphate buffer with a conductivity of 1.5 S/m (A) or 0.4 S/m (B) after PEF treatment. The electric field strength was 2.5 V/μm, the pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C. The inoculum for the 1.5-S/m buffer contained 3.3 × 107 CFU/ml, and the inoculum for the 0.4-S/m buffer contained 4.4 × 108 CFU/ml. The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. T, time; Q, energy input.
Membrane permeabilization of L. plantarum after heat treatment.
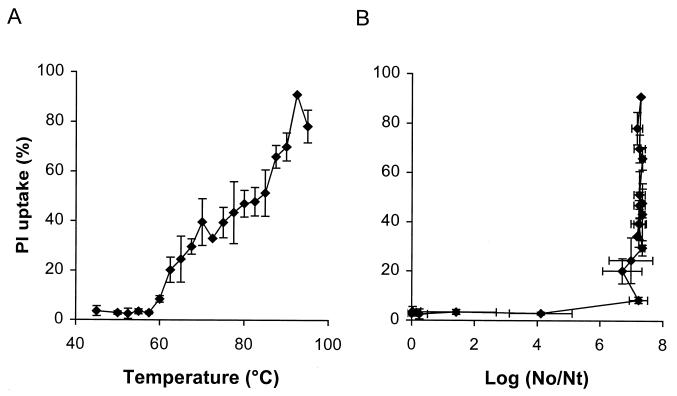
In order to investigate whether the observed membrane damage after PEF treatment could be due to the temperature generated during the PEF treatment, PI uptake after different heat treatments of L. plantarum LA 10-11 was investigated (Fig. 7A). After 3 min of heat treatment at temperatures ranging from 45 to 60°C, no significant PI uptake was observed, although increasing the temperature up to 95°C resulted in gradual increases in PI uptake. At temperatures above 65°C the standard deviations became considerably larger. The properties of binding of PI to the DNA and therefore the fluorescent signal might have been influenced by the melting point of the DNA, which is around 65°C. The relationship between PI uptake and inactivation of L. plantarum LA 10-11 after heat treatment is shown in Fig. 7B. From the results we concluded that there was no correlation between membrane permeabilization and heat inactivation, since the cells were completely inactivated by heat before significant membrane permeabilization was observed. Furthermore, the observed membrane permeabilization after PEF treatments that resulted in temperatures up to 58°C was probably mainly due to the electric field effect.
FIG. 7.
PI uptake by stationary-growth-phase L. plantarum LA 10-11 cells after 3-min heat treatments at different temperatures between 40 and 95°C (A) and membrane permeabilization in relation to inactivation after the heat treatments (B). The PI concentration was 0.5 μg/ml. The heat experiments were performed in pH 4.3 phosphate buffer having a conductivity of 1.5 S/m at 23°C The results are means based on data from two independent experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; No, number of cells before treatment.
Comparison of inactivation and membrane permeabilization of two lactobacilli that differ in PEF resistance.
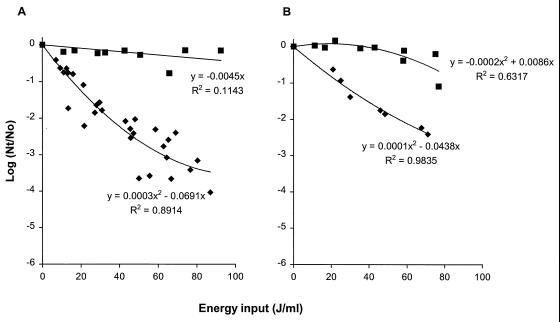
During pilot plant PEF studies with tomato paste, a strain of L. fermentum was isolated that survived the PEF treatment used. PEF inactivation of this strain, L. fermentum PW7, was compared to inactivation of L. plantarum LA 10-11 both in phosphate buffer and in tomato juice having a pH 4.3 and a conductivity of 1.5 S/m (Fig. 8). Experiments were performed with an electric field strength of 2.5 V/μm, different levels of energy input, a pulse length of 2.3 μs, a flow rate of 3.6 liters/h, and an initial temperature of 30°C. Inactivation of both strains increased as the energy input increased, but this effect was noticeably more pronounced for L. plantarum than for L. fermentum (Fig. 8A). A PEF treatment consisting of four pulses with an electric field strength of 2.5 V/μm, corresponding to an energy input of 80 J/ml, resulted in an approximately 3.4-log reduction for L. plantarum, whereas it resulted in only a 0.3-log reduction for L. fermentum (Fig. 8A). Thus, L. fermentum was more PEF resistant than L. plantarum both in phosphate buffer and in tomato juice (Fig. 8). The level of inactivation of L. plantarum in tomato juice was slightly lower (up to 0.8 log at an energy input of 70 J/ml) than the level of inactivation in phosphate buffer. To assess if a difference in resistance to PEF inactivation was related to membrane permeabilization in other lactobacilli, the PI uptake data for L. plantarum and L. fermentum were compared after different PEF treatments (Fig. 9). Membrane permeabilization of L. plantarum or L. fermentum was plotted versus the energy input (in joules per milliliter) during a 2.5-V/μm PEF treatment. Increasing the energy input resulted in more PI uptake by L. plantarum, whereas increasing the energy levels hardly affected the amount of PI taken up by L. fermentum cells (Fig. 9A). This observation indicated that PEF treatment caused more membrane permeabilization in L. plantarum than in L. fermentum. By plotting PI uptake versus the number of inactivated cells, we found that L. fermentum was hardly inactivated, whereas a correlation between PEF inactivation and membrane permeabilization was demonstrated for L. plantarum (Fig. 9B). The more PEF-sensitive L. plantarum strain showed more membrane damage than the more PEF-resistant L. fermentum strain, which suffered minor membrane damage.
FIG. 8.
Effect of energy input on inactivation of stationary-growth-phase L. plantarum LA 10-11 (⧫) and L. fermentum PW7 (■) cells after PEF treatment in phosphate buffer (A) and tomato juice (B). Experiments were performed in pH 4.3 phosphate buffer having a conductivity of 1.5 S/m at 23°C. The electric field strength was 2.5 V/μm, the pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C. The L. plantarum inoculum contained 2.4 × 107 CFU/ml, and the L. fermentum inoculum contained 4.6 × 107 CFU/ml. Nt, number of survivors after treatment; No, number of cells before treatment. Regression analysis of inactivation of L. plantarum was done with data from five independent experiments, and regression analysis of inactivation of L. fermentum was done, with data, from two independent experiments.
FIG. 9.
Comparison of membrane permeabilization of stationary-growth-phase L. plantarum LA 10-11 (⧫) and L. fermentum PW7 (■) cells after PEF treatment (A) and membrane permeabilization in relation to inactivation of both strains after PEF treatment (B). The PI concentration was 0.5 μg/ml. The PEF experiments were performed in pH 4.3 phosphate buffer having a conductivity of 1.5 S/m at 23°C. The electric field strength was 2.5 V/μm, the pulse length was 2.3 μs, the flow rate was 3.6 liters/h, and the start temperature was 30°C. Regression analysis of inactivation of L. plantarum was done with data from three independent experiments, and regression analysis of inactivation of L. fermentum was done with data from two independent experiments. Nt, number of survivors after treatment; No, number of cells before treatment.
DISCUSSION
In this study, we found that there is a relationship between field strength and the number of permeabilized L. plantarum cells, so that greater field strengths resulted in permeabilization of more cells, as demonstrated by PI uptake determined with a flow cytometer. Further evidence that induced membrane permeabilization occurred was the leakage of ATP that was observed after PEF treatment of L. plantarum cells (data not shown). A correlation was observed between membrane permeabilization and inactivation of L. plantarum after PEF treatment with an electric field strength of 1.5 or 2.5 V/μm. Besides an increase in the number of fluorescent cells after a severe PEF treatment, a small increase in the number of cell fragments was observed by the FCM technique. These fragments were located outside our specified region of bacterial cells and therefore did not interfere with the fluorescence measured as a result of PI uptake. However, this could mean that the amount of membrane permeabilization or cell disruption could be slightly higher than that determined by PI uptake.
The number of membrane-permeabilized cells increased as the conductivity of the treatment medium decreased at a constant energy input. However, as the conductivity of the treatment medium affected the energy input, more pulses were applied to the 0.4-S/m buffer than to the 1.5-S/m buffer to keep the energy input the same. As a result, no significant effect of conductivity was observed for a constant treatment time. Furthermore, the relationship between membrane permeabilization and inactivation was dependent on the conductivity at an electric field strength of 2.5 V/μm. Jayaram et al. (15) studied inactivation in a batch PEF system with a set number of pulses and speculated that the increased inactivation of Lactobacillus brevis observed in a medium with low conductivity was due to an increase in membrane permeabilization. In contrast, in our continuous-PEF system a low-conductivity medium allows more pulses to be applied and thus generates more membrane permeabilization and eventually more inactivation. Therefore, it is more advantageous to treat products with low conductivity. The effects of the ionic composition and ionic strength of the treatment suspension on the uptake of PI by electropermeabilized plasma membranes of mammalian cells have been studied by Djuzenova et al. (7). They found that electric field-induced incorporation of PI into reversibly permeabilized cells was almost independent of the ionic composition and ionic strength of the treatment suspension but that PI incorporation increased dramatically with decreasing medium conductivity at a fixed field strength. In our studies with a gram-positive microorganism and a continuous-PEF system we also observed the latter phenomenon but only at a fixed energy input.
Similar levels of membrane permeabilization were found after PEF treatment in treatment media having pH values of 4.5 and 6.8, whereas inactivation of L. plantarum was greater at pH 4.5 than at pH 6.8. Presumably, membrane permeabilization caused an influx of H+-protons, and as a result the cells that were in a low-pH environment were more rapidly inactivated. Some evidence that supports this hypothesis is the observation of Simpson et al. (32) that PEF treatment reduced the ability of Listeria monocytogenes to maintain a pH gradient. These investigators found that the H+-ATPase activity was not affected, suggesting that this enzyme is not a primary site of bacterial inactivation during PEF treatment.
PEF treatment of exponential- and stationary-phase cells, which resulted in similar levels of membrane permeabilization, resulted in higher inactivation levels in exponentially growing cells than in stationary-phase cells. These results may indicate that factors other than membrane permeabilization are involved in determining inactivation. As cells enter the stationary growth phase, they adapt to ensure that they are able to survive under stress conditions (20, 28). The resulting physiological changes may explain their increased resistance to PEF treatment. Further research must elucidate which specific changes are responsible for the increased PEF resistance.
It is generally assumed that the induced membrane potential causes membrane permeabilization during PEF treatment (19). The membrane potential (ΔΨ) can be calculated with the following equation: ΔΨ = −f · g · r · E · cos θ(M)(1 − e−t/τ), where M is the point on the cell surface considered, E is the intensity of the electric field, f is a factor reflecting the shape of the cell, g is controlled by the electric permeability of the membrane, r is the size of the pulsed cell, θ(M) is the angle between the direction of the field and the normal of the cell surface at M pointing out of the cell, t is the time after the field is turned on, and τ is a characteristic time constant (in the microsecond range). Pore formation or membrane permeabilization begins at the moment when the induced transmembrane voltage exceeds a certain critical value, ΔΨc, which depends on the nature of the cell (41). The equation given above shows that cell size and shape can affect the induced membrane potential. Indeed, cell size was also demonstrated to be one of the parameters that affect inactivation of bacteria and yeast cells (10, 14). In the latter studies, cells of different sizes were obtained by using either different species or different strains; therefore, not only the absolute cell size but also, for example, cellular structures and membrane compositions may have differed. Our data based on cell size and shape differences in a single culture provides experimental evidence that cell morphology plays a role in determining the number of permeabilized cells and consequently in determining the number of inactivated cells. However, we found that treatment time is also an important factor, because the effect of cell size and shape gradually disappeared when treatment times were long enough.
Membrane permeabilization was studied after different heat treatments in order to investigate whether the heat generated during PEF treatment could influence the membrane permeabilization observed. No correlation was observed between inactivation and membrane permeabilization after heat treatment alone. A similar conclusion was drawn from an assessment of the viability of heat-treated cells of Lactococcus lactis subsp. lactis ML3 in which the probes carboxyfluorescein (a fluorescent probe to measure cell viability) and PI were used (3). Carboxyfluorescein and PI could distinguish between live and dead cells only in mixtures of cells treated at a relatively high temperature (70°C) and nontreated cells. The information obtained in these studies strongly suggests that during PEF treatment membrane permeabilization is caused mainly by the electric field. However, the possibility that heat has an additive effect cannot be excluded.
We compared the membrane permeabilization of two Lactobacillus strains after PEF inactivation. We used high-acid conditions because we previously demonstrated that low pH and PEF have a synergistic effect on inactivation of vegetative microorganisms (39). The L. fermentum strain was found to be more PEF resistant, both in a buffer system and in a food product (tomato juice), and showed less membrane permeabilization than the more PEF-sensitive strain, the L. plantarum strain. Thus, membrane permeabilization is probably important in determining PEF inactivation.
This study provided evidence that permeabilization of the membrane is involved in determining inactivation of vegetative cells during PEF treatment. Consequently, differences in membrane composition or properties may be important factors in determining inactivation of microorganisms by PEF treatment. Understanding the role of membrane composition or properties in inactivation resistance is important, especially since in this study it was observed that some microorganisms can be quite resistant to PEF treatment. Therefore, in order to use PEF treatment as an alternative, safe method for preserving food products, more knowledge is required about the mechanisms underlying PEF sensitivity so that conditions under which microorganisms can be optimally inactivated can be designed.
ACKNOWLEDGMENTS
This research was supported in part by a grant from EU FAIR project 97-3044 on high electric field pulses and food safety, quality, and critical process parameters.
The experimental assistance of Saskia van Rosmalen and Florence Abram and the technical assistance with the PEF apparatus of Alex Volanschi, Sebo Poel, and Jan Siebesma are gratefully acknowledged. Gerard van Dalen is thanked for assistance with the image analysis, and Stanley Brul, Leon Gorris, and Huub Lelieveld are thanked for critically reading the manuscript.
REFERENCES
- 1.Barbosa-Cánovas G V, Góngora M M, Pothakamury U R, Swanson B G. Preservation of foods with pulsed electric fields. San Diego, Calif: Academic Press; 1999. [Google Scholar]
- 2.Brown R E, Bartoletti D C, Harrison G I, Gamble T R, Bliss J G, Powell K T, Weaver J C. Multiple-pulse electroporation: uptake of a macromolecule by individual cells of Saccharomyces cerevisiae. Bioelectrochem Bioenerg. 1992;28:235–245. [Google Scholar]
- 3.Bunthof C J, van den Braak S, Breeuwer P, Rombouts F M, Abee T. Rapid fluorescence assessment of the viability of stressed Lactococcus lactis. Appl Environ Microbiol. 1999;65:3681–3689. doi: 10.1128/aem.65.8.3681-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushnell, A. H., J. E. Dunn, and R. W. Clark. September 1991. High pulsed voltage system for extending the shelf life of pumpable food products. U.S. patent 5,048,404.
- 5.Davey H M, Davey C L, Kell D B. On the determination of the cell size of microbial cells using flow cytometry. In: Lloyd D, editor. Flow cytometry in microbiology. London, United Kingdom: Springer Verlag; 1993. pp. 49–65. [Google Scholar]
- 6.Davey H M, Kell D B. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djuzenova C S, Zimmermann U, Frank H, Sukhorukov V L, Richter E, Fuhr G. Effect of medium conductivity and composition on the uptake of propidium iodide into electropermeabilized myeloma cells. Biochim Biophys Acta. 1996;1284:143–152. doi: 10.1016/s0005-2736(96)00119-8. [DOI] [PubMed] [Google Scholar]
- 8.Evrendilek G A, Zhang Q H, Richter E R. Inactivation of Escherichia coli O157:H7 and Escherichia coli 8739 in apple juice by pulsed electric fields. J Food Prot. 1999;62:793–796. doi: 10.4315/0362-028x-62.7.793. [DOI] [PubMed] [Google Scholar]
- 9.Ganeva V, Galutov B, Teissié J. Electric field mediated loading of macromolecules in intact yeast cells is critically controlled at the wall level. Biochim Biophys Acta. 1995;1240:229–236. doi: 10.1016/0005-2736(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 10.Gášková D, Sigler K, Janderová B, Plášek J. Effect of high-voltage electric field pulses on yeast cell: factors influencing the killing efficiency. Bioelectrochem Bioenerg. 1996;39:195–202. [Google Scholar]
- 11.Hamilton W A, Sale A J H. Effects of high electric fields on microorganisms. II. Killing of bacteria and yeasts. Biochim Biophys Acta. 1967;148:789–800. [Google Scholar]
- 12.Heinz V, Phillips S T, Zenker M, Knorr D. Inactivation of Bacillus subtilis by high intensity pulsed electric fields under close to isothermal conditions. Food Biotechnol. 1999;13:155–168. [Google Scholar]
- 13.Ho S Y, Mittal G S. Electroporation of cell membranes: a review. Crit Rev Biotechnol. 1996;16:349–362. doi: 10.3109/07388559609147426. [DOI] [PubMed] [Google Scholar]
- 14.Hülsheger H, Potel J, Niemann E G. Electric field effects on bacteria and yeast cells. Radiat Environ Biophys. 1983;22:149–162. doi: 10.1007/BF01338893. [DOI] [PubMed] [Google Scholar]
- 15.Jayaram S, Castle G S P, Margaritis A. The effects of high field DC pulse and liquid medium conductivity on survivability of Lactobacillus brevis. Appl Microbiol Biotechnol. 1993;40:117–122. [Google Scholar]
- 16.Jin Z T, Zhang Q H. Pulsed electric field inactivation of microorganisms and preservation of quality of cranberry juice. J Food Process Preserv. 1999;23:481–497. [Google Scholar]
- 17.Julià O, Comas J, Vives-Rego J. Second-order functions are the simplest correlations between flow cytometric light scatter and bacterial diameter. J Microbiol Methods. 2000;40:57–61. doi: 10.1016/s0167-7012(99)00132-3. [DOI] [PubMed] [Google Scholar]
- 18.Kaneshiro E S, Wyder M A, Wu Y P, Cushion M T. Reliability of calcein acetoxy methyl ester and ethidium homodimer or propidium iodide for viability assessment of microbes. J Microbiol Methods. 1993;17:1–16. [Google Scholar]
- 19.Kinosita K, Jr, Tsong T Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977;268:438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- 20.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 21.Kooiman W J, Geers J M. Simple and accurate technique for determination of heat resistance of bacterial spores. J Appl Bacteriol. 1975;38:185–189. doi: 10.1111/j.1365-2672.1975.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 22.Muraji M, Tatebe W, Konishi T, Fujii T, Berg H. Effect of electrical energy on the electropermeabilization of yeast cells. Bioelectrochem Bioenerg. 1993;31:77–84. [Google Scholar]
- 23.Muraji M, Tatebe W, Berg H. The influence of extracellular alkali and alkaline-earth ions on electropermeabilization of Saccharomyces cerevisiae. Bioelectrochem Bioenerg. 1998;46:293–295. [Google Scholar]
- 24.Muraji M, Taniguchi H, Tatebe W, Berg H. Examination of the relationship between parameters to determine electropermeability of Saccharomyces cerevisiae. Bioelectrochem Bioenerg. 1999;48:485–488. doi: 10.1016/s0302-4598(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 25.Nebe-von Caron G, Stephens P, Badley R A. Assessment of bacterial viability by flow cytometry and single cell sorting. J Appl Microbiol. 1994;84:988–998. doi: 10.1046/j.1365-2672.1998.00436.x. [DOI] [PubMed] [Google Scholar]
- 26.Qin B-L, Barbosa-Cánovas G V, Swanson B G, Pedrow P D, Olsen R G. Inactivating microorganisms using a pulsed electric field continuous treatment system. IEEE Trans Ind Applic. 1998;34:43–50. [Google Scholar]
- 27.Qiu X, Sharma S, Tuhela L, Jia M, Zhang Q H. An integrated PEF pilot plant for continuous nonthermal pasteurization of fresh orange juice. Trans ASAE (Am Soc Agric Eng) 1998;41:1069–1074. [Google Scholar]
- 28.Rees C E D, Dodd C E R, Gibson P T, Booth I R, Stewart G S A B. The significance of bacteria in stationary phase to food microbiology. Int J Food Microbiol. 1995;28:263–275. doi: 10.1016/0168-1605(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 29.Reina L D, Jin T, Zhang Q H, Yousef A E. Inactivation of Listeria monocytogenes in milk by pulsed electric field. J Food Prot. 1998;61:1203–1206. doi: 10.4315/0362-028x-61.9.1203. [DOI] [PubMed] [Google Scholar]
- 30.Rols M P, Dahhou F, Mishra K P, Teissié J. Control of electric field induced cell membrane permeabilization by membrane order. Biochemistry. 1990;29:2960–2966. doi: 10.1021/bi00464a011. [DOI] [PubMed] [Google Scholar]
- 31.Sale A J H, Hamilton W A. Effect of high electric fields on microorganisms. III. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968;163:37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 32.Simpson R K, Whittington R, Earnshaw R G, Russell N J. Pulsed high electric field causes ‘all or nothing’ membrane damage in Listeria monocytogenes and Salmonella typhimurium, but membrane H+-ATPase is not a primary target. Int J Food Microbiol. 1999;48:1–10. doi: 10.1016/s0168-1605(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 33.Sixou S, Eynard N, Escoubas J M, Werner E, Teissié J. Optimized conditions for electrotransformation of bacteria related to the extent of electropermeabilization. Biochim Biophys Acta. 1991;1088:135–138. doi: 10.1016/0167-4781(91)90163-g. [DOI] [PubMed] [Google Scholar]
- 34.Tsong T Y. On electropration of cell membranes and some related phenomena. Bioelectrochem Bioenerg. 1990;24:271–295. [Google Scholar]
- 35.Ueckert J E, Breeuwer P, Abee T, Stephens P, Nebe von-Caron G, ter Steeg P F. Flow cytometry in physiological study and detection of foodborne microorganisms. Int J Food Microbiol. 1995;28:317–326. doi: 10.1016/0168-1605(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 36.Weaver J C, Harrison G I, Bliss J G, Mourant J R, Powell K T. Electroporation: high frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988;229:30–34. doi: 10.1016/0014-5793(88)80791-9. [DOI] [PubMed] [Google Scholar]
- 37.Weaver J C, Chizmadzhev Y A. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41:135–160. [Google Scholar]
- 38.Wouters P C, Smelt J P P M. Inactivation of microorganisms with pulsed electric fields: potential for food preservation. Food Biotechnol. 1997;11:193–229. [Google Scholar]
- 39.Wouters P C, Dutreux N, Smelt J P P M, Lelieveld H L M. Effects of pulsed electric fields on inactivation kinetics of Listeria innocua. Appl Environ Microbiol. 1999;65:5364–5371. doi: 10.1128/aem.65.12.5364-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin, Y., Q. H. Zhang, and S. K. Sastry. November 1997. High voltage pulsed electric field treatment chambers for the preservation of liquid food products. U.S. patent 5,690,978.
- 41.Zimmermann U, Pilwat G, Riemann F. Dielectric breakdown of cell membranes. Biophys J. 1974;14:881–899. doi: 10.1016/S0006-3495(74)85956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]