Abstract
Background:
The MSK nomogram combined both gastroesophageal junction (GEJ) and gastric cancer patients and was created in an era from patients who generally did not receive neoadjuvant chemotherapy. We sought to re-evaluate the MSK nomogram in the era of multidisciplinary treatment for GEJ and gastric cancer.
Study design:
Using data on patients who underwent R0 resection for GEJ or gastric cancer between 2002 and 2016, the C-index of prediction for disease-specific survival (DSS) was compared between the MSK nomogram and the AJCC 8th edition staging system after segregating patients by tumor location (GEJ or gastric cancer) and neoadjuvant treatment. A new nomogram was created for the group for which both systems poorly predicted prognosis.
Results:
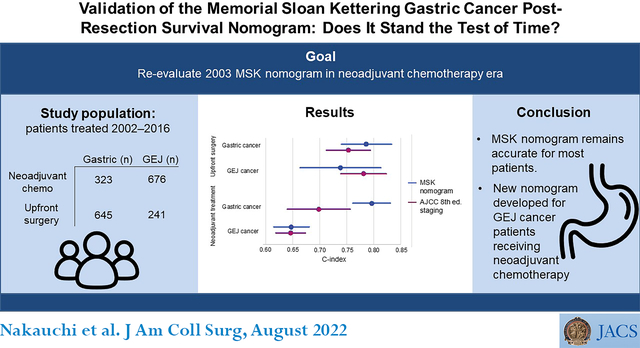
During the study period, 886 patients (645 gastric and 241 GEJ cancer) underwent upfront surgery, and 999 patients (323 gastric and 676 GEJ) received neoadjuvant treatment. Compared with the AJCC staging system, the MSK nomogram demonstrated a comparable C-index in gastric cancer patients undergoing upfront surgery (0.786 vs. 0.753) and a better C-index in gastric cancer patients receiving neoadjuvant treatment (0.796 vs. 0.698). In GEJ cancer patients receiving neoadjuvant chemotherapy, neither the MSK nomogram nor the AJCC staging system performed well (C-indices 0.647 and 0.646). A new GEJ nomogram was created based on multivariable Cox regression analysis and was validated with a C-index of 0.718.
Conclusions:
The MSK gastric cancer nomogram’s predictive accuracy remains high. We developed a new GEJ nomogram that can effectively predict DSS in patients receiving neoadjuvant treatment.
Keywords: prognostic prediction, preoperative chemotherapy
Graphical Abstract
Précis:
Re-evaluation of the 2003 Memorial Sloan Kettering nomogram revealed continued predictive accuracy for all gastric cancer patients and gastroesophageal junction (GEJ) cancer patients undergoing upfront operation. A new nomogram was created and validated for GEJ cancer patients receiving neoadjuvant chemotherapy.
Introduction
Gastric and GEJ cancers are one of the most common cancer and leading cause of cancer-related deaths in the world (1) and have a heterogenous presentation.(2) Perioperative chemotherapy with or without radiotherapy is now the standard treatment for locally advanced gastric and GEJ cancer based on the results of several phase III trials demonstrating benefit over surgery alone in Western countries.(3–6) Predicting prognosis for patients with cancer takes an important role in treatment planning and patient counseling.
The prognosis of patients with gastric or GEJ cancer is generally estimated according to the American Joint Committee on Cancer (AJCC) staging system,(2, 7) which consists of tumor depth, nodal status, and presence of metastasis. Although survival curves by stage separate well, individual outcomes vary widely, especially among patients with pathological stage II or III disease.(8) To improve the accuracy of prognostic prediction, a nomogram for gastric cancer was first developed by researchers at Memorial Sloan Kettering Cancer Center (MSK) in 2003, based on data from 1173 patients who underwent curative resection between 1985 and 2002.(8) This nomogram included 8 variables: sex, age, primary site, Lauren classification, tumor size, number of positive nodes, number of negative nodes, and pathological tumor depth. Several studies have since validated the utility of this nomogram at other institutions and internationally.(9–11) Since then other centers, mostly from Asian countries, have published variations on this nomogram, including at least 13 for gastric (12–24) and 2 for GEJ cancer,(25, 26) which incorporate different combinations of variables.
The inclusion of both GEJ and gastric cancer patients in the MSK nomogram contrasts with other prognostic tools. AJCC guidelines recommend using the staging system for esophageal adenocarcinoma for GEJ adenocarcinomas with an epicenter located within 2 cm of the anatomical GEJ and the gastric cancer staging system for any tumors with an epicenter located greater than 2 cm below the anatomical GEJ.(7) Nomograms from Asian countries have included only gastric cancer patients(12, 14–19, 21) because the incidence of GEJ cancer in Asia is low.
In addition, few patients included in the development of the MSK nomogram received neoadjuvant chemotherapy, as it was created before widespread adoption of perioperative therapy in 2005.(4) Nomograms from Asian countries are not likely to be more accurate, as they are based on data from patients who rarely receive neoadjuvant chemotherapy(12, 14–19, 21) because adjuvant chemotherapy following curative resection is the standard treatment for advanced disease in these countries.(27, 28) Given the significant changes in the treatment and outcomes of patients with GEJ and gastric cancers in the 18 years since the publication of the original MSK nomogram, we aimed to evaluate the predictive value of the nomogram in the era of multidisciplinary treatment. Our hypothesis was that because the nomogram was based on post-resection pathological variables, the introduction of neoadjuvant therapy would not affect its prognostic value.
Methods
Patient characteristics and clinicopathological data
All patients were treated in accordance with the Declaration of Helsinki, and this retrospective review was approved by the MSK institutional review board (protocol #19-111). Demographic, clinicopathological, and treatment information was collected from the prospectively maintained surgical GEJ and gastric cancer database and electronic medical records. Inclusion criteria were histologically confirmed GEJ or gastric adenocarcinoma and curative-intent resection between January 2002 and December 2016. Exclusion criteria were pathological stage IV disease, resection for remnant GEJ or gastric cancer, non-curative resection, and wedge resection without lymph node dissection.
Tumor location was classified as GEJ or gastric in the final pathological report by a dedicated gastrointestinal pathologist in accordance with the 8th edition of the AJCC staging system; tumors with an epicenter located < 2 cm into the gastric cardia were classified as GEJ cancer.(7) GEJ cancers with > 75% of the tumor located above or below the anatomical GEJ were classified as upper or lower GEJ tumors, respectively; others were classified as a middle GEJ cancer. Tumor depth (T stage), lymph node status (N status), and TNM stage were classified according to the 8th edition of the AJCC staging system.(2, 7) Tumor size and vascular invasion were collected from the final pathologic report. CT scan of the chest, abdomen, and pelvis, as well as endoscopic ultrasonography and PET scans when available, were used for clinical staging. Patients underwent staging laparoscopy before treatment to rule out occult metastatic disease, classified as biopsy-proven peritoneal carcinomatosis or positive peritoneal cytology.
In general, GEJ and gastric cancer patients with clinical T ≥ 3 and/or node-positive disease were offered neoadjuvant treatment. The regimen of neoadjuvant treatment was selected on the basis of guideline recommendations or trial regimens. For patients with GEJ cancer, a platinum-based doublet regimen (e.g. carboplatin/paclitaxel, cisplatin/paclitaxel, or cisplatin/irinotecan) with or without concurrent radiotherapy of 41.4–50.4 Gy or an epirubicin-based triplet regimen (e.g. epirubicin/cisplatin/5-fluorouracil, epirubicin/cisplatin/capecitabine, or epirubicin/oxaliplatin/capecitabine) was administered. For patients with gastric cancer, an epirubicin-based triplet regimen or FOLFOX (5-fluorouracil/oxaliplatin/leucovorin) was predominantly administered. A transthoracic approach including Ivor-Lewis esophagectomy with two-field lymphadenectomy was commonly performed for patients with GEJ cancer. A transabdominal approach with one-field lymphadenectomy was performed for patients with GEJ cancer mainly located at the abdominal esophagus or distally. In terms of extent of abdominal lymphadenectomy, D2 dissection, indicated for patients with advanced cancer, included perigastric nodes and nodes along the celiac trunk, left gastric artery, common hepatic artery, splenic artery, proper hepatic artery, and portal vein. D1 dissection, indicated for patients with clinical T1N0 cancer, included perigastric nodes. D2 dissection was commonly indicated for patients with gastric cancer, and D1 or D1+ dissection was indicated for patients with early gastric cancer. Among GEJ cancer patients, mediastinal lymphadenectomy included periesophageal, infracarinal, and hilar nodes when a transthoracic approach was used (with upper paratracheal and/or cervical lymphadenectomy if the tumor extended proximally to the mid esophagus); lower mediastinal nodes up to the level of the proximal margin and pericardial nodes were included with a transabdominal approach. Pathological chemotherapy response was assessed by an experienced gastric cancer pathologist on a scale from 0 to 100%. Neutrophil-to-lymphocyte ratio (NLR) and hemoglobin and albumin were measured prior to initiation of any treatment for GEJ or gastric cancer.
Follow-up
Follow-up after resection consisted of visits to the outpatient department and included blood tests including complete blood count and chemistry panel, as well as a CT scan of the chest, abdomen, and pelvis every 3–6 months for the first 2 years and annually for years 3–5 after surgery. Survival was measured from the date of surgery to the date of death from any cause or last follow-up, whichever occurred first. For disease-specific survival (DSS), death from recurrence of primary GEJ or gastric cancer was considered an event and death from other causes was considered as censored.
Statistical analysis
Patients were divided into 4 groups according to gastric vs. GEJ cancer and receipt or non-receipt of neoadjuvant treatment. Categorical variables were compared using chi-square test or Fisher’s exact test, and continuous variables using a Mann-Whitney U test. The accuracy of survival predictions was compared between our previous nomogram and the AJCC 8th edition staging system for each group.(2, 7) Survival curves were estimated by Kaplan-Meier methods and compared by log-rank test. Univariable and multivariable analyses for survival were performed by Cox regression analysis. Variables with p values of < 0.2 in univariable analysis were included in the multivariable analysis.
As the prior nomogram did not include patients receiving neoadjuvant treatment, which can lead to downstaging, we built a new nomogram using independently significant variables in the multivariable analysis and clinically important variables, for which the dataset was divided into a training and validation set at a 3 to 1 ratio.
Data were expressed as median (interquartile range [IQR]), odds ratio (OR; 95% confidence interval), or hazard ratio (HR; 95% confidence interval), unless otherwise stated. Missing variables were indicated as unknown and multivariable analysis did not include cases in which variables in the model were missing. P values of < 0.05 (two-tailed) were considered to be statistically significant. Statistical analyses were performed using SPSS® software version 25 (IBM, Armonk, New York, USA) or R version 3.6 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
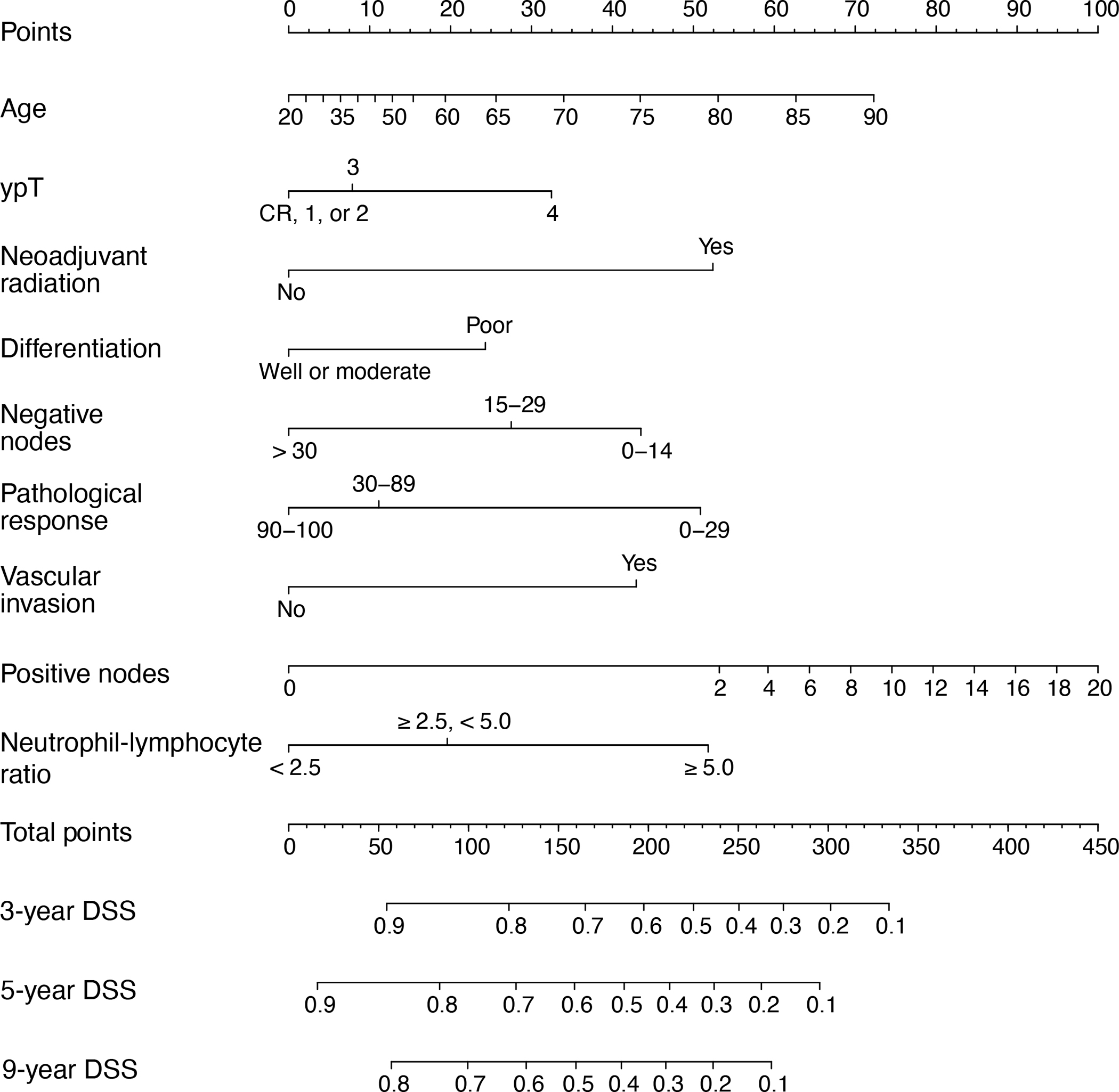
Of the 2,028 patients who underwent surgery for GEJ or gastric adenocarcinoma between January 2002 and December 2016, 1,885 patients were included in this study. Of these, 886 patients (645 [73%] gastric cancer and 241 [27%] GEJ cancer patients) underwent upfront surgery and 999 patients (323 [32%] gastric cancer and 676 [68%] GEJ cancer patients) received neoadjuvant treatment (Supplemental Digital Content 1). Approximately half of GEJ tumors were located in the middle of the anatomical GEJ and approximately half of gastric tumors were located in the lower third of the stomach (Table 1). In patients receiving neoadjuvant treatment, chemoradiotherapy was given almost exclusively to GEJ patients (84%), with only a very small number of gastric cancer patients receiving chemoradiotherapy (n = 4, 1.2%). The use of neoadjuvant chemoradiotherapy was more frequent in GEJ patients with tumors in the upper or middle GEJ (71% vs. 36% among those with tumors in the lower GEJ). GEJ cancer patients had lower T and N status compared to gastric cancer patients regardless of neoadjuvant treatment, as well as more differentiated tumors, less vascular invasion, less perineural invasion, and higher NLR, hemoglobin, and albumin (p < 0.001 for all; Table 1). The distribution of TNM stages in the 4 subgroups is shown in Supplemental Digital Content 2.
Table 1.
Patient Characteristics
| Characteristic | Entire (n = 1,885) | Upfront operation (n = 846) | Neoadjuvant treatment (n = 999) | p Value | ||
|---|---|---|---|---|---|---|
| Gastric (n = 645) | GEJ (n = 241) | Gastric (n = 323) | GEJ (n = 676) | |||
| Sex, m, n (%) | 1,283 (68.1) | 344 (53.3) | 198 (82.2) | 182 (56.3) | 559 (82.7) | <0.001 |
| Age, y, median (IQR) | 65 (56–73) | 68 (56–77) | 68 (60–74) | 63 (53–70) | 62 (56–69) | <0.001 |
| Race, n (%) | <0.001 | |||||
| White | 1,519 (80.6) | 456 (70.7) | 223 (92.5) | 218 (67.5) | 622 (92.0) | |
| Asian | 181 (9.6) | 106 (16.4) | 8 (3.3) | 44 (13.6) | 23 (3.4) | |
| Black | 98 (5.2) | 48 (7.4) | 3 (1.2) | 35 (10.8) | 12 (1.8) | |
| Other/unknown | 87 (4.6) | 35 (5.4) | 7 (2.9) | 26 (8.0) | 19 (2.8) | |
| Location, n (%) | - | |||||
| Upper GEJ | 151 (8.0) | - | 38 (15.8) | - | 113 (16.7) | |
| Middle GEJ | 546 (29.0) | - | 118 (49.0) | - | 428 (63.3) | |
| Lower GEJ | 220 (11.7) | - | 85 (35.3) | - | 135 (20.0) | |
| Upper stomach | 105 (5.6) | 55 (8.5) | - | 50 (15.5) | - | |
| Middle stomach | 337 (17.9) | 214 (33.2) | - | 123 (38.1) | - | |
| Lower stomach | 479 (25.4) | 334 (51.8) | - | 145 (44.9) | - | |
| Whole stomach | 47 (2.5) | 42 (6.5) | - | 5 (1.5) | - | |
| Neoadjuvant therapy, n (%) | ||||||
| Chemotherapy only | 425 (22.5) | 0 (0) | 0 (0) | 319 (98.8) | 106 (15.7) | |
| Chemoradiotherapy | 574 (30.5) | 0 (0) | 0 (0) | 4 (1.2) | 570 (84.3) | |
| None | 886 (47.0) | 645 (100) | 241 (100) | 0 (0) | 0 (0) | |
| Adjuvant treatment, n (%) | <0.001 | |||||
| Chemotherapy only | 290 (15.4) | 84 (13.0) | 19 (7.9) | 136 (42.1) | 51 (7.5) | |
| Chemoradiotherapy | 94 (5.0) | 48 (7.4) | 21 (8.7) | 16 (5.0) | 9 (1.3) | |
| Radiotherapy only | 6 (0.3) | 0 (0) | 0 (0) | 4 (1.2) | 2 (0.3) | |
| None | 1,495 (79.3) | 513 (79.5) | 201 (83.4) | 167 (51.7) | 614 (90.8) | |
| Type of operation, n (%) | <0.001 | |||||
| Ivor-Lewis | 780 (41.4) | 0 (0) | 181 (75.1) | 0 (0) | 599 (88.6) | |
| Total gastrectomy | 408 (21.6) | 167 (25.9) | 51 (21.2) | 113 (35.0) | 77 (11.4) | |
| Proximal gastrectomy | 23 (1.2) | 10 (1.6) | 9 (3.7) | 4 (1.2) | 0 (0) | |
| Distal gastrectomy | 674 (35.8) | 468 (72.6) | 0 (0) | 206 (63.8) | 0 (0) | |
| Pathological T stage, n (%) | <0.001 | |||||
| 1a | 264 (14.0) | 171 (26.5) | 52 (21.6) | 14 (4.3) | 27 (4.0) | |
| 1b | 412 (21.9) | 178 (27.6) | 103 (42.7) | 32 (9.9) | 99 (14.6) | |
| 2 | 261 (13.8) | 60 (9.3) | 32 (13.3) | 44 (13.6) | 125 (18.5) | |
| 3 | 544 (28.9) | 111 (17.2) | 39 (16.2) | 112 (34.7) | 282 (41.7) | |
| 4a | 227 (12.0) | 116 (18.0) | 15 (6.2) | 83 (25.7) | 13 (1.9) | |
| 4b | 18 (1.0) | 9 (1.4) | 0 (0) | 7 (2.2) | 2 (0.3) | |
| Complete response | 159 (8.4) | - | - | 31 (9.6) | 128 (18.9) | |
| Pathological N status, n (%) | <0.001 | |||||
| 0 | 1,099 (58.3) | 406 (62.9) | 160 (66.4) | 152 (47.1) | 381 (56.4) | |
| 1 | 353 (18.7) | 91 (14.1) | 48 (19.9) | 59 (18.3) | 155 (22.9) | |
| 2 | 246 (13.1) | 73 (11.3) | 16 (6.6) | 51 (15.8) | 106 (15.7) | |
| 3a | 154 (8.2) | 59 (9.1) | 16 (6.6) | 48 (14.9) | 31 (4.6) | |
| 3b | 33 (1.8) | 16 (2.5) | 1 (0.4) | 13 (4.0) | 3 (0.4) | |
| No. of dissected nodes, median (IQR) | 22 (17–30) | 21 (16–31) | 22 (16–30) | 25 (18–33) | 22 (17–28) | <0.001 |
| No. of positive nodes, median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 1 (0–5) | 0 (0–2) | <0.001 |
| Tumor size, cm, median (IQR) | 2.5 (1.4–4.5) | 2.6 (1.3–4.6) | 2.2 (1.5–3.5) | 3.5 (2.0–6.0) | 2.5 (0.7–4.2) | <0.001 |
| Pathological response, %, median (IQR) | - | - | - | 40 (10–80) | 80 (40–95) | <0.001 |
| Lauren type, n (%) | <0.001 | |||||
| Intestinal | 956 (50.7) | 308 (47.8) | 137 (56.8) | 146 (45.2) | 365 (54.0) | |
| Diffuse | 381 (20.2) | 209 (32.4) | 16 (6.6) | 104 (32.2) | 52 (7.7) | |
| Mixed | 285 (15.1) | 121 (18.8) | 25 (10.4) | 68 (21.1) | 71 (10.5) | |
| Unknown | 263 (14.0) | 7 (1.1) | 63 (26.1) | 5 (1.5) | 188 (27.8) | |
| Differentiation, n (%) | <0.001 | |||||
| Well | 113 (6.0) | 66 (10.2) | 21 (8.7) | 6 (1.9) | 20 (3.0) | |
| Moderate | 862 (45.7) | 228 (35.3) | 146 (60.6) | 98 (30.3) | 390 (57.7) | |
| Poor | 822 (43.6) | 344 (53.3) | 66 (27.4) | 207 (64.1) | 205 (30.3) | |
| Unknown | 88 (4.7) | 7 (1.1) | 8 (3.3) | 12 (3.7) | 61 (9.0) | |
| Vascular invasion, n (%) | <0.001 | |||||
| No | 1,143 (60.6) | 372 (57.7) | 147 (61.0) | 163 (50.5) | 461 (68.2) | |
| Yes | 733 (38.9) | 270 (41.9) | 94 (39.0) | 156 (48.3) | 213 (31.5) | |
| Unknown | 9 (0.5) | 3 (0.5) | 0 (0) | 4 (1.2) | 2 (0.3) | |
| Neutrophil-lymphocyte ratio, median (IQR) | 2.8 (2.0–3.9) | 2.5 (1.8–3.5) | 2.6 (2.1–3.7) | 2.5 (1.9–3.4) | 3.3 (2.3–4.8) | <0.001 |
| Hemoglobin, median (IQR) | 12.7 (11.4–13.9) | 12.7 (11.4–13.8) | 13.6 (12.4–14.6) | 12.0 (10.9–13.3) | 12.8 (11.4–14.0) | <0.001 |
| Albumin, median (IQR) | 4.2 (3.9–4.4) | 4.2 (4.0–4.4) | 4.3 (4.1–4.5) | 4.1 (3.8–4.3) | 4.2 (3.9–4.4) | <0.001 |
p Values compare gastric vs GEJ cancer patients.
IQR, interquartile range; GEJ, gastroesophageal junction
Disease-specific survival
After a median follow-up of 42 months (IQR 20–70), 5-year DSS was 81% and 76% for gastric and GEJ cancer patients undergoing upfront surgery, respectively. For patients receiving neoadjuvant therapy, 5-year DSS was 67% and 53% for gastric and GEJ cancer patients, respectively (Fig. 1). DSS in each group according to AJCC TNM stage is shown in Supplemental Digital Content 3 and 4. In gastric cancer patients receiving neoadjuvant treatment, 31 (9.6%) patients had pathological complete response at the primary site (ypTCR). Five-year DSS for such patients was 92% (Supplemental Digital Content 4a). In GEJ cancer patients receiving neoadjuvant treatment, 128 (18.9%) patients had ypTCR.
Figure 1.
Disease-specific survival (DSS) according to tumor site (gastroesophageal junction [GEJ] vs. gastric) and receipt of neoadjuvant treatment (Tx).
Evaluation of previous MSK nomogram and AJCC staging system
The C-index of the MSK nomogram for predicting DSS in the entire cohort was 0.756 (95% CI 0.736–0.776, Figure 2). In the upfront surgery cohort, the MSK nomogram demonstrated a slightly higher C-index in gastric cancer patients compared with AJCC staging, whereas AJCC staging demonstrated a somewhat higher C-index in GEJ cancer patients compared with the MSK nomogram, though 95% CIs overlapped for both comparisons. In patients receiving neoadjuvant treatment, the MSK nomogram again had a higher C-index in gastric cancer patients compared with AJCC staging, while both the AJCC staging and the MSK nomogram demonstrated low predictive value in GEJ cancer patients (C-index 0.647 and 0.646, respectively, Fig. 2).
Figure 2.
Concordance index (C-index) of the Memorial Sloan Kettering (MSK) gastric cancer nomogram and the American Joint Committee on Cancer (AJCC) 8th edition TNM staging system for gastroesophageal junction (GEJ) and gastric cancer patients who did or did not receive neoadjuvant treatment. Bars represent 95% CIs.
Revised nomogram for GEJ patients receiving neoadjuvant treatment
From the 676 GEJ cancer patients who received neoadjuvant treatment, training and validation sets were created. There was no significant difference in clinicopathological characteristics between the two groups (Supplemental Digital Content 5). In the training set, independently significant predictors for worse DSS identified by multivariable Cox regression analysis included number of positive nodes (HR 1.08, p = 0.003), poorly differentiated tumors (HR 1.48, p = 0.018), vascular invasion (HR 1.78, p = 0.003), neoadjuvant radiation (HR 1.88, p = 0.005), and NLR ≥ 5.0 (HR 1.62, p = 0.036). Predictors of improved DSS included ≥ 30 negative nodes (HR 0.54, p = 0.026) and pathological treatment response ≥ 30% (HR 0.60, p = 0.014) and ≥ 90% (HR 0.52, p = 0.031) (Table 2).
Table 2.
Factors Associated with Disease-Specific Survival in the Training Set
| Factor | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.01 | 1.00–1.02 | 0.189 | 1.01 | 1.00–1.03 | 0.071 |
| Sex, f | 1.12 | 0.79–1.57 | 0.523 | - | - | - |
| Race | ||||||
| White | 1 | - | - | 1 | - | - |
| Non-White | 0.69 | 0.39–1.21 | 0.191 | 0.88 | 0.48–1.62 | 0.672 |
| Tumor location | ||||||
| Upper GEJ | 1 | - | - | - | - | - |
| Middle GEJ | 1.02 | 0.67–1.55 | 0.936 | - | - | - |
| Lower GEJ | 1.04 | 0.64–1.69 | 0.881 | - | - | - |
| ypT | ||||||
| 1 | 1 | - | - | 1 | - | - |
| 2 | 1.16 | 0.71–1.89 | 0.556 | 0.77 | 0.45–1.33 | 0.343 |
| 3 | 1.78 | 1.19–2.65 | 0.005 | 0.90 | 0.54–1.50 | 0.683 |
| 4 | 4.07 | 1.93–8.57 | <0.001 | 1.29 | 0.51–3.27 | 0.596 |
| Complete response | 0.88 | 0.53–1.46 | 0.616 | 0.67 | 0.33–1.34 | 0.256 |
| No. of positive lymph nodes | 1.12 | 1.08–1.16 | <0.001 | 1.08 | 1.03–1.13 | 0.003* |
| No. of negative lymph nodes | ||||||
| <15 | 1 | - | - | 1 | - | - |
| ≥15, <30 | 0.59 | 0.43–0.82 | 0.001 | 0.86 | 0.58–1.28 | 0.468 |
| ≥30 | 0.36 | 0.23–0.57 | <0.001 | 0.54 | 0.32–0.93 | 0.026* |
| Differentiation | ||||||
| Well/moderately | 1 | - | - | 1 | - | - |
| Poorly | 1.31 | 0.99–1.75 | 0.064 | 1.48 | 1.07–2.05 | 0.018* |
| Vascular invasion | 2.36 | 1.79–3.10 | <0.001 | 1.78 | 1.23–2.60 | 0.003 |
| Tumor size, cm | 1.09 | 1.03–1.15 | 0.004 | 1.00 | 0.92–1.09 | 0.993 |
| Neoadjuvant therapy | ||||||
| Chemotherapy only | 1 | - | - | 1 | - | - |
| Chemoradiotherapy | 1.34 | 0.91–1.97 | 0.137 | 1.88 | 1.21–2.94 | 0.005* |
| Adjuvant therapy | ||||||
| None | 1 | - | - | - | - | - |
| Chemotherapy only | 0.89 | 0.54–1.45 | 0.631 | - | - | - |
| Chemoradiotherapy/radiotherapy | 0.93 | 0.38–2.26 | 0.871 | - | - | - |
| NAC response rate | ||||||
| <30% | 1 | - | - | 1 | - | - |
| ≥30%, <90% | 0.61 | 0.43–0.86 | 0.005 | 0.60 | 0.40–0.90 | 0.014* |
| ≥90% | 0.41 | 0.28–0.58 | <0.001 | 0.52 | 0.29–0.94 | 0.031* |
| Neutrophil-lymphocyte ratio | ||||||
| <2.5 | 1 | - | - | 1 | - | - |
| ≥ 2.5, <5.0 | 1.38 | 0.98–1.94 | 0.065 | 1.30 | 0.90–1.88 | 0.156 |
| ≥ 5.0 | 1.75 | 1.18–2.59 | 0.005 | 1.62 | 1.03–2.54 | 0.036* |
Statistically significant
GEJ, gastroesophageal junction; NAC, neoadjuvant chemotherapy
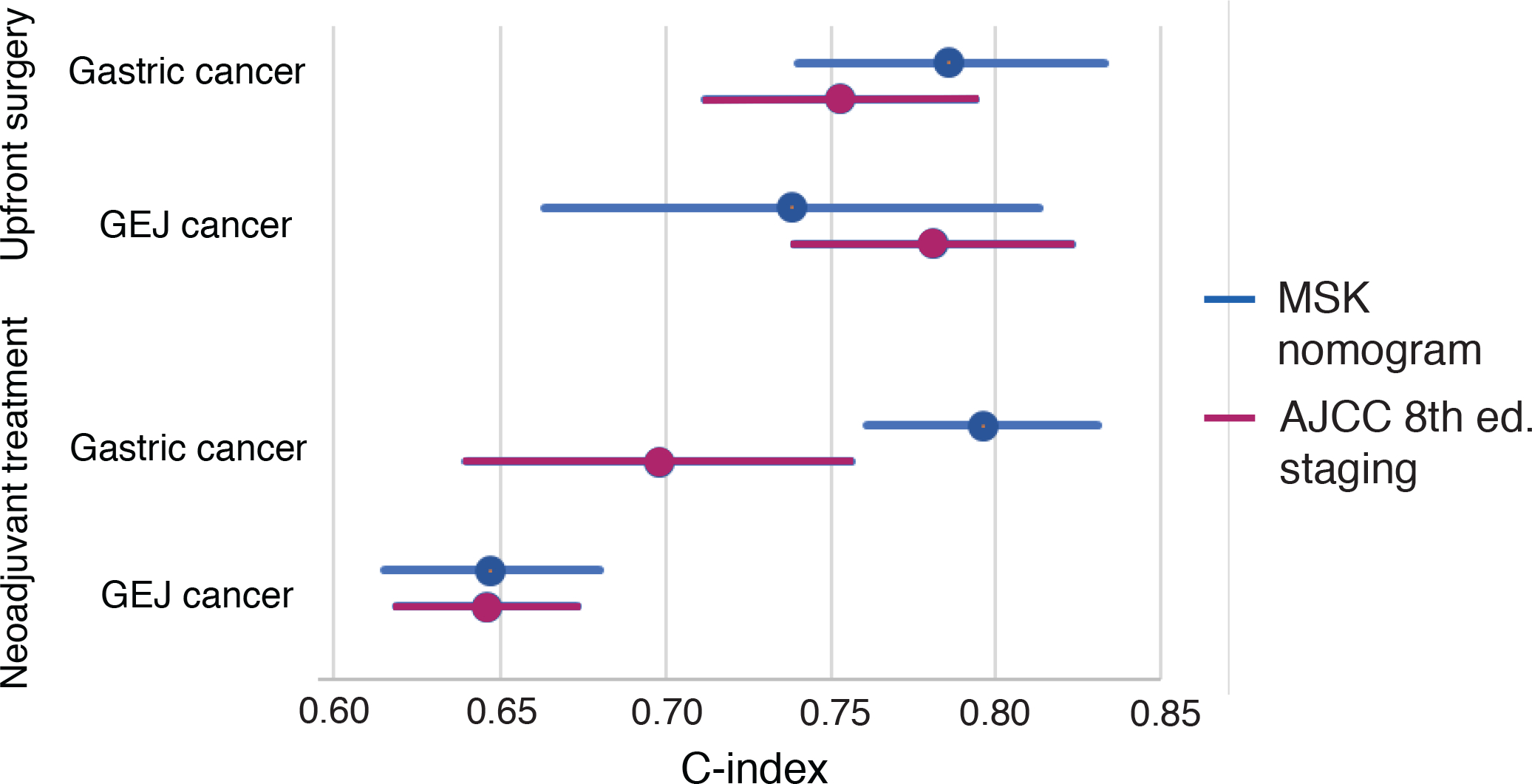
The C-index for the prediction model incorporating these 7 variables was 0.665 [95% CI 0.635–0.696]. A nomogram was created that added age and ypT status to these factors, resulting in a model with 9 variables and a C-index of 0.669 [95% CI 0.619–0.673] in the training set and 0.718 [95% CI 0.672–0.764] in the validation set (Fig. 3), both higher than the C-indices for the AJCC staging system in each set. The calibration plot of the new nomogram showed a high degree of similarity between the actual and estimated DSS at 3, 5, and 9 years after surgery (Fig. 4).
Figure 3.
New nomogram for gastroesophageal junction cancer patients receiving neoadjuvant treatment. CR, complete response; DSS, disease-specific survival
Figure 4.
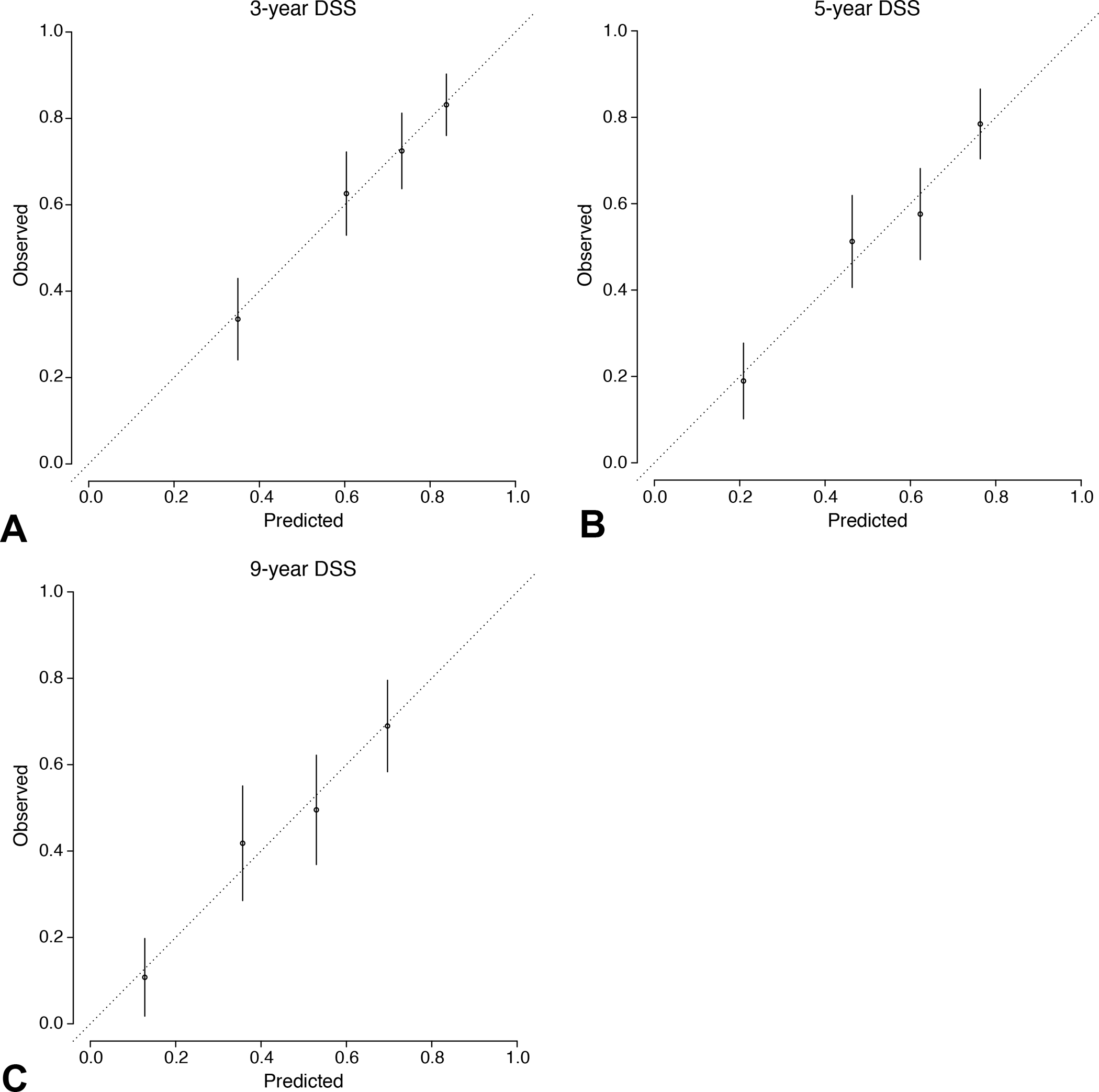
Calibration curve comparing the nomogram’s prediction of survival vs observed survival (mean, 95% CI for each quartile) at (A) 3, (B) 5, and (C) 9 years after operation. DSS, disease-specific survival.
Discussion
Management of gastric and GEJ cancers has changed significantly since the publication of the classic MSK nomogram in 2003, particularly with the addition of neoadjuvant therapy prior to resection for most patients. We therefore sought to re-evaluate the prognostic and predictive value of the MSK nomogram for patients treated under the current standard of care and compared it to that of the AJCC 8th edition staging system. The MSK nomogram prevailed in providing reliable prognostic prediction for DSS in patients who did not receive neoadjuvant treatment, with a C-index of 0.786 and 0.738 in gastric and GEJ cancer patients, respectively, within the range of C-indices of other published nomograms for gastric cancer, 0.68 to 0.87.(12, 14, 15, 17–19) This similarity likely reflects its inclusion of many of the same clinicopathological variables(8) as other nomograms, namely age, sex, tumor location, tumor size, number or status of metastatic lymph nodes, and pathological tumor depth.(12, 14–16, 18)
For GEJ cancer patients treated with upfront surgery, the AJCC staging system provided relatively better prediction compared with the MSK nomogram. This superior accuracy likely reflects the AJCC system’s incorporation of 10 categories (i.e., Stage 0 to IVB), including tumor grade for Stage IA to IIA disease,(7) which accounted for 60.2% of GEJ cancer patients in this study.
The previous MSK nomogram showed significantly better performance in gastric cancer patients receiving neoadjuvant treatment when compared with the AJCC staging system despite the fact that it was created in an era when neoadjuvant treatment was not widely applied (C-index 0.796 vs. 0.698). Only one nomogram for such patients was reported from China, which consisted of body mass index, tumor location, pathological T stage, and pathological N status and had a C-index of 0.74,(24) lower than that of the MSK nomogram in the current study. Although neither the Chinese nor the MSK nomogram include variables reflecting the effect of neoadjuvant treatment, they predict prognosis fairly accurately. Pathological response after neoadjuvant chemotherapy has been reported not to be an independent predictor of overall survival in gastric cancer patients.(4) Thus, post-resection variables such as ypT and N status predict survival regardless of receipt of neoadjuvant chemotherapy.
The survival of gastric cancer patients with pathological complete response (ypTCR) following neoadjuvant chemotherapy cannot be estimated using either the MSK nomogram or the AJCC staging system. The present study showed a very good prognosis for ypTCR patients; however, only 31 patients with ypTCR were identified, limiting the applicability of this finding. As more such patients are identified, the nomogram could be further revised in the future.
We created a new nomogram for GEJ cancer patients receiving neoadjuvant treatment and demonstrated its prognostic accuracy (C-index 0.718), comparable to that of the AJCC staging system. It proved more accurate compared with another nomogram for GEJ cancer patients treated with neoadjuvant chemoradiotherapy, which was developed using data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and had a C-index of 0.61.(26) Its accuracy cannot be compared with the other GEJ cancer nomogram reported by Zhou et al., also based on SEER data, because it did not include information on neoadjuvant treatment.(25) In addition, these 2 nomograms predicted overall survival, whereas our study focused on DSS, an endpoint that is not available from administrative databases.
In this study, we also analyzed NLR as a candidate variable given the results of multiple studies including our previous study,(29) demonstrating an association between high NLR and poor survival in several cancers.(30) NLR was an independent predictor for DSS in GEJ cancer patients receiving neoadjuvant treatment, similar to Choi et al.’s findings in a nomogram for gastric cancer patients.(21) NLR is a readily available marker in clinical practice, as it can be calculated easily from a complete blood count.
The previous MSK nomogram and new GEJ nomogram reported here, similar to most published nomograms, are based on regression models, partially overcoming the problem of heterogeneity in stage.(31) These models are limited by their focus on specific variables, as they cannot account for the effects of combination. More sophisticated approaches using artificial intelligence can now account for combinatorial interactions,(31–33) including a recent nomogram that predicts the number of lymph node metastases in locally advanced gastric cancer,(34) which could improve the performance of prognostic prediction models.
There are several limitations to this study. Selection bias is inherent in any retrospective study design. Patients received a number of neoadjuvant chemotherapy regimens because recommendations changed during the 15-year study period, and chemotherapy dose intensity was not available. In addition, improved efficacy of newer chemotherapy regimens, in addition to the use of targeted therapy and checkpoint blockade for patients who experienced recurrence during the follow-up period, may have affected disease-specific outcomes. Increasing use of these new regimens, including in the neoadjuvant setting, could affect survival, calling for future re-assessment of the previous MSK nomogram and the new GEJ cancer nomogram. The new nomogram for GEJ in the present study was created and validated using data from the same group of 676 patients and should be externally validated using data from a larger cohort in the future. Finally, because more than 90% of the patients in the cohort used to develop the new GEJ cancer nomogram were white, it should be validated in cohorts of other ethnic backgrounds.
Conclusions
This study shows that the classic MSK gastric cancer nomogram continues to provide accurate prognostic information for patients treated with modern regimens that include neoadjuvant chemotherapy and chemoradiotherapy. Additionally, we developed a new GEJ nomogram that can more effectively predict DSS in patients receiving neoadjuvant treatment and help to individualize prognostic assessment and clinical decisions.
Supplementary Material
Supplemental Digital Content 3. Disease-specific survival (DSS) according to tumor stage (American Joint Committee on Cancer 8th edition) in patients undergoing upfront operation. (A) Gastric and (B) gastroesophageal junction cancer.
Supplemental Digital Content 4. Disease-specific survival (DSS) according to tumor stage (American Joint Committee on Cancer 8th edition) in patients receiving neoadjuvant treatment. (A) Gastric and (B) gastroesophageal junction cancer.
Supplemental Digital Content 5. Clinicopathological Characteristics in the Training and Validation Sets for the New Gastroesophageal Junction Cancer Nomogram
Supplemental Digital Content 1. Consolidated Standards of Reporting Trials diagram of the study population. GEJ, gastroesophageal junction.
Supplemental Digital Content 2. Distribution of American Joint Committee on Cancer Stage According to Tumor Site and Receipt of Neoadjuvant Treatment
Acknowledgement
We gratefully acknowledge Jessica Moore, MS, of Memorial Sloan Kettering Cancer Center for editing this manuscript. We also thank Elvira Vos, MD, PhD, Geoffrey Ku, MD, David H. Ilson, MD, and Sam S. Yoon, MD, for critical review of the manuscript.
Support:
This research was supported in part by the NIH/National Cancer Institute Cancer Center Support grant [P30 CA008748]. Dr Brennan is supported by the De Beaumont Foundation. Dr Janjigian is supported by Cycle for Survival, Fred’s Team, the US Department of Defense [W81XWH-16-1-0341], and the National Cancer Institute [R01 CA244233].
Footnotes
Disclosures outside the scope of this work: Dr Janjigian received research funding from RGENIX, Boehringer Ingelheim, Bayer, Genentech/Roche, Bristol Myers Squibb, Eli Lilly, and Merck; served on advisory boards for RGENIX, Merck Serono, Bristol Myers Squibb, Eli Lilly, Pfizer, Bayer, Imugene, Merck, Daiichi-Sankyo, Basilea Pharmaceutica and AstraZeneca; and is a paid consultant to Michael J Hennessy Associates, Paradigm Medical Communications, and Seagen Zymeworks, Inc. Dr Ku received honoraria payments and research funding from Merck, Bristol Myers Squibb, and Pieris, and research funding from AstraZeneca, Zymeworks, and Daiichi Sankyo. Dr Ilson has received research funding from and served on advisory boards for Astellas, Eli Lilly, Pieris, and Taiho, and served on advisory boards for AstraZeneca, Amgen, Bayer, Bristol-Myers Squibb, and Roche. Dr Maron has received research support from Genentech and Guardant Health, receives honoraria payments from Basilea Pharmaceutica, Natera, and Daiichi-Sankyo, Bicara, Novartis, owned stock in Calithera, and receives travel support from Bayer. Dr. Molena is a paid consultant to AstraZeneca, Johnson & Johnson, Boston Scientific, and Bristol Myers Squibb. Other authors have nothing to disclose.
Disclosure Information: Nothing to disclose.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. Nov;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016. Oct;14(10):1286–312. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015. Sep;16(9):1090–8. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006. Jul 6;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017. Mar;18(3):357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019. May 11;393(10184):1948–57. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019. Jul 1;17(7):855–83. [DOI] [PubMed] [Google Scholar]
- 8.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003. Oct 1;21(19):3647–50. [DOI] [PubMed] [Google Scholar]
- 9.Novotny AR, Schuhmacher C, Busch R, et al. Predicting individual survival after gastric cancer resection: validation of a U.S.-derived nomogram at a single high-volume center in Europe. Ann Surg. 2006. Jan;243(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Jiang B, Xing J, et al. Validation of the memorial Sloan-Kettering Cancer Center nomogram to predict disease-specific survival after R0 resection in a Chinese gastric cancer population. PLoS One. 2013;8(10):e76041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou ML, Wang L, Wang JZ, et al. Validation of the Memorial Sloan Kettering Cancer Center nomogram to predict disease-specific survival in a Chinese gastric cancer population receiving postoperative chemoradiotherapy after an R0 resection. Oncotarget. 2016. Oct 4;7(40):64757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012. Nov 1;30(31):3834–40. [DOI] [PubMed] [Google Scholar]
- 13.Dikken JL, Baser RE, Gonen M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann Surg Oncol. 2013. May;20(5):1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol. 2014. Jun;25(6):1179–84. [DOI] [PubMed] [Google Scholar]
- 15.Song KY, Park YG, Jeon HM, Park CH. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer. 2014. Apr;17(2):287–93. [DOI] [PubMed] [Google Scholar]
- 16.Eom BW, Ryu KW, Nam BH, et al. Survival nomogram for curatively resected Korean gastric cancer patients: multicenter retrospective analysis with external validation. PLoS One. 2015;10(2):e0119671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Geng Q, Chen S, et al. Nomogram based on systemic inflammatory response markers predicting the survival of patients with resectable gastric cancer after D2 gastrectomy. Oncotarget. 2016. Jun 21;7(25):37556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Sun Z, Deng JY, et al. A novel nomogram individually predicting disease-specific survival after D2 gastrectomy for advanced gastric cancer. Cancer Commun (Lond). 2018. May 15;38(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng ZF, Lu J, Wang W, et al. Development and External Validation of a Simplified Nomogram Predicting Individual Survival After R0 Resection for Gastric Cancer: An International, Multicenter Study. Ann Surg Oncol. 2018. Aug;25(8):2383–90. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Rao H, Liu J, et al. Lymph nodes ratio based nomogram predicts survival of resectable gastric cancer regardless of the number of examined lymph nodes. Oncotarget. 2017. Jul 11;8(28):45585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JH, Suh YS, Choi Y, et al. Comprehensive Analysis of the Neutrophil-to-Lymphocyte Ratio for Preoperative Prognostic Prediction Nomogram in Gastric Cancer. World J Surg. 2018. Aug;42(8):2530–41. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Kim HS, Seo WY, et al. External validation of nomogram for the prediction of recurrence after curative resection in early gastric cancer. Ann Oncol. 2012. Feb;23(2):361–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang PF, Du ZD, Wen F, et al. Development and validation of a nomogram for predicting overall survival of gastric cancer patients after D2R0 resection. Eur J Cancer Care (Engl). 2020. Sep;29(5):e13260. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Xiao Q, Wang Y, et al. A Modified ypTNM Staging System-Development and External Validation of a Nomogram Predicting the Overall Survival of Gastric Cancer Patients Received Neoadjuvant Chemotherapy. Cancer Manag Res. 2020;12:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Zhang H, Xu Z, et al. Nomogram predicted survival of patients with adenocarcinoma of esophagogastric junction. World J Surg Oncol. 2015. Jun 10;13:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Zhou R, Jiang F, et al. Proposal of a Nomogram for Predicting Survival in Patients with Siewert Type II Adenocarcinoma of the Esophagogastric Junction After Preoperative Radiation. Ann Surg Oncol. 2019. May;26(5):1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007. Nov 1;357(18):1810–20. [DOI] [PubMed] [Google Scholar]
- 28.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012. Jan 28;379(9813):315–21. [DOI] [PubMed] [Google Scholar]
- 29.Wang SC, Chou JF, Strong VE, et al. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Resectable Gastroesophageal Junction and Gastric Adenocarcinoma. Ann Surg. 2016. Feb;263(2):292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014. Jun;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 31.Brennan M, Gonen M. The Problem of -Heterogeneity Within Stage. 2020.
- 32.Huang B, Tian S, Zhan N, et al. Accurate diagnosis and prognosis prediction of gastric cancer using deep learning on digital pathological images: A retrospective multicentre study. EBioMedicine. 2021. Nov;73:103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Wang X, Li L, et al. Machine Learning-Based Model for the Prognosis of Postoperative Gastric Cancer. Cancer Manag Res. 2022. Jan;7(14):135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong D, Fang MJ, Tang L, et al. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multi-center study. Ann Oncol. 2020. Jul;31(7):912–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 3. Disease-specific survival (DSS) according to tumor stage (American Joint Committee on Cancer 8th edition) in patients undergoing upfront operation. (A) Gastric and (B) gastroesophageal junction cancer.
Supplemental Digital Content 4. Disease-specific survival (DSS) according to tumor stage (American Joint Committee on Cancer 8th edition) in patients receiving neoadjuvant treatment. (A) Gastric and (B) gastroesophageal junction cancer.
Supplemental Digital Content 5. Clinicopathological Characteristics in the Training and Validation Sets for the New Gastroesophageal Junction Cancer Nomogram
Supplemental Digital Content 1. Consolidated Standards of Reporting Trials diagram of the study population. GEJ, gastroesophageal junction.
Supplemental Digital Content 2. Distribution of American Joint Committee on Cancer Stage According to Tumor Site and Receipt of Neoadjuvant Treatment


