Abstract
Less-common fruits from Cornus spp. (Cornaceae), also named dogwoods, have shown antidiabetic, antibacterial and anti-allergic properties and are thus considered a source of phytochemicals that are beneficial to human health. The study aimed to compare the chemical compositions of the aqueous and ethanolic extracts of lyophilized fresh-picked and commercially available dried fruits of Cornus mas (Cm, cornelian cherry) and Cornus alba (Ca) fruits using HPLC-DAD-MS/MS method. Simultaneously, the a-amylase and pancreatic lipase (PL) inhibitory activities of the prepared extracts were compared by in vitro fluorescence assay based on the kinetic hydrolysis of starch or oleate ester of 4-methylumbelliferone (MUO), respectively. Additionally, a bio-assay guided identification of compounds potentially responsible for the inhibition of pancreatic enzymes was performed. Iridoids (loganic acid, cornuside) and anthocyanins (pelargonidin 3-O-galactoside) were identified in the Cmfruit extracts. Flavonoids, such as quercetin and kaempferol derivatives, were detected in the Ca fruit extracts. The chromatographic separation of the constituents of Ca fruit provided a fraction containing phenolic acids derivatives, which inhibited PL activity by 69.9 ± 4.5% at a concentration of 7.5 μg·mL−1. The IC50 of hydroxytyrosol glucoside, isolated from the most active Ca fraction, was 0.99 ± 0.10 mg·mL−1 indicating other constituents responsible for the fraction activity. The most active subfraction from Cm fruit (7.5 μg·mL−1), which inhibited PL activity by 28.3 ± 1.5%, contained pelargonidin 3-O-galactoside. Loganic acid and cornuside in highly pure form did not inhibit lipase activity. The phytochemical constituents of Cm, and particularly of Ca fruit extracts, can inhibit pancreatic enzymes and thus might be considered effective preparations in the prevention and control of hyperlipidemia related diseases.
Keywords: Anthocyanins, Digestive enzymes, Iridoids, Metabolic syndrome, Phenolic compounds
1. Introduction
Less-common fruits including cornelian cherry are grown in specific rural areas around the world under poor cultivation conditions, and these fruits are of considerable significance as a prominent source of phytochemicals that are important for human health and wellbeing. Fruits from Cornus spp. (Cornaceae), commonly named dogwoods, have been traditionally used to improve liver and kidney functions [1,2]. Cornus mas L. (Cm), also named cornelian cherry, is a species of dogwood native to southern Europe and Southwest Asia and has been used in the treatment of gastrointestinal disorders and diarrhea. Due to its flavor and high content of antioxidants, juices, liqueurs, wines and jams from cornelian cherry fruits are commonly consumed and are considered potential phytomedicines [2]. In randomized clinical studies, an amelioration of lipid profile, concentrations of apolipoproteins as well as intracellular and vascular cell adhesion molecules by the Cm fruits were observed in dyslipidemic children and adolescents [3]. Fruit of Cm significantly reduced blood glucose levels and increased insulin levels in the diabetic rats as well as regulated β-cell function in mice fed a high-fat diet [4,5]. Despite some data on the antidiabetic and lipid-modifying effects of cornelian cherry fruits [6], their glucose- and lipid-lowering mechanisms are not well understood. It is believed that anthocyanins and other phenolic compounds likely protect pancreatic β-cells via their antioxidant activity and stimulate insulin secretion [7]. To date, anthocyanins, such as cyanidin and pelargonidin derivatives, as well as flavonoids, particularly quercetin glycosides like, kaempferol-3-O-galactoside and aromadendrin-7-O-glucoside, and iridoids, such as loganic acid and cornuside, have been detected in the fruits of Cm[8,9]. On the other hand, the compounds identified in another dogwood species, Cornus alba L. (Ca), were flavonoids, such as quercetin-3-O-glucuronide, kaempferol and quercetin 3-O-glucosides, as well as hydrolysable tannins, including 1,2,3,4,6-penta-O-galloylglucoside (PGG), and cornusiins A and B [10]. According to the available data, β-PGG is considered a non-competitive inhibitor of human salivary amylase. Both the amino acids residues of the enzyme as well as galloyl units of β-PGG are considered crucial factors for amylases inhibition [11].
Therefore, a comparative study of these two Cornus species with different phytochemical profiles to define the potency and utility of their anti-amylase and anti-lipase activities is justified. The most common antidiabetic and anti-obesity drugs, such as acarbose and orlistat, target digestion and absorption through the inhibition of enzymes such as pancreatic lipase (PL), a-amylase or a-glucosidase [12–14]. However, management of hyperlipidemia and associated diseases with drugs that do not have side effects remains a challenge, and searching of effective digestive enzyme inhibitors from natural sources, which are characterized by lower systemic adverse effects rates, should be considered.
Thus, the aim of this study was to investigate and compare the in vitro pancreatic lipase and a-amylase inhibitory activities of the aqueous and ethanolic extracts from both lyophilized C. mas fresh-picked fruits and dried fruits purchased commercially as well as C. alba fruits picked fresh. Additionally, the phytochemical compositions of the preparations from both species of Cornus were analyzed. To determine which compounds might be responsible for the activity of C. mas extracts as well as the extracts of less-common C. alba fruits, a bio-assay guided identification was conducted based on the pancreatic lipase inhibition.
2. Materials
2.1. Chemicals
Acetonitrile (MeCN, UHPLC-grade), n-butanol (BuOH), chloroform (CHCl3), ethyl acetate (EtOAc), ethanol (EtOH) and methanol (MeOH) for extraction were obtained from POCH (Gliwice, Poland). Natural product reagent A (diphenylboric acid 2-aminoethyl ester) and quercetin 3-O-glucoside (iso-quercitrin) were purchased from Carl Roth GmbH (Karlsruhe, Germany). Formic acid (HCOOH) for uses as an additive in the UHPLC-MS eluent, orlistat, 4-methylumbelliferyl oleate (MUO), acarbose and pancreatin from porcine pancreas were purchased from Sigma–Aldrich Chemie GmbH (Steinheim, Germany). The standards of kaempferol 3-O-glucoside (astragalin), quercetin 3-O-rhamnoside (quercitrin) and quercetin 3-O-rutinoside (rutin) were purchased from Sigma–Aldrich Chemie GmbH (Steinheim, Germany). Quercetin 3-O-galactoside (hyperoside) was purchased from HWI Analytik GmbH (Rheinzaberner, Germany). The standards of quercetin 3-O-glucuronide, kaempferol 3-O-glucuronide, quercetin 3-O-β-D-(6″-O-malonyl)-glucoside and hydroxytyrosol were isolated in the Department of Pharmacognosy and Molecular Basis of Phytotherapy, Medical University of Warsaw (Poland) [15–17]. An EnzChek™ Ultra Amylase Assay Kit (Invitrogen, Pailey, UK) was used. Tris–HCl buffer was prepared as follows: 13 mM Tris–HCl (Promega Corporation, Madison, USA), 150 mM NaCl (POCH, Gliwice, Poland), and 1.3 mM CaCl2 (POCH, Gliwice, Poland). Water was obtained using Millipore Simfilter Simplicity UV (Molsheim, France) water purification system.
2.2. Plant material
C. mas fruits were collected in September 2016 in Dąbrowa Chotomowska in the Masovian District in Poland (52°25′34″N, 20°51′50″E), and C. alba fruits were collected in the Ursynów District of Warsaw, Poland (52°09′01″N, 21°03′01″E). Specimens (No FW25_20160914_CM, No FW25_20160929_CA) are available in the herbarium of the Department of Pharmacognosy and Molecular Basis of Phytotherapy, Medical University of Warsaw. The plant material was identified by Monika E. Czerwińska supported by Konrad Woliński (biologist, MSc) from the Botanical Garden – Center for Biological Diversity Conservation in Powsin (Polish Academy of Sciences, Poland) according to Rutkowski’s plant guidebook [18]. The fresh-picked fruits were lyophilized before extractions. Commercially available samples of dried Cm fruits distributed by Dary Natury (CmDN; series No 01.01.2017) and Eko Herba (CmEH; series No 139/10.2018) were purchased from herbal stores. A sample of each plant material was used to prepare extracts.
2.3. Preparation and fractionation of extracts and isolation of the constituents
Preparation of the ethanolic extracts of the fruits: A 10-g portion of powdered plant material was extracted under reflux (95 °C) four times with aqueous ethanol (60%, v/v) in a ratio of 1:10 for 30 min each time. The collected ethanolic extracts were concentrated under reduced pressure and lyophilized. The obtained dry weights of the ethanolic extracts from the fruit samples were 3.31 g (Cm), 4.24 g (CmDN), 4.55 g (CmEH) and 3.13 g (Ca).
Preparation of the aqueous extracts of the fruits: A 5-g of powdered plant material was extracted three times with boiling water in a ratio of 1:40 for 15 min each time. The collected aqueous extracts were concentrated under reduced pressure and lyophilized. The obtained dry weights of the aqueous extracts from the fruit samples were 1.62 g (Cm), 2.05 g (CmDN), 2.07 g (CmEH) and 1.18 g (Ca).
Isolation of compounds: A 71-g portion of powdered Cm fruits and 150 g of Ca fruits were macerated three times with aqueous methanol (70%, v/v) (ratio 1:10) for 1 h each time. The organic solvent was evaporated under reduced pressure at 40 °C. The obtained dry weights of the methanolic extracts from the fruits of Cm and Ca were 5.26 g and 6.33 g, respectively. Next, the residues of Cm and Ca fruits were suspended in water and partitioned between chloroform, ethyl acetate and n-butanol saturated with water, and each extraction was conducted with 1:1 ratio of residue and solvent. The obtained Cm fractions were evaporated to dryness under reduced pressure or lyophilized giving 0.01 g, 1.13 g and 0.69 g of residue, respectively. The obtained Ca fractions were evaporated under reduced pressure to dryness or lyophilized giving 0.15 g, 2.10 g and 4.04 g of residue, respectively.
Based on the PL inhibition results, the selected fractions, CmEtOAc, CaEtOAc and CaBuOH were subjected to column chromatography on Sephadex LH-20 (2.5 cm × 130 cm), eluted with MeOH(70%, v/v) to give 144 fractions each. These fractions were combined into 3 (CmEtOAcA – CmEtOAcC) and 4 (CaEtOAcA – CaEtOAcD and CaBuOHA – CaBuOHD) main subfractions based on the TLC profiles (EtOAc:HCOOH:acetic acid:water (100:11:11:26, v/v/v/v) after derivatization with 1% Natural product reagent A. The subfractions CmEtOAcA, CmEtOAcB and CaEtOAcB were subjected to preparative HPLC system (Shimadzu LC10vp, Japan, Kinetex XB-C18 – 5 μm, 150 mm × 21.2 mm, Agilent, CA, USA, 280 nm, flow 20mL·min−1,mobile phase: 0.1% HCOOH in water (A) and 0.1% HCOOH in acetonitrile (B); elution program: 0% B – 45% B (0–45 min). Fractions were collected based on UV–Vis chromatograms to give pure compound 2 (10 mg) from the subrfraction CmEtOAcA, compound 12 (1.5 mg) from the subfraction CmEtOAcB and compound 16 (6.6 mg) from the subfraction CaEtOAcB. Isolated compounds were identified based on UV–Vis, MS and 1H NMR spectra. Additionally, acid hydrolysis of compound 16 was performed as follows: approximately 1 mg of compound was heated in 1MHCl for 5 h in a water bath at 95 °C and afterwards the mixture was cooled down and extracted with diethyl ether (3×5mL); the combined organic layers were evaporated and prepared for the HPLC analysis, and compared with a standard of hydroxytyrosol.
2.4. Phytochemical analysis by an HPLC-DAD-MSn method
HPLC-DAD-MSn analysis was performed on a UHPLC-3000 RS system (Dionex, Germany) with DAD and an AmaZon SL ion trap mass spectrometer with an ESI interface (Bruker Daltonik GmbH, Germany). Separations were performed on a Zorbax SB-C18 column (150 × 2.1 mm, 1.9 μm) (Agilent, USA). The column temperature was 25 °C. For preliminary phytochemical analyses of the extracts and fractions, mobile phase A was 0.1% HCOOH in water and mobile phase B was 0.1% HCOOH in acetonitrile. The gradient program was as follows: 0–5 min. 2–5% B; 5–40 min. 5–26% B; 40–52 min. 26–60% B; 55–60 min. 95–2% B. The flow rate was 0.2 mL·min−1. The column was equilibrated for 10 min between injections. UV spectra were recorded 200–800 nm range, and chromatograms were acquired at 240, 280, 325, 350 nm or 520 nm. The LC eluate was introduced directly into the ESI interface without splitting. The nebulizer pressure was 40 psi; dry gas flow was 9 L·min−1; the oven temperature was 300 °C; and capillary voltage was 4.5 kV. Analyses were carried out scanning from m/z 200 to 2200. Compounds were analyzed in negative and positive ion mode. The MS2 fragmentation patterns were obtained for the most abundant ion.
2.5. Anthocyanins contents and total phenols content
To determine the total anthocyanins contents (ACC) in the Cm and Ca fruit extracts, the assay was performed as follows: 0.25 g of the extract was extracted with 80 mL of 0.1% HCl (v/v) solution in methanol for 30 min in the dark. After decantation, the residue was extracted twice with 0.1% methanolic HCl (v/v) solution (50mL) for 15min in the dark. The extracts were combined and diluted to 200 mL with 0.1% methanolic HCl (v/v) solution. The solution was diluted by a factor of 5, and the absorbance of the resulting solution was measured at 528 nm in a spectrophotometer (Shimadzu UV-160 A) [19]. The results are expressed as cyanidin chloride equivalents (CCE mg·g−1 of extract).
The total phenols content (TPC) was determined using a modified spectrophotometric method with Folin-Ciocalteu reagent [20]. The assay was performed in 96-well plates: a 10-μL aliquot of a solution of the extract (5 mg·mL−1 in 50% methanol, v/v), 105 μL of 10% Folin-Ciocalteu reagent (diluted in distilled water) and 85 μL of Na2CO3 (1 M) were mixed and incubated for 15 min at room temperature in darkness. The absorbance was measured at 765 nm in a microplate reader (Synergy 4, BioTek, USA) and the results are expressed as gallic acid equivalents (GAE mg·g−1 of extract).
2.6. Pancreatic lipase inhibition
A previously described enzymatic in vitro assay based on the hydrolysis kinetics of the oleate ester of 4-methylumbelliferone (0.5 mM) was used to determine the PL activity and inhibitory potential of the extracts, fractions and subfractions [14]. Porcine pancreas powder (0.5 mg·mL−1 in Tris–HCl buffer, pH 8.0) was used as the enzyme source. The extracts, fractions and subfractions were dissolved in dimethylsulfoxide (DMSO). The IC50 values of the extracts were determined. The fractions and subfractions were tested at concentrations of 15 μg·mL−1 and 7.5 μg·mL−1, respectively. The enzyme and substrate solutions as well as the test samples were prepared immediately before use. The fluorescence of 4-methylumbelliferone was measured at excitation and emission wavelengths of 360 nm and 465 nm, respectively, at 37 °C in a microplate reader (Synergy 4, Biotek, USA). Orlistat was used as a positive control. A control without test extracts or orlistat represented 100% PL activity.
2.7. α-Amylase inhibition
An EnzChek™ Ultra Amylase Assay Kit was used to determine the a-amylase activity as described previously [14]. The fluorescence method was based on the hydrolytic cleavage of a modified starch derivative (DQ™ starch from corn, BODIPY® FL conjugate, 200 μg·mL−1). Porcine pancreas powder (0.5 mg·mL−1 in Tris–HCl buffer, pH 8.0) was used as the enzyme source. The extracts and fractions were dissolved in DMSO. The IC50 values of the extracts and fractions were determined. The enzyme and substrate solutions as well as the test samples were prepared immediately before use. The fluorescence of the starch derivative was measured at excitation and emission wavelengths of 485 nm and 535 nm, respectively, at 37 °C in a microplate reader (Synergy 4, Biotek, USA). Acarbose was used as a positive control. A control without test samples or acarbose represented 100% a-amylase activity.
2.8. Statistical analysis
The results are expressed as means ± SD. Each sample of extract, fraction or compound was tested in triplicate in three independent experiments. Statistical significance of the differences between means was established by testing an homogeneity of variance and a normality of distribution followed by ANOVA with Tukey’s post hoc test. The nonparametric methods (Kruskal–Wallis test) were used if neither the homogeneity of variance nor the normality of distribution had been established. The P values below 0.05 were considered statistically significant. All analyses were performed using Statistica 10 (StatSoft, Poland).
3. Results
3.1. Phytochemical analysis of the extracts
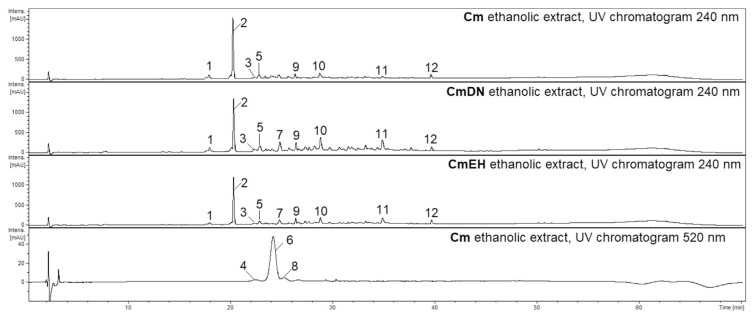
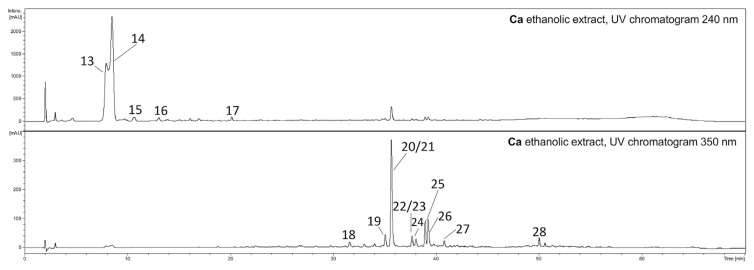
The preliminary phytochemical analysis of the ethanolic and aqueous extracts from the fruits of Cm (Fig. 1) and Ca (Fig. 2) were conducted to compare their compositions. The most abundant compound in the ethanolic and aqueous extracts of Cm fruit from both lyophilized fresh-picked and dried commercial samples was loganic acid (2, Rt = 20.2 min) (Fig. 1). The main ions in the MS spectrum were [2 M–H]− (m/z 751) and [M–H]− (m/z 375) in the negative ESI mode (Table 1). The major MS2 ion in negative ionization mode was [M-H-Glc]− (m/z 213). Another iridoid, cornuside (12, Rt = 39.7 min), was also detected in all extracts of Cm fruits. The main ion in the MS spectrum was [M–H]− (m/z 541), whereas the main ion in the MS2 pattern in negative ESI mode was [M-H-Glc]− (m/z 379). The MS2 fragmentation pattern of cornuside showed signals at m/z 347, 277 and 169 in the negative ionization mode. Additionally, the another iridoid (9, Rt = 26.4 min), characterized by an [M + HCOOH–H]− ion at m/z 435 in negative ESI mode, was detected in the Cm extracts. The ions from the MS2 fragmentation pattern of this iridoid were m/z 389 and 335. The most abundant anthocyanin was pelargonidin-3-O-galactoside (6, Rt = 25.0 min). The major ion in its MS spectrum was [M+H]+ (m/z 433) in positive ESI mode. The major MS2 fragmentation pattern of pelargonidin-3-O-galactoside showed a signal at m/z 271. The [M+H]+ peak of cyanidin 3-O-galactoside (4, Rt = 22.5 min) at m/z 449 as well as the [M+H]+ peak of pelargonidin 3-O-robinobioside (7, Rt = 25.3 min) at m/z 579 in the positive ESI mode were also identified based on a comparison to literature data [9]. On the other hand, the phytochemical composition of the extracts of Ca fruits (Fig. 2) were completely different from the extracts of Cm. In particular, the iridoids and anthocyanins identified in Cmfruit extracts were not detected in Ca fruit extracts. The screening of their constituents allowed us to identify flavonols such as derivatives of quercetin and kaempferol (Table 1). The most intense peaks were assigned to quercetin 3-O-glucuronide (20, Rt = 35.9 min, [M–H]− m/z 477) and quercetin 3-O-glucoside (21, isoquercitrin, Rt = 35.9 min, [M–H]− m/z 463) as well as kaempferol hexoside (25, Rt = 39.2 min, [M–H]− m/z 447) and kaempferol 3-O-glucuronide (26, Rt = 39.4 min, [M–H]− m/z 461). The compounds were identified by comparison of their retention times and MS/MS fragmentation patterns to those of standard compounds.
Fig. 1.
HPLC chromatograms of the ethanolic extracts from fruits of Cm (10 mg·mL−1) acquired at 240 nm and 520 nm. HPLC conditions: Zorbax SB-C18 (150 × 2.1 mm, 1.9 μm), mobile phase A: 0.1% HCOOH/H2O; B: 0.1% HCOOH/MeCN, and the gradient was as follows: 0–5min. 2–5% B; 5–40 min. 5–26% B; 40–52 min. 26–60% B; 55–60 min. 95–2% B. Cm – Cornus mas picked fresh, CmDN – Cornus mas Dary Natury, CmEH – Cornus mas Eko Herba.
Fig. 2.
HPLC chromatograms of the ethanolic extracts from fruits of Ca (10 mg·mL−1) acquired at 240 nm and 350 nm. HPLC conditions: Zorbax SB-C18 (150 × 2.1 mm, 1.9 μm), mobile phase A: 0.1% HCOOH/H2O; B: 0.1% HCOOH/MeCN, and the gradient was as follows: 0–5min. 2–5% B; 5–40 min. 5–26% B; 40–52 min. 26–60% B; 55–60 min. 95–2% B. Ca – Cornus alba picked fresh.
Table 1.
Retention times, UV–Vis spectra, and MS/MS data in the negative ion mode for compounds identified in the extracts from fruits of Cm and Ca.
| Peak no. | Proposed compounds/analyte | Retention time [min] | UV λmax [nm] | [M–H]− m/z | Fragmentary ions | Extract |
|---|---|---|---|---|---|---|
| Extracts of Cm fruit (Fig. 1) | ||||||
| 1 | Digalloyl glucose | 18.1 | 270sh | 483 | 465, 3310, 271, 193 | Cm, CmDN, CmEH |
| 2 | Loganic acid | 20.2 | 235 | 751a | 473, 375, 213 | Cm, CmDN, CmEH |
| 3 | Loganic acid glucuronide | 22.2 | 280 | 549 | 530, 451, 375, 307, 213 | Cm, CmDN, CmEH |
| 4 | Cyanidin 3-O-galactoside | 22.5 | 280, 517 | [M+H]+ 449 | [M+H]+ 287 | Cm |
| 5 | Gallic acid derivative | 22.9 | 270, 330 | 785 | 765, 708, 633, 483, 419, 301 | Cm, CmDN, CmEH |
| 6 | Pelargonidin 3-O-galactoside | 24.0 | 275, 500 | [M+H]+ 433 | [M+H]+ 271 | Cm |
| 7 | Unidentified glucuronide | 24.7 | 268 | 589 | 491, 413, 341 | CmDN, CmEH |
| 8 | Pelargonidin-3-O-robinobioside | 25.3 | 275, 500 | [M+H]+ 579 | [M+H]+271 | Cm |
| 9 | Iridoid | 26.4 | 227 | 435b | 389, 335, 273, 227 | Cm, CmDN, CmEH |
| 10 | Digalloyl HHDP-glucose | 28.7 | 268 | 785 | 767, 700, 634, 483, 419, 301, 249 | Cm, CmDN, CmEH |
| 11 | Tetragalloyl glucose | 34.8 | 275, 360sh | 787 | 465, 313 | Cm, CmDN, CmEH |
| 12 | Cornuside | 39.7 | 280 | 541 | 379, 347, 277, 169 | Cm, CmDN, CmEH |
| Extract of Ca fruit (Fig. 2) | ||||||
| 13 | Iridoid | 7.8 | 231 | 361b | 315 | |
| 14 | Iridoid | 8.7 | 230 | 361b | 315, 179 | |
| 15 | Unidentified | 10.7 | 216 | 417 | 391, 338 | |
| 16 | Hydroxytyrosol glucoside | 13.0 | 290 | 631 | 315 | |
| 17 | Unidentified | 20.0 | 280sh | 632 | 315, 277 | |
| 18 | Unidentified | 31.8 | 260 | 479 | 457, 442 | |
| 19 | Quercetin 3-O-galactoside (hyperoside) | 35.1 | 260, 356 | 463 | 441, 301, 343, 151 | |
| 20 | Quercetin 3-O-glucuronide | 35.9 | overlapped 256, 353 | 477 | 301, 179 | |
| 21 | Quercetin 3-O-glucoside (isoquercitrin) | 35.9 | overlapped 256, 353 | 463 | 301 | |
| 22 | Kaempferol 3-O-glucoside (astragalin) | 37.8 | overlapped 256, 354 | 447 | 419, 285, 255, 151 | |
| 23 | Quercetin 3-O-rhamnoside (quercitrin) | 37.8 | overlapped 256, 354 | 433 | 301 | |
| 24 | Quercetin 3-O-β-D-(6″-O-malonyl)-glucoside | 38.3 | 256, 360 | 549 | 505, 463, 387, 173 | |
| 25 | Kaempferol hexoside | 39.2 | 264, 344 | 447 | 285 | |
| 26 | Kaempferol 3-O-glucuronide | 39.4 | 264, 349 | 461 | 285 | |
| 27 | Kaempferol malonylhexoside | 40.9 | 260, 360 | 533 | 489, 435, 373, 296 | |
| 28 | Unidentified | 50.3 | 280, 343 | 337 | 322, 217, 177 | |
[2 M–H]−.
[M + HCOOH–H]−; sh – shoulder; Cm – Cornus mas picked fresh, CmDN – Cornus mas Dary Natury, CmEH – Cornus mas Eko Herba, Ca – Cornus alba picked fresh.
3.2. Quantitative determination of anthocyanins content (ACC) and total phenols content (TPC)
Based on quantitative analysis of the ACC, extracts prepared from fruits of Cm picked fresh contained more anthocyanins than the Cm fruits acquired commercially (Table 2). The ACC values were 8.8 ± 0.1 cyanidin chloride equivalents (CCE) mg·g−1 and 10.0 ± 0.1 CCE mg·g−1 in the aqueous and ethanolic extracts of Cm, respectively.
Table 2.
Total anthocyanins (ACC) and phenols (TPC) content in the extracts from fruits of Cm and Ca.
| ACC ± SD [CCE mg·g−1] | ||||
|---|---|---|---|---|
|
| ||||
| Dried fruit | Cm | CmDN | CmEH | Ca |
| Aqueous extract | 8.8 ± 0.1 | 4.4 ± 0.5 | 4.4 ± 0.5 | 3.9 ± 0.1 |
| Ethanolic extract | 10.0 ± 0.1 | 5.2 ± 0.1 | 4.7 ± 0.1 | 4.0 ± 0.1 |
| Methanolic extract | 12.8 ± 0.1 | – | – | 4.3 ± 0.1 |
|
| ||||
| TPC ± SD [GAE mg·g−1] | ||||
|
| ||||
| Dried fruit | Cm | CmDN | CmEH | Ca |
|
| ||||
| Aqueous extract | 41.5 ± 3.4 | 69.8 ± 8.5 | 49.1 ± 4.6 | 28.6 ± 2.1 |
| Ethanolic extract | 50.5 ± 5.9 | 75.5 ± 5.6 | 51.1 ± 4.2 | 39.6 ± 2.3 |
| Methanolic extract | 36.0 ± 5.6 | – | – | 42.5 ± 1.8 |
Cm – Cornus mas picked fresh, CmDN – Cornus mas Dary Natury, CmEH – Cornus mas Eko Herba, Ca – Cornus alba picked fresh.
The quantitative analysis of the extracts showed that the ethanolic extracts were characterized by higher contents of phenolic compounds than the aqueous extracts, the exception to this was the extracts from dried fruits of Cm manufactured by Eko Herba (CmEH). The highest concentration of phenols was found in the ethanolic extract from dried fruits of Cm manufactured by Dary Natury (CmDN) (75.5 ± 5.6 gallic acid equivalents (GAE) mg·g−1 of extract) as well as in the aqueous extract of CmDN (69.8 ± 8.5 GAE mg g−1 of extract). The aqueous and ethanolic extracts of Ca contained 28.6 ± 2.1 GAE mg g−1 and 39.6 ± 2.3 GAE mg g−1, respectively (Table 2). Thus, the extracts from Cm fruits were characterized by higher TPC values than the extracts from Ca fruits.
3.3. Inhibition of digestive enzymes
The IC50 values of a-amylase inhibition for the Cm aqueous and ethanolic extracts were 134 ± 6.4 μg·mL−1 and 92.5 ± 16.6 μg·mL−1, respectively. Similarly, the IC50 values for a-amylase inhibition for the Ca aqueous and ethanolic extracts were 136 ± 13.1 μg·mL−1 and 110 ± 5.6 μg·mL−1, respectively (Table 3). The aqueous and ethanolic extracts from the commercial dried fruits of Cm exhibited higher IC50 values above 200 μg·mL−1, and thus they were established as not active. On the other hand, all tested extracts were stronger PL activity inhibitors than a-amylase activity inhibitors. The Cm ethanolic extract (IC50 = 15.2 ± 3.9 μg·mL−1) was the most active PL inhibitor, whereas the IC50 of the Cmaqueous extract was 34.2 ± 4.2 μg·mL−1. The aqueous and ethanolic extracts of Ca showed comparable IC50 values; 22.6 ± 3.0 μg·mL−1 and 25.3 ± 2.0 μg·mL−1, respectively (Table 3). On the other hand, the IC50 values of the aqueous and ethanolic preparations of commercially available fruits ranged from 61.4 to 112 μg·mL−1 and 57.7–59.0 μg·mL−1 in the PL assay, respectively. However, all tested extracts were less active than the PL inhibitor orlistat (1.3 ± 0.3 ng·mL−1; 2.6 ± 0.5 nM) and a-amylase inhibitor acarbose (2.4 ± 0.4 μg·mL−1; 3.7 ± 0.6 μM).
Table 3.
IC50 values (μg·mL−1) of the extracts from different sources of fruits of Cm and Ca for the inhibition of pancreatic lipase and α-amylase.
| Pancreatic lipase IC50 ± SD [μg·mL−1] | ||||
|---|---|---|---|---|
|
| ||||
| Dried fruit | Cm | CmDN | CmEH | Ca |
| Aqueous extract | 34.2 ± 4.2a | 112 ± 18.0* | 61.4 ± 4.3 a–d | 22.6 ± 3.0c |
| Ethanolic extract | 15.2 ± 3.9b | 57.7 ± 8.6a–c | 59.0 ± 9.6a–d | 25.3 ± 2.0d |
| Methanolic extract | 48.3 ± 12a,b | n.d. | n.d. | 13.4 ± 3.6a |
| Orlistat | 2.6 ± 0.5 nM (1.3 ± 0.3 ng·mL−1) | |||
|
| ||||
| α-Amylase IC50 ± SD [μg·mL−1] | ||||
|
| ||||
| Dried fruit | Cm | CmDN | CmEH | Ca |
|
| ||||
| Aqueous extract | 134 ± 6.4† | n.a. | n.a. | 136 ± 13.1† |
| Ethanolic extract | 92.5 ± 16.6 | n.a. | n.a. | 110 ± 5.6† |
| Methanolic extract | 79.0 ± 0.1 | n.d. | n.d. | 112 ± 2.3† |
| Acarbose | 3.7 ± 0.6 μM (2.4 ± 0.4 μg·mL−1) | |||
Cm – Cornus mas picked fresh, CmDN – Cornus mas Dary Natury, CmEH – Cornus mas Eko Herba, Ca – Cornus alba picked fresh; n.a. – not active (IC50 > 200 μg·mL−1), n.d. – not determined.
Statistical differences between PL IC50 values of extracts are labeled with the same letter a–d (P < 0.05):
P < 0.05 vs. Ca methanolic extract;
P < 0.05 vs. Cm ethanolic extract,
P < 0.05 vs. Ca aqueous extract,
P < 0.05 vs. Ca ethanolic extract;
P < 0.05 vs. all extracts (pancreatic lipase). Statistical differences between a-amylase IC50 values:
P < 0.05 vs. Cm methanolic extract.
Due to the fact that the extracts of lyophilized fresh-picked fruits were characterized by higher a-amylase and PL inhibitory activity than the commercially available ones, the fresh-picked plant materials were selected for further investigations.
3.4. Phytochemical analysis and pancreatic enzymes inhibitory activity of the methanolic extract
Due to the potential thermal instability of the constituents of Cm, the extraction with methanol was conducted by macerating the plant material at room temperature to avoid chemical degradation during extraction process. The TPC and ACC values of Cm methanolic extract were 36.0 ± 5.6 GAE mg·g−1 and 12.8 ± 0.1 CCE mg·g−1 of extract, respectively. Indeed, the higher ACC in the methanolic extract, compared to the aqueous and ethanolic ones, confirmed the negative influence of high-temperature processing on the stability of the anthocyanins. The Ca methanolic extract contained 42.5 ± 1.8 GAE mg·g−1 and 4.3 ± 0.1 CCE mg·g−1 of extract (Table 2). Additionally, the abilities of the crude methanolic extracts to inhibit a-amylase and PL activity were studied. The Ca methanolic extract showed a lower IC50 (13.4 ± 3.6 μg·mL−1) than Cm methanolic extract (48.3 ± 12.0 μg·mL−1) in the PL assay (Table 3). The anti-amylase activity of methanolic extracts turned out to be much lower in comparison to their anti-lipase activity. Thus, the methanolic extracts were fractionated only based on the anti-lipase activity. Taking into consideration both the higher ACC concentration in Cm methanolic extract and the most relevant anti-lipase activity of Ca methanolic extract, the methanolic preparations were selected for further investigation with a bio-assay guided isolation of compounds.
3.5. Bio-assay guided isolation and identification of compounds
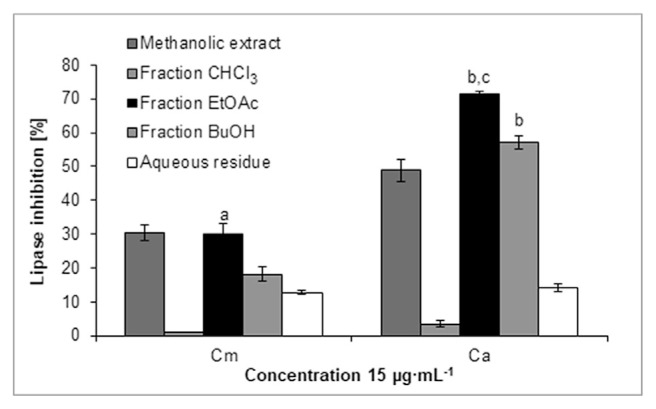
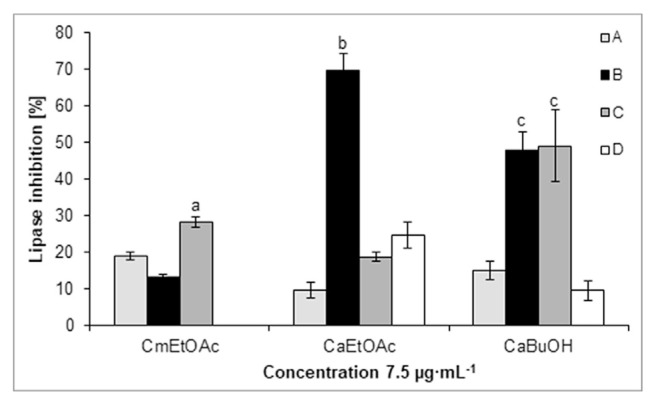
Among the fractions obtained from the methanolic extracts of Cm and Ca fruit, the CmEtOAc, CaEtOAc and CaBuOH fractions at 15 μg·mL−1 reduced the PL activity by 30.2 ± 3.2%, 71.4 ± 0.9% and 57.0 ± 1.9%, respectively (Fig. 3). Based on HPLC-DAD-MS/MS analysis, loganic acid (2), cornuside (12) as well as pelargonidin-3-O-galactoside (6) were detected in the CmEtOAc fraction based on the comparison with the available data [8,9]. On the other hand, derivatives of kaempferol and quercetin, such as quercetin 3-O-rutinoside (rutin, Rt = 34.0 min, [M–H]− m/z 609), quercetin 3-O-galactoside (19, hyperoside) and quercetin 3-O-glucuronide (20) as well as kaempferol 3-O-glucoside (22, astragalin) were identified in the CaEtOAc and CaBuOH fractions by comparison of their retention time and spectral data with those of used standards. As the most active lipase inhibitors, the CmEtOAc, CaEtOAc and CaBuOH fractions were selected for further investigation, and their constituents were separated by column chromatography and preparative HPLC. The subfraction CaEtOAcB at a concentration of 7.5 μg·mL−1 significantly inhibited lipase activity by 69.9 ± 4.5% and was the most active of all obtained subfractions of Cm and Ca (Fig. 4). In the subfraction CaEtOAcB, the phenolic compounds, and more specifically phenolic acids derivatives, were detected. Hydroxytyrosol glucoside (16) genereating peak at m/z 315 ([M–H]−, Rt = 13.0 min) was isolated from this subfraction. The IC50 value of pure hydroxytyrosol glucoside (16) was 0.99 ± 0.10 mg·mL−1 in the PL assay. The other compounds of the subfraction CaEtOAcB generated peaks at m/z 433 ([M–H]−, Rt = 10.1 min) as well as overlapped peaks at m/z 503 and 441 ([M–H]−, Rt = 24.7 min) in trace amounts. Among the subfractions of CmEtOAc, subfraction C more significantly reduced lipase activity (28.3 ± 1.5%) than other subfractions at a concentration of 7.5 μg·mL−1. The most abundant compound in CmEtOAcC was pelargonidin 3-O-galactoside. Subfractions CmEtOAcA and CmEtOAcB contained mainly loganic acid (2), and cornuside (12). The final isolation of these compounds allowed us to confirm their structures by NMR spectroscopy. However, we established that neither loganic acid (2) nor cornuside (12) in highly pure forms inhibit PL activity.
Fig. 3.
Pancreatic lipase inhibitory activity of the methanolic extracts from Cm and Ca as well as of the obtained fractions: chloroform (CHCl3), ethyl acetate (EtOAc), butanol (BuOH) and aqueous residue. aP < 0.001 vs. the CHCl3, BuOH and aqueous fractions; bP < 0.001 vs. the CHCl3 and aqueous fractions; cP < 0.05 vs. the BuOH fraction.
Fig. 4.
Pancreatic lipase inhibitory activity of the ethyl acetate subfractions from Cm (CmEtOAc) and the ethyl acetate (CaEtOAc) and butanolic subfractions from Ca (CaBuOH). aP < 0.001 vs. subfractions A and B; bP < 0.001 vs. subfractions A, C and D; cP < 0.001 vs. subfractions A and D.
Loganic acid (2). 1H NMR (300 MHz, CD3OD) δ 7.40 (s, H-3), 5.27 (d, J = 4.5 Hz, H-1), 4.56 (d, J = 7.6 Hz, H-1′), 4.05 (m, H-7), 3.85 (d, J = 10.4 Hz, H-6′b), 3.68 (m, H-6′a), 3.34 (overlapping H-3′, H-5′), 3.24 (m, H-4′), 3.12 (d, J = 9.0 Hz, H-2′), 3.05 (m, H-5), 2.12 (dd, J = 13.5, 8.0 Hz, H-6b), 1.98 (m, H-9), 1.82 (m, H-8), 1.64 (dd, J = 12.0 Hz, 5.8, H-1), 1.05 (d, J = 6.8 Hz, H-10). The structure of loganic acid was confirmed by comparing its 1H NMR spectrum with the spectral data available in the literature [21].
Cornuside (12). 1H NMR (300 MHz, CD3OD) δ 7.50 (s, H-3), 7.05 (s, galloyl group), 5.80 (dd, J = 18.0, 8.0 Hz, H-8), 5.58 (d, J = 6.6 Hz, H-1), 5.32 (d, J = 18.6 Hz, H-10), 5.27 (d, J = 10.9 Hz H-10), 4.71 (d, J = 7.8 Hz, H-1′), 4.26 (m, H-7) 3.89 (m, H-6′b), 3.66 (m, H-6′a), 3.60 (s, carboxymethyl group), 3.34 (m, H-3′), 3.24 (d, J = 11.3 Hz, H-4′), 3.18 (d, J = 9.0 Hz, H-2′), 2.96 (m, H-5), 2.68 (m, H-9), 2.10 (dd, J = 14.0, 7.0 Hz, H-6), 1.92 (dd, J = 13.9, 7.0 Hz, H-6). The structure of cornuside was confirmed by comparing its 1H NMR spectrum with the spectral data available in the literature [22].
Hydroxytyrosol glucoside ((3,4-dihydroxyphenyl)-ethyl-β-d-glucopyranoside) (16) 1H NMR (300 MHz, CD3OD) δ 6.62 (d, J = 2.1 Hz, H-2), 6.59 (d, J = 7.8 Hz, H-5), 6.48 (dd, J = 7.8, 2.1 Hz, H-6), 4.32 (d, J = 7.8 Hz, H-1′), 4.05 (m, H-8a), 3.87 (dd, J = 11.9, 2.0 Hz, H-6′a), 3.66–3.73 (overlapping H-5′, H-6′b, H-8b), 3.28–3.38 (overlapping H-2′, H-3′), 3.20 (dd, J = 8.0, 7.0 Hz, H-4′), 2.80 (t, J = 7.0 Hz, H-7). The structure of hydroxytyrosol glucoside was confirmed by comparing its 1H NMR spectrum with the spectral data available in the literature [23].
4. Discussion
Our study revealed the significant anti-amylase and anti-lipase activities of extracts from fruits of Cm and Ca. In particular, this study allowed us to determine the biological activities and phytochemical composition of Ca fruits, which has not previously been possible due to the limited number of reports on this species. The bio-assay guided identification of constituents revealed that the flavonoids and phenolic acid derivatives in Ca are likely to inhibit the PL activity to a greater extent than the components of Cm, such as anthocyanins and iridoids. Considering the activity of Cm, we established that anthocyanins rather than iridoids might be responsible for the inhibition of pancreatic enzymes inhibition. However, the ultimate effect of this plant material may result from the synergistic activity of the anthocyanins and iridoids. Additionally, our results showed that extracts from commercially available dried fruits were less active than these from lyophilized fresh-picked fruit probably due to the lower quantity of anthocyanins. It may be concluded that the conditions used to process the plant material for commercial use severely reduce the biological potential of fruits, and these results indicate the consumption of fresh fruits has health benefits. Neither the anti-amylase nor the anti-lipase activity of Cm fruit extracts containing phytochemicals, such as loganic acid, pelargonidin-3-O-galactoside and cornuside, have been established to date.
Among iridoids, which are monoterpenoid compounds, some are characterized by valuable biological activity. Notably, these compounds are often found in leaves and young stems, but they seldom occur in fruits. Loganic acid (2) and cornuside (12) have previously been identified in the fruits of Cm [9,24,25].We attempted to assess the anti-lipase activity of iridoid compound such as loganic acid (2) and cornuside (12) for the first time. However, the iridoid compounds turned out to be not active in the studied biological assays. The another iridoid (9), characterized by an [M + HCOOH–H]− ion at m/z 435 in negative ESI mode, was detected. It is supposed that the compound 9 is loganin, which plays of crucial role in formation of iridoid glycosides in plant materials, but its detailed identification was not possible due to trace amount compared to loganic acid and the lack of enough data acquired in our study. In addition to the iridoids considered to be the active constituents of Cm, anthocyanins, particularly pelargonidin and cyanidin derivatives, were identified in the extracts of lyophilized fresh-picked fruits, but not from the commercial fruit samples. In contrast to previous studies, we detected neither the peaks of delphinidin 3-O-galactoside nor those of cyanidin-3-O-robinobioside [9,26]. Additionally, hydrolysable tannins, such as galloyl esters of glucose have been identified in the extracts of Cm fruits (Table 1) based on the data provided by Kucharska [27]. Among the flavonoids in the Cm extracts quercetin 3-O-glucuronide and kaempferol 3-O-glucoside (astragalin) were detected in trace amounts compared to other classes of compounds. In previous reports, only quercetin 3-O-glucuronide was identified in the extracts of Cm fruits [8]. Notably, only three flavonoids, namely, quercetin-3-O-glucuronide, quercetin-3-O-glucoside and kaempferol-3-O-glucoside, have previously been isolated from Ca [10]. However, it should be highlighted that the authors did not provide accurate information regarding which part of the Ca contained the twelve known compounds. Therefore, in our study we have identified flavonoids, in particular hyperoside, rutin and kaempferol hexoside, in the fruits of Ca for the first time. In contrast to the recent results of Park et al. (2016), we detected neither PGG nor cornusiins. On the other hand, this is the first report on isolation of hydroxytyrosol glucoside in this plant material.
The TPC in the extract of Cm was estimated to be in the 41.5 to 69.8 GAE mg·g−1 range in our study, whereas in previous studies the TPC in an aqueous extract was 12.8 ± 0.8 GAE mg·g−1 [28]. In our study, we determined the TPC in extracts from fruits of Ca for the first time. Although anthocyanins are believed to contribute to the TPC [2], our study shows a non-significant correlation between the anthocyanins content and the total phenols in Cm. The differences between the TPC and ACC in CmDN indicated that both anthocyanins and tannins contribute to the total phenols content. Anthocyanins are a class of naturally occurring phenols and water-soluble pigments. Their biological activities make them important alternatives to synthetic dyes. The color of the anthocyanin depends on the structural configuration it usually takes. However, their high reactivities mean they are often converted undesirable colorless or brown compounds. Notably, the stability of anthocyanins is influenced by many factors, especially temperature. However, other factors such as exposure to light, environmental pH, and the presence of oxygen, hydrogen peroxide, enzymes, sugars and ascorbic acid may also affect the stability of anthocyanins [29–32]. The lower ACC values in the extracts from the commercial Cm fruit samples may be a result of their degradation kinetics during storage or thermal processing, which can cause changes in food products containing the anthocyanins. It is worth to note that the content of compounds characterized by labile chemistry also change in the extraction process due to the acid hydrolysis, which occurs in the aqueous extract of plant material rich in organic acids. Thus, using organic solvents such as ethanol in fruit preparation allows to limit this process.
Inhibition of digestive enzymes, which are involved in the breakdown of fat and starch, is a mechanism for the treatment of obesity and related diseases due to the reduction of fat absorption as well as a postprandrial increase of blood glucose [33–35]. In our study, it was shown that among preparations often used by patients the concentrated ethanolic extracts revealed more significant inhibitory potential for a-amylase and PL activities than the aqueous extracts. Thus, a correlation between the TPC and digestive enzymes inhibition was identified. The lowest IC50 values of the extracts from lyophilized fresh-picked fruits of both Cm and Ca are noted (Table 3). All tested extracts were stronger PL activity inhibitors than a-amylase activity inhibitors. This is in agreement with previous studies that shown preparations rich in phenolic compounds were less potent a-amylase than PL activity inhibitors [36]. On the other hand, the ethanolic extract of Ficus carica fruit, characterized by the presence of 5-hydroxymethylfurfural, fatty acids and β-sitosterol, has exerted the similar effect on these enzymes [37]. Some reports indicate fruits rich in anthocyanins have potential as hypolipidemic natural products for regulating abnormalities in blood glucose [38,39], which is consistent with the biological influence seen in our study. The possible mechanisms of action assigned to anthocyanins include cell stimulation of glucose uptake, glycogen synthesis, and insulin secretion as well as protection of β-cells from oxidative stress and inhibition of enzymes, such as maltase, sucrase and a-glucosidase [40,41]. Previous studies on the inhibition of carbohydrate digestive enzymes, which support the modulation of diabetes mellitus, suggest that phenolic compounds are also potent inhibitors of these enzymes [36]. In our study, hydroxytyrosol glucoside (16) isolated from the most active anti-lipase Ca subfraction did not inhibit significantly enzyme activity (IC50 = 0.99 ± 0.10 mg·mL−1). Thus, the other constituents of subfraction CaEtOAcB seems to be also active phytochemicals, and the total PL inhibitory effect is likely to be synergistic effect of all compounds present in this subfraction. However, a combined formulation sometimes seems to be desirable to prevent excessive inhibition of enzymes. It is worth to note that drugs used in the therapy of obesity and diabetes type 2, such as orlistat and acarbose, lead to a wide range of adverse effects linked with the accumulation of undigested lipids and carbohydrates, including flatulence, diarrhea and abdominal distension. It is suggested that a combination therapy of conventional drugs with plant-derived compounds might at least attenuate these side effects. The recent study showed the inhibition of a-amylase activity by different combinations of gallic acid and acarbose [42].
In conclusion, the extracts from fruits of species belonging to the genus Cornus can be considered valuable and effective inhibitors of digestive enzymes correlated with prevention and control of hyperlipidemia related diseases. Thus, our study opens up the possibility for development of preparations from edible fruits such as Cm fruits. Their potential use as a novel food ingredient, nutraceutical or dietary supplement should not be excluded. In addition, the relevance of phenolic phytochemicals of Ca fruits rather than iridoids and anthocyanins of Cm fruits for the inhibition of enzymes activity should be noted. However, the complex composition of extracts seems to be more effective due to the potential synergistic or additive activity of their constituents. Further investigations to identify and isolate the bioactive phytochemicals in Ca that are responsible for the significant lipase activity inhibition are justified and required.
Acknowledgments
The authors would like to thank Roman Rudecki (technical assistance, Medical University of Warsaw) for the collection of the plantmaterial. This work was financially supported by the National Science Centre, Poland (grant No 2016/23/D/NZ7/00958), and partially supported by the Medical University of Warsaw, Poland (mini-grant No FW25/NM3/17). The project was carried out with the use of CePT infrastructure financed by the European Regional Development Fund within the Operational Programme ‘Innovative economy’ for 2007–2013.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.06.005.
Funding Statement
This work was financially supported by the National Science Centre, Poland (grant No 2016/23/D/NZ7/00958), and partially supported by the Medical University of Warsaw, Poland (mini-grant No FW25/NM3/17).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- 1. Vareed SK, Reddy MK, Schutzki RE, Nair MG. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus Florida fruits with health benefits. Life Sci. 2006;78:777–84. doi: 10.1016/j.lfs.2005.05.094. [DOI] [PubMed] [Google Scholar]
- 2. Popović BM, Štajner D, Slavko K, Sandra B. Antioxidant capacity of cornelian cherry (Cornus mas L.) – comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012;134:734–41. doi: 10.1016/j.foodchem.2012.02.170. [DOI] [PubMed] [Google Scholar]
- 3. Asgary S, Kelishadi R, Rafieian-Kopaei M, Najafi S, Najafi M, Sahebkar A. Investigation of the lipid-modifying and anti inflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr Cardiol. 2013;34:1729–35. doi: 10.1007/s00246-013-0693-5. [DOI] [PubMed] [Google Scholar]
- 4. Shamsi F, Asgari S, Rafieian M, Kazemi S, Adelnia A. Effects of Cornus mas L. on blood glucose, insulin and histopathology of pancreas in alloxan-induced diabetic rats. Abstract. J Isfahan Med School. 2011;29:929–38. [Google Scholar]
- 5. Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) J Agric Food Chem. 2006;54:243–8. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 6. Sozański T, Kucharska AZ, Szumny A, Magdalan J, Bielska K, Merwid-Ląd A, et al. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARalpha activation in hypercholesterolemic rabbits. Phytomedicine – Int J Phytother Phytopharmacol. 2014;21:1774–84. doi: 10.1016/j.phymed.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 7. Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 8. Pawlowska AM, Camangi F, Braca A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010;119:1257–61. [Google Scholar]
- 9. Kucharska AZ, Szumny A, Sokół-Łętowska A, Piórecki N, Klymenko SV. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J Food Comp Anal. 2015;40:95–102. [Google Scholar]
- 10. Park KH, Yin J, Yoon KH, Hwang YJ, Lee MW. Antiproliferative effects of new dimeric ellagitannin from Cornus alba in prostate cancer cells including apoptosis-related S-phase arrest. Molecules. 2016;21:137. doi: 10.3390/molecules21020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gyemant G, Zajacz A, Becsi B, Ragunath C, Ramasubbu N, Erdodi F, et al. Evidence for pentagalloyl glucose binding to human salivary alpha-amylase through aromatic amino acid residues. Biochim Biophys Acta. 2009;1794:291–6. doi: 10.1016/j.bbapap.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12. Amiri A, Azemi ME, Khodayar MJ, Namjoyan F. In vitro α-amylase and α-glucosidases inhibitory effects of some plant extracts. Int J Pharmacogn Phytochem Res. 2015;7:315–8. [Google Scholar]
- 13. Gholamhoseinian A, Shahouzehi B, Sharifi-Far F. Inhibitory effect of some plant extracts on pancreatic lipase. Int J Pharmacol. 2010;6:18–24. [Google Scholar]
- 14. Buchholz T, Melzig MF. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus – screening for pancreatic lipase and α-amylase inhibition. Phytother Res. 2016;30:260–6. doi: 10.1002/ptr.5525. [DOI] [PubMed] [Google Scholar]
- 15. Piwowarski J, Waltenberger B, Stuppner H, Kiss A, Granica S. The analysis of phenolic compounds from the aerial parts of Eupatorium cannabinum L. subsp. cannabinum. Biochem Sys Ecol. 2018;79:37–43. [Google Scholar]
- 16. Kiss AK, Mańk M, Melzig MF. Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. J Ethnopharmacol. 2008;120:220–5. doi: 10.1016/j.jep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 17. Granica S, Czerwińska M, Żyżyńska-Granica B, Kiss A. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia. 2013;91:180–8. doi: 10.1016/j.fitote.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowski L. Klucz do oznaczania roślin naczyniowych Polski niżowej. 2 ed. Warszawa: Wydawnictwo Naukowe PWN; 2011. [Google Scholar]
- 19. Markiewicz M. Właściwości antyoksydacyjne owoców bażyny czarnojagodowej, borówki brusznicy, latorośli winnej, żurawiny błotnej a zawartośćzwiązków polifenolowych i witaminy C. Akademia Medyczna Warszawa. 2000 [Google Scholar]
- 20. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999;299:152–78. [Google Scholar]
- 21. Di L, Zu L, Wang K, Zhao Y, Wang Z. Three new iridoid glucosides from the roots Patrinia scabra. Bull Korean Chem Soc. 2011;32:3251–4. [Google Scholar]
- 22. Hatano T, Yasuhara T, Abe R, Okuda T. A galloylated monoterpene glucoside and a dimeric hydrolysable tannin from Cornus officinalis. Phytochemistry. 1990;29:2975–8. [Google Scholar]
- 23. Shimomura H, Sashida Y, Adachi T. Phenolic glucosides from Prunus grayana. Phytochemistry. 1987;26:249–51. [Google Scholar]
- 24. Deng S, West BJ, Jensen CJ. UPLC-TOF-MS characterization and identification of bioactive iridoids in Cornus mas fruit. J Anal Methods Chem. 2013;2013:710972. doi: 10.1155/2013/710972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. West BJ, Deng S, Jensen CJ, Palu AK, Berrio LF. Antioxidant, toxicity, and iridoids tests of processed Cornelian cherry fruits. Int J Food Sci Technol. 2012;47:1392–7. [Google Scholar]
- 26. Seeram NP, Schutzki R, Chandra A, Nair MG. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J Agric Food Chem. 2002;50:2519–23. doi: 10.1021/jf0115903. [DOI] [PubMed] [Google Scholar]
- 27.Kucharska AZ. Związki aktywne owoców derenia (Cornus mas L. Wrocław: Wrocław University of Environmental and Life Sciences; 2012. [Google Scholar]
- 28. Stankovic MS, Zia-Ul-Haq M, Bojovic BM, Topuzovic MD. Total phenolics, flavonoid content and antioxidant power of leaf, flower and fruits from cornelian cherry (Cornus mas L.) Bulg J Agric Sci. 2014;20:358–63. [Google Scholar]
- 29. Moldovan B, David L. Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules. 2014;19:8177–88. doi: 10.3390/molecules19068177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Özkan M, Yemenicioglu A, Asefi N, Cemeroglu B. Degradation kinetics of anthocyanins from sour cherry, pomegranate, and strawberry juices by hydrogen peroxide. J Food Sci. 2002;67:525–9. [Google Scholar]
- 31. Cevallos-Casalas B, Cisneros-Zevallos L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004;86:69–77. [Google Scholar]
- 32. Moldovan B, David L, Chişbora C, Cimpoiu C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. Effects of temperature, pH and storage solvent. Molecules. 2012;17:11655–66. doi: 10.3390/molecules171011655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans, and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10:266–75. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- 34. Moreno DA, Ilic N, Poulev A, Raskin I. Effects of Arachis hypogaea nutshell extract on lipid metabolic enzymes and obesity parameters. Life Sci. 2006;78:2797–803. doi: 10.1016/j.lfs.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35. Sebban-Kreuzer C, Ayvazian L, Juhel C, Salles JP, Chapus C, Kerfelec B. Inhibitory effect of the pancreatic lipase C-terminal domain on intestinal lipolysis in rats fed a high-fat diet: chronic study. Int J Obes Relat Metab Disord – J Int Assoc Study Obesity. 2003;27:319–25. doi: 10.1038/sj.ijo.0802245. [DOI] [PubMed] [Google Scholar]
- 36. Tan Y, Chang SKC. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J Func Foods. 2017;38:644–55. [Google Scholar]
- 37. Mopuri R, Ganjayi M, Meriga B, Koorbanally NA, Islam MS. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J Food Drug Anal. 2018;26:201–10. doi: 10.1016/j.jfda.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon SH, Ahn IS, Kim SO, Kong CS, Chung HY, Do MS, et al. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J Med Food. 2007;10:552–6. doi: 10.1089/jmf.2006.147. [DOI] [PubMed] [Google Scholar]
- 39. Valcheva-Kuzmanova S, Kuzmanov K, Tancheva S, Belcheva A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol. 2007;29:101–5. doi: 10.1358/mf.2007.29.2.1075349. [DOI] [PubMed] [Google Scholar]
- 40. Jurgoński A, Juśkiewicz J, Zduńczyk Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods Hum Nutr. 2008;63:176–82. doi: 10.1007/s11130-008-0087-7. [DOI] [PubMed] [Google Scholar]
- 41.Haq MZU, Riaz M, Saad B. Anthocyanins and human health: biomolecular and therapeutic aspects. Springer; 2016. [Google Scholar]
- 42. Oboh G, Ogunsuyi OB, Ogunbadejo MD, Adefegha SA. Influence of gallic acid on alpha-amylase and alpha-glucosidase inhibitory properties of acarbose. J Food Drug Anal. 2016;24:627–34. doi: 10.1016/j.jfda.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]